Abstract
Objective:
The aim of this study was to test the effectiveness of a tailored quitline tobacco treatment (‘Quitlink’) among people receiving support for mental health conditions.
Methods:
We employed a prospective, cluster-randomised, open, blinded endpoint design to compare a control condition to our ‘Quitlink’ intervention. Both conditions received a brief intervention delivered by a peer researcher. Control participants received no further intervention. Quitlink participants were referred to a tailored 8-week quitline intervention delivered by dedicated Quitline counsellors plus combination nicotine replacement therapy. The primary outcome was self-reported 6 months continuous abstinence from end of treatment (8 months from baseline). Secondary outcomes included additional smoking outcomes, mental health symptoms, substance use and quality of life. A within-trial economic evaluation was conducted.
Results:
In total, 110 participants were recruited over 26 months and 91 had confirmed outcomes at 8 months post baseline. There was a difference in self-reported prolonged abstinence at 8-month follow-up between Quitlink (16%, n = 6) and control (2%, n = 1) conditions, which was not statistically significant (OR = 8.33 [0.52, 132.09] p = 0.131 available case). There was a significant difference in favour of the Quitlink condition on 7-day point prevalence at 2 months (OR = 8.06 [1.27, 51.00] p = 0.027 available case). Quitlink costs AU$9231 per additional quit achieved.
Conclusion:
The Quitlink intervention did not result in significantly higher rates of prolonged abstinence at 8 months post baseline. However, engagement rates and satisfaction with the ‘Quitlink’ intervention were high. While underpowered, the Quitlink intervention shows promise. A powered trial to determine its effectiveness for improving long-term cessation is warranted.
Keywords: Tobacco treatment, smoking cessation, quitline, telephone counselling, peer worker, mental ill-health, severe mental ill-health, cost analysis
Introduction
Although tobacco control measures, particularly when implemented comprehensively, have resulted in declines in smoking across many parts of the world, not all population groups have benefitted to the same degree (Dai et al., 2022). In Australia, tobacco smoking rates among people experiencing mental health conditions remain between two and five times higher than those without mental health conditions, with prevalence of smoking increasing with severity of mental health conditions (Australian Bureau of Statistics, 2018, 2021). Similar disparities in tobacco smoking rates have been reported in the United States (Dickerson et al., 2018; Szatkowski and McNeill, 2015). Although people experiencing mental health conditions are just as likely to make quit attempts and are even more likely to use cessation aids, they report less success in their quitting efforts (Twyman et al., 2014; Schwindt et al., 2017). Consequently, many people living with mental health conditions experience poorer quality of life (Dixon et al., 2007) and die prematurely from smoking-caused diseases (Bandiera et al., 2015).
There is accumulating evidence that people experiencing mental health conditions benefit from telephone-delivered interventions for health behaviour change, including tobacco smoking, though there is a paucity of evidence of cost-effectiveness (Baker et al., 2018). Proactive telephone counselling for tobacco dependence from quitlines has demonstrated effectiveness in the general population (Matkin et al., 2019). A recent review argued that quitlines could have high impact among people with mental health conditions who smoke, given their potential reach, utility and effectiveness when they are tailored to meet the special needs of this group (Berg, 2021). In existing randomised controlled trials (RCTs) in smokers with mental health conditions, tailoring of quitline interventions has included adding a mood management component among people with a past history of depression (Van der Meer et al., 2010); community-based group counselling focussing on smoking cessation among people attending community mental health centres (Morris et al., 2011) and more post-quit sessions for veterans attending mental health clinics (Rogers et al., 2016). Despite this accumulating evidence, few people who smoke, including those with mental health conditions, contact quitlines (Berg, 2021; Greenhalgh et al., 2022). To address this, we developed a tailored quitline intervention (known as ‘Quitlink’). Quitlink added dedicated counsellors plus 8 weeks combination nicotine replacement therapy (cNRT) to Quitline’s existing service in the state of Victoria, Australia, for people experiencing mental health conditions. Quitline’s existing service encourages use of mood management strategies that dually aid cessation and monitor nicotine withdrawal and medication side effects to help distinguish temporary withdrawal symptoms from psychiatric symptoms and to facilitate targeted treatment (Segan et al., 2017). We also engaged peer researchers, with their own experience of recovery from mental health conditions and tobacco smoking, and skills obtained from formal mental health peer support worker and research training (Byrne et al., 2021; Meagher and Stratford, 2018). Peer researchers facilitated recruitment, delivered the control intervention and proactively referred participants to quitline if randomly allocated to ‘Quitlink’ (Baker et al., 2019). A detailed description of the protocol that includes the rationale for a tailored approach has been previously reported (Baker et al., 2019).
This paper reports on the effectiveness and cost-effectiveness of Quitlink for cessation of tobacco smoking among people experiencing mental health conditions. It was hypothesised that Quitlink would be associated with higher rates of prolonged abstinence from tobacco smoking since the end of the treatment period (i.e. 6 months sustained abstinence) at 8-month post-baseline follow-up relative to the control condition. Secondary aims were to examine the effect of Quitlink on 7-day point prevalence abstinence and effects on cigarette consumption, quitting behaviours, other substance use, mental health and health-related quality of life (HRQL).
Methods
Design
We used a prospective, cluster-randomised, open, blinded endpoint (PROBE) design to compare a control condition with Quitlink.
Inclusion/exclusion criteria
Participants were required to be smoking at least 10 cigarettes a day and accessing treatment or support from participating mental health agencies in Victoria (Mind Australia and St Vincent’s Mental Health). Exclusion criteria were current engagement in Quitline Victoria’s callback service; no ready access to a telephone; inability to complete informed consent and the screening survey; acute suicidality; contraindications to NRT and pregnancy. When online recruitment commenced (described below), inclusion criteria were expanded to include anyone in Victoria accessing treatment or support, including from their general practitioner, for a mental health, and alcohol or other drug use condition.
Recruitment and consent
Study recruitment occurred between March 2019 and April 2021, with the 8-month post-baseline follow-up finalised in December 2021. As described in detail elsewhere (Baker et al., 2019; Sweeney et al., 2019), peer-facilitated recruitment strategies were adapted from face-to-face to direct mail (postcard) and online due to lower than expected recruitment, in part due to the impact of COVID-19 pandemic restrictions (Baker et al., 2022). Following provision of informed consent, baseline data were collected at enrolment within Research Electronic Data Capture (REDCap; Harris et al., 2009, 2019) via an iPad. Participants recruited online self-completed their baseline assessment at enrolment and follow-up assessments, with the exception of follow-up safety and diagnostic data which were collected via telephone. Participants received an AU$40 gift card on completion of baseline and each completed follow-up assessment.
Randomisation
Procedures for blinding and randomisation were as described previously (Baker et al., 2019). Cluster randomisation was used in residential services where risk of contamination was higher, stratified by short- or long-term residence, with 1:1 allocation. Individual randomisation was used in community-based services where contamination risk was lower, via permutated block sizes of 4 and 6 to avoid incomplete blocks, stratified for site. Randomisation was managed by an independent statistician and the randomisation module was embedded in REDCap.
Control condition
The control condition consisted of a peer researcher-delivered brief intervention (following baseline assessment and prior to randomisation) that included advice to quit, encouragement to use cNRT and to call Quitline, and provision of a Quit pack of written materials to motivate a quit attempt and support self-management. With consent, a letter was sent by the research team to health professionals and the participant nominated with information about the person’s trial participation, and a link to Australia’s smoking cessation guidelines for health professionals, including a list of medications affected by smoking.
