Abstract
Spheroids and organoids are promising models for biomedical applications ranging from human disease modeling to drug discovery. A main goal of these 3D cell-based platforms is to recapitulate important physiological parameters of their in vivo organ counterparts. One way to achieve improved biomimetic architectures and functions is to culture cells at higher density and larger total numbers. However, poor nutrient and waste transport lead to low stability, survival, and functionality over extended periods of time, presenting outstanding challenges in this field. Fortunately, important improvements in culture strategies have enhanced the survival and function of cells within engineered microtissues/organs. Here, we first discuss the challenges of growing large spheroids/organoids with a focus on mass transport limitations, then highlight recent tools and methodologies that are available for producing and sustaining functional 3D in vitro models. This information points toward the fact that there is a critical need for the continued development of novel cell culture strategies that address mass transport in a physiologically relevant human setting to generate long-lasting and large-sized spheroids/organoids.
I. INTRODUCTION
Spheroids and organoids are three-dimensional (3D) structures that are expanding in popularity as platforms for the study of human development, pathophysiology, and drug screening. Since the structure and function of spheroids/organoids resemble cellular microenvironments of human organs, they have been broadly used in a diverse range of biomedical applications.1 Henry Van Peters Wilson established the first successful 3D cell culture in 1907 when he showed that sponge cells could fuse and form aggregates after dissociation.2 Several decades later, in 1971, Sutherland and associates grew an immortalized lung cell line in a spinner flask and showed that these cells were able to form multicellular spheroids morphologically resembling the nodules seen in animal and human lung carcinomas.3 As early as 1989, researchers used a reconstituted basement membrane matrix (derived from Engelbreth-Holm-Swarm murine tumors, later commercialized as MatrigelTM) to culture epithelial cells, which highlighted the importance of physiologically relevant culture systems in promoting the organization and formation of 3D in vitro culture models.4 In the early 21st century, cell culture systems began to improve drastically via implementation of new technological approaches. For example, in 2003 individual 3D cell–packed spheroids could be formed in high throughput method by using gravity to form hanging drops suspended from a microtiter plate.5 Subsequently, in 2006 96 well round (U) or conical (V) bottom plates, precoated with poly-HEMA, were utilized to better control the rapid generation of 3D human breast cancer (MDA-MB-231) in vitro spheroid models.6
Currently, spheroids/organoids are emerging components of medical industry and are increasingly used in technological applications, such as in vitro disease models and for drug screening. However, the mentioned approaches experience low cell survival and functional readouts when cultured for any length of time, thus limiting their usefulness.7 Most researchers agree that the factor most contributing to these limitations is poor nutrient and gas transport.8–11 In this context, this review will highlight emerging approaches to overcome these limitations, focusing on strategies that utilize engineered microenvironments to create larger 3D microtissues and better recapitulate organs with enhanced structures and physiologic relevance.
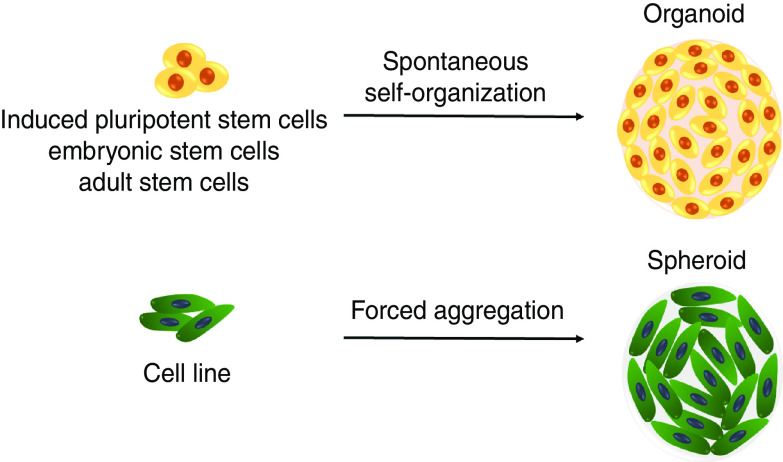
A. Terminology
In foundational work by Weiswald and co-workers,12 these authors highlighted various terminologies that are most often used to describe models of cancer cell aggregates and emphasized the need for more consistent terminologies in this field. For example, 3D structures made of many cells are often interchangeably referred to as ‘spheroids’ or ‘organoids’ because they can recapitulate some tissue-specific functions. However, these two terms should not be used as direct corollaries to one another as they describe two distinct types of in vitro cell aggregate models. The term “organoid” or “mini-organs” should be applied to 3D cell aggregates derived from stem cells or progenitor cells that exhibit lineage-specific differentiation and self-assembly, whereas spheroids are formed from cell lines (one or more) or patient tissue biopsies and are inherently less complex (Fig. 1). Compared to spheroids, the level of self-organization that occurs in organoids is quite remarkable and often approaches structures and functions found in mature organ systems.13 Comparatively, it has been shown that in spheroid models cells integrate into compact aggregates via induction of cell–cell adhesion proteins, namely E-cadherin, that associate with the cytoskeletal to ultimately lead to the formation of epithelial sheets.14 Another important difference between spheroids and organoids is the fact that spheroids often require specific methodological and technological approaches to form stable spherical shapes. In contrast, organoids possess the intrinsic ability to form in chemically well-defined cell culture medium and maintain their structural complexity and organization for longer time spans.15 Although organoids are suitable for studying complicated processes, it might often take months or longer to produce them, whereas spheroids tend to need less time to develop. It is worth noting that upon long-term expansion, organoids often stall in their development and introduce variation, which is less likely in spheroids, therefore making spheroids more reproducible.16
FIG. 1.
Schematic comparing organoid and spheroid 3D cell cultures comparing cell source and mechanism of formation.
B. Cell sourcing
3D in vitro models are generated from human pluripotent stem cells (hPSCs), adult stem cells (ASCs), or immortalized cell lines, and are often employed in disease modeling, drug screening, and transplantation.17 ASCs possess both limited self-renewal and differentiation potential in vitro, whereas PSCs can differentiate into nearly every cell type and possess unlimited self-renewal.18 hPSCs include both induced PSCs (iPSCs), and embryonic stem cells (ESCs), which are often utilized in the development of 3D culture models.19 As early as 2004, Gerecht and associates were one of the first to describe the formation of differentiating human embryoid bodies (hEBs) in rotating bioreactors.20 Cell origin is the main ethical concern in conducting research using human ESCs (hESCs). Nevertheless, in the literature there are examples of embryonic-derived gastruloids for exploring early human embryonic development in vitro.1,21 These 3D in vitro multicellular aggregates can differentiate to form the three germ layers without additional extra-embryonic tissues22 and only with the use of appropriate culture conditions, including signaling proteins. Moris et al.23 presented a human gastruloid model by culturing ESCs in Nutristem supplemented with CHIR99021 (Chiron). Aggregates showed an elongated morphology at 72 h as well as enhanced expression of Nodal and WNT signaling. Moreover, in a recent study conducted by Girgin and Lutolf,24 hydrogel microwell arrays were used for gastruloid culture in order to study peri-gastrulation development in mouse PSCs, with a focus on the formation of anterior neural tissue. They found that elongation and patterning of aggregates was highly dependent on initial aggregate size and endogenous WNT activity in vitro. These two studies confirm the critical role of the signaling environment in establishing both the morphology and the patterning of human gastruloids. A major criticism of utilizing human gastruloids is the ethical restrictions, which currently require human gastruloids to be cultured for a limited period of time (which some governing bodies define as up to 14 days).25 However, some have argued that 14 days can be extended to 28 days because no functional neural connections or sensory systems will form within 28 days, making it impossible for the embryo to experience sentience or pain.26
Alternatively, iPSCs enhance the potential of generating ESC-like cells (with high plasticity similar to ESC) from adult somatic cells without the ethical sourcing concerns, which makes them an ideal candidate for studies.27 In the past decade iPSC-derived organoids have risen to increased prominence in clinical and translational applications.28 The remarkable cellular complexity of in vitro models generated from PSCs, including their intricate architectures and functions are similar to their in vivo organ counterparts.29 Moreover, iPSCs allow establishment of laboratory models specific to an individual, which has led to the emergent field of personalized medicine and the possibility of testing of therapies on patient-specific models.30
Another multi-potent stem cell type commonly used for 3D cellular models is mesenchymal stem cells (MSCs), a well-characterized ASC type. These multipotent cells are easy to collect and are abundantly present in bone marrow, muscle, liver, and adipose tissues.31 In 2016, Lee et al.32 developed an adipose-derived MSC spheroid system to promote stem cell bioactivity via secretion of hypoxia-induced angiogenic cytokines, preservation of ECM components (laminin and fibronectin), and regulation of apoptotic signals in a culture time and spheroid size–dependent manner.
More recently, considerable attention has been paid on forming organoids using mixtures of cell sources to increase their complexity for improved 3D in vitro models, with a focus on enhancing vascularization in vitro. For instance, one of the first examples of 3D PSC differentiation into hepatic endoderm spheroids with subsequent formation into hepatoblast spheroids is presented by Ramli and co-workers.33 They cultured two hPSC cell sources, human ESCs and iPSCs, with a chemically defined and a matrix-free environment to induce formation of hepatoblast spheroids. In another illustrative example, Song et al.34 developed pre-vascularized brain organoids in vitro through the fusion of cortical spheroids, vascular spheroids, and mesenchymal cells to study neurovascular interactions. In the first step, hiPSCs was differentiated into endothelial cells (ECs) and neural progenitor cells (NPCs). Then cells were cultured in U-bottom ultra-low-attachment 96-well plates to form spheroids. On day 14 EC spheroids were transferred into the wells containing NPC spheroids to allow aggregate fusion followed by the addition of hMSCs after 7 days. They observed that direct contact among NPCs, ECs, and hMSCs accelerated the development of 3D cortical tissue structures (containing vasculature-like structures) while leading to enhanced E-cadherin expression, cytokine secretion and Notch-1 expression. In a similar co-culture study, iPSC-derived hepatocytes were combined with human umbilical vein endothelial cells (HUVECs) and hMSCs that enabled formation of liver buds with vascular-like networks.35 Finally, Varzideh et al.36 developed a strategy to form mature human cardiac organoids using human ESC-derived cardiac progenitor cells (CPCs), combined with ECs and MSCs. Spontaneously beating structures were created and subsequently transplanted into mice, which successfully induced neovascularization and upregulated genes coding for the contractile apparatus, Ca2+ handling and ion channels as compared to implanted 2D cultures.
Another source of cells for making 3D in vitro models are immortalized cell lines. For over 65 years, immortalized tumor cell lines have been the most common source for in vitro experimentation. They represent the backbone of basic cancer research worldwide but suffer from the inability to form complex model systems.37 The potential of spheroids to overcome this challenge in cancer research was recently shown by successfully using the breast cancer cell line (MDA-MB-231) to make spheroids, often seen as challenging to spontaneously form spheroids without scaffolds. On day 6, spheroids were co-cultured with immune cells from the peripheral blood, which are able to infiltrate into the 3D spheroid. This platform allowed study of the crosstalk between breast cancer cells and immune cells in vitro to accelerate discovery of new immunotherapeutic strategies.38 Antunes et al.39 also established a model of prostate cancer/human osteoblast (PC-3/hOB) to mimic prostate cancer-to-bone metastasis. In this study cells were incapsulated in methacrylated hyaluronic acid and gelatin methacryloyl microgels. Further analysis showed minimal cell death as well as increased cellular metabolic activity and calcium matrix deposit over time. In another study, a highly metastatic human ovarian cancer cell line (HO-8910PM) was cultured in a synthetic amphiphilic peptide hydrogel (RADA16-I) to mimic the structure of native microtumor tissues. This study confirmed significantly higher viable cell aggregate growth, cell proliferation rate, and chemoresistance to cisplatin and paclitaxel when compared to 2D monolayer cell culture.40
This brief overview of spheroid models used in cancer research supports their increased physiological relevance over monolayer culture to allow for more beneficial and ultimately relevant data. Table I summarizes the results of various studies where immortalized cell lines were used as mono or co-culture systems to form cell aggregate spheroids.
TABLE I.
Utilization of immortalized cell lines for spheroid formation.
