Abstract
Light has broad applications in medicine as a tool for diagnosis and therapy. Recent advances in optical technology and bioelectronics have opened opportunities for wearable, ingestible, and implantable devices that use light to continuously monitor health and precisely treat diseases. In this review, we discuss recent progress in the development and application of light-based bioelectronic devices. We summarize the key features of the technologies underlying these devices, including light sources, light detectors, energy storage and harvesting, and wireless power and communications. We investigate the current state of bioelectronic devices for the continuous measurement of health and on-demand delivery of therapy. Finally, we highlight major challenges and opportunities associated with light-based bioelectronic devices and discuss their promise for enabling digital forms of health care.
I. INTRODUCTION
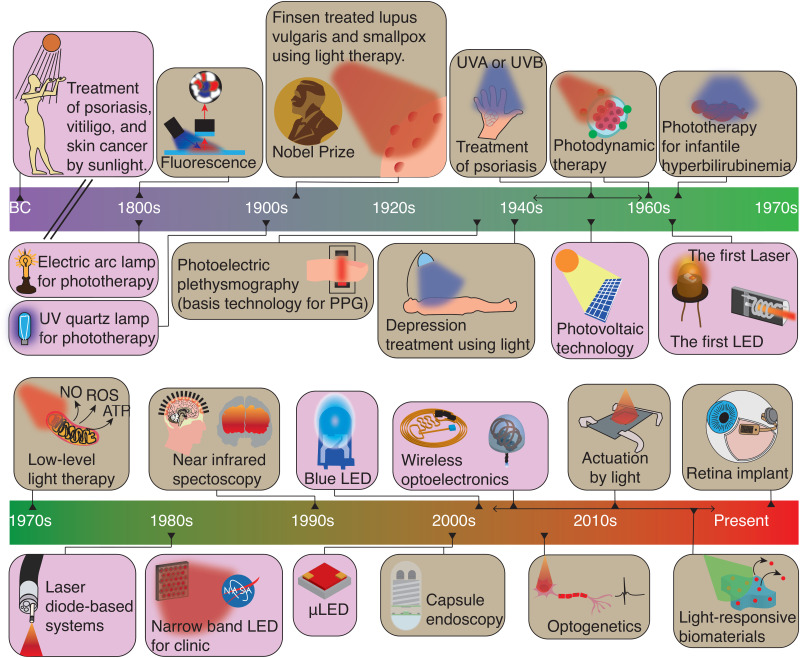
The development of many important tools in modern medicine has been driven by advances in technology to generate and detect light. An early example was the successful treatment of lupus vulgaris using concentrated ultraviolet (UV) light from quartz lamps, a discovery for which Niels Finsen was awarded the Nobel prize in 1903 (Fig. 1).1,2 The invention of lasers and light-emitting diodes (LEDs) in the 1960s laid the foundation for therapies that exploit the ability to generate light with high intensities, short pulses, and precise wavelengths. Examples include laser surgery in ophthalmology3,4 and dermatology,5,6 blue light therapy for neonatal jaundice,7,8 and photodynamic therapy (PDT) for oncology.9,10 Advances in light detection have similarly led to optical sensing tools that are used along the entire spectrum of diagnostic medicine. Today, pulse oximetry—which is based on optical absorption differences measured by semiconductor photodiodes—is universally used in emergency medicine,11–13 while optical image sensors are routinely used by clinicians to examine cavities and guide surgery.14
FIG. 1.
Major milestones in the use of light in medicine and in bioelectronic devices. Sunlight has been used to treat skin diseases since ancient times.1,2,174 The invention of artificial light sources led to the emergence of modern phototherapies, such as treatment of lupus vulgaris with UV light by Niels Finsen who was awarded a Nobel Prize in Physiology and Medicine.1,174 Advances in optoelectronics have paved the way for the use of light in wearable, implantable, and ingestible devices.25 Major examples include photoplethysmography (PPG),15,16 which is universal in emergency medicine, and capsule endoscopy,17 which is widely used for the diagnosis of small bowel diseases. Recent progress in material science,36 electronics,291 energy storage,292 and wireless technology31,279 has enabled new light-based diagnostic and therapeutic tools, including brain–computer interfaces,186 wireless optogenetic implants,37 drug delivery devices,262 and retinal implants.286,289 Milestones in the use of light in medicine are indicated in brown,293–295 and key advances in optical technologies are shown in purple.40
The miniaturization of electronics over the past few decades has opened opportunities for the application of light outside of traditional clinical settings. With integrated light sources and detectors, optical technologies can be incorporated into wearable, ingestible, and implantable devices that operate during daily life. As examples, wrist-worn optoelectronics can track heart rate and oxygen saturation,15,16 swallowable endoscopes can allow minimally invasive optical imaging of the gastrointestinal tract,17–19 and skin-mounted optical devices can monitor blood flow and sweat.20–22 Because such devices can facilitate the collection of rich datasets about a person's health, they could be the basis for a more personalized and cost-effective form of health care.23–25 Therapeutic applications of light can also harness the capabilities of electronics to deliver more precise and personalized care. Wearable near-infrared (NIR) light sources, for example, have shown potential for wound healing, hair growth, and pain therapy in home settings.26,27 With recent advances in light-activated nanomedicine28,29 and optogenetics,30–32 innovations in miniaturized light delivery implants are opening new opportunities to target diseases in regions that have previously been neglected due to the lack of suitable light sources.33–35
In this review, we summarize the basic components of light-based bioelectronic devices, including light sources, photodetectors, energy storage, and powering strategies. We next investigate major applications of these devices in both sensing and therapy, focusing on the requirements that they impose on the performance of the various components and current solutions. Finally, we discuss the challenges in the clinical application of light-based bioelectronics and their promise in enabling new forms of digital health care.
II. LIGHT-BASED BIOELECTRONIC COMPONENTS
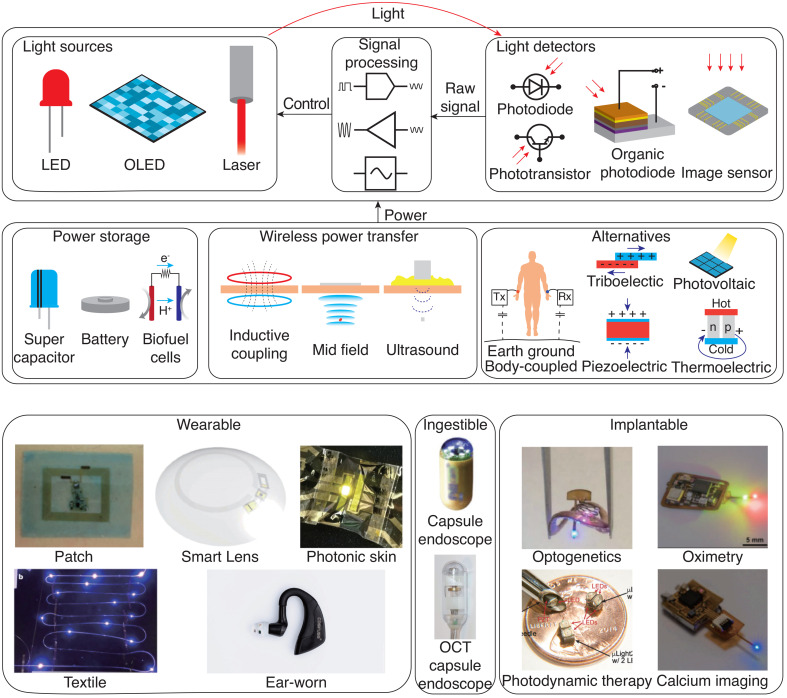
Bioelectronic devices that exploit light–tissue interactions combine a variety of functional components, including optical sources and detectors, energy storage and harvesting solutions, and wireless communication and power transfer interfaces (Fig. 2). These components need to be integrated into a wearable, ingestible, or implantable package that is biocompatible over its desired operational lifetime. Advances in optoelectronics and materials have enabled the optical interface with biological tissue to take a wide range of forms, such as skin-conformal patches,36 ingestible pills,17 injectable probes,37 and miniature implants.28 Progress in energy storage and harvesting solutions as well as wireless power transfer have paved the way for the miniaturization of such devices and new modes of operation. In this section, we briefly introduce the fundamental components of light-based bioelectronic devices, describe their use in current clinical practice, and highlight emerging directions of research.
FIG. 2.
Components of light-based bioelectronic devices. Devices consist of a light sources and detectors, electronic components for signal acquisition and processing, and power management components. Wearable: Reprinted with permission from Kim et al., Sci. Adv. 2, e1600418 (2016). Copyright 2016 Authors, licensed under a Creative Commons Attribution (CC BY) License;78 Reprinted with permission from Park et al., Sci. Adv. 4, eaap9841 (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) License;296 Reprinted with permission from Jinno et al., Nat. Commun. 12, 2234 (2021). Copyright 2021 Authors, licensed under a Creative Commons Attribution (CC BY) License;297 Reprinted with permission from Rein et al., Nature 560, 214–218 (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) License;298 Reprinted with permission from Baldinger et al., IEEE Pervasive Comput. 20, 58–62 (2021). Copyright 2021 Authors, licensed under a Creative Commons Attribution (CC BY) License;23 Ingestible: Reprinted with permission from Ciuti et al., IEEE Rev. Biomed. Eng. 4, 59–72 (2011). Copyright 2011 Authors, licensed under a Creative Commons Attribution (CC BY) License;99 Reprinted with permission from Liang et al., Optica 5, 36–43 (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) License;209 Implantable: Reprinted with permission from Shin et al., Neuron 93, 509–521 (2017). Copyright 2017 Authors licensed under a Creative Commons Attribution (CC BY) License;31 Reprinted with permission from Zhang et al., Sci. Adv. 5, eqqw0873 (2019). Copyright 2019 Authors, licensed under a Creative Commons Attribution (CC BY) License;299 Reprinted with permission from Kim et al., Sci. Rep. 9, 1395 (2019). Copyright 2019 Authors, licensed under a Creative Commons Attribution (CC BY) License;29 Reprinted with permission from Burton et al., Proc. Natl. Acad. Sci. U. S. A. 117, 1835–1845 (2020). Copyright 2020 Authors, licensed under a Creative Commons Attribution (CC BY) License.88
A. Light delivery
The use of light in bioelectronics requires an integrated optical source whose wavelength, intensity, and pulse duration can be precisely selected. Today, light delivery for bioelectronics relies on various forms of semiconductor lasers and LEDs to meet requirements in optical emission, power consumption, form factor, and cost.
1. Inorganic light-emitting devices
Inorganic LEDs based on crystalline semiconductors are among the most mature of light sources and achieve the lowest power consumption. Light emission in inorganic LEDs occurs through the recombination of electrons and electron holes, with the specific wavelength of emission corresponding to the energy bandgap of the semiconductor.38 Depending on the desired emission wavelength, choices for the semiconductor include aluminum nitride (<400 nm), indium gallium nitride (400–500 nm), gallium phosphide (500–760 nm), and gallium arsenide (>610 nm).39 Inorganic LEDs are currently used in a wide range of wearable healthcare devices, including pulse oximeters and wrist-based heart rate trackers.40,41 They are also widely used within the body in clinical endoscopic, ingestible, and implantable devices, where their electrical efficiency, small dimensions, and ability to withstand humidity are major advantages. State-of-the-art inorganic LEDs can achieve quantum efficiencies exceeding 50%, which is about twofold larger than possible with alternatives based on organic materials.42–44 However, inorganic LEDs are inherently rigid and therefore pose challenges when directly interfacing with soft tissues.45 This risk can be mitigated using LEDs with microscale dimensions. For example, indium gallium nitride LEDs with an area smaller than 100 × 100 μm2 and the thickness of several micrometers have been used for applications in optogenetics37,46 and PDT.29 LED arrays formed with flexible interconnects can also be used to achieve macroscopic conformability with curved tissue surfaces.47
2. Organic light-emitting devices (OLEDs)
Organic LEDs (OLEDs) consist of a multilayer structure in which a film of organic compound is sandwiched between two conductors.25,48 They are promising alternatives to crystalline semiconductor LEDs in a wide range of bioelectronic applications due to their mechanical flexibility,49,50 low weight,51,52 and broad tunability,53 although the maximum irradiation and spectral selectivity are generally less than can be achieved with inorganic LEDs.54 The operating principle of OLEDs is identical to that of inorganic LEDs except that the emission wavelength is determined by energy gaps associated with the highest and lowest occupied molecular orbital of the organic compound. Typically, at least one of the electrodes consists of a transparent conductor such as indium titanium oxide. OLED's organic materials and unique structures enable the following advantages: mechanical robustness, lightweight, and a lack of heating effect, resulting in reliable operation in dynamic environments without thermal damage to tissues.49,54 Ultra-flexible, conformable polymer OLEDs with a thickness of 3 μm can be utilized to display and sense physiological signals.49 The wavelength of emission can be tuned by vertically stacking multiple LEDs with different emitter layers, which is important for both sensing and therapy applications that require multi-wavelength operation.55 The longevity (beyond 10,000 h) and durability of OLEDs in humid environments also remain challenges that need to be overcome;56–58 in this regard, encapsulation strategies are critical to extending their lifetimes.59–61
3. Quantum-dot light-emitting devices (QLEDs)
Quantum-dot LEDs (QLEDs) are of interest due to their high efficiency, mechanical flexibility, and wavelength tunability.62,63 Structurally, QLEDs resemble OLEDs, but use a layer of solution-processed colloidal quantum dots for light emission. QLEDs have been explored for use in phototherapies requiring multiple wavelengths in which the tunability of the emission wavelength is a major advantage.64 For example, a wearable QLED-based device operated by a coin cell battery was shown to provide an antimicrobial effect via the emission of blue light with intensities and spectral characteristics comparable to an inorganic LED.65 Bioelectronic applications of QLEDs have been hindered by their limited lifetimes due to charge balance issues and existence of non-radiative pathways.66 Techniques that balance charge injection and disrupt non-radiative recombination processes have shown the potential to improve device stability.67,68
4. Lasers
Compared with LEDs, lasers can produce coherent light emission with significantly higher intensity, narrower linewidth, and shorter pulses. These properties can enable a wide range of optical imaging modalities, including optical coherence tomography (OCT), diffuse optical tomography, and photoacoustic imaging.69 Recent work has explored the integration of miniaturized pulsed laser sources for wearable time-domain diffuse optical imaging and photoacoustic imaging. Using complementary metal-oxide semiconductor (CMOS) circuits to drive a laser diode, ∼100 ps pulses can be generated using an integrated source with dimension 6 × 12 mm2.70 Laser-based light delivery could be used by swallowable endoscopes for tomographic imaging,71 by microrobots to perform surgery,72,73 or by light-activated actuators to trigger drug release.74 However, challenges in safety, reliability, and power consumption, particularly when there is physiological motion, will need to be addressed before clinical applications are feasible.
B. Light detection
Light detectors can be used in combination with integrated or ambient light sources to probe physiological changes in the optical properties of tissue.75–77 When integrated into a bioelectronic device, they can extend the diagnostic use of light beyond the clinical setting and enable the real-time assessment of health. Light detector technologies are based on either inorganic or organic materials and are used in applications such as measurement hemodynamic activity,12,78,79 assessment of skin lesions,21 and imaging of the gastrointestinal tract.17,18,24 They can cater to a wide range of optical wavelengths, from visible light to the NIR and UV regions, and can achieve both broadband or narrowband operation.
1. Inorganic photodetectors
Inorganic photodetectors are the sensing counterparts of inorganic LEDs and are based on the same set of crystalline semiconductors. Owing to their maturity, low cost, and ease of integration with other electronic components, they are the widely used type of photodetector in bioelectronic applications.80,81 Photodiodes consisting of a semiconductor p–n junction represent the simplest and most commonly used variety. Other types include phototransistors, which do not require an external amplifier but suffer from larger dark current.82 Most sensing applications operate photodiodes in the photoconductive mode (reverse bias) to achieve a large linear range of operation.82 They can also be operated in the photovoltaic mode (forward bias) to improve the detection of low-intensity light or for applications in optical energy harvesting. Current clinical devices such as pulse oximeters and heart rate trackers use inorganic photodetectors integrated in rigid packaging. Recent research has explored ways to interface photodetectors intimately with biological tissue for improved sensing. For example, reducing the film thickness to 400 μm can yield flexible crystalline photodetectors.83,84 A photodetector based on graphene oxide and zinc oxide on 25-μm-thick mica substrate was used for UV dosimetry and exhibited 4% photocurrent deviation over 500 bending cycles.85 The low optical absorptivity of two-dimensional materials can be overcome by crumpling: a photodetector based on crumpled graphene achieved 12.5 times higher optical extinction and 370% higher photoresponsivity.86 Micro-scale photodetectors have also been mounted on injectable probes for calcium imaging of deep brain neural activity in rodents.87,88 Bioresorbable photodetectors based on silicon nanomembranes can dissolve in physiological environments via hydrolysis. For example, an injectable bioresorbable spectrometer based on comb-structured silicon membranes with zinc electrodes was demonstrated for cerebral monitoring in mice.89
2. Organic photodetectors
Photodetectors based on organic semiconductors have several potential advantages over inorganic photodetectors, including mechanical flexibility,90 tunability of the absorption band through chemical modifications,91 low cost, and ease of processing. Their mechanical flexibility results from both the intrinsic properties of the organic film and the ability to achieve a high extinction coefficient in thin films that are less than 200 nm. Flexible OLED devices can be achieved by bonding organic photodiodes to thin and transparent substrates, such as polyimide or Parylene-C, via transfer printing.92,93 OLEDs have been used to develop a variety of flexible optoelectronic sensors with mechanical properties comparable to that of human skin. For instance, flexible NIR-sensitive organic photodetectors have been used to provide continuous monitoring of heart rate variability, enabling more precise pulse pressure tracking than conventional pulse oximeters during user activity.94 Multilayered films can be applied on OLEDs for achieving spectral selectivity, which can reduce noise from environment illumination.95,96 Noise due to dark current can be mitigated using charge blocking layers between the active layer and the electrodes.97 Applications of OLEDs within the body will require further advances in their lifetime and robustness in humid and hot environments. Encapsulation strategies based on flexible ultrathin passivation layers have shown promise in prolonging OLED lifetimes.81
3. Image sensors
Image sensors are arrays of photodetectors in which each detector (pixel) can be read out to form an image.80 Image sensors can be classified into two types: charge-coupled device (CCD) sensors that deliver analog signals directly and CMOS sensors that convert analog signals on each pixel to digital signals.98,99 While CCD sensors can produce higher quality images than CMOS sensors, the latter is frequently preferred due to its low power consumption and low manufacturing cost.100 CMOS sensors are also compatible with CMOS-based circuits and benefit from the miniaturization of transistors.99,100 A miniaturized wireless CMOS sensor with dimensions of 300 × 300 μm2 and a thickness of 25 μm, for example, has shown potential for capturing fluorescence images for optical recording of genetically encoded calcium indicators in the brain.101 Capsule endoscopes have been made possible by advances in CMOS sensors. Clinically used capsule endoscopes vary the frame rate varies depending on the target application.99 For example, a low frame rate of 2–4 fps is appropriate for small-bowel or colonoscopy, which occurs over about 10 h, whereas esophagogastroduodenoscopy uses a high frame rate of 18 fps that limits the battery life to only 30 min.102
A limitation of inorganic image sensors is that their broad absorption spectrum necessitates the use of color filters, which introduce optical losses. Organic image sensors, in contrast, do not require color filters because their active layers absorb selectively in certain narrow color bands.103 Furthermore, they can detect NIR light and x rays in a narrowband manner, which has applications in medical imaging.104 For instance, by exploiting the differential transmittance of muscle and fat to 1200 nm light, a 4 × 4 NIR organic photodiode array was used to improve the contrast between organs and surrounding tissues during laparoscopic surgery.105 Organic NIR photodiode arrays have also been used as retinal prosthetics that convert pulsed NIR (880–915 nm) light into photocurrent for neural stimulation.106 These pixel arrays can be monolithically stacked, allowing for high spatial resolution without increasing the pixel size.107 Fabrication methods based on solution-processing, such as inkjet printing and spray coating, have the potential to create flexible, large-area, and low-cost organic sensors.108,109 Recent work has demonstrated solution-processed organic phototransistor arrays with high photoconductive gain and dynamic range.91,110
C. Energy storage and harvesting
Electrical power is a fundamental requirement for bioelectronic devices that determines their operational lifetime and range of functionalities. Light-based bioelectronic devices generally require significant power (from 1 mW to more than 100 mW) for both sensing and therapy. To meet the power requirements for long-term use, a wide range of solutions for energy storage and harvesting are being explored.
