Abstract
The fission yeast Schizosaccharomyces pombe starts sexual development when starved for nutrients and simultaneously activated by mating pheromones. We have identified a new gene regulating the onset of this process. This gene, called nrd1+, encodes a typical RNA binding protein that preferentially binds poly(U). Deletion of nrd1+ causes cells to initiate sexual development without nutrient starvation. We have found that the biological role of nrd1+ is to block the onset of sexual development by repressing the Ste11-regulated genes essential for conjugation and meiosis until cells reach a critical level of starvation.
In the fission yeast Schizosaccharomyces pombe, co-occurrence of nutrient starvation and the mating-pheromone availability triggers the onset of sexual development (10, 23). This complex biological process is initiated by conjugation that takes place between opposite-mating-type cells, leading to the formation of diploid cells. These diploid cells are short-lived and immediately undergo meiosis and subsequent sporulation to complete sexual development.
The Ste11 transcriptional factor plays a central role in the initiation and progression of this process (45). Nutritional starvation, particularly nitrogen starvation, leads to induction of the ste11+ gene, which in turn induces many genes needed for conjugation and meiosis (20, 45). fus1+ and sxa2+ are among those induced and required for conjugation, whereas mei2+, mat1-Pm, and rep1+ are among those induced and required for meiosis (1, 17, 39, 46, 50). Many Ste11-regulated genes additionally require a mating-pheromone signal for their induction, but others do not. Among the genes mentioned above, mei2+ can be nearly fully induced without mating-pheromone signals but the rest require pheromone signals for full induction (1, 17, 39, 46, 50).
mei2+, which is absolutely required for meiosis, is rapidly induced by nitrogen starvation (43). However, the Mei2 protein itself is held largely inactive until conjugation takes place. This is achieved by the action of the Pat1 kinase, which directly inactivates Mei2 by phosphorylation (51). Conjugation activates the mei3+ gene, which encodes an inhibitor of Pat1 kinase. The induced Mei3 protein inactivates Pat1, thereby rescuing Mei2 to initiate meiosis (28). In addition, the G1 arrest-inducing ability of Ste11 is modulated by direct phosphorylation by Pat1 kinase, although its ability to promote conjugation does not seem to be influenced (24). Thus, the main role of Pat1 kinase is to block the onset of meiosis until conjugation takes place. Inactivation of Pat1 in heterothallic haploid cells therefore induces unconditional meiosis and inevitable cell death (7). This cell death can effectively be suppressed by inactivating mei2+ or ste11+, since cells cannot commit lethal meiosis without either (45). Consequently, any factors that repress ste11+ or mei2+ or inhibit their function are likely to suppress the Pat1 phenotype.
Cyclic AMP (cAMP) is a major second messenger mediating a carbon source signal and controlling sexual differentiation (14, 25, 26). gpa2+ encodes the α subunit of the heterotrimeric G protein responsible for the activation of adenylate cyclase (18). Cells with this factor deleted are unable to elevate the cAMP level upon stimulation with glucose, resulting in constitutive activation of sexual development and glyconeogenesis despite the absence of nutritional starvation (14, 18). Similarly, chromosomal deletion of either cyr1+ or pka1+, which encode adenylate cyclase and the catalytic subunit for the cAMP-dependent protein kinase, respectively, causes derepression of sexual development (25, 26). In these strains, the level of ste11+ mRNA is highly elevated despite the presence of abundant nutrient (45, 53). Conversely, addition of a high concentration of cAMP to the medium inhibits mating (33).
Starvation for the nitrogen source is the most effective condition promoting sexual development in fission yeast. Nitrogen starvation preferentially arrests cells in G1 and induces ste11+ mRNA (35, 45). The cAMP-Pka1 pathway mediates at least some nitrogen signals (29), and Rcd1, a newly identified factor that is highly conserved among eukaryotes, is required for nitrogen starvation-responsive ste11+ induction under the regular differentiation conditions (38). Nevertheless, our understanding of how the onset of sexual development is regulated by nutrient starvation signals is still far from complete.
Several other signal pathways also control sexual development. As we reported previously, cell cycle “start” genes, such as res1+ and res2+, inhibit sexual differentiation (35). The Cig2/Cyc17 B-type cyclin mediates this inhibition (35). Meanwhile, the function of this cyclin is negatively regulated by Srw1, a WD repeat protein that plays a key role in switching between differentiation and proliferation in response to at least nitrogen starvation (55). A stress signal mediated by the Wis1-Sty1/Spc1/Phh1 mitogen-activated protein kinase kinase (MAPKK)-MAPK positively regulates sexual differentiation by inducing ste11+ via the Atf1 transcriptional factor (19, 44, 54). Several other genes are known to influence differentiation. Among those are pac2+ and puc1+ (9, 21). pac2+ encodes a protein with no homology to any proteins in the database, whereas puc1+ encodes a protein similar to the budding-yeast G1 cyclins, although how they influence differentiation is not known. Thus, multicascades mediating the signals for nutrient, the cell cycle start, and stress positively and negatively regulate the initiation of differentiation.
In a search for new genes controlling the onset of differentiation in fission yeast, we have identified one such gene named nrd1+, which encodes a typical RNA binding protein. In this paper, we report the structure and function of the Nrd1 RNA binding protein, whose role seems to be to block the onset of differentiation by repressing ste11+-regulated genes until the cells reach a critical level of nutrient starvation.
MATERIALS AND METHODS
Strains and media.
The strains of S. pombe used in this study are listed in Table 1. Media were prepared as described previously (6, 13, 30, 34, 37).
TABLE 1.
S. pombe strains used in this study
| Strain | Relevant genotype |
|---|---|
| L968 | h90 |
| L972 | h− |
| K150-A6 | h−leu1-32 |
| K153-B8 | h90 leu1-32 |
| D1 | h−/h+ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 |
| 4DD25 | h−/h+ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 nrd1+/nrd1::ura4+ |
| 14-1 | h90 ura4-D18 nrd1::ura4+ |
| 109-1 | h−ura4-D18 nrd1::ura4+ |
| 4DH63 | h90 leu1-32 ura4-D18 nrd1::ura4+ |
| 4DH61 | h−leu1-32 ura4-D18 nrd1::ura4+ |
| SO6 | h−pat1-114 leu1-32 |
| KT23 | h−pat1-114 leu1-32 ura4-D18 nrd1::ura4+ |
| TI-150 | h−pat1-114 leu1-32 ura4-294 cyc17::ura4+ |
| KT76 | h−pat1-114 leu1-32 ura4-D18 pka1::ura4+ |
| KT56 | h90 his1-102 wis1::his1+ |
| KT55 | h−his1-102 wis1::his1+ |
| KT86 | h90 ura4-D18 nrd1::ura4+his1-102 wis1::his1+ |
| KT34 | h−ura4-D18 nrd1::ura4+his1-102 wis1::his1+ |
| 639 | h90 ura4-D18 rcd1::ura4+ |
| KT96 | h90 ura4-D18 rcd1::ura4+nrd1::ura4+ |
Libraries and vectors.