| Cell type | Culture method | Spheroid Size | Remarks | Ref. |
|---|---|---|---|---|
| Human dermal fibroblasts | Rotational culture | 240 μm | Aggregates were inoculated on a scaffold of polyglycolic acid after the formation and could develop a new type of tissue engineered skin. | 41 |
| Lung adenocarcinoma (H358 and A549 cells) | 96-well plates coated with type I rat tail collagen | 60 μm | The 3D spheroids were more resistant to treatment with higher IC50 values for A549 and H358 cell lines compared to 2D. | 42 |
| MCF7 human breast cell | Encapsulation of cells in PEG-fibrinogen hydrogel microspheres made via hanging droplet | 100–400 μm | Cells encapsulated in gel-based microspheres had higher nuclear masses, a greater degree of disorganization, and enhanced tumorigenic morphology compared to those in spheroids. | 43 |
| Human fibroblasts, endothelial cells, and colon cell line (CRC and CCD841 CoN) | Low attachment 96-well U-bottom plate | 350–400 μm | Variations in drug combination efficacy between the cell types, cell ratios, and culture systems were observed. | 44 |
| Primary human hepatocytes (PHH) and Kupffer cells (KCs) | 96-well spheroid plates | 300– 350 μm | Inflammatory responses were recapitulated in co-culture spheroids, and 3D PHH spheroids with repeated dosing were more sensitive than 2D monolayer. | 45 |
| Normal human keratinocytes (NHKs) | 96-well round-bottom plate coated with a polymerized mixture of agarose (1.4%) and KSFM-scm | N/A | Spheroid-derived NHKs were enriched for a P63/K14 double-positive population that formed holoclonal colonies and reassembled into multicellular spheroids during 3D suspension subculture. | 46 |
| Ovarian cancer cells (OV-MZ-6, SKOV-3) | PEG-based hydrogels | > 50 μm | Spheroid formation was observed exclusively in 3D when cells were embedded within hydrogels. Proliferation in 3D was dependent on cell-integrin engagement and the ability of cells to proteolytically remodel their extracellular microenvironment. | 47 |
| LNCaP prostate cancer (PCa) cells | Hyaluronic acid (HA)–based bilayer hydrogel | 85 μm | HA-based bilayer platform supported the growth of prostate tumoroids, modeled paracrine interactions in the tumor microenvironment, and led to the production of pro-angiogenic signals in growing tumoroids. | 48 |
II. CLINICAL/COMMERCIAL APPLICATIONS OF HUMAN CELL–BASED MODELS
One of the most widely used applications of 3D cell cultures for commercial purposes to date is drug screening. Translating discovery to clinical practice requires significant investments in capital, time, and effort. To improve identification and validation of viable drug candidates during preclinical stages, the pharmaceutical industry needs to improve the robustness and reliability of their drug development methods.49 Many researchers have expressed doubts about animal studies as a reliable method for use in early-stage clinical trials.50 A major criticism of animal studies is that drug pharmacokinetics/dynamics and gene expressional networks differ considerably between animal models and humans.51 Of specific concern is a group of enzymes [known as cytochrome P450 (CYP 450)], responsible for metabolizing more than 30 types of medications. These metabolic enzymes are found at the highest concentrations in the human liver and have a specific role in converting lipid-soluble drugs into water-soluble compounds that can then be absorbed by the blood stream.52 This further complicates the suitability of animal models that mimic human CYP activity patterns53 and has been partially addressed by the development of humanized transgenic models. In response, humanized mice have been bioengineered with human genes, tissues, cells, and immune systems to improve human relatability of animal models.54 Although this approach is interesting, the cost of production as well as the variability between individual mice do not allow widespread application.55 Despite these improved animal models, even if a medication is demonstrated to be safe in animals, humans may experience toxicity or adverse immune reactions because of expressional variations, thus precipitating the need for long, federally sanctioned clinical trials. Moreover, animal models often fail to take into account the contribution of animal species' specific pathways for drug metabolism and cannot avoid undesirable species-specific contributions of components in every single process.56 Any reduction in animal testing is not only ethically desirable, but would also reduce the overall costs of the drug discovery pipeline.57 A less lengthy as well as costly alternative to animal studies is 2D human cell culture systems; however, an unnatural cellular environment leads to false drug response data. Such misleading results can lead to failure and higher attrition of drug candidates moving on to clinical stages.58 Thus, human-cell based 3D cell culture models provide an attractive alternative for drug screening by providing microenvironments that allow the expression of tissue-like phenotypes. This technology further improves upon previous methods by providing direct cell–cell interactions and recapitulation of the functions and structures of the native human organ of interest.59
A. Large‐scale 3D tissue models and their challenges
To achieve organ-level physiologically meaningful functions in vitro, as well as to improve the screening of therapeutic candidates in the early stages of the drug development process, cultures with larger numbers of cells at higher densities must be performed.60 Heterogeneity in cell type and variation of spheroid shapes are two important factors leading to different responses to treatment in large spheroid models.61 This is attributed to the existence of several subpopulations that coexist in large spheroids: a proliferative subpopulation and a quiescent or even apoptotic subpopulation.62 As a result, each subpopulation responds differently to a potential treatment. This, coupled with large variations in the assembled spheroid shape, affects sensitivity of the cell subpopulations to different therapeutic agents.63 For example, Mulholland et al.64 investigated cancer cell spheroids of varied sizes and their related responses when treated with cisplatin. Specifically, human prostate cancer cell–based spheroids cultured for 12 days demonstrated that larger spheroids (∼250 μm) were more sensitive to cisplatin than smaller ones (∼50 μm). Given this, large scale human-based 3D models have attracted more attention than smaller ones,65,66 and spheroids between 500 and 1500 μm in diameter are widely considered to be “large” in size and are thus recommended for pharmaceutical applications.67
To date, widespread application of larger cell aggregates has been largely hindered by the lack of nutrient/waste transport properties, which is accomplished by their in vivo counterparts via vascularization. Indeed, previous studies have demonstrated that spheroids with sizes greater than 500 μm in diameter possess a necrotic core surrounded by a viable rim.68,69 In our own work with large cell spheroids in vitro we have shown that the hypoxia and necrotic core formation is a direct result of poor oxygen partial pressures in the central regions of spheroids.70 Diffusion limitations of nutrients and waste ultimately dictates overall spheroid size and affects cell function and viability within the aggregates. Diffusion of oxygen is most vital since oxidative processes are necessary for vital cellular processes and cell signaling pathways.11,71 As such, a significant amount of effort has gone toward improving cell culture techniques where species mass transport (chief of which is oxygen) can be controlled precisely.72 The delivery of nutrients, oxygen, and removal of waste becomes more difficult when scaling up large 3D tissue models, as size and cell density are increased.73 The role of oxygen diffusion as a limiting factor in tissue culture was first proposed by Dr. August Krogh nearly a century ago. He proved that gasses diffuse slowly through aqueous medium and highlighted the need for more effective oxygen delivery systems to maintain cell viability.74 The foundational scientific principle for this is that matter in a solid phase has less kinetic energy that impedes mixture with higher energy gas phases. This rule also applies to in vitro cell culture systems where gas and nutrients must diffuse from media into a solid phase composed of extracellular matrix and cellular components. Oxygen diffusion limitations can be further explained by a constitutive relationship, Fick's first law of diffusion, J = D × ΔC/Δx. Based on this law, the rate of diffusion or flux (J) of a gas through a medium is directly proportional to the diffusion coefficient of the dissolved solute through a media (D) and inversely proportional to the distance/thickness. Based on this natural law, very small distance (Δx) steepens the slope of the concentration gradient (ΔC/Δx), where C is species concentration, leading to a greater flux. In contrast, when diffusion distances increase, or resistance is greater, diffusion becomes limited.75
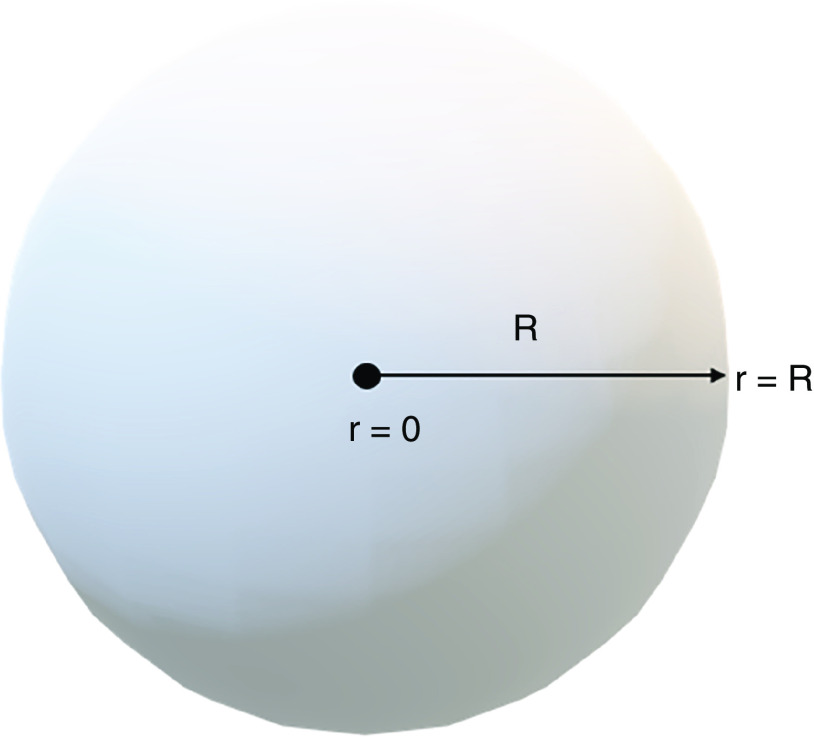
Increasingly complex models of species mass transport are also available and have recently been applied to the field of spheroids and organoids. For example, the modeling of species mass transport in 3D cellular constructs with active metabolism has been discussed in detail by McMurtrey.76 Utilizing a constitutive approach to model species diffusion in a spherical tissue construct with a homogenous metabolic consumption rate of φ, the associated governing equation is
| (1) |
In Eq. (1), C is concentration of a single chemical species (e.g., O2 or a nutrient), s is shape (for sphere s = 3), r is radius, D is the characteristic diffusion coefficient, and t is time. To achieve a solution, initial and boundary conditions are stated (Fig. 2). To streamline the approach, averaging of species concentration in the media with time ( ) is used, as adjusted by volume ratio between the construct (Vc) and media around the construct (Vm) as follows:
| = initial species concentration at the interface of source and construct | |
| = average species concentration | |
| R= outer radius of 3D construct | |
| r = radial distance | |
| t= time | |
| = volume of 3D tissue construct | |
| = volume of media | |
| φ = metabolic consumption rate (constant in the construct) |
FIG. 2.
Schematic representation of geometry, initial and boundary conditions.
Based on this setup the average concentration profile solution can be derived via standard mathematical techniques,
| (2) |
Defining maximal radius as a function of time as , the final solution for the unsteady state concentration profile can be written as
| (3) |
This mathematical solution is useful to enhance our fundamental understanding of chemical species diffusion in 3D cellular tissues and is useful for determining parameters that are vital for maintaining spheroid/organoid cultures in vitro.
More complex mass transport equations and models are also discussed in detail by this author in another paper with a focus on cerebral organoids.77 Based on these analyses, and the impacts of cell density, lower rates of metabolic oxygen consumption, the study reports a diameter of about 1.4 mm as the maximal predicted diameter of cerebral organoids (spontaneously organizing and likely containing more than one brain region) without central cell death. In experimental work by Paşca's group,78 they reported the development of human cortical organoids from hiPSCs without ECM (i.e., Matrigel), using only well-defined biochemical stimuli with specific timing. These organoids grew up to 4 mm in diameter and contained both stratified deep and superficial cortical neurons, and after approximately 2.5 months, the organoids matured to resemble the developing in vivo human prenatal brain. It is important to keep in mind that brain organoids take a long time to mature, and contain many more cell types/sub-types than other organoids, and thus encompass more complex interactions.79 This might partially explain why different approaches, such as dynamic culture and transplantation into animals, have been utilized to better ensure proper oxygen and nutrient supply.80 To directly address diffusion distances of chemical species, Rothenbücher et al.81 introduced a 3D printed 12 × 12 mm polycaprolactone scaffold to enable the creation of flat brain organoids (efBOs). hPSCs were seeded together with Matrigel onto the flat scaffold resulting in controlled and reproducible self-organization. Using this technique, the authors were able to better supply oxygen and nutrients to the tissue and observe a consistent formation of neuroepithelial folding in vitro. Previously Karzbrun and co-workers82 had published on folding and wrinkling of brain organoids using a different microfabricated device; however, Rothenbücher and associates' groundbreaking work is the first demonstrating folding via cell intrinsic processes.
An important finding from the modeling of oxygen and glucose transport concludes that if avascular constructs grow beyond the limits of both oxygen diffusion and metabolic consumption, a central necrotic core will be formed within the cultured spheroids.76 One simple way to overcome this limitation is by increasing the nutrient concentration in the surrounding fluid. This view is supported by Heywood et al. who argued that glucose availability is a critical parameter regulating oxygen tension within tissue engineered constructs, as oxygen consumption rate is known to increase with low glucose availability. They recommended maintaining a tissue glucose concentration of ≥ 2.7 mM in bioreactors to minimize oxygen gradients within tissue engineered cartilage.83 This phenomenon is further supported by work using 2D and 3D collagen-based culture methods for MSCs. By providing higher concentrations of glucose (from 0.5 to 25.0 mM), Deorosan and Nauman were able to push the cells toward a more anaerobic state. The results highlighted that in 2D culture supplemented with 10% serum, the lactate-to-pyruvate ratio elevated from 4.83 to 152.36 with the stated increase in the starting culture glucose concentration. However, in the 3D system elevating glucose concentration did not produce marked increases in the metabolic ratio within 6 days, indicating that the 3D culture was less anaerobic then the 2D culture.84 It is worth noting that a major drawback to using high concentrations of glucose is enhanced production of mitochondrial reactive oxygen species (ROS).85 Also, the ‘safe’ level of glucose is heavily dependent on the cell type. For example, it has been reported that high glucose (∼4.5 g/L, or ∼25 mM) interferes with epidermal growth factor receptor (EGFR)–based signaling pathways, leads to increased cell apoptosis and reduced multipotency of MSCs after 5 days of culture.86 In hepatocytes the effect of high glucose (44.8 mM) and normal glucose concentration (5.5 mM) on lipid accumulation and malondialdehyde (MDA) generation were studied. These tests revealed a 35% increase in lipid accumulation and 50% induction in MDA accumulation compared to normal condition group after treatment with high glucose.87 This supports the recommendation that rather than simply increasing the glucose concentration, it might be more useful to consider other major parameters that affect species mass transport in 3D cell culture to maintain glucose concentration at organ physiologic levels for long-term culture.88,89 One parameter that can be added to improve mass transport processes in cell culture is intentional convection via engineered approaches, such as perfusion systems and stirred or rotating bioreactors. As such, the next section discusses current trends and developments in advanced cell culture technologies under dynamic and static conditions for large 3D cell culture to remove mass transport limitations within these in vitro models.
III. TECHNOLOGIES AND APPROACHES TO ENHANCE NUTRIENT MASS TRANSPORT IN LARGE SPHEROIDS/ORGANOIDS
A. Bioreactor-based cell culture
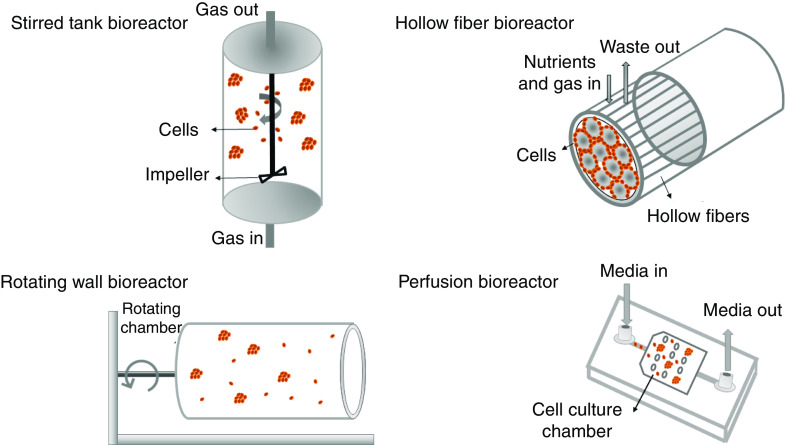
Dynamic suspension culture allows for frequent and gentle mixing of fluids providing a 3D homogenous culture environment to enable precise control of culture parameters.90 Bioreactors are closed vessels for cell cultivation, which enable high control over environmental and operational conditions, including nutrient distribution and external stresses.91,92 They can be used for drug discovery to study drug metabolism and toxicity responses,93 in tissue engineering to maintain cellular survival and structural support within tissue constructs,94 or for clinical applications to provide physiologically relevant mechanical cues on the growing grafts (namely, bone) with relevant sizes and shapes.95 In terms of bioreactors for cell culture, nutrient supply together with waste removal are the most important factors that ensure cell survival throughout 3D engineered constructs.96 Furthermore, bioreactors allow for tuning and enhancement of oxygen mass transport. In 2017 Martínez-Corona and co-workers97 developed an optical approach for measuring the oxygen mass transfer coefficient in a stirred tank bioreactor, quantifying the capacity of a bioreactor to provide oxygen to cultured cells. In their methodology, the dynamics of the air injection into water and the bubbles created were photographed. Eventually they showed that the oxygen transfer rate increases as the aeration (air flux) and agitation rate (stirring) increases. The culture environment created by bioreactors allows cells to perform at naturally functioning levels with enhanced long-term viability, as compared to other methods.98 Therefore, the physical environment provided by bioreactors can provide more favorable conditions for the formation of spheroids/organoids and their subsequent survival and functionality.89 The first study to successfully use a bioreactor for cell culture took place nearly 30 years ago when Rozga et al.99 showed that liver cells (hepatocytes) do not lose important metabolic and phenotypic functions when seeded onto the outer surfaces of hollow‐fiber bioreactors. Building upon this, Naruse and co-workers100 created a bioreactor composed of a non-woven fabric in 1996. Hepatocytes isolated from pig livers were immobilized in this bioreactor and successfully formed spheroids that were functionally superior to hepatocytes grown in monolayer. Since their introduction, considerable effort has been made by researchers to optimize bioreactor design features through increasing mass transfer abilities while supporting a low-shear environment,101 since low shear is needed to maintain cell aggregation in 3D cultures.
Among the different models that are currently available (Fig. 3), a stirred tank design is the most widely used type of bioreactor and can be as simple as the implementation of a spinner flask.102 The operation of this bioreactor is based on a flowing system that provides enhanced nutrient circulation, as well as enhanced metabolic waste elimination to support intensive cell expansion.103 A recent study conducted by Schwedhelm et al.104 showed the application of a fully monitored stirred tank reactor system integrated into a custom-designed and fully automated incubator for the culture of hiPSC-based aggregates. This bioreactor allowed real-time assessment of glucose, lactose, glutamine, ammonia, and lactate dehydrogenase activity, as well as being equipped with optical sensor spots for measuring pH and dissolved oxygen concentration. Still, several practical issues arise when dealing with stirred tank bioreactors, such as collisions of the cells with the impeller, the onset of turbulent flow that can also lead to direct cell damage, and aggregate dissociation. One modification to commercially available spinning bioreactors is multi-well spinning microbioreactors (SpinΩ) where each well acts as a miniature spinner flask to provide a suspension environment with improved oxygen delivery to the organoids. In this novel tissue culture approach, the driving force to spin the wells originates from a single electric motor. In addition, wells were connected and rotated in synchrony by interconnecting gears.105 Romero-Morales and co-workers106 further implemented this approach with a design that reduced the chances of contamination and mechanical failure (using magnetic forces) and termed it Spinfinity (Spin∞). Cerebral organoids were cultured in this microbioreactor for more than 200 days without motor change or any sign of contamination.
FIG. 3.
Main classes of bioreactors for dynamic 3D cell culture to improve nutrient transport.