1. Batteries and supercapacitors
Wearable bioelectronic devices can take advantage of lithium-ion batteries developed for consumer applications, which achieve among the highest energy densities at low cost.111–113 For implantable and ingestible applications, standard battery technologies are associated with safety concerns, such as risk of toxicity and self-ignition, and thus, more specialized systems are used. Silver oxide batteries are preferred for capsule endoscopes,99 and lithium iodine batteries are the main option for implantable cardiac pacemakers. Because batteries usually occupy a large part of the device volume, improvements in their properties and performance are important to facilitate miniaturization and enable long-term use. Stretchable batteries made by layering intrinsically flexible active materials on such as substrates like polymers or by interconnecting arrays of rigid islands with serpentine wires can mitigate the mechanical mismatch with soft tissues.114 Intrinsically flexible batteries based on ceramic, organic polymer, or gel polymer electrolytes have shown promise for biomedical applications.115 Solid-state batteries have also been explored for implantable devices owing to their mechanical strength, thermal stability, and increased energy density.112 For example, a millimeter-scale and flexible thin-film battery weighing just 236 μg was used to power a smart dental brace that delivers 640 nm light to accelerate bone regeneration and protect enamel health.116
Bioresorbable batteries are useful technologies for short-term implanted devices because they eliminate the need for additional surgery to remove implanted devices after therapy or monitoring is completed.117 While magnesium or zinc metal-based galvanic cells have been used in the development of bioresorbable batteries due to their good biosafety and electrochemical activity, they are difficult to use in implantable devices due to their unstable output power and uncontrollable lifetime.118,119 A possible solution is a battery composed of an anode filament based on a zinc microparticle network coated with chitosan and aluminum oxide double shells. Its lifetime can be adjusted from 80 to 200 h by adjusting the filament length from 5 to 15 mm.120
Supercapacitors are used in bioelectronic applications requiring many rapid charging and discharging cycles. They can support significantly higher power densities than batteries, at the expense of 3–30 times lower energy density.121–123 The charge storage mechanism can be based on electrostatic double layers using carbon electrodes, electrochemical capacitance using metal oxide or conducting polymer electrodes, or a combination of both.124 Supercapacitors can facilitate pulsatile delivery of high-intensity light for therapy or actuation.124–126 They can also mitigate issues related to the intermittency of energy harvesting or wireless power transfer, providing a stable power supply despite variations in the power supply over the storage timescale.127–129 For instance, flexible supercapacitors have been used in wearable and implantable devices to store power collected from piezoelectrics, photovoltaics, and antennas.130,131 Supercapacitors based on graphene and other nanomaterials can also be woven into textiles for wearable energy storage.121,132 Supercapacitors can also be made flexible and bioabsorbable, such as by layering defective amorphous molybdenum oxide flakes on molybdenum foil.133
2. Energy harvesting
The human body presents opportunities to harvest mechanical and thermal energy to power bioelectronic devices. One of the most significant natural sources of energy in the human body is physical movements, for example, walking with a full 5-cm stroke can generate up to 67 W of power, while arm lift consumes up to 60 W.134 This energy can be directly converted using piezoelectric materials such as lead zirconate titanate, which achieve among the highest mechanical-to-electrical conversion efficiencies. Up to 2 mW, for example, can be harvested by using a piezoelectric harvester in the insole of a shoe during running. Owing to issues such as brittleness and toxicity,135 more biocompatible alternatives such as zinc niobate, lithium niobate, and barium titanate are being explored as alternatives.136 Because these sources are generally intermittent, energy harvesters need to be combined with energy storage components to provide a steady power supply.
Triboelectric generators covert mechanical motion to electricity through frictional charge transfer between two materials.137–139 The interfaces between a wide variety of materials can be used, including polytetrafluoroethylene and silicone for attaining net negative triboelectric charges and nylon and metals like aluminum and gold for net positive charges.140 Using a nanostructured polytetrafluoroethylene (50 μm) and aluminum layer (100 μm), the motion of a beating heart was used to charge a flexible battery and power a commercial pacemaker implanted in a pig.141,142 Material strategies to overcome their sensitivity to humidity and increase power density are being explored.
Physiological thermal gradients can also be harnessed for electrical power generation via thermoelectrics, which include materials such as bismuth telluride, lead telluride, and calcium manganese oxide.123,143 They have been widely explored for wearable applications where relatively a large temperature difference exists between the surface of the body and the surrounding environment. For instance, thermoelectric materials integrated into clothing have been used to harvest up to 4 mW between the skin and the ambient environment.144 They have also been investigated for implantable devices, but the lack of large thermal gradients within the body has limited performance.145,146
D. Wireless power and communications
Wireless communication and power transfer allow light-based bioelectronic devices to transmit sensor data and deliver therapies on demand. Various forms of energy—including radio frequency fields, acoustic waves, and light—can be used to transfer energy and data to and from a bioelectronic device. These forms of energy interact differently with biological tissue and lead to systems with different form factors, operating characteristics, and safety considerations.
1. Radio-frequency
Radio-frequency wireless communication and power transfer technologies are standard in many clinical devices owing to their relative maturity, safety, and ease of use. Leveraging technologies developed for consumer electronics, wireless protocols adapted for medical frequency bands can support digital communication with high data rates, such as for video streaming from ingestible endoscopes. Proper design of the antenna is essential because the human body profoundly affects the performance of the antenna. For example, the size of the antenna needs to be on the scale of a quarter of the wavelength in biological tissue to achieve high radiation efficiency and absorption in tissue must be minimized for thermal safety.
Near-field wireless power based on pairs of inductively coupled coils can be used to operate implantable devices and avoid complications associated with surgery to replace batteries. They generally require that the separation distance be comparable to the coil size in order to achieve high efficiency.147,148 Operating at frequencies less than 100 MHz, up to 10 W can be safely transferred through the human chest wall with this approach.149 Near-field systems have also been adapted for use in freely moving rodents, where the tether-free mode operation can open many opportunities for light-based sensing and therapy. Implantable LEDs powered by near-field systems, for example, have been used to enable rodents to freely move within a circular region of 30 × 60 cm2 for closed-loop optogenetic experiments.150 Flexible, near-field powered devices have been used to interface with the musculoskeletal system as long-term biological interfaces for applications such as phototherapeutic stimulation for bone regeneration and optogenetic control of muscle contraction.151 Near-field systems can also support short-range, low data rate communications in parallel with power transfer. For example, battery-free optoelectronic skin patches can be operated by smartphones using near-field communication (NFC) to monitor heart rate, oxygen saturation, and skin color.78,79
Radiative wireless power transfer techniques can overcome certain limitations in the operating range and miniaturization of the device.152 For instance, mid-field wireless power operating between 1 and 5 GHz can focus energy and power millimeter-sized devices several centimeters deep in tissue.37,153,154 Using this approach, miniaturized (<15 mm3) wireless LEDs were activated 4 cm deep at light intensities exceeding 1 mW cm−2 for PDT.28 Owing to increased tissue absorption, radiative wireless power systems typically operate near consumer limits for the specific absorption rate, although medical applications will require judicious assessment of the risks and potential benefits.
2. Ultrasound
Ultrasound—widely used for diagnostic imaging—can also be a modality for wireless communication and power. Compared to radio frequency waves, key advantages of ultrasound include millimeter-scale wavelengths, which can facilitate miniaturization, and high penetration depth through tissue, potentially exceeding 10 cm.155,156 Ultrasonically operated devices use a miniaturized piezoelectric transducer to harvest energy from ultrasound transmitted into the body. Efficient power transfer requires good acoustic coupling between the transducers and skin, minimal reflection at hard–soft tissue interfaces, and alignment between the transducer and the incident acoustic wave.157 Wireless LED-based devices with dimensions as small as 2 × 4 × 2 mm3 have achieved an output light intensity of 6.5 mW cm−2 when illuminated with an acoustic power density of 185 mW cm2.29 Ultrasound-based millimeter-sized devices with μLEDs have also been developed for optogenetics of peripheral and brain neurons.158,159 Bidirectional ultrasound links can also be established using backscattering techniques. A wireless battery-free millimeter-sized (4.5 mm3) device implanted at 10-cm-deep inside tissue has the potential to prevent vascular complications after solid organ transplantation by sending the oxygen level information from deep tissues with low power consumption and high resolution.160
3. Optical
Light can be harnessed to power bioelectronic devices using photovoltaics.161–163 Indoor ambient light can provide a power density of 100 mW cm−2 and direct sunlight up to 500 mW cm−2.164 To maximize the area of illumination, photovoltaics based on flexible165–167 or textile materials168 can be used. The penetration of light through biological tissue can be maximized in the near-infrared window (650–1350 nm) to power or communicate with implantable devices.169 Using deep-red light at 638 or 660 nm, an organic electrolytic photocapacitor was powered 10 mm below the surface of the skin using a laser to stimulate the sciatic nerve in rats.170 Optically powered devices can achieve microscale dimensions if the power density in tissue is sufficient. Serially connected photovoltaic diodes integrated into a device with dimensions 220 × 220 μm2, for example, were used to convert infrared light (810 nm) to visible red light (630 nm) for optogenetics.171
4. Tissue conduction
The ionic conductivity of biological tissue can be exploited for wireless communication. In this approach, an electrode in contact with tissue creates an alternating voltage with amplitude and frequency outside of the window of physiological effect, which can be detected by an electrode mounted elsewhere on the body. For example, an electrode forming 0.3-mm-long dipole with a 2.5-mm-radius skirt was used in an ingestible sensor to communicate with a patch mounted on the abdomen in order to measure medical adherence.172 Power transfer through tissue conduction has also been demonstrated using capacitive coupling between 30 and 90 MHz. Using a skin-coupled transmitter that injects 1.2 mW into the body, about 2 μW was transferred at a distance of 160 cm, which was sufficient to operate a digital calculator. The efficiency of transfer can be 35 dB higher than radio frequency communication when the path between the transmitter and receiver is obstructed by the body.173
III. LIGHT-BASED BIOELECTRONIC DIAGNOSTICS
Probing light scattering and absorption by tissue constituents can enable the measurement of physiological parameters. Light-based bioelectronic devices exploit this capability to track diagnostic markers continuously and reveal insight about a person's health.
A. Hemodynamic monitoring
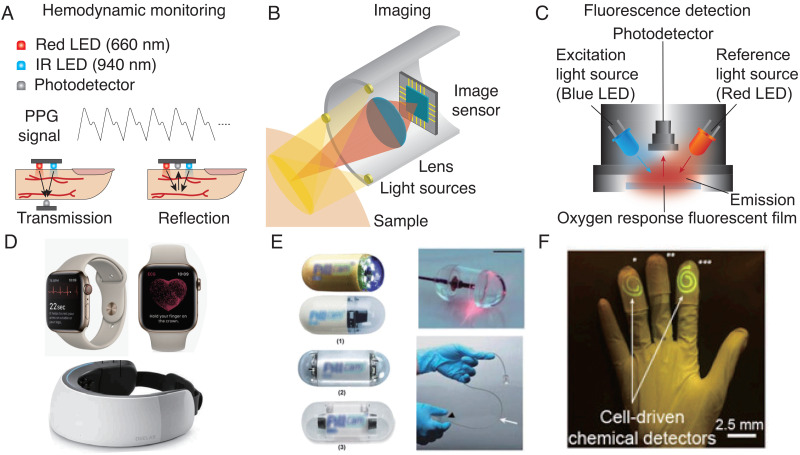
The distinct absorption spectra of oxygenated and deoxygenated hemoglobin provide the basis for optical hemodynamic monitoring.21,77,174 Photoplethysmography (PPG) is the most prominent example that is universally used in emergency medicine to track blood oxygen saturation. In the clinical setting, PPG is typically obtained using a pulse oximeter that measures differential absorption of red (typically 660 nm) and infrared light (typically 940 nm) transmitted through the finger [Fig. 3(a)]; modern wearables such as smartwatches are also able to acquire PPG signals through differential reflection measurements from the skin [Fig. 3(d)].175–177 Heart rate trackers are based on a similar principle and typically use the reflection of green light (520 nm),75,178,179 which is preferred due to its high absorption by hemoglobin and shallow penetration depth.175,180,181 Hemodynamic monitoring during daily life can be challenging because of motion artifacts and interference from ambient light. Mounting optoelectronic components against the skin in the form of a conformal patch can greatly reduce noise and increase the reliability of tracking. Using near-field wireless power transfer, a binodal network of soft optical and electrophysiological sensors was used to track oxygen saturation, heart rate, and pulse transit times in neonates.182 NFC can also be used to operate PPG sensors just a few millimeters in size using a smartphone.78,79,182 Combining PPG sensors with pneumatic actuators can enable the estimation of a person's blood pressure by measuring the pressure at which inflow and outflow are balanced.12 The design of the encapsulation layers is important to reduce noise: black elastomeric layers can reduce interference due to ambient light.21 Radiative cooling implemented using a polymer structured with nano- and micro-voids has also been used to improve outdoor performance by reflecting sunlight while maximizing thermal radiation.79 Ingestible and implantable devices can also provide localized hemodynamic monitoring using optical techniques. A telemetric gastric sensor, for example, could detect gastric bleeding in porcine studies using differential absorption at 415 and 720 nm with >80% sensitivity and specificity.183
FIG. 3.
Bioelectronics for light-based diagnosis. (a) Architecture for heart rate monitoring and two different types of hemodynamic mechanisms. (b) Simple schematic of ingestible imaging devices. (c) Mechanism of fluorescence detection. (d) Portable fNIRS device and Apple watch. (e) Capsule endoscopes and OCT capsule endoscope. (f) Glove type wearable fluorescence sensor. (d) Reprinted with permission from Saghir et al., Cardiovasc. Digital Health J. 1, 30–36 (2020). Copyright 2020 Authors, licensed under a Creative Commons Attribution (CC BY) license;15 Reprinted with permission from Kwon et al., Front. Neurol. 898, (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) license;193 (e) Reprinted with permission from Ciuti et al., IEEE Rev. Biomed. Eng. 4, 59–72 (2011). Copyright 2011 Authors, licensed under a Creative Commons Attribution (CC BY) license;99 Reprinted with permission from Gora et al., Nat. Med. 19, 238–240 (2013). Copyright (2013) Authors, licensed under a Creative Commons Attribution (CC BY) License;207 (f) Reprinted with permission from Liu et al., Proc. Natl. Acad. Sci. U. S. A. 114, 2200–2205 (2017). Copyright 2017 Authors, licensed under a Creative Commons Attribution (CC BY) License.222
Functional near-infrared spectroscopy (fNIRS) is a noninvasive tool for monitoring brain activity based on inferring cortical hemodynamic activity through hemoglobin absorption [Fig. 3(d)].184–186 Although the utility of fNIRS is limited to the cortical surface,187 its simplicity compared to other brain imaging techniques has motivated applications in brain computer interfaces, hypoxia studies, and functional connectivity estimation.188–190 Wearable fNIRS devices have many applications in both research and clinical diagnostics due to their ability to monitor brain activity during everyday life.191 A wireless fNIRS patch consisting of a pair of LEDs and multiple photodiodes, for example, can monitor systemic and cerebral hemodynamics in pediatric patients with and without congenital central hypoventilation syndrome.192 Commercially available, wearable fNIRS devices with hundreds of channels have shown promise for early detection of stroke193 and Alzheimer's disease.194 Wearable fNIRS devices have also been explored for a wide range of other applications, including neurodevelopment studies, social perception and interaction research, and augmented and virtual reality applications.195
B. Optical imaging
Bioelectronic devices can be used to image regions of body that are otherwise difficult to access. Endoscopic imaging, for example, has had a major impact on the way that surgeries are performed, enabling procedures to be less invasive and precise despite lack of direct visual access.196–198 Capsule endoscopes integrate image sensors, lenses, LEDs, microprocessors, and antennas in an ingestible form factor to enable wireless video transmission from the gastrointestinal track [Fig. 3(b)].199,200 Current clinically used capsule endoscopes have typical dimensions of 26 × 11 mm2 and can operate for up to 12 h at 2 fps with a resolution of 1920 × 1080 pixels.19 Over two decades of clinical data provide evidence of its benefits for the diagnosis of occult gastrointestinal bleeding and assessment of Crohn's disease after negative ileoscopy.201 The design of capsule endoscopes requires a balance of achieving high-resolution images at a high frame rate for better diagnostic performance while ensuring adequate battery life in a small form factor that minimizes the risk of retention.19,202,203 Because conventional white-light imaging may miss certain abnormalities, the integration of other surface imaging techniques such as chromoendoscopy, virtual chromoendoscopy, magnification endoscopy, and autofluorescence imaging has been investigated.19 Combining optical imaging with temperature, pressure, and pH sensors may also improve diagnostic accuracy. The broad success of artificial intelligence in medical image analysis has also motivated efforts to incorporate deep learning in imaging devices to detect diseases automatically.204 Convolutional neural networks applied to capsule endoscopy, for example, have shown promise for automatic detection of protruding lesions205 and angioectasia.206
Optical coherence tomography (OCT) is an established technique for label-free, high-resolution imaging. OCT can provide near-histological resolution (10–30 μm) at up to 3 mm depth and is the standard of care in ophthalmic applications and intracoronary imaging [Fig. 3(e)].207,208 OCT-based capsule endoscopes can provide 30 μm lateral and 7 μm axial resolution, although the scanning speed is limited to about 20 fps.207 Using a distal scanner, scanning speeds of up to 250 fps have been demonstrated with 8.5 μm axial and 30 μm transverse resolution.208,209
Optical imaging has also been used in wearable devices to track skin color changes over time. Using reflection measurements with three LEDs across the range of melanin-sensitive wavelengths, a wearable device can distinguish between three different skin tones.78 This technology could have applications in monitoring gradual changes in skin tone linked to liver and kidney-related diseases.