The S. pombe genomic library was constructed by inserting HindIII-digested wild-type (L972) genomic DNA into the HindIII-digested pALSK+ vector (37). The expression vectors pcL, pREP1, and pFLAG2 (IBI) for epitope tagging have been described elsewhere (16, 27, 32). The S. pombe cDNA library was constructed with mRNA prepared from the L972 wild-type cells grown to mid-log phase and with the pcD vector (5) as described previously (36).
Cloning of nrd1+ and DNA sequencing.
The nrd1+ gene was isolated as described previously (37) with h− pat1-114 leu1-32 (SO6) as a cloning host. After transformation with the S. pombe genomic library, cells were spread on minimal medium agar plates and incubated at 23°C for 24 h. The plates were then incubated at 32.2°C for 3 to 5 days to select complemented cells. The long 5′-flanking region of the 3.6-kb nrd1+ gene isolated was trimmed down to 0.1 kb upstream and downstream of the nrd1+ open reading frame with exonuclease III, and cloned into the pALSK+ or pcL vector. DNA was sequenced by the dideoxynucleotide method (42) after being subcloned into M13-driven vectors and pBluescriptII KS+ (Stratagene).
RNA binding analysis.
Synthetic poly(A), poly(U), poly(C), and poly(G) homopolymers were labeled with [γ-32P]ATP and polynucleotide kinase. The h− ura4-D18 leu1-32 nrd1::ura4+ cells were transformed with the pREP1 vector containing C-FLAG Nrd1 or no insert and grown to log phase, and cell extracts were prepared (3). The cell extracts (9.5 μg of protein) were then incubated at room temperature for 10 min in a total 20-μl reaction mixture containing fivefold-diluted buffer H, 10 μg of Escherichia coli tRNA, and 3 × 105 cpm of a labeled homopolymer. The reaction mixtures were then irradiated for 10 min on ice with a germicidal light placed 4 cm above the samples. The irradiated solutions were diluted with 1 ml of buffer H, incubated with a 25-μl suspension of anti-FLAG M2 affinity gel (IBI) at room temperature for 1 h, and centrifuged at 5,000 rpm for 2 min in a microcentrifuge with a TMA-6 rotor (Tomy). Immunoprecipitates were washed six times with buffer H containing 1 mg of bovine serum albumin per ml and 150 mM NaCl, and the radioactivity in the precipitates was determined. In a competition assay, excess unlabeled homopolymer was added to the initial reaction mixture.
Deletion analysis.
The truncated nrd1 genes were generated by PCR with appropriate primers and inserted in the pcL vector. The deletion mutant and the full-length nrd1+ genes inserted into the vector were transfected into a pat1-114 mutant and incubated on minimal medium agar plates at 23°C for 24 h and then at 32.5°C for 4 days or incubated at 23°C for 6 days to determine the numbers of both rescued and stably transfected cells. The suppressor activities were calculated by dividing the number of colonies formed at 32.5°C by the number of colonies formed at 23°C.
Gene disruption.
The mutant strain containing a null nrd1 gene was constructed as follows. The 6.4-kb genomic fragment containing nrd1+ was isolated from an S. pombe EcoRI partially digested genomic library by colony hybridization. The 2.0-kb SphI-SpeI region containing 92% of the nrd1+ open reading frame was replaced with the 1.8-kb HindIII genomic DNA fragment of the ura4+ gene (pALSK-nrd1::ura4+). Inactivation of nrd1+ in this construct was confirmed by its inability to rescue the pat1-114 mutant. A diploid strain (D1) was transformed with the nrd1::ura4+ DNA excised from pALSK-nrd1::ura4+ with EcoRI and XhoI endonucleases. Stable ura+ transformants were selected, and the nrd1+ locus was analyzed by Southern blot hybridization with the SphI fragment of nrd1+ as a probe. One transformant (4DD25) successfully disrupted for one nrd1+ allele was induced to undergo sporulation. After ethanol treatment, random spores were tested for the Ura+ phenotype.
Conjugation assay.
The mating frequencies of h90 (L968) and h90 ura4-D18 nrd1::ura4+ (14-1) were assayed as follows. The cells were grown to mid-log phase in pombe minimum (PM) medium (containing 0.5% NH4Cl and 2% glucose), washed, inoculated into NH4Cl-free PM medium or PM medium with the indicated concentrations of NH4Cl and glucose at a density of 3 × 106 to 1 × 107 cells/ml, and incubated at 27 or 30°C. At the indicated times, 1 ml each of cell suspension was collected and sonicated gently and the number of zygotes were counted under the microscope. The percent mating frequencies were calculated by dividing the number of zygotes (one zygote counted as two cells) by the number of total cells.
Northern blot analysis.
Total RNA was prepared and Northern blot analysis was performed as described previously (8, 31). The DNA probes used were the 1.3-kb PvuII fragment for ste11+ (45), the 3.2-kb ClaI fragment for mei2+ (50), the 1.9-kb cDNA fragment for rep1+ (46), and the 0.7-kb HindIII fragment for sxa2+ (17).
RESULTS
Isolation of the nrd1+ gene.