Intervention
The Quitlink intervention consisted all of the above and the following:
A proactive referral to Quitline immediately following the brief intervention.
Tailored and manual-guided Quitline counselling for people experiencing mental health conditions. A team of six Quitlink counsellors was trained to deliver the intervention. Specific to the Quitlink intervention, Quitlink offered continuity of care through having one counsellor allocated to each participant. Procedures for training and supervision of quitline counsellors and peer researchers have been reported previously (Baker et al., 2019). The counselling was based on cognitive behavioural principles and offered up to seven calls within an 8 week period (with additional calls allowed to deal with relapse crises and beyond if needed) and provision of written feedback to treatment providers at the end of the Quitline counselling programme.
As in the control condition, with consent, a letter was sent to the person’s GP or psychiatrist. In addition, for the intervention condition, peer-reviewed articles that provide practical advice to assist doctors in helping people with mental illness to quit smoking were included.
In addition, participants received a Quit Victoria brochure for carers and a Quitting Mood and Experiences Diary.
Participants were offered up to 8 weeks of cNRT (patches plus an oral form of NRT [Baker et al., 2019]).
Outcome measures
The complete list of outcome measures and assessment schedule can be found in Table 1. Key demographic, smoking (including 7-day point prevalence abstinence), alcohol and cannabis use, mental health and HRQL outcome measures are reported in this paper.
Table 1.
Outcomes and assessment schedule.
| Baseline | 2 months | 5 months | 8 months | |
|---|---|---|---|---|
| Demographics | X | |||
| Mental ill-health or AOD diagnosis | ||||
| Self-report – Have you ever received a diagnosis of a mental health or drug and alcohol problem? | X | |||
| MINI (diagnostic interview) (Sheehan et al., 1998) | T | * | * | |
| McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD) (Zanarini et al., 2003) | T | * | * | |
| Medications | ||||
| Current medications | X | |||
| Smoking measures: | ||||
| Current smoking and quit attempts | X | X | X | X |
| 7-day point prevalence abstinence | X | X | X | |
| 6-month prolonged abstinence (primary outcome) | X | |||
| Heaviness of smoking index (Heatherton et al., 1989; Kozlowski et al., 1994) | X | S | S | S |
| Tobacco types | X | |||
| Expenditure on cigarettes | X | S | S | S |
| History (age first smoked) | X | |||
| Social influences on smoking, e.g., lives with other smokers | X | |||
| Cravings (Herd and Borland, 2009; Herd et al., 2009) – Currently, how often do you get strong cravings to smoke tobacco? | X | X | X | X |
| Situations not allowed to smoke | X | X | X | |
| Goal | X | |||
| Motivation to quit (Crittenden et al., 1994) | X | S | S | S |
| Confidence to quit – How confident are you that you can stop smoking for good in the next 2 months if you wanted to? | X | |||
| Self-efficacy (Perkins et al., 2012) – How confident are you that you will not smoke at all tomorrow? | X | X | X | |
| Products/services to help quit (including NRT, Quitline) | X | X | X | X |
| Nicotine replacement products (helpfulness, concern) | X | |||
| Counselling preferences | X | |||
| Mental health: | ||||
| Psychological distress (Kessler-10) (Kessler et al., 2003) | X | X | X | X |
| Substance use: | ||||
| Alcohol (AUDIT-C) (Bush et al., 1998) | X | X | X | X |
| Cannabis use with tobacco question | X | X | X | X |
| Cannabis (First question of CUDIT [Adamson et al., 2010]) | X | X | X | X |
| HRQOL: | ||||
| EQ-5D (Herdman et al., 2011) + four AQol-8D (Richardson et al., 2014) psychosocial bolt-on questions^ | X | X | X | X |
| Medications – NRT/cessation: | ||||
| Process measure (i.e. provided by intervention) | E | |||
| Perceived support – GP, psychiatrist, other health professional | X | |||
| Quitline use: | ||||
| Number, length, content and timing of and satisfaction with calls | E | |||
| Service use | ||||
| Hospitalisations and other intensive health service use | X | X | ||
| Time off from work and usual duties | X | X | X | |
| Financial stress questions adapted from Siahpush and Carlin (2006) | X | X | X | X |
| Therapeutic alliance: | X | |||
| WAIT-3 (Warlick et al., 2018) | X# | |||
| Peer worker brief intervention question | X | |||
| PBS/MBS cost data | E |
AUDIT-C: Alcohol Use Disorders Identification Test – Brief; AQoL-8D: Assessment of Quality of Life-8D; CUDIT-R: Cannabis Use Disorders Identification Test – Revised; CO: carbon monoxide; EQ-5D: EuroQoL 5-deimension; HRQL: Health-related quality of life; Kessler-10: Kessler Psychological Distress Scale; MINI: Mini International Neuropsychiatric Interview; NRT: nicotine replacement therapy; GP – general practitioner; WAIT-3: Working Alliance Inventory for Tobacco-3; MBS: Medicare Benefits Scheme; PBS: Pharmaceutical Benefits Scheme.
Key
– If not captured at previous assessment.
E – Extracted data.
S – Current smokers.
# – For those who used Quitline.
T – Via telephone if a participant recruited online.
– To minimise participant survey burden as we moved away from face-to-face recruitment, we transitioned from capturing HRQL using the AQoL-8D instrument, which has strong psychosocial dimension properties, to the EQ-5D-5L plus four AQol-8D bolt-on questions (Baker et al., 2022). These can be used in combination to calculate HRQL utilities and has been shown to be comparable to the AQOL-8D (Chen and Olsen, 2020)
Primary outcome
Due to COVID-19 restrictions on in-person contact, the primary outcome was modified from biochemically (carbon monoxide, CO) verified to self-reported prolonged abstinence from tobacco smoking since the end of the treatment period (i.e. 6 months sustained abstinence, with no relapse, defined as seven or more days of continuous tobacco smoking and no reported tobacco smoking in the prior week) at 8-month post-baseline follow-up.
Secondary outcomes
Secondary outcomes were assessed by research assistants blinded to group allocation and intervention content via telephone. The assessment was abbreviated to reduce participant burden (removed assessment of smoking use motives [Cooper et al., 1992; Spencer et al., 2002], nicotine withdrawal symptoms [Toll et al., 2007] and changed HRQL instrument – see Table 1 note) as we transitioned from face-to-face to telephone then online recruitment due to COVID-19 restrictions.
Adverse events were coded according to the Medical Dictionary for Regulatory Activities codes (MedDRA®). MedDRA is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
Sample size and statistical analysis
Based on our previous study (Baker et al., 2006; Segan et al., 2011) and rates of cessation among those with more severe mental health conditions (Baker et al., 2006), we anticipated that prolonged abstinence would occur in 1% of the control arm vs 8% in Quitlink. To detect this effect with 80% power at p = 0.05, we required 134 participants per arm. We expected ~30% attrition and thus planned to recruit 382 smokers over 36 months.
All statistical analyses were completed using SAS v9.4 (SAS Institute Inc. Cary, NC, USA [SAS Institute, 2013]). Statistical significance was set a priori at p < 0.05.
Analysis of primary and secondary outcomes was carried out using a cluster randomised trial framework where the individuals recruited from community-based services were treated as clusters that contributed only one person, while individuals recruited from residential programmes were clustered together, i.e., called a split-plot design (Goulão et al., 2018). There was a total of 95 clusters (11 residential and 84 community-based participants of whom 14 were from St Vincent’s Hospital, 56 from Mind Centres for Mental Health and Wellbeing and 14 recruited online, equally distributed across both conditions). Of the 55 in the control condition, 42 participants were individual clusters and 13 were recruited across 6 other clusters. Of the 54 in the intervention condition, 42 were individual clusters and 12 were recruited across 5 other clusters.
