Abstract
Estrogen-induced progression through G1 phase of the cell cycle is preceded by increased expression of the G1-phase regulatory proteins c-Myc and cyclin D1. To investigate the potential contribution of these proteins to estrogen action, we derived clonal MCF-7 breast cancer cell lines in which c-Myc or cyclin D1 was expressed under the control of the metal-inducible metallothionein promoter. Inducible expression of either c-Myc or cyclin D1 was sufficient for S-phase entry in cells previously arrested in G1 phase by pretreatment with ICI 182780, a potent estrogen antagonist. c-Myc expression was not accompanied by increased cyclin D1 expression or Cdk4 activation, nor was cyclin D1 induction accompanied by increases in c-Myc. Expression of c-Myc or cyclin D1 was sufficient to activate cyclin E-Cdk2 by promoting the formation of high-molecular-weight complexes lacking the cyclin-dependent kinase inhibitor p21, as has been described, following estrogen treatment. Interestingly, this was accompanied by an association between active cyclin E-Cdk2 complexes and hyperphosphorylated p130, identifying a previously undefined role for p130 in estrogen action. These data provide evidence for distinct c-Myc and cyclin D1 pathways in estrogen-induced mitogenesis which converge on or prior to the formation of active cyclin E-Cdk2-p130 complexes and loss of inactive cyclin E-Cdk2-p21 complexes, indicating a physiologically relevant role for the cyclin E binding motifs shared by p130 and p21.
Estrogenic steroids elicit mitogenic responses in a variety of cell types, particularly those of female reproductive tissues, including uterus and mammary gland tissues. In addition, estrogens have well-described mitogenic actions on neoplastic breast epithelial cells both in vivo (55) and in vitro (25), and this effect has been linked to the established role of estrogens in the development and progression of the majority of human breast cancers (16). Estrogenic steroids, e.g., 17β-estradiol (E2), stimulate resting (G0-phase) cells to enter the cell cycle and accelerate G1-S-phase progression (23, 58). Advances in the understanding of molecular mechanisms controlling cell cycle progression (31, 50, 51, 66) have identified cyclin-dependent kinases (CDKs) as potential targets of E2-induced mitogenesis (2, 12, 39, 42).
Sensitivity to mitogenic stimulation is limited to G1 phase of the cell cycle, transit through which is regulated by the activities of Cdk4, Cdk6, and Cdk2. These CDKs are activated by cyclin binding: Cdk4 and Cdk6 by D-type cyclins (50) and Cdk2 by cyclin E (22). Additional control of cyclin-CDK activity is achieved by phosphorylation/dephosphorylation of specific residues conserved among CDKs and by interaction with two families of CDK inhibitors: the INK4 family, of which p16INK4A is prototypic, and the p21WAF1,CIP1,SDI1/p27KIP1/p57KIP2 family (reviewed in references 31 and 51). Other factors, such as the activity of Cdc25 phosphatases that catalyze the removal of inhibitory phosphates on CDKs (31), identify a further degree of complexity in CDK regulation. G1-phase progression induced by a variety of mitogens is associated with specific effects on these CDK regulatory mechanisms (38, 49). Current evidence suggests that G1-phase cyclin-CDK complexes promote S-phase entry by phosphorylating key protein substrates that include pRB (the product of the retinoblastoma susceptibility gene) and other members of the pocket protein family, p107 and p130. Hypophosphorylated pocket proteins bind and repress the transcriptional activity of the E2F/DP family of proteins, and phosphorylation of these pocket proteins by CDKs releases E2F/DP, with consequent activation of transcription of genes whose products are required for S-phase progression (46, 66).
E2-induced G1-S-phase progression in MCF-7 breast cancer cells has recently been linked to increased cyclin D1 expression, cyclin D1-Cdk4 complex formation, and cyclin D1-Cdk4 activation (2, 12, 39, 42), suggesting that cyclin D1 may mediate E2 effects. This is supported by studies demonstrating that overexpression of cyclin D1 in breast cancer cells is sufficient to overcome antiestrogen-induced G1-phase arrest (67) and also by the prevention of E2-induced G1-S-phase progression following microinjection of cyclin D1 antibodies or the Cdk4 inhibitor p16INK4A (protein or cDNA) (26). However, mice carrying a null mutation of both cyclin D1 alleles exhibit normal mammary gland ductal development and pregnancy-related uterine hyperplasia (11, 53). These processes are E2 dependent, indicating the presence of cyclin D1-independent mechanisms by which E2 can stimulate cell proliferation.
Another target of estrogen action on cell proliferation is the proto-oncogene product c-Myc, which is rapidly induced in target cells following E2 treatment (10, 32). c-Myc antisense oligonucleotides inhibit E2-stimulated breast cancer cell proliferation (64), and therefore c-Myc is likely to play a key role in estrogen action. In fibroblasts, c-Myc is both necessary and sufficient for G1-S-phase progression (17). In these cells, activation of conditional alleles of c-myc is followed by the activation of both cyclin D1-Cdk4 and cyclin E-Cdk2 (36, 48, 56). A number of mechanisms have been defined for cyclin E-Cdk2 activation by c-Myc and include conversion of cyclin E-Cdk2 complexes to forms that can be activated by Cdc25 phosphatase (56), an increase in cyclin E protein levels (20, 36), and prevention of the association between the CDK inhibitor p27 and cyclin E-Cdk2 (36, 63). In MCF-7 breast cancer cells, E2 treatment also activates cyclin E-Cdk2 (12, 39, 42). We and others have presented evidence that activation of cyclin E-Cdk2 results from the failure of such complexes to bind the CDK inhibitor p21 (39, 42). Active cyclin E-Cdk2 complexes induced by E2 in MCF-7 cells are relatively deficient in both p21 and p27 (42). Furthermore, following E2 treatment there is a decrease in inhibitory activity toward cyclin E-Cdk2. This inhibitory activity is predominantly due to p21, not p27 (39, 42), and is accompanied by a decrease in the ability of p21 to associate with recombinant cyclin E-Cdk2 (42). While the mechanisms underlying the redistribution of p21 are undefined, there are parallels with the effect of c-Myc on p27 and cyclin E-Cdk2 association. It is therefore possible that E2-induced activation of cyclin E-Cdk2 via p21 redistribution is mediated by the preceding increase in c-Myc expression.
To evaluate the potential contributions of c-Myc and cyclin D1 to the proliferative effect of E2, we constructed MCF-7 cell lines that expressed either protein under the control of the Zn-inducible metallothionein promoter. Zn-induced expression of c-Myc or cyclin D1 was, like that of E2, sufficient to promote S-phase entry in cells that had been previously arrested in G1 phase by the antiestrogen ICI 182780. Expression of c-Myc or cyclin D1 also mimicked the effect of E2 on activation of cyclin E-Cdk2 via formation of active cyclin E-Cdk2-p130 complexes at the expense of inactive cyclin E-Cdk2-p21 complexes.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies (in parentheses) directed against the following proteins were used: c-Myc (9E10; American Type Culture Collection, Manassas, Va.), cyclin D1 (DCS-6; Novacastra Laboratories Ltd., Newcastle-upon-Tyne, United Kingdom), cyclin E (HE12; Santa Cruz Biotechnology Inc., Santa Cruz, Calif.), pRB (G3-245; PharMingen, San Diego, Calif.), p21 (catalog no. C24420; Transduction Laboratories, Lexington, Ky.), p27 (catalog no. K25020, Transduction Laboratories), and glutathione S-transferase (GST) (B-14; Santa Cruz Biotechnology).
Rabbit polyclonal antibodies against cyclin E (C-19), Cdk4 (H-22), Cdk2 (M2), p21 (C-19), p107 (C-18), and p130 (C-20) and their corresponding immunogenic peptides were obtained from Santa Cruz Biotechnology. Rabbit antiserum to cyclin D1 has been described previously (34). A purified rabbit polyclonal antibody against a pRB-derived peptide (phosphorylated on the amino acid corresponding to Ser-780) was a gift from Y. Taya, National Cancer Center Research Institute, Tokyo, Japan, and has been recently described (21).
Plasmid construction.