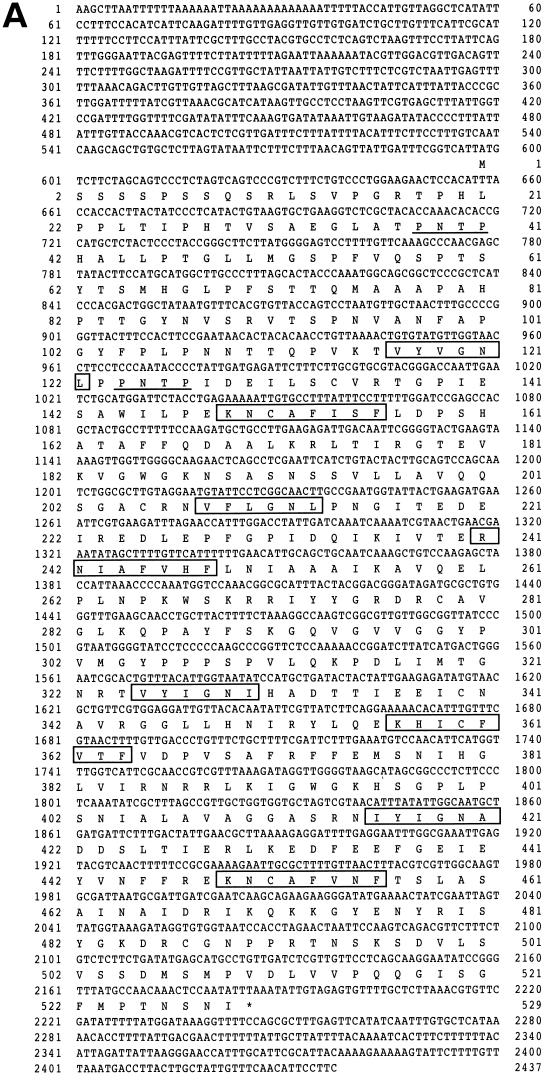
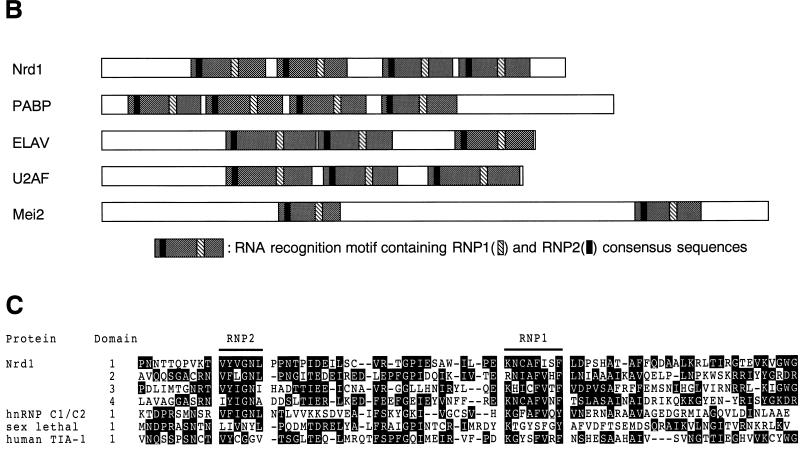
To isolate new genes controlling the onset of differentiation of S. pombe, a genomic library of this yeast was screened for genes that could suppress the temperature-sensitive pat1-114 mutant. One such clone, called nrd1+ (negative regulator of differentiation), contained a 3.6-kb HindIII genomic DNA insert. It rescued the lethality of the pat1-114ts mutant at nonpermissive temperatures up to 35°C. The nucleotide and predicted amino acid sequences of the nrd1+ gene were determined from both the genomic DNA and a corresponding cDNA that was isolated subsequently (Fig. 1A). nrd1+ contains an open reading frame capable of encoding a 529-amino-acid protein with a calculated molecular mass of 57,760 Da. The predicted Nrd1 protein has four repeats of the typical RNA recognition motif (RRM) containing two semiconserved sequences called RNP1 and RNP2. This class of RNA binding proteins includes the Saccharomyces cerevisiae poly(A) binding protein (41) and differs from others in the number of RRMs (Fig. 1B). Unlike Nrd1, Mei2, which is essential for meiosis, contains two RRMs (52). The four RRMs in Nrd1 show a mild level of amino acid homology to one another and to the motif of other RNA binding proteins (Fig. 1C). In addition, Nrd1 possesses two potential MAPK phosphorylation sites in the very amino-terminal region (Fig. 1A).
FIG. 1.
(A) Nucleotide sequence of nrd1+ and the predicted amino acid sequence of the encoded protein. The conserved amino acid sequences of RNP1 and RNP2 are boxed. The MAPK phosphorylation consensus sequences are underlined. (B) Schematic illustration of the structure of Nrd1 protein, the S. cerevisiae poly(A) binding protein (PABP) (41), Drosophila melanogaster ELAV (40), human U2AF (56), and S. pombe Mei2 (52). Nrd1 carries four RRMs. Each RRM includes two highly conserved sequences designated RNP1 and RNP2. (C) The four RRM regions of Nrd1 are aligned with those carried by other RNA binding proteins, which include human hnRNP C1/C2 (4), the Drosophila Sx1 gene product (2), and human TIA-1 (47). Amino acid residues identical among these proteins are highlighted.
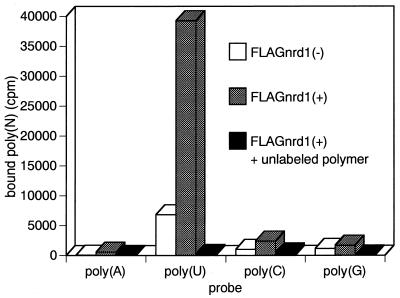
As indicated by its structure, Nrd1 has an RNA binding activity. When assayed for in vitro binding of RNA homopolymers, Nrd1 preferentially bound poly(U), displaying a character as a typical polypyrimidine tract-binding protein (Fig. 2).
FIG. 2.
Nrd1 has RNA binding activity. Extracts prepared from h− Δnrd1/empty pREP1 or h− Δnrd1/pREP1C-FLAG-Nrd1 cells were incubated with 32P-labeled poly(A), poly(U), poly(C), or poly(G) in the presence or absence of a 100-fold excess of unlabeled homopolymers. The RNA-protein complex was UV cross-linked and immunoprecipitated with anti-FLAG affinity gel. The radioactivity of precipitates was counted.
RNA binding domains are essential for Nrd1 function.
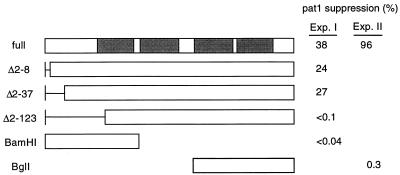
To define the region essential for function, several deletion mutants of the nrd1+ gene were constructed and examined for their ability to rescue the pat1 mutation as a conventional assay. In some of these constructs, 2 to 123 amino acids spanning from the second amino acid toward the C terminus were progressively deleted. Deletion of 7 or 36 amino acids only slightly decreased the activity. However, when 122 amino acids (Δ2–123), which included RNP2 in the first RRM, were deleted, nrd1+ was completely inactivated (Fig. 3). In addition, neither the fragment containing the N-terminal first RRM nor the one containing the C-terminal two RRMs was active. These results indicate that all the RRMs are likely to be essential for Nrd1 activity.
FIG. 3.
Deletion analysis of nrd1+ and identification of functionally essential regions. Truncated or full-length nrd1+ genes were cloned into the pcL (Exp. I) or pALSK vector (Exp. II) and transfected into h− pat1-114 leu1-32 cells. The percent suppression was calculated by dividing the number of colonies formed at 32.5°C by the number of colonies formed at 23°C. Shaded boxes indicate the RRMs.
Cells with nrd1+ deleted conjugate without nutrient starvation.
To investigate the physiological role of nrd1+, nrd1 disruptants were constructed by one-step gene replacement. The SphI-SpeI region in the nrd1+ gene covering 92% of the coding region was replaced with the 1.8-kb ura4+ gene. Complete inactivation of nrd1+ function in the construct was confirmed by its inability to rescue the pat1-114ts mutant. A Ura− diploid strain was then transformed with the 5.1-kb EcoRI-XhoI fragment containing the disrupted nrd1+ gene, and stable Ura+ transformants were selected. Cells with one nrd1+ allele deleted were identified by Southern blotting and subsequently sporulated to obtain haploid segregants. All the Δnrd1 segregants were viable. To eliminate possible second mutations, they were backcrossed with the wild-type strain several times before being subjected to further analysis.