Outcomes were modelled using generalised linear mixed models with linear regressions used for continuous outcomes, logistic regressions for dichotomous outcomes and ordinal logistic regression for ordinal outcomes. Mixed models handled the cluster and repeated measures at baseline, 2-, 5- and 8-month post-baseline follow-up periods. Within the models, individuals and clusters were modelled as random effects to account for the non-independence of measurements from the same individual and cluster, with group assignment and study time point – and their interaction – as fixed effects.
Mixed models allow for missing data under a missing at random assumption, but a sensitivity analysis using a worst-case scenario for smoking outcomes (intention to treat [ITT], missing imputed as smoking in the case of abstinence outcomes and not using NRT for the NRT use outcome) was also performed. In the case of continuous abstinence, participants who were smoking at 2 months post baseline were unable to meet the required criteria for abstinence at either the 5- or 8-month follow-up and as such were counted as not continuously abstinent even if not followed up.
When modelling continuous abstinence and 7-day point prevalence abstinence, study design effects (stratification and clustering) were not controlled for, due to lack of model convergence because of the high proportion of single-case clusters. As such, presented results for these outcomes should be interpreted cautiously.
Safety analysis used chi-square tests to compare percentage of participants in the Quitlink and control conditions experiencing adverse events by System Organ Class (SOC).
Cost-effectiveness analysis
As described previously (Sweeney et al., 2019), a within-trial cost-effectiveness analysis (CEA) was conducted, estimating the incremental cost-effectiveness ratios (ICERs) for (1) additional quit (primary outcome) and (2) quality-adjusted life years (QALYs) gained. Full details are presented in Supplementary Materials – CEA. QALYs were estimated by multiplying the HRQL utility scores by the number of months since last surveyed (2 or 3 months). The underpowered sample required some deviations from the planned protocol (discussed in Supplementary Materials) (Sweeney et al., 2019). Most notably, the within-trial analysis takes a more focused intervention implementation perspective, excluding indirect health resource utilisation costs incurred during the 8 months of follow-up. Costs associated with control and Quitlink were determined for each person using Quitline (staff time and call costs) and cNRT costs. We also included postcard mail outs as costs, as this is a likely strategy for reaching potential clients beyond the trial (see Baker et al., 2022). Costs were calculated in AUD 2021. Sensitivity analyses were conducted for both ICERs, and longer-term cost-effectiveness was also modelled (see Supplementary materials for further details and analyses).
Results
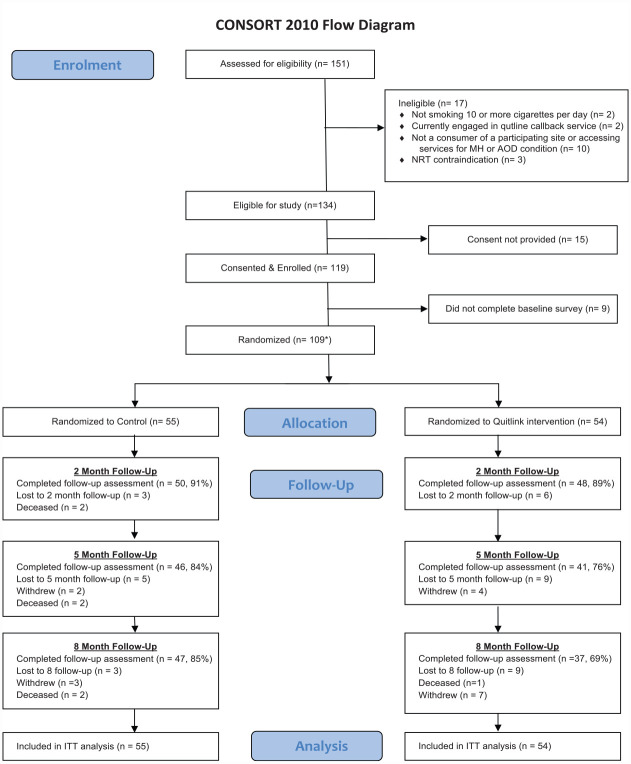
A total of 110 of our target sample of 382 people completed consent procedures and baseline assessments and were randomised (29 from face-to-face, 67 from direct mail postcard and 14 from online advertisement). One person subsequently withdrew from the study (including their baseline data), leaving a total sample of 109 people. Recruitment ended after 26 months in line with our grant timeline prior to achieving our target sample. As can be seen in Figure 1, significantly fewer participants assigned to the Quitlink condition completed the 8-month post-baseline follow-up compared to those in the control condition (p = 0.036). Participants who completed the 8-month follow-up were slightly older and somewhat more likely to have been recruited from a community-based service (Supplementary Table 1).
Figure 1.
Consort diagram.
Demographic and baseline clinical variables of the two conditions are shown in Table 2. About half the sample was female, with an average age of approximately 45 years, and most were receiving disability support benefits. Most had a diagnosis of a psychotic disorder, about half consumed alcohol above national guideline recommendations, while a quarter had used cannabis in the previous month. Moderate-to-high tobacco dependence on the HSI (Heatherton et al., 1989; Kozlowski et al., 1994) was evidenced by mixed tobacco consumption with people smoking around 20 cigarettes per day. Although almost all had made a quit attempt at some time, with about half trying in the past year, confidence in quitting was low (see Table 2). As described earlier, when online recruitment commenced, inclusion criteria were expanded to include anyone in Victoria accessing treatment or support, including from their general practitioner, for a mental health, and alcohol or other drug use condition. However, 9 of the 14 participants recruited online reported an alcohol or other drug use condition (n = 7 had a co-occurring mental health condition).
Table 2.
Baseline participant characteristics by intervention allocation.
