Abstract
Current guidelines recommend a deferred testing approach in low-risk patients presenting with stable chest pain. After simulating a deferred testing approach using the PROMISE Minimal Risk Score to identify 915 minimal risk participants with cost data from the PROMISE trial, a deferred testing strategy was associated with an adjusted cost savings of -$748.74 (95% CI: −1646.97, 158.06) per participant and 74.6% of samples had better clinical outcomes and lower mean cost. This supports the current guideline recommended deferred testing approach in low-risk patients with stable chest pain.
Low-risk patients presenting with stable chest pain often receive diagnostic testing to aid in identifying the few with obstructive coronary artery disease (CAD). However, the 2021 AHA/ACC Guideline for the Evaluation and Diagnosis of Chest Pain recommends deferring testing in patients with a low pretest probability (PTP) for obstructive CAD (Class 1, LOE B-NR)1. This recommendation is based on observational data demonstrating a favorable prognosis for low-risk patients in general, most of whose management was informed by testing, rather than prospective testing of a strategy with specific cut-points in comparison to usual testing.
Accurate identification of low-risk patients is essential to any deferred testing strategy. The Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) minimal-risk score (PMRS) identifies stable chest pain patients with very low rates of any plaque or cardiovascular events, and is cited by the Chest Pain Guideline as a potential decision aid1, 2. To explore the possible impact of deferring testing on low risk patients with stable chest pain, we used the PMRS to identify a minimal risk cohort in the PROMISE trial population among those randomized to either functional testing or CTA, assuming that the lowest risk 10% of PROMISE participants (corresponding to a PMRS score >0.46) could safely receive a deferred testing strategy. We then compared actual clinical events and costs in the functional participants vs actual events and modelled costs in the anatomical ones to represent a deferred testing group (derived by removing initial testing costs in 80% (assuming that 20% would eventually undergo anatomic testing)3. Clinical outcomes included one-year death, myocardial infarction (MI) and non-obstructive CAD on catheterization using a Cox model. Direct or modelled medical costs were also compared, as previously described4. Bootstrap resampling was used to estimate uncertainty around estimates of clinical and cost differences. We performed two sensitivity analyses which would be expected to minimize the advantage of deferred testing: 1) using a higher PMRS threshold (lowest 20% risk) and 2) higher percentage of deferred testing patients (30%) eventually undergoing testing;
Of the 994 minimal risk participants, 915 (97%) had cost data available (usual testing: N=468, CTA/deferred testing: N=447). Adverse clinical outcomes were infrequent and included 15 in the usual testing arm (1 death, 0 MI, 14 catheterizations), compared to 9 in the simulated deferred testing arm (1 death, 1 MI, 7 catheterizations). For the composite clinical outcomes, the deferred testing:usual testing hazard ratio was 0.62 (95% CI: 0.16, 1.81). As modelled, deferred testing had an adjusted cost savings of -$748.74 (95% CI: −1646.97, 158.06) per participant. As shown in Figure 1, 74.6% of samples had better clinical outcomes and lower mean cost using a modelled deferred testing strategy (lower left-hand quadrant). Results were consistent in both sensitivity analyses using a higher PMRS threshold (20%) and higher percentage of deferred testing patients (30%) who eventually undergoing testing. With the shift to a higher PMRS threshold of 20%, the deferred testing:usual testing hazard ratio remained directionally consistent but the beneficial effect magnitude was halved at 0.84 (95% CI: 0.39, 1.72), and cost savings were mildly reduced: -$676.63 (95% CI: −1333.17, 70.70). Estimating that testing would be performed in a higher percentage of deferred testing patients (30%) had little effect, with a similar hazard ratio of 0.62 (95% CI: 0.16, 1.81) and adjusted cost savings of -$709.73 (95% CI: −1611.03, 209.27).
Figure 1.