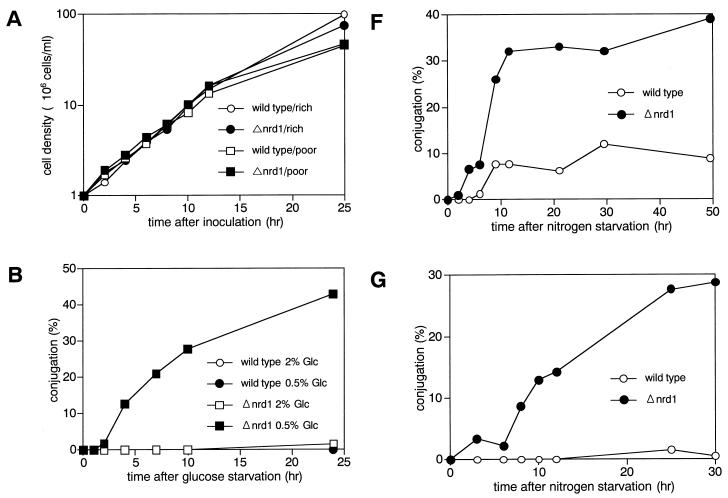
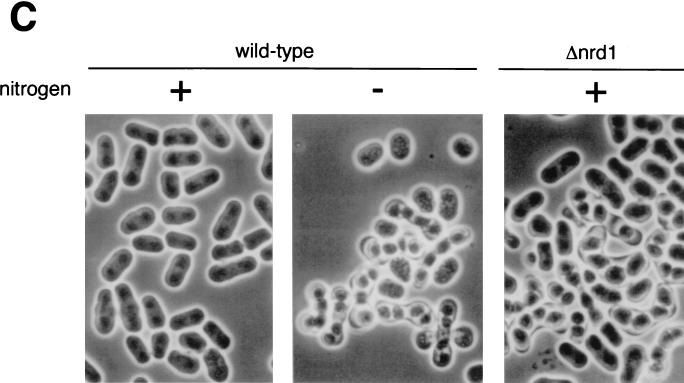
Heterothallic Δnrd1 cells were indistinguishable from the wild-type strain in growth properties and morphology under the various culture conditions tested. They arrested and resumed growth in response to nitrogen or glucose starvation and refeeding, respectively. As shown in Fig. 4A, in both rich and poor synthetic media, they grew at the same rates as nrd1+ cells. Nonetheless, in low-glucose but high-ammonium chloride medium, homothallic h90 Δnrd1 cells behaved as if they were starved for nitrogen and actively underwent conjugation. In growth medium containing 2% glucose and 0.5% ammonium chloride as the sole carbon and nitrogen sources, they were indistinguishable from the wild-type cells, staying uncommitted to differentiation. However, when the glucose concentration was lowered to 0.5%, the disruptant began to conjugate and undergo meiosis very efficiently, reaching a frequency of 40% conjugation after a 24-h incubation (Fig. 4B). The cell of the disruptant performing conjugation was relatively long, and its size was similar to that of the wild-type cells that were fully fed with nitrogen (Fig. 4C), which is consistent with conjugation being performed without nitrogen starvation. Under these conditions, wild-type cells scarcely conjugated.
FIG. 4.
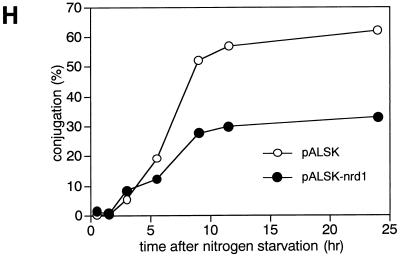
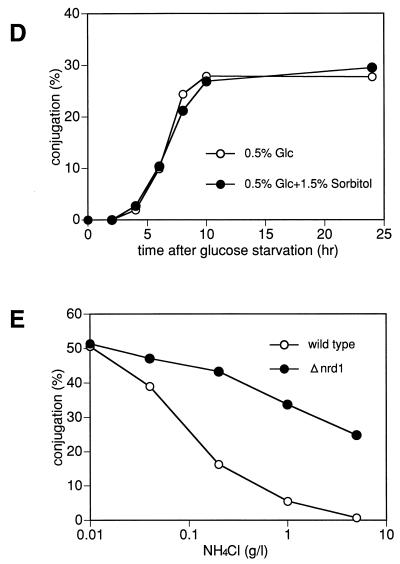
(A) Δnrd1 cells are indistinguishable from wild-type cells in their proliferation ability in rich and poor medium. The h− (open symbols) and h− Δnrd1 (solid symbols) cells were grown in PM medium to mid-log phase. Each strain was then inoculated into PM medium containing 0.5% NH4Cl and 2% glucose (circles), or PM medium containing only 0.1% NH4Cl and 0.5% glucose (squares) at 106 cells/ml and incubated at 30°C for the indicated times, and the number of cells was counted. (B) Δnrd1 cells mate without nitrogen starvation. The h90 nrd1+ and h90 Δnrd1 cells were grown in PM medium to mid-log phase. Each strain was then inoculated in PM medium or PM medium containing 0.5% glucose at 3 × 106 cells/ml and incubated at 30°C for the indicated times, and the number of zygotes formed was counted. The percent population of zygotes was calculated as described in Materials and Methods. (C) Cell morphology of Δnrd1 cells. Each strain was incubated in 0.5% glucose medium with or without nitrogen at 30°C for 24 h. (D) Effect of osmotic pressure on enhanced conjugation. The h90 Δnrd1 cells were grown in complete PM medium and then incubated in PM medium containing only 0.5% glucose or 0.5% glucose plus 1.5% sorbitol for the indicated times, and the number of zygotes formed was counted. (E) Conjugation of Δnrd1 cells at various nitrogen concentrations. The h90 and h90 Δnrd1 cells were grown in PM medium to mid-log phase. Each strain (5 × 106 cells) was inoculated into 1 ml of PM medium containing 0.5% glucose and the indicated concentrations of nitrogen and incubated at 27°C for 40 h, and the number of zygotes was counted. (F and G) Conjugation of Δnrd1 cells in nitrogen-starved, high-glucose medium. The h90 and h90 Δnrd1 cells were grown in PM medium to mid-log phase. Each strain was then inoculated in NH4Cl-free PM medium (F) or PM medium containing 0.01% NH4Cl (G) at a concentration of 1 × 107 and 5 × 106 cells/ml, respectively, and incubated at 30°C for the indicated times, and the number of zygotes was counted. (H) Overexpression of nrd1+ inhibits the conjugation of wild-type cells. Exponentially growing h90 leu1-32 cells harboring pALSK or pALSKnrd1+ were incubated for the indicated times in nitrogen-free PM medium containing 0.5% glucose, and the number of zygotes was counted.
The induction of conjugation of the disruptants by a shift to low glucose concentrations was not caused by a change in osmotic pressure. Adjustment of the osmotic pressure by addition of 1.5% sorbitol to the medium failed to block the onset of conjugation (Fig. 4D). These results were fully confirmed in a separate experiment, in which the glucose concentration was fixed at 0.5% and the concentration of ammonium chloride, used as the sole nitrogen source, was varied (Fig. 4E). As shown in Fig. 4E, 0.5% ammonium chloride (5 g/liter) was generally sufficient to block the sexual development of wild-type cells but allowed the disruptant to perform conjugation at half the maximum level (Fig. 4E). At this glucose concentration fixed to 0.5%, nitrogen starvation increased its conjugation frequency, but only twofold, and the fully induced frequency was the same as that of the wild-type cells. Thus, the disruptant was the same as the wild-type cells in full conjugation ability but was largely defective in regulation of the start of sexual development.
