Abstract
Corollary discharge (CD) allows organisms to discriminate external sensory inputs from self-generated cues. However, the underlying synaptic and molecular mechanisms are not well understood. A new study identified a tyraminergic CD signal that extrasynaptically modulates chemosensory neurons in C. elegans.
Keywords: Behavior, Chemotaxis, Circuit, CO2, BAG
Animals and humans must distinguish external sensory stimuli from self-generated cues in order to respond appropriately. For example, our body can readily tell tickles applied by others from those by ourselves. Such an ability is important for sensory-motor harmony. However, the underlying neural mechanisms remain poorly understood. Corollary discharge (CD) is a mechanism by which animals and humans distinguish external from self-produced sensory stimuli. Since the development of the CD theory in the 1950s, this phenomenon has been discovered in many different species and found to adopt a similar circuit motif1–3 (Fig 1A). However, the underlying synaptic and molecular mechanisms are poorly understood. Using C. elegans as a model, a new study from Riedl et al. has now identified a neural circuit for CD and dissected its synaptic and molecular mechanisms at the single neuron resolution, demonstrating that even simple organisms are able to tell self from the outside world using this mechanism4.
Figure 1.
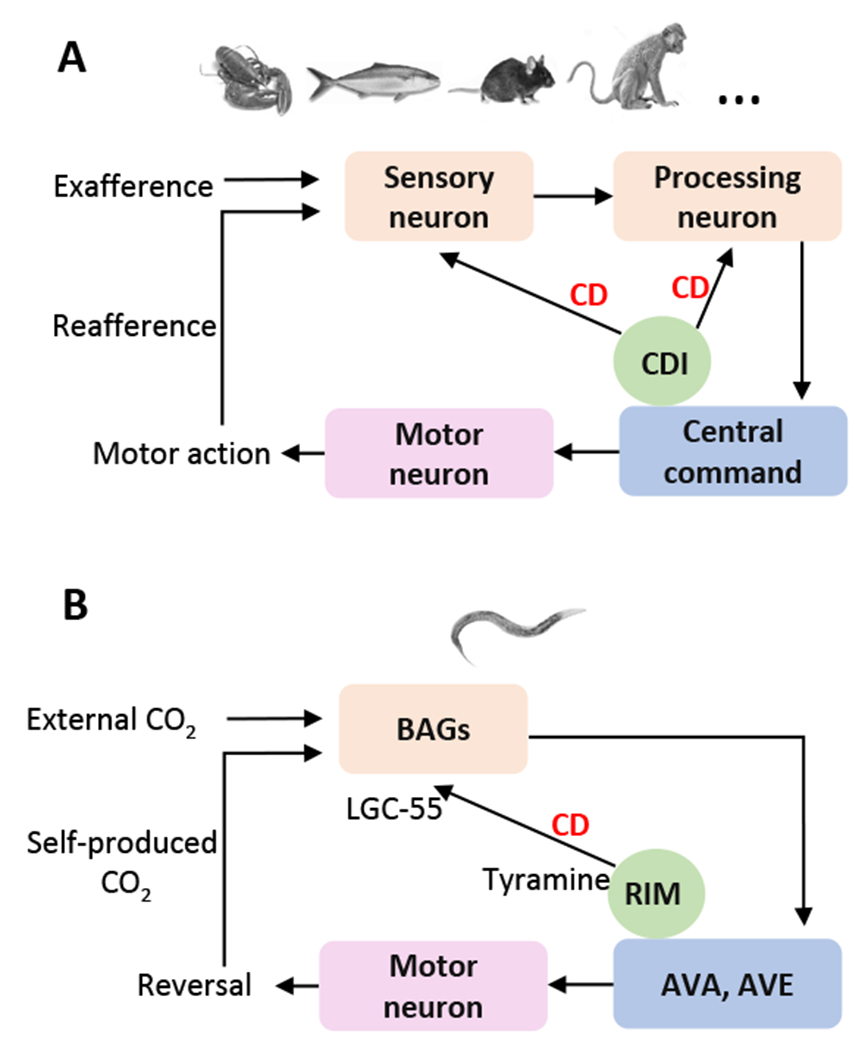
Corollary discharge circuits. A. Schematic of a general corollary discharge circuit found in diverse organisms such as crayfish, fish, mouse, monkey, etc. CDI: corollary discharge interneuron. CD: corollary discharge. B. A corollary discharge circuit in C. elegans chemosensation pathway.
