Abstract
Objectives
To understand young people’s expectations of, and experience with sexual healthcare in New Zealand.
Methods
Online survey of 15–24 year olds in a region with high socioeconomic deprivation, with selected outcomes compared for Māori and Europeans.
Results
Of 500 respondents, 60% had received sexual healthcare (74.3% in general practice) and 81% were happy with care received. Fewer Māori and people not in education, employment, or training reported positive experiences of sexual healthcare on arrival and in the consultation.
Conclusions
Findings highlight the need for equitable delivery of youth-friendly, culturally safe, sexual and reproductive healthcare in general practice settings.
Keywords: General practice, indigenous Māori, patient satisfaction, sexual healthcare, survey, young people
Introduction
To maintain good sexual health and wellbeing, young people need universal access to culturally safe, nonjudgmental healthcare that meets their unique needs as individuals (Curtis et al., 2019; World Health Organisation, 2017). Young people are known to face barriers to healthcare access generally (Health Quality & Safety Commission New Zealand, 2019; Matich et al., 2015; Peiris-John et al., 2020), so services and staff need to be approachable and actively support young people to develop independent health-seeking skills (Tylee et al., 2007). Young people accessing care for their sexual and reproductive health (SRH) may experience additional complexities due to persistent societal negative attitudes surrounding disclosure of sexual activity and stigma surrounding sexually transmitted infections (STIs) (Bender & Fulbright, 2013; Cunningham et al., 2002; Denison et al., 2017; Kennedy et al., 2013; Morris & Rushwan, 2015). Patient satisfaction with sexual healthcare service provision is determined by factors related to accessibility, acceptability, effectiveness and confidentiality (Hathorn et al., 2011; Weston et al., 2010). These factors are also among those deemed to be important indicators of “youth-friendly” healthcare by the World Health Organization (World Health Organization, 2012).
Past research (Denison et al., 2018; Turner et al., 2017; Tylee et al., 2007) has found fear that a sexual health visit will not be kept confidential, and real or perceived stigma and discrimination from health providers can prevent young people seeking care or revealing the actual reason for presentation when they do present (e.g., possible STI symptoms). Stigma, discrimination, and implicit or unconscious biases held by clinicians are internationally recognized as impacting on sexual healthcare provision particularly for marginalized groups and indigenous populations (FitzGerald & Hurst, 2017; MacLean, 2018). For these reasons, it is often young, gender and sexuality diverse, indigenous (Māori in the case of New Zealnd [NZ]), and young people not in education, employment, or training (NEET) who are among those facing challenges accessing acceptable sexual healthcare, and who are disproportionately affected by negative sexual health outcomes (Clark et al., 2020; Tipene & Green, 2017).
The inability to routinely access SRH services can have serious short- and long-term health and wellbeing consequences. For example, inability to access contraception or a preferred method of contraception influences the ability to avoid, plan, or space pregnancies. Unwanted hormone-related side effects or troublesome bleeding patterns associated with nonpreferred contraceptive methods can reduce quality of life and result in method discontinuation (Polis et al., 2018). Without early access to sexually transmitted infection (STI) information, testing, and treatment, bacterial infections like chlamydia and gonorrhea can lead to adverse reproductive, pregnancy, and neonatal outcomes and can facilitate the transmission of HIV and HPV (World Health Organization, 2016). In NZ, SRH services are delivered in a range of settings, but most often in primary care or general practice clinics. Māori and Pacific health clinics, Family Planning clinics, Youth One Stop Shops, and student and school health clinics also provide SRH services. Appointment costs for SRH visits vary by patient age within and between services but are often free in general practice for under 20-year-olds, and free for under 22-year-old NZ residents in Family Planning clinics that are available in 15 cities and towns. For “older” young people, costs may be up to NZ$45 (approximately USD33). Specialist sexual health clinics are mostly located in main cities (or in smaller towns with limited hours), offering free consultations but some prioritize higher risk groups and not all offer contraceptive services, so specialist sexual health clinics provide for only small proportions of young people’s SRH needs.