Rotating bioreactors are now commonly used to overcome the limitations of stirred tank devices, including high mechanical shear forces and bubbles generated by the impeller that can damage cells.107 Rotating wall vessel (RWV) bioreactors can provide efficient platforms for culturing vulnerable cell types by providing unique microenvironments and biophysical stimulation. The fundamental characteristic of a rotating-wall bioreactor is minimized shear and turbulence, which creates laminar flow in the culture vessel, leading to reduced mechanical damage to cultured cells.108 Compared with intact PSC-derived retinal organoids in static culture, retinal organoids grown in a RWV bioreactor showed enhanced growth and differentiation, closely recapitulating the spatiotemporal development of the retina in vivo. Varley et al.109 studied the role of combining two axes of rotation (periodic oscillation and orbital motion) on cell growth in culture with a goal of showing that imposed rotation of culture media enhances mass transport of oxygen and nutrients in the bioreactor. Fetal human osteoblasts (fHObs) were seeded within collagen–glycosaminoglycan scaffolds and then cultured in a RWV bioreactor for 21 days. No differences were found between single axis and dual axis rotation bioreactors; however, the study did show significant cell proliferation due to a better supply of oxygen, nutrients, and removal of waste, as compared to the static control group.
Hollow fiber bioreactors are another alternative that help to minimize cell damage due to shear stresses. With a hollow fiber bioreactor, cell aggregation starts on the surface between the spaces of the packed fibers. Culture medium is pumped through the lumens of the hollow fibers, allowing nutrients and metabolic products to diffuse in both directions across the fiber walls to offer an in vivo–like environment, with the fibers mimicking blood capillaries. After passing through the fibers, medium can either be oxygenated and recycled or collected while fresh medium is introduced. In 2017 a crossed hollow fiber bioreactor was used to make and grow liver spheroids.110 Results of this study showed a shear stress–reduced microenvironment with continuous feeding, minimal mass transfer limitations, and 3D cell interactions, thus creating a desirable environment for the formation of human liver organoids with long-term maintenance of liver-specific activities. Similarly, a hollow alginate fiber bioreactor was constructed using a multilayer coaxial laminar flow microfluidic system by Zhu and co-workers111 for the purpose of improving cerebral organoid generation. hPSC-based EBs were formed in a low attachment well plate, then dispersed in Matrigel, gently injected into fibers, and grown for 21 days in the device. Due to the large specific surface area, the fibers facilitated sufficient exchange of nutrients and oxygen, and removal of wastes during human cerebral organoid culture, hence continuously improving long-term 3D culture, in situ differentiation, and formation of 3D brain organoids. Real time monitoring of the organoid growth in the bioreactor revealed rapid neuroepithelial expansion as compared to established techniques.
The final main category of bioreactors is perfusion systems that utilize a pressure gradient to move media, thereby enhancing gas and nutrient exchange.112 A perfusion microbioreactor usually consists of a peristaltic pump with low pulsation channels to facilitate better cell culture medium transport, an air bubble trapper, oxygenator, and culture medium reservoir.113 In one study, a perfusion microbioreactor was made using 3D printing and was parallelizable up to four microbioreactors, including a peristaltic pump, and oxygen measurement instrumentation, which was assembled into one system. A highly automated cell seeding procedure together with real time measurement of oxygen allowed high homogeneity and viability, with improved reproducibility.114 The Vunjak-Novakovic group115 also hypothesized that direct perfusion of cultured constructs could reduce diffusional distances for mass transport to enhance control of oxygen and nutrients in the cell microenvironment, thereby increasing the spatial uniformity of engineered cardiac muscle. To test their hypothesis, 3D neonatal rat cardiac myocytes and fibrous polyglycolic acid (PGA) scaffolds were cultured in a perfusion-based bioreactor. Due to the improved control of local microenvironmental conditions within the forming tissue, the spatial uniformity of cell distribution and the expression of cardiac-specific markers were enhanced by perfusion culture. In total, these studies point to the fact that direct perfusion brings culture medium of a desired composition in closer contact with cells throughout a construct volume, therefore directly reducing species mass transport limitations between surface and the innermost regions.
B. Organs-on-a-chip
Bioreactors generally have the drawback of requiring large amounts of media and cells to support 3D cell growth. Organs-on-a-chip directly address this issue by utilizing microfluidic technologies that allow for small-volume reactions with higher throughput for biological experiments.116 Similar to computer microchips, organ chips are fabricated using lithography methods. These miniaturized bioreactors contain hollow chambers and operate by controlled and continuous infusion of fluids, which can be designed to create physiologically relevant organ microenvironments and desired cell–cell interactions.117 Due to this reason, cell culture chips can be harnessed for a variety of applications, especially 3D in vitro cultures. According to Dr. Konry's group,118 microfluidics culture chips allow for the generation, maintenance, stimulation, and analysis of multicellular spheroids in a single platform. As such, they fabricated alginate droplets containing MCF-7 breast cancer cells and maintained them up to 14 days on-chip. They contended that the broad range of pore sizes in alginate hydrogels permitted rapid and efficient transport of oxygen, nutrients, and drugs to cells in the spheroids, and cells were more susceptible to drug treatment in a 2D environment compared to 3D. Another study conducted by Achberger and associates119 utilized a microfluidic chip to generate improved conditions for the generation of retinal organoids from hiPSCs. They were able to recapitulate the complex stratified and interconnected tissue architectures found in the human retina, as well as in vivo–like physiological processes such as outer segment phagocytosis and calcium dynamics. Bauer et al.120 also proposed a two-organ chip for the co-culture of human pancreatic islet microtissues and liver spheroids. They showed that islet–liver crosstalk was maintained during the 15-day culture period with stable insulin levels (4.3 ± 1.1 nM). A more engineering-focused platform was presented by Bovard et al.121 to co-culture 3D bronchial and liver spheroid tissues in a single chip to increase the complexity and physiological relevance of tissue responses following exposure to inhaled toxicants. This chip retained metabolic activity of both lung and liver tissues for up to 28 days. Another recent study on this topic122 found that when hiPSCs are encapsulated and grown in hydrogels made of Na‐alginate (NaA) and chitosan (CS), the proper permeability of the hybrid scaffold allows for the exchange of nutrients, gases, and metabolites. Here, an all‐in‐water droplet microfluidic system was used to fabricate hybrid hydrogel capsules that enable 3D culture and generation of islet organoid. They validated the generation of islet‐specific α‐ and β‐like cells with high expression of pancreatic hormone–specific genes and proteins within the organoids.
Microfluidic devices can be also modified by ECs, which is often referred to as endothelialized microfluidic technology. In this technique, adhesive ECM proteins are deposited within the microchannels to provide stable endothelial cell anchorage followed by endothelial cells perfusion into the device where they adhere across the wall.123 On-chip microvasculature provides perfusable channels for the transport of small molecules, especially nutrients and oxygen, while allowing deposition of the flowing cells.124 Chonan and co-workers125 utilized a 3D microfluidic platform to recapitulate the brain tumor microenvironment constituted of engineered blood vessels in type I collagen. They found out the invasive capacity of glioma cell populations into type I collagen gel was increased by the presence of HUVECs in close proximity. Similarly, Zhang et al.126 formed a pulmonary alveolus model using microfluidic platform and verified that the alveolar epithelium interacted with the microvascular endothelium synergistically strengthening the chemical resistance of the pulmonary alveolus system to the exogenous pollutants due to better functionality.
A rapid rise in the use of microfluidic platforms allows for noninvasive monitoring of microenvironmental properties, such as oxygen concentration, pH, and temperature in real time with direct coupling to analysis systems.127 For instance, in 2017 Shin and co-workers128 developed a human liver-on-a-chip microfluidic platform that could monitor cell‐secreted soluble biomarkers from the organoids continuously up to 7 days. Automation allows advantages of long-term monitoring without human intervention with continual monitoring of biomarkers. In similar fashion, Schuster et al.129 developed an automated organoid culture platform for dynamic and combinatorial drug screening of human-derived pancreatic tumor organoids. The 3D culture chamber device was connected to a custom software for automated and programmable experimental control and live-cell time-lapse fluorescence microscopy, which enabled robust experimental analyses of organoids.
In summary, in vitro models involving the culture of human cells inside microfluidic chips, are increasingly demonstrating their value as research tools for studying human health and disease by creating relevant physiologic features, enabling enhanced nutrients, better removal of waste, and real-time monitoring of cellular behaviors in 3D cell culture.
C. Vascularization
While bioreactors and microfluidic chips support 3D cell culture survival mainly through improved supply of nutrients and removal of wastes, they do not fully imitate in vivo vasculature. In fact, one of the biggest challenges in bioengineering today is generating clinically relevant vascularized tissues. Vascularization allows cells that are within a tissue to gain access to oxygen and nutrients for cell survival and functions.130 The study of vascularization, both the fundamental aspects and bioengineered approaches, has been well documented and discussed previously,131 and it is recognized as being the most important and challenging step in the process of engineering complex organs.132,133
There are different strategies to induce vascularization within spheroids and organoids or other engineered 3D constructs. One preferred approach utilizes vascular endothelial growth factor (VEGF) signaling to target nearby established vasculature and/or stem cells and progenitors. VEGF is widely considered to be one of the most important factors in normal and abnormal angiogenesis both in vitro and in vivo.134–136 In this regard, Remuzzi's group developed an approach for the generation of vascularized renal organoids from single-cell suspensions.137 They pretreated them with VEGF and then implanted these organoids below the kidney capsule of a living rat host. The results of this study highlighted the improved effect of VEGF pretreatment on growth and maturation of implanted organoids. In 2020 Ham and associates138 also developed blood vessel–like structures that offered similar properties of the blood–brain barrier (BBB) in cerebral organoids by adding VEGF to the media during the embryoid body formation (day 1–6) and throughout neural induction (day 6–10). They are among the first to show co-differentiation of cerebral organoids and blood vessel-like structures is potentially possible with this approach. According to the authors, the main downside of this study is a decrease in the density of vascular-like structures in long-term cultures (more than 36 days) and the fact that these vessels were not functional in terms of circulating medium. One should note that there are still few known reports of formation of robust functioning in vitro vascular networks combined with organoids/spheroids without in vivo transplantation into a host species.139 For instance, in a study the authors provided a detailed methodology to transplant hPSC-derived human intestinal organoids in an animal model to generate mature, functional human intestinal tissues in vivo.140 Organoids were transplanted under the kidney capsule of immunocompromised mice, like the study mentioned earlier with renal organoids. Within 8 weeks engrafted organoids formed a laminated intestinal tissue with an epithelium supported by the host vasculature.
In terms of bioengineering approaches, capillary-like structures are formed either by self-organization initiated by crosstalk between cells in 3D culture or via developing geometric platforms to encourage vasculogenesis.141 This approach includes the use of channeled scaffolds, which offer potential for vascular cell alignment, reduce the distance for oxygen diffusion, and also increase the formation of nutrients gradients within scaffolds.142 Given these points, vascularization strategies are discussed in the Secs. below. Table II summarizes key examples for the generation of vascular networks for enhancing nutrient and oxygen delivery.
TABLE II.
Examples of modeling vascularization in vitro using different types of engineering methods.
| Method | Polymer+ photo initiator + cell type | Ref. |
|---|---|---|
| Stereolithography | • PEGDA + LAP + human non–small-cell lung cancer | 143 |
| • PEGDA+ TPO+ MCF-7 + L929 cells | 144 | |
| • PEGDA + Irgacure 2959 + OP‐9 marrow stromal cells | 145 | |
| Sacrificial networks | Sacrificial template + polymer + cell type | 146 |
| • PVOH - calcium acetate template + polyacrylamide, PEG, alginate + neuroepithelial cells | ||
| • Gelatin + transglutaminase mixed with collagen I + iPSCs | 147 | |
| • Sucrose + polycaprolactone-PU and poly (glycerol sebacate urethane) + H9c2 cell line | 148 | |
| Endothelial cell co-culture | Polymer + cell type + strategy | 149 |
| • Methacrylated hyaluronic acid (HA-MA) and fibrinogen + endothelial cell, smooth muscle cell + composite hydrogels | ||
| • Fibrin gel + human umbilical vein endothelial cells (HUVEC) and normal human lung fibroblasts (LF) + microfluidic device based on PDMS | 150 | |
| • Collagen gel + magnetically labeled B16F1 and human umbilical vein endothelial cells (HUVECs) + pin-holder device | 151 | |
| Bioprinting | Scaffold-based | 152 |
| • Agarose rods + CHO (Chinese hamster ovary) spheroids | ||
| • Alginate hydrogel + 50% HUVECs and 50% hMSCs | 153 | |
| • Gelatin+ MCF-7 | 154 | |
| Scaffold-free | ||
| • Needle array + cardiac spheroids (HUVECs and NHDFs) | 155 | |
| • Tubular permeable alginate capsules + chondrocytes | 156 |
1. Sacrificial networks
As mentioned above, one approach to form vascular networks in situ is coculturing with ECs in 3D structures. Rouwkema et al.157 developed a spheroid coculture model with human MSCs and HUVECs. The cells self-organized into a pre-vascular network in vitro with fourfold upregulation of the osteogenic marker alkaline phosphatase. However, it seems this strategy is restricted to relatively thin constructs to permit gas and nutrient exchange. In addition, most of the reported vascular networks made by the co-culture of ECs are not perfusable and leaky due to the lack of functional connections between lumina.158 To better assure interconnection and perfusibility, the preferred research strategy is focused on the use of sacrificial templates followed by cell seeding to form vascular networks.
A main focus of research on sacrificial networks is currently on printing fine resolution filamentous network structures.159 There are several examples of sacrificial inks used for the printing of these structures, including PLA dissolved by chloroform,160 Pluronic F127 liquefied at low temperatures,161 agarose liquefied at high temperature (80–95 °C),162 gelatin that dissolves in water,163 and alginate removed via exchange reactions with monovalent cations.164 After printing a sacrificial template using the proper ink and embedding then casting the hydrogels, the sacrificial template is melted or dissolved to form branched vascular-like channeled scaffolds.165 Miller and co-workers166 reported a biocompatible sacrificial glass made from mixtures of inexpensive carbohydrates (glucose, sucrose, and dextran). They printed rigid filament networks using this material to make a perfusable scaffold with channels and junctions and then successfully showed that it has the ability to dissolve rapidly and safely in the presence of living cells. In their study, the maintenance of cellular metabolic activity was associated with better nutrient transport through the channeled scaffold. In another study, Negrini et al.167 produced a prevascular channel in tissue-mimicking crosslinked gelatin hydrogels for adipose tissue engineering. They used aligned alginate microbeads (20 μm in diameter) as the sacrificial component, which left hollow channels within 3D printed gelatin hydrogel filaments. Similarly, agarose was used as a sacrificial vascular network material that was successfully printed then surrounded by HepG2/C3A cell-loaded GelMA bulk hydrogels.168 The whole construct was then exposed to UV light for cross-linking, then the agarose fibers were manually removed to create hollow microchannels. HUVECs were finally seeded inside the hollow channels to create an endothelial layer on the inner surface for the tissue-engineered liver constructs. This approach allowed for the creation of more physiologically accurate vascularized models in bulk constructs. Tseng and team169 used a similar approach where they extruded a glucose-sensitive hydrogel based on poly(ethylene glycol) diacrylate (PEGDA) as the sacrificial layer consisting of branched tubular channels. The PEGDA network was embedded in a non–glucose-sensitive hydrogel (fibrin or chitosan gel) containing neural stem cells (NSCs). By soaking the construct in the culture media, the glucose-sensitive component was rapidly dissolved and formed the channels inside the other non–glucose-sensitive hydrogel. Vascular ECs seeded in the channels, migrated in the non-sacrificial hydrogel, and formed capillary networks. The results of this study showed considerable cell proliferation (∼160%) within the construct, and during long-term culture (∼14 days) the ECs formed capillary-like structures (vascular networks) while NSCs formed neurosphere-like structures (indicative of neural development) in the constructs. Shao et al.170 reported a new sacrificial microgel-laden bioink for directly bioprinting constructs with mesoscale pore networks in order to enhance nutrient delivery. Their bioink containing GelMA and gelatin microgels was first bioprinted into temporally stable structures using a reversible thermo–cross-linking mechanism. Next, permanent photo–cross-linking of the GelMA phase via UV exposure was followed by the dissolution of the gelatin microgels inside the printed constructs to form an inter-connected porous network. This methodology allowed for effective oxygen/nutrient diffusion to osteoblasts and HUVECs encapsulated in the constructs to facilitate formation of complex tissue constructs. In another study, a fibrin-based composite hydrogel including fibrinogen, gelatin, aprotinin, glycerol, and hyaluronic acid was used as a bioink.171 Primary cardiomyocytes suspended in the bioink were sequentially printed with a sacrificial hydrogel (gelatin, glycerol, and hyaluronic acid) and a supporting polymeric frame made of polycaprolactone (PCL). Upon removal of the sacrificial hydrogel phase, open spaces were created, which enabled better gas and nutrient transport. Finally, bioprinted cardiac tissue was formed with uniformly aligned, dense, and electromechanically coupled cardiac cells and developed spontaneous synchronous contraction in culture.