| Variable | Control | Quitlink | Total |
|---|---|---|---|
| (n = 55) | (n = 54) | (N = 109) | |
| Gender (female), n (%) | 29 (52.7) | 27 (50.0) | 56 (51.4) |
| Age (years), M (SD) | 43.56 (13.26) | 46.26 (12.82) | 44.89 (13.05) |
| Married/de facto, n (%) | 9 (16.4) | 7 (13.0) | 16 (14.7) |
| Unemployed, n (%) | 36 (65.5) | 28 (51.9) | 64 (58.7) |
| Receiving disability support benefit, n (%) | 35 (63.6) | 32 (59.3) | 67 (61.5.0) |
| Left school at or before 16 years, n (%) | 22 (40.0) | 13 (24.1) | 35 (32.1) |
| Supported residential accommodation, n (%) | 13 (23.6) | 12 (22.2) | 25 (22.9) |
| Age started smoking (years), M (SD) | 16.11 (4.34) | 17.02 (7.03) | 16.65 (5.83) |
| Types of tobacco used, n (%) | |||
| Cigarettes | 43 (78.2) | 40 (74.1) | 83 (76.1) |
| Pouch | 24 (43.6) | 30 (55.6) | 54 (49.5) |
| Bulk | 16 (29.1) | 11 (20.4) | 27 (24.8) |
| Butts left behind | 10 (18.2) | 15 (27.8) | 25 (22.9) |
| Cigarettes per day, M (SD) | 21.29 (9.99) | 20.30 (9.56) | 20.80 (9.75) |
| HSI addiction category, n (%) | |||
| Low | 6 (10.9) | 7 (13.0) | 13 (11.9) |
| Moderate | 37 (67.3) | 35 (64.8) | 72 (65.1) |
| High | 12 (21.8) | 12 (22.2) | 24 (22.0) |
| Quit attempt (ever), n (%) | 45 (81.8) | 51 (94.4) | 96 (88.1) |
| Quit attempt in last year, n (%) | 23 (41.8) | 27 (50.0) | 50 (45.9) |
| Currently trying to cut down, n (%) | 46 (83.6) | 41 (75.9) | 87 (79.8) |
| Motivation to quit, n (%) | |||
| Not at all | 3 (5.5) | 2 (3.7) | 5 (4.6) |
| A little | 2 (3.6) | 4 (7.4) | 6 (5.5) |
| Some | 10 (18.2) | 8 (14.8) | 18 (16.5) |
| Very much | 40 (72.7) | 40 (74.1) | 80 (73.4) |
| Main goal to quit smoking in next 2 months | 36 (65.5) | 34 (63.0) | 70 (64.2) |
| Confidence in quitting in next 2 months, n (%) | |||
| Not at all | 19 (34.5) | 14 (25.9) | 33 (30.3) |
| Somewhat | 13 (23.6) | 13 (24.1) | 26 (23.8) |
| Moderately | 12 (21.8) | 17 (31.5) | 29 (26.6) |
| Very | 3 (5.5) | 7 (13.0) | 10 (9.2) |
| Extremely | 8 (14.5) | 3 (5.5) | 11 (10.1) |
| How helpful will NRT be in helping quit? n (%) | |||
| Not at all | 10 (18.2) | 4 (7.3) | 14 (12.8) |
| Somewhat helpful | 21 (38.2) | 23 (42.6) | 44 (40.4) |
| Moderately helpful | 11 (20.0) | 16 (29.6) | 27 (24.8) |
| Extremely helpful | 13 (23.6) | 11 (20.4) | 24 (22.1) |
| Likelihood of using NRT products in the long term, n (%) | |||
| Not at all likely | 18 (32.7) | 12 (22.2) | 30 (27.5) |
| Somewhat likely | 11 (20.0) | 13 (24.1) | 24 (22.1) |
| Moderately likely | 14 (25.5) | 12 (22.2) | 26 (23.9) |
| Extremely likely | 12 (21.8) | 17 (31.5) | 29 (26.6) |
| Preference for in-person or telephone counselling for smoking, n (%) | |||
| Face-to-face | 16 (29.1) | 16 (29.6) | 32 (29.4) |
| Telephone | 20 (36.4) | 21 (38.9) | 41 (37.6) |
| No preference | 19 (34.5) | 17 (31.5) | 36 (33.1) |
| K10, M (SD) | 29.04 (8.33) (n = 54) | 26.39 (8.05) (n = 49) | 27.78 (8.27) (N = 103) |
| MINI diagnosis (psychotic disorder), n (%) | 32 (65) (n = 49) | 27 (62.8) (n = 43) | 53 (64.13) (N = 92) |
| AUDIT C % excessive drinker, n (%) (available case) | 22 (41.0) (n = 54) | 28 (52.8) (n = 53) | 50 (46.7) (N = 107) |
| Cannabis use, n (%) | |||
| Never | 39 (72.2) (n = 54) | 41 (75.9) (n = 54) | 80 (74.1) (N = 108) |
| Monthly or less | 8 (14.8) | 4 (7.4) | 12 (11.1) |
| 2–4 times a month | 3 (5.6) | 6 (11.1) | 9 (8.3) |
| 2–3 times a week | 0 (0) | 0 (0) | 0 (0) |
| 4 or more times a week | 4 (7.4) | 3 (5.6) | 7 (6.5) |
| Always or nearly always mix tobacco with cannabis, n (%) | 12 (80.0) | 9 (69.2) | 21 (75.0) |
| HRQOL (ITT), M (SD) | 0.50 (0.20) | 0.55 (0.20) | 0.52 (0.20) |
HSI: Heaviness of Smoking Index (Heatherton et al., 1989; Kozlowski et al., 1994); MINI: Mini International Neuropsychiatric Interview (Sheehan et al., 1998); K10: Kessler Psychological Distress Scale (Kessler et al., 2003); AUDIT C: Alcohol Use Disorders Identification Test-Brief (Bush et al., 1998); HRQL: Health-related quality of life
Descriptive characteristics of interventions received
Of the 54 participants allocated to the Quitlink intervention condition, 48 agreed to proactive referral to quitline and 45 received Quitlink calls. However, 6 of the 55 participants allocated to the control condition (11%) contacted Victoria’s Quitline and received Quitline’s standard tailored counselling for people with mental health conditions (no cNRT or dedicated counsellor). Number and duration of and satisfaction with quitline calls and the peer researcher brief intervention are presented in Table 3. All participants in the Quitlink condition were interested in receiving free NRT.
Table 3.
Number and duration of and satisfaction with quitline calls and the peer researcher brief intervention.
| Characteristic | Condition | Variables | ||
|---|---|---|---|---|
| Total number and duration of quitline calls and days from first to last call between baseline and 2-month follow-up assessment | Call number | Minutes | Days | |
| M (SD), | M (SD), | M (SD), | ||
| Range | Range | Range | ||
| Control | 2.20 (1.30) | 29.00 (15.75) | 6.75 (4.11) | |
| 1–4 (n = 5) | 11–52 (n = 5) | 3–12 (n = 4) | ||
| Quitlink | 5.20 (3.16) | 173.27 (131.04) | 40.05 (17.07) | |
| 1–15 (n = 45) | 13–673 (n = 44) | 1–66 (n = 38) | ||
| Total number and duration of extra quitline calls received and days from the first to last extra call between the 2- and 8-month assessment dates | Call number | Minutes | Days | |
| M (SD), | M (SD), | M (SD), | ||
| Range | Range | Range | ||
| Control | 2.67 (1.53) | 64.33 (22.03) | 60.00 (32.60) | |
| 1–4 (n = 3) | 39–79 (n = 3) | 26–91 (n = 3) | ||
| Quitlink | 3.83 (3.26) | 86.89 (123.05) | 112.00 (56.40) | |
| 1–12 (n = 18) | 2–509 (n = 18) | 56.00–245.00 | ||
| Total number and duration of calls received from baseline to 8 months | Call number | Minutes | ||
| M (SD), | M (SD), | |||
| Range | Range | |||
| Control | 3.33 (2.07) | 56.50 (43.19) | ||
| 1–7 (n = 6) | 11–131 (n = 6) | |||
| Quitlink | 6.73 (5.22) | 208.86 (189.13) | ||
| 1–22 (n = 45) | 13–827 (n = 44) | |||
| Total number of unsuccessful call attempts from baseline to 8 months | M (SD) | |||