The commitment of the nrd1 disruptant to sexual development without nitrogen starvation does not necessarily mean that the function of this gene is specific to nitrogen starvation. In fact, Δnrd1 cells were markedly enhanced in conjugation in medium containing high glucose concentrations but no nitrogen. When wild-type cells were exposed to PM medium containing 2% glucose and no nitrogen, conjugation was significantly suppressed, reaching a conjugational frequency of only 10% versus the regular conjugation frequencies of 30 to 50% because of blocking by the high concentration of glucose (Fig. 4F). Under these conditions, the Δnrd1 cells conjugated much more efficiently. They started to conjugate earlier and reached a conjugation frequency of 40%, a value that wild-type cells can generally reach under regular mating conditions (medium containing a low glucose concentration [0.5%] and no nitrogen). Thus, high glucose concentrations failed to effectively inhibit the conjugation of Δnrd1 cells. This phenotype became more evident in PM medium containing 0.01% ammonium chloride and 2% glucose (Fig. 4G). In this medium, wild-type cells were unable to commit conjugation whereas the disruptant efficiently underwent conjugation, resembling the behavior in low-glucose, high-ammonium chloride medium. These results indicate that the ability of nrd1+ to inhibit the onset of sexual development is not specifically associated with particular nutrients. Consistent with the differentiation-inhibitory action of nrd1+, overexpressed nrd1+ mildly but significantly inhibited the conjugation of wild-type cells even under severe nutrient starvation for full induction of sexual development (Fig. 4H).
ste11+-regulated genes are derepressed in Δnrd1 cells and repressed in nrd1+-overexpressed cells.
The next question we examined is how nrd1+ inhibits differentiation. As is known for the budding yeast, the factors controlling the cell cycle start greatly influence differentiation. We therefore examined possible roles for nrd1+ in this control, but as described above, we failed to detect any such roles in the cell cycle start. Moreover, the lack of any apparent defects in growth ability of the nrd1 disruptant also indicates that this gene is not involved in general nutrient metabolism or in nutrient-sensing signal pathways.
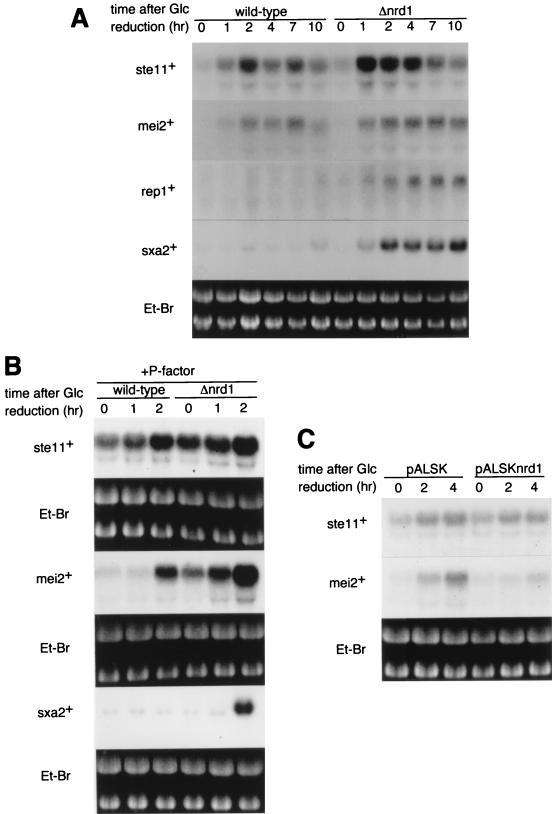
nrd1+ was isolated as a multicopy suppressor of the lethal pat1 mutation. This ability of nrd1+ is very probably attributable to its ability to block meiosis. Blocking meiosis could be achieved by the inhibition of mei2+ or other genes specifically required for meiosis. Alternatively, it could be achieved by the inactivation of Ste11 function, which is essential for mei2+ induction (45). As described above, a defect in the control of the onset of conjugation, not meiosis, is apparent for Δnrd1 cells. We therefore reasoned that nrd1+ might be involved in the regulation of ste11+ expression itself or its activity, and so we examined the effect of nrd1+ deletion on the expression of ste11+ and typical ste11+-regulated genes, such as mei2+, rep1+, and sxa2+. rep1+ and sxa2+ encode a zinc finger protein required for the onset of premeiotic DNA synthesis and a protease thought to degrade the mating pheromone P-factor, respectively, and both genes contain the Ste11-responsive element in their promoters (20, 46). The levels of the mei2+, rep1+, and sxa2+ transcripts expressed in homothallic nrd1+ and Δnrd1 cells were examined after the shift from 2 to 0.5% glucose in nitrogen-rich medium. As shown in Fig. 5A, ste11+ and mei2+ were induced upon the shift in both cell types, although induction was higher in Δnrd1 cells. A more significant difference between the two cell types, however, was seen in rep1+ and sxa2+ induction. These genes were markedly induced in Δnrd1 cells but remained largely repressed in nrd1+ cells. The induction of sxa2+ (and perhaps also rep1+) in Δnrd1 cells was not the consequence of conjugation. A similar assay was done for ste11+, mei2+, and sxa2+ mRNAs in the h− heterothallic cells stimulated with the mating pheromone P-factor, which mimics the presence of a mating partner (Fig. 5B). In this experiment, mei2+ was induced in both nrd1+ and Δnrd1 cells, although it was expressed to a certain extent in the disruptant before the shift to low glucose concentrations. Again, sxa2+ was induced only in Δnrd1 cells. Thus, when an abundance of the nitrogen source was available, nrd1+ repressed mating pheromone-dependent Ste11-regulated genes.
FIG. 5.
ste11+-regulated genes are derepressed in Δnrd1 cells and repressed by nrd1+ overexpression. (A) Expression of ste11+, mei2+, rep1+, and sxa2+ mRNA during glucose reduction. The homothallic h90 nrd1+ or h90 Δnrd1 cells were grown in PM medium to mid-log phase and then incubated for the indicated times in PM medium containing 0.5% glucose, and cellular RNA was prepared. Total RNA (20 μg each) was applied to each lane and analyzed by Northern blotting. (B) Expression of ste11+, mei2+, and sxa2+ mRNA in heterothallic cells during glucose reduction in the presence of P-factor. Exponentially growing h− nrd1+ and h− Δnrd1 cells were incubated for the indicated times in complete PM medium or PM medium with 0.5% glucose, both containing 2 μg of synthetic P-factor per ml. Total RNA was prepared and analyzed by Northern blotting. (C) Inhibition of mei2+ induction by overexpression of nrd1+. Exponentially growing h− leu1-32 cells harboring pALSK or pALSKnrd1+ were incubated for the indicated times in PM medium containing 0.5% glucose. The level of mei2+ transcript was analyzed by Northern blotting. Et-Br, ethidium bromide.
nrd1+ was also able to repress genes which do not require mating-pheromone signals for induction. As described above, when the glucose concentration was lowered from 2 to 0.5%, ste11+ and mei2+ were induced slightly more in the Δnrd1 cells than in the nrd1+ cells (Fig. 5A). However, repression was particularly noticeable when Nrd1 was overproduced. Overexpression of nrd1+ in h− cells significantly repressed the induction of mei2+ obtained by the glucose shift (Fig. 5C). A similar result was obtained with h90 cells (data not shown). These data suggest that Nrd1 represses Ste11-regulated genes, particularly those that require mating-pheromone signaling for their induction.