Currently, there is a lack of data about the extent to which SRH care aligns with young people's expectations of youth-friendly healthcare in NZ. The present study surveyed 15–24 year olds about their expectations and experience of sexual healthcare with responses compared for Māori and European ethnic groups. The survey was undertaken in the Hawkes Bay region in the North Island of NZ which is home to a high proportion of people living in areas of greater deprivation (Ministry of Health, 2021). NZDep2018 is an area-based measure of deprivation where Quintile 1 (NZDep scores 1–2) is a low deprivation community and Quintile 5 (NZDep scores 8–10) a high-deprivation community (Atkinson et al., 2019). Just over half the Hawkes Bay population live in the two most deprived quintiles, whereas in the Wellington region in the lower North Island, over half the population lives in the two least deprived quintiles (Ministry of Health, 2021). Hawkes Bay has a higher than average proportion of Māori − 37% of people aged 15–24 are Māori compared with 21% of 15–24 year olds in NZ overall (Statistics New Zealand, 2021). The region includes two cities, a number of smaller towns and rural settlements and has around 27 general practices services, one Youth One Stop Shop, one sexual health clinic (but no Family Planning clinic). Hawkes Bay has disproportionately high population rates of chlamydia and gonorrhea than many other regions in New Zealand (Environmental Science & Research Ltd, 2021), so it is particularly important to know whether SRH service provision is meeting the needs of young people in this area.
Materials and methods
Participants and recruitment
Survey respondents were recruited via social media advertising (Facebook and Instagram) over five weeks (during August–October 2020). Sponsored posts were presented to people meeting the inclusion criteria (15–24 years and resident in Hawkes Bay, NZ). The text on the advertisement read “Hawkes Bay 15–24 year olds. Share your views.” The image included a graphic of four young people, with a rainbow colored wash, Māori artwork and a University logo in the background. The tagline read “Tell us what you think about health care for young people and be in to win $100! (USD72), 5–10 min anonymous survey.” Sexual healthcare was only mentioned once people clicked through to the information sheet as we wanted to hear from people who had, and had not previously accessed care for their sexual health. The information sheet stated that participants had the chance to enter a prize draw for one of three NZ$100 cash prizes by sending an email to the researchers asking to be entered into the draw once the survey was complete (an email link was provided at the end of the survey). We ran two prize draws and sent a total of six people one NZ$100 voucher each (upon receipt of their name and a Hawkes Bay postal address). Ethical approval was granted by the University of Otago Human Ethics Committee Health (ref H19/154).
Sample size
A sample of at least 370 participants was deemed necessary to provide a 5% margin of error (ie a confidence interval of ±5 percentage points around the proportion of responses to a given survey item). The target demographic group included an estimated 24,000 individuals who met the inclusion criteria. Sample size calculation performed using the sample size for a descriptive study function on OpenEpi.com.
Survey development and data collection
This cross-sectional, descriptive survey included questions that were customized for the current study but drew on past research (Bender & Fulbright, 2013; Howarth et al., 2017; Mazur et al., 2018; Sutcliffe et al., 2011; Weston et al., 2010). To ensure the survey was appropriate for, and relevant to, the target study population, the draft questionnaire was reviewed by two focus groups (12 males, eight females, aged 14–24 years, including Māori) who were given NZ$20 (USD15) vouchers for their contribution. Questions and response options were modified following focus group feedback. The recruitment strategy, advertisement image and wording followed recommendations provided by the groups.
The survey was delivered using the Qualtrics online survey platform, with questions formatted as multiple choice, Likert/rating scales, and matrices. Questions that led to branching required a response to continue, but otherwise respondents could skip questions they did not wish to answer. Respondents were asked about access to sexual healthcare (when and where they had been, reasons for choice of place for sexual healthcare), the importance of factors describing attributes of the staff/clinic, access and consultation, and overall satisfaction and experience of sexual healthcare. Near the beginning of the survey, sexual health was defined as including “talking about staying safe when sexually active, sexually transmitted infections (STIs), an STI or symptom check, contraception or anything else you think is related to your sexual health and wellbeing.” Demographic information was collected at the end of the survey including age, gender, self-identified ethnicity, sexual orientation, education/employment status and place of residence. Potential respondents who clicked on the advertisement link were taken to the information sheet describing the study that included the statement “Agreement to take part (consent): starting the survey will tell us that you agree to take part. You can stop the survey at any time.”