Plasmid pΔMTcycD1, which has a metal-inducible metallothionein promoter (6) upstream of the cDNA sequence of human cyclin D1, has been described previously (33). The same procedure was used to clone a cDNA encoding human c-Myc into the SalI site of pΔMT (pΔMTmyc). The integrity of this construct was confirmed by sequencing the entire coding region. c-Myc cDNA was obtained from Jerry Adams, Walter and Eliza Hall Institute, Parkville, Victoria, Australia.
Transfection, cell culture, and DNA flow cytometry.
MCF-7 cells were obtained from the EG & G Mason Research Institute (Worcester, Mass.) and were maintained as previously described (57). MCF-7.7, a clonal MCF-7 cell line derived by limiting dilution (8), was transfected with either pΔMT, pΔMTmyc, or pΔMTcycD1 by electroporation or calcium phosphate precipitation procedures that have been previously described (33, 67). Expansion of individual G418-resistant colonies generated clonal cell lines containing either pΔMT (MCF-7.7mt), pΔMTmyc (MCF-7.7myc), or pΔMTcycD1 (MCF-7.7D1). Pools of G418-resistant cells were also generated by expanding multiple colonies together.
Exponentially proliferating cells were growth arrested by pretreatment for 48 h with 10 nM steroidal antiestrogen ICI 182780 {7α-[9-(4,4,5,5,5-pentafluropentylsulfinyl)nonyl]estra-1,3,5,(10)-triene-3,17β-diol; from Alan Wakeling, Zeneca Pharmaceuticals, Macclesfield, United Kingdom} and then treated with either 100 nM E2 as described previously (42) or Zn (as ZnSO4) as described previously (67). Unless otherwise indicated, the final concentration of Zn was 50 μM. Vehicle controls for E2 and Zn were absolute ethanol and water, respectively. In some experiments, the specific Cdk2 inhibitor roscovitine (Calbiochem-Novabiochem, Alexandria, New South Wales, Australia) was added directly to cell culture medium 30 min prior to the addition of either E2, Zn, or vehicle. Working dilutions of roscovitine were prepared in dimethyl sulfoxide at 1,000-fold the required final concentration in cell culture medium. Analysis of cell cycle phase distribution by DNA flow cytometry was performed as described previously (67), with minor modifications: the final concentration of ethidium bromide was 50 μg/ml, mithramycin was omitted, and RNase A was added to a final concentration of 0.4 mg/ml 1 to 24 h prior to analysis.
Immunoblotting, immunoprecipitation, and protein kinase assays.
Immunoblotting and immunoprecipitation were performed as described previously (42). Kinase assays for Cdk4 and cyclin E-associated activity were performed by the methods described previously (42), with minor modifications to the Cdk4 assay as follows. Cdk4 complexes were immunoprecipitated by incubating lysates containing 400 μg of protein with 5 μl of a rabbit polyclonal Cdk4 antibody (H-22; Santa Cruz Biotechnology) for 1 h at 4°C. The complexes were recovered by the addition of 7.5 μl of protein A-Sepharose beads (Zymed, San Francisco, Calif.) per sample and further incubation for 30 min at 4°C. The final kinase reaction mixture contained 10 μg of bovine serum albumin.
Gel filtration.
Cell lysates were fractionated on a HiLoad 16/60 Superdex 200 column (Pharmacia Biotech, Uppsala, Sweden) as previously described (42). Proteins were eluted at 1.2 ml/min at 4°C in a buffer consisting of 20 mM HEPES (pH 7.5), 250 mM NaCl, 1 mM EDTA, 0.1% (vol/vol) β-mercaptoethanol, and 0.01% (vol/vol) Tween 20. The column void volume was ∼45 ml, and 10 3-ml fractions were collected between 55 and 84 ml (termed fractions 1 to 10). Column calibration was performed as described previously (42).
Binding of p21 to recombinant cyclin E-Cdk2.
Assays designed to determine the ability of p21 in cell lysates to bind to recombinant GST-cyclin E-Cdk2 were performed by incubating either recombinant GST-cyclin E-Cdk2 complexes (42) or GST with cell lysates for either 2 h at 4°C or 30 min at 30°C. GST-cyclin E-Cdk2 complexes were retrieved with glutathione-agarose beads added for 1 h at 4°C and washed three times with lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride, 200 μM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM NaF, 1 mM dithiothreitol). p21 bound to recombinant GST-cyclin E-Cdk2 was detected following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
RESULTS
Antiestrogen-induced G1-phase arrest can be reversed by inducible expression of either c-Myc or cyclin D1.
E2-induced G1-S-phase progression in MCF-7 cells is associated with increased expression of c-Myc (10, 42) and cyclin D1 (2, 12, 39, 42). To assess the ability of either protein to promote G1-S-phase progression, MCF-7.7 cell lines that contained stably integrated c-Myc cDNA (MCF-7.7myc) or cyclin D1 cDNA (MCF-7.7D1) under the control of the Zn-inducible metallothionein promoter were derived. Pooled and clonal cell lines were growth arrested by 48 h of pretreatment with the steroidal antiestrogen ICI 182780 and tested for inducible expression of c-Myc and cyclin D1 following treatment with 50 μM Zn. For both MCF-7.7myc and MCF-7.7D1 cell lines, there was a wide range of both basal and Zn-inducible expression of the exogenous proteins (Fig. 1A). Zn treatment had no detectable effect on c-Myc or cyclin D1 expression in control cell lines transfected with vector alone (MCF-7.7mt).
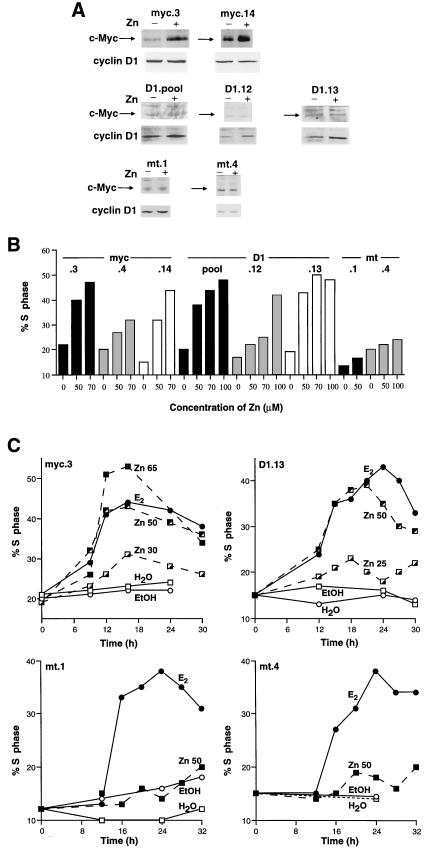
FIG. 1.
Generation of MCF-7 cell lines with Zn-inducible c-Myc or cyclin D1. MCF-7.7 cell lines stably transfected with the Zn-inducible pΔMT vector containing c-Myc cDNA (myc), cyclin D1 cDNA (D1), or no cDNA (mt) were growth arrested for 48 h with 10 nM antiestrogen ICI 182780. (A) Cells were treated at time zero with either 50 μM Zn (+) or vehicle (−). After 6 to 8 h, cell lysates were prepared and immunoblotted with antibodies against c-Myc and cyclin D1. (B) Cells were treated at time zero with the indicated concentration (micromolar) of Zn. Cells were harvested (18 h for myc cells; 21 h for D1 and mt cells) and stained for DNA content, and the proportion of cells in S phase was determined by flow cytometry. (C) Cells were treated at time zero with either the indicated concentration (micromolar) of Zn, 100 nM 17β-estradiol (E2) or vehicle (ethanol [EtOH]). At intervals thereafter, cells were harvested and stained for DNA content, and the proportion of cells in S phase was determined by flow cytometry.