2. Endothelial cell (EC)–spheroid/organoid co-culture
Since vasculature is a recognized important component of solid tissues, there is directed effort working to add ECs and to encourage them to form tubular-like structures within spheroids and organoids.172,173 Takahashi et al.174 generated vascularized islets in vitro by co-culture of pancreatic islets isolated from human and mouse with HUVECs and human MSCs. They obtained satisfactory results from in vivo study proving that vessels in human islets connected to the host vessels and made non-leaky functional vascular network after transplantation. Pham and associates175 showed that coating human cerebral organoids with iPSC-derived ECs leads to a vasculature-like network in organoids within 5 weeks in vitro. They hypothesized that upon transplantation, these cerebral organoids would send signals to promote ingrowth of blood vessels from the host. To test it the pre-vascularized and non-vascularized cerebral organoids were used for transplantation in the brain resection cavity of a mouse. They observed that non-vascularized organoids did not survive 2 weeks after the transplantation, whereas there was robust vascularization of the outer and some penetration of host vessel into the pre-vascularized human organoids. Unlike previous research studies, they were unable to see functional connections with the pre-vasculature and the host microvasculature. The findings from these two studies point toward the idea that even using a host animal cannot guarantee the formation of functional vessels. This has led scientists to investigate organoid vascularization in physiologically relevant conditions such as by using a microfluidic device.176 An example of this is the methodology presented by Osaki et al.,177 who generated 3D networks by co-culturing human ES-derived motor neuron (MN) spheroids and iPS-derived ECs in a microfluidic device. For this purpose, a collagen gel with iPS-ECs and MN neurospheres (diameter <150 μm) was injected into the channels, followed by cross-linking for 10 min. After 2 days, EC networks formed followed by MNs extending neurites outward from the spheroids. In 5 days, they could obtain vascular lumens with an average diameter of ∼60 μm. Homan et al.178 also developed an in vitro method for culturing vascularized and mature (hPSC)-derived human kidney organoids under flow in a 3D-printed lab-on-a-chip. For this purpose, a layer of engineered ECM that included fibrin, HUVECs, and human neonatal dermal fibroblast cells (HNDFs) was cast in the 3D-printed chip. Then a subsequent layer of renal organoids was achieved by seeding into the perfusable millifluidic chip such that they adhered to the ECM coating. As a result of inter-lineage endothelial–epithelial communication, kidney organoids cultured in this device under perfusion supported better glomerular vascularization compared to organoids cultured under static condition, which generated limited vasculature with immature gene expression profiles. Moreover, to examine the extent of perfusable vasculature, the authors added fluorescent beads into the flowing media and subsequently acquired confocal images of the organoids. They confirmed the presence of beads in the vessels together with some sprout-like structures, suggestive of angiogenesis. According to the author's claim, there is still ambiguity if the microvascular networks present in these kidney organoids will be readily perfusable. Recently, the term “inside-out” was used by Zhang and co-workers179 to refer to vasculature networks formed within the organoid that expand and grow into the surrounding matrix, either a channel in a microfluidic device or a neighboring hydrogel. In this strategy, first pre-vascularized organoids are formed by encapsulating them in a hydrogel containing ECs followed by embedding them in microfluidic channels that were also vascularized. Upon embedding, established vessels in the channels penetrated into the organoids and interconnected to the networks. In an innovative approach, Huh's group180 developed engineered perfusable channels housed in an open-top microfluidic device. Needle templates were used to generate perfusable hydrogel cell-culture scaffolds. Next, a fibrinogen solution mixed with a suspension of primary HUVECs and fibroblasts was injected into the channels. Once enzymatic gelation was completed, the needles were removed from the scaffold to generate hollow microchannels. HUVECs were then seeded and cultured in the created microchannels, allowing the side channels to form perfusable networks with endothelial tubes in the hydrogels. They went on to show the application of this system for constructing models of vascularized human adipose tissue, the blood−retinal barrier, and a 3D organotypic model of vascularized human lung adenocarcinoma. They observed that during a period of 7 days, lung spheroids maintained their spherical shape as well as structural integrity and appeared to be integrated with their surrounding microvessels, thereby making perfusable networks in and around the cancer spheroids. In light of this information, the use of ECs is an effective way to produce perfusable microvascular constructs when it is used along with a hybrid microfluidic platform that also contains perfusable microchannels. Taken together, considerable progress has been made to remove mass transport limitations by using ECs as a common strategy for promoting vascularization; however, significant progress is still required to demonstrate functionality, including the ability to flow media (or blood) and recapitulate in vivo functions.
3. Bioprinting
Recent advances in 3D extrusion-based printing have enabled bioprinting of increasingly complex vascular conduits with improved structural integrity and functionality. The most notable feature of extrusion-based bioprinting is the ability to perform simultaneous deposition of cells and biomaterials in a layer-by-layer fashion to form well-organized structures similar to complex biological tissue architectures.181 There are two main approaches for printing vascular networks via 3D printing. One is the generation of interconnected vessel systems and channels via a sacrificial template (indirect printing, as discussed above), while the second is the generation of free-standing individual vascular conduits (direct printing).182 For all 3D printed approaches, fabrication of perfusable channel systems enable O2 and CO2 exchange and nutrient supply within the bulk of 3D printed tissues.183 For instance, in one study hollow alginate filaments were 3D printed by a coaxial nozzle, offering built-in microchannels to deliver additional nutrients for enhanced cell growth. Hollow filaments created had an average inner and outer diameter of 892 and 1192 μm, respectively. It was shown that L929 mouse fibroblasts cultured within the constructs showed higher viability than cells encapsulated in alginate structures without built-in microchannels.184 In their 2018 paper, Zhao and co-workers185 presented a novel platform of airflow-assisted 3D bioprinting for producing spiral-based spheroids with sophisticated vessel-like microarchitectures. Their printing setup utilized a sodium alginate solution proportionally extruded out from a PDMS microchip and rotated by the adjustable airflow allowing the flows to be stretched into spiral structures during the rotation period. They studied osteogenesis and angiogenesis in this artificial sphere-shaped organoid by co-culturing HUVECs and human MSCs. The viability of the encapsulated cells exceeded 75%, and osteogenic nodules were found, suggesting that this technique was successful for building functional organoids in vitro. They concluded that the arrangement of cells and the resulting morphologies of printed microarchitectures had the greatest impact on nutrient diffusion and in vitro angiogenesis.
A main challenge of constructing free-standing 3D vascular networks is the requirement of the printed structure to be supported during printing so that cells can establish structural integrity for 3D tissue engineering.186 In a recent paper by Yang et al.,187 a photocurable hydrogel (including sodium alginate, photoinitiator, and PEGDA) was printed to form a self-supporting matrix with the grooves of internal channels. In the next step a sacrificial ink, PF127, was printed within the previously formed hydrogel to fill the internal grooves and to facilitate fabrication of perfusable channels. Next, it was immersed in a CaCl2 solution to cross-link the structure, and then the sacrificial ink was removed by water to form 3D hollow channels. In another 2018 study, experiments on printability of fibrin/fibrinogen demonstrated how to overcome the problematic printability of fibrin and make use of its “glue-like” behavior to generate in vitro blood vessel models.188 For this purpose, the researchers mixed HUVECs with a sacrificial material (gelatin) before printing and then later casting the mixture of fibrin and collagen around the sacrificial material to prepare a simple vascular structure that was lined by a single layer of endothelial cells. They observed over 83% cell viability as well as the expression of VE-cadherin, smooth muscle actin, and collagen IV indicating angiogenesis. This study established the ability to form perfusable complex structures with a functional endothelial lining. In another study, a branched vascular model to improve delivery of O2 and removal CO2 was developed via a novel double-nozzle assembly technology.189 In this study, adipose-derived stromal cells (ADSC) were combined within a gelatin/alginate/fibrinogen hydrogel to form a vascular-like network, and hepatocytes combined in the gelatin/alginate/chitosan were placed around these networks. During a 2-week culture, the hepatocytes inside the construct performed enhanced liver metabolic functions, such as albumin and urea secretion, and the ADSCs at the periphery of the vascular-like network demonstrated EC-like properties. In a different study, Zheng et al.190 studied a combination of PEG and silk as a self-standing printable bioink. The strength and toughness of the silk/PEG ink after curing was sufficient to support 3D structures without the need of additional supporting materials. These silk/PEG bioink gels at varied concentrations from 3.75% to 10% w/v were loaded with hMSCs then cultured in vitro, and the results showed that the presence of cells did not change the printability and self-standing properties of the bioink. Due to a more porous structure formed in the gel matrix to support nutrient transport, cells grew faster in the higher concentration (10% w/v) gels, as compared to the lower concentration ones (7.5% and 5%, w/v). Interestingly, the printed cell-loaded 10% w/v constructs maintained their shape during 12 weeks of culture. Another method for improving nutrient and oxygen diffusion in printed constructs was presented by Kang and co-workers.191 This work also demonstrated methods to overcome limitations associated with structural integrity and vascularization of bioprinted tissue constructs. As such, mechanically stable human-scale tissue constructs (bone, ear, and muscle) were developed by printing cell-laden hydrogels (consisting of a mixture of gelatin, fibrinogen, HA, and glycerol) together with biodegradable polymers (poly(ε-caprolactone) in integrated patterns and anchored on sacrificial hydrogels (Pluronic F-127). The incorporation of microchannels into the tissue constructs facilitated improved diffusion of nutrients to printed cells and provided a favorable microenvironment for osteogenic differentiation of human amniotic fluid–derived stem cells (hAFSCs), and ultimately the generation of human ear–shaped tissue constructs or highly oriented myofiber bundles. Freeman et al.192 also demonstrated a 3D rotary bioprinter with a new bioink formulation based on fibrinogen and gelatin for vessel bioprinting. To prepare a cell-laden bioink, they mixed HNDFs into the gelatin–fibrinogen bioink before 3D printing, then crosslinked the construct by the addition of thrombin. The authors went on to show that the density of cells in the bioink influenced printability and tissue volumetric changes of the printed vessel constructs during cultures. SEM micrographs of the blended bioink demonstrated a highly porous structure, which would provide better culture conditions for cells to grow and assemble into vessel-like structures. A more engineering-based approach was presented recently where a microfluidic device was combined with 3D bioprinting to mimic vascular-like networks.193 An elastin-like polypeptide (ELP)–RGD hydrogel was prepared by mixing ELP with a diluted solution of the amine-reactive crosslinker tetrakis(hydroxymethyl)phosphonium chloride (THPC) in a 4:1 volumetric ratio, which was then used as a bioink for nervous tissue engineered 3D in vitro models with on-chip vascular-like channels. Neural cell survival was reported as 88.9% after bioprinting, and analysis of the endothelialized channels demonstrated distribution of endothelial cells along the entire lumen of the channel.
Another common 3D bioprinting technique is stereolithography, where a laser-assisted method is utilized to achieve in vitro vascularization. This detailed 3D bioprinting process involves computer-driven and spatially controlled irradiation of a liquid resin, which enables structures with precise microscale features (∼20–100 μm) to be prepared directly from a computer drawing.194 For instance, Magalhães et al.195 employed stereolithography technology to create PEGDA and GelMA vascularized structures with more than five layers and resolutions between 42 and 83 μm. Notability, this technique is well suited for manufacture of complex structures since it does not require a sacrificial material while demonstrating high accuracy for the printing of cell-laden hydrogels.196,197 For generating vascularized systems, stereolithography allows a suspension of endothelial cells in a photoink/resin, thereby facilitating precise deposition of cells in desired locations and spatial patterns. ECs can be either incorporated in a photocrosslinkable biomaterial or post-seeded on the outer surface of the scaffolds after preparation.198 The Harris group199 developed a photocurable resin consist of Bisphenol A (BPA)–ethoxylated-diacrylate, lauryl acrylate and isobornylacrylate with Irgacure 184 (0.5 and 1 wt. %) and used it to print vascular networks. Their main focus in this study was to improve design rules for complex artificial vascular networks using stereolithography. After preparation, the branched networks were embedded in GelMA scaffolds loaded with HUVECS, hASCs, and pericytes. Their measured cell death rates showed a sharp drop (from 55% to 27%) due to better nutrient supply via the vascular-like networks.
Finally, the recent evolution of 4D printing offers additional temporal response or control as well as exciting possibilities to the field of in vitro vascularization. Scaffolds made by 4D printing respond to the microenvironment by changing their chemical or physical nature. Smart materials used in this approach can enable scaffolds to mimic the dynamic nature of tissues to a greater extent.200 One interesting example is related to Senatov's group201 that 3D printed a scaffold based on polylactide (PLA)/hydroxyapatite (HA) with average pore size and porosity of 700 μm and 30 vol. %, respectively. The shape recovery during compression-heating-compression cycles was about 96%, suggesting the approach as a good candidate as a self-fitting implant for small bone defect replacement. Moreover, the porosity and a suitable pore size provides favorable culture conditions for cell seeding while enhancing nutrient diffusion throughout the structure.
D. Oxygenating scaffolds and microparticles for cell culture
The last main approach researchers have utilized to support the formation of large 3D cell models with enhanced functions is via methods that directly enhance local oxygenation, as summarized in Fig. 4.202 One common research strategy to enhance oxygen transport within engineered microenvironments is by using oxygen-permeable membranes at the bottom of culture dishes.203,204 This planar configuration has been shown to enable oxygen transport from the top and the bottom of the culture, thereby increasing the overall oxygen concentration in the media. Some authors have also suggested that similar gas permeable wells/scaffolds can be used in combination with oxygen-generating compounds, and such an approach has been used to reduce necrotic cell death in vitro. In one study, porcine neonatal pancreatic cell clusters were encapsulated in PDMS + CaO2 scaffolds.205 This approach makes use of the fact that CaO2 eventually dissociates into molecular oxygen via spontaneous reaction with water. PDMS rings with incorporated CaO2 were able to gradually generate oxygen under contact with the culture medium, and results showed that this system could effectively enhance viability while decreasing hypoxic cell expression patterns and ROS levels. In another study, the synergic effects of oxygen-permeable microwells combined with an antioxidant (AA2P) on pancreatic β-cell spheroids were examined.206 The results suggested that additional oxygen, together with removal of ROS, may lead to a better approach to prepare more viable and functional bioartificial pancreatic islets. Importantly, this method addressed a major concern for the use of oxygen-generating compounds regarding elevated levels of reactive oxygen species (ROS) generated inside cells. Besides contributing to cell death, ROS is also known to play a role in β-cell dysfunction in diabetes.
FIG. 4.