Data cleaning and analysis
Data were exported into Microsoft Excel for collation and analysis. A number of measures were undertaken to check the legitimacy of responses. IP addresses (that are unique to a computer or device) were checked for duplication, and the geolocation of responses reviewed. The patterning of responses was checked to ensure they told a logical story, and free text fields (not reported on here) checked for random keystrokes and inappropriate comments that might indicate “mischievous responders.” Survey completion time was checked for outliers. Emails sent by participants wishing to enter the prize draw were reviewed for duplication and those randomly drawn for one of six prizes were asked to provide a name and postal address.
Responses were reviewed for completeness and a decision made to include partially completed surveys only if the first block of questions in each branch (4–5 items) had been answered in full. Demographic data were re-coded by combining selected responses, for respondents reporting more than one ethnic group, ethnicity was re-coded using prioritization as per standardized protocols (Ministry of Health, 2017). Response frequencies were tabulated with number, percentages and 95% confidence intervals (CI) calculated where appropriate. Cross-tabulated tables were populated for selected questions to compare responses by demographic characteristics (primarily ethnicity, but also employment/education, gender and sexuality diversity). Chi-square tests for significance were performed for post hoc comparisons between demographic groups (with p values reported in results where relevant). All statistical tests were performed using R 4.0 (R Institute, Vienna, Austria).
Results
Participant characteristics and survey completion
The advertisement received 1,187 clicks, and of those who clicked through to the survey, 560 submitted a partially or fully completed survey (47.2%). Fully completed surveys were received from 439 individuals (78.4%, 469/560), and 121 were partially completed (of which 61 were sufficiently complete to be included in the analysis), resulting in a total sample of 500 respondents. Around half the respondents (277) asked to be included in the prize draw. Following completion of checks for response legitimacy, we were satisfied that responses included in the sample for analysis represented those of respondents who had participated with good intent. None of the completed surveys violated checks for duplication, ineligibility (location), or response patterning. Denominators varied for individual questions due to the inclusion of incomplete surveys, the branched nature of the survey and participants’ ability to skip questions. The majority of respondents who reached the end of the survey completed all of the questions they were presented with (that is, few questions had missing data).
Table 1 presents the characteristics of the 500 respondents, together with the number and proportion (95% CI) who had ever received sexual healthcare. Survey respondents were spread across the study region, with 58.8% identifying as female, a quarter self-identified as Māori (25.4%), 42.6% were in school or studying, 39.4% in employment and 13% were NEET. Of those who answered gender identity and sexual orientation questions, 21.4% of participants were lesbian, gay, bisexual, transgender, queer (LGBTQ+) (107/500). Around 11% of respondents included in the analysis did not reach the end of the survey so did not provide any demographic data.
Table 1.
Demographic characteristics of all survey respondents (n = 500), and numbers (%, 95% CI) who had ever received sexual healthcare.