Nrd1 acts independently of the cAMP-Pka1, Wis1-Phh1/Sty1, and Rcd1 pathways.
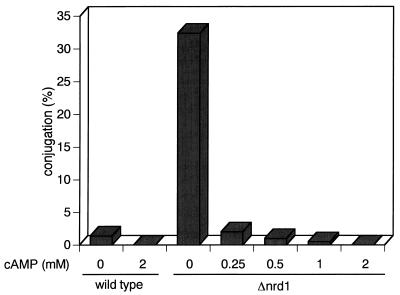
The data described above suggest that Nrd1 is not associated with particular nutrients or extracellular signals. This was supported by its functional independence from known pathways. The cAMP-Pka1 pathway mediates carbon and nitrogen signals (29). Therefore, disruption of any point in the cAMP-Pka1 pathway causes cells to commit to sexual development without nutritional starvation (14, 18, 25, 26). Conversely, enforced activation of the pathway, such as by adding a high concentration of cAMP to the medium, inhibits mating (33). Similarly, the addition of various concentrations of cAMP to the medium effectively inhibited the low-glucose-induced mating of Δnrd1 cells (Fig. 6), which was complete at cAMP levels as low as 0.5 mM, indicating that the inhibition of conjugation by cAMP does not require Nrd1.
FIG. 6.
The low-glucose-induced conjugation of Δnrd1 cells is inhibited by exogenously added cAMP. Exponentially growing h90 nrd1+ and h90 Δnrd1 cells were cultured at 30°C for 48 h in PM medium containing 0.5% glucose, the indicated concentrations of cAMP, and 1 mM caffeine, and examined for conjugation efficiencies.
As noted previously, pat1 suppression is a convenient way to assay epistatic relations among the genes controlling the onset of sexual development (35, 38). In this assay, nrd1+ rescued the pat1-induced lethality in the absence of pka1+ (Table 2). Reciprocally, pka1+ rescued the pat1-induced lethality without nrd1+, providing additional evidence to support the above result.
TABLE 2.
Nrd1 function is independent of Pka1 and Cig2a
| Mutant | % Suppressionb after transformation with:
|
|||
|---|---|---|---|---|
| pcL-x | pcLnrd1+ | pcLcig2+ | pcLpka1+ | |
| pat1-114 | <0.04 | 14 | 61 | 39 |
| pat1-114 Δnrd1 | <0.07 | 9 | 72 | 37 |
| pat1-114 Δcig2 | <0.06 | 5 | 106 | 55 |
| pat1-114 Δpka1 | 0.4 | 17 | 0.8 | 53 |
The pat1-114, pat1-114 Δnrd1, pat1-114 Δcyc17, or pat1-114 Δpka1 mutant cells were transformed with the indicated plasmids and selected at the minimum restriction temperatures for each strain: 32.5°C for pat1-114, 32.2°C for pat1-114 Δnrd1, 31.8°C for pat1-114 Δcyc17, and 29.5°C for pat1-114 Δpka1.
The percent suppression was calculated by dividing the number of colonies formed at each restriction temperature by the number of colonies formed at 23°C.
Using the same pat1 rescue assay, we also examined a possible interaction between Nrd1 and Cig2 cyclin that negatively regulates the onset of sexual development at a step downstream of ste11+ induction (35, 55). nrd1+ rescued pat1-114 without cig2+ and vice versa, indicating that Nrd1 was also independent of Cig2 cyclin. Interestingly, Pka1 was absolutely required for suppression of the pat1-induced lethality by cig2+.
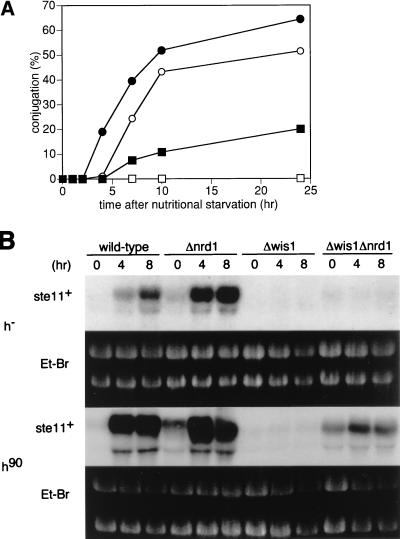
The stress signal mediated by the Wis1-Sty1/Spc1/Phh1 MAPKK-MAPK pathway is also essential for ste11+ induction during conjugation (19, 44, 54). We examined a possible relationship between nrd1+ and this pathway by analyzing the phenotype of a Δwis1 Δnrd1 double disruptant (Fig. 7A). The sterility of Δwis1 cells was partially suppressed by the deletion of nrd1+, with concomitant partial restoration of ste11+ expression, which was seen in mating pheromone-activated homothallic cells (Fig. 7B), again indicating that nrd1+ was independent of the Wis1-Phh1 pathway.
FIG. 7.
nrd1+ is independent of the Wis1-Phh1 stress signal pathway. (A) The h90 (open circles), h90 Δnrd1 (solid circles), h90 Δwis1 (open squares), and h90 Δnrd1 Δwis1 (solid squares) cells were grown in PM medium to mid-log phase. Each strain was then inoculated into nitrogen-free PM medium plus 0.5% glucose at a concentration of 107 cells/ml and incubated at 30°C for the indicated times, and the number of zygotes was counted. (B) Expression of ste11+ mRNA during nutritional starvation in h90 and h− cells with the wild-type, Δnrd1, Δwis1, or Δnrd1Δwis1 genotype. Logarithmically growing cells of each strain were inoculated into nitrogen-free PM medium plus 0.5% glucose at a concentration of 107 cells/ml and incubated at 30°C for the indicated times. Total RNA was prepared, and 20 μg was applied to each lane for Northern blot analysis. Et-Br, ethidium bromide.
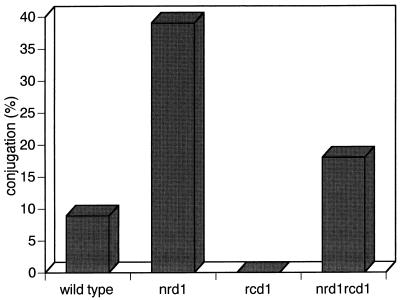
nrd1+ was also independent of rcd1+, which is specifically required for nitrogen starvation-induced ste11+ expression under the regular mating conditions (38). The sterility of the rcd1 disruptant was largely suppressed by the deletion of nrd1+ (Fig. 8).
FIG. 8.