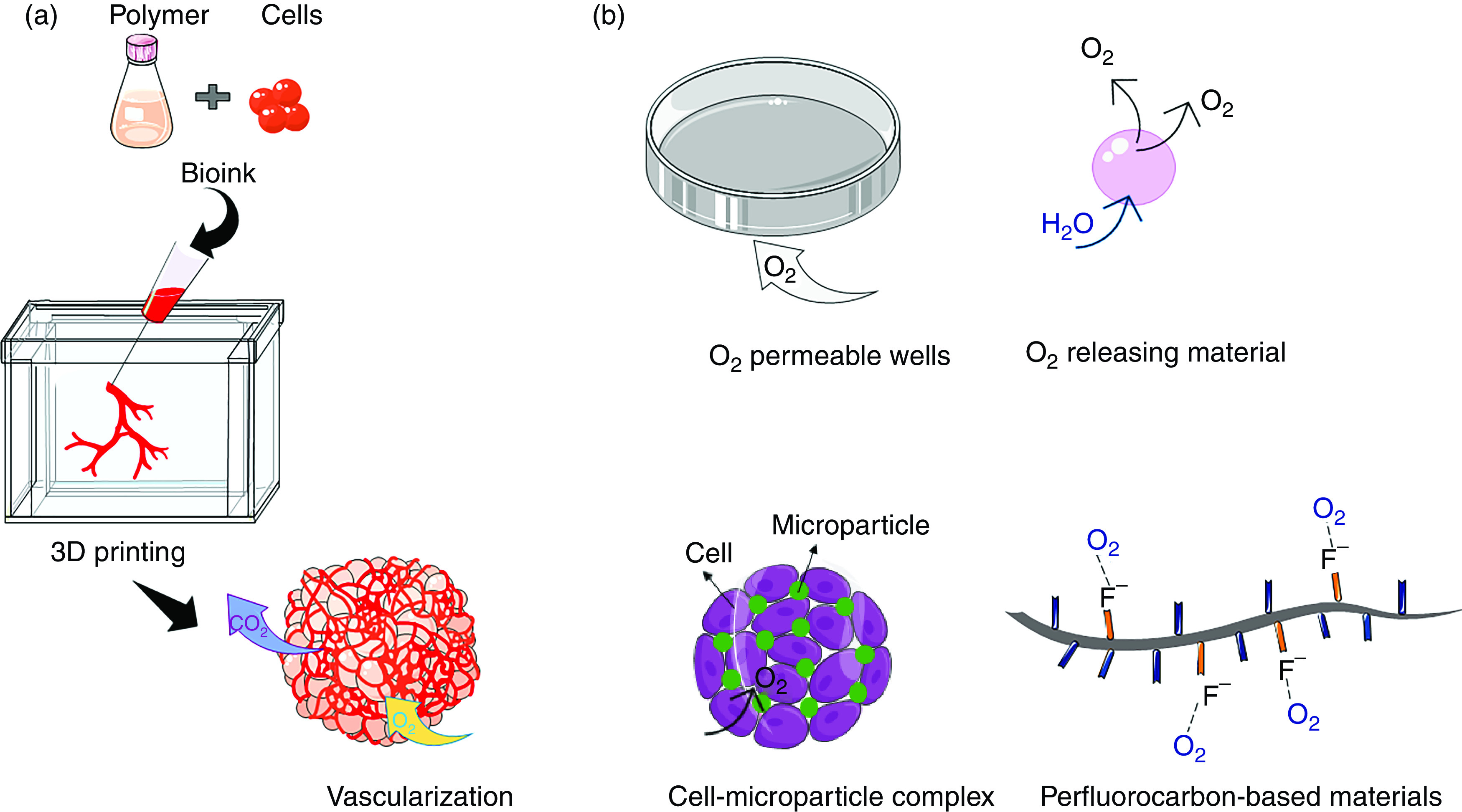
Techniques to improve static culture conditions in 3D cell culture models via (a) vascularization and (b) direct oxygenation approaches.
The use of hydrogel beads has also been studied as a tool to regulate the structure and packing of multicellular spheroids to promote enhanced oxygenation. In this approach, hydrogel beads are added to the cell aggregates to obtain “densely packed” structures with microchannels enabling enhanced gas/nutrient exchange. This theory is supported by a range of in vitro cell-mixing experiments.207 For instance, in one study gelatin microparticles (sized 5 to 63 μm) were employed to form spheroids with adipose-derived MSCs. It was shown that cell viability and proliferation increased due to increased space availability for diffusion of nutrients.208 In another study, Mihara and colleagues209 reported a similar concept for improving the 3D culture of HepG2 cell-based spheroids. They demonstrated that using hydrogel beads in combination with a gas-permeable plate leads to enhanced oxygen supply to improve spheroid formation. The results revealed that 20 μm diameter hydrogel beads helped to best distribute oxygen internally and to suppress cell death within the central regions of spheroids as compared with spheroids comprised of only cells.
Another potentially effective strategy to overcome insufficient oxygen supply is to synthesize 3D scaffolds with oxygen-generating capability. In this regard, Newland et al.210 draw our attention to the synthesis of PEGDA/CaO2 microspheres using photocrosslinking methods. They showed that upon sphere contact with water, highly cytotoxic hydrogen peroxide is produced. Eventually, they partially solved this issue by adding a catalase to breakdown excess hydrogen peroxide. Their results demonstrated that the addition of oxygen-producing spheres to SH-SY5Y cells and MSCs increased oxygen levels, with the conclusion that oxygen-producing spheres could compensate for cellular oxygen consumption levels. Similarly, oxygen-generating microparticles based on PCL, pluronic F-127, and calcium peroxide (CPO) were fabricated through an electrospraying process.211 To evaluate cell viability responses, rat chondrocytes were encapsulated in GelMA hydrogels with various concentrations of microparticles. It was shown that these microparticles released oxygen in a sustained manner for up to 7 days, maintaining high cell viability. A main weakness of both studies (and similar reaction-based approaches) is that the generation of oxygen is exhaustible and can lead to increased levels of cytotoxic ROS, as mentioned above. Moreover, oxygen delivery in both cases is triggered upon contact with water, which leads to the burst release of oxygen and subsequent cell toxicity responses. Finally, it is worth mentioning that the generation of oxygen from peroxides is an exothermic reaction, which further increases the chance of cell death due to overheating.212
Perfluorocarbons (PFCs) offer another strategy for improving oxygen transport and offer a solution to the finiteness and the toxic side effects of oxygen-generating materials.213 Highly electronegative fluorine groups in PFCs dissolve O2 according to Henry's law.214 Due to the hydrophobic nature of PFCs, emulsion systems have mainly been used to overcome their solubility issues. Early on in their discovery and application, PFCs were pursued extensively for use as blood substitutes215 as well as a support for pulmonary gas exchange216 in vivo; however, their clinical utilization has largely been discontinued due to excessive organ retention and long clearance times. Moreover, PFC emulsions suffer from insufficient long-term stability and handling limitations, which require significant validation prior to clinical use.217 Interestingly, the CO2-extraction abilities of PFCs (via similar gas dissolving mechanisms as O2) has been much less investigated than oxygen transport. CO2 is a major waste product of metabolism that is more soluble in PFCs as compared to O2. Thus, PFCs could hypothetically be employed to act as scavengers of gaseous waste compounds and can likely perform this function better than supplying oxygen.218 As a result, the known and long regarded ability of PFCs to enhance oxygen levels could be directly related to the elimination of CO2 from the region of interest.219
To address several shortcomings of PFC emulsions and free PFCs, they can be covalently attached to polymer chains then formulated into microparticles or scaffolds. For instance, White and co-workers220 showed that incorporation of 7% perfluorooctyl bromide into alginate gels increased oxygen transport mainly due to oxygen solubility in PFC‐containing gels.
Our group has also reported a strategy for covalently immobilizing PFCs to biopolymers as side chains enabling the creation of hydrogels with oxygen-carrying capabilities.70,221–226 The biopolymer of choice has mainly consisted of chitosan modified with linear PFCs. The lack of chemical bonds between O2 and PFC molecules allows for the efficient release of gases when sinks (e.g., metabolically active cells) are present, thereby enhancing the gradient/driving force for oxygen transport. For the synthesis of our polymer, PFC chains are bonded to methacrylamide chitosan via Schiff base nucleophilic substitution.221 Afterward, radical polymerization is used to form hydrogels. We have observed generally that the material with the most fluorines per substitution shows the greatest uptake and release of oxygen, while also supporting in vitro cultures resulting in the highest number of viable cells with the greatest metabolic activity.227 In a recent paper of ours70 we extended this work to spheroids, where we made large-sized spheroids (> 500 μm) with severe oxygen transport limitations. By formulating PFC-conjugated chitosan-based microgels (∼20 μm diameter) that were subsequently added during spheroid agglomeration, we demonstrated the unique ability of PFC microgels to drive oxygen transport internally to reduce the oxygen gradient to the center of spheroids and subsequently reduce cell hypoxia responses.
In summary, in vitro cultures require both a constant supply of oxygen and an effective mechanism to remove waste gasses such as carbon dioxide, facts that are compounded by 3D cultures lacking sufficient perfusion. Due to their unique chemistry, PFCs can be utilized to facilitate efficient gas exchange to restore adequate oxygen delivery to hypoxic areas, thereby offering a chance to bridge the need for vascularization of 3D in vitro constructs.
IV. CONCLUSIONS AND FUTURE DIRECTIONS
In vitro spheroid/organoid models (or tissue-engineered constructs) lack functioning vasculature, which stymies tissue growth after a certain growth stage due to insufficient nutrient and waste transport. To directly address this need, technological approaches are being pursued to capture in vivo complexity more closely and ultimately in vivo tissue functions. These platforms allow control over the adhesion of cells, architectures formed, mechanical as well as chemical properties of cell microenvironments, and therefore increase scientists' ability to develop better in vitro models. However, there is still room to overcome common limitations of spheroids/organoids, such as inadequate nutrient/oxygen supply, removal of cell waste products, and the ultimate loss of cell/organ specific functions over long-term cultures. These issues can be solved, or reduced in impact, by continuing to integrate emerging knowledge from cell biology with novel engineering approaches (summarized in Table III).
TABLE III.
Summary of technologies used to remove mass transport limitations of spheroids/organoids.
| Method | Principle | Advantage | Disadvantage | Scalability | Long-term cell viability |
|---|---|---|---|---|---|
| Bioreactor | Efficient distribution of culture medium and oxygen throughout constructs | Improved oxygenation | Cell death due to shear stress | 250 ml to 5000 l | < 50 days |
| Uniform media delivery | Increased heterogeneity by increasing the size | ||||
| Closely monitored and tightly controlled environmental and operating conditions | High cell density and large volume of media | ||||
| Bulk media metabolism analyses | High cost of media, cell, and operation | ||||
| Limited number of experiments possible within a certain time frame | |||||
| Microfluidics | Continuous pumping of fresh media and removal of waste | Small-scale cultivation; spatial control over cell behavior | Cell adhesion to PDMS surfaces | ½″ to 4″ (∼1 − 10 cm) | < 3 weeks |
| Mimicking flow of vessels in vivo | Connections between different cell populations through the device | Difficulty in removing the samples for further analysis | |||
| Improved mass transfer due to laminar media flow condition | |||||
| Real time detection of reactions using noninvasive measurements | |||||
| Vascularization | VEGF-induced vascularization | Enhanced cell viability and mass transfer by developing capillary‐like structures within the 3D tissue constructs | Short half‐life of VEGF | Lumen diameters 100 μm to 1 mm | < 2 weeks |
| Co-culture with endothelial cells | No functional perfusion in vivo | ||||
| Formation of bioengineered channels | |||||
| Oxygenating scaffolds | Generation of oxygen upon contact with water | Enhanced oxygenation | Presence of cytotoxic free radicals | Nanoparticles to bulk hydrogels | < 1 week |
| Diffusion of dissolved oxygen from PFC modified scaffolds | |||||
| Exothermic reactions | |||||
| Finite supply not suitable for extended culture |
In this review paper, we attempted to highlight the need for increasing the complexity and size of current cell-based structures for more relevant, reproducible, and quantitative studies to be used in emerging fields such as personalized medicine, therapy, and screening. This is evidenced by the fact that large 3D model systems are not always easy to generate and culture due to a lack of efficient oxygenation, nutrient uptake, and waste extraction. To date, methodologies for adding complexity to 3D in vitro cell structures have improved drastically with enhanced potential to uncover more accurate cellular interactions that may have otherwise been missed. Advances in bioreactors and microfabrication techniques have been shown to be effective in improving the consistency and reproducibility of larger sized spheroids/organoids in research. Moreover, in terms of addressing paramount oxygen transport limitations, PDMS-based well-plates, oxygen-generating peroxides, and PFC polymer–based systems that either enhance oxygenation or reduce gas transport limitations of large size 3D models in culture systems offer informative examples. In total, the studies highlighted in this review demonstrate the growing need for generating larger and more complex spheroids/organoids and maintaining them for long-term studies. Furthermore, this body of work supports the desire of researchers to use more physiologically relevant 3D in vitro models for areas such as disease modeling, drug screening, and other preclinical protocols.
It is worth noting that while bioengineered microenvironments offer interesting opportunities to study complex cell structures that more accurately model human physiology, there are still unoptimized factors to consider continuing to move the field forward. For instance, 3D in vitro human models do not capture the unique genetic characteristics of each patient that could lead to different responses during disease modeling or therapeutics testing. Therefore, the status of 3D in vitro technologies and their applications in personalized medicine remains largely unexplored. As one key direction, future efforts will doubtlessly improve the complexity of mini-organs by connecting more than one human organ system such as through incorporating immune system elements derived from individual patients, which may later serve as an extraordinary platform to screen immunotherapy drugs. In conclusion, rapid technical advances in the field suggest that spheroids and organoids will provide unprecedented opportunities to advance the impact on human health and disease in the near future.
DATA AVAILABILITY
Data sharing is not applicable as no new data were created or analyzed in this article.
References
- 1. Corrò C., Novellasdemunt L., and Li V. S. W., “ A brief history of organoids,” Am. J. Physiol. - Cell Physiol 319(1), C151–C165 (2020). 10.1152/ajpcell.00120.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson H. V., “ A new method by which sponges may be artificially reared,” Science 25(649), 912–915 (1907). 10.1126/science.25.649.912 [DOI] [PubMed] [Google Scholar]
- 3. Sutherland R. M., McCredie J. A., and Inch W. R., “ Growth of multicell spheroids in tissue culture as a model of nodular carcinomas,” J. Natl. Cancer Inst. 46(1), 113–120 (1971). 10.1093/jnci/46.1.113 [DOI] [PubMed] [Google Scholar]
- 4. Barcellos-Hoff M. H., Aggeler J., Ram T. G., and Bissell M. J., “ Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane,” Development 105(2), 223–235 (1989). 10.1242/dev.105.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelm J. M., Timmins N. E., Brown C. J., Fussenegger M., and Nielsen L. K., “ Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types,” Biotechnol. Bioeng. 83(2), 173–180 (2003). 10.1002/bit.10655 [DOI] [PubMed] [Google Scholar]
- 6. Ivascu A. and Kubbies M., “ Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis,” SLAS Discov. 11(8), 922–932 (2006). 10.1177/1087057106292763 [DOI] [PubMed] [Google Scholar]
- 7. Knight E. and Przyborski S., “ Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro,” J. Anatomy 227(6), 746–756 (2015). 10.1111/joa.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edmondson R., Broglie J. J., Adcock A. F., and Yang L., “ Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors,” Assay Drug Develop. Technol. 12(4), 207–218 (2014). 10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rouwkema J., Koopman B. F., Van Blitterswijk C. A., Dhert W. J., and Malda J., “ Supply of nutrients to cells in engineered tissues,” Biotechnol. Genet. Eng. Rev 26(1), 163–178 (2009). 10.5661/bger-26-163 [DOI] [PubMed] [Google Scholar]
- 10. Rademakers T., Horvath J. M., van Blitterswijk C. A., and LaPointe V. L. S., “ Oxygen and nutrient delivery in tissue engineering: Approaches to graft vascularization,” J. Tissue Eng. Regenerative Med. 13(10), 1815–1829 (2019). 10.1002/term.2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fathollahipour S., Patil P. S., and Leipzig N. D., “ Oxygen regulation in development: Lessons from embryogenesis towards tissue engineering,” Cells Tissues Organs 205(5–6), 350–371 (2018). 10.1159/000493162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiswald L.-B., Bellet D., and Dangles-Marie V., “ Spherical cancer models in tumor biology,” Neoplasia 17(1), 1–15 (2015). 10.1016/j.neo.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lancaster M. A. and Knoblich J. A., “ Organogenesis in a dish: Modeling development and disease using organoid technologies,” Science 345(6194), 1247125 (2014). 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- 14. Stadler M., Scherzer M., Walter S., Holzner S., Pudelko K., Ridel A., Unger C., Kramer N., Weil B., Neesen J., Hengstschlaeger M., and Dolznig H., “ Exclusion from spheroid formation identifies loss of essential cell-cell adhesion molecules in colon cancer cells,” Sci. Rep. 8(1), 1–16 (2018). 10.1038/s41598-018-19384-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foglietta F., Canaparo R., Muccioli G., Terreno E., and Serpe L., “ Methodological aspects and pharmacological applications of three-dimensional cancer cell cultures and organoids,” Life Sci. 254, 117784 (2020). 10.1016/j.lfs.2020.117784 [DOI] [PubMed] [Google Scholar]
- 16. Huch M. and Koo B. K., “ Modeling mouse and human development using organoid cultures,” Development (Cambridge) 142(18), 3113–3125 (2015). 10.1242/dev.118570 [DOI] [PubMed] [Google Scholar]
- 17. Okamoto R., Shimizu H., Suzuki K., Kawamoto A., Takahashi J., Kawai M., Nagata S., Hiraguri Y., Takeoka S., Sugihara H. Y., Yui S., and Watanabe M., “ Organoid-based regenerative medicine for inflammatory bowel disease,” Regen. Ther. 13, 1–6 (2020). 10.1016/j.reth.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Do J. T. and Hong Y. J., “ Neural lineage differentiation from pluripotent stem cells to mimic human brain tissues,” Front. Bioeng. Biotechnol. 7, 400 (2019). 10.3389/fbioe.2019.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orqueda A. J., Giménez C. A., and Pereyra-Bonnet F., “ iPSCs: A minireview from bench to bed, including organoids and the CRISPR system,” Stem Cells Int. 2016, 5934782. 10.1155/2016/5934782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerecht-Nir S., Cohen S., and Itskovitz-Eldor J., “ Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation,” Biotechnol. Bioeng. 86(5), 493–502 (2004). 10.1002/bit.20045 [DOI] [PubMed] [Google Scholar]
- 21. van den Brink S. C. et al. , “ Publisher correction: Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids,” Nature 579(7799), E11 (2020). 10.1038/s41586-020-2113-3 [DOI] [PubMed] [Google Scholar]
- 22. Yu L. et al. , “ Blastocyst-like structures generated from human pluripotent stem cells,” Nature 591(7851), 620–626 (2021). 10.1038/s41586-021-03356-y [DOI] [PubMed] [Google Scholar]
- 23. Moris N. et al. , “ An in vitro model of early anteroposterior organization during human development,” Nature 582(7812), 410–415 (2020). 10.1038/s41586-020-2383-9 [DOI] [PubMed] [Google Scholar]
- 24. Girgin M. U. and Lutolf M. P., “ Gastruloids generated without exogenous wnt activation develop anterior neural tissues,” bioRxiv:10.10.334326 (2020). [DOI] [PMC free article] [PubMed]
- 25.See https://www.isscr.org/ for SSCR Guidelines Updates Task Force, “ Guidelines for stem cell research and clinical translation,” 2016.