| Total sample |
Ever received sexual healthcare |
||||
|---|---|---|---|---|---|
| Characteristics | n | % | n | % | 95% CI |
| Total | 500 | 100 | 300 | 60.0 | [55.6−64.3] |
| Age | |||||
| 15–17 years | 151 | 30.2 | 42 | 27.8 | [20.8–35.7] |
| 18–21 years | 147 | 29.4 | 100 | 68.0 | [59.8−75.5] |
| 22–24 years | 149 | 29.8 | 121 | 81.2 | [74.0−87.1] |
| Not stated | 53 | 10.6 | 37 | 69.8 | [55.7−81.7] |
| Ethnicity (prioritized) | |||||
| Māori | 127 | 25.4 | 87 | 68.5 | [59.7−76.5] |
| Pacific | 14 | 2.8 | 9 | 64.3 | [35.1−87.2] |
| Europeana | 281 | 56.2 | 158 | 56.2 | [50.2−62.1] |
| Asian | 18 | 3.6 | 6 | 33.3 | [13.3−59.0] |
| Middle Eastern, Latin American, African | 4 | 0.8 | 2 | 50.0 | [6.8−93.2] |
| Not stated | 56 | 11.2 | 38 | 67.9 | [54.0−79.7] |
| Genderb | |||||
| Female | 294 | 58.8 | 214 | 72.8 | [67.3−77.8] |
| Male | 138 | 27.6 | 42 | 30.4 | [22.7−38.6] |
| Transgender and gender diverse | 11 | 2.2 | 4 | 36.4 | [10.9−69.2] |
| Not stated | 57 | 11.4 | 40 | 70.2 | [56.6−81.6] |
| Sexual orientation | |||||
| Heterosexual or straight | 315 | 63 | 180 | 57.1 | [51.3−62.6] |
| Bisexual | 72 | 14.4 | 50 | 69.4 | [57.5−79.8] |
| Gay or lesbian | 27 | 5.4 | 13 | 48.1 | [27.8−68.7] |
| Other | 11 | 2.2 | 7 | 63.6 | [32.3−83.7] |
| Don't know | 9 | 1.8 | 5 | 55.6 | [21.2−86.3] |
| Not stated | 66 | 13.2 | 45 | 68.2 | [55.6−79.1] |
| LGBTQ+c | 107 | 21.4 | 68 | 63.6 | [53.7−72.6] |
| Education/employment | |||||
| School or studying | 213 | 42.6 | 89 | 41.8 | [35.6−49.4] |
| Working full/part-timed | 197 | 39.4 | 135 | 68.5 | [61.5−74.9] |
| Not in employment, education, or training | 65 | 13.0 | 50 | 76.9 | [62.5−84.5] |
| Caring for child(ren)/someone else | 27 | 5.4 | 20 | 74.1 | [62.6−95.3] |
| Not stated | 58 | 11.6 | 40 | 69.0 | [57.8−82.7] |
| Usual place for general healthcare | |||||
| General practice | 432 | 86.4 | 255 | 59.0 | [54.2−63.7] |
| Māori or Pacific clinic | 11 | 2.2 | 7 | 63.6 | [30.8−89.1] |
| School/student health clinic | 25 | 5.0 | 13 | 52.0 | [31.3−72.2] |
| Youth One Stop Shop clinic | 13 | 2.6 | 10 | 76.9 | [46.2−95.0] |
| After hours clinic | 4 | 0.8 | 4 | 100 | [47.3−100] |
| Emergency department | 3 | 0.6 | 3 | 100 | [36.8−100] |
| Other/Don't know/Not stated | 12 | 2.4 | 4 | 33.3 | [9.9−65.1] |
| Sexual healthcare access | |||||
| Ever tested for STIs | 192 | 38.4 | 192 | 100 | [98.5−100] |
| Ever treated for STIs | 79 | 15.8 | 79 | 100 | [96.3−100] |
Note. STI = sexually transmitted infections.
aIncludes 277 NZ European and 4 “other European” groups.
bTransgender and gender diverse group includes: 2 transfemales, 3 transmales, 2 gender fluid, 1 gender apathetic and 3 did not further define “gender diverse.”
cLGBTQ+ is used as an umbrella term that includes individuals who identified with gender and/or sexual orientation sub-categories including lesbian, gay, queer, bisexual, and/or transgender or gender diverse (note we did not ask participants whether they identified as “LGBTQ+”).
dPeople in job training or apprenticeships are included with those in full/part-time work. Respondents could select more than one option here so column percentages sum to more than 100%.
Receipt of sexual healthcare
Sixty percent of all respondents had ever received sexual healthcare (see Table 1). Significant differences in characteristics of those who had received sexual healthcare were observed. Older respondents, females, Māori, and those NEET were more likely to report experience of sexual healthcare. For a third of the sample, sexual healthcare was accessed in the past 3-months (32.7%, 98/300), 19.7% within 3–6-months (59/300), 23% within 6–12-months (69/300), 22% over 12-months ago (66/300), and 2.7% responded “don’t know” (eight people).