The ability of c-Myc or cyclin D1 to rescue cells arrested in G1 phase by ICI 182780 was then tested in multiple cell lines. Induced expression of c-Myc or cyclin D1 in these cell lines was sufficient to promote G1-S-phase progression (Fig. 1B). In all cell lines, there was a good correlation between ectopic protein expression and S-phase entry (Fig. 2B and data not shown). The kinetics of S phase entry were studied in the clonal cell lines MCF-7.7D1.13 (D1.13) and MCF-7.7myc.3 (myc.3) because these cell lines had the lowest basal expression of c-Myc and cyclin D1, and high expression of the ectopic genes was achieved with relatively low concentrations of Zn (Fig. 2B). Following E2 or Zn treatment of D1.13 cells, there were substantial increases in the proportion of cells in S phase by 15 to 16 h and maximum levels were reached between 21 and 24 h (Fig. 1C). The kinetics of S-phase entry were similar in myc.3 cells, although S-phase entry was somewhat earlier, with substantial increases in the proportion of cells in S phase by 12 h and maximum levels reached at 16 to 18 h. Interestingly, the doubling time for all myc cell lines was less than that for D1 and mt cell lines (data not shown), which may indicate slightly enhanced cell proliferation due to leaky c-Myc expression from the metallothionein promoter. As described above, Zn-induced S-phase entry was concentration dependent in D1.13 and myc.3 cells and in both, 50 μM Zn stimulated degrees of S-phase entry similar to that induced by E2 (Fig. 1C). E2 treatment of control cell lines (mt.1 and mt.4) caused an increase in the proportion of cells in S phase similar to that in the other cell lines, but 50 μM Zn treatment had little effect on G1-S-phase progression (Fig. 1C), consistent with the negligible effect of Zn on c-Myc and cyclin D1 protein expression in these control clones (Fig. 1A). Unless stated otherwise, subsequent experiments used the clonal MCF-7.7 cell lines myc.3, D1.13, and mt.4 and rescue from ICI 182780 arrest with either 50 μM Zn or 100 nM E2.
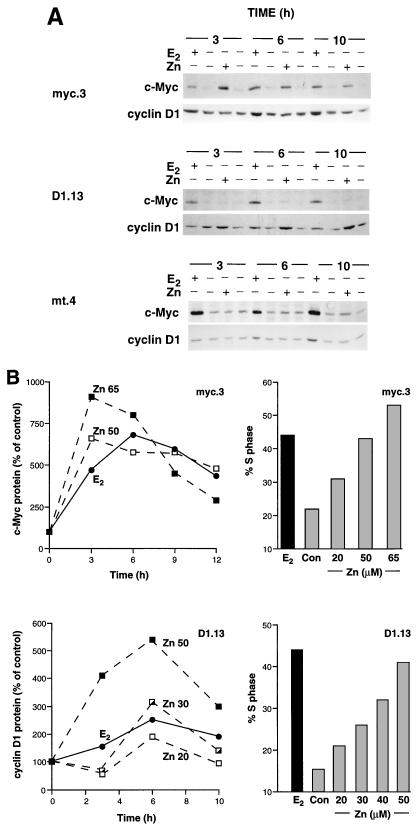
FIG. 2.
c-Myc and cyclin D1 protein expression following E2 treatment or Zn induction of c-Myc or cyclin D1. Three of the clonal MCF-7.7 cell lines used for Fig. 1 (myc.3, D1.13, and mt.4) were growth arrested with 10 nM ICI 182780 for 48 h. (A) Cells were treated at time zero with 50 μM Zn or 100 nM E2 (+) or with vehicle (−). Whole-cell lysates were prepared at intervals thereafter (shown in hours). Lysates were immunoblotted with antibodies against c-Myc and cyclin D1. (B) Cells were treated at time zero with either the indicated concentration (micromolar) of Zn, 100 nM E2, or vehicle (Con). At intervals thereafter, cell lysates were prepared and immunoblotted with antibodies against c-Myc or cyclin D1. Autoradiographs were quantitated by densitometry and expressed relative to time-matched controls. After 18 h (myc.3) or 21 h (D1.13), cells were harvested and stained for DNA content, and the proportion of cells in S phase was determined by flow cytometry. Data for protein and S phase are from the same experiment.
Increased expression of c-Myc failed to induce cyclin D1, and vice versa.
c-Myc has been proposed to either increase (5), decrease (20, 37), or have no effect on (18, 19, 54) cyclin D1 gene expression in fibroblasts. E2-induced expression of c-Myc protein by 30 to 120 min (42, 64) and cyclin D1 by 120 to 240 min (2, 12, 39, 42) in MCF-7 cells is consistent with the possibility that E2 induction of c-Myc is a prerequisite for expression of cyclin D1. This was investigated by comparing the temporal changes in expression of these proteins during G1-phase progression following E2 treatment with their expression following Zn-induced expression of either c-Myc or cyclin D1. E2 treatment increased expression of c-Myc and cyclin D1 in all cell lines examined (Fig. 2A), in agreement with previously published data (2, 12, 39, 42, 64). Changes in both cyclin D1 levels and the proportion of cells in S phase following E2 treatment were smaller than we have reported previously (42), probably reflecting less marked cell cycle synchrony in these clonal cell lines. Zn induction of c-Myc in myc.3 cells had no effect on the expression of cyclin D1 from 3 to 24 h (Fig. 2A and data not shown). Similarly, Zn-induced expression of cyclin D1 in D1.13 cells had no effect on the expression of c-Myc from 3 to 24 h (Fig. 2A and data not shown). Furthermore, induced expression of c-Myc or cyclin D1 did not affect the expression of the other gene product in any other MCF-7.7 cell lines examined (Fig. 1A and data not shown).
The concentration-dependent induction of c-Myc and cyclin D1 by E2 or Zn and the degree of S-phase entry were examined in representative cell lines. Treatment of myc.3 cells with 100 nM E2 or 50 μM Zn resulted in similar levels of both S-phase entry (Fig. 1C and 2B) and c-Myc protein expression (Fig. 2). Similarly, treatment of D1.13 cells with E2 or 50 μM Zn resulted in similar levels of S-phase entry (Fig. 1C and 2B). However, in marked contrast to the situation with c-Myc, a greater than twofold-higher level of cyclin D1 protein was required following Zn treatment to elicit the same degree of S-phase entry as that induced by E2 treatment (Fig. 2). When Zn concentrations were adjusted to induce a level of cyclin D1 protein expression similar to that induced by 100 nM E2, i.e., 30 μM Zn, the increase in S phase was only ∼40% of that induced by E2 (Fig. 2B). These data are consistent with a model of E2 action in which E2-induced expression of c-Myc, but not cyclin D1, is sufficient to account quantitatively for the subsequent S-phase entry. However, it is clear that the E2-induced expression of cyclin D1 can still make a substantial contribution to S-phase entry.
Cdk4 is activated by induction of cyclin D1 but not c-Myc.
Although increased expression of c-Myc was without effect on cyclin D1 expression, it is possible that the c-Myc pathway can activate cyclin D1-associated CDKs (56). The major contribution to cyclin D1-associated kinase activity in MCF-7 cells is from cyclin D1-Cdk4 complexes since in these cells cyclin D1-Cdk2 complexes are inactive and cyclin D1-Cdk6 complexes are in low abundance (59). E2 treatment of all cell lines resulted in similar increases in the level of Cdk4 activity (Fig. 3A and data not shown). Zn treatment of D1.13 cells, but not myc.3 cells, was accompanied by early activation of Cdk4 (Fig. 3A), paralleling the changes in cyclin D1 protein expression in these cell lines (Fig. 2A). Similarly, Cdk4-specific phosphorylation of pRB detected by immunoblot analysis with an antibody specific for a Ser-780 Cdk4 phosphorylation site on pRB (21) was substantially increased following Zn treatment of D1.13 cells (Fig. 3B). In contrast, in both myc.3 and mt.4 cells there were only small changes in Ser-780 pRB phosphorylation following Zn treatment (Fig. 3B), indicating that Zn treatment had minor effects on this parameter and c-Myc expression had no effect. E2 treatment of all cell lines resulted in similar levels of Cdk4-specific phosphorylation of pRB at 16 h (Fig. 3B).
FIG. 3.