Typically, self-produced sensory stimuli (reafference) mingle with external signals (efference) from the outside world. To coordinate sensory-motor functions, the central command generates CD (motor copy) to facilitate, inhibit or modulate the sensory pathways (Fig 1A). In this process, CD interneurons (CDI) enable the central command to suppress sensory neurons and/or sensory processing neurons1, 2. For example, in crayfish, environmental cues activate arrays of hair cells and trigger a tail-flipping escape behavior to avoid the threat. Meanwhile, the motor action generates a reafferent input from repeated water turbulence, which may also activate the circuit that drives the escape behavior. In this process, CD is employed to distinguish these two types of sensory information to minimize false detection of escape cues from repeated reafferent inputs. The lateral giant command neurons send motor-related signals to CDI that suppress the sensory pathway in hair cells1, 5. This mechanism filters out reafferent inputs to ensure smooth execution of escape responses. Similar circuit motifs have been reported in many other organisms to coordinate different sensory modalities, including visual, mechanosensory, and electrosensory systems1, 2. However, due to the complexity of the nervous system in most of these organisms, it remains a challenge to delineate the underlying neural and molecular mechanisms.
C. elegans offers an excellent model to study CD. Despite possessing a small nervous system, C. elegans executes a complex array of sensorimotor behaviors to communicate with both the outside and internal world6. The completely resolved connectome makes it an ideal system to map neural circuits and dissect cellular and molecular mechanisms underpinning behavior at the single neuron resolution7. Notably, a CD signal from head motor neurons to interneurons mediates head movement regulation in C. elegans8. However, whether CD is involved in the regulation of sensory modalities in this simple organism remains elusive. Now Riedl et al. has provided a nice example showing that CD is employed by C. elegans to coordinate chemosensory responses to CO24.
CO2 is a repellent cue detected by BAG, a pair of sensory neurons. Activation of BAG neurons triggers an escape behavior (reversal) by activating the downstream motor command neurons AVA and AVE and the A/AS-type motor neurons (Fig 1B). Riedl et al. first developed a calcium imaging assay to record BAG activity in animals freely moving in a defined CO2 gradient landscape. They found that the current motor state strongly affects the calcium activity of BAG. When worms move forward, BAG activity is correlated with CO2 concentrations. In contrast, BAG becomes unresponsive to CO2 when worms move backward (reversal). Interestingly, BAG activity even decreases during reversals when animals moved up the CO2 gradient, indicating that these neurons are inhibited in this process. To uncover the neural mechanism by which BAG activity is suppressed during reversals, Riedl et al. searched for the molecules and neurons that regulate reversal behavior and identified the neurotransmitter tyramine, which is released from the interneuron RIM that plays an important role in suppressing BAG activity (Fig 1B). In tdc-1 mutants that fail to produce tyramine or mutants lacking lgc-55 that encodes a tyramine-gated inhibitory chloride channel, RIM is unable to inhibit BAG during up-gradient reversals. This points to a model that tyramine released from RIM inhibits BAG via the tyramine-gated chloride channel LGC-55. Notably, the activity pattern in RIM represents an internal motor state copy, similar to those in the reversal command neurons9. This suggests that the internal motor state may suppress BAG activity via release of tyramine from RIM, a mechanism similar to CD. To verify this, they recorded the activity of BAG and RIM simultaneously in immobilized worms. They found that, in the absence of motor execution, BAG activity is still suppressed by the internal motor command signals from RIM. Thus, modulation of BAG activity by behavioral states is an intrinsic property of the circuitry, which operates independently of motor feedback, thus representing a typical form of CD.
These results suggest a CD circuit that regulates CO2 sensation in C. elegans (Fig 1B). During forward crawling, external CO2 activates BAG neurons, which in turn activate the central command neurons AVA and AVE to trigger reversal. RIM then acts as a CD interneuron (CDI) to inhibit BAG sensory neurons through tyramine and its receptor LGC-55, thereby regulating CO2 sensation. This CD circuit is remarkably similar to that found in other organisms (Fig 1A). It thus appears that CD regulation of sensory functions adopts a similar circuit mechanism across species. Notably, RIM does not form synaptic connections with BAG, indicating that this tyraminergic CD signaling extrasynaptically modulates BAG neuron function. This extrasynaptic mechanism is distinct from those synaptic GABA and glycine signals in the CD inhibition of reafferent electrical- and mechanical sensory pathways found in mormyrid fish and hatchling xenopus, respectively10, 11. The identification of an extrasynaptic CD signal in C. elegans expands the molecular repertoire of CD.