Accessing services and choice of clinic
The majority of participants (74.3%, 223/300) went to a general practice clinic for their sexual healthcare. Small proportions went to the sexual health service (8.7%, 26/300), a school or student health service (8.7%) the youth health clinic (3.7%), after-hours primary care clinic (1.0%), the hospital emergency department (1.3%).
Reasons for choice of clinic
The majority of respondents reported that they went to (or in the case of those who had not yet accessed sexual healthcare, would want to go to) the same place for all their healthcare needs including sexual health (72.6%, 363/500). This finding did not differ between Māori and European participants. A higher proportion of LGBTQ+ participants who had not before received any care for their sexual health, indicated they would not want to go to their usual healthcare provider for sexual health in the future (41%, 16/39 versus 21%, 31/144 of the heterosexual/cisgender group, p < .05). For those who indicated they prefer to go somewhere different for their sexual health (26.6%, 133/500), reasons relating to ease of access were common, with 45.1% selecting because its free (60/133), 26.3% because it’s easier to get to (35/133) and 31.6% because its quicker to get an appointment (42/133). Privacy reasons were also commonly selected, 43.6% indicated they don’t want to talk to their usual nurse/doctor about their sexual health (58/133), 19.5% don’t want to go where their family go (26/133) and 15% don’t want to see people they know in the waiting room (20/133). Factors relating to staff at their preferred place for sexual healthcare included: they’re nonjudgmental (19.5%, 26/133), the staff know more about sexual health (26.3%, 35/133), and the staff are more welcoming of gender and sexuality diverse young people (18.8%, 25/133).
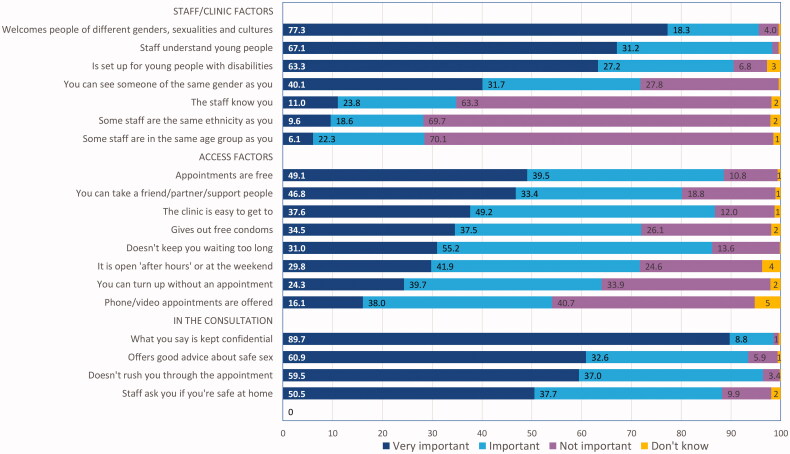
Factors deemed important when accessing sexual healthcare
Respondents were asked to rank the importance of various positive aspects of service provision relating to staff/clinic, access and consultation attributes (see Figure 1). Items most frequently ranked as very important (by two thirds or more participants) included trusting their discussion is confidential, staff being welcoming of different genders, sexualities and cultures and seeing staff that understand young people. Those less often ranked by the majority as being important included seeing staff that know you, that are the same ethnicity or in the same age group and being able to access phone or video (telehealth) appointments.
Figure 1.
Perceived importance of factors related to access, the staff/clinic and the consultation when receiving sexual healthcare.
When comparing responses for Māori and European participants (see Table 2), Māori were significantly more likely than European respondents to rank selected items as being very important including access-related factors (turning up without an appointment, being easy to get to and open after hours). A number of staff/clinic-related factors were more often ranked by Māori than European as being very important. Those that reached statistical significance included being set up for young people with disabilities, staff that know you and who are the same ethnicity as you (p < .05). Higher proportions of LGBTQ+ young people ranked confidentiality as “very important” (104/107, 97% versus 293/336, 87% of heterosexual/cisgender young people, p < .05). Similarly, 91% (97/107) of LGBTQ+ young people ranked staff who were welcoming of different genders, sexualities and cultures as very important (versus 250/336, 74% of heterosexual/cisgender young people, p < .05).
