Abstract
Urachal cancer is a rare malignancy of the urachus that is treated with surgical resection if localized and systemic chemotherapy for metastatic disease. Circulating tumor DNA (ctDNA) is a single-stranded or double-stranded DNA released by tumor cells into the blood and harbored the mutations of the original tumor, shedding new light on molecular diagnosis and monitoring of cancer. We report a case of resected localized urachal cancer with clear surgical margins and negative lymph node dissection but elevated ctDNA that progressed to metastatic disease. We also highlight the possibility of using ctDNA levels to assist in adjuvant therapy.
Keywords: circulating tumor DNA, urachal cancer, urachal remnant, urachus
Introduction
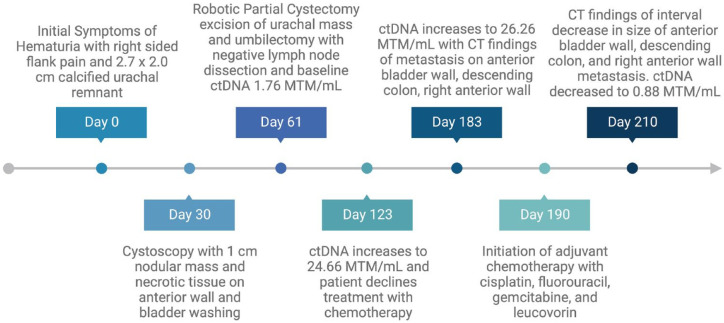
The urachus is a fibrous cord that connects the urinary bladder to the umbilicus. 1 The urachus is formed during fetal development and whose remnant may persist throughout life. After birth, the urachus is known as the median umbilical ligament that joins the umbilicus to the dome of the bladder. 1 This median umbilical ligament can transform into carcinoma, also referred to as urachal cancer. Urachal cancer comprises 0.3% of all invasive bladder cancers along with urachal cancer accounting for approximately 0.01% of all cancers diagnosed. 2 Circulating tumor DNA (ctDNA) is a single-stranded or double-stranded DNA released by tumor cells into the blood and harbored the mutations of the original tumor, shedding new light on molecular diagnosis and monitoring of cancer. 3 Subgroup analysis of IMVIGOR010 is a phase III trial comparing adjuvant atezolizumab versus observation showed significant benefit with adjuvant atezolizumab in disease-free survival and overall survival (OS) in ctDNA-positive patients but no other subgroup in muscle-invasive urothelial carcinoma.4,5 This was the first study highlighting a potential benefit of ctDNA as a guide in adjuvant treatment in genitourinary cancers. Hereafter, we report a case of resected localized urachal cancer with clear surgical margins and negative lymph node dissection but elevated ctDNA that progressed to metastatic disease. We also highlight the possibility of using ctDNA levels to assist in adjuvant treatment in urachal cancer (Figure 1). CtDNA was obtained using the personalized Signatera assay, similar to IMVIGOR 010, and was evaluated every month. The next-generation tissue sequencing was obtained using the Foundation One comprehensive diagnostic gene panel which is used for all solid tumors. In addition, the reporting of this study conforms to the CARE statement. 6
Figure 1.
Timeline of the case report.
Case report
A 58-year-old Caucasian male with a past medical and social history notable for active smoking and alcohol use presented to the emergency department with a sudden episode of hematuria with blood clots in an intermittent right flank pain radiating to his right lower quadrant. His family history was notable for his mother being diagnosed with multiple malignancies, including breast cancer, endometrial cancer, and mesothelioma. The patient underwent computed tomography (CT) of the abdomen and pelvis, which demonstrated a 2.7 × 2.0 cm calcified urachal remnant with a vesicourachal diverticulum stone [Figure 2(a)]. Cystoscopy found a 1 cm nodular mass with calcifications and necrotic tissue on the anterior wall. Bladder washings demonstrated rare atypical transitional cell groups.
Figure 2.