| Range | ||||
| Control | 5.00 (5.10) | |||
| 1–15 (n = 6) | ||||
| Quitlink | 10.85 (6.28) | |||
| 2–27 (n = 48) | ||||
| Total number of calls and duration of calls to a health practitioner from baseline to 8 months | Call number | Call duration | ||
| M (SD), | M (SD), | |||
| Range | Range | |||
| Quitlink only | 2.00 (0.82) | 15.0 (8.03) | ||
| 1–3 (n = 4) | 4.39–23 (n = 4) | |||
| Response | Controln (%) | Quitlinkn (%) | ||
| Satisfaction with quitline (2 months) | (n = 4) | (n = 32) | ||
| Counsellor provided quitting information and strategies that were relevant | Not at all | 0 (0.0) | 2 (6.3) | |
| A little | 1 (25.0) | 3 (9.4) | ||
| Moderately | 2 (50.0) | 3 (9.4) | ||
| Mostly | 0 (0.0) | 4 (12.5) | ||
| Very much so | 1 (25.0) | 20 (62.5) | ||
| Satisfaction with the service received from quitline | Not at all | 0 (0.0) | 3 (9.4) | |
| A little | 0 (0.0) | 0 (0.0) | ||
| Moderately | 2 (50.0) | 2 (6.3) | ||
| Mostly | 1 (25) | 3 (9.4) | ||
| Very much so | 1 (25) | 24 (75.0) | ||
| Would call the quitline in future if wanting help with quitting | No | 1 (25.0) | 6 (19.0) | |
| Yes | 3 (75.0) | 25 (78.0) | ||
| Don’t know | 0 (0.0) | 1 (3.1) | ||
| Would recommend quitline to others | Definitely not | 0 (0.0) | 2 (6.3) | |
| Probably not | 0 (0.0) | 2 (6.3) | ||
| Maybe | 0 (0.0) | 1 (3.1) | ||
| Probably | 2 (50.0) | 2 (6.3) | ||
| Definitely | 2 (50.0) | 25 (78.1) | ||
| Level of comfort discussing smoking over the phone | Not at all | 0 (0.0) | 1 (3.1) | |
| Somewhat | 1 (25.0) | 1 (3.1) | ||
| Moderately | 0 (0.0) | 4 (12.5) | ||
| Mostly | 0 (0.0) | 6 (18.8) | ||
| Completely | 3 (75.0) | 20 (62.5) | ||
| Helpfulness of conversation 2 months ago with peer worker in encouraging you to try to stop smoking or cut down | Not at all helpful | 1 (2.5) | 0 (0.0) | |
| A little helpful | 8 (20.0) | 5 (13.2) | ||
| Moderately helpful | 8 (20.0) | 6 (15.8) | ||
| Very helpful | 23 (57.5) | 27 (71.1) | ||
| n = 40 | n = 38 | |||
As can be seen in Table 3, between baseline and 8 months, Quitlink callers who received proactive quitline calls received twice the number and duration of calls compared to the small number (n = 6) in the control condition who chose to contact quitline. Table 3 also shows that satisfaction with quitline counsellors was higher in the Quitlink condition. Of those who had a conversation with a peer researcher (n = 78), most participants (82%) also rated their conversation (brief intervention) with a peer researcher as moderately or very helpful in encouraging change. As can be seen in Table 4, 36% in the control condition and 48% in the intervention condition reported using NRT in the past week at 2-month post-baseline follow-up (p = 0.243).
Table 4.
Smoking outcomes.
| Measure | Assessment occasion | Control, n (%) | Quitlink, n (%) | OR (95% CI) between conditions | p |
|---|---|---|---|---|---|
| Continuous abstinence (N = 91) (available case^) | 5 months (i.e. 3 months continuous) | 1 (2) | 6 (14) | 8.33 [0.52, 132.09] | 0.131 |
| 8 months (i.e. 6 months continuous) | 1 (2) | 6 (16) | 8.33 [0.52, 132.09] | 0.131 | |
| 7-day point prevalence abstinence (N = 102) (available case) | 2 months | 2 (4) | 11 (22) | 8.06 [1.27, 51.00] | 0.027 |
| 5 months | 6 (13) | 10 (24) | 2.21 [0.52, 9.41] | 0.284 | |
| 8 months | 6 (13) | 9 (24) | 2.12 [0.48, 9.39] | 0.32 | |
| Using NRT within the last week (ITT) | Baseline | 11 (20) | 10 (19) | ||
| 2 months | 18 (36) n = 50 | 23 (48) n = 48 | 1.80 [0.67, 4.81] | 0.243 | |
| 5 months | 16 (35) n = 46 | 14 (34) n = 41 | 0.96 [0.33, 2.82] | 0.945 | |
| 8 months | 11 (23) n = 47 | 8 (22) n = 37 | 0.96 [0.29, 3.23] | 0.953 | |
| Sensitivity analysis# | |||||
| 1.51 [0.58, 3.90] | 0.395 | ||||
| 0.79 [0.28, 2.18] | 0.643 | ||||
| 0.70 [0.22, 2.23] | 0.54 | ||||
| Using NRT or pharmacotherapy (e-cigarettes or stop smoking medication) within the last week (ITT) | Baseline | 14 (25) | 14 (26) | ||
| 2 months | 23 (46) n = 50 | 24 (50) n = 48 | 1.17 [0.47, 2.89] | 0.739 | |
| 5 months | 19 (41) n = 46 | 17 (41) n = 41 | 0.92 [0.35, 2.45] | 0.87 | |
| 8 months | 15 (32) n = 47 | 8 (22) n = 37 | 0.57 [0.19, 1.73] | 0.323 | |
| Sensitivity analysis# | |||||
| 1.02 [0.43, 2.44] | 0.965 | ||||
| 0.78 [0.31, 1.96] | 0.589 | ||||
| 0.42 [0.15, 1.24] | 0.116 | ||||
| HSI category (ITT) | Baseline | n = 55 | n = 54 | (N = 109) | |
| Low | 6 (10.9) | 8 (14.8) | |||
| Moderate | 37 (67.3) | 34 (63.0) | |||
| High | 12 (21.8) | 12 (22.2) | |||
| Quit smoking | 0 | 0 | |||
| 2 months | n = 46 | n = 30 | 0.38 [0.11, 1.41]* | 0.148 | |
| Low | 16 (34.8) | 14 (46.7) | |||
| Moderate | 21 (45.7) | 13 (43.4) | |||
| High | 9 (19.6) | 3 (10.0) | |||
| Quit smoking | 2 (4.0) | 12 (25.0) | |||
| 5 months | n = 36 | n = 29 | 1.56 [0.38, 6.42]* | 0.534 | |
| Low | 15 (41.7) | 12 (41.4) | |||
| Moderate | 15 (41.7) | 14 (48.3) | |||
| High | 6 (16.7) | 3 (10.3) | |||
| Quit smoking | 7 (15.0) | 11 (27.0) | |||
| 8 months | n = 37 | n = 25 | 0.96 [0.23, 3.97]* | 0.953 | |
| Low | 14 (37.8) | 11 (44.0) | |||
| Moderate | 14 (37.8) | 12 (48.0) | |||
| High | 9 (24.3) | 2 (8.0) | |||
| Quit smoking | 7 (15.0) | 9 (25.0) | |||
| Cigarettes per day (all participants) (ITT) | Baseline | 21.31 (9.97) | 20.48 (9.37) | ||
| 2 months | 15.96 (13.00) (n = 50) | 10.33 (11.09) (n = 49) | −4.76 [−9.48, −0.04] | 0.048 | |
| 5 months | 15.05 (15.25) (n = 46) | 10.98 (10.74) (n = 41) | −2.49 [−7.36, 2.38] | 0.315 | |
| 8 months | 13.53 (14.30) (n = 47) | 11.24 (11.4) (n = 37) | −1.02 [−5.94, 3.90] | 0.683 | |
| Cigarettes per day (Continuing smokers) (ITT) | Baseline | 21.31 (9.97) | 20.48 (9.37) | ||
| 2 months | 16.62 (12.84) (n = 48) | 13.68 (10.81) (n = 37) | −3.12 [−8.02, 1.78] | 0.211 | |
| 5 months | 17.77 (15.04) (n = 39) | 15.01 (9.83) (n = 30) | −0.19 [−5.43, 5.04] | 0.942 | |
| 8 months | 15.90 (14.23) (n = 40) | 14.86 (10.90) (n = 28) | 0.86 [−4.45, 6.17] | 0.75 |
HSI: Heaviness of Smoking Index (Heatherton et al., 1989);
Odds ratios (ORs) and associated 95% confidence intervals (CIs) with missing data classified as non-abstinent.