Table 2.
Comparison of Māori and European respondents ranking statements describing aspects of care as “very important.”
| Aspect of care | Māori (n = 127) |
European (n = 281) |
p value | ||||
|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | ||
| Staff/clinic factors | |||||||
| Welcomes people of different genders, sexualities and cultures | 105 | 82.7 | (75.0−88.8] | 214 | 76.2 | (70.7−81.0] | .178 |
| Is set up for young people with disabilities | 92 | 72.4 | (63.8−80.0] | 164 | 58.4 | (52.4−64.2] | .009 |
| Staff understand young people | 87 | 68.5 | (59.7−76.5] | 182 | 64.8 | (58.9−70.3] | .532 |
| You can see someone of the same gender as you | 57 | 44.9 | (36.1−54.0] | 106 | 37.7 | (32.0−43.7] | .208 |
| The staff know you | 22 | 17.3 | (11.2−25.0] | 22 | 7.8 | (5.0−11.6] | .007 |
| Some staff are the same ethnicity as you | 20 | 15.7 | (9.9−23.3] | 17 | 6.0 | (3.6−9.5] | .003 |
| Some staff are in the same age group as you | 12 | 9.4 | (5.0−15.9] | 12 | 4.3 | (2.2−7.3] | .067 |
| Access factors | |||||||
| Appointments are free | 72 | 56.7 | (47.6−65.5] | 131 | 46.6 | (40.7−52.6] | .076 |
| You can take a friend/partner/support people | 70 | 55.1 | (46.0−63.9] | 119 | 42.3 | (36.5−48.4] | .022 |
| The clinic is easy to get to | 56 | 44.1 | (35.3−53.2] | 94 | 33.5 | (28.0−39.3] | .051 |
| It is open “after hours” or at the weekend | 47 | 37.0 | (28.6−46.0] | 69 | 24.6 | (19.6−30.0] | .014 |
| Gives out free condoms | 47 | 37.0 | (28.6−46.0] | 91 | 32.4 | (26.9−38.2] | .423 |
| Doesn't keep you waiting too long | 46 | 36.2 | (27.9−45.2] | 75 | 26.7 | (21.6−32.3] | .067 |
| You can turn up without an appointment | 40 | 31.5 | (23.5−40.3] | 56 | 19.9 | (15.4−25.1] | .015 |
| Phone/video appointments are offered | 25 | 19.7 | (13.2−27.7] | 40 | 14.2 | (10.4−18.9] | .212 |
| In the consultation | |||||||
| What you say is kept confidential | 114 | 89.8 | (83.1−94.4] | 255 | 90.7 | (86.7−93.9] | .896 |
| Doesn’t rush you through the appointment | 86 | 67.7 | (58.8−75.7] | 158 | 56.2 | (50.2−62.1] | .037 |
| Offers good advice about safe sex | 77 | 60.6 | (51.6−69.2] | 169 | 60.1 | (54.2−65.9] | 1.00 |
| Staff ask you if you’re safe at home | 74 | 58.3 | (49.2−67.0] | 129 | 45.9 | (40.0−51.9] | .027 |
Experience of sexual healthcare
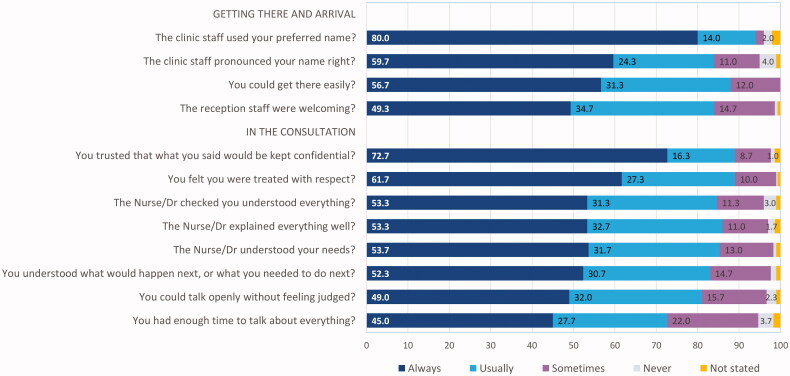
Figure 2 presents the frequency (proportions) with which participant’s experiences aligned with each of 12 statements describing positive aspects of care. Overall, the collective experience of the 300 young people who had received sexual healthcare was positive, with three quarters or more reporting that care was always or usually as described in statements presented, with the exception of having enough time to talk about everything (72.7% always or usually experienced this).