C. Biochemical sensing
Bioelectronic devices can incorporate optical detectors to sense the molecular constituents of biological fluids and tissues.210 These devices typically exploit fluorescent sensors in combination with an excitation source (LED or laser), a photodetector, optical filters, and electronics for signal processing and data transmission [Fig. 3(c)].211 The design of the fluorescent sensor is challenging because the molecular probe needs to be capable of real-time, continuous tracking of analyte concentrations. Oxygen and pH sensors based on fluorescein derivatives and platinum porphyrins are among the most widely investigated of real-time fluorescent sensors. Their interaction with biological substrates can be controlled by physically or chemically trapping the probes in polymer matrices such as silicone, polystyrene, cellulose derivatives, and polyurethane hydrogels.212 Ratiometric measurements of the intensity ratio or lifetime of two different fluorescent sensors can improve the stability and accuracy by subtracting interference from sources such as dye concentration, fluctuations in the source intensity, and background fluorescence.213,214 Fluorescent calcium indicators can be used to monitor neural dynamics in animals for neuroscientific applications. A subdermally implanted, battery-free photometer consisting of a micro-scale blue LED and photodiode mounted on an injectable probe was used to record neural activity during freely moving behavioral experiments.88 The recent development of an integrated circuit consisting of 36 × 40 pixel array, a laser driver, a power management unit demonstrates the technological feasibility of wireless, implantable fluorescence imaging.215
Bioluminescent probes enable optical sensing without the need for a light excitation source.216 For instance, the luciferase reporter, which emits broad-spectrum light centered around 560 nm, is a commonly used tool for studies of gene expression and tumorigenesis in animal models.217 The lack of endogenous bioluminescence in mammalian tissues results in little background interference, enabling measurements with relatively high signal-to-noise ratio (SNR).217,218 A portable bioluminescent detector consisting of an injectable luciferase-based probe and a sensitive light detector has been developed to facilitate functional bioluminescent studies in large animals.219 Miniaturization of the light detector is enabled by the use of a 1-cm2 silicon photodiode operated in the photovoltaic mode instead of a conventional cooled CCD camera. Time-resolved bioluminescence within the body can also be measured using an implantable device. By tuning the quality factor of a resonant circuit with a photodiode, bioluminescence from engineered luciferase in a rodent brain was measured by locally modulating the magnetic resonance imaging signal.220
Microorganisms, which are innately resilient in physiological environments, can also be used to transduce biochemical signals for readout by a bioelectronic device.221 Bacterial biosensors trapped in hydrogel-elastomer hybrids have been used to create wearable bioelectronic patches and gloves that can detect rhamnose, β-D-1-thiogalactopyranoside, and N-acyl homoserine lactones [Fig. 3(f)].222 After 4 h of contact with the biomarkers of interest, the devices produce fluorescence visible to the naked eye. An ingestible bacterial-electronic sensor has also been developed to detect gastric bleeding. The sensor is based on engineered probiotic Escherichia coli that provides a luminescence output upon exposure to extracellular heme, which is read out by phototransistors operated by an integrated luminometer chip with a microprocessor and wireless transmitter.223 Synthetic biology methods could enable the engineering of more complex genetic circuits to implement sensing logic.224,225 Challenges in the clinical use of bacterial sensors include the need for immobilization, low sensitivity, and slow response times.226–228
IV. LIGHT-BASED BIOELECTRONIC THERAPY
A. Antimicrobial therapy
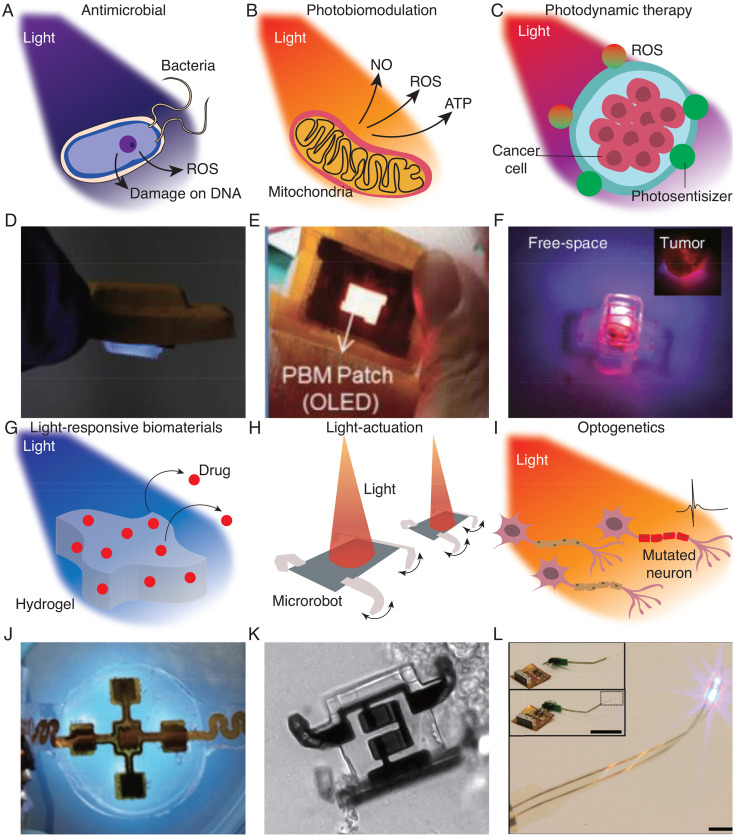
Blue light (400–500 nm) has been shown to be effective for treating a variety of bacterial infections, including Propionibacterium acnes associated with acne vulgaris and Helicobacter pylori associated with gastritis.229–231 Studies in animal wound models have also demonstrated their efficacy in treating antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA). The therapeutic effect arises from light-induced activation of endogenous porphyrins, which produces cytotoxic reactive oxygen species (ROS) [Fig. 4(a)].232–234 Efficient blue LEDs based on gallium nitride can enable the integration of antimicrobial light sources into wearable form factors. For example, electronic bandages that emit blue light with optical intensities up to 44.5 mW cm−2 have been shown to provide complete extermination of MRSA after 2 h of irradiation in in vitro studies.230 Microneedles can act as optical waveguides to deliver therapeutic light beyond the blue-light penetration depth [Fig. 4(d)].235 A light-emitting dental implant producing 15 mW cm−2 of 410 nm light has been shown to provide a stronger antimicrobial effect compared to calcium hydroxide, which is the standard of care, in an Enterococcus faecalis-infected root canal.236
FIG. 4.
Bioelectronics for light-based therapeutics. Schematic of antimicrobial (a), photobiomodulation (b), photodynamic therapy (c), light-triggered drug delivery (g), micro-robot actuation (h), and neural stimulation mechanisms with light (i). Examples of each application, UV antibacterial skin patch (d), OLED-based stretchable photobiomodulation skin patch (e), wireless photodynamic therapy devices for cancer treatments (f), hydrogel-based UV triggered drug release skin patch (j), light-actuated microrobot (k), and flexible optotrode for optogenetics (l). (d) Reprinted with permission from Zhang et al., Adv. Funct. Mater. 31, 2100576 (2021). Copyright 2021 Authors, licensed under a Creative Commons Attribution (CC BY) license;235 (e) Reprinted with permission from Jeon et al., Adv. Mater. Technol. 3, 1700391 (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) License;26 (f) Reprinted with permission from Bansal et al., Proc. Natl. Acad. Sci. U. S. A. 115, 1469–1474 (2018). Copyright 2018 Authors, licensed under a Creative Commons Attribution (CC BY) License;28 (j) Reprinted with permission from Pang et al., Adv. Sci. 7, 1902673 (2020). Copyright 2020 Authors, licensed under a Creative Commons Attribution (CC BY) License;260 (k) Reprinted with permission from Miskin et al., Nature 584, 557–561 (2020). Copyright 2020 Authors, licensed under a Creative Commons Attribution (CC BY) License;268 (l) Reprinted with permission from Kim et al., Science 340, 211–216 (2013). Copyright 2013 Authors, licensed under a Creative Commons Attribution (CC BY) License.37
B. Photobiomodulation
Photobiomodulation (PBM), also called low-level light therapy, uses red and NIR light (600–1000 nm) for applications related to stimulating or enhancing cell function.237 PBM generally involves fluence of 1–10 J cm−2 at intensities of 3–90 mW cm−2 and has shown therapeutic potential for wound healing,231,238 tissue repair,239 anti-inflammation therapy,240 and pain therapy.241,242 The mechanisms of PBM are thought to arise from the absorption of light by cytochrome C oxidase in mitochondria, which dissociates inhibitory nitric oxide and leads to increased adenosine triphosphate synthesis [Fig. 4(b)].243 Other potential mechanisms include the generation of ROS and the modulation of gene transcription. Because these mechanisms are still not well understood, the clinical use of PBM remains controversial. Regardless, a wide range of portable and wearable applicators are commercially available, including handheld sources for pain relief244 and LED-integrated helmets for promoting hair growth.245 OLEDs, in particular, have been used to develop PBM applicators with thicknesses less than 1 mm and weight less than 1 g. Using OLEDs with light emission tunable between 600 and 700 nm, a patch delivering 10 mW cm−2 was shown to promote wound healing in animal models [Fig. 4(e)].26 Flexible μLEDs utilizing pulsed stimulation of 650 nm at 10 Hz can facilitate hair removal without thermal damage to tissues.27
C. Photodynamic therapy
PDT uses light and a photosensitizer in combination with cellular oxygen to selectively eliminate diseased cells. Following the absorption of light by the photosensitizer, cell death is elicited by the conversion of cellular oxygen to cytotoxic ROS [Fig. 4(c)]. PDT is a clinically established treatment of a range of cancers—including bladder, skin, esophageal, bile ducts, and brain—where the ability to target diseased cells while sparing healthy cells can provide advantages over systemic treatments such as chemotherapy.246,247 Current use of PDT relies on light sources such as lamps or fiber optics, which limits its use to clinical settings.248 Wearable PDT devices based on inorganic LEDs or OLEDs, which have the advantage of being more lightweight, can enable ambulatory PDT with lower irradiance, longer exposure times, and greater convenience. Clinical studies have shown that ambulatory PDT of non-melanoma skin cancer based on LEDs resulted in comparable clearance rates and lower pain scores compared to conventional PDT, and is preferred by most patients.249,250
PDT is currently limited to treating lesions less than a centimeter in depth because of the low tissue penetration depth of light. Implantable light delivery devices have the potential to overcome this limitation and enable PDT to be repeatably performed deep in the body. These devices need to be miniaturized in order to be implanted during standard procedures, such as during biopsy of the tumor or during surgical resection to combat tumor recurrence. LED-based devices for PDT powered by ultrasound as well as near-field and mid-field wireless power transfer have been successfully demonstrated in rodent cancer models.28,29,251 In particular, mid-field wireless power transfer was used to operate a device less than 30 mg in weight and 15 mm3 in volume with an output light intensity of 1 mW cm−2 at 5 cm depth in a pig model [Fig. 4(f)].28 Using multiple LEDs, multiwavelength emission can be tailored to the absorption peaks of the photosensitizer.28,251 Flexible devices can be attached on tumor surfaces using tissue adhesives to increase the efficacy of PDT.252 Artificial intelligence techniques can be used to perform multiple PDT studies in parallel.253
The effective depth of PDT can also be enhanced using upconversion nanoparticles based on lanthanide- or actinide-doped transition metals, which convert more deeply penetrating NIR light to visible wavelengths suitable for photosensitizer activation.254 An implantable optical guide containing upconversion nanoparticles was used in combination with an external NIR laser to suppress glioblastoma multiforme (GBM) tumor growth in a mouse model.255
D. Light-triggered drug release
Materials engineered to respond to light can be used for on-demand, spatiotemporally targeted drug release [Fig. 4(g)].256 The photo-responsive properties of such materials can be based on a wide range of wavelengths and chemistries. UV light can trigger changes in material structure and properties through photoisomerization, photocleavage, or photopolymerization for applications in drug release and tissue adhesives.257–259 For instance, a flexible LED-based device emitting 365 nm light was integrated with a UV-cleavable hydrogel for programmable antibiotic wound therapy [Fig. 4(j)].260 A bioelectronic-tissue adhesive combining UV-photocurable covalent networks with ionic networks can also provide interfaces that allow optical, electrical, and chemical exchange.261 NIR light can activate changes in the properties of materials incorporating photothermal nanoparticles. Hydrogels comprising polydopamine nanoparticles and graphene aerogel, for example, can be programmed for drug release via volume shrinkage when irradiated with a 808 nm laser.262
Gold nanoparticles have broad therapeutic applications owing to their versatile functionalization capabilities and unique optical properties. In particular, they support plasmonic resonances, which can be broadly tuned by their size and shape, that lead to strong photothermal effects at specific wavelengths.263,264 Gold–silica nanoshells coated with a drug-eluting hydrogel, for example, can be designed for thermally triggered drug release when exposed to NIR light.265 Using intratumorally injected gold nanostars coated with an active layer of trans-1,2-bi-(4-pyridyl) ethylene (BPE) and polyethylene glycol (PEG), a wireless NIR-emitting device was shown to prolong survival in a rat model of GBM.266
Light can also potentially be used to manipulate micro-robots for applications in drug delivery and surgery [Fig. 4(h)]. A wide range of manipulation mechanisms have been demonstrated in vitro, including optical trapping, photothermal actuation, and photo-induced electrophoresis.73,267 Photolithographic fabrication can enable mass production of microrobots and facilitate integration with other semiconductor devices for expanded functionality. For example, more than 1 × 106 light-controllable electrochemical actuator-based microrobots were fabricated on a 4-in. wafer with a 9% yield [Fig. 4(k)].268
E. Neural modulation
Light can provide a spatiotemporally targeted stimulus to control neural activity for clinical and scientific applications.269 Photothermal neuromodulation uses pulsed infrared light (1800–2100 nm) at high intensities for stimulation through the induction of membrane-capacitive current or low intensities for inhibition through thermal suppression of axon conductance.270 Auditory nerve responses, for example, can be modulated by illumination at wavelengths of 1844–2120 nm using pulse lengths between 0.035 and 1.6 ms and stimulation thresholds of 1.6–27 mJ cm−2.271 The efficiency and selectivity of photothermal neuromodulation can be further enhanced using nanoparticles with optical properties, such as those comprising of gold, carbon, or polymers. A study found that gold nanoparticles increased the neuronal responsivity of a rat sciatic nerve to NIR light by 5.7 times.272 The mechanisms of photothermal neuromodulation, however, are still not well understood and demonstrations have been largely limited to animal studies, in part due to the need for careful control of light intensities to prevent thermal damage.270,271
Optogenetics enables genetically targeted, optical control over cellular activity through the genetic integration of light-gated ion channels [Fig. 4(i)].273 In a typical protocol, target neurons are genetically modified to express depolarizing or hyperpolarizing microbial opsins, which are activated at visible wavelengths at intensities above 1 mW cm−2.274–276 Optogenetics has revolutionized the study of neural circuits and revealed many insights with far-reaching clinical impact, such the design of deep-brain stimulation protocols for Parkinson's.277,278 To enable optogenetic behavioral investigations in freely moving animals, a variety of wireless optogenetic devices have been developed. μLEDs mounted on injectable probes can be stereotactically placed in the brain and wirelessly activated with light intensities up to 17.7 mW cm−2 using an headset-integrated antenna [Fig. 4(l)].37 Fully implantable optogenetic devices capable of targeting the peripheral nervous system as well as the brain have been demonstrated using self-tracking wireless powering techniques to enable millimeter-scale miniaturization34,279 or using stretchable design strategies to facilitate implantation in soft tissues.30,280 Battery-free NFC systems can be used to realize a range of sophisticated computation-based functionalities, such as programmability, independent operation of multiple devices, and closed-loop sensing and control.281
Optogenetics is being actively developed for clinical use, although significant challenges associated with the need for genetic transfection and light delivery through thick tissues remain. Applications in vision restoration are currently undergoing clinical trials (NCT02556736282,283 and NCT03326336284) in which the target cells are accessible to both light and transgene delivery. Studies in non-human primates have suggested that clinical applications may necessitate the activation of a larger number of neurons compared to rodents, which may be achieved by using higher light intensities, optical diffusers, or longer wavelengths.285 Wearable patches and wirelessly powered implants have been suggested as potential light delivery solutions.32,166
F. Retinal prostheses
Retinal prostheses can partially restore vision in blind patients via electrical stimulation of preserved neurons in the visual system. In a decade-long clinical trial, epiretinal prostheses consisting of an implanted microelectrode array and a wirelessly linked camera mounted on glasses (Argus II) demonstrated improvement in patients' functional vision, although the development has since ceased, in part due to the limited quality of artificial vision.286 Photovoltaic retinal prosthetics (PRIMA; Pixium Vision, Paris, France) have been developed to overcome limitations in the electrode density resulting from electrical feedlines.106,287,288 A 2 × 2 mm2, 30-μm-thick photovoltaic array with 378 pixels provided augmented prosthetic vision in patients with age-related macular degeneration using a glass-mounted NIR projector.289
V. OUTLOOK
Bioelectronic devices can harness the versatile role of light in medicine to enable sensing and therapy outside of traditional clinical settings. Current commercially available devices are predominantly based on rigid, inorganic semiconductor LEDs and photodetectors owing to their high quantum efficiencies, ability to be miniaturized, and low power consumption. These devices, however, pose challenges for interfacing with soft biological tissues. Optical sources and detectors based on organic semiconductors have enabled a wide variety of flexible wearable and implantable devices with skin-like mechanical properties, and significant advances have been made in increasing their efficiency and stability through improved materials and encapsulation techniques. Strategies for energy harvesting and wireless power transfer offer the possibility of miniaturizing devices and prolonging their lifetime beyond that of existing battery-powered devices. Energy harvesters have shown significant potential for powering light-based bioelectronic devices through physical movements of the human body, but biocompatibility and intermittency remain major challenges. Advanced wireless power transfer techniques based on radio frequency and ultrasonic modalities can enable battery-free operation of millimeter-scale devices, but the robustness and safety of the systems during clinical use needs to be addressed.