Cdk4 activity following E2 treatment or Zn induction of c-Myc or cyclin D1. The experimental design was as described for Fig. 2A. (A) Cdk4 immunoprecipitates were assayed for kinase activity toward a GST-pRB773-928 substrate. Autoradiographs were quantitated by densitometry and expressed relative to time-matched controls. E2 treatment of all cell lines resulted in similar levels of Cdk4 activity and is represented by results from myc.3 cells. Points shown represent the means of two independent experiments. (B) Total cell lysates were immunoblotted with antibodies against a pRB-derived phosphopeptide that contains a Cdk4-specific target (phospho-Ser 780). The immunoreactive band labeled with an asterisk is nonspecific since it was not detected in pRB immunoprecipitates (data not shown).
Induction of c-Myc or cyclin D1 leads to activation of cyclin E-Cdk2 and hyperphosphorylation of pocket proteins.
The effect of c-Myc or cyclin D1 induction on cyclin E-Cdk2 activity was next examined since both have been reported to activate cyclin E-Cdk2 (34, 36, 48, 56). Activation of cyclin E-Cdk2 occurs relatively early after E2 treatment (12, 39, 42), suggesting a particular importance for this kinase in E2-induced G1-S-phase progression. E2 treatment of all cell lines and Zn induction of c-Myc or cyclin D1 were followed by activation of cyclin E-Cdk2, beginning with minor increases at 3 h (∼20% [Fig. 4A]) and increasing thereafter. Cyclin E-Cdk2 activity reached levels ∼4-fold above control levels at 16 h in cells treated with E2 and ∼3.5-fold above control levels at 16 h following Zn induction of c-Myc (Fig. 4A). In contrast, cyclin E-Cdk2 activity reached maximum levels at 6 h (∼3-fold above control levels) following Zn induction of cyclin D1 and thereafter remained constant (Fig. 4A). Zn treatment of mt.4 control cells had little effect on cyclin E-Cdk2 activity. These results indicate that the E2-activated c-Myc and cyclin D1 pathways converge at or prior to cyclin E-Cdk2 activation.
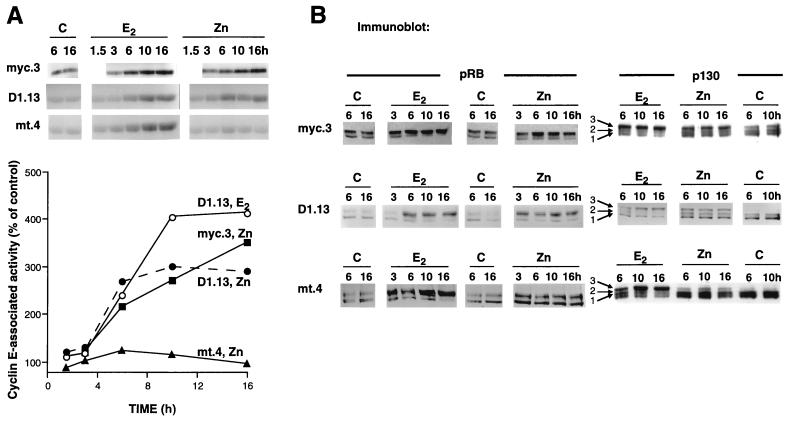
FIG. 4.
Cyclin E-Cdk2 activation and hyperphosphorylation of pocket proteins following E2 or Zn treatment. The experimental design was as described for Fig. 2A. Control lanes (C) represent results from cells treated with vehicle. (A) Cyclin E immunoprecipitates were assayed for kinase activity toward histone H1 substrate. A 1.5-h time point is included for D1.13 and mt.4 cells. For each cell line, the results shown are from the same autoradiograph. Autoradiographs were quantitated by densitometry, and results are expressed relative to those for time-matched controls. E2 treatment of all cell lines resulted in similar levels of cyclin E-associated kinase activity and is represented by results for D1.13 cells. Points shown for 3 to 16 h represent the means of two independent experiments. (B) Cell lysates were immunoblotted with either pRB or p130 antibodies. Three distinct phosphorylated species of p130 are indicated. For each cell line and antibody the results shown are from the same autoradiograph.
Since pocket proteins are in vivo substrates for G1 CDKs, we next examined the phosphorylation of pRB, p130, and p107 by immunoblotting following E2 treatment or Zn induction of c-Myc or cyclin D1. As expected from previous studies (42, 65), a significant proportion of pRB was hypophosphorylated (most mobile form) following antiestrogen pretreatment (Fig. 4B). p130 was mainly present as hypophosphorylated form 1 and phosphorylated form 2 (27, 28), characteristic of G0-phase cells. E2 treatment of all cell lines resulted in an increase in the total amount of hyperphosphorylated, less mobile pRB and p130 (form 3) (Fig. 4B) and an increase in the hyperphosphorylated/hypophosphorylated ratio of pRB and p130. Zn induction of c-Myc in myc.3 cells resulted in similar phosphorylation of pRB but less pronounced phosphorylation of p130 (Fig. 4B). In contrast, Zn induction of cyclin D1 in D1.13 cells resulted in earlier phosphorylation of pRB and p130 (3 to 6 h) than E2 treatment (6 to 10 h), consistent with the more rapid effects of Zn treatment on cyclin D1 protein expression (Fig. 2A), Cdk4 activity, and Cdk4-specific phosphorylation of pRB (data not shown). Similar to the effects of ectopic gene expression on pRB and p130, p107 phosphorylation was evident by 3 h in D1.13 cells, 6 h in myc.3 cells, and not at all in mt.4 cells following Zn treatment (data not shown). For all cell lines studied, E2 treatment resulted in an increased degree of p107 phosphorylation that was evident by 6 h (data not shown).
Cells subjected to E2- and c-Myc-induced, but not those subjected to cyclin D1-induced, G1-S-phase progression have similar sensitivities to inhibition by roscovitine.
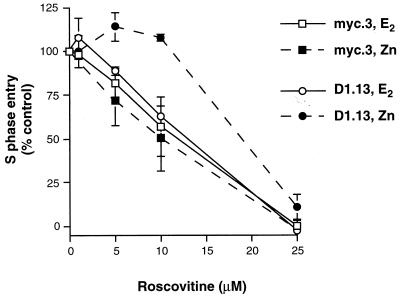
The above experiments showed that similar degrees of G1-S-phase progression following E2 treatment or ectopic expression of c-Myc or cyclin D1 were preceded by induction of similar levels of cyclin E-Cdk2 activity. The dependence of G1-S-phase progression on cyclin E-Cdk2 activity was next determined by examining sensitivity to the Cdk2-specific chemical inhibitor roscovitine (7, 29). The maximum increases in E2- or Zn-induced S-phase entry were compared following treatment with different concentrations of roscovitine. Both myc.3 and D1.13 cell lines had similar sensitivities to roscovitine inhibition of E2-induced G1-S-phase progression, with ∼40% inhibition of S-phase entry with 10 μM roscovitine and ∼100% inhibition with 25 μM roscovitine (Fig. 5). G1-S-phase progression following Zn induction of c-Myc in myc.3 cells was also inhibited with a similar sensitivity (Fig. 5). However, G1-S-phase progression following Zn induction of cyclin D1 in D1.13 cells was markedly less sensitive to roscovitine, such that concentrations of roscovitine as high as 10 μM had no inhibitory effect (Fig. 5). These results demonstrate that G1-S-phase progression stimulated by E2 and c-Myc was similarly dependent on Cdk2 activity. However, the G1-S-phase progression stimulated by cyclin D1 was markedly different, being less dependent on Cdk2 activity despite similar levels of cyclin E-Cdk2 activation. It is possible that the early increase in cyclin D1-Cdk4 activity following cyclin D1 induction (Fig. 3A) compensates for the loss of Cdk2 activity and thereby accounts for the relative resistance to roscovitine.
FIG. 5.
Inhibition of E2-, c-Myc-, or cyclin D1-induced G1-S-phase progression by the Cdk2-specific inhibitor roscovitine. The experimental design was as described for Fig. 2A except that cells were pretreated with the indicated concentration of roscovitine 30 min prior to treatment with Zn, E2, or vehicle. After 18 h (myc.3) or 21 h (D1.13), cells were harvested and stained for DNA content, and the proportion of cells in S phase was determined by flow cytometry. For each concentration of roscovitine the increase in S phase with either Zn or E2 (above the vehicle-treated control level) was expressed as a percentage of the increase in S phase with 0 μM roscovitine. Points represent the means of three (myc.3) or four to five separate experiments (D1.13), and error bars indicate the standard errors of the means.
c-Myc- and cyclin D1-induced activation of cyclin E-Cdk2 is associated with loss of p21 and association with p130.