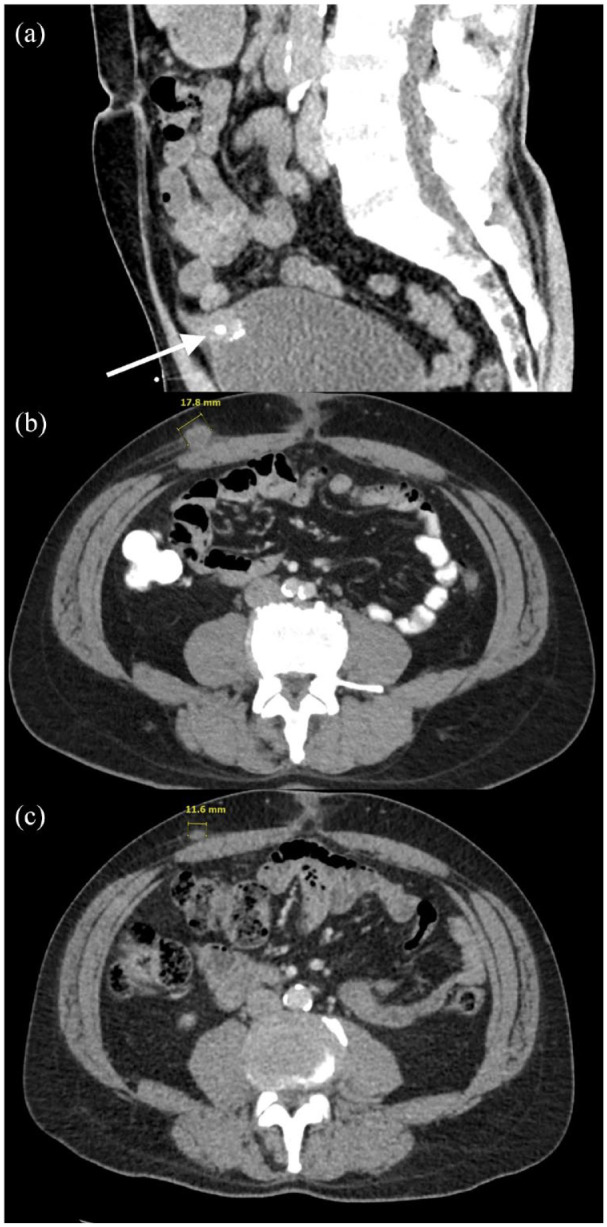
CT of the abdomen demonstrates urachus. (a) Sagittal view, respectively, of 2.7 × 2.0 cm peripherally calcified urachal remnant with vesicourachal diverticulum stone with respective arrows at the time of initial hematuria and right flank pain. (b) Axial CT imaging denoting peripherally enhancing subcutaneous mass along the right anterior abdominal wall 4 months after partial cystectomy and excision of urachal mass. (c) Axial CT views of the abdomen demonstrating interval decreased size of metastasis of peripherally enhancing subcutaneous mass along the right anterior abdominal wall after one cycle of Gem-FLP.
CT, computed tomography; FLP-Gem, gemcitabine, cisplatin, 5-FU, and leucovorin.
Given these findings, he underwent transurethral resection of the bladder tumor 2 weeks later without subsequent complications. The pathology from the surgical resection showed sections of moderately differentiated adenocarcinoma involving the muscularis propria without lymphovascular invasion. Immunohistochemistry was positive for CK20 and CKX2 and negative for CK7, CK903, GATA3, PAX8, and NKX3, concerning urachal or colorectal cancer. He underwent a colonoscopy 2 weeks later with no findings to suggest colorectal cancer.
A positron-electron transmission (PET) scan demonstrated no signs of F-fluorodeoxyglucose (FDG) avid metastatic disease. Since colon adenocarcinoma was deemed less likely and urachal cancer was the suspected diagnosis, he underwent a robotic partial cystectomy, excision of urachal mass, and umbilectomy without any complications. The pathology was notable for a moderately differentiated enteric type urachal adenocarcinoma with invasion into the bladder and negative resection margins, no lymphovascular invasion, and a lymph node dissection was notable for no lymph node involvement in 11 lymph nodes. As such, the patient was formally diagnosed with Sheldon system stage IIIA urachal cancer. He was offered adjuvant chemotherapy given his young age and lack of comorbidities and requested active surveillance in the adjuvant setting due to concern for potential adverse effects. Further evaluation with next-generation sequencing was notable for microsatellite status – stable, programmed death ligand-1 expression of less than 1% with immunohistochemical staining of 30%, tumor marker burden of 5 mutations per megabase, BRAF–G469R mutation, CHEK2-splice site 444 + IG > A mutation, PTEN–D24_L25 deletion, TP53–Y220C mutation, FLT3 amplification, PPP2R2A loss, SMAD4–A118V mutation, CEBPA–P97S mutation, PPARG–R212L mutation, and KEL–R404H mutation. Baseline ctDNA using the Signatera assay kit was detectable post-cystectomy at 1.76 MTM/mL (mean tumor molecules per mL) (Figure 1).