The use of bioelectronics and integrated optical technology will play an increasingly important role as the paradigm of digital medicine shifts the focus of health care away from episodic care and toward continuous monitoring and intervention.290 Capsule endoscopy and wrist-worn PPG are prominent examples of light-based bioelectronic devices already in wide clinical use. Technologies that exploit the molecular specificity of light for biochemical sensing are emerging, but challenges associated with the robustness, longevity, and form factor of the device still need to be addressed. Many exciting therapies based on the remote delivery of light with bioelectronic devices have been demonstrated, and efforts are underway to clinically translate them. Progress in optical technology, energy storage, materials science, and wireless techniques as well as recent translational efforts demonstrate the potential of bioelectronics to use light to improve human health.
ACKNOWLEDGMENTS
The authors acknowledge support from the National Research Foundation Singapore (Grant No. NRFF2017–07), Ministry of Education Singapore (Grant Nos. MOE2016-T3-1-004 and A-0009047-00-00), the Institute for Health Innovation and Technology, and the SIA-NUS Digital Aviation Corporate Laboratory.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Author Contributions
Han-Joon Kim: Writing – original draft (equal); Writing – review & editing (equal). Weni Sritandi: Writing – original draft (equal). Ze Xiong: Writing – review & editing (equal). John S Ho: Conceptualization (equal); Funding acquisition (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Grzybowski A., Sak J., and Pawlikowski J., “ A brief report on the history of phototherapy,” Clin. Dermatol. 34, 532–537 (2016). 10.1016/j.clindermatol.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 2. McDonagh A. F., “ Phototherapy: From ancient Egypt to the new millennium,” J. Perinatol. 21, S7–S12 (2001). 10.1038/sj.jp.7210625 [DOI] [PubMed] [Google Scholar]
- 3. Pantanelli S. M., Lin C. C., Al-Mohtaseb Z., Rose-Nussbaumer J. R., Santhiago M. R., Steigleman W. A. III, and Schallhorn J. M., “ Intraocular lens power calculation in eyes with previous excimer laser surgery for myopia: A report by the American Academy of Ophthalmology,” Ophthalmology 128, 781–792 (2021). 10.1016/j.ophtha.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 4. Khalkhal E., Rezaei-Tavirani M., Zali M. R., and Akbari Z., “ The evaluation of laser application in surgery: A review article,” J. Lasers Med. Sci. 10, S104 (2019). 10.15171/jlms.2019.S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Passeron T., Genedy R., Salah L., Fusade T., Kositratna G., Laubach H.-J., Marini L., and Badawi A., “ Laser treatment of hyperpigmented lesions: Position statement of the European Society of Laser in Dermatology,” J. Eur. Acad. Dermatol. Venereol. 33, 987–1005 (2019). 10.1111/jdv.15497 [DOI] [PubMed] [Google Scholar]
- 6. Krenitsky A., Ghamrawi R. I., and Feldman S. R., “ Phototherapy: A review and update of treatment options in dermatology,” Curr. Dermatol. Rep. 9, 10–21 (2020). 10.1007/s13671-020-00290-6 [DOI] [Google Scholar]
- 7. Hansen T. W. R., Maisels M. J., Ebbesen F., Vreman H. J., Stevenson D. K., Wong R. J., and Bhutani V. K., “ Sixty years of phototherapy for neonatal jaundice–from serendipitous observation to standardized treatment and rescue for millions,” J. Perinatol. 40, 180–193 (2020). 10.1038/s41372-019-0439-1 [DOI] [PubMed] [Google Scholar]
- 8. Inamori G., Kamoto U., Nakamura F., Isoda Y., Uozumi A., Matsuda R., Shimamura M., Okubo Y., Ito S., and Ota H., “ Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals,” Sci. Adv. 7, eabe3793 (2021). 10.1126/sciadv.abe3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McFarland S. A., Mandel A., Dumoulin-White R., and Gasser G., “ Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology?,” Curr. Opin. Chem. Biol. 56, 23–27 (2020). 10.1016/j.cbpa.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tampa M., Sarbu M.-I., Matei C., Mitran C.-I., Mitran M.-I., Caruntu C., Constantin C., Neagu M., and Georgescu S.-R., “ Photodynamic therapy: A hot topic in dermato-oncology,” Oncol. Lett. 17, 4085–4093 (2019). 10.3892/ol.2019.9939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah S., Majmudar K., Stein A., Gupta N., Suppes S., Karamanis M., Capannari J., Sethi S., and Patte C., “ Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization,” Acad. Emerg. Med. 27, 681–692 (2020). 10.1111/acem.14053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fortin J., Rogge D. E., Fellner C., Flotzinger D., Grond J., Lerche K., and Saugel B., “ A novel art of continuous noninvasive blood pressure measurement,” Nat. Commun. 12, 1387 (2021). 10.1038/s41467-021-21271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luks A. M. and Swenson E. R., “ Pulse oximetry for monitoring patients with COVID-19 at home. Potential pitfalls and practical guidance,” Ann. Am. Thorac. Soc. 17, 1040–1046 (2020). 10.1513/AnnalsATS.202005-418FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ajlan R. S., Desai A. A., and Mainster M. A., “ Endoscopic vitreoretinal surgery: Principles, applications and new directions,” Int. J. Retina Vitreous 5, 15 (2019). 10.1186/s40942-019-0165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saghir N., Aggarwal A., Soneji N., Valencia V., Rodgers G., and Kurian T., “ A comparison of manual electrocardiographic interval and waveform analysis in lead 1 of 12-lead ECG and apple watch ECG: A validation study,” Cardiovasc. Digital Health J. 1, 30–36 (2020). 10.1016/j.cvdhj.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee Y., Chung J. W., Lee G. H., Kang H., Kim J.-Y., Bae C., Yoo H., Jeong S., Cho H., Kang S.-G. et al. , “ Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system,” Sci. Adv. 7, eabg9180 (2021). 10.1126/sciadv.abg9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steiger C., Abramson A., Nadeau P., Chandrakasan A. P., Langer R., and Traverso G., “ Ingestible electronics for diagnostics and therapy,” Nat. Rev. Mater. 4, 83–98 (2019). 10.1038/s41578-018-0070-3 [DOI] [Google Scholar]
- 18. Gora M. J., Suter M. J., Tearney G. J., and Li X., “ Endoscopic optical coherence tomography: Technologies and clinical applications,” Biomed. Opt. Express 8, 2405–2444 (2017). 10.1364/BOE.8.002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummins G., Cox B. F., Ciuti G., Anbarasan T., Desmulliez M. P., Cochran S., Steele R., Plevris J. N., and Koulaouzidis A., “ Gastrointestinal diagnosis using non-white light imaging capsule endoscopy,” Nat. Rev. Gastroenterol. Hepatol. 16, 429–447 (2019). 10.1038/s41575-019-0140-z [DOI] [PubMed] [Google Scholar]
- 20. Gao W., Emaminejad S., Nyein H. Y. Y., Challa S., Chen K., Peck A., Fahad H. M., Ota H., Shiraki H., Kiriya D. et al. , “ Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis,” Nature 529, 509–514 (2016). 10.1038/nature16521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim J., Gutruf P., Chiarelli A. M., Heo S. Y., Cho K., Xie Z., Banks A., Han S., Jang K.-I., Lee J. W. et al. , “ Miniaturized battery-free wireless systems for wearable pulse oximetry,” Adv. Funct. Mater. 27, 1604373 (2017). 10.1002/adfm.201604373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H.-J., Jin Y., Achavananthadith S., Lin R., and Ho J. S., “ A wireless optoelectronic skin patch for light delivery and thermal monitoring,” iScience 24, 103284 (2021). 10.1016/j.isci.2021.103284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baldinger M., Heinrich A., Adams T., Martens E., Dommasch M., Müller A., Siegmann A., and Schmidt G., “ Telecovid: Remote vital signs monitoring of COVID-19 risk patients in home isolation with an in-ear wearable,” IEEE Pervasive Comput. 20, 58–62 (2021). 10.1109/MPRV.2021.3066825 [DOI] [Google Scholar]
- 24. Yun S. H. and Kwok S. J., “ Light in diagnosis, therapy and surgery,” Nat. Biomed. Eng. 1, 0008 (2017). 10.1038/s41551-016-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee G.-H., Moon H., Kim H., Lee G. H., Kwon W., Yoo S., Myung D., Yun S. H., Bao Z., and Hahn S. K., “ Multifunctional materials for implantable and wearable photonic healthcare devices,” Nat. Rev. Mater. 5, 149–165 (2020). 10.1038/s41578-019-0167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeon Y., Choi H.-R., Lim M., Choi S., Kim H., Kwon J. H., Park K.-C., and Choi K. C., “ A wearable photobiomodulation patch using a flexible red-wavelength OLED and its in vitro differential cell proliferation effects,” Adv. Mater. Technol. 3, 1700391 (2018). 10.1002/admt.201700391 [DOI] [Google Scholar]
- 27. Lee H. E., Lee S. H., Jeong M., Shin J. H., Ahn Y., Kim D., Oh S. H., Yun S. H., and Lee K. J., “ Trichogenic photostimulation using monolithic flexible vertical AlGaInP light-emitting diodes,” ACS Nano 12, 9587–9595 (2018). 10.1021/acsnano.8b05568 [DOI] [PubMed] [Google Scholar]
- 28. Bansal A., Yang F., Xi T., Zhang Y., and Ho J. S., “ In vivo wireless photonic photodynamic therapy,” Proc. Natl. Acad. Sci. U. S. A. 115, 1469–1474 (2018). 10.1073/pnas.1717552115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim A., Zhou J., Samaddar S., Song S. H., Elzey B. D., Thompson D. H., and Ziaie B., “ An implantable ultrasonically-powered micro-light-source (μlight) for photodynamic therapy,” Sci. Rep. 9, 1395 (2019). 10.1038/s41598-019-38554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park S. I., Brenner D. S., Shin G., Morgan C. D., Copits B. A., Chung H. U., Pullen M. Y., Noh K. N., Davidson S., Oh S. J. et al. , “ Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics,” Nat. Biotechnol. 33, 1280–1286 (2015). 10.1038/nbt.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin G., Gomez A. M., Al-Hasani R., Jeong Y. R., Kim J., Xie Z., Banks A., Lee S. M., Han S. Y., Yoo C. J. et al. , “ Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics,” Neuron 93, 509–521 (2017). 10.1016/j.neuron.2016.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim C. Y., Ku M. J., Qazi R., Nam H. J., Park J. W., Nam K. S., Oh S., Kang I., Jang J.-H., Kim W. Y. et al. , “ Soft subdermal implant capable of wireless battery charging and programmable controls for applications in optogenetics,” Nat. Commun. 12, 535 (2021). 10.1038/s41467-020-20803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S., Cortese A. J., Gandhi A. P., Agger E. R., McEuen P. L., and Molnar A. C., “ A 250 μm× 57 μm microscale opto-electronically transduced electrodes (motes) for neural recording,” IEEE Trans. Biomed. Circuits Syst. 12, 1256–1266 (2018). 10.1109/TBCAS.2018.2876069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montgomery K. L., Yeh A. J., Ho J. S., Tsao V., Iyer S. M., Grosenick L., Ferenczi E. A., Tanabe Y., Deisseroth K., Delp S. L. et al. , “ Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice,” Nat. Methods 12, 969–974 (2015). 10.1038/nmeth.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Y., Wu M., Vázquez-Guardado A., Kim J., Zhang X., Avila R., Kim J.-T., Deng Y., Yu Y., Melzer S. et al. , “ Wireless multi-lateral optofluidic microsystems for real-time programmable optogenetics and photopharmacology,” Nat. Commun. 13, 5571 (2022). 10.1038/s41467-022-32947-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Z., Obaid S. N., and Lu L., “ Recent advances in organic optoelectronic devices for biomedical applications,” Opt. Mater. Express 9, 3843–3856 (2019). 10.1364/OME.9.003843 [DOI] [Google Scholar]
- 37. Kim T.-I., McCall J. G., Jung Y. H., Huang X., Siuda E. R., Li Y., Song J., Song Y. M., Pao H. A., Kim R.-H. et al. , “ Injectable, cellular-scale optoelectronics with applications for wireless optogenetics,” Science 340, 211–216 (2013). 10.1126/science.1232437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pearsall T. P., Photonics Essentials ( McGraw-Hill Education, 2003). [Google Scholar]
- 39. Dutta Gupta S. and Jatothu B., “ Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis,” Plant Biotechnol. Rep. 7, 211–220 (2013). 10.1007/s11816-013-0277-0 [DOI] [Google Scholar]
- 40. Opel D. R., Hagstrom E., Pace A. K., Sisto K., Hirano-ALi S. A., Desai S., and Swan J., “ Light-emitting diodes: A brief review and clinical experience,” J. Clin. Aesthet. Dermatol. 8, 36–44 (2015), see https://jcadonline.com/light-emitting-diodes-a-brief-review-and-clinical-experience/. [PMC free article] [PubMed] [Google Scholar]
- 41. Calderhead R. G., “ The photobiological basics behind light-emitting diode (LED) phototherapy,” Laser Ther. 16, 97–108 (2007). 10.5978/islsm.16.97 [DOI] [Google Scholar]
- 42. Huang H., Huang J., Lin C., Lee K., Hsu H., Yu C., and Kuo H.-C., “ Efficiency improvement of GaN-based leds with a SiO2 nanorod array and a patterned sapphire substrate,” IEEE Electron Device Lett. 31, 582–584 (2010). 10.1109/LED.2010.2045472 [DOI] [Google Scholar]
- 43. Chiu C., Yen H., Chao C., Li Z., Yu P., Kuo H.-C., Lu T.-C., Wang S., Lau K. M., and Cheng S.-J., “ Nanoscale epitaxial lateral overgrowth of gan-based light-emitting diodes on a SiO2 nanorod-array patterned sapphire template,” Appl. Phys. Lett. 93, 081108 (2008). 10.1063/1.2969062 [DOI] [Google Scholar]
- 44. Pietryga J. M., Park Y.-S., Lim J., Fidler A. F., Bae W. K., Brovelli S., and Klimov V. I., “ Spectroscopic and device aspects of nanocrystal quantum dots,” Chem. Rev. 116, 10513–10622 (2016). 10.1021/acs.chemrev.6b00169 [DOI] [PubMed] [Google Scholar]
- 45. Goncalves S., Ribeiro J., Silva A., Costa R., and Correia J., “ Design and manufacturing challenges of optogenetic neural interfaces: A review,” J. Neural Eng. 14, 041001 (2017). 10.1088/1741-2552/aa7004 [DOI] [PubMed] [Google Scholar]
- 46. Mondello S., Pedigo B., Sunshine M., Fischedick A., Horner P., and Moritz C., “ A micro-led implant and technique for optogenetic stimulation of the rat spinal cord,” Exp. Neurol. 335, 113480 (2021). 10.1016/j.expneurol.2020.113480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sim J. Y., Haney M. P., Park S. I., McCall J. G., and Jeong J.-W., “ Microfluidic neural probes: In vivo tools for advancing neuroscience,” Lab Chip 17, 1406–1435 (2017). 10.1039/C7LC00103G [DOI] [PubMed] [Google Scholar]
- 48. Song J., Lee H., Jeong E. G., Choi K. C., and Yoo S., “ Organic light-emitting diodes: Pushing toward the limits and beyond,” Adv. Mater. 32, 1907539 (2020). 10.1002/adma.201907539 [DOI] [PubMed] [Google Scholar]
- 49. Yokota T., Zalar P., Kaltenbrunner M., Jinno H., Matsuhisa N., Kitanosako H., Tachibana Y., Yukita W., Koizumi M., and Someya T., “ Ultraflexible organic photonic skin,” Sci. Adv. 2, e1501856 (2016). 10.1126/sciadv.1501856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qian Y., Zhang X., Xie L., Qi D., Chandran B. K., Chen X., and Huang W., “ Stretchable organic semiconductor devices,” Adv. Mater. 28, 9243–9265 (2016). 10.1002/adma.201601278 [DOI] [PubMed] [Google Scholar]
- 51. Yin D., Feng J., Ma R., Liu Y.-F., Zhang Y.-L., Zhang X.-L., Bi Y.-G., Chen Q.-D., and Sun H.-B., “ Efficient and mechanically robust stretchable organic light-emitting devices by a laser-programmable buckling process,” Nat. Commun. 7, 11573 (2016). 10.1038/ncomms11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bansal A. K., Hou S., Kulyk O., Bowman E. M., and Samuel I. D., “ Wearable organic optoelectronic sensors for medicine,” Adv. Mater. 27, 7638–7644 (2015). 10.1002/adma.201403560 [DOI] [PubMed] [Google Scholar]
- 53. Heo J. S., Eom J., Kim Y.-H., and Park S. K., “ Recent progress of textile-based wearable electronics: A comprehensive review of materials, devices, and applications,” Small 14, 1703034 (2018). 10.1002/smll.201703034 [DOI] [PubMed] [Google Scholar]
- 54. Triana M. A., Restrepo A. A., Lanzafame R. J., Palomaki P., and Dong Y., “ Quantum dot light-emitting diodes as light sources in photomedicine: Photodynamic therapy and photobiomodulation,” J. Phys. Mater. 3, 032002 (2020). 10.1088/2515-7639/ab95e8 [DOI] [Google Scholar]
- 55. Jeon Y., Noh I., Seo Y. C., Han J. H., Park Y., Cho E. H., and Choi K. C., “ Parallel-stacked flexible organic light-emitting diodes for wearable photodynamic therapeutics and color-tunable optoelectronics,” ACS Nano 14, 15688–15699 (2020). 10.1021/acsnano.0c06649 [DOI] [PubMed] [Google Scholar]
- 56. Zhang H. and Rogers J. A., “ Recent advances in flexible inorganic light emitting diodes: From materials design to integrated optoelectronic platforms,” Adv. Opt. Mater. 7, 1800936 (2019). 10.1002/adom.201800936 [DOI] [Google Scholar]
- 57. Scholz S., Kondakov D., Lussem B., and Leo K., “ Degradation mechanisms and reactions in organic light-emitting devices,” Chem. Rev. 115, 8449–8503 (2015). 10.1021/cr400704v [DOI] [PubMed] [Google Scholar]
- 58. Aziz H., Popovic Z., Tripp C. P., Hu N.-X., Hor A.-M., and Xu G., “ Degradation processes at the cathode/organic interface in organic light emitting devices with mg: Ag cathodes,” Appl. Phys. Lett. 72, 2642–2644 (1998). 10.1063/1.121442 [DOI] [Google Scholar]
- 59. Pode R., “ Organic light emitting diode devices: An energy efficient solid state lighting for applications,” Renewable Sustainable Energy Rev. 133, 110043 (2020). 10.1016/j.rser.2020.110043 [DOI] [Google Scholar]
- 60. Sun L., Kurosawa Y., Ito H., Makishima Y., Kita H., Yoshida T., and Suzuri Y., “ Solution processing of alternating PDMS/SiOx multilayer for encapsulation of organic light emitting diodes,” Org. Electron. 64, 176–180 (2019). 10.1016/j.orgel.2018.10.027 [DOI] [Google Scholar]
- 61. Yu Y., “ Package structure of flexible OLED device and display device,” U.S. patent Application 15/310,101 (1 June 2016).