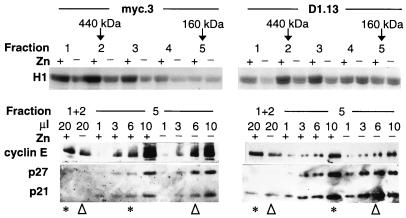
The E2-stimulated c-Myc and cyclin D1 pathways appeared to converge on or just prior to cyclin E-Cdk2 activation. Therefore, the mechanisms of cyclin E-Cdk2 activation were investigated to determine whether they were the same following c-Myc or cyclin D1 induction. In whole-cell lysates and cyclin E immunoprecipitates from myc.3 or D1.13 cells treated with E2 or Zn, there were no changes in the levels of cyclin E, Cdk2, p21, or p27 from 0 to 16 h (data not shown), consistent with observations made following E2 treatment of MCF-7 cells (39, 42). In these cells, cyclin E-Cdk2 activation is associated with the formation of high-specific-activity, high-molecular-weight cyclin E-Cdk2 complexes lacking CDK inhibitors p21 and p27 (42). Gel filtration of cell lysates was therefore performed to determine if similar changes occurred following induction of c-Myc or cyclin D1. Zn treatment of myc.3 or D1.13 cells induced an increase in cyclin E-associated kinase activity that eluted between 400 and 500 kDa (fractions 1 and 2 [Fig. 6]). Subsequent experiments demonstrated less marked changes in cyclin E-associated kinase activity eluting at predicted molecular masses higher than 500 kDa (data not shown). These changes in the elution profile of cyclin E-associated kinase activity following c-Myc or cyclin D1 expression are similar to the changes following E2 treatment of these clones (data not shown) and parental MCF-7 cells (42). The relatively greater proportion of high-molecular-weight cyclin E-associated kinase activity following expression of c-Myc compared to that following cyclin D1 expression is likely to indicate some differences in complex composition. In contrast, in all clones most of the cyclin E protein eluted at ∼160 kDa (fraction 5) [Fig. 6 and 7B and data not shown]) as previously described (42). Consequently the specific activity of the 400- to 500-kDa cyclin E complexes was ∼12-fold greater than that of the ∼160-kDa complexes, demonstrating that the activity of the total cyclin E-Cdk2 pool was due to a small number of highly active high-molecular-weight complexes.
FIG. 6.
Mechanism of activation of cyclin E-Cdk2 by E2 treatment or Zn induction of c-Myc or cyclin D1. The experimental design was as described for Fig. 2A. Lysates from myc.3 and D1.13 cells were prepared 10 h after treatment with Zn and fractionated on a HiLoad 16/60 Superdex 200 gel filtration column. Cyclin E complexes were immunoprecipitated from 3-ml fractions and then either assayed for histone (H1) kinase activity (as described for Fig. 3A) or resuspended in 20 μl of sample buffer, separated by SDS-PAGE, transferred to a nitrocellulose filter, and sequentially blotted with the indicated antibodies. Fraction 5 (which contained high levels of cyclin E) was loaded in variable amounts in order to permit comparison of the relative levels of coimmunoprecipitating proteins with those in fractions 1 and 2 (combined). Lanes containing similar levels of cyclin E protein are indicated with either an asterisk (Zn treated) or an arrowhead (vehicle treated). The elution volumes for marker proteins of known molecular weight are indicated at the top.
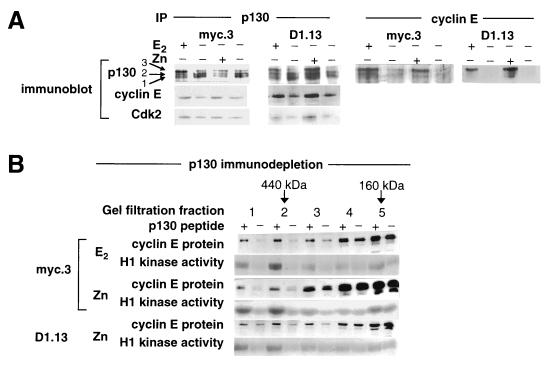
FIG. 7.
Increased association of cyclin E with p130 follows E2 treatment or Zn induction of c-Myc or cyclin D1. The experimental design was as described for Fig. 2A. Lysates were prepared 10 h after treatment. (A) Cyclin E or p130 immunoprecipitates (IP) from total cell lysates were immunoblotted with the indicated antibodies. (B) p130 was immunoprecipitated from lysates with p130 antibodies in the presence (+) or absence (−) of immunizing peptide. The supernatant was fractionated on a gel filtration column as described for Fig. 6. Representative cyclin E protein immunoblots and cyclin E histone (H1) kinase assays are shown following E2 and Zn treatment of myc.3 cells and Zn treatment of D1.13 cells. The elution volumes for marker proteins of known molecular weight are indicated.
The composition of the 400- to 500-kDa cyclin E complexes was compared with that of the relatively inactive ∼160-kDa complexes. Cyclin E immunoprecipitates were prepared from fractions 1 and 2 (400 to 500 kDa) or fraction 5 (∼160 kDa), resuspended in 20 μl of sample buffer, and then separated by SDS-PAGE. Since there was relatively little cyclin E protein eluting at 400 to 500 kDa compared to that eluting at ∼160 kDa, different amounts of fraction 5 (∼1, 3, 6, and 10 μl) were loaded on the gel to facilitate comparison of cyclin E/CDK inhibitor ratios. The relatively high level of cyclin E protein eluting at ∼160 kDa is clearly evident (compare cyclin E protein in fractions 1 and 2 with all 4 lanes from fraction 5). In myc.3 and D1.13 cells, the 400- to 500-kDa cyclin E complexes were relatively deficient in p21 and p27 compared with lanes containing similar levels of cyclin E in the ∼160-kDa complexes. These differences are likely to contribute to the high specific activity of the 400- to 500-kDa cyclin E complexes. Zn induction of c-Myc or cyclin D1 increased the levels of cyclin E eluting at 400 to 500 kDa without altering the levels of cyclin E-associated p21 or p27, indicating an increase in high-molecular-weight cyclin E complexes that were not associated with these CDK inhibitors. Consistent with this, there was an increase in the relative abundance of the more mobile, CAK (CDK-activating kinase)-phosphorylated form of cyclin E-associated Cdk2 species (13) in the 400- to 500-kDa complexes (data not shown), since CAK phosphorylation of Cdk2 is prevented by association of p21 and p27 with Cdk2 (3, 40). These changes in cyclin E complex composition were identical to the changes following E2 treatment of these clones (data not shown) and MCF-7 cells (42).
The composition of the active cyclin E-Cdk2 complexes was investigated further by examining interactions between cyclin E and proteins previously found to associate with cyclin E. Significant interactions were detected between cyclin E and the pocket protein p130. In cyclin E immunoprecipitates, p130 was present predominantly in the hyperphosphorylated form 3, with little hypophosphorylated protein detectable (Fig. 7A). Following E2 treatment, or Zn induction of c-Myc or cyclin D1, the levels of p130 associated with cyclin E increased. Consistent with these data, p130 immunoprecipitates contained increased levels of cyclin E and Cdk2 following all treatments (Fig. 7A). Taken together, these results indicated an increase in protein complexes containing cyclin E-Cdk2 and hyperphosphorylated p130. No significant interactions between cyclin E and the other pocket protein p107 or pRB were detected in similar experiments (data not shown). Cyclin E-Cdk2-p130 complexes eluted from the gel filtration column coincident with active cyclin E complexes (data not shown), suggesting that the active complexes may contain p130. Immunodepletion of p130 was sufficient to remove the majority of the cyclin E protein and cyclin E-Cdk2 activity that eluted at 400 to 500 kDa following E2 and induction of c-Myc or cyclin D1 (Fig. 7B). This finding indicates that cyclin E-p130 complexes constitute the majority of 400- to 500-kDa cyclin E complexes, and these complexes contribute most of the total cyclin E-Cdk2 activity. In summary, activation of cyclin E-Cdk2, whether by E2, c-Myc, or cyclin D1, was invariably associated with the formation of high-molecular-weight cyclin E-Cdk2 complexes that were relatively deficient in both p21 and p27 and contained p130 and CAK-phosphorylated Cdk2.