Deletion of nrd1+ suppresses the sterility of Δrcd1 cells. The h90, h90 Δnrd1, h90 Δrcd1, and h90 Δnrd1 Δrcd1 cells were grown in PM medium to mid-log phase. Each strain was then inoculated into nitrogen-free PM medium plus 2% glucose at a concentration of 107 cells/ml and incubated at 30°C for 50 h, and the number of zygotes was counted.
DISCUSSION
Nrd1 is a typical RNA binding protein with four conserved motifs, which has an ability to bind uridine-rich sequences. At least the first motif, residing 100 amino acids from the N terminus, as well as the third and fourth motifs is essential for activity. The most noticeable phenotype of the cells lacking this gene is commitment to conjugation despite the absence of nutrient starvation (Fig. 4A and G). In medium with either the NH4Cl or glucose concentration reduced, nrd1 disruptants actively conjugated despite the presence of enough nutrients to sustain the growth and inhibit the mating of wild-type cells. This phenotype indicates that the function of nrd1+ is not specifically associated with a particular nutrient. Consistently, epistatic analysis shows that the activity of nrd1+ is independent of any of the pathways mediating carbon, nitrogen, and even stress signals. These data, taken together, suggest that the biological role of nrd1+ may be to control a threshold of cellular responses to nutrient starvation specifically for commitment to sexual development. The presence of only mild inhibition of conjugation by nrd1+ overexpression under the regular mating conditions is certainly consistent with this possibility.
The ultimate, if not the direct, targets for Nrd1 action are the genes regulated by the Ste11 transcriptional factor. There seem to be two types of Ste11-regulated genes, one largely inducible without a mating-pheromone signal and the other inducible only with a mating-pheromone signal (1, 17, 39, 46, 50). Both types of genes are targets for nrd1+ action. sxa2+ (and rep1+) represents the second type (17, 46). Nrd1 effectively blocks the expression of this type of gene when a sufficient concentration of nutrient is present. Regulation of the first type of gene, represented by mei2+, was less clear but became noticeable when nrd1+ was overexpressed. Overexpression of nrd1+ markedly reduced the induction of mei2+ triggered by a shift to low glucose concentrations.
Little information is available regarding the molecular mechanism by which Nrd1 blocks the induction of these genes. However, the possibility that Nrd1 directly regulates a mating-pheromone pathway seems to be remote, since we did not observe any significant signs of overactivation of the mating-signal pathway in the cells lacking nrd1+, which is typically seen in those carrying the constitutively active ras gene (11). Moreover, deletion of nrd1+ did not influence sterility caused by the ste1, ste5, and ste6 mutations in the pheromone signal pathway (unpublished observation). We therefore believe that the primary function of Nrd1 is probably to control Ste11-regulated gene expression. Nrd1 might interfere with the ability of Ste11 protein to bind the TR-box, a Ste11-responsive cis element present in both types of genes including ste11+ itself (1, 39, 45). Alternatively, Nrd1 might inhibit the production of the active Ste11 molecule in a posttranscriptional step, such as by decreasing ste11+ mRNA stability or its translation. These possibilities remain to be investigated.
If Nrd1 is indeed involved in controlling a threshold of cellular responses to nutrient starvation for commitment to sexual development, is Nrd1 regulated? So far we have no definitive evidence indicating that it is. The nrd1+ gene is constitutively expressed during nutrient starvation (unpublished observation). However, the activity of this protein might be regulated possibly by a MAPK. Nrd1 contains two MAPK phosphorylation consensus sequences. Replacement of Thr126 in the second MAPK phosphorylation consensus site by Asp mimicking phosphorylation, but not by Ala, markedly reduced its ability to rescue the pat1-114 mutant when ste11+ was massively induced by the deletion of the pka1+ gene (unpublished data).
Is Nrd1 evolutionarily conserved? The answer is likely to be yes. We recently isolated human and rat genes that seem to be functional homologs of nrd1+ by using the same screening strategy as the one for the isolation of nrd1+ (55a). Their amino acid homology to Nrd1 is low, but, like Nrd1, they contain four RNA binding motifs. They are indistinguishable from Nrd1 in biological activities in fission yeast. This protein is highly expressed in hematopoietic cells, particularly T cells and B cells. A factor called TCF-1 is essential for the terminal differentiation of T cells (49). Interestingly, TCF-1 is structurally homologous to Ste11 (48). Just as in fission yeast, cAMP and p38, a homolog of the Sty1/Spc1/Phh1 MAPK, are also involved in the regulation of growth and differentiation of hematopoietic cells (12, 22). In addition, many eukaryotes including mammals contain a highly conserved structural homolog of rcd1+ (38). Thus, the entire differentiation control system involving Ste11, Nrd1, Rcd1, cAMP, and the Phh1 stress MAPK might be conserved up to mammals.
ACKNOWLEDGMENTS
We thank Peter Fantes for the wis1− strain, Masayuki Yamamoto for the pka1− cells and sxa2+ gene, and Chikashi Shimoda for sharing unpublished information with us. We also thank Tomoko Obara-Ishihara, Tomohisa Kato, Jr., and Noriko Okazaki for the plasmids and yeast strains used in this study, and we thank K. Tanaka for valuable discussions.
This work was supported by research grants from the Ministry of Science, Education and Culture of Japan and HFSP.