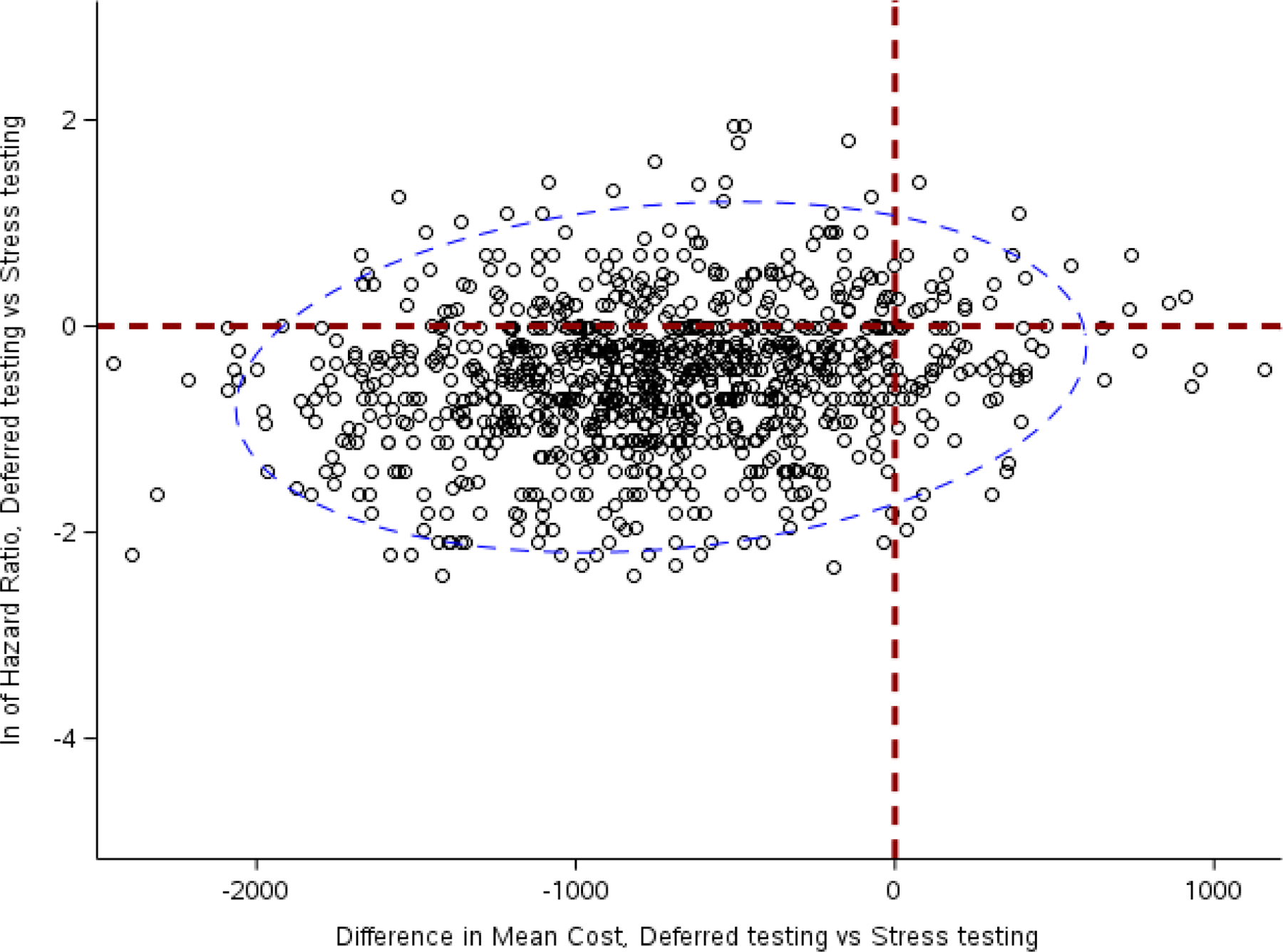
Scatterplot of natural log (ln) of clinical hazard ratios vs. differences in cost in dollars among individuals assigned to a modelled deferred testing strategy (with 20% eventually receiving anatomic testing) and functional testing in PROMISE, both of which favor the deferred testing arm. The dashed blue ellipse represents the 95% confidence interval around the scattered plots.
This simulation uses prospective trial data to expand our understanding of the implications of deferring testing and quantify the implications of Guideline recommendations. It also has important implications for trial design comparing diagnostic strategies in minimal-risk patients. By using the PROMISE randomized arms, we were able to create comparable groups within an overall negative trial. We assumed that the testing in PROMISE did not alter management in important ways relative to an actual deferred testing strategy. However, it is possible this may have introduced bias into the simulation. For example, medical treatments may have been intensified in those who underwent testing based on those test results, which wouldn’t necessarily occur in a deferred testing population. Given the very low event rate observed in this cohort (2 MI, 1 death), the margin for dramatic improvements in outcomes from intensified medical therapy based on diagnostic testing is small. Indeed, the low event rate demonstrated by the present analysis of the PROMISE trial data minimal risk cohort highlights the challenges in achieving adequate precision in the comparison of different management strategies for these very low risk patients. This is part of the rationale examining clinically important non-traditional outcomes, such as catheterization without obstructive disease, which have important implications for risk exposure, health-related quality of life and cost.5–7 It should also be noted that catheterization was more common in the anatomical arm, an unlikely finding in a true deferred testing arm and which may have reduced the differences between groups in both the clinical and cost endpoints. Interestingly, although we did not formally evaluate the optimal cut-point for deferred testing in this modelled cohort, a higher PMRS cut-point (deferred testing in 20%) reduced potential clinical benefit. Prior data comparing functional testing vs. no testing in low-risk patients are limited to observational studies but support the Chest Pain Guideline recommendation for deferral of non-invasive testing in minimal risk patients8. Thus, there is a need for a randomized trial prospectively defining and evaluating a deferred testing strategy in patients at minimal risk.