- 62. Supran G. J., Shirasaki Y., Song K. W., Caruge J.-M., Kazlas P. T., Coe-Sullivan S., Andrew T. L., Bawendi M. G., and Bulović V., “ QLEDs for displays and solid-state lighting,” MRS Bull. 38, 703–711 (2013). 10.1557/mrs.2013.181 [DOI] [Google Scholar]
- 63. Choi M. K., Yang J., Hyeon T., and Kim D.-H., “ Flexible quantum dot light-emitting diodes for next-generation displays,” npj Flexible Electron. 2, 10 (2018). 10.1038/s41528-018-0023-3 [DOI] [Google Scholar]
- 64. Chen H., He J., Lanzafame R., Stadler I., Hamidi H. E., Liu H., Celli J., Hamblin M. R., Huang Y., Oakley E. et al. , “ Quantum dot light emitting devices for photomedical applications,” J. Soc. Inf. Disp. 25, 177–184 (2017). 10.1002/jsid.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen H., Yeh T.-H., He J., Zhang C., Abbel R., Hamblin M. R., Huang Y., Lanzafame R. J., Stadler I., Celli J. et al. , “ Flexible quantum dot light-emitting devices for targeted photomedical applications,” J. Soc. Inf. Disp. 26, 296–303 (2018). 10.1002/jsid.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun Y., Jiang Y., Sun X. W., Zhang S., and Chen S., “ Beyond oled: Efficient quantum dot light-emitting diodes for display and lighting application,” Chem. Rec. 19, 1729–1752 (2019). 10.1002/tcr.201800191 [DOI] [PubMed] [Google Scholar]
- 67. Dai X., Zhang Z., Jin Y., Niu Y., Cao H., Liang X., Chen L., Wang J., and Peng X., “ Solution-processed, high-performance light-emitting diodes based on quantum dots,” Nature 515, 96–99 (2014). 10.1038/nature13829 [DOI] [PubMed] [Google Scholar]
- 68. Yang Y., Zheng Y., Cao W., Titov A., Hyvonen J., Manders J. R., Xue J., Holloway P. H., and Qian L., “ High-efficiency light-emitting devices based on quantum dots with tailored nanostructures,” Nat. Photonics 9, 259–266 (2015). 10.1038/nphoton.2015.36 [DOI] [Google Scholar]
- 69. Rinaldi F., “ Laser: A review,” Clin. Dermatol. 26, 590–601 (2008). 10.1016/j.clindermatol.2007.09.014 [DOI] [PubMed] [Google Scholar]
- 70. Sieno L. D., Nissinen J., Hallman L., Martinenghi E., Contini D., Pifferi A., Kostamovaara J., and Mora A. D., “ Miniaturized pulsed laser source for time-domain diffuse optics routes to wearable devices,” J. Biomed. Opt. 22, 085004 (2017). 10.1117/1.JBO.22.8.085004 [DOI] [PubMed] [Google Scholar]
- 71. Yun S. H., Tearney G. J., Vakoc B. J., Shishkov M., Oh W. Y., Desjardins A. E., Suter M. J., Chan R. C., Evans J. A., Jang I.-K. et al. , “ Comprehensive volumetric optical microscopy in vivo,” Nat. Med. 12, 1429–1433 (2006). 10.1038/nm1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim M.-S., Lee H.-T., and Ahn S.-H., “ Laser controlled 65 μm long microrobot made of Ni-Ti shape memory alloy,” Adv. Mater. Technol. 4, 1900583 (2019). 10.1002/admt.201900583 [DOI] [Google Scholar]
- 73. Bunea A.-I., Martella D., Nocentini S., Parmeggiani C., Taboryski R., and Wiersma D. S., “ Light-powered microrobots: Challenges and opportunities for hard and soft responsive microswimmers,” Adv. Intell. Syst. 3, 2000256 (2021). 10.1002/aisy.202000256 [DOI] [Google Scholar]
- 74. Goodman A. M., Neumann O., Nørregaard K., Henderson L., Choi M.-R., Clare S. E., and Halas N. J., “ Near-infrared remotely triggered drug-release strategies for cancer treatment,” Proc. Natl. Acad. Sci. U. S. A. 114, 12419–12424 (2017). 10.1073/pnas.1713137114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Allen J., “ Photoplethysmography and its application in clinical physiological measurement,” Physiol. Meas. 28, R1 (2007). 10.1088/0967-3334/28/3/R01 [DOI] [PubMed] [Google Scholar]
- 76. Fonseca P., Weysen T., Goelema M. S., Møst E. I., Radha M., Lunsingh Scheurleer C., van den Heuvel L., and Aarts R. M., “ Validation of photoplethysmography-based sleep staging compared with polysomnography in healthy middle-aged adults,” Sleep 40, 28838130 (2017). 10.1093/sleep/zsx097 [DOI] [PubMed] [Google Scholar]
- 77. Sola J., Proença M., Ferrario D., Porchet J.-A., Falhi A., Grossenbacher O., Allemann Y., Rimoldi S. F., and Sartori C., “ Noninvasive and nonocclusive blood pressure estimation via a chest sensor,” IEEE Trans. Biomed. Eng. 60, 3505–3513 (2013). 10.1109/TBME.2013.2272699 [DOI] [PubMed] [Google Scholar]
- 78. Kim J., Salvatore G. A., Araki H., Chiarelli A. M., Xie Z., Banks A., Sheng X., Liu Y., Lee J. W., Jang K.-I. et al. , “ Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin,” Sci. Adv. 2, e1600418 (2016). 10.1126/sciadv.1600418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kang M. H., Lee G. J., Lee J. H., Kim M. S., Yan Z., Jeong J.-W., Jang K.-I., and Song Y. M., “ Outdoor-useable, wireless/battery-free patch-type tissue oximeter with radiative cooling,” Adv. Sci. 8, 2004885 (2021). 10.1002/advs.202004885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jansen-van Vuuren R. D., Armin A., Pandey A. K., Burn P. L., and Meredith P., “ Organic photodiodes: The future of full color detection and image sensing,” Adv. Mater. 28, 4766–4802 (2016). 10.1002/adma.201505405 [DOI] [PubMed] [Google Scholar]
- 81. Chow P. C. and Someya T., “ Organic photodetectors for next-generation wearable electronics,” Adv. Mater. 32, 1902045 (2020). 10.1002/adma.201902045 [DOI] [PubMed] [Google Scholar]
- 82. Häberlin H., Photovoltaics: System Design and Practice ( John Wiley & Sons, 2012). [Google Scholar]
- 83. Song Y. M., Xie Y., Malyarchuk V., Xiao J., Jung I., Choi K.-J., Liu Z., Park H., Lu C., Kim R.-H. et al. , “ Digital cameras with designs inspired by the arthropod eye,” Nature 497, 95–99 (2013). 10.1038/nature12083 [DOI] [PubMed] [Google Scholar]
- 84. Jung I., Xiao J., Malyarchuk V., Lu C., Li M., Liu Z., Yoon J., Huang Y., and Rogers J. A., “ Dynamically tunable hemispherical electronic eye camera system with adjustable zoom capability,” Proc. Natl. Acad. Sci. U. S. A. 108, 1788–1793 (2011). 10.1073/pnas.1015440108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. An J., Le T.-S. D., Lim C. H. J., Tran V. T., Zhan Z., Gao Y., Zheng L., Sun G., and Kim Y.-J., “ Single-step selective laser writing of flexible photodetectors for wearable optoelectronics,” Adv. Sci. 5, 1800496 (2018). 10.1002/advs.201800496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kang P., Wang M. C., Knapp P. M., and Nam S., “ Crumpled graphene photodetector with enhanced, strain-tunable, and wavelength-selective photoresponsivity,” Adv. Mater. 28, 4639–4645 (2016). 10.1002/adma.201600482 [DOI] [PubMed] [Google Scholar]
- 87. Lu L., Gutruf P., Xia L., Bhatti D. L., Wang X., Vazquez-Guardado A., Ning X., Shen X., Sang T., Ma R. et al. , “ Wireless optoelectronic photometers for monitoring neuronal dynamics in the deep brain,” Proc. Natl. Acad. Sci. U. S. A. 115, E1374–E1383 (2018). 10.1073/pnas.1718721115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Burton A., Obaid S. N., Vázquez-Guardado A., Schmit M. B., Stuart T., Cai L., Chen Z., Kandela I., Haney C. R., Waters E. A. et al. , “ Wireless, battery-free subdermally implantable photometry systems for chronic recording of neural dynamics,” Proc. Natl. Acad. Sci. U. S. A. 117, 2835–2845 (2020). 10.1073/pnas.1920073117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bai W., Shin J., Fu R., Kandela I., Lu D., Ni X., Park Y., Liu Z., Hang T., Wu D. et al. , “ Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity,” Nat. Biomed. Eng. 3, 644–654 (2019). 10.1038/s41551-019-0435-y [DOI] [PubMed] [Google Scholar]
- 90. Wang Y. and Zhan X., “ Layer-by-layer processed organic solar cells,” Adv. Energy Mater. 6, 1600414 (2016). 10.1002/aenm.201600414 [DOI] [Google Scholar]
- 91. Pierre A. and Arias A. C., “ Solution-processed image sensors on flexible substrates,” Flexible Printed Electron. 1, 043001 (2016). 10.1088/2058-8585/1/4/043001 [DOI] [Google Scholar]
- 92. Kaltenbrunner M., White M. S., Głowacki E. D., Sekitani T., Someya T., Sariciftci N. S., and Bauer S., “ Ultrathin and lightweight organic solar cells with high flexibility,” Nat. Commun. 3, 770 (2012). 10.1038/ncomms1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park S., Fukuda K., Wang M., Lee C., Yokota T., Jin H., Jinno H., Kimura H., Zalar P., Matsuhisa N. et al. , “ Ultraflexible near-infrared organic photodetectors for conformal photoplethysmogram sensors,” Adv. Mater. 30, 1802359 (2018). 10.1002/adma.201802359 [DOI] [PubMed] [Google Scholar]
- 94. Xu H., Liu J., Zhang J., Zhou G., Luo N., and Zhao N., “ Flexible organic/inorganic hybrid near-infrared photoplethysmogram sensor for cardiovascular monitoring,” Adv. Mater. 29, 1700975 (2017). 10.1002/adma.201700975 [DOI] [PubMed] [Google Scholar]
- 95. Lin Q., Armin A., Burn P. L., and Meredith P., “ Filterless narrowband visible photodetectors,” Nat. Photonics 9, 687–694 (2015). 10.1038/nphoton.2015.175 [DOI] [Google Scholar]
- 96. García de Arquer F. P., Armin A., Meredith P., and Sargent E. H., “ Solution-processed semiconductors for next-generation photodetectors,” Nat. Rev. Mater. 2, 16100 (2017). 10.1038/natrevmats.2016.100 [DOI] [Google Scholar]
- 97. Zhou Y., Fuentes-Hernandez C., Shim J., Meyer J., Giordano A. J., Li H., Winget P., Papadopoulos T., Cheun H., Kim J. et al. , “ A universal method to produce low–work function electrodes for organic electronics,” Science 336, 327–332 (2012). 10.1126/science.1218829 [DOI] [PubMed] [Google Scholar]
- 98. Cave D. R., Fleischer D. E., Leighton J. A., Faigel D. O., Heigh R. I., Sharma V. K., Gostout C. J., Rajan E., Mergener K., Foley A. et al. , “ A multicenter randomized comparison of the Endocapsule and the Pillcam SB,” Gastrointest. Endosc. 68, 487–494 (2008). 10.1016/j.gie.2007.12.037 [DOI] [PubMed] [Google Scholar]
- 99. Ciuti G., Menciassi A., and Dario P., “ Capsule endoscopy: From current achievements to open challenges,” IEEE Rev. Biomed. Eng. 4, 59–72 (2011). 10.1109/RBME.2011.2171182 [DOI] [PubMed] [Google Scholar]
- 100. Gouveia L. C. P. and Choubey B., “ Advances on CMOS image sensors,” Sens. Rev. 36, 231 (2016). 10.1108/SR-11-2015-0189 [DOI] [Google Scholar]
- 101. Zhang J., Khalifa A., Spetalnick S., Alemohammad M., Rattray J., Thakur C. S., Eisape A., and Etienne-Cummings R., “ A miniature wireless silicon-on-insulator image sensor for brain fluorescence imaging,” in 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) ( IEEE, 2020), pp. 3403–3406. [DOI] [PubMed] [Google Scholar]
- 102. Moglia A., Menciassi A., Dario P., and Cuschieri A., “ Capsule endoscopy: Progress update and challenges ahead,” Nat. Rev. Gastroenterol. Hepatol. 6, 353–361 (2009). 10.1038/nrgastro.2009.69 [DOI] [PubMed] [Google Scholar]
- 103. Konstantatos G., Clifford J., Levina L., and Sargent E. H., “ Sensitive solution-processed visible-wavelength photodetectors,” Nat. Photonics 1, 531–534 (2007). 10.1038/nphoton.2007.147 [DOI] [Google Scholar]
- 104. Rauch T., Böberl M., Tedde S. F., Fürst J., Kovalenko M. V., Hesser G., Lemmer U., Heiss W., and Hayden O., “ Near-infrared imaging with quantum-dot-sensitized organic photodiodes,” Nat. Photonics 3, 332–336 (2009). 10.1038/nphoton.2009.72 [DOI] [Google Scholar]
- 105. Wu Z., Zhai Y., Yao W., Eedugurala N., Zhang S., Huang L., Gu X., Azoulay J. D., and Ng T. N., “ The role of dielectric screening in organic shortwave infrared photodiodes for spectroscopic image sensing,” Adv. Funct. Mater. 28, 1805738 (2018). 10.1002/adfm.201805738 [DOI] [Google Scholar]
- 106. Mathieson K., Loudin J., Goetz G., Huie P., Wang L., Kamins T. I., Galambos L., Smith R., Harris J. S., Sher A. et al. , “ Photovoltaic retinal prosthesis with high pixel density,” Nat. Photonics 6, 391–397 (2012). 10.1038/nphoton.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Simone G., Di Carlo Rasi D., de Vries X., Heintges G. H., Meskers S. C., Janssen R. A., and Gelinck G. H., “ Near-infrared tandem organic photodiodes for future application in artificial retinal implants,” Adv. Mater. 30, 1804678 (2018). 10.1002/adma.201804678 [DOI] [PubMed] [Google Scholar]
- 108. Azzellino G., Grimoldi A., Binda M., Caironi M., Natali D., and Sampietro M., “ Fully inkjet-printed organic photodetectors with high quantum yield,” Adv. Mater. 25, 6829–6833 (2013). 10.1002/adma.201303473 [DOI] [PubMed] [Google Scholar]
- 109. Tedde S. F., Kern J., Sterzl T., Furst J., Lugli P., and Hayden O., “ Fully spray coated organic photodiodes,” Nano Lett. 9, 980–983 (2009). 10.1021/nl803386y [DOI] [PubMed] [Google Scholar]
- 110. Chow P. C., Matsuhisa N., Zalar P., Koizumi M., Yokota T., and Someya T., “ Dual-gate organic phototransistor with high-gain and linear photoresponse,” Nat. Commun. 9, 4546 (2018). 10.1038/s41467-018-06907-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Drews J., Fehrmann G., Staub R., and Wolf R., “ Primary batteries for implantable pacemakers and defibrillators,” J. Power Sources 97, 747–749 (2001). 10.1016/S0378-7753(01)00649-8 [DOI] [Google Scholar]
- 112. Bock D. C., Marschilok A. C., Takeuchi K. J., and Takeuchi E. S., “ Batteries used to power implantable biomedical devices,” Electrochim. Acta 84, 155–164 (2012). 10.1016/j.electacta.2012.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nathan M., “ Microbattery technologies for miniaturized implantable medical devices,” Curr. Pharm. Biotechnol. 11, 404–410 (2010). 10.2174/138920110791233334 [DOI] [PubMed] [Google Scholar]
- 114. Lee J. W., Xu R., Lee S., Jang K.-I., Yang Y., Banks A., Yu K. J., Kim J., Xu S., Ma S. et al. , “ Soft, thin skin-mounted power management systems and their use in wireless thermography,” Proc. Natl. Acad. Sci. U. S. A. 113, 6131–6136 (2016). 10.1073/pnas.1605720113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fan L., Wei S., Li S., Li Q., and Lu Y., “ Recent progress of the solid-state electrolytes for high-energy metal-based batteries,” Adv. Energy Mater. 8, 1702657 (2018). 10.1002/aenm.201702657 [DOI] [Google Scholar]
- 116. Kutbee A. T., Bahabry R. R., Alamoudi K. O., Ghoneim M. T., Cordero M. D., Almuslem A. S., Gumus A., Diallo E. M., Nassar J. M., Hussain A. M. et al. , “ Flexible and biocompatible high-performance solid-state micro-battery for implantable orthodontic system,” npj Flexible Electron. 1, 7 (2017). 10.1038/s41528-017-0008-7 [DOI] [Google Scholar]
- 117. Zvezdin A., Mauro E. D., Rho D., Santato C., and Khalil M., “ En route toward sustainable organic electronics,” MRS Energy Sustainability 7, 16 (2020). 10.1557/mre.2020.16 [DOI] [Google Scholar]
- 118. Peng F., Li H., Wang D., Tian P., Tian Y., Yuan G., Xu D., and Liu X., “ Enhanced corrosion resistance and biocompatibility of magnesium alloy by Mg–Al-layered double hydroxide,” ACS Appl. Mater. Interfaces 8, 35033–35044 (2016). 10.1021/acsami.6b12974 [DOI] [PubMed] [Google Scholar]
- 119. Nadeau P., El-Damak D., Glettig D., Kong Y. L., Mo S., Cleveland C., Booth L., Roxhed N., Langer R., Chandrakasan A. P. et al. , “ Prolonged energy harvesting for ingestible devices,” Nat. Biomed. Eng. 1, 0022 (2017). 10.1038/s41551-016-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dong Y., Li J., Yang F., Wang Y., Zhang Z., Wang J., Long Y., and Wang X., “ Bioresorbable primary battery anodes built on core–double-shell zinc microparticle networks,” ACS Appl. Mater. Interfaces 13, 14275–14282 (2021). 10.1021/acsami.1c00602 [DOI] [PubMed] [Google Scholar]