The patient underwent repeat ctDNA testing 2 months following his initial visit with medical oncology, with a rise in ctDNA to 24.66 MTM/mL (Figure 1). Imaging studies re-demonstrated no evidence of disease. Adjuvant chemotherapy was offered again and highly encouraged given the ctDNA findings. The patient ultimately decided that he did not want to pursue adjuvant chemotherapy as he remained concerned about potential adverse effects. Two months later, his ctDNA increased to 26.26 MTM/mL, and a CT chest, abdomen, and pelvis found multiple peripherally enhancing foci along the anterior bladder, medial to the descending colon, the presacral fat, and right anterior abdominal wall [Figure 2(b)]. The lack of abdominopelvic lymphadenopathy raised concern for local recurrence, prompting soft tissue biopsy of the most accessible abdominal wall lesion. Pathology analysis demonstrated adenocarcinoma with immunohistochemical staining notable for CDX2 and CK20 positivity with PD-L1 expression <1% in the tumor cells consistent with recurrence of urachal cancer.
The patient was subsequently treated with palliative gemcitabine, cisplatin, 5-FU, and leucovorin (Gem-FLP) chemotherapy regimen: cisplatin (20 mg/m2 on days 1–5), fluorouracil (200 mg/m2/day on day 1), gemcitabine (200 mg/m2 day 1 and 5), leucovorin (10 mg/m2 on day 1) with growth factor support. He underwent a CT abdomen and pelvis on cycle 1 day 20 of chemotherapy notable for an interval decrease in the soft tissue masses and subcutaneous abdominal wall masses [Figure 2(c)]. After completing two cycles of Gem-FLP, his ctDNA level was 0.88 and 0.00 MTM/mL after his third cycle. At the time of publication, the patient has transitioned to maintenance immunotherapy with Avelumab.
Discussion
In recent demographic studies of patients with urachal cancer, 56.51% were male with a mean age of 57.98 years and were non-Hispanic white. 7 Standard work-up for urachal cancer includes a cystoscopy to identify tumor localization. 8 Imaging evaluation for staging is traditionally performed with PET scans. 9 Our patient had typical demographics for an individual diagnosed with urachal cancer and had a conventional PET scan to rule out radiographic evidence of metastatic disease.
Traditionally, staging is performed with the Sheldon staging system that consists of a numeric system with stage 1 tumors limited to urachal mucosa, stage 2 pertaining to invasion confined to urachal remnant, stage 3 with local extension in nearby organs, and stage 4 with metastasis. 10 The median OS among all patients is approximately 76 months. For individuals with localized or in situ diseases, regional diseases, and metastatic diseases, the median OS is approximately 76, 83, and 19 months, respectively. 7 Advanced tumor stage, failure to perform umbilectomy, primary radiation therapy, late symptom presentation, and high tumor grade are all poor prognostic factors. 11
Guidelines for the treatment of urachal carcinoma are limited, with the only known consensus statement released by the Canadian Urological Association with the Genitourinary Medical Oncologists of Canada. 12 Further clinical equipoise is needed with a randomized multicenter clinical trial to determine the optimal treatment of urachal carcinoma in the neoadjuvant and adjuvant settings. The only known recruiting prospective trial is evaluating the use of first-line modified FOLFIRINOX in a single center in South Korea, which has been open to accrual since 2020 with an accrual goal of 35 patients (ClinicalTrials.gov identifier: NCT04611724). Localized cancer is primarily treated with partial or complete cystectomy with en bloc resection of the urachal ligament with umbilicus and lymph node dissection. For patients with node-positive disease or metastatic disease, combination chemotherapy with a 5-fluorouracil (5-FU)-based regimen of Gem-FLP, FOLFOX (oxaliplatin, leucovorin, and 5-FU), or TIP (paclitaxel, ifosfamide, and cisplatin) remains the mainstay of treatment. 13 As of recently, there is an increasing prevalence in the usage of gemcitabine as a foundational component of the treatment regimen. 14 There are also several studies about the future possibility of response to precision oncology treatment modalities such as epidermal growth factor inhibition, Kirsten rat sarcoma virus gene inhibition, myc inhibition, and several other targetable pathways.15,16
In our patient who had an invasive disease to the bladder with negative resected margins and no lymph node involvement on lymphadenectomy, a partial or complete cystectomy with en bloc resection without adjuvant chemotherapy is endorsed by National Comprehensive Cancer Network guidelines. 13 Experts have provided clinical guidance in the adjuvant setting and offer adjuvant treatment to patients who wish to take an aggressive approach to their cancer and have a high likelihood of relapse due to the presence of positive margins, lymph node, or peritoneal involvement, and when the umbilicus was not resected en-bloc with the urachal ligament and bladder.15,17 Although this framework can be helpful, there are also additional prognostic factors to take into consideration such as the fraction of patients with detectable ctDNA, which can vary with the tumor type and stage.