– Sensitivity analysis using the worst-case scenario (not using NRT) was performed in the case of missing follow-up.
– Not including those who had quit.
– The available case analysis includes the N study participants whose smoking status (continuous abstinence) was measured at 8 months post-baseline follow-up, plus N participants lost to 8-month follow-up, but who had been assessed as a non-successful quit at 2 or 5 months post-baseline follow-up, confirming them as non-successful quits at 8 months under the study definition of a successful quit as 6 months continual abstinence (Quitlink = N and control = N = 91).
Primary outcome
As seen in Table 4, in an available case analysis (see Table 4 footnote for definition), there was a large but not statistically significant difference in self-reported prolonged abstinence at 8-month post-baseline follow-up between the Quitlink (16%, n = 6) and control (2%, n = 1) conditions (OR = 8.33 [0.52, 132.09] p = 0.131, n = 91). After worst-case sensitivity analysis (ITT) with missing data treated as not continuously abstinent, the intervention effect at the 8-month timepoint remained large (OR = 6.72 [0.45, 100.27] p = 0.165, N = 109) and not statistically significant (Table 4). The number of people successfully quit was the same at 5-and 8-month follow-ups.
Secondary outcomes
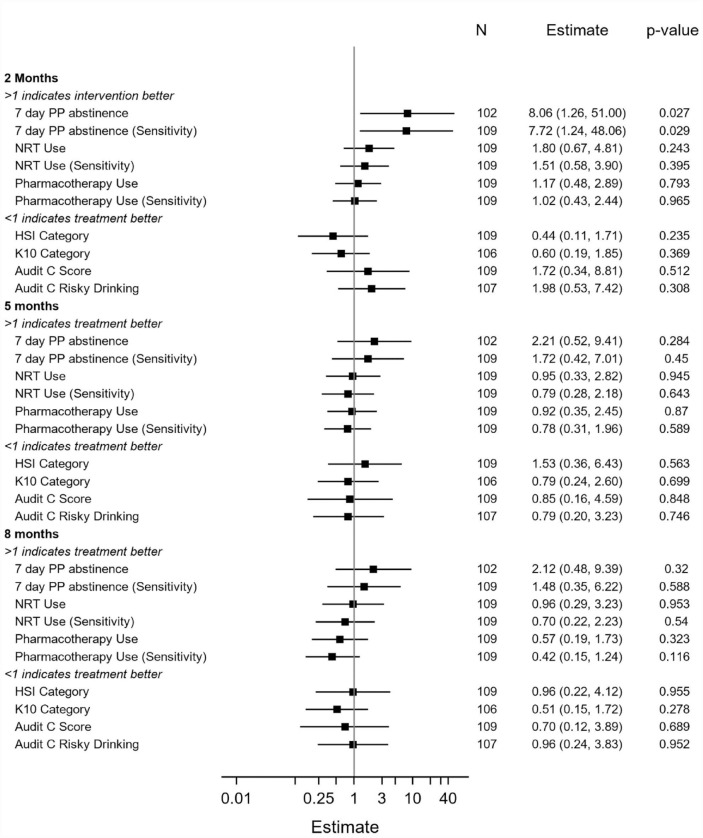
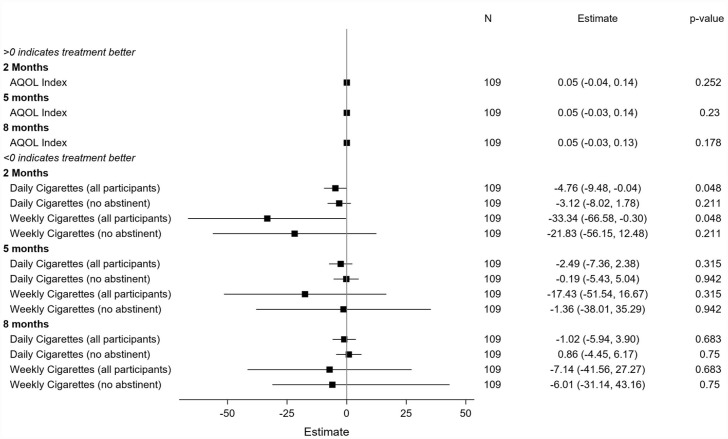
Data describing secondary outcomes are displayed in Table 4 (and Supplementary Table 2). Figures 2 and 3 present summaries of secondary outcome modelling. For categorical variables, ORs with 95% CIs, and p-values, are presented.
Figure 2.
Secondary outcome modelling for categorical variables.
Figure 3.
Secondary outcome modelling for continuous variables.
At the 2-month post-baseline follow-up (Table 4), there were significant differences in the odds of 7-day point prevalence abstinence between the Quitlink (11/49, 22.4%) and control (2/50, 4.0%) conditions (OR = 8.06 [1.27, 51.00] p = 0.027). There were no significant differences in this outcome at subsequent follow-ups. The overall interaction between intervention arm and study time period was not significant (p = 0.411), so this should be interpreted with caution. Table 4 shows that Quitlink participants achieved a statistically significant mean decrease in the number of cigarettes they smoked per day at the 2-month follow-up compared to control participants. There were no significant differences between conditions at subsequent follow-ups. There were no significant differences between intervention and control conditions in psychological distress (K10) or alcohol use (AUDIT) at any of the follow-up time points.
Over half of the participants (30 [56%] Quitlink and 31 [56%] control participants) reported experiencing at least one adverse event coded to SOC (Supplementary Table 3). During the trial, 86 serious adverse events (SAEs) were recorded (affecting 48 participants: 29 in the control group and 19 in the intervention group). Seven SAEs were rated by the study physician as possibly caused by the study and NRT, all psychiatric events (four events for three participants in the control arm; three events for three participants in the intervention arm). No differences were seen between the Quitlink and control conditions in the percentage of participants reporting adverse events or SAEs. ‘Psychiatric Disorders’ was the most frequently presenting SOC.
The estimated incremental cost per additional quit (6 months abstinence) at 8 months was between AU$9231 (N = 91, available case) and AU$11,333 (N = 109, worst case). The incremental cost per QALY over the 8-month period was estimated at AU$57,456 and has an approximately 48% chance of meeting a cost-effectiveness threshold of AU$50,000 AUD (Wang et al., 2018) in the short run (within-trial). When the available case sample was modelled over 50 years, the estimated cost per QALY gained was AU$54,361 (see Supplementary Materials for further details on assumptions, model parameters and analyses).
Discussion
This trial did not find a significant difference on the primary outcome measure of self-reported prolonged abstinence at 8-month post-baseline follow-up between the Quitlink and control conditions. Point prevalence abstinence and cigarettes smoked per day favoured the Quitlink condition significantly at our first follow-up (2 months post baseline).
No secondary or sensitivity outcome showed any evidence of Quitlink being inferior. Further testing is required to determine if Quitlink could potentially be a relatively cost-effective way to help people experiencing mental health conditions quit smoking, at least in the short term (Barnett et al., 2008, 2015). Psychological distress scores did not worsen, and rates of psychiatric adverse events did not differ between conditions, supporting previous findings that addressing tobacco smoking does not worsen mental health (Anthenelli et al., 2016). Furthermore, any observed reduction in cigarette use may have important financial benefits for this generally economically disadvantaged population. Cigarettes in Australia are highly taxed (average pack is approximately AUD40), and 40% (42/109) of participants reported at baseline that their spending on cigarettes had resulted in having insufficient money for household essentials, such as food, in the previous month.