- 121. El-Kady M. F., Strong V., Dubin S., and Kaner R. B., “ Laser scribing of high-performance and flexible graphene-based electrochemical capacitors,” Science 335, 1326–1330 (2012). 10.1126/science.1216744 [DOI] [PubMed] [Google Scholar]
- 122. El-Kady M. F., Ihns M., Li M., Hwang J. Y., Mousavi M. F., Chaney L., Lech A. T., and Kaner R. B., “ Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage,” Proc. Natl. Acad. Sci. U. S. A. 112, 4233–4238 (2015). 10.1073/pnas.1420398112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chong Y.-W., Ismail W., Ko K., and Lee C.-Y., “ Energy harvesting for wearable devices: A review,” IEEE Sens. J. 19, 9047–9062 (2019). 10.1109/JSEN.2019.2925638 [DOI] [Google Scholar]
- 124. Zhong C., Deng Y., Hu W., Qiao J., Zhang L., and Zhang J., “ A review of electrolyte materials and compositions for electrochemical supercapacitors,” Chem. Soc. Rev. 44, 7484–7539 (2015). 10.1039/C5CS00303B [DOI] [PubMed] [Google Scholar]
- 125. Wentz C. T., Bernstein J. G., Monahan P., Guerra A., Rodriguez A., and Boyden E. S., “ A wirelessly powered and controlled device for optical neural control of freely-behaving animals,” J. Neural Eng. 8, 046021 (2011). 10.1088/1741-2560/8/4/046021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huggins R. A., “ Supercapacitors and electrochemical pulse sources,” Solid State Ionics 134, 179–195 (2000). 10.1016/S0167-2738(00)00725-6 [DOI] [Google Scholar]
- 127. Gilshteyn E. P., Amanbaev D., Silibin M. V., Sysa A., Kondrashov V. A., Anisimov A. S., Kallio T., and Nasibulin A. G., “ Flexible self-powered piezo-supercapacitor system for wearable electronics,” Nanotechnology 29, 325501 (2018). 10.1088/1361-6528/aac658 [DOI] [PubMed] [Google Scholar]
- 128. Deng F., Qiu H., Chen J., Wang L., and Wang B., “ Wearable thermoelectric power generators combined with flexible supercapacitor for low-power human diagnosis devices,” IEEE Trans. Ind. Electron. 64, 1477–1485 (2016). 10.1109/TIE.2016.2613063 [DOI] [Google Scholar]
- 129. Yue Y., Yang Z., Liu N., Liu W., Zhang H., Ma Y., Yang C., Su J., Li L., Long F. et al. , “ A flexible integrated system containing a microsupercapacitor, a photodetector, and a wireless charging coil,” ACS Nano 10, 11249–11257 (2016). 10.1021/acsnano.6b06326 [DOI] [PubMed] [Google Scholar]
- 130. Lv F., Ma H., Shen L., Jiang Y., Sun T., Ma J., Geng X., Kiran A., and Zhu N., “ Wearable helical molybdenum nitride supercapacitors for self-powered healthcare smartsensors,” ACS Appl. Mater. Interfaces 13, 29780–29787 (2021). 10.1021/acsami.1c05247 [DOI] [PubMed] [Google Scholar]
- 131. Maitra A., Bera R., Halder L., Bera A., Paria S., Karan S. K., Si S. K., De A., Ojha S., and Khatua B. B., “ Photovoltaic and triboelectrification empowered light-weight flexible self-charging asymmetric supercapacitor cell for self-powered multifunctional electronics,” Renewable Sust. Energy Rev. 151, 111595 (2021). 10.1016/j.rser.2021.111595 [DOI] [Google Scholar]
- 132. Chen K., Wang Q., Niu Z., and Chen J., “ Graphene-based materials for flexible energy storage devices,” J. Energy Chem. 27, 12–24 (2018). 10.1016/j.jechem.2017.08.015 [DOI] [Google Scholar]
- 133. Sheng H., Zhou J., Li B., He Y., Zhang X., Liang J., Zhou J., Su Q., Xie E., Lan W. et al. , “ A thin, deformable, high-performance supercapacitor implant that can be biodegraded and bioabsorbed within an animal body,” Sci. Adv. 7, eabe3097 (2021). 10.1126/sciadv.abe3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zou Y., Bo L., and Li Z., “ Recent progress in human body energy harvesting for smart bioelectronic system,” Fundam. Res. 1, 364–382 (2021). 10.1016/j.fmre.2021.05.002 [DOI] [Google Scholar]
- 135. Anton S. R. and Sodano H. A., “ A review of power harvesting using piezoelectric materials (2003–2006),” Smart Mater. Struct. 16, R1 (2007). 10.1088/0964-1726/16/3/R01 [DOI] [Google Scholar]
- 136. Song P., Yang G., Lang T., and Yong K.-T., “ Nanogenerators for wearable bioelectronics and biodevices,” J. Phys. D: Appl. Phys. 52, 023002 (2018). 10.1088/1361-6463/aae44d [DOI] [Google Scholar]
- 137. Ha M., Park J., Lee Y., and Ko H., “ Triboelectric generators and sensors for self-powered wearable electronics,” ACS Nano 9, 3421–3427 (2015). 10.1021/acsnano.5b01478 [DOI] [PubMed] [Google Scholar]
- 138. Zhu G., Zhou Y. S., Bai P., Meng X. S., Jing Q., Chen J., and Wang Z. L., “ A shape-adaptive thin-film-based approach for 50% high-efficiency energy generation through micro-grating sliding electrification,” Adv. Mater. 26, 3788–3796 (2014). 10.1002/adma.201400021 [DOI] [PubMed] [Google Scholar]
- 139. Seung W., Gupta M. K., Lee K. Y., Shin K.-S., Lee J.-H., Kim T. Y., Kim S., Lin J., Kim J. H., and Kim S.-W., “ Nanopatterned textile-based wearable triboelectric nanogenerator,” ACS Nano 9, 3501–3509 (2015). 10.1021/nn507221f [DOI] [PubMed] [Google Scholar]
- 140. Wu C., Wang A. C., Ding W., Guo H., and Wang Z. L., “ Triboelectric nanogenerator: A foundation of the energy for the new era,” Adv. Energy Mater. 9, 1802906 (2019). 10.1002/aenm.201802906 [DOI] [Google Scholar]
- 141. Ma Y., Zheng Q., Liu Y., Shi B., Xue X., Ji W., Liu Z., Jin Y., Zou Y., An Z. et al. , “ Self-powered, one-stop, and multifunctional implantable triboelectric active sensor for real-time biomedical monitoring,” Nano Lett. 16, 6042–6051 (2016). 10.1021/acs.nanolett.6b01968 [DOI] [PubMed] [Google Scholar]
- 142. Zheng Q., Shi B., Fan F., Wang X., Yan L., Yuan W., Wang S., Liu H., Li Z., and Wang Z. L., “ In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator,” Adv. Mater. 26, 5851–5856 (2014). 10.1002/adma.201402064 [DOI] [PubMed] [Google Scholar]
- 143. Olivo J., Carrara S., and De Micheli G., “ Energy harvesting and remote powering for implantable biosensors,” IEEE Sens. J. 11, 1573–1586 (2010). 10.1109/JSEN.2010.2085042 [DOI] [Google Scholar]
- 144. Leonov V., “ Thermoelectric energy harvesting of human body heat for wearable sensors,” IEEE Sens. J. 13, 2284–2291 (2013). 10.1109/JSEN.2013.2252526 [DOI] [Google Scholar]
- 145. Stark I. and Stordeur M., “ New micro thermoelectric devices based on bismuth telluride-type thin solid films,” in Eighteenth International Conference on Thermoelectrics, Proceedings, ICT'99 ( IEEE, 1999), pp. 465–472. [Google Scholar]
- 146. Strasser M., Aigner R., Lauterbach C., Sturm T., Franosch M., and Wachutka G., “ Micromachined cmos thermoelectric generators as on-chip power supply,” Sens. Actuators, A 114, 362–370 (2004). 10.1016/j.sna.2003.11.039 [DOI] [Google Scholar]
- 147. Fotopoulou K. and Flynn B. W., “ Wireless power transfer in loosely coupled links: Coil misalignment model,” IEEE Trans. Magn. 47, 416–430 (2010). 10.1109/TMAG.2010.2093534 [DOI] [Google Scholar]
- 148. Soma M., Galbraith D. C., and White R. L., “ Radio-frequency coils in implantable devices: Misalignment analysis and design procedure,” IEEE Trans. Biomed. Eng. BME-34, 276–282 (1987). 10.1109/TBME.1987.326088 [DOI] [PubMed] [Google Scholar]
- 149. Waters B. H., Sample A. P., Bonde P., and Smith J. R., “ Powering a ventricular assist device (VAD) with the free-range resonant electrical energy delivery (free-d) system,” Proc. IEEE 100, 138–149 (2011). 10.1109/JPROC.2011.2165309 [DOI] [Google Scholar]
- 150. Mickle A. D., Won S. M., Noh K. N., Yoon J., Meacham K. W., Xue Y., McIlvried L. A., Copits B. A., Samineni V. K., Crawford K. E. et al. , “ A wireless closed-loop system for optogenetic peripheral neuromodulation,” Nature 565, 361–365 (2019). 10.1038/s41586-018-0823-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cai L., Burton A., Gonzales D. A., Kasper K. A., Azami A., Peralta R., Johnson M., Bakall J. A., Barron Villalobos E., Ross E. C., et al. , “ Osseosurface electronics–thin, wireless, battery-free and multimodal musculoskeletal biointerfaces,” Nat. Commun. 12, 6707 (2021). 10.1038/s41467-021-27003-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kim S., Ho J. S., and Poon A. S., “ Midfield wireless powering of subwavelength autonomous devices,” Phys. Rev. Lett. 110, 203905 (2013). 10.1103/PhysRevLett.110.203905 [DOI] [PubMed] [Google Scholar]
- 153. Ho J. S., Yeh A. J., Neofytou E., Kim S., Tanabe Y., Patlolla B., Beygui R. E., and Poon A. S., “ Wireless power transfer to deep-tissue microimplants,” Proc. Natl. Acad. Sci. U. S. A. 111, 7974–7979 (2014). 10.1073/pnas.1403002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Agarwal K., Jegadeesan R., Guo Y.-X., and Thakor N. V., “ Wireless power transfer strategies for implantable bioelectronics,” IEEE Rev. Biomed. Eng. 10, 136–161 (2017). 10.1109/RBME.2017.2683520 [DOI] [PubMed] [Google Scholar]
- 155. Ozeri S. and Shmilovitz D., “ Ultrasonic transcutaneous energy transfer for powering implanted devices,” Ultrasonics 50, 556–566 (2010). 10.1016/j.ultras.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 156. Piech D. K., Johnson B. C., Shen K., Ghanbari M. M., Li K. Y., Neely R. M., Kay J. E., Carmena J. M., Maharbiz M. M., and Muller R., “ A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication,” Nat. Biomed. Eng. 4, 207–222 (2020). 10.1038/s41551-020-0518-9 [DOI] [PubMed] [Google Scholar]
- 157. Pinton G., Aubry J.-F., Bossy E., Muller M., Pernot M., and Tanter M., “ Attenuation, scattering, and absorption of ultrasound in the skull bone,” Med. Phys. 39, 299–307 (2012). 10.1118/1.3668316 [DOI] [PubMed] [Google Scholar]
- 158. Hosseini S., Laursen K., Rashidi A., Mondal T., Corbett B., and Moradi F., “ S-mrut: Sectored-multiring ultrasonic transducer for selective powering of brain implants,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 68, 191–200 (2020). 10.1109/TUFFC.2020.3001084 [DOI] [PubMed] [Google Scholar]
- 159. Charthad J., Chang T. C., Liu Z., Sawaby A., Weber M. J., Baker S., Gore F., Felt S. A., and Arbabian A., “ A mm-sized wireless implantable device for electrical stimulation of peripheral nerves,” IEEE Trans. Biomed. Circuits Syst. 12, 257–270 (2018). 10.1109/TBCAS.2018.2799623 [DOI] [PubMed] [Google Scholar]
- 160. Sonmezoglu S., Fineman J. R., Maltepe E., and Maharbiz M. M., “ Monitoring deep-tissue oxygenation with a millimeter-scale ultrasonic implant,” Nat. Biotechnol. 39, 855–864 (2021). 10.1038/s41587-021-00866-y [DOI] [PubMed] [Google Scholar]
- 161. Murakawa K., Kobayashi M., Nakamura O., and Kawata S., “ A wireless near-infrared energy system for medical implants,” IEEE Eng. Med. Biol. Mag. 18, 70–72 (1999). 10.1109/51.805148 [DOI] [PubMed] [Google Scholar]
- 162. Yu G., Gao J., Hummelen J. C., Wudl F., and Heeger A. J., “ Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions,” Science 270, 1789–1791 (1995). 10.1126/science.270.5243.1789 [DOI] [Google Scholar]
- 163. Jung D., Song K., Ju H., Park H., Han J. H., Kim N., Kim J., and Lee J., “ Sustainably powered, multifunctional flexible feedback implant by the bifacial design and si photovoltaics,” Adv. Healthcare Mater. 10, 2001480 (2021). 10.1002/adhm.202001480 [DOI] [PubMed] [Google Scholar]
- 164. Khan Y., Ostfeld A. E., Lochner C. M., Pierre A., and Arias A. C., “ Monitoring of vital signs with flexible and wearable medical devices,” Adv. Mater. 28, 4373–4395 (2016). 10.1002/adma.201504366 [DOI] [PubMed] [Google Scholar]
- 165. Gong S. and Cheng W., “ Toward soft skin-like wearable and implantable energy devices,” Adv. Energy Mater. 7, 1700648 (2017). 10.1002/aenm.201700648 [DOI] [Google Scholar]
- 166. Jeong J., Jung J., Jung D., Kim J., Ju H., Kim T., and Lee J., “ An implantable optogenetic stimulator wirelessly powered by flexible photovoltaics with near-infrared (NIR) light,” Biosens. Bioelectron. 180, 113139 (2021). 10.1016/j.bios.2021.113139 [DOI] [PubMed] [Google Scholar]
- 167. Song K., Han J. H., Lim T., Kim N., Shin S., Kim J., Choo H., Jeong S., Kim Y.-C., Wang Z. L. et al. , “ Subdermal flexible solar cell arrays for powering medical electronic implants,” Adv. Healthcare Mater. 5, 1572–1580 (2016). 10.1002/adhm.201600222 [DOI] [PubMed] [Google Scholar]
- 168. Cho S. H., Lee J., Lee M. J., Kim H. J., Lee S.-M., and Choi K. C., “ Plasmonically engineered textile polymer solar cells for high-performance, wearable photovoltaics,” ACS Appl. Mater. Interfaces 11, 20864–20872 (2019). 10.1021/acsami.9b05048 [DOI] [PubMed] [Google Scholar]
- 169. Troy T. L. and Thennadil S. N., “ Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm,” J. Biomed. Opt. 6, 167–176 (2001). 10.1117/1.1344191 [DOI] [PubMed] [Google Scholar]
- 170. Silverå Ejneby M., Jakešová M., Ferrero J. J., Migliaccio L., Sahalianov I., Zhao Z., Berggren M., Khodagholy D., Đerek V., Gelinas J. N. et al. , “ Chronic electrical stimulation of peripheral nerves via deep-red light transduced by an implanted organic photocapacitor,” Nat. Biomed. Eng. 6, 741 (2021). 10.1038/s41551-021-00817-7 [DOI] [PubMed] [Google Scholar]
- 171. Ding H., Lu L., Shi Z., Wang D., Li L., Li X., Ren Y., Liu C., Cheng D., Kim H. et al. , “ Microscale optoelectronic infrared-to-visible upconversion devices and their use as injectable light sources,” Proc. Natl. Acad. Sci. U. S. A. 115, 6632–6637 (2018). 10.1073/pnas.1802064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hafezi H., Robertson T. L., Moon G. D., Au-Yeung K.-Y., Zdeblick M. J., and Savage G. M., “ An ingestible sensor for measuring medication adherence,” IEEE Trans. Biomed. Eng. 62, 99–109 (2014). 10.1109/TBME.2014.2341272 [DOI] [PubMed] [Google Scholar]
- 173. Li J., Dong Y., Park J. H., Lin L., Tang T., and Yoo J., “ Body-area powering with human body-coupled power transmission and energy harvesting ICS,” IEEE Trans. Biomed. Circuits Syst. 14, 1263–1273 (2020). 10.1109/TBCAS.2020.3039191 [DOI] [PubMed] [Google Scholar]
- 174. Sun Y. and Thakor N., “ Photoplethysmography revisited: From contact to noncontact, from point to imaging,” IEEE Trans. Biomed. Eng. 63, 463–477 (2015). 10.1109/TBME.2015.2476337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tamura T., “ Current progress of photoplethysmography and SPO2 for health monitoring,” Biomed. Eng. Lett. 9, 21–36 (2019). 10.1007/s13534-019-00097-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lee H., Ko H., and Lee J., “ Reflectance pulse oximetry: Practical issues and limitations,” ICT Express 2, 195–198 (2016). 10.1016/j.icte.2016.10.004 [DOI] [Google Scholar]
- 177. Chung H. U., Rwei A. Y., Hourlier-Fargette A., Xu S., Lee K., Dunne E. C., Xie Z., Liu C., Carlini A., Kim D. H. et al. , “ Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units,” Nat. Med. 26, 418–429 (2020). 10.1038/s41591-020-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Jones D. P., “ Medical electro-optics: Measurements in the human microcirculation,” Phys. Technol. 18, 79 (1987). 10.1088/0305-4624/18/2/305 [DOI] [Google Scholar]
- 179. Webster J. G., Design of Pulse Oximeters ( CRC Press, 1997). [Google Scholar]
- 180. Lee J., Matsumura K., Yamakoshi K.-i., Rolfe P., Tanaka S., and Yamakoshi T., “ Comparison between red, green and blue light reflection photoplethysmography for heart rate monitoring during motion,” in 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) ( IEEE, 2013), pp. 1724–1727. [DOI] [PubMed] [Google Scholar]
- 181. Maeda Y., Sekine M., and Tamura T., “ The advantages of wearable green reflected photoplethysmography,” J. Med. Syst. 35, 829–834 (2011). 10.1007/s10916-010-9506-z [DOI] [PubMed] [Google Scholar]