CtDNA has been shown to predict response and prognosis and guide oncologists in determining the duration of treatment of various types of cancer.18–21 CtDNA is also used to detect minimal residual disease (MRD). Our patient had an elevated ctDNA level of 24.66 MTM/mL postoperatively and was offered chemotherapy but declined due to concern about adverse effects. Two months later, he was found to have metastatic disease with an increasing ctDNA to 26.26 MTM/mL. While Signatera does not report the standard deviation of the ctDNA levels, it is important to note that ctDNA has shown evidence of being a sensitive and reliable molecular indicator of true progression and that clearance has been associated with a strong predictor of progression-free survival and OS.22,23 While it would be helpful to understand if this increase is within the standard deviation, we are hopeful that Signatera will eventually have this data and understand if increased ctDNA levels correlate with a higher disease burden.
Given the rarity of urachal cancer, there is a paucity of data to support adjuvant therapy; however, there remains a possibility of benefiting a subset of patients who have a high risk of recurrence, are ‘chemotherapy fit’, and are willing to take an aggressive approach to their cancer. This case is unique in that, to our knowledge, it is the first documented case report to suggest ctDNA’s prognostic value in urachal cancer.
In addition to ctDNA, serum tumor marker levels are useful tools for follow-up. In a retrospective analysis of patients with urachal and urothelial carcinoma, serum tumor markers were evaluated for diagnosis, prognosis, and monitoring, and in particular carcinoembryonic antigen (CEA) combined with cancer antigen 72-4 expression levels were significantly higher in the urachal cancer group than in the urothelial carcinoma group.24,25 An additional study noted high clinical value in CEA, cancer antigen 19-9, cancer antigen 125, and cancer antigen 15-3 in surgically treated urachal cancer. 26 These studies suggest a prognostic clinical value in serum tumor markers and raise the idea that utilizing a combination of serum tumor markers and ctDNA may provide a benefit as a predictive tool for the guidance of treatment and prognostic value.
Current consensus guidelines do not include formal guidance on the use of postoperative ctDNA. Our case suggests the possibility of using ctDNA’s prognostic value to guide adjuvant treatment after surgical resection in localized urachal cancer with a negative lymph node dissection. Given the findings in this rare case, we propose that positive MRD detected by ctDNA can be used as an additional decision-making factor for consideration of adjuvant therapy.
Conclusion
Urachal cancer is a rare genitourinary malignancy originating from the median umbilical ligament connecting to the bladder dome. It is imperative to surgically treat patients with localized disease. In addition, patients with metastatic disease or positive lymph nodes might benefit from systemic treatment with chemotherapy. It is also important to consider additional prognostic markers such as ctDNA when guiding adjuvant systemic treatment. Based on the clinical picture highlighted in the case report, we propose that adjuvant treatment for urachal cancer can be guided with ctDNA in addition to other high-risk features for recurrence.
Acknowledgments
None.
Footnotes
ORCID iD: Christopher Ryan Grant  https://orcid.org/0009-0001-6432-5635
https://orcid.org/0009-0001-6432-5635
Contributor Information
Christopher Ryan Grant, Department of Medicine, University of California Irvine Medical Center, UC Irvine Health, 200 S Manchester Avenue, Room 423, Orange, CA 92868, USA.
David J. Benjamin, Hoag Family Cancer Institute, Newport Beach, CA, USA
Scott Cramer, Department of Radiology, University of California Irvine Medical Center, Orange, CA, USA.
Arash Rezazadeh Kalebasty, Division of Hematology/Oncology, Department of Medicine, University of California Irvine Medical Center, Orange, CA, USA.
Declarations
Ethics approval and consent to participate: Consent to participate was obtained in the form of verbal and written consent for participation. The ethics committee at the sponsoring institution waived the requirement for approval given this is a case report with one patient.
Consent for publication: Consent for publication was obtained in the form of written consent for publication and has been uploaded to the SAGE Journals portal.
Author contributions: Christopher Ryan Grant: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
David J. Benjamin: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
Scott Cramer: Data curation; Formal analysis; Writing – review & editing.
Arash Rezazadeh Kalebasty: Conceptualization; Data curation; Formal analysis; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
DJB has the following disclosures: Consulting: Seagen. Speakers’ Bureau: Merck. Travel & Accommodations: Merck. ARK has the following disclosures: Stock and Other Ownership Interests: ECOM Medical. Consulting or Advisory Role: Exelixis, AstraZeneca, Bayer, Pfizer, Novartis, Genentech, Bristol Myers Squibb, EMD Serono, Immunomedics, Gilead Sciences. Speakers’ Bureau: Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, Bristol Myers Squibb, Amgen, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas, Myovant Sciences, Gilead Sciences, AVEO. Research Funding: Genentech, Exelixis, Janssen, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Macrogenics, Astellas Pharma, BeyondSpring Pharmaceuticals, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, Epizyme. Travel, Accommodations, Expenses: Genentech, Prometheus, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, AstraZeneca.