- 26. Appleby J. B. and Bredenoord A. L., “ Should the 14‐day rule for embryo research become the 28‐day rule?,” EMBO Mol. Med 10(9), e9437 (2018). 10.15252/emmm.201809437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Markou M. et al. , “ Tissue engineering using vascular organoids from human pluripotent stem cell derived mural cell phenotypes,” Front. Bioeng. Biotechnol. 8, 278 (2020). 10.3389/fbioe.2020.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amit M. et al. , “ Suspension culture of undifferentiated human embryonic and induced pluripotent stem cells,” Stem Cell Rev. Rep. 6(2), 248–259 (2010). 10.1007/s12015-010-9149-y [DOI] [PubMed] [Google Scholar]
- 29. Pacitti D., Privolizzi R., and Bax B. E., “ Organs to cells and cells to organoids: The evolution of in vitro central nervous system modelling,” Front. Cell. Neurosci. 13, 129 (2019). 10.3389/fncel.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCauley H. A. and Wells J. M., “ Pluripotent stem cell-derived organoids: Using principles of developmental biology to grow human tissues in a dish,” Development 144(6), 958–962 (2017). 10.1242/dev.140731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hribar K. C. et al. , “ Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture,” Lab on a Chip 15(11), 2412–2418 (2015). 10.1039/C5LC00159E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee J. H., Han Y. S., and Lee S. H., “ Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells,” Biomol. Ther. 24(3), 260–267 (2016). 10.4062/biomolther.2015.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bin Ramli M. N. et al. , “ Human pluripotent stem cell–derived organoids as models of liver disease,” Gastroenterology 159(4), 1471–1486 (2020). 10.1053/j.gastro.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 34. Song L. et al. , “ Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues,” Sci. Rep. 9, 5977 (2019). 10.1038/s41598-019-42439-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takebe T. et al. , “ Vascularized and functional human liver from an iPSC-derived organ bud transplant,” Nature 499(7459), 481–484 (2013). 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- 36. Varzideh F. et al. , “ Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants,” Biomaterials 192, 537–550 (2019). 10.1016/j.biomaterials.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 37. Hickman J. A. et al. , “ Three‐dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo,” Biotechnol. J. 9(9), 1115–1128 (2014). 10.1002/biot.201300492 [DOI] [PubMed] [Google Scholar]
- 38. Saraiva D. P., Matias A. T., Braga S., Jacinto A., and Cabral M. G., “ Establishment of a 3D co-culture with MDA-MB-231 breast cancer cell line and patient-derived immune cells for application in the development of immunotherapies,” Front. Oncol. 10, 1543 (2020). 10.3389/fonc.2020.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Antunes J. et al. , “ In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening,” Acta Biomater. 94, 392–409 (2019). 10.1016/j.actbio.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 40. Song H., Cai G. H., Liang J., Ao D. S., Wang H., and Yang Z. H., “ Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials,” J. Nanobiotechnology 18(1), 90 (2020). 10.1186/s12951-020-00646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furukawa K. S., Ushida T., Sakai Y., Suzuki M., Tanaka J., and Tateishi T., “ Formation of human fibroblast aggregates (spheroids) by rotational culture,” Cell Transplant. 10(4–5), 441–445 (2001). 10.3727/000000001783986503 [DOI] [PubMed] [Google Scholar]
- 42. Meenach S. A., Tsoras A. N., McGARRY R. C., Mansour H. M., Hilt J. Z., and Anderson K. W., “ Development of three-dimensional lung multicellular spheroids in air- and liquid-interface culture for the evaluation of anticancer therapeutics,” Int. J. Oncol. 48(4), 1701–1709 (2016). 10.3892/ijo.2016.3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pradhan S., Clary J. M., Seliktar D., and Lipke E. A., “ A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres,” Biomaterials 115, 141–154 (2017). 10.1016/j.biomaterials.2016.10.052 [DOI] [PubMed] [Google Scholar]
- 44. Zoetemelk M., Rausch M., Colin D. J., Dormond O., and Nowak-Sliwinska P., “ Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma,” Sci. Rep. 9(1), 1–14 (2019). 10.1038/s41598-019-42836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li F., Cao L., Parikh S., and Zuo R., “ 3D Spheroids with Primary Human Liver Cells and Differential Roles of Kupffer Cells in Drug-Induced Liver Injury,” J. Pharm. Sci. 143, 1–8 (2020). 10.1016/j.jphs.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 46. Woappi Y., Altomare D., Creek K. E., and Pirisi L., “ Self-assembling 3D spheroid cultures of human neonatal keratinocytes have enhanced regenerative properties,” Stem Cell Res. 49, 102048 (2020). 10.1016/j.scr.2020.102048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loessner D., Stok K. S., Lutolf M. P., Hutmacher D. W., Clements J. A., and Rizzi S. C., “ Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells,” Biomaterials 31(32), 8494–8506 (2010). 10.1016/j.biomaterials.2010.07.064 [DOI] [PubMed] [Google Scholar]
- 48. Xu X., Gurski L. A., Zhang C., Harrington D. A., Farach-Carson M. C., and Jia X., “ Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids,” Biomaterials 33(35), 9049–9060 (2012). 10.1016/j.biomaterials.2012.08.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rezaee R. and Abdollahi M., The Importance of Translatability in Drug Discovery ( Taylor & Francis, 2017). [DOI] [PubMed] [Google Scholar]
- 50. Breslin S. and O'Driscoll L., “ Three-dimensional cell culture: The missing link in drug discovery,” Drug Discov. Today 18(5–6), 240–249 (2013). 10.1016/j.drudis.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 51. Eliasof S. et al. , “ Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle,” Proc. Natl. Acad. Sci 110(37), 15127–15132 (2013). 10.1073/pnas.1309566110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sadler N. C. et al. , “ Hepatic cytochrome P450 activity, abundance, and expression throughout human development,” Drug Metab. Dispos. 44(7), 984–991 (2016). 10.1124/dmd.115.068593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Turpeinen M., Ghiciuc C., Opritoui M., Tursas L., Pelkonen O., and Pasanen M., “ Predictive value of animal models for human cytochrome P450 (CYP)-mediated metabolism: A comparative study in vitro,” Xenobiotica 37(12), 1367–1377 (2007). 10.1080/00498250701658312 [DOI] [PubMed] [Google Scholar]
- 54. Gonzalez L., Strbo N., and Podack E. R., “ Humanized mice: Novel model for studying mechanisms of human immune-based therapies,” Immunol. Res. 57(1–3), 326–334 (2013). 10.1007/s12026-013-8471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scheer N. and Wilson I. D., “ A comparison between genetically humanized and chimeric liver humanized mouse models for studies in drug metabolism and toxicity,” Drug Discov. Today 21(2), 250–263 (2016). 10.1016/j.drudis.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 56. Yong K. S. M., Her Z., and Chen Q., “ Humanized mice as unique tools for human-specific studies,” Arch. Immunol. Ther. Ex. 66(4), 245–266 (2018). 10.1007/s00005-018-0506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arora T. et al. , “ Substitute of animals in drug research: An approach towards fulfillment of 4R's,” Indian J. Pharm. Sci. 73(1), 1 (2011). 10.4103/0250-474X.89750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Antoni D., Burckel H., Josset E., and Noel G., “ Three-dimensional cell culture: A breakthrough in vivo,” Int. J. Mol. Sci. 16(3), 5517–5527 (2015). 10.3390/ijms16035517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Langhans S. A., “ Three-dimensional in vitro cell culture models in drug discovery and drug repositioning,” Front. Pharmacol. 9, 6 (2018). 10.3389/fphar.2018.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leclerc E., Sakai Y., and Fujii T., “ Microfluidic PDMS (polydimethylsiloxane) bioreactor for large‐scale culture of hepatocytes,” Biotechnol. Prog. 20(3), 750–755 (2004). 10.1021/bp0300568 [DOI] [PubMed] [Google Scholar]
- 61. Benning L., Peintner A., Finkenzeller G., and Peintner L., “ Automated spheroid generation, drug application and efficacy screening using a deep learning classification: A feasibility study,” Sci. Rep. 10(1), 11071 (2020). 10.1038/s41598-020-67960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muciño-Olmos E. A. et al. , “ Unveiling functional heterogeneity in breast cancer multicellular tumor spheroids through single-cell RNA-seq,” Sci. Rep. 10(1), 12728 (2020). 10.1038/s41598-020-69026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gao Q. et al. , “ Cell culture system for analysis of genetic heterogeneity within hepatocellular carcinomas and response to pharmacologic agents,” Gastroenterology 152(1), 232–242 (2017). 10.1053/j.gastro.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 64. Mulholland T. et al. , “ Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient,” Sci. Rep. 8(1), 1–12 (2018). 10.1038/s41598-018-33055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nunes A. S., Barros A. S., Costa E. C., Moreira A. F., and Correia I. J., “ 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs,” Biotechnol. Bioeng. 116(1), 206–226 (2019). 10.1002/bit.26845 [DOI] [PubMed] [Google Scholar]
- 66. Zanoni M. et al. , “ 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained,” Sci. Rep. 6(1), 1–11 (2016). 10.1038/srep19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liew C. V., Chua S. M., and Heng P. W. S., “ Elucidation of spheroid formation with and without the extrusion step,” AAPS PharmSciTech 8(1), E70–E81 (2007). 10.1208/pt0801010 [DOI] [PubMed] [Google Scholar]
- 68. Lin R. and Chang H., “ Recent advances in three‐dimensional multicellular spheroid culture for biomedical research,” Biotechnol. J. Healthc. Nutr. Technol. 3(9‐10), 1172–1184 (2008). 10.1002/biot.200700228 [DOI] [PubMed] [Google Scholar]
- 69. Curcio E., Salerno S., Barbieri G., De Bartolo L., Drioli E., and Bader A., “ Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system,” Biomaterials 28(36), 5487–5497 (2007). 10.1016/j.biomaterials.2007.08.033 [DOI] [PubMed] [Google Scholar]
- 70. Patil P. S., Mansouri M., and Leipzig N. D., “ Fluorinated chitosan microgels to overcome internal oxygen transport deficiencies in microtissue culture systems,” Adv. Biosyst. 4(8), 1900250 (2020). 10.1002/adbi.201900250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wrzesinski K. and Fey S. J., “ Metabolic reprogramming and the recovery of physiological functionality in 3D cultures in micro-bioreactors,” Bioengineering 5(1), 22 (2018). 10.3390/bioengineering5010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lagies S. et al. , “ Cells grown in three-dimensional spheroids mirror in vivo metabolic response of epithelial cells,” Commun. Biol. 3(1), 1–10 (2020). 10.1038/s42003-020-0973-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ehsan S. M. and George S. C., “ Nonsteady state oxygen transport in engineered tissue: Implications for design,” Tissue Eng. Part A 19(11–12), 1433–1442 (2013). 10.1089/ten.tea.2012.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krogh A., “ The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion,” J. Physiol. 52(6), 391–408 (1919). 10.1113/jphysiol.1919.sp001838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Place T. L., Domann F. E., and Case A. J., “ Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research,” Free Radic. Biol. Med. 113, 311–322 (2017). 10.1016/j.freeradbiomed.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McMurtrey R. J., “ Roles of Diffusion Dynamics in Stem Cell Signaling and Three-Dimensional Tissue Development,” Stem Cells Dev. 26(18), 1293–1303 (2017). 10.1089/scd.2017.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McMurtrey R. J., “ Analytic models of oxygen and nutrient diffusion, metabolism dynamics, and architecture optimization in three-dimensional tissue constructs with applications and insights in cerebral organoids,” Tissue Eng. Part C Methods 22(3), 221–249 (2016). 10.1089/ten.tec.2015.0375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pasca A. M. et al. , “ Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture,” Nat. Methods 12(7), 671–678 (2015). 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Simunovic M. and Brivanlou A. H., “ Embryoids, organoids and gastruloids: New approaches to understanding embryogenesis,” Development (Cambridge) 144(6), 976–985 (2017). 10.1242/dev.143529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Qian X., Song H., and Ming G. L., “ Brain organoids: Advances, applications and challenges,” Development (Cambridge) 146(8), dev166074 (2019). 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rothenbücher T. S. P., Gürbüz H., Pereira M. P., Heiskanen A., Emneus J., and Martinez-Serrano A., “ Next generation human brain models: Engineered flat brain organoids featuring gyrification,” Biofabrication 13(1), 011001 (2021). 10.1088/1758-5090/abc95e [DOI] [PubMed] [Google Scholar]
- 82. Karzbrun E., Kshirsagar A., Cohen S. R., Hanna J. H., and Reiner O., “ Human brain organoids on a chip reveal the physics of folding,” Nat. Phys. 14(5), 515–522 (2018). 10.1038/s41567-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Heywood H. K., Bader D. L., and Lee D. A., “ Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration,” J. Cell. Physiol. 206(2), 402–410 (2006). 10.1002/jcp.20491 [DOI] [PubMed] [Google Scholar]
- 84. Nauman E. A. and Deorosan B., “ The role of glucose, serum, and three-dimensional cell culture on the metabolism of bone marrow-derived mesenchymal stem cells,” Stem Cells Int. 2011, 1–12. 10.4061/2011/429187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Guo Y. et al. , “ Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction,” GeroScience 42(2), 547–561 (2020). 10.1007/s11357-020-00179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stolzing A., Coleman N., and Scutt A., “ Glucose-induced replicative senescence in mesenchymal stem cells,” Rejuvenation Res. 9(1), 31–35 (2006). 10.1089/rej.2006.9.31 [DOI] [PubMed] [Google Scholar]
- 87. Lu Y., Zhang G., Shen C., Uygun K., Yarmush M. L., and Meng Q., “ A novel 3D liver organoid system for elucidation of hepatic glucose metabolism,” Biotechnol. Bioeng. 109(2), 595–604 (2012). 10.1002/bit.23349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tao T. et al. , “ Engineering human islet organoids from iPSCs using an organ-on-chip platform,” Lab on a Chip 19(6), 948–958 (2019). 10.1039/C8LC01298A [DOI] [PubMed] [Google Scholar]
- 89. Phelan M. A., Lelkes P. I., and Swaroop A., “ Mini and customized low-cost bioreactors for optimized high-throughput generation of tissue organoids,” Stem Cell Investig. 5, 33 (2018). 10.21037/sci.2018.09.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Massai D. et al. , “ A versatile bioreactor for dynamic suspension cell culture. Application to the culture of cancer cell spheroids,” PLoS One 11(5), e0154610 (2016). 10.1371/journal.pone.0154610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Naing M. W. and Williams D. J., “ Three-dimensional culture and bioreactors for cellular therapies,” Cytotherapy 13(4), 391–399 (2011). 10.3109/14653249.2011.556352 [DOI] [PubMed] [Google Scholar]