- 182. Chung H. U., Kim B. H., Lee J. Y., Lee J., Xie Z., Ibler E. M., Lee K., Banks A., Jeong J. Y., Kim J. et al. , “ Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care,” Science 363, eaau0780 (2019). 10.1126/science.aau0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Schostek S., Zimmermann M., Keller J., Fode M., Melbert M., Prosst R. L., Gottwald T., and Schurr M. O., “ Pre-clinical study on a telemetric gastric sensor for recognition of acute upper gastrointestinal bleeding: The ‘hemopill monitor’,” Surg. Endosc. 34, 888–898 (2020). 10.1007/s00464-019-06845-4 [DOI] [PubMed] [Google Scholar]
- 184. Jobsis F. F., “ Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters,” Science 198, 1264–1267 (1977). 10.1126/science.929199 [DOI] [PubMed] [Google Scholar]
- 185. Villringer A. and Chance B., “ Non-invasive optical spectroscopy and imaging of human brain function,” Trends Neurosci. 20, 435–442 (1997). 10.1016/S0166-2236(97)01132-6 [DOI] [PubMed] [Google Scholar]
- 186. Ferrari M. and Quaresima V., “ A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application,” Neuroimage 63, 921–935 (2012). 10.1016/j.neuroimage.2012.03.049 [DOI] [PubMed] [Google Scholar]
- 187. Chance B., Leigh J., Miyake H., Smith D., Nioka S., Greenfeld R., Finander M., Kaufmann K., Levy W., and Young M., “ Comparison of time-resolved and-unresolved measurements of deoxyhemoglobin in brain,” Proc. Natl. Acad. Sci. U. S. A. 85, 4971–4975 (1988). 10.1073/pnas.85.14.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Piper S. K., Krueger A., Koch S. P., Mehnert J., Habermehl C., Steinbrink J., Obrig H., and Schmitz C. H., “ A wearable multi-channel fNIRS system for brain imaging in freely moving subjects,” Neuroimage 85, 64–71 (2014). 10.1016/j.neuroimage.2013.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bozkurt A., Rosen A., Rosen H., and Onaral B., “ A portable near infrared spectroscopy system for bedside monitoring of newborn brain,” Biomed. Eng. Online 4, 29 (2005). 10.1186/1475-925X-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Balardin J. B., Zimeo Morais G. A., Furucho R. A., Trambaiolli L., Vanzella P., C. Biazoli, Jr. , and Sato J. R., “ Imaging brain function with functional near-infrared spectroscopy in unconstrained environments,” Front. Hum. Neurosci. 11, 258 (2017). 10.3389/fnhum.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pinti P., Aichelburg C., Gilbert S., Hamilton A., Hirsch J., Burgess P., and Tachtsidis I., “ A review on the use of wearable functional near-infrared spectroscopy in naturalistic environments,” Jpn. Psychol. Res. 60, 347–373 (2018). 10.1111/jpr.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Rwei A. Y., Lu W., Wu C., Human K., Suen E., Franklin D., Fabiani M., Gratton G., Xie Z., Deng Y. et al. , “ A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care,” Proc. Nat. Acad. Sci. U. S. A. 117, 31674–31684 (2020). 10.1073/pnas.2019786117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kwon H., Kim K., Jo Y. H., Park M. J., Ko S.-B., Kim T. J., Kang J., Bae H.-M., and Lee J. E., “ Early detection of cerebral infarction with middle cerebral artery occlusion with functional near-infrared spectroscopy: A pilot study,” Front. Neurol. 9, 898 (2018). 10.3389/fneur.2018.00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yu J.-W., Lim S.-H., Kim B., Kim E., Kim K., Park S. K., Byun Y. S., Sakong J., and Choi J.-W., “ Prefrontal functional connectivity analysis of cognitive decline for early diagnosis of mild cognitive impairment: A functional near-infrared spectroscopy study,” Biomed. Opt. Express 11, 1725–1741 (2020). 10.1364/BOE.382197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Pinti P., Tachtsidis I., Hamilton A., Hirsch J., Aichelburg C., Gilbert S., and Burgess P. W., “ The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience,” Ann. N. Y. Acad. Sci. 1464, 5–29 (2020). 10.1111/nyas.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ojidu H., Palmer H., Lewandowski J., Hampton J., Blakeborough T., Epstein O., and McAlindon M. E., “ Patient tolerance and acceptance of different colonic imaging modalities: An observational cohort study,” Eur. J. Gastroenterol. Hepatol. 30, 520–525 (2018). 10.1097/MEG.0000000000001090 [DOI] [PubMed] [Google Scholar]
- 197. Thygesen M. K., Baatrup G., Petersen C., Qvist N., Kroijer R., and Kobaek-Larsen M., “ Screening individuals' experiences of colonoscopy and colon capsule endoscopy; a mixed methods study,” Acta Oncol. 58, S71–S76 (2019). 10.1080/0284186X.2019.1581372 [DOI] [PubMed] [Google Scholar]
- 198. Loeve A., Breedveld P., and Dankelman J., “ Scopes too flexible… and too stiff,” IEEE Pulse 1, 26–41 (2010). 10.1109/MPUL.2010.939176 [DOI] [PubMed] [Google Scholar]
- 199. Chetcuti Zammit S. and Sidhu R., “ Capsule endoscopy–recent developments and future directions,” Expert Rev. Gastroenterol. Hepatol. 15, 127–137 (2021). 10.1080/17474124.2021.1840351 [DOI] [PubMed] [Google Scholar]
- 200. Romero-Vázquez J., Argüelles-Arias F., García-Montes J. M., Caunedo-Álvarez Á., Pellicer-Bautista F. J., and Herrerías-Gutiérrez J. M., “ Capsule endoscopy in patients refusing conventional endoscopy,” World J. Gastroenterol. 20, 7424 (2014). 10.3748/wjg.v20.i23.7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Gerson L. B., “ Use and misuse of small bowel video capsule endoscopy in clinical practice,” Clin. Gastroenterol. Hepatol. 11, 1224–1231 (2013). 10.1016/j.cgh.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 202. Kav T. and Sivri B., “ Is enteroscopy necessary for diagnosis of celiac disease?,” World J. Gastroenterol. 18, 4095 (2012). 10.3748/wjg.v18.i31.4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Tajiri H., “ Advances in endoscopic imaging and diagnosis: Toward molecular imaging,” in New Challenges in Gastrointestinal Endoscopy ( Springer, 2008), pp. 29–39. [Google Scholar]
- 204. Soffer S., Klang E., Shimon O., Nachmias N., Eliakim R., Ben-Horin S., Kopylov U., and Barash Y., “ Deep learning for wireless capsule endoscopy: A systematic review and meta-analysis,” Gastrointest. Endosc. 92, 831–839 (2020). 10.1016/j.gie.2020.04.039 [DOI] [PubMed] [Google Scholar]
- 205. Saito H., Aoki T., Aoyama K., Kato Y., Tsuboi A., Yamada A., Fujishiro M., Oka S., Ishihara S., Matsuda T. et al. , “ Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network,” Gastrointest. Endosc. 92, 144–151 (2020). 10.1016/j.gie.2020.01.054 [DOI] [PubMed] [Google Scholar]
- 206. Tsuboi A., Oka S., Aoyama K., Saito H., Aoki T., Yamada A., Matsuda T., Fujishiro M., Ishihara S., Nakahori M. et al. , “ Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images,” Dig. Endosc. 32, 382–390 (2020). 10.1111/den.13507 [DOI] [PubMed] [Google Scholar]
- 207. Gora M. J., Sauk J. S., Carruth R. W., Gallagher K. A., Suter M. J., Nishioka N. S., Kava L. E., Rosenberg M., Bouma B. E., and Tearney G. J., “ Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure,” Nat. Med. 19, 238–240 (2013). 10.1038/nm.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Liang K., Traverso G., Lee H.-C., Ahsen O. O., Wang Z., Potsaid B., Giacomelli M., Jayaraman V., Barman R., Cable A. et al. , “ Ultrahigh speed en face OCT capsule for endoscopic imaging,” Biomed. Opt. Express 6, 1146–1163 (2015). 10.1364/BOE.6.001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Liang K., Wang Z., Ahsen O. O., Lee H.-C., Potsaid B. M., Jayaraman V., Cable A., Mashimo H., Li X., and Fujimoto J. G., “ Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo,” Optica 5, 36–43 (2018). 10.1364/OPTICA.5.000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Schäferling M., “ The art of fluorescence imaging with chemical sensors,” Angew. Chem. Int. Ed. 51, 3532–3554 (2012). 10.1002/anie.201105459 [DOI] [PubMed] [Google Scholar]
- 211. Gartia M. R., Hsiao A., Sivaguru M., Chen Y., and Liu G. L., “ Enhanced 3D fluorescence live cell imaging on nanoplasmonic substrate,” Nanotechnology 22, 365203 (2011). 10.1088/0957-4484/22/36/365203 [DOI] [PubMed] [Google Scholar]
- 212. Tian Y., Shumway B. R., Youngbull A. C., Li Y., Jen A. K.-Y., Johnson R. H., and Meldrum D. R., “ Dually fluorescent sensing of pH and dissolved oxygen using a membrane made from polymerizable sensing monomers,” Sens. Actuators, B 147, 714–722 (2010). 10.1016/j.snb.2010.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Song A., Parus S., and Kopelman R., “ High-performance fiber-optic pH microsensors for practical physiological measurements using a dual-emission sensitive dye,” Anal. Chem. 69, 863–867 (1997). 10.1021/ac960917+ [DOI] [PubMed] [Google Scholar]
- 214. Lee S., Ibey B. L., Coté G. L., and Pishko M. V., “ Measurement of pH and dissolved oxygen within cell culture media using a hydrogel microarray sensor,” Sens. Actuators, B 128, 388–398 (2008). 10.1016/j.snb.2007.06.027 [DOI] [Google Scholar]
- 215. Rabbani R., Najafiaghdam H., Ghanbari M. M., Papageorgiou E. P., Zhao B., Roschelle M., Stojanovic V., Muller R., and Anwar M., “ Towards an implantable fluorescence image sensor for real-time monitoring of immune response in cancer therapy,” in 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) ( IEEE, 2021), pp. 7399–7403. [DOI] [PubMed] [Google Scholar]
- 216. Benaron D. A., Contag P. R., and Contag C. H., “ Imaging brain structure and function, infection and gene expression in the body using light,” Philos. Trans. R. Soc. London B 352, 755–761 (1997). 10.1098/rstb.1997.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sadikot R. T. and Blackwell T. S., “ Bioluminescence imaging,” Proc. Am. Thorac. Soc. 2, 537–540 (2005). 10.1513/pats.200507-067DS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Contag P. R., Olomu IN., Stevenson D. K., and Contag C. H., “ Bioluminescent indicators in living mammals,” Nat. Med. 4, 245–247 (1998). 10.1038/nm0298-245 [DOI] [PubMed] [Google Scholar]
- 219. Yevtodiyenko A., Bazhin A., Khodakivskyi P., Godinat A., Budin G., Maric T., Pietramaggiori G., Scherer S. S., Kunchulia M., Eppeldauer G. et al. , “ Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals,” Nat. Commun. 12, 2680 (2021). 10.1038/s41467-021-22892-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hai A., Spanoudaki V. C., Bartelle B. B., and Jasanoff A., “ Wireless resonant circuits for the minimally invasive sensing of biophysical processes in magnetic resonance imaging,” Nat. Biomed. Eng. 3, 69–78 (2019). 10.1038/s41551-018-0309-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Strianese M., Staiano M., Ruggiero G., Labella T., Pellecchia C., and Auria S. D., “ Fluorescence-based biosensors,” in Spectroscopic Methods of Analysis ( Springer, 2012), pp. 193–216. [DOI] [PubMed] [Google Scholar]
- 222. Liu X., Tang T.-C., Tham E., Yuk H., Lin S., Lu T. K., and Zhao X., “ Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells,” Proc. Natl. Acad. Sci. U. S. A. 114, 2200–2205 (2017). 10.1073/pnas.1618307114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mimee M., Nadeau P., Hayward A., Carim S., Flanagan S., Jerger L., Collins J., McDonnell S., Swartwout R., Citorik R. J. et al. , “ An ingestible bacterial-electronic system to monitor gastrointestinal health,” Science 360, 915–918 (2018). 10.1126/science.aas9315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Khalil A. S. and Collins J. J., “ Synthetic biology: Applications come of age,” Nat. Rev. Genet. 11, 367–379 (2010). 10.1038/nrg2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Karig D. K., “ Cell-free synthetic biology for environmental sensing and remediation,” Curr. Opin. Biotechnol. 45, 69–75 (2017). 10.1016/j.copbio.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 226. Moraskie M., Roshid M. H. O., O'Connor G., Dikici E., Zingg J.-M., Deo S., and Daunert S., “ Microbial whole-cell biosensors: Current applications, challenges, and future perspectives,” Biosens. Bioelectron. 191, 113359 (2021). 10.1016/j.bios.2021.113359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Reyes-Caballero H., Campanello G. C., and Giedroc D. P., “ Metalloregulatory proteins: Metal selectivity and allosteric switching,” Biophys. Chem. 156, 103–114 (2011). 10.1016/j.bpc.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kumari A., Pasini P., and Daunert S., “ Detection of bacterial quorum sensing n-acyl homoserine lactones in clinical samples,” Anal. Bioanal. Chem. 391, 1619–1627 (2008). 10.1007/s00216-008-2002-3 [DOI] [PubMed] [Google Scholar]
- 229. Stern R. S., “ Psoralen and ultraviolet a light therapy for psoriasis,” New Engl. J. Med. 357, 682–690 (2007). 10.1056/NEJMct072317 [DOI] [PubMed] [Google Scholar]
- 230. Greenberg M., Sharan R., Galbadage T., Sule P., Smith R., Lovelady A., Cirillo J. D., Glowczwski A., and Maitland K. C., “ In vitro results of flexible light-emitting antimicrobial bandage designed for prevention of surgical site infections,” Proc. SPIE 10479, 104790M (2018). 10.1117/12.2290743 [DOI] [Google Scholar]
- 231. Percival S. L., Francolini I., and Donelli G., “ Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing,” Future Microbiol. 10, 255–272 (2015). 10.2217/fmb.14.109 [DOI] [PubMed] [Google Scholar]
- 232. Dai T., Gupta A., Murray C. K., Vrahas M. S., Tegos G. P., and Hamblin M. R., “ Blue light for infectious diseases: Propionibacterium acnes, helicobacter pylori, and beyond?,” Drug Resistance Updates 15, 223–236 (2012). 10.1016/j.drup.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Halstead F. D., Thwaite J. E., Burt R., Laws T. R., Raguse M., Moeller R., Webber M. A., and Oppenheim B. A., “ Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms,” Appl. Environ. Microbiol. 82, 4006–4016 (2016). 10.1128/AEM.00756-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Agrawal T., Avci P., Gupta G. K., Rineh A., Lakshmanan S., Batwala V., Tegos G. P., and Hamblin M. R., “ Harnessing the power of light to treat staphylococcal infections focusing on MRSA,” Curr. Pharm. Des. 21, 2109–2121 (2015). 10.2174/1381612821666150310102318 [DOI] [PubMed] [Google Scholar]
- 235. Zhang H., Zhao H., Zhao X., Xu C., Franklin D., Vázquez-Guardado A., Bai W., Zhao J., Li K., Monti G. et al. , “ Biocompatible light guide-assisted wearable devices for enhanced UV light delivery in deep skin,” Adv. Funct. Mater. 31, 2100576 (2021). 10.1002/adfm.202100576 [DOI] [Google Scholar]
- 236. Zhang L., Li Y., Zhang Q., Du N., Li X., Zhang Q., Yuan L., Dong F., Jiang Y., Tang J. et al. , “ Antimicrobial activity of an implantable wireless blue light-emitting diode against root canal biofilm in vitro,” Photobiomodulation Photomed., Laser Surg. 38, 694–702 (2020). 10.1089/photob.2020.4821 [DOI] [PubMed] [Google Scholar]
- 237. Hamblin M. R. and Demidova T. N., “ Mechanisms of low level light therapy,” Proc. SPIE 6140, 614001 (2006). 10.1117/12.646294 [DOI] [Google Scholar]
- 238. Zhang L., Jiang X., Jiang W., Li S., Chi Y., Liu H., Zhang M., Li J., Fang M., Pan B. et al. , “ Infrared skin-like active stretchable electronics based on organic–inorganic composite structures for promotion of cutaneous wound healing,” Adv. Mat. Technol. 4, 1900150 (2019). 10.1002/admt.201900150 [DOI] [Google Scholar]
- 239. de Freitas L. F. and Hamblin M. R., “ Proposed mechanisms of photobiomodulation or low-level light therapy,” IEEE J. Sel. Top. Quantum Electron. 22, 348–364 (2016). 10.1109/JSTQE.2016.2561201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hamblin M. R., “ Mechanisms and applications of the anti-inflammatory effects of photobiomodulation,” AIMS Biophys. 4, 337 (2017). 10.3934/biophy.2017.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Chow R. T. and Armati P. J., “ Photobiomodulation: Implications for anesthesia and pain relief,” Photomed. Laser Surg. 34, 599–609 (2016). 10.1089/pho.2015.4048 [DOI] [PubMed] [Google Scholar]