To address this data gap and provide clinicians with a prospectively tested care pathway for implementation of deferred testing, the Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization (PRECISE; NCT03702244)9 is assessing clinical and cost outcomes following randomization to usual testing vs a precision evaluation strategy which assigns minimal risk, stable chest pain patients to deferred testing and higher risk patients to CTA +/− FFRCT..9 Because randomization is stratified by PROMISE minimal-risk score, PRECISE will directly compare deferred testing with usual testing in a minimal-risk subset.
Conflict of Interest Disclosures:
Nanna MG: Dr. Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Yow E: Grant support to institution from HeartFlow
Vemulapalli S: Grants / contracts: American College of Cardiology, Society of Thoracic Surgeons, National Institutes of Health (SBIR and R01), Food and Drug Administration (NESTcc), Abbott Vascular, Boston Scientific, Cytokinetics. Consulting / Advisory Board: HeartFlow, Janssen, American College of Physicians, Edwards LifeSciences
Mark DB: Grant support to institution from HeartFlow and Merck. Consulting/Advisory Board: Novartis
Kelsey M: Dr. Kelsey is supported by 5T32HL069749-18.
Patel MR: Research Grants/Advisory Board: Amgen, Bayer, Janssen, Heartflow; Research Grants; NHLBI, Novartis.
Al-Khalidi HR: Grant support to institution from HeartFlow
Rogers C: Employee of HeartFlow: Salary and equity.
Udelson JE: Research funding from HeartFlow.
Douglas PS: Grant support to institution from HeartFlow
REFERENCES:
- 1.Gulati M, Levy PD, Mukherjee D, et al. 2021. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 0(0):CIR.0000000000001029. doi:doi: 10.1161/CIR.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 2.Fordyce CB, Douglas PS, Roberts RS, et al. Identification of Patients With Stable Chest Pain Deriving Minimal Value From Noninvasive Testing: The PROMISE Minimal-Risk Tool, A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. Apr 1 2017;2(4):400–408. doi: 10.1001/jamacardio.2016.5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas PS, Hoffmann U. Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. Jul 2 2015;373(1):91. doi: 10.1056/NEJMc1505594 [DOI] [PubMed] [Google Scholar]
- 4.Mark DB, Federspiel JJ, Cowper PA, et al. Economic Outcomes With Anatomical Versus Functional Diagnostic Testing for Coronary Artery Disease. Ann Intern Med. Jul 19 2016;165(2):94–102. doi: 10.7326/m15-2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastwood JA, Doering LV, Dracup K, Evangelista L, Hays RD. Health-related quality of life: The impact of diagnostic angiography. Heart Lung. Mar-Apr 2011;40(2):147–55. doi: 10.1016/j.hrtlng.2010.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas PS, De Bruyne B, Pontone G, et al. 1-Year Outcomes of FFRCT-Guided Care in Patients With Suspected Coronary Disease: The PLATFORM Study. J Am Coll Cardiol. Aug 2 2016;68(5):435–445. doi: 10.1016/j.jacc.2016.05.057 [DOI] [PubMed] [Google Scholar]
- 7.Maurovich-Horvat P, Bosserdt M, Kofoed KF, et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N Engl J Med. Apr 28 2022;386(17):1591–1602. doi: 10.1056/NEJMoa2200963 [DOI] [PubMed] [Google Scholar]
- 8.Mesnier J, Ducrocq G, Danchin N, et al. International Observational Analysis of Evolution and Outcomes of Chronic Stable Angina: The Multinational CLARIFY Study. Circulation. 2021;144(7):512–523. doi:doi: 10.1161/CIRCULATIONAHA.121.054567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanna MG, Vemulapalli S, Fordyce CB, et al. The prospective randomized trial of the optimal evaluation of cardiac symptoms and revascularization: Rationale and design of the PRECISE trial. Am Heart J Mar 2022;245:136–148. doi: 10.1016/j.ahj.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