- 92. Stephenson M. and Grayson W., “ Recent advances in bioreactors for cell-based therapies [version 1; referees: 2 approved],” F1000Research 7, 517 (2018). 10.12688/f1000research.12533.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ginai M., Elsby R., Hewitt C. J., Surry D., Fenner K., and Coopman K., “ The use of bioreactors as in vitro models in pharmaceutical research,” Drug Discov. Today 18(19–20), 922–935 (2013). 10.1016/j.drudis.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 94. Pörtner R., Nagel-Heyer S., Goepfert C., Adamietz P., and Meenen N. M., “ Bioreactor design for tissue engineering,” J. Biosci. Bioeng. 100(3), 235–245 (2005). 10.1263/jbb.100.235 [DOI] [PubMed] [Google Scholar]
- 95. Ravichandran A., Liu Y., and Teoh S. H., “ Review: Bioreactor design towards generation of relevant engineered tissues: Focus on clinical translation,” J. Tissue Eng. Regen. Med. 12(1), e7–e22 (2018). 10.1002/term.2270 [DOI] [PubMed] [Google Scholar]
- 96. Yang S.-T., Bioprocessing for Value-Added Products from Renewable Resources: New Technologies and Applications ( Elsevier, 2011). [Google Scholar]
- 97. Martínez-Corona J. I., Cisneros-Garza R. R., Robledo-Padilla F., Parra-Saldívar R., Treviño-Martínez A. S., and Alvarez A. J., “ Optical approach for measuring oxygen mass transfer in stirred tank bioreactors,” Int. J. Chem. React. Eng. 15(4), 20160209 (2017). 10.1515/ijcre-2016-0209 [DOI] [Google Scholar]
- 98. Kawada M. et al. , “ Massive culture of human liver cancer cells in a newly developed radial flow bioreactor system: Ultrafine structure of functionally enhanced hepatocarcinoma cell lines,” Vitr. Cell Dev. Biol. Anim. 34(2), 109–115 (1998). 10.1007/s11626-998-0092-z [DOI] [PubMed] [Google Scholar]
- 99. Rozga J. et al. , “ Development of a bioartificial liver: Properties and function of a hollow-fiber module inoculated with liver cells,” Hepatology 17(2), 258–265 (1993). 10.1002/hep.1840170216 [DOI] [PubMed] [Google Scholar]
- 100. Naruse K., Sakai Y., Nagashima I., Jiang G. X., Suzuki M., and Muto T., “ Comparisons of porcine hepatocyte spheroids and single hepatocytes in the non-woven fabric bioartificial liver module,” Int. J. Artif. Organs 19(10), 605–609 (1996). 10.1177/039139889601901008 [DOI] [PubMed] [Google Scholar]
- 101. Garcia-Ochoa F. and Gomez E., “ Prediction of gas-liquid mass transfer coefficient in sparged stirred tank bioreactors,” Biotechnol. Bioeng. 92(6), 761–772 (2005). 10.1002/bit.20638 [DOI] [PubMed] [Google Scholar]
- 102. Tostoes R. M. et al. , “ Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing,” Hepatology 55(4), 1227–1236 (2012). 10.1002/hep.24760 [DOI] [PubMed] [Google Scholar]
- 103. Chaicharoenaudomrung N., Kunhorm P., and Noisa P., “ Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling,” World J. Stem Cells 11(12), 1065 (2019). 10.4252/wjsc.v11.i12.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schwedhelm I. et al. , “ Automated real-time monitoring of human pluripotent stem cell aggregation in stirred tank reactors,” Sci. Rep. 9(1), 12297 (2019). 10.1038/s41598-019-48814-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qian X., Jacob F., Song M. M., Nguyen H. N., Song H., and Ming G., “ Generation of human brain region–specific organoids using a miniaturized spinning bioreactor,” Nat. Protoc. 13(3), 565 (2018). 10.1038/nprot.2017.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Romero-Morales A. I., O'Grady B. J., Balotin K. M., Bellan L. M., Lippmann E. S., and Gama V., “ Spin∞: An updated miniaturized spinning bioreactor design for the generation of human cerebral organoids from pluripotent stem cells,” HardwareX 6, e00084 (2019). 10.1016/j.ohx.2019.e00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kazemzadeh A., Elias C., Tamer M., and Ein-Mozaffari F., “ Hydrodynamic performance of a single-use aerated stirred bioreactor in animal cell culture: Applications of tomography, dynamic gas disengagement (DGD), and CFD,” Bioprocess. Biosyst. Eng. 41(5), 679–695 (2018). 10.1007/s00449-018-1902-7 [DOI] [PubMed] [Google Scholar]
- 108. Hammond T. G. and Hammond J. M., “ Optimized suspension culture: The rotating-wall vessel,” Am. J. Physiol. Ren. Physiol. 281(1), F12–F25 (2001). 10.1152/ajprenal.2001.281.1.F12 [DOI] [PubMed] [Google Scholar]
- 109. Varley M. C., Markaki A. E., and Brooks R. A., “ Effect of rotation on scaffold motion and cell growth in rotating bioreactors,” Tissue Eng. Part A 23(11–12), 522–534 (2017). 10.1089/ten.tea.2016.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ahmed H. M. M., Salerno S., Piscioneri A., Khakpour S., Giorno L., and De Bartolo L., “ Human liver microtissue spheroids in hollow fiber membrane bioreactor,” Colloids Surf. B Biointerfaces 160, 272–280 (2017). 10.1016/j.colsurfb.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 111. Zhu Y. et al. , “ A hollow fiber system for simple generation of human brain organoids,” Integr. Biol. 9(9), 774–781 (2017). 10.1039/C7IB00080D [DOI] [PubMed] [Google Scholar]
- 112. Blose K. J., Krawiec J. T., Weinbaum J. S., and Vorp D. A., “ Bioreactors for tissue engineering purposes,” in Regenerative Medicine Applications in Organ Transplantation ( Elsevier, 2014), pp. 177–185. [Google Scholar]
- 113. Kim S. S., Penkala R., and Abrahimi P., “ A perfusion bioreactor for intestinal tissue engineering,” J. Surg. Res. 142(2), 327–331 (2007). 10.1016/j.jss.2007.03.039 [DOI] [PubMed] [Google Scholar]
- 114. Schmid J. et al. , “ A perfusion bioreactor system for cell seeding and oxygen-controlled cultivation of three-dimensional cell cultures,” Tissue Eng. Part C Methods 24(10), 585–595 (2018). 10.1089/ten.tec.2018.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carrier R. L., Rupnick M., Langer R., Schoen F. J., Freed L. E., and Vunjak-Novakovic G., “ Perfusion improves tissue architecture of engineered cardiac muscle,” Tissue Eng. 8(2), 175–188 (2002). 10.1089/107632702753724950 [DOI] [PubMed] [Google Scholar]
- 116. Ingber D. E., “ Developmentally inspired human ‘organs on chips’” Development 145(16), dev156125 (2018). 10.1242/dev.156125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bhatia S. N. and Ingber D. E., “ Microfluidic organs-on-chips,” Nat. Biotechnol. 32(8), 760–772 (2014). 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]
- 118. Sabhachandani P., Motwani V., Cohen N., Sarkar S., Torchilin V., and Konry T., “ Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform,” Lab on a Chip 16(3), 497–505 (2016). 10.1039/C5LC01139F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Achberger K. et al. , “ Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform,” Elife 8, e46188 (2019). 10.7554/eLife.46188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bauer S. et al. , “ Publisher correction: Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model,” Sci. Rep. 8(1), 1672 (2018). 10.1038/s41598-018-20340-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bovard D. et al. , “ A lung/liver-on-a-chip platform for acute and chronic toxicity studies,” Lab on a Chip 18(24), 3814–3829 (2018). 10.1039/C8LC01029C [DOI] [PubMed] [Google Scholar]
- 122. Liu H. et al. , “ A droplet microfluidic system to fabricate hybrid capsules enabling stem cell organoid engineering,” Adv. Sci. 7(11), 1903739 (2020). 10.1002/advs.201903739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mannino R. G., Qiu Y., and Lam W. A., “ Endothelial cell culture in microfluidic devices for investigating microvascular processes,” Biomicrofluidics 12(4), 042203 (2018). 10.1063/1.5024901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tsvirkun D., Grichine A., Duperray A., Misbah C., and Bureau L., “ Microvasculature on a chip: Study of the endothelial surface layer and the flow structure of red blood cells,” Sci. Rep 7(1), 1–11 (2017). 10.1038/srep45036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chonan Y., Taki S., Sampetrean O., Saya H., and Sudo R., “ Endothelium-induced three-dimensional invasion of heterogeneous glioma initiating cells in a microfluidic coculture platform,” Integr. Biol. (United Kingdom) 9(9), 762–773 (2017). 10.1039/C7IB00091J [DOI] [PubMed] [Google Scholar]
- 126. Zhang F. et al. , “ Investigation of environmental pollutant-induced lung inflammation and injury in a 3D coculture-based microfluidic pulmonary alveolus system,” Anal. Chem. 92(10), 7200–7208 (2020). 10.1021/acs.analchem.0c00759 [DOI] [PubMed] [Google Scholar]
- 127. Ahmed S., Chauhan V. M., Ghaemmaghami A. M., and Aylott J. W., “ New generation of bioreactors that advance extracellular matrix modelling and tissue engineering,” Biotechnol. Lett. 41(1), 1–25 (2019). 10.1007/s10529-018-2611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shin S. R. et al. , “ Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes,” Adv. Sci. 4(5), 1600522 (2017). 10.1002/advs.201600522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schuster B. et al. , “ Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids,” Nat. Commun. 11(1), 1–12 (2020). 10.1038/s41467-020-19058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ho B. X., Pek N. M. Q., and Soh B.-S., “ Disease modeling using 3D organoids derived from human induced pluripotent stem cells,” Int. J. Mol. Sci 19(4), 936 (2018). 10.3390/ijms19040936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yang G., Mahadik B., Choi J. Y., and Fisher J. P., “ Vascularization in tissue engineering: Fundamentals and state-of-art,” Prog. Biomed. Eng. 2(1), 012002 (2020). 10.1088/2516-1091/ab5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pellegata A. F., Tedeschi A. M., and De Coppi P., “ Whole organ tissue vascularization: Engineering the tree to develop the fruits,” Front. Bioeng. Biotechnol. 6, 56 (2018). 10.3389/fbioe.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mastrullo V., Cathery W., Velliou E., Madeddu P., and Campagnolo P., “ Angiogenesis in tissue engineering: As nature intended?,” Front. Bioeng. Biotechnol. 8, 188 (2020). 10.3389/fbioe.2020.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Guo D. et al. , “ Vascular endothelial growth factor signaling requires glycine to promote angiogenesis,” Sci. Rep. 7(1), 1–10 (2017). 10.1038/s41598-017-15246-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Haro H., Kato T., Komori H., Osada M., and Shinomiya K., “ Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption,” J. Orthop. Res. 20(3), 409–415 (2002). 10.1016/S0736-0266(01)00150-4 [DOI] [PubMed] [Google Scholar]
- 136. Roy H., Bhardwaj S., and Ylä-Herttuala S., “ Biology of vascular endothelial growth factors,” FEBS Lett. 580(12), 2879–2887 (2006). 10.1016/j.febslet.2006.03.087 [DOI] [PubMed] [Google Scholar]
- 137. Xinaris C. et al. , “ In vivo maturation of functional renal organoids formed from embryonic cell suspensions,” J. Am. Soc. Nephrol. 23(11), 1857–1868 (2012). 10.1681/ASN.2012050505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ham O., Jin Y. B., Kim J., and Lee M. O., “ Blood vessel formation in cerebral organoids formed from human embryonic stem cells,” Biochem. Biophys. Res. Commun. 521(1), 84–90 (2020). 10.1016/j.bbrc.2019.10.079 [DOI] [PubMed] [Google Scholar]
- 139. Balikov D. A., Neal E. H., and Lippmann E. S., “ Organotypic neurovascular models: Past results and future directions,” Trends Mol. Med. 26(3), 273–284 (2020). 10.1016/j.molmed.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 140. Watson C. L. et al. , “ An in vivo model of human small intestine using pluripotent stem cells,” Nat. Med. 20(11), 1310–1314 (2014). 10.1038/nm.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shoval H. et al. , “ Tumor cells and their crosstalk with endothelial cells in 3D spheroids,” Sci. Rep. 7(1), 1–11 (2017). 10.1038/s41598-017-10699-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lovett M., Lee K., Edwards A., and Kaplan D. L., “ Vascularization strategies for tissue engineering,” Tissue Eng. Part B Rev. 15(3), 353–370 (2009). 10.1089/ten.teb.2009.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Xue D., Wang Y., Zhang J., Mei D., Wang Y., and Chen S., “ Projection-based 3D printing of cell patterning scaffolds with multiscale channels,” ACS Appl. Mater. Interfaces 10(23), 19428–19435 (2018). 10.1021/acsami.8b03867 [DOI] [PubMed] [Google Scholar]
- 144. Yang W. et al. , “ Mask-free generation of multicellular 3D heterospheroids array for high-throughput combinatorial anti-cancer drug screening,” Mater. Des. 183, 108182 (2019). 10.1016/j.matdes.2019.108182 [DOI] [Google Scholar]
- 145. Lu Y., Mapili G., Suhali G., Chen S., and Roy K., “ A digital micro‐mirror device‐based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds,” J. Biomed. Mater. Res. 77A(2), 396–405 (2006). 10.1002/jbm.a.30601 [DOI] [PubMed] [Google Scholar]
- 146. McNulty J. D. et al. , “ Micro-injection molded, poly (vinyl alcohol)-calcium salt templates for precise customization of 3D hydrogel internal architecture,” Acta Biomater. 95, 258–268 (2019). 10.1016/j.actbio.2019.04.050 [DOI] [PubMed] [Google Scholar]
- 147. Skylar-Scott M. A. et al. , “ Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels,” Sci. Adv. 5(9), eaaw2459 (2019). 10.1126/sciadv.aaw2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lei D. et al. , “ 3D printing of biomimetic vasculature for tissue regeneration,” Mater. Horiz. 6(6), 1197–1206 (2019). 10.1039/C9MH00174C [DOI] [Google Scholar]
- 149. Zuo X. et al. , “ Spheroids of endothelial cells and vascular smooth muscle cells promote cell migration in hyaluronic acid and fibrinogen composite hydrogels,” Research 2020, 8970480. 10.34133/2020/8970480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hayashi T., Takigawa-Imamura H., Nishiyama K., Shintaku H., Kotera H., Miura T., and Yokokawa R., “ Vascular network formation for a long-term spheroid culture by co-culturing endothelial cells and fibroblasts,” in 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), January, 2015. (IEEE), pp. 476–479. 10.1109/MEMSYS.2015.7050995 [DOI] [Google Scholar]
- 151. Yamamoto S., Hotta M. M., Okochi M., and Honda H., “ Effect of vascular formed endothelial cell network on the invasive capacity of melanoma using the in vitro 3D co-culture patterning model,” PLoS One 9(7), e103502 (2014). 10.1371/journal.pone.0103502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Norotte C., Marga F. S., Niklason L. E., and Forgacs G., “ Scaffold-free vascular tissue engineering using bioprinting,” Biomaterials 30(30), 5910–5917 (2009). 10.1016/j.biomaterials.2009.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tan Y. et al. , “ 3D printing facilitated scaffold-free tissue unit fabrication,” Biofabrication 6(2), 24111 (2014). 10.1088/1758-5082/6/2/024111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ling K. et al. , “ Bioprinting-based high-throughput fabrication of three-dimensional MCF-7 human breast cancer cellular spheroids,” Engineering 1(2), 269–274 (2015). 10.15302/J-ENG-2015062 [DOI] [Google Scholar]
- 155. Arai K. et al. , “ Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer,” PLoS One 13(12), e0209162 (2018). 10.1371/journal.pone.0209162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yu Y. et al. , “ Three-dimensional bioprinting using self-assembling scalable scaffold-free ‘tissue strands’ as a new bioink,” Sci. Rep. 6, 28714 (2016). 10.1038/srep28714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rouwkema J., Boer J. D., and Van Blitterswijk C. A., “ Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct,” Tissue Eng. 12(9), 2685–2693 (2006). 10.1089/ten.2006.12.2685 [DOI] [PubMed] [Google Scholar]
- 158. Nashimoto Y. et al. , “ Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid,” Biomaterials 229, 119547 (2020). 10.1016/j.biomaterials.2019.119547 [DOI] [PubMed] [Google Scholar]