- 242. Hamblin M. R., Huang Y.-Y., and Heiskanen V., “ Non-mammalian hosts and photobiomodulation: Do all life-forms respond to light?,” Photochem. Photobiol. 95, 126–139 (2019). 10.1111/php.12951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Passarella S. and Karu T., “ Absorption of monochromatic and narrow band radiation in the visible and near ir by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation,” J. Photochem. Photobiol. B 140, 344–358 (2014). 10.1016/j.jphotobiol.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 244. Aisaiti A., Zhou Y., Wen Y., Zhou W., Wang C., Zhao J., Yu L., Zhang J., Wang K., and Svensson P., “ Effect of photobiomodulation therapy on painful temporomandibular disorders,” Sci. Rep. 11, 9049 (2021). 10.1038/s41598-021-87265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Yoon J. S., Ku W. Y., Lee J. H., and Ahn H. C., “ Low-level light therapy using a helmet-type device for the treatment of androgenetic alopecia: A 16-week, multicenter, randomized, double-blind, sham device-controlled trial,” Medicine 99, e21181 (2020). 10.1097/MD.0000000000021181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Dolmans D. E., Fukumura D., and Jain R. K., “ Photodynamic therapy for cancer,” Nat. Rev. Cancer 3, 380–387 (2003). 10.1038/nrc1071 [DOI] [PubMed] [Google Scholar]
- 247. Pervaiz S. and Olivo M., “ Art and science of photodynamic therapy,” Clin. Exp. Pharmacol. 33, 551–556 (2006). 10.1111/j.1440-1681.2006.04406.x [DOI] [PubMed] [Google Scholar]
- 248. Li X., Lovell J. F., Yoon J., and Chen X., “ Clinical development and potential of photothermal and photodynamic therapies for cancer,” Nat. Rev. Clin. Oncol. 17, 657–674 (2020). 10.1038/s41571-020-0410-2 [DOI] [PubMed] [Google Scholar]
- 249. Ibbotson S. H. and Ferguson J., “ Ambulatory photodynamic therapy using low irradiance inorganic light-emitting diodes for the treatment of non-melanoma skin cancer: An open study,” Photodermatol., Photoimmunol. Photomed. 28, 235–239 (2012). 10.1111/j.1600-0781.2012.00681.x [DOI] [PubMed] [Google Scholar]
- 250. Attili S., Lesar A., McNeill A., Camacho-Lopez M., Moseley H., Ibbotson S., Samuel I., and Ferguson J., “ An open pilot study of ambulatory photodynamic therapy using a wearable low-irradiance organic light-emitting diode light source in the treatment of nonmelanoma skin cancer,” Br. J. Dermatol. 161, 170–173 (2009). 10.1111/j.1365-2133.2009.09096.x [DOI] [PubMed] [Google Scholar]
- 251. Sun B., Bte Rahmat J. N., Kim H. J., Mahendran R., Esuvaranathan K., Chiong E., Ho J. S., Neoh K. G., and Zhang Y., “ Wirelessly activated nanotherapeutics for in vivo programmable photodynamic-chemotherapy of orthotopic bladder cancer,” Adv. Sci. 9, 2200731 (2022). 10.1002/advs.202200731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Yamagishi K., Kirino I., Takahashi I., Amano H., Takeoka S., Morimoto Y., and Fujie T., “ Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy,” Nat. Biomed. Eng. 3, 27–36 (2019). 10.1038/s41551-018-0261-7 [DOI] [PubMed] [Google Scholar]
- 253. Kim W. S., Khot M. I., Woo H.-M., Hong S., Baek D.-H., Maisey T., Daniels B., Coletta P. L., Yoon B.-J., Jayne D. G. et al. , “ AI-enabled, implantable, multichannel wireless telemetry for photodynamic therapy,” Nat. Commun. 13, 2178 (2022). 10.1038/s41467-022-29878-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Idris N. M., Gnanasammandhan M. K., Zhang J., Ho P. C., Mahendran R., and Zhang Y., “ In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers,” Nat. Med. 18, 1580–1585 (2012). 10.1038/nm.2933 [DOI] [PubMed] [Google Scholar]
- 255. Teh D. B. L., Bansal A., Chai C., Toh T. B., Tucker R. A. J., Gammad G. G. L., Yeo Y., Lei Z., Zheng X., Yang F. et al. , “ A flexi-PEGDA upconversion implant for wireless brain photodynamic therapy,” Adv. Mater. 32, 2001459 (2020). 10.1002/adma.202001459 [DOI] [PubMed] [Google Scholar]
- 256. Municoy S., Álvarez Echazú M. I., Antezana P. E., Galdopórpora J. M., Olivetti C., Mebert A. M., Foglia M. L., Tuttolomondo M. V., Alvarez G. S., Hardy J. G. et al. , “ Stimuli-responsive materials for tissue engineering and drug delivery,” Int. J. Mol. Sci. 21, 4724 (2020). 10.3390/ijms21134724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Bandara H. D. and Burdette S. C., “ Photoisomerization in different classes of azobenzene,” Chem. Soc. Rev. 41, 1809–1825 (2012). 10.1039/C1CS15179G [DOI] [PubMed] [Google Scholar]
- 258. Bao C., Zhu L., Lin Q., and Tian H., “ Building biomedical materials using photochemical bond cleavage,” Adv. Mater. 27, 1647–1662 (2015). 10.1002/adma.201403783 [DOI] [PubMed] [Google Scholar]
- 259. Hillel A. T., Unterman S., Nahas Z., Reid B., Coburn J. M., Axelman J., Chae J. J., Guo Q., Trow R., Thomas A. et al. , “ Photoactivated composite biomaterial for soft tissue restoration in rodents and in humans,” Sci. Transl. Med. 3, 93ra67–93ra67 (2011). 10.1126/scitranslmed.3002331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Pang Q., Lou D., Li S., Wang G., Qiao B., Dong S., Ma L., Gao C., and Wu Z., “ Smart flexible electronics-integrated wound dressing for real-time monitoring and on-demand treatment of infected wounds,” Adv. Sci. 7, 1902673 (2020). 10.1002/advs.201902673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Yang Q., Wei T., Yin R. T., Wu M., Xu Y., Koo J., Choi Y. S., Xie Z., Chen S. W., Kandela I. et al. , “ Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues,” Nat. Mater. 20, 1559–1570 (2021). 10.1038/s41563-021-01051-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Lajunen T., Viitala L., Kontturi L.-S., Laaksonen T., Liang H., Vuorimaa-Laukkanen E., Viitala T., Guével X. L., Yliperttula M., Murtomäki L. et al. , “ Light induced cytosolic drug delivery from liposomes with gold nanoparticles,” J. Controlled Release 203, 85–98 (2015). 10.1016/j.jconrel.2015.02.028 [DOI] [PubMed] [Google Scholar]
- 263. Vines J. B., Yoon J.-H., Ryu N.-E., Lim D.-J., and Park H., “ Gold nanoparticles for photothermal cancer therapy,” Front. Chem. 7, 167 (2019). 10.3389/fchem.2019.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Huang X. and El-Sayed M. A., “ Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy,” J. Adv. Res. 1, 13–28 (2010). 10.1016/j.jare.2010.02.002 [DOI] [Google Scholar]
- 265. Strong L. E. and West J. L., “ Hydrogel-coated near infrared absorbing nanoshells as light-responsive drug delivery vehicles,” ACS Biomater. Sci. Eng. 1, 685–692 (2015). 10.1021/acsbiomaterials.5b00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Arami H., Kananian S., Khalifehzadeh L., Patel C. B., Chang E., Tanabe Y., Zeng Y., Madsen S. J., Mandella M. J., Natarajan A., Peterson E. E., Sinclair R., Poon A. S. Y., and Gambhir S. S., “ Remotely controlled near-infrared triggered photothermal treatment of brain tumors in freely-behaving mice using gold nanostars,” Nat. Nanotechnol. 17, 1015 (2022). 10.1038/s41565-022-01189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Palagi S., Singh D. P., and Fischer P., “ Light-controlled micromotors and soft microrobots,” Adv. Opt. Mater. 7, 1900370 (2019). 10.1002/adom.201900370 [DOI] [Google Scholar]
- 268. Miskin M. Z., Cortese A. J., Dorsey K., Esposito E. P., Reynolds M. F., Liu Q., Cao M., Muller D. A., McEuen P. L., and Cohen I., “ Electronically integrated, mass-manufactured, microscopic robots,” Nature 584, 557–561 (2020). 10.1038/s41586-020-2626-9 [DOI] [PubMed] [Google Scholar]
- 269. Shapiro M. G., Homma K., Villarreal S., Richter C.-P., and Bezanilla F., “ Infrared light excites cells by changing their electrical capacitance,” Nat. Commun. 3, 736 (2012). 10.1038/ncomms1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Alt M. T., Fiedler E., Rudmann L., Ordonez J. S., Ruther P., and Stieglitz T., “ Let there be light–optoprobes for neural implants,” Proc. IEEE 105, 101–138 (2016). 10.1109/JPROC.2016.2577518 [DOI] [Google Scholar]
- 271. Thompson A. C., Stoddart P. R., and Jansen E. D., “ Optical stimulation of neurons,” Curr. Mol. Imaging 3, 162–177 (2014). 10.2174/2211555203666141117220611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Eom K., Kim J., Choi J. M., Kang T., Chang J. W., Byun K. M., Jun S. B., and Kim S. J., “ Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods,” Small 10, 3853–3857 (2014). 10.1002/smll.201400599 [DOI] [PubMed] [Google Scholar]
- 273. Zhao H., “ Recent progress of development of optogenetic implantable neural probes,” Int. J. Mol. Sci. 18, 1751 (2017). 10.3390/ijms18081751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Nagel G., Brauner M., Liewald J. F., Adeishvili N., Bamberg E., and Gottschalk A., “ Light activation of channel rhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses,” Curr. Biol. 15, 2279–2284 (2005). 10.1016/j.cub.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 275. Zhang F., Wang L.-P., Brauner M., Liewald J. F., Kay K., Watzke N., Wood P. G., Bamberg E., Nagel G., Gottschalk A. et al. , “ Multimodal fast optical interrogation of neural circuitry,” Nature 446, 633–639 (2007). 10.1038/nature05744 [DOI] [PubMed] [Google Scholar]
- 276. Mohanty S. K. and Lakshminarayananan V., “ Optical techniques in optogenetics,” J. Mod. Opt. 62, 949–970 (2015). 10.1080/09500340.2015.1010620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Gradinaru V., Mogri M., Thompson K. R., Henderson J. M., and Deisseroth K., “ Optical deconstruction of parkinsonian neural circuitry,” Science 324, 354–359 (2009). 10.1126/science.1167093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Krook-Magnuson E., Armstrong C., Oijala M., and Soltesz I., “ On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy,” Nat. Commun. 4, 1376 (2013). 10.1038/ncomms2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Ho J. S., Tanabe Y., Iyer S. M., Christensen A. J., Grosenick L., Deisseroth K., Delp S. L., and Poon A. S., “ Self-tracking energy transfer for neural stimulation in untethered mice,” Phys. Rev. Appl. 4, 024001 (2015). 10.1103/PhysRevApplied.4.024001 [DOI] [Google Scholar]
- 280. Park S. I., Shin G., McCall J. G., Al-Hasani R., Norris A., Xia L., Brenner D. S., Noh K. N., Bang S. Y., Bhatti D. L. et al. , “ Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics,” Proc. Natl. Acad. Sci. U. S. A. 113, E8169–E8177 (2016). 10.1073/pnas.1611769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Gutruf P., Krishnamurthi V., Vázquez-Guardado A., Xie Z., Banks A., Su C.-J., Xu Y., Haney C. R., Waters E. A., Kandela I. et al. , “ Fully implantable optoelectronic systems for battery-free, multimodal operation in neuroscience research,” Nat. Electron. 1, 652–660 (2018). 10.1038/s41928-018-0175-0 [DOI] [Google Scholar]
- 282. Badger H. and Allergan P., “ Phase I/IIA, open-label, dose-escalation study of safety and tolerability of intravitreal rst-001 in patients with advanced retinitis pigmentosa (rp),” NCT02556736 (2022).
- 283. Simunovic M., Shen W., Lin J., Protti D., Lisowski L., and Gillies M., “ Optogenetic approaches to vision restoration,” Exp. Eye Res. 178, 15–26 (2019). 10.1016/j.exer.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 284. Taiel M. and Roux M., “ A phase 1/2a, open-label, non-randomized, dose-escalation study to evaluate the safety and tolerability of gs030 in subjects with retinitis pigmentosa,” NCT02556736 (2021).
- 285. Shen Y., Campbell R. E., Côté D. C., and Paquet M.-E., “ Challenges for therapeutic applications of opsin-based optogenetic tools in humans,” Front. Neural Circuits 14, 41 (2020). 10.3389/fncir.2020.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. da Cruz L., Dorn J. D., Humayun M. S., Dagnelie G., Handa J., Barale P.-O., Sahel J.-A., Stanga P. E., Hafezi F., Safran A. B. et al. , “ Five-year safety and performance results from the Argus II retinal prosthesis system clinical trial,” Ophthalmology 123, 2248–2254 (2016). 10.1016/j.ophtha.2016.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Palanker D., Mer Y. L., Mohand-Said S., Muqit M., and Sahel J. A., “ Photovoltaic restoration of central vision in atrophic age-related macular degeneration,” Ophthalmology 127, 1097–1104 (2020). 10.1016/j.ophtha.2020.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Lorach H., Goetz G., Smith R., Lei X., Mandel Y., Kamins T., Mathieson K., Huie P., Harris J., Sher A. et al. , “ Photovoltaic restoration of sight with high visual acuity,” Nat. Med. 21, 476–482 (2015). 10.1038/nm.3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Palanker D., Le Mer Y., Mohand-Said S., and Sahel J.-A., “ Simultaneous perception of prosthetic and natural vision in AMD patients,” Nat. Commun. 13, 513 (2022). 10.1038/s41467-022-28125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Kvedar J. C., Fogel A. L., Elenko E., and Zohar D., “ Digital medicine's march on chronic disease,” Nat. Biotechnol. 34, 239–246 (2016). 10.1038/nbt.3495 [DOI] [PubMed] [Google Scholar]
- 291. Soref R. A., “ Silicon-based optoelectronics,” Proc. IEEE 81, 1687–1706 (1993). 10.1109/5.248958 [DOI] [Google Scholar]
- 292. Lee Y.-H., Kim J.-S., Noh J., Lee I., Kim H. J., Choi S., Seo J., Jeon S., Kim T.-S., Lee J.-Y. et al. , “ Wearable textile battery rechargeable by solar energy,” Nano Lett. 13, 5753–5761 (2013). 10.1021/nl403860k [DOI] [PubMed] [Google Scholar]
- 293. Goldberg D. J., Laser Dermatology: Pearls and Problems ( John Wiley & Sons, 2011). [Google Scholar]
- 294. Agostinis P., Berg K., Cengel K. A., Foster T. H., Girotti A. W., Gollnick S. O., Hahn S. M., Hamblin M. R., Juzeniene A., Kessel D. et al. , “ Photodynamic therapy of cancer: An update,” Cancer J. Clin. 61, 250–281 (2011). 10.3322/caac.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Maisels M. J. and McDonagh A. F., “ Phototherapy for neonatal jaundice,” N. Engl. J. Med. 358, 920–928 (2008). 10.1056/NEJMct0708376 [DOI] [PubMed] [Google Scholar]
- 296. Park J., Kim J., Kim S.-Y., Cheong W. H., Jang J., Park Y.-G., Na K., Kim Y.-T., Heo J. H., Lee C. Y. et al. , “ Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays,” Sci. Adv. 4, eaap9841 (2018). 10.1126/sciadv.aap9841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Jinno H., Yokota T., Koizumi M., Yukita W., Saito M., Osaka I., Fukuda K., and Someya T., “ Self-powered ultraflexible photonic skin for continuous bio-signal detection via air-operation-stable polymer light-emitting diodes,” Nat. Commun. 12, 2234 (2021). 10.1038/s41467-021-22558-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Rein M., Favrod V. D., Hou C., Khudiyev T., Stolyarov A., Cox J., Chung C.-C., Chhav C., Ellis M., Joannopoulos J. et al. , “ Diode fibres for fabric-based optical communications,” Nature 560, 214–218 (2018). 10.1038/s41586-018-0390-x [DOI] [PubMed] [Google Scholar]
- 299. Zhang H., Gutruf P., Meacham K., Montana M. C., Zhao X., Chiarelli A. M., Vázquez-Guardado A., Norris A., Lu L., Guo Q. et al. , “ Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry,” Sci. Adv. 5, eaaw0873 (2019). 10.1126/sciadv.aaw0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.