The small sample leaves significant uncertainty around estimates of intervention cost-effectiveness. Recruiting people with mental health conditions remains a real challenge, making conducting such trials expensive, probably requiring cooperation of a wider range of services. However, as they are a group that clearly need augmented assistance if they are to quit smoking, it is an investment that is likely worthwhile to determine what levels and nature of supports they need and to provide such services when there is sufficient evidence of their effectiveness. Supporting this investment, we present some evidence suggesting the Quitlink model may be cost-effective but more corroborating evidence is needed to be certain (Lightwood and Glantz, 1997; Rejas-Gutiérrez et al., 2017; Weber et al., 2021).
There are now two RCTs reporting sizeable prolonged self-reported abstinence rates following a quitline intervention among a sample with over half of participants experiencing psychotic disorders (Morris et al., 2011). However, sample size was limited in both studies. Future studies should investigate the effectiveness of quitline interventions among larger samples of people experiencing mental health conditions, including psychotic disorders.
Quitline counselling was acceptable in the present trial. Quitlink participants received more and longer calls than the few participants in the control condition who contacted quitline, and the Quitlink calls were rated more highly on satisfaction. It is likely that the additional training Quitlink counsellors received, the availability of a treatment manual and dedicated counsellors assigned to each participant all contributed to positive ratings. Both the Quitlink and control counsellors received regular supervision and feedback regarding their counselling, assuring quality across both conditions. This is consistent with our nested qualitative study that found compassionate support offered by the quitline counsellors was appreciated by participants and acknowledged how commonly this population experiences marginalisation and complex recovery trajectories (McCarter et al., 2022).
Limitations
This study has several limitations. First and foremost, the study was underpowered due to recruitment challenges faced by peer researchers and exacerbated by COVID restrictions, as previously described (Baker et al., 2022). Due to this lack of power, we are unable to draw firm conclusions about the potential benefit of the Quitlink intervention.
There were significantly fewer people at the 8-month follow-up in the intervention group compared to the control group. It is possible that there was a difference in responsiveness to the intervention, with some being receptive to the more intensive intervention arm and others being actively put off, the latter being more likely to drop out. This would have inflated our effect size, except that the use of mixed models (which imputes missing data) and worst case and ITT sensitivity analyses mitigates such an effect. COVID restrictions also meant that CO verification of self-reported abstinence was not undertaken. Hence, our abstinence rates may be overestimated, potentially more so in the Quitlink condition as participants received more intervention and may therefore have been more inclined to feel social pressure to report quitting but as outcomes were assessed by people not engaged in clinical delivery, we think this unlikely. As we did not collect frequency of cNRT use, we were unable to relate this to effectiveness. A major strength of this study was its pragmatic design and minimal exclusion criteria, with findings likely representative of people receiving mental health services in the community.
Conclusion
This study developed and tested peer researcher facilitated referral to a tailored quitline intervention (‘Quitlink’) for people receiving mental health services who smoke. The Quitlink intervention did not result in significantly higher rates of prolonged abstinence at 8 months post baseline. Participants had significantly higher 7-day point prevalence rates at 2 months and were more satisfied with the quitline service they received. Despite our lack of power, this provides important information about both effect size and acceptability for a subsequent trial. There is suggestive evidence that the intervention would be a relatively cost-effective way to help people experiencing mental health conditions to quit, at least in the short term.
Supplemental Material
Supplemental material, sj-docx-1-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-2-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-3-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-4-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Acknowledgments
The authors thank the staff of participating services, particularly Dr Laura Hayes, Dr Elise Davies, Nadine Cocks and Melissa McKinlay. They also thank Dr Catherine Brasier for her research assistance and consumers of participating services.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the National Health and Medical Research Council (APP1139125).
Ethics Approval Statement: The protocol was approved by Ethics Committees of St Vincent’s Hospital, Melbourne (HREC/18/SVHM/154), the University of Newcastle (H-2018-0192) and the Cancer Council Victoria (HREC 1807).
Trial sponsor: University of Newcastle, NSW, Australia
Clinical Trial Registration: The trial is registered with ANZCTR (www.anzctr.org.au): ACTRN12619000244101 prior to the accrual of the first participant and updated regularly as per registry guidelines.
Data Availability Statement: Deidentified data from this study may be made available to investigators pending ethics approval and agreement from trial chief investigators.
ORCID iDs: Kristen McCarter  https://orcid.org/0000-0002-2638-6381
https://orcid.org/0000-0002-2638-6381
Anna L Wrobel  https://orcid.org/0000-0002-1864-0394
https://orcid.org/0000-0002-1864-0394
Supplemental material: Supplemental material for this article is available online.
References
- Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. (2010) An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug and Alcohol Dependence 110: 137–143. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, et al. (2016) Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. The Lancet 387: 2507–2520. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics (2018) 4364.0.55.001 – National Health Survey: First Results, 2017-18. Available at: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/national-health-survey-first-results/latest-release (accessed 19 June 2022).
- Australian Bureau of Statistics (2021) TableBuilder. Available at: http://www.abs.gov.au/websitedbs/d3310114.nsf/home/about+tablebuilder(accessed 19 June 2022).