- 159. Greco Song H. H., Rumma R. T., Ozaki C. K., Edelman E. R., and Chen C. S., “ Vascular tissue engineering: Progress, challenges, and clinical promise,” Cell Stem Cell 22(4), 608 (2018). 10.1016/j.stem.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pimentel C. R. et al. , “ Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network,” Acta Biomater. 65, 174–184 (2018). 10.1016/j.actbio.2017.10.047 [DOI] [PubMed] [Google Scholar]
- 161. Gong J. et al. , “ Complexation-induced resolution enhancement of 3D-printed hydrogel constructs,” Nat. Commun. 11(1), 1267 (2020). 10.1038/s41467-020-14997-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Tomasina C., Bodet T., Mota C., Moroni L., and Camarero-Espinosa S., “ Bioprinting vasculature: Materials, cells and emergent techniques,” Materials 12(17), 2701 (2019). 10.3390/ma12172701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang Y. et al. , “ Recent advances in 3D bioprinting of vascularized tissues,” Mater. Des. 199, 109398 (2021). 10.1016/j.matdes.2020.109398 [DOI] [Google Scholar]
- 164. Lee K. Y. and Mooney D. J., “ Alginate: Properties and biomedical applications,” Prog. Polym. Sci. (Oxford) 37(1), 106–126 (2012). 10.1016/j.progpolymsci.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kang Y. and Chang J., “ Channels in a porous scaffold: A new player for vascularization,” Regen. Med. 13(6), 705–715 (2018). 10.2217/rme-2018-0022 [DOI] [PubMed] [Google Scholar]
- 166. Miller J. S. et al. , “ Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues,” Nat. Mater. 11(9), 768–774 (2012). 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Negrini N. C., Bonnetier M., Giatsidis G., Orgill D. P., Farè S., and Marelli B., “ Tissue-mimicking gelatin scaffolds by alginate sacrificial templates for adipose tissue engineering,” Acta Biomater. 87, 61–75 (2019). 10.1016/j.actbio.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 168. Massa S. et al. , “ Bioprinted 3D vascularized tissue model for drug toxicity analysis,” Biomicrofluidics 11(4), 44109 (2017). 10.1063/1.4994708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tseng T.-C., Hsieh F.-Y., Theato P., Wei Y., and Hsu S., “ Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs,” Biomaterials 133, 20–28 (2017). 10.1016/j.biomaterials.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 170. Shao L. et al. , “ Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks,” Bio-Des. Manuf 3(1), 30–39 (2020). 10.1007/s42242-020-00062-y [DOI] [Google Scholar]
- 171. Wang Z., Lee S. J., Cheng H.-J., Yoo J. J., and Atala A., “ 3D bioprinted functional and contractile cardiac tissue constructs,” Acta Biomater. 70, 48 (2018). 10.1016/j.actbio.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Teixeira F. C., Chaves S., Torres A. L., Barrias C. C., and Bidarra S. J., “ Engineering a vascularized 3D hybrid system to model tumor-stroma interactions in breast cancer,” Front. Bioeng. Biotechnol. 9, 647031 (2021). 10.3389/fbioe.2021.647031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kwak T. J. and Lee E., “ In vitro modeling of solid tumor interactions with perfused blood vessels,” Sci. Rep. 10(1), 1–9 (2020). 10.1038/s41598-020-77180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Takahashi Y., Sekine K., Kin T., Takebe T., and Taniguchi H., “ Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation,” Cell Rep. 23(6), 1620–1629 (2018). 10.1016/j.celrep.2018.03.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pham M. T. et al. , “ Generation of human vascularized brain organoids,” Neuroreport 29(7), 588–593 (2018). 10.1097/WNR.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Shirure V. S., Hughes C. C. W., and George S. C., “ Engineering vascularized organoid-on-a-chip models,” Annu. Rev. Biomed. Eng. (published online). 10.1146/annurev-bioeng-090120-094330 [DOI] [PubMed] [Google Scholar]
- 177. Osaki T., Sivathanu V., and Kamm R. D., “ Engineered 3D vascular and neuronal networks in a microfluidic platform,” Sci. Rep. 8(1), 5168 (2018). 10.1038/s41598-018-23512-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Homan K. A. et al. , “ Flow-enhanced vascularization and maturation of kidney organoids in vitro,” Nat. Methods 16(3), 255–262 (2019). 10.1038/s41592-019-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zhang S., Wan Z., and Kamm R. D., “ Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature,” Lab on a Chip 21(3), 473–488 (2021). 10.1039/D0LC01186J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Paek J. et al. , “ Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues,” ACS Nano 13(7), 7627–7643 (2019). 10.1021/acsnano.9b00686 [DOI] [PubMed] [Google Scholar]
- 181. Maiullari F. et al. , “ A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes,” Sci. Rep. 8(1), 13532 (2018). 10.1038/s41598-018-31848-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Hann S. Y. et al. , “ Recent advances in 3D printing: Vascular network for tissue and organ regeneration,” Translational Res. 211, 46–63 (2019). 10.1016/j.trsl.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Maina R. M. et al. , “ Generating vascular conduits: From tissue engineering to three-dimensional bioprinting,” Innov. Surg. Sci. 3(3), 203–213 (2018). 10.1515/iss-2018-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gao Q., He Y., Fu J.-Z., Liu A., and Ma L., “ Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery,” Biomaterials 61, 203–215 (2015). 10.1016/j.biomaterials.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 185. Zhao H. et al. , “ Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle,” Small 14(39), 1802630 (2018). 10.1002/smll.201802630 [DOI] [PubMed] [Google Scholar]
- 186. Richards D., Jia J., Yost M., Markwald R., and Mei Y., “ 3D bioprinting for vascularized tissue fabrication,” Ann. Biomed. Eng. 45(1), 132–147 (2017). 10.1007/s10439-016-1653-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yang S., Tang H., Feng C., Shi J., and Yang J., “ The research on multi-material 3D vascularized network integrated printing technology,” Micromachines 11(3), 237 (2020). 10.3390/mi11030237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Schöneberg J. et al. , “ Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique,” Sci. Rep. 8(1), 1–13 (2018). 10.1038/s41598-018-28715-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Li S., Xiong Z., Wang X., Yan Y., Liu H., and Zhang R., “ Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology,” J. Bioact. Compat. Polym. 24(3), 249–265 (2009). 10.1177/0883911509104094 [DOI] [Google Scholar]
- 190. Zheng Z. et al. , “ 3D bioprinting of self‐standing silk‐based bioink,” Adv. Healthc. Mater. 7(6), 1701026 (2018). 10.1002/adhm.201701026 [DOI] [PubMed] [Google Scholar]
- 191. Kang H. W., Lee S. J., Ko I. K., Kengla C., Yoo J. J., and Atala A., “ A 3D bioprinting system to produce human-scale tissue constructs with structural integrity,” Nat. Biotechnol. 34(3), 312–319 (2016). 10.1038/nbt.3413 [DOI] [PubMed] [Google Scholar]
- 192. Freeman S. et al. , “ A bioink blend for rotary 3D bioprinting tissue engineered small-diameter vascular constructs,” Acta Biomater. 95, 152–164 (2019). 10.1016/j.actbio.2019.06.052 [DOI] [PubMed] [Google Scholar]
- 193. Campos D. F. D. et al. , “ Bioprinting cell-and spheroid-laden protein-engineered hydrogels as tissue-on-chip platforms,” Front. Bioeng. Biotechnol. 8, 374 (2020). 10.3389/fbioe.2020.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Skoog S. A., Goering P. L., and Narayan R. J., “ Stereolithography in tissue engineering,” J. Mater. Sci. Mater. Med. 25(3), 845–856 (2014). 10.1007/s10856-013-5107-y [DOI] [PubMed] [Google Scholar]
- 195. Magalhães L. S. S. M. et al. , “ Printing 3D hydrogel structures employing low-cost stereolithography technology,” J. Funct. Biomater. 11(1), 12 (2020). 10.3390/jfb11010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., and Xing M., “ 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances,” Bioactive Mater. 3(2), 144–156 (2018). 10.1016/j.bioactmat.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Aisenbrey E. A. et al. , “ A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair,” Macromol. Biosci. 18(2), 1700267 (2018). 10.1002/mabi.201700267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sarker M. D., Naghieh S., Sharma N. K., Ning L., and Chen X., “ Bioprinting of vascularized tissue scaffolds: Influence of biopolymer, cells, growth factors, and gene delivery,” J. Heal. Eng. 2019, 9156921. 10.1155/2019/9156921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Han X. et al. , “ Optimized vascular network by stereolithography for tissue engineered skin,” Int. J. Bioprinting 4(2), 134 (2018). 10.18063/IJB.v4i2.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Tamay D. G., Dursun Usal T., Alagoz A. S., Yucel D., Hasirci N., and Hasirci V., “ 3D and 4D printing of polymers for tissue engineering applications,” Front. Bioeng. Biotechnol. 7, 164 (2019). 10.3389/fbioe.2019.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Senatov F. S., Niaza K. V., Zadorozhnyy M. Y., Maksimkin A. V., Kaloshkin S. D., and Estrin Y. Z., “ Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds,” J. Mech. Behav. Biomed. Mater. 57, 139–148 (2016). 10.1016/j.jmbbm.2015.11.036 [DOI] [PubMed] [Google Scholar]
- 202. Anada T., Fukuda J., Sai Y., and Suzuki O., “ An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids,” Biomaterials 33(33), 8430–8441 (2012). 10.1016/j.biomaterials.2012.08.040 [DOI] [PubMed] [Google Scholar]
- 203. DiMarco R. L., Su J., Yan K. S., Dewi R., Kuo C. J., and Heilshorn S. C., “ Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids,” Integr. Biol. 6(2), 127–142 (2014). 10.1039/C3IB40188J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lee E. M. et al. , “ Effect of an oxygen‐generating scaffold on the viability and insulin secretion function of porcine neonatal pancreatic cell clusters,” Xenotransplantation 25(2), e12378 (2018)., 10.1111/xen.12378 [DOI] [PubMed] [Google Scholar]
- 205. Myasnikova D., Osaki T., Onishi K., Kageyama T., Molino B. Z., and Fukuda J., “ Synergic effects of oxygen supply and antioxidants on pancreatic β-cell spheroids,” Sci. Rep. 9(1), 1–10 (2019). 10.1038/s41598-018-38011-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Motoyama W., Sayo K., Mihara H., Aoki S., and Kojima N., “ Induction of hepatic tissues in multicellular spheroids composed of murine fetal hepatic cells and embedded hydrogel beads,” Regen. Ther. 3, 7–10 (2016). 10.1016/j.reth.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ahrens C. C., Dong Z., and Li W., “ Engineering cell aggregates through incorporated polymeric microparticles,” Acta Biomater. 62, 64–81 (2017). 10.1016/j.actbio.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 208. Kim Y., Baipaywad P., Jeong Y., and Park H., “ Incorporation of gelatin microparticles on the formation of adipose-derived stem cell spheroids,” Int. J. Biol. Macromol. 110, 472–478 (2018). 10.1016/j.ijbiomac.2018.01.046 [DOI] [PubMed] [Google Scholar]
- 209. Mihara H. et al. , “ Improved oxygen supply to multicellular spheroids using a gas-permeable plate and embedded hydrogel beads,” Cells 8(6), 525 (2019). 10.3390/cells8060525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Newland H., Eigel D., Rosser A. E., Werner C., and Newland B., “ Oxygen producing microscale spheres affect cell survival in conditions of oxygen-glucose deprivation in a cell specific manner: Implications for cell transplantation,” Biomater. Sci. 6(10), 2571–2577 (2018). 10.1039/C8BM00490K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Morais A. I. et al. , “ Electrospraying oxygen-generating microparticles for tissue engineering applications,” Int. J. Nanomedicine 15, 1173–1186 (2020). 10.2147/IJN.S237334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Tatsuoka T. and Koga N., “ Energy diagram for the catalytic decomposition of hydrogen peroxide,” J. Chem. Educ. 90(5), 633–636 (2013). 10.1021/ed400002t [DOI] [Google Scholar]
- 213. Spiess B. D., “ Perfluorocarbon emulsions as a promising technology: A review of tissue and vascular gas dynamics,” J. Appl. Physiol 106(4), 1444–1452 (2009). 10.1152/japplphysiol.90995.2008 [DOI] [PubMed] [Google Scholar]
- 214. Suvarnapathaki S., Wu X., Lantigua D., Nguyen M. A., and Camci-Unal G., “ Breathing life into engineered tissues using oxygen-releasing biomaterials,” NPG Asia Mater. 11(1), 65 (2019). 10.1038/s41427-019-0166-2 [DOI] [Google Scholar]
- 215. Jägers J., Wrobeln A., and Ferenz K. B., “ Perfluorocarbon-based oxygen carriers: From physics to physiology,” Pflugers Archiv Eur. J. Physiology 473(2), 139–150 (2021). 10.1007/s00424-020-02482-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tawfic Q. A. and Kausalya R., “ Liquid ventilation,” Oman Med. J. 26(1), 4–9 (2011). 10.5001/omj.2011.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lambert E., Gorantla V. S., and Janjic J. M., “ Pharmaceutical design and development of perfluorocarbon nanocolloids for oxygen delivery in regenerative medicine,” Nanomedicine 14(20), 2697–2712 (2019). 10.2217/nnm-2019-0260 [DOI] [PubMed] [Google Scholar]
- 218. Pilarek M., “ Liquid perfluorochemicals as flexible and efficient gas carriers applied in bioprocess engineering: An updated overview and future prospects,” Chem. Process Eng. 35(4), 463–487 (2014). 10.2478/cpe-2014-0035 [DOI] [Google Scholar]
- 219. Mayer D. and Ferenz K. B., “ Perfluorocarbons for the treatment of decompression illness: How to bridge the gap between theory and practice,” Eur. J. Appl. Physiology 119(11–12), 2421–2433 (2019). 10.1007/s00421-019-04252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. White J. C., Godsey M. E., and Bhatia S. R., “ Perfluorocarbons enhance oxygen transport in alginate‐based hydrogels,” Polym. Adv. Technol. 25(11), 1242–1246 (2014). 10.1002/pat.3296 [DOI] [Google Scholar]
- 221. Wijekoon A., Fountas-Davis N., and Leipzig N. D., “ Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing,” Acta Biomater. 9(3), 5653–5664 (2013). 10.1016/j.actbio.2012.10.034 [DOI] [PubMed] [Google Scholar]
- 222. Li H., Wijekoon A., and Leipzig N. D., “ Encapsulated neural stem cell neuronal differentiation in fluorinated methacrylamide chitosan hydrogels,” Ann. Biomed. Eng. 42(7), 1456–1469 (2014). 10.1007/s10439-013-0925-0 [DOI] [PubMed] [Google Scholar]
- 223. Patil P. S. et al. , “ Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability,” Acta Biomater. 36, 164–174 (2016). 10.1016/j.actbio.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Patil P. S. and Leipzig N. D., “ Fluorinated methacrylamide chitosan sequesters reactive oxygen species to relieve oxidative stress while delivering oxygen,” J. Biomed. Mater. Res. - Part A 105(8), 2368–2374 (2017). 10.1002/jbm.a.36079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Patil P. S. et al. , “ Fluorinated methacrylamide chitosan hydrogel dressings enhance healing in an acute porcine wound model,” PLoS One 13(9), e0203371 (2018). 10.1371/journal.pone.0203371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Patil P. S. et al. , “ Fluorinated methacrylamide chitosan hydrogel dressings improve regenerated wound tissue quality in diabetic wound healing,” Adv. Wound Care 8(8), 374–385 (2019). 10.1089/wound.2018.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Akula S., Brosch I. K., and Leipzig N. D., “ Fluorinated methacrylamide chitosan hydrogels enhance cellular wound healing processes,” Ann. Biomed. Eng. 45(11), 2693–2702 (2017). 10.1007/s10439-017-1893-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no new data were created or analyzed in this article.