Having dissected the synaptic and molecular mechanisms of CD in CO2 sensation, the authors went on to identify the behavioral function of this circuit. They found that, when CD is disrupted, worms execute excessive avoidance responses in a sudden CO2 upshift assay, revealing an important role of CD in maintaining appropriate sensory-motor behavior. To further explore its role, Riedl et al. monitored BAG activity in a more natural aqueous condition. They observed calcium transients in BAG neurons, which are presumably activated by self-produced CO2 plumes and O2 sinks when animals reverse into their own path even without application of external CO2. Notably, in addition to CO2, BAG neurons are also sensitive to O212. Combined with their simulation results, this suggests that self-produced CO2/O2 plumes may act as reafferent stimuli to activate BAG neurons (Fig 2B). Importantly, such stimuli are countered by the tyraminergic CD signal released from RIM, as in tdc-1 and lgc-55 mutants, self-produced CO2/O2 plumes evoke a much more robust calcium activity in BAG neurons during reversals. Disrupting CD also impairs the initial phase of perception of exafferent stimuli, rendering BAG neurons insensitive to the rise of external CO2 concentration in the first six second after switching to forward movement. Taking together, this RIM-mediated CD cancels out the effect of self-produced CO2 (reafference) during reversals and coordinates external CO2 (exafference) perception during forward movement (Fig 1B).
In summary, Riedl et al. identified an extrasynaptic tyramine signaling mechanism for corollary discharge (CD) in C. elegans, expanding the molecular repertoire of CD. As extrasynaptic signaling is generally believed to act relatively slowly compared to synaptic signaling, future work may investigate how this extrasynaptic signaling mediates the observed rapid inhibition of BAG activity via LGC-55. Interestingly, LGC-55 is also expressed in several other sensory neurons; it would be interesting to examine if tyramine released from RIM functions as a CD signal to regulate these sensory neurons. Owing to the small nervous system and fully mapped neural connectivity, C. elegans has become an increasingly attractive model for neural circuit research. A growing number of circuit motifs have been identified to process sensorimotor signals at the synaptic and molecular levels in C. elegans13, 14. The CD circuit for chemosensation discovered by Riedl et al. represents a nice example of using C. elegans as a model to dissect the circuit and molecular mechanisms of CD. A recent report from Ji et al. also identified a CD circuit that modulates thermosensory behavior in C. elegans15. These studies together suggest that CD may be widely employed by C. elegans to coordinate sensory behaviors. Notably, C. elegans relies on many other sensory modalities to perceive the outside world, such as touch sensation, light sensation and auditory sensation16–20. It would be interesting to test if CD is also involved in coordinating these sensory modalities.
Reference:
- 1.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9(8):587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukutomi M, Carlson BA. A History of Corollary Discharge: Contributions of Mormyrid Weakly Electric Fish. Front Integr Neurosci. 2020;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950;43(6):482–9. [DOI] [PubMed] [Google Scholar]
- 4.Riedl J, Fieseler C, Manuel Zimmer M, . Tyraminergic corollary discharge filters reafferent perception in a chemosensory neuron. Curr Biol. 2022. [DOI] [PubMed] [Google Scholar]
- 5.Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci. 1999;22(4):153–61. [DOI] [PubMed] [Google Scholar]
- 6.Iliff AJ, Xu XZS. C. elegans: a sensible model for sensory biology. J Neurogenet. 2020;34(3-4):347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. [DOI] [PubMed] [Google Scholar]
- 8.Hendricks M, Ha H, Maffey N, Zhang Y. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature. 2012;487(7405):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S, Kaplan HS, Schrodel T, Skora S, Lindsay TH, Yemini E, et al. Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell. 2015;163(3):656–69. [DOI] [PubMed] [Google Scholar]
- 10.Mugnaini E, Maler L. Cytology and immunocytochemistry of the nucleus of the lateral line lobe in the electric fish Gnathonemus petersii (Mormyridae): evidence suggesting that GABAergic synapses mediate an inhibitory corollary discharge. Synapse. 1987;1(1):32–56. [DOI] [PubMed] [Google Scholar]
- 11.Knogler LD, Drapeau P. Sensory gating of an embryonic zebrafish interneuron during spontaneous motor behaviors. Front Neural Circuits. 2014;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, Marletta MA, et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61(6):865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450(7166):63–70. [DOI] [PubMed] [Google Scholar]
- 14.Li ZY, Liu J, Zheng MH, Xu XZS. Encoding of Both Analog- and Digital-like Behavioral Outputs by One C. elegans Interneuron. Cell. 2014;159(4):751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji N, Venkatachalam V, Rodgers HD, Hung W, Kawano T, Clark CM, et al. Corollary discharge promotes a sustained motor state in a neural circuit for navigation. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Hagan R, Chalfie M. Mechanosensation in Caenorhabditis elegans. Int Rev Neurobiol. 2006;69:169–203. [DOI] [PubMed] [Google Scholar]
- 17.Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6(8):e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward A, Liu J, Feng Z, Xu XZ. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat Neurosci. 2008;11(8):916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J, Yuan Y, Ward A, Kang L, Zhang B, Wu Z, et al. The C. elegans Taste Receptor Homolog LITE-1 Is a Photoreceptor. Cell. 2016;167(5):1252–63 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliff AJ, Wang C, Ronan EA, Hake AE, Guo Y, Li X, et al. The nematode C. elegans senses airborne sound. Neuron. 2021;109(22):3633–46 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