- Baker A, Richmond R, Haile M, et al. (2006) A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. The American Journal of Psychiatry 163: 1934–1942. [DOI] [PubMed] [Google Scholar]
- Baker AL, Borland R, Bonevski B, et al. (2019) ‘Quitlink’—A randomized controlled trial of peer worker facilitated quitline support for smokers receiving mental health services: Study protocol. Frontiers in Psychiatry 10: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AL, McCarter K, Brophy L, et al. (2022) Adapting peer researcher facilitated strategies to recruit people receiving mental health services to a tobacco treatment trial. Frontiers in Psychiatry 13: 869169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AL, Turner A, Beck A, et al. (2018) Telephone-delivered psychosocial interventions targeting key health priorities in adults with a psychotic disorder: Systematic review. Psychological Medicine 48: 2637–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandiera FC, Anteneh B, Le T, et al. (2015) Tobacco-related mortality among persons with mental health and substance abuse problems. PLoS ONE 10: e0120581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett PG, Wong W, Hall S. (2008) The cost-effectiveness of a smoking cessation program for out-patients in treatment for depression. Addiction 2008; 103: 834–840. [DOI] [PubMed] [Google Scholar]
- Barnett PG, Wong W, Jeffers A, et al. (2015) Cost-effectiveness of smoking cessation treatment initiated during psychiatric hospitalization: Analysis from a randomized, controlled trial. The Journal of Clinical Psychiatry 76: e1285–e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ. (2021) Quitline reach and effectiveness among populations disproportionately affected by tobacco use: Future directions. Journal of Health Care for the Poor and Underserved 32: 1188–1198. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, et al. (1998) The AUDIT Alcohol Consumption Questions (AUDIT-C): An effective brief screening test for problem drinking. Archives of Internal Medicine 158: 1789–1795. [DOI] [PubMed] [Google Scholar]
- Byrne L, Wang L, Roennfeldt H, et al. (2021) National Lived Experience Workforce Guidelines. Sydney, NSW, Australia: National Mental Health Commission. [Google Scholar]
- Chen G, Olsen JA. (2020) Filling the psycho-social gap in the EQ-5D: The empirical support for four bolt-on dimensions. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation 29: 3119–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, et al. (1992) Development and validation of a three-dimensional measure of drinking motives. Psychological Assessment 4: 123–132. [Google Scholar]
- Crittenden KS, Manfredi C, Lacey L, et al. (1994) Measuring readiness and motivation to quit smoking among women in public health clinics. Addictive Behaviors 19: 497–507. [DOI] [PubMed] [Google Scholar]
- Dai X, Gakidou E, Lopez AD. (2022) Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tobacco Control 31: 129–137. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Schroeder J, Katsafanas E, et al. (2018) Cigarette smoking by patients with serious mental illness, 1999-2016: An increasing disparity. Psychiatric Services 69: 147–153. [DOI] [PubMed] [Google Scholar]
- Dixon L, Medoff DR, Wohlheiter K, et al. (2007) Correlates of severity of smoking among persons with severe mental illness. The American Journal on Addictions 16: 101–110. [DOI] [PubMed] [Google Scholar]
- Goulão B, MacLennan G, Ramsay C. (2018) The split-plot design was useful for evaluating complex, multilevel interventions, but there is need for improvement in its design and report. Journal of Clinical Epidemiology 96: 120–125. [DOI] [PubMed] [Google Scholar]
- Greenhalgh EM, Brennan E, Segan C, et al. (2022) Monitoring changes in smoking and quitting behaviours among Australians with and without mental illness over 15 years. Australian and New Zealand Journal of Public Health 46: 223–229. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Minor BL, et al. (2019) The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, et al. (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. (1989) Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction 84: 791–799. [DOI] [PubMed] [Google Scholar]
- Herd N, Borland R. (2009) The natural history of quitting smoking: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction (Abingdon, England) 104: 2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd N, Borland R, Hyland A. (2009) Predictors of smoking relapse by duration of abstinence: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction (Abingdon, England) 104: 2088–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman M, Gudex C, Lloyd A, et al. (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, et al. (2003) Screening for serious mental illness in the general population. Archives of General Psychiatry 60: 184–189. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, et al. (1994) Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence 34: 211–216. [DOI] [PubMed] [Google Scholar]
- Lightwood JM, Glantz SA. (1997) Short-term economic and health benefits of smoking cessation: Myocardial infarction and stroke. Circulation 96: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J. (2019) Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 5: CD002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter K, McKinlay ML, Cocks N, et al. (2022) The value of compassionate support to address smoking: A qualitative study with people who experience severe mental illness. Frontiers in Psychiatry 13: 868032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher J, Stratford A, Jackson F, et al. (2018) Peer Work in Australia – a New Future for Mental Health. Heidelberg VIC, Australia: Flourish Australia. [Google Scholar]
- Morris CD, Waxmonsky JA, May MG, et al. (2011) Smoking reduction for persons with mental illnesses: 6-month results from community-based interventions. Community Mental Health Journal 47: 694–702. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Parzynski C, Mercincavage M, et al. (2012) Is self-efficacy for smoking abstinence a cause of, or a reflection on, smoking behavior change. Experimental and Clinical Psychopharmacology 20: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejas-Gutiérrez J, Bruguera E, Cedillo S. (2017) Modelling a budgetary impact analysis for funding drug-based smoking cessation therapies for patients with major depressive disorder in Spain. European Psychiatry: The Journal of the Association of European Psychiatrists 45: 41–49. [DOI] [PubMed] [Google Scholar]
- Richardson J, Iezzi A, Khan MA, et al. (2014) Validity and reliability of the assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. The Patient 7: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ES, Smelson DA, Gillespie CC, et al. (2016) Telephone smoking-cessation counseling for smokers in mental health clinics: A patient-randomized controlled trial. American Journal of Preventive Medicine 50: 518–527. [DOI] [PubMed] [Google Scholar]
- SAS Institute (2013) SAS/ACCESS® 9.4 Interface to ADABAS. Cary, NC: SAS Institute. [Google Scholar]
- Schwindt R, Hudmon KS, Knisely M, et al. (2017) Impact of tobacco quitlines on smoking cessation in persons with mental illness: A systematic review. Journal of Drug Education 47: 68–81. [DOI] [PubMed] [Google Scholar]
- Segan CJ, Baker AL, Turner A, et al. (2017) Nicotine withdrawal, relapse of mental illness, or medication side-effect? Implementing a monitoring tool for people with mental illness Into Quitline counseling. Journal of Dual Diagnosis 13: 60–66. [DOI] [PubMed] [Google Scholar]
- Segan CJ, Borland R, Wilhelm KA, et al. (2011) Helping smokers with depression to quit smoking: Collaborative care with Quitline. Medical Journal of Australia 195: S7–S11. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998) The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry 59: 22–33; quiz 34. [PubMed] [Google Scholar]
- Siahpush M, Carlin JB. (2006) Financial stress, smoking cessation and relapse: Results from a prospective study of an Australian national sample. Addiction (Abingdon, England) 101: 121–127. [DOI] [PubMed] [Google Scholar]
- Spencer C, Castle D, Michie PT. (2002) Motivations that maintain substance use among individuals with psychotic disorders. Schizophrenia Bulletin 28: 233–247. [DOI] [PubMed] [Google Scholar]
- Sweeney R, Moodie M, Baker AL, et al. (2019) Protocol for an economic evaluation of the quitlink randomized controlled trial for accessible smoking cessation support for people with severe mental illness. Frontiers in Psychiatry 10: 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski L, McNeill A. (2015) Diverging trends in smoking behaviors according to mental health status. Nicotine & Tobacco Research 17: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O’Malley SS, McKee SA, et al. (2007) Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychology of Addictive Behaviors 21: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman L, Bonevski B, Paul C, et al. (2014) Perceived barriers to smoking cessation in selected vulnerable groups: A systematic review of the qualitative and quantitative literature. BMJ Open 4: e006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer RM, Willemsen MC, Smit F, et al. (2010) Effectiveness of a mood management component as an adjunct to a telephone counselling smoking cessation intervention for smokers with a past major depression: A pragmatic randomized controlled trial. Addiction 105: 1991–1999. [DOI] [PubMed] [Google Scholar]
- Wang S, Gum D, Merlin T. (2018) Comparing the ICERs in medicine reimbursement submissions to NICE and PBAC – does the presence of an explicit threshold affect the ICER proposed. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research 21: 938–943. [DOI] [PubMed] [Google Scholar]
- Warlick C, Richter KP, Catley D, et al. (2018) Two brief valid measures of therapeutic alliance in counseling for tobacco dependence. Journal of Substance Abuse Treatment 86: 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MF, Sarich PEA, Vaneckova P, et al. (2021) Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. International Journal of Cancer 149: 1076–1088. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Vujanovic AA, Parachini EA, et al. (2003) A screening measure for BPD: The McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Journal of Personality Disorders 17: 568–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-2-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-3-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry
Supplemental material, sj-docx-4-anp-10.1177_00048674231181039 for ‘Quitlink’: Outcomes of a randomised controlled trial of peer researcher facilitated referral to a tailored quitline tobacco treatment for people receiving mental health services by Amanda L Baker, Kristen McCarter, Alyna Turner, Catherine Segan, David Castle, Lisa Brophy, Ron Borland, Peter J Kelly, Billie Bonevski, Donita Baird, Sacha Filia, John Attia, Stuart Szwec, Kerrin Palazzi, Sarah L White, Jill M Williams, Anna L Wrobel, Andrew Ireland, Karinna Saxby, Peter Ghijben, Dennis Petrie and Rohan Sweeney in Australian & New Zealand Journal of Psychiatry