E2, c-Myc, and cyclin D1 decrease the association between p21 and recombinant cyclin E-Cdk2.
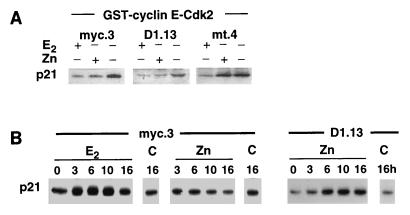
The decrease in p21 association with cyclin E-Cdk2 complexes in vivo following E2 treatment has been proposed as a major factor contributing to the relief of inhibition of cyclin E-Cdk2 (39, 42). The association between p21 and recombinant cyclin E-Cdk2 in vitro is also inhibited following E2 treatment (42) and was therefore investigated in the current paradigm. Zn induction of c-Myc or cyclin D1 was accompanied by decreased association of p21 with cyclin E-Cdk2 complexes in vivo (Fig. 6) and reduced association between p21 and recombinant GST-cyclin E-Cdk2 in vitro (Fig. 8A). Control experiments showed no association between p21 and GST, indicating that the binding was specific for cyclin E-Cdk2 (data not shown). These results indicate that decreased association of p21 with cyclin E-Cdk2 is a common activating mechanism for cyclin E-Cdk2 shared by E2, c-Myc, and cyclin D1. p21 levels do not change at the time of early activation of cyclin E-Cdk2 following E2 treatment of MCF-7 cells (42). Similarly, levels of p21 did not alter following Zn induction of c-Myc or cyclin D1 (data not shown), and therefore decreased abundance of p21 does not appear to account for the decreased association of p21 with cyclin E-Cdk2. An alternative explanation for this effect is that p21 is sequestered by other proteins and thus is unavailable for binding to cyclin E-Cdk2. It has been suggested that cyclin D1-Cdk4 performs this role following E2 treatment of MCF-7 cells (39). Examination of cyclin D1 immunoprecipitates revealed that there was increased association of cyclin D1 with p21 following E2 treatment or Zn induction of cyclin D1 but not following Zn induction of c-Myc (Fig. 8B). This finding indicates that cyclin D1, but not c-Myc, may contribute to the activation of cyclin E-Cdk2 by sequestering p21 into cyclin D1 complexes.
FIG. 8.
Effects of E2 treatment or Zn induction of c-Myc or cyclin D1 on the binding of p21 to recombinant cyclin E-Cdk2. The experimental design was as described for Fig. 2A. (A) Lysates prepared 10 h after treatment were incubated with GST-cyclin E-Cdk2 complexes or GST. GST proteins were recovered on glutathione-agarose beads and then immunoblotted for p21. (B) Cyclin D1 immunoprecipitates were immunoblotted for p21. Immunoblots of control nonimmune rabbit antiserum immunoprecipitates failed to detect p21. Control lanes (C) represent results from cells treated with vehicle. For each cell line the results shown are from the same radiograph.
DISCUSSION
E2-induced G1-phase progression can be mimicked by c-Myc or cyclin D1.
The proliferative effect of estrogens is of major importance in the development and normal physiological function of female reproductive organs and in breast cancer initiation and progression. We and others have used the estrogen-responsive human breast cancer cell line MCF-7 to investigate the underlying molecular mechanisms for the proliferative effect. This study has focused on the roles of c-Myc and cyclin D1 in this process, since both gene products can stimulate cell cycle progression (17, 33, 44, 45) and the expression of both genes is rapidly induced following E2 treatment (2, 10, 12, 39, 42). Our results demonstrate that ectopic expression of either c-Myc or cyclin D1 induced S-phase entry in MCF-7 cells previously arrested in G1 phase by pretreatment with antiestrogen. c-Myc is therefore sufficient to initiate G1-S-phase progression in this epithelial cell model, extending previous findings in other cell types. These data also indicate a potential role for c-Myc in clinical antiestrogen resistance, similar to the one we have suggested previously for cyclin D1 (67). In this model, c-Myc did not induce expression of cyclin D1 protein. Similarly, others have demonstrated that activation of conditional c-Myc alleles (MycER) does not activate cyclin D1 transcription (54), despite some conflicting earlier reports (5, 20). However, MycER activates cyclin D1-dependent CDKs in rat fibroblasts (43, 56), but the effect is relatively small in contrast to the large changes in both cyclin E-Cdk2 activity and G1-S-phase progression. In our system, Zn induction of c-Myc did not increase Cdk4 activity, and conversely, Zn induction of cyclin D1 and subsequent Cdk4 activation did not induce expression of c-Myc. These results demonstrate that E2-induced G1-phase progression is likely to be mediated by initially distinct c-Myc and cyclin D1 pathways. It remains possible that the cyclin D1 pathway upregulates the activity of the c-Myc pathway at some point other than c-Myc protein expression.
Comparison of protein expression and S-phase entry following E2 treatment or inducible expression of c-Myc or cyclin D1 suggested that the effects of E2 in these cells were quantitatively more closely mimicked by induction of c-Myc than by induction of cyclin D1, indicating that E2-induced expression of c-Myc may be sufficient for G1-S-phase progression. Consistent with a predominant role for c-Myc over cyclin D1 in E2-induced cell proliferation, S-phase entry induced by E2 or c-Myc, but not by cyclin D1, was equally sensitive to inhibition by the Cdk2 antagonist roscovitine. c-Myc expression is apparently necessary for E2-dependent G1-S-phase progression in breast cancer cells, since c-Myc antisense oligonucleotides can prevent E2-dependent MCF-7 cell proliferation (64). However, this observation does not preclude a requirement for cyclin D1 in E2- and c-Myc-induced G1-S-phase progression. In fibroblasts, cyclin D1 antibodies prevent c-Myc-induced S-phase entry (47), and the Cdk4/6 inhibitor p16 inhibits c-Myc-dependent transformation (14). Therefore, the functional consequences of c-Myc expression appear to be dependent on cyclin D1 expression. In MCF-7 cells, cyclin D1 protein levels are reduced by only 50% following antiestrogen pretreatment (42, 65) and may be sufficient for subsequent c-Myc action. Indeed, inhibition of cyclin D1 function in MCF-7 cells also inhibits E2-dependent S-phase entry (26), although it remains to be determined whether the role of cyclin D1-Cdk4 complexes involves Cdk4 activity, CDK inhibitor sequestration, or some other function.
Cyclin E-Cdk2 is activated following c-Myc or cyclin D1 expression.
Activation of cyclin E-Cdk2 is necessary for G1-S-phase progression (35, 60, 62) and is inhibited in antiestrogen-arrested MCF-7 cells by association with p21 (39, 42). Expression of c-Myc or cyclin D1 resulted in early activation of cyclin E-Cdk2, and therefore the E2-induced expression of c-Myc and cyclin D1 is likely to activate pathways that converge at or prior to this point. Activation of cyclin E-Cdk2 by E2 or following c-Myc or cyclin D1 expression was associated with decreased p21 in high-molecular-weight, high-specific-activity cyclin E-Cdk2 complexes, suggesting a common mechanism of activation involving formation of cyclin E-Cdk2 complexes deficient in p21. Further evidence for such a mechanism is provided by increased CAK-phosphorylated Cdk2 in high-specific-activity cyclin E-Cdk2 complexes since CDK inhibitors directly prevent CAK phosphorylation of Cdk2 (3, 40). CAK activity (measured as Cdk7 activity) was unchanged following E2 treatment of MCF-7 cells (42) and therefore is unlikely to account for the increase in CAK-phosphorylated Cdk2. Moreover, peptide motifs shared by p130 and p21 ensure that their binding to cyclin E-Cdk2 is mutually exclusive (1, 52), which suggests that active cyclin E-Cdk2 complexes which contain p130 are deficient in p21. Therefore, competition between proteins with these shared peptide motifs plays a major role in determining cyclin E-Cdk2 complex formation, activity, and substrate preference following E2 treatment. To our knowledge, this is the first description of competition between p21 and p130 for association with cyclin E-Cdk2 in a physiologically relevant model.