Availability of data and materials: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Writing assistance: No assistance in writing was utilized.
References
- 1. Moreira I, Coelho S, Rodrigues Â, et al. Urachal carcinoma: a case of a rare neoplasm. Curr Probl Cancer 2021; 45: 100711. [DOI] [PubMed] [Google Scholar]
- 2. Collins DC, Velázquez-Kennedy K, Deady S, et al. National incidence, management and survival of urachal carcinoma. Rare Tumors 2016; 8: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget 2016; 7: 48832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021; 595: 432–437. [DOI] [PubMed] [Google Scholar]
- 5. Grunewald CM, Niegisch G, Albers P. Using circulating tumor DNA to guide adjuvant therapy in bladder cancer: IMvigor010 and IMvigor011. Eur Urol Focus 2022; 8: 646–647. [DOI] [PubMed] [Google Scholar]
- 6. Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013; 2: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin DJ, Shrestha A, Fellman D, et al. Association of sociodemographic characteristics with survival among patients with urachal cancer in California from 1988 to 2019. JAMA Oncol 2022; 8: 1505–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shao G, Xu C, Liu J, et al. Clinical, pathological, and prognostic analysis of urachal carcinoma. Urol Int 2022; 106: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stokkel LE, Stokkel MPM, Donswijk ML, et al. The diagnostic value of FDG-PET/CT for urachal cancer. Clin Genitourin Cancer 2021; 19: 373–380. [DOI] [PubMed] [Google Scholar]
- 10. Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984; 131: 1–8. [DOI] [PubMed] [Google Scholar]
- 11. Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer 2006; 107: 712–720. [DOI] [PubMed] [Google Scholar]
- 12. Hamilou Z, North S, Canil C, et al. Management of urachal cancer: a consensus statement by the Canadian Urological Association and Genitourinary Medical Oncologists of Canada. Can Urol Assoc J 2020; 14: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flaig TW, Spiess PE, Chair V, et al. NCCN guidelines version 2.2023 bladder cancer rick bangs, MBA ¥ patient advocate, https://www.nccn.org/home/member- (2023, accessed 15 May 2023).
- 14. Mylonas KS, O’Malley P, Ziogas IA, et al. Malignant urachal neoplasms: a population-based study and systematic review of literature. Urol Oncol 2017; 35: 33.e11–33.e19. [DOI] [PubMed] [Google Scholar]
- 15. Collazo-Lorduy A, Castillo-Martin M, Wang L, et al. Urachal carcinoma shares genomic alterations with colorectal carcinoma and may respond to epidermal growth factor inhibition. Eur Urol 2016; 70: 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varadi M, Nagy N, Reis H, et al. Clinical sequencing identifies potential actionable alterations in a high rate of urachal and primary bladder adenocarcinomas. Cancer Med 2023; 12: 9041–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siefker-Radtke A. Urachal adenocarcinoma: a clinician’s guide for treatment. Semin Oncol 2012; 39: 619–624. [DOI] [PubMed] [Google Scholar]
- 18. Moding EJ, Liu Y, Nabet BY, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer 2020; 1: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanz-Garcia E, Zhao E, Bratman SV, et al. Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: current research, opportunities, and challenges. Sci Adv 2022; 8: 8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M, Huang C, Zhou H, et al. Circulating tumor DNA predicts the outcome of chemotherapy in patients with lung cancer. Thorac Cancer 2022; 13: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6: 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer 2020; 1: 873–881. [DOI] [PubMed] [Google Scholar]
- 23. Signatera™ is a personalized, tumor-informed assay for ultrasensitive detection of molecular residual disease (MRD). [Google Scholar]
- 24. Ke C, Hu Z, Yang C. Preoperative accuracy of diagnostic evaluation of urachal carcinoma. Cancer Med 2023; 12: 9106–9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: The M. D. Anderson Cancer Center Experience. J Urol 2003; 169: 1295–1298. [DOI] [PubMed] [Google Scholar]
- 26. Stokkel LE, van Rossum HH, van de Kamp MW, et al. Clinical value of preoperative serum tumor markers CEA, CA19-9, CA125, and CA15-3 in surgically treated urachal cancer. Urol Oncol 2023; 41: 326.e17–326.e24. [DOI] [PubMed] [Google Scholar]