REFERENCES
- 1.Aono T, Yanai H, Miki F, Davey J, Shimoda C. Mating pheromone-induced expression of the mat1-Pm gene of Schizosaccharomyces pombe: identification of signaling components and characterization of upstream controlling elements. Yeast. 1994;10:757–770. doi: 10.1002/yea.320100607. [DOI] [PubMed] [Google Scholar]
- 2.Bell L R, Maine E M, Schedl P, Cline T W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55:1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 3.Booher R N, Alfa C E, Hyams J S, Beach D. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- 4.Burd C G, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- 7.Egel R, Nielsen O, Weilguny D. Sexual differentiation in fission yeast. Trends Genet. 1990;6:369–373. doi: 10.1016/0168-9525(90)90279-f. [DOI] [PubMed] [Google Scholar]
- 8.Elder R T, Loh E Y, Davis R W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci USA. 1983;80:2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsburg S L, Nurse P. Identification of a G1-type cyclin puc1+ in the fission yeast Schizosaccharomyces pombe. Nature. 1991;351:245–248. doi: 10.1038/351245a0. [DOI] [PubMed] [Google Scholar]
- 10.Fukui Y, Kaziro Y, Yamamoto M. Mating pheromone-like diffusible factor released by Schizosaccharomyces pombe. EMBO J. 1986;5:1991–1993. doi: 10.1002/j.1460-2075.1986.tb04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukui Y, Kozasa T, Kaziro Y, Takeda T, Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986;44:329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- 12.Galcheva-Gargova Z, Dèrijard B, Wu I-H, Davis R J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 13.Gutz H, Heslot H, Leupold U, Loprieno N. Schizosaccharomyces pombe. In: King R C, editor. Handbook of genetics. Vol. 1. New York, N.Y: Plenum Press; 1974. pp. 395–446. [Google Scholar]
- 14.Hoffman C S, Winston F. Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev. 1991;5:561–571. doi: 10.1101/gad.5.4.561. [DOI] [PubMed] [Google Scholar]
- 15.Hughes D A, Yabana N, Yamamoto M. Transcriptional regulation of a Ras nucleotide-exchange factor gene by extracellular signals in fission yeast. J Cell Sci. 1994;107:3635–3642. doi: 10.1242/jcs.107.12.3635. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1+-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Yamamoto M. Schizosaccharomyces pombe sxa1+ and sxa2+ encode putative proteases involved in the mating response. Mol Cell Biol. 1992;12:1827–1834. doi: 10.1128/mcb.12.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isshiki T, Mochizuki N, Maeda T, Yamamoto M. Characterization of a fission yeast gene, gpa2, that encodes a Gα subunit involved in the monitoring of nutrition. Genes Dev. 1992;6:2455–2462. doi: 10.1101/gad.6.12b.2455. [DOI] [PubMed] [Google Scholar]
- 19.Kato T, Jr, Okazaki K, Murakami H, Stettler S, Fantes P A, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- 20.Kjærulff S, Dooijes D, Clevers H, Nielsen O. Cell differentiation by interaction of two HMG-box proteins: Mat1-Mc activates M cell-specific genes in S. pombe by recruiting the ubiquitous transcription factor Ste11 to weak binding sites. EMBO J. 1997;16:4021–4033. doi: 10.1093/emboj/16.13.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunitomo H, Sugimoto A, Wilkinson C R, Yamamoto M. Schizosaccharomyces pombe pac2+ controls the onset of sexual development via a pathway independent of the cAMP cascade. Curr Genet. 1995;28:32–38. doi: 10.1007/BF00311879. [DOI] [PubMed] [Google Scholar]
- 22.Lalli E, Sassone-Corsi P, Ceredig R. Block of T lymphocyte differentiation by activation of the cAMP-dependent signal transduction pathway. EMBO J. 1996;15:528–537. [PMC free article] [PubMed] [Google Scholar]
- 23.Leupold U. Sex appeal in fission yeast. Curr Genet. 1987;12:543–545. [Google Scholar]
- 24.Li P, McLeod M. Molecular mimicry in development: identification of ste11+ as a substrate and mei3+ as a pseudosubstrate inhibitor of ran1+ kinase. Cell. 1996;29:869–880. doi: 10.1016/s0092-8674(00)81994-7. [DOI] [PubMed] [Google Scholar]
- 25.Maeda T, Mochizuki N, Yamamoto M. Adenylyl cyclase is dispensable for vegetative cell growth in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1990;87:7814–7818. doi: 10.1073/pnas.87.20.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda T, Watanabe Y, Kunitomo H, Yamamoto M. Cloning of the pka1 gene encoding the catalytic subunit of the cAMP-dependent protein kinase in Schizosaccharomyces pombe. J Biol Chem. 1994;269:9632–9637. [PubMed] [Google Scholar]
- 27.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 28.McLeod M, Beach D. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature. 1988;332:509–514. doi: 10.1038/332509a0. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki N, Yamamoto M. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol Gen Genet. 1992;233:17–24. doi: 10.1007/BF00587556. [DOI] [PubMed] [Google Scholar]
- 30.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 31.Nagata A, Igarashi M, Jinno S, Suto K, Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991;3:959–968. [PubMed] [Google Scholar]
- 32.Nakashima N, Tanaka K, Sturm S, Okayama H. Fission yeast Rep2 is a putative transcriptional activator subunit for the cell cycle ‘start’ function of Res2-Cdc10. EMBO J. 1995;14:4794–4802. doi: 10.1002/j.1460-2075.1995.tb00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen O. Signal transduction during mating and meiosis in S. pombe. Trends Cell Biol. 1993;3:60–65. doi: 10.1016/0962-8924(93)90162-t. [DOI] [PubMed] [Google Scholar]
- 34.Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- 35.Obara-Ishihara T, Okayama H. A B-type cyclin negatively regulates conjugation via interacting with cell cycle ‘start’ genes in fission yeast. EMBO J. 1994;13:1863–1872. doi: 10.1002/j.1460-2075.1994.tb06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okayama H, Kawaichi M, Brownstein M, Lee F, Yokota T, Arai K. High-efficiency cloning of full-length cDNA; construction and screening of cDNA expression libraries for mammalian cells. Methods Enzymol. 1987;154:3–29. doi: 10.1016/0076-6879(87)54067-8. [DOI] [PubMed] [Google Scholar]
- 37.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki N, Okazaki K, Watanabe Y, Kato-Hayashi M, Yamamoto M, Okayama H. Novel factor highly conserved among eukaryotes controls sexual development in fission yeast. Mol Cell Biol. 1998;18:887–895. doi: 10.1128/mcb.18.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen J, Weilguny D, Egel R, Nielsen O. Characterization of fus1 of Schizosaccharomyces pombe: a developmentally controlled function needed for conjugation. Mol Cell Biol. 1995;15:3697–3707. doi: 10.1128/mcb.15.7.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinow S, Campos A R, Yao K M, White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988;242:1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- 41.Sachs A B, Bond M W, Kornberg R D. A single gene from yeast for both nuclear and cytoplasmic polyadenylate-binding proteins: domain structure and expression. Cell. 1986;45:827–835. doi: 10.1016/0092-8674(86)90557-x. [DOI] [PubMed] [Google Scholar]
- 42.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimoda C, Uehira M, Kishida M, Fujioka H, Iino Y, Watanabe Y, Yamamoto M. Cloning and analysis of transcription of the mei2 gene responsible for initiation of meiosis in the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1987;169:93–96. doi: 10.1128/jb.169.1.93-96.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto A, Iino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama A, Tanaka K, Okazaki K, Nojima H, Okayama H. A zinc finger protein controls the onset of premeiotic DNA synthesis of fission yeast in a Mei2-independent cascade. EMBO J. 1994;13:1881–1887. doi: 10.1002/j.1460-2075.1994.tb06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Q, Streuli M, Saito H, Schlossman S F, Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67:629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 48.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991;10:123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald H R, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe Y, Iino Y, Furuhata K, Shimoda C, Yamamoto M. The S. pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 1988;7:761–767. doi: 10.1002/j.1460-2075.1988.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe Y, Shinozaki-Yabana S, Chikashige Y, Hiraoka Y, Yamamoto M. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature. 1997;387:187–190. doi: 10.1038/386187a0. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe Y, Yamamoto M. S. pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell. 1994;78:487–498. doi: 10.1016/0092-8674(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe Y, Yamamoto M. Schizosaccharomyces pombe pcr1+ encodes a CREB/ATF protein involved in regulation of gene expression for sexual development. Mol Cell Biol. 1996;16:704–711. doi: 10.1128/mcb.16.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson M G, Samuels M, Takeda T, Toone W M, Shieh J C, Toda T, Millar J B A, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi S, Murakami H, Okayama H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Yamamoto, H., et al. Unpublished data.
- 56.Zamore P D, Patton J G, Green M R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]