There are a number of potential mechanisms for the formation of cyclin E-Cdk2 complexes that are deficient in p21 and contain p130. These include changes to any one of the proteins involved (cyclin E, Cdk2, p21, and p130) such that cyclin E-Cdk2-p130 complex formation is favored over cyclin E-Cdk2-p21 complex formation. Our in vitro binding studies demonstrate that binding of p21 to recombinant cyclin E-Cdk2 is decreased following E2 treatment, c-Myc expression, or cyclin D1 expression. Therefore, it is likely that changes in p21 rather than changes in cyclin E-Cdk2 account for the alteration in complex formation. Furthermore, changes to p130 are unlikely to account for the alteration because binding of p21 to recombinant cyclin E-Cdk2 is not altered by p130 immunodepletion (41). These data argue that E2 treatment, c-Myc expression, or cyclin D1 expression may instead target p21 and prevent its association with cyclin E-Cdk2, for example, by phosphorylation/sequestration of p21 or decreased production or increased destruction of the pool of p21 capable of binding to cyclin E-Cdk2. Increased binding of p21 to cyclin D1-Cdk4 occurred following E2 treatment and cyclin D1 induction, and cyclin D1-Cdk4 may therefore sequester p21 from cyclin E-Cdk2. Our observation that c-Myc can promote G1-S-phase progression in the absence of an increase in Cdk4 activity may indicate that the major role for cyclin D1 in E2 action is sequestration of p21 rather than activation of Cdk4, as has been suggested by others (39). However, binding of p21 to cyclin D1-Cdk4 did not increase following c-Myc induction indicating a different activating mechanism by c-Myc. These observations are similar to those made for rat fibroblasts, in which c-Myc activated cyclin E-Cdk2 by inhibiting association with p27 and without sequestration of p27 by cyclin D1 (36, 63). c-Myc has also been reported to abrogate the inhibitory action of p21 on Cdk2 activity in fibroblasts (18). Potentially, c-Myc may target all members of the p21/p27 class of CDK inhibitors and prevent their association with cyclin E-Cdk2 by a common mechanism.
Cyclin E-Cdk2 complexes activated by E2 treatment, or expression of c-Myc or cyclin D1, are associated with p130.
The c-Myc and cyclin D1 pathways also converged on p130 phosphorylation. Following antiestrogen pretreatment, p130 was present as the faster-migrating phosphorylated forms 1 and 2 and formed complexes that contained E2F-4 but lacked cyclin E-Cdk2 (41). Similar complexes and phosphorylated forms of p130 are typical of G0-phase cells derived from populations of normal and immortalized cells and from cancer cell lines (4, 27, 28, 30, 61). Following E2 treatment, p130 was phosphorylated to the more slowly migrating form 3. This pattern is similar to that following cell cycle reentry stimulated by serum (27). These observations are consistent with earlier observations on E2 action (reviewed in reference 58) which demonstrate both recruitment of noncycling cells into the cell cycle and acceleration of G1-phase progression. Phosphorylation of p130 is likely to be due to cyclin E-Cdk2 since p130 phosphorylation coincided with both activation of cyclin E-Cdk2 and formation of cyclin E-Cdk2-p130 complexes. Cdk2 can phosphorylate p130 in vitro (28, 68), and both cyclin E-Cdk2 and cyclin A-Cdk2 are capable of phosphorylating associated p130 (15, 24, 69). The p130-containing complexes contributed substantially to cyclin E-Cdk2 histone kinase activity since p130 immunodepletion of lysates prior to gel filtration resulted in a significant diminution of cyclin E-associated kinase activity. Others have reported increases in p130-associated histone kinase activity during G1-phase progression (27, 68). However, p130 has also recently been reported to inhibit cyclin E-Cdk2 histone kinase activity (9, 69) and to redirect cyclin E-Cdk2 substrate specificity from histone to pocket protein family members (15), although the degree of phosphorylation of p130 in these studies was undefined. It is possible that phosphorylated p130 is less potent than hypophosphorylated p130 at inhibiting cyclin E-Cdk2 histone kinase activity, and this could account for the histone kinase activity associated with cyclin E-Cdk2-p130 complexes following E2 treatment and following c-Myc or cyclin D1 expression.
Finally, these results support the presence of an undefined G1-phase rate-limiting step in these cells, as the timing of S-phase entry was not closely tied to CDK activation or pocket protein phosphorylation. Cdk4 activation had increased by 3 to 6 h following cyclin D1 induction and did not increase at all following c-Myc induction. Pocket protein phosphorylation was almost complete by 3 to 6 h following cyclin D1 induction and by 10 to 16 h following either E2 treatment or c-Myc induction, but the timing of S-phase entry was approximately the same following all treatments. Conversely, cyclin E-Cdk2 activation occurred by 3 to 6 h with all treatments, but cells did not enter S phase until 9 to 12 h after cyclin E-Cdk2 activation. Taken together, these data demonstrate that S-phase entry was still delayed despite the completion of a number of known rate-limiting steps including c-Myc expression, G1-phase CDK activation, and pocket protein phosphorylation. A recent report demonstrates that cell size in fibroblasts is a requirement for S-phase entry despite c-Myc-induced activation of CDKs and hyperphosphorylation of pRB (43). A similar requirement for a critical cell size may represent the undefined G1-phase rate-limiting step identified here, and this requires further investigation.
In conclusion, we have identified that in antiestrogen-arrested MCF-7 cells, increased expression of c-Myc and that of cyclin D1 are separate events activating pathways that are initially distinct. It is likely that both of these pathways contribute to E2-induced G1-S-phase progression, and our results support a predominant role for c-Myc in mediating estrogenic actions. The convergence of these pathways on the formation of active cyclin E-Cdk2 complexes deficient in p21 highlights a fundamental role for p21 in E2 action.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) and the New South Wales State Cancer Council. Owen Prall is a recipient of a Medical Postgraduate Scholarship from the NHMRC.
We thank Boris Sarcevic for providing the recombinant cyclin E-Cdk2 proteins and Gillian Lehrbach and Alex Swarbrick for their contributions to some experimental procedures. We are indebted to the late Lisa Porter for construction of plasmid pΔMTmyc.
REFERENCES
- 1.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 3.Aprelikova O, Xiong Y, Liu E T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- 4.Cobrinik D, Whyte P, Peeper D S, Jacks T, Weinberg R A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- 5.Daksis J I, Lu R Y, Facchini L M, Marhin W W, Penn L J. Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9:3635–3645. [PubMed] [Google Scholar]
- 6.Daly R J, Harris W H, Wang D Y, Darbre P D. Autocrine production of insulin-like growth factor II using an inducible expression system results in reduced estrogen sensitivity of MCF-7 human breast cancer cells. Cell Growth Differ. 1991;2:457–464. [PubMed] [Google Scholar]
- 7.De Azevedo W F, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim S H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 8.deFazio A, Chiew Y-E, McEvoy M, Watts C K W, Sutherland R L. Antisense estrogen receptor RNA expression increases epidermal growth factor receptor expression in breast cancer cells. Cell Growth Differ. 1997;8:903–911. [PubMed] [Google Scholar]
- 9.De Luca A, MacLachlan T K, Bagella L, Dean C, Howard C M, Paolo Claudio P, Baldi A, Khalili K, Giordano A. A unique domain of pRb2/p130 acts as an inhibitor of Cdk2 kinase activity. J Biol Chem. 1997;272:20971–20974. doi: 10.1074/jbc.272.34.20971. [DOI] [PubMed] [Google Scholar]
- 10.Dubik D, Dembinski T C, Shiu R P C. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47:6517–6521. [PubMed] [Google Scholar]
- 11.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 12.Foster J S, Wimalasena J. Estrogen regulates activity of cyclin-dependent kinases and retinoblastoma protein phosphorylation in breast cancer cells. Mol Endocrinol. 1996;10:488–498. doi: 10.1210/mend.10.5.8732680. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas K, Staller P, Geisen C, Bartek J, Eilers M, Moroy T. Mutual requirement of Cdk4 and Myc in malignant transformation: evidence for cyclin D1/Cdk4 and p16INK4A as upstream regulators of Myc. Oncogene. 1997;15:179–192. doi: 10.1038/sj.onc.1201171. [DOI] [PubMed] [Google Scholar]
- 15.Hauser P J, Agrawal D, Chu B, Pledger W J. p107 and p130 associated cyclin A has altered substrate specificity. J Biol Chem. 1997;272:22954–22959. doi: 10.1074/jbc.272.36.22954. [DOI] [PubMed] [Google Scholar]
- 16.Henderson B, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation Award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 17.Henriksson M, Luscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 18.Hermeking H, Funk J O, Reichert M, Ellwart J W, Eick D. Abrogation of p53-induced cell cycle arrest by c-Myc: evidence for an inhibitor of p21WAF1/CIP1/SDI1. Oncogene. 1995;11:1409–1415. [PubMed] [Google Scholar]
- 19.Huang T S, Duyster J, Wang J Y. Biological response to phorbol ester determined by alternative G1 pathways. Proc Natl Acad Sci USA. 1995;92:4793–4797. doi: 10.1073/pnas.92.11.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen-Durr P, Meichle A, Steiner P, Pagano M, Finke K, Botz J, Wessbecher J, Draetta G, Eilers M. Differential modulation of cyclin gene expression by MYC. Proc Natl Acad Sci USA. 1993;90:3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 22.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts J M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 23.Leung B S, Potter A H. Mode of estrogen action on cell proliferation in CAMA-1 cells. II. Sensitivity of G1 phase population. J Cell Biochem. 1987;34:213–225. doi: 10.1002/jcb.240340307. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Graham C, Lacy S, Duncan A M, Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- 25.Lippman M E, Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature (London) 1975;256:592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- 26.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D–cyclin-dependent kinase–pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayol X, Garriga J, Grana X. Cell cycle-dependent phosphorylation of the retinoblastoma-related protein p130. Oncogene. 1995;11:801–808. [PubMed] [Google Scholar]
- 28.Mayol X, Garriga J, Grana X. G1 cyclin/CDK-independent phosphorylation and accumulation of p130 during the transition from G1 to G0 lead to its association with E2F-4. Oncogene. 1996;13:237–246. [PubMed] [Google Scholar]
- 29.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 30.Moberg K, Starz M A, Lees J A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan D O. Principles of CDK regulation. Nature (London) 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 32.Murphy L J, Murphy L C, Friesen H G. Estrogen induction of N-myc and c-myc proto-oncogene expression in the rat uterus. Endocrinology. 1987;120:1882–1888. doi: 10.1210/endo-120-5-1882. [DOI] [PubMed] [Google Scholar]
- 33.Musgrove E A, Lee C S L, Buckley M F, Sutherland R L. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musgrove E A, Sarcevic B, Sutherland R L. Inducible expression of cyclin D1 in T-47D human breast cancer cells is sufficient for CDK2 activation and pRB hyperphosphorylation. J Cell Biochem. 1996;60:363–378. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C363::AID-JCB8%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Roger I, Solomon D L, Sewing A, Land H. Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27(Kip1) binding to newly formed complexes. Oncogene. 1997;14:2373–2381. doi: 10.1038/sj.onc.1201197. [DOI] [PubMed] [Google Scholar]
- 37.Philipp A, Schneider A, Vasrik I, Finke K, Xiong Y, Beach D, Alitalo K, Eilers M. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol. 1994;14:4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pines J. Cyclins and cyclin-dependent kinases: a biochemical view. Biochem J. 1995;308:697–711. doi: 10.1042/bj3080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Planas-Silva M D, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 41.Prall, O. W. J. Unpublished data.
- 42.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 43.Pusch O, Bernaschek G, Eilers M, Hengstschlager M. Activation of c-Myc uncouples DNA replication from activation of G1-cyclin dependent kinases. Oncogene. 1997;15:649–656. doi: 10.1038/sj.onc.1201236. [DOI] [PubMed] [Google Scholar]
- 44.Quelle D E, Ashmun R A, Shurtleff S A, Kato J-Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 45.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riley D J, Lee E Y-H P, Lee W-H. The retinoblastoma protein: more than a tumor suppressor. Annu Rev Cell Biol. 1994;10:1–29. doi: 10.1146/annurev.cb.10.110194.000245. [DOI] [PubMed] [Google Scholar]
- 47.Roussel M F, Theodoras A M, Pagano M, Sherr C J. Rescue of defective mitogenic signaling by D-type cyclins. Proc Natl Acad Sci USA. 1995;92:6837–6841. doi: 10.1073/pnas.92.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 49.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 50.Sherr C J. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 51.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 52.Shiyanov P, Bagchi S, Adami G, Kokontis J, Hay N, Arroyo M, Morozov A, Raychaudhuri P. p21 disrupts the interaction between cdk2 and the E2F-p130 complex. Mol Cell Biol. 1996;16:737–744. doi: 10.1128/mcb.16.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 54.Solomon D L, Philipp A, Land H, Eilers M. Expression of cyclin D1 mRNA is not upregulated by Myc in rat fibroblasts. Oncogene. 1995;11:1893–1897. [PubMed] [Google Scholar]
- 55.Soule H D, McGrath C M. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- 56.Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, Bartek J, Eilers M. Identification of a Myc-dependent step during the formation of active G1 cyclin-cdk complexes. EMBO J. 1995;14:4814–4826. doi: 10.1002/j.1460-2075.1995.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland R L, Hall R E, Taylor I W. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983;43:3998–4006. [PubMed] [Google Scholar]
- 58.Sutherland R L, Reddel R R, Green M D. Effects of oestrogens on cell proliferation and cell cycle kinetics. A hypothesis on the cell cycle effects of antioestrogens. Eur J Cancer Clin Oncol. 1983;19:307–318. doi: 10.1016/0277-5379(83)90127-x. [DOI] [PubMed] [Google Scholar]
- 59.Sweeney K J, Swarbrick A, Sutherland R L, Musgrove E A. Lack of relationship between CDK activity and G1 cyclin expression in breast cancer cells. Oncogene. 1997;16:2865–2878. doi: 10.1038/sj.onc.1201814. [DOI] [PubMed] [Google Scholar]
- 60.Tsai L H, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- 61.Vairo G, Livingston D M, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 62.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 63.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 64.Watson P H, Pon R T, Shiu R P C. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer Res. 1991;51:3996–4000. [PubMed] [Google Scholar]
- 65.Watts C K W, Brady A, Sarcevic B, deFazio A, Musgrove E A, Sutherland R L. Antiestrogen inhibition of cell cycle progression in breast cancer cells is associated with inhibition of cyclin-dependent kinase activity and decreased retinoblastoma protein phosphorylation. Mol Endocrinol. 1995;9:1804–1813. doi: 10.1210/mend.9.12.8614416. [DOI] [PubMed] [Google Scholar]
- 66.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 67.Wilcken N R C, Prall O W J, Musgrove E A, Sutherland R L. Inducible overexpression of cyclin D1 in breast cancer cells reverses the growth-inhibitory effects of antiestrogens. Clin Cancer Res. 1997;3:849–854. [PubMed] [Google Scholar]
- 68.Wolf D A, Hermeking H, Albert T, Herzinger T, Kind P, Eick D. A complex between E2F and the pRb-related protein p130 is specifically targeted by the simian virus 40 large T antigen during cell transformation. Oncogene. 1995;10:2067–2078. [PubMed] [Google Scholar]
- 69.Woo M S, Sanchez I, Dynlacht B D. p130 and p107 use a conserved domain to inhibit cellular cyclin-dependent kinase activity. Mol Cell Biol. 1997;17:3566–3579. doi: 10.1128/mcb.17.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]