Figure 2.
Frequency (%) with which respondents “always” or “usually” experienced positive aspects of sexual healthcare described in statements presented.
To determine whether experiences of care differed between demographic groups, proportions of always and usually responses (combined) were compared by ethnic group, gender/sexual identity and education/employment status. No statistically significant differences were observed by gender/sexual identity on any of the statements when always and usually responses were combined. However proportionately fewer LGBTQ+ participants reported that reception staff were always welcoming (42.7% versus 51% of the heterosexual/cisgender comparison group), and fewer always trusted what they said would be kept confidential (68.7% versus 75% for the comparison group). Table 3 presents the results of comparisons by ethnic group and education/employment status. Māori were significantly less likely than European participants to always or usually experience care described in most of these statements. Differences that reached statistical significance included fewer Māori always or usually: feeling welcomed by reception staff, having their name pronounced correctly, and feeling the nurse/doctor understood their needs (p < .05). Young people who were NEET were less likely to respond always or usually on many of the statements compared with those who were employed/in education or training. Note that Māori were over-represented in this group with half of those in the NEET group self-identifying as Māori.
Table 3.
Number (% and 95% CIs) of respondents who responded “always” or “usually” to statements about their experience of sexual health care presented by ethnic group and education/employment status.
| Statement about overall experience of sexual healthcare | Ethnic group |
Education/employment status |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Māori (n = 87) |
European (n = 158) |
p value | NEET (n = 50) |
Work, study (n = 211) |
p value | |||||||||
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |||
| Getting there and arrival | ||||||||||||||
| The clinic staff used your preferred name? | 79 | 91.9 | [83.9−96.7] | 150 | 94.9 | [90.3−97.8] | .499 | 43 | 87.8 | [75.2−95.4] | 200 | 95.7 | [92.0−98.0] | .043 |
| You could get there easily? | 72 | 82.8 | [73.2−90.0] | 144 | 91.1 | [85.6−95.1] | .082 | 37 | 74.0 | [59.7−85.4] | 190 | 90.0 | [85.2−93.7] | .005 |
| The reception staff were welcoming? | 66 | 75.9 | [65.5−84.4] | 140 | 88.6 | [82.6−93.1] | .015 | 39 | 78.0 | [64.0−88.5] | 178 | 84.4 | [78.7−89.0] | .384 |
| The clinic staff pronounced your name right? | 63 | 73.3 | [62.6−82.2] | 142 | 89.9 | [84.1−94.1] | .001 | 35 | 70.0 | [55.4−82.1] | 181 | 86.2 | [80.8−90.6] | .011 |
| In the consultation | ||||||||||||||
| You trusted what you said would be kept confidential? | 74 | 85.1 | [75.8−91.8] | 144 | 91.1 | [85.6−95.1] | .214 | 39 | 78.0 | [64.0−88.5] | 191 | 91.0 | [86.2−94.5] | .02 |
| The Nurse/Dr explained everything well? | 72 | 82.8 | [73.2−90.0] | 138 | 87.3 | [81.1−92.1] | .429 | 44 | 88.0 | [75.7−95.5] | 179 | 84.8 | [79.3−89.4] | .727 |
| You felt you were treated with respect? | 72 | 82.8 | [73.2−90.0] | 145 | 91.8 | [86.3−95.5] | .056 | 40 | 80.0 | [66.3−90.0] | 189 | 89.6 | [84.6−93.3] | .106 |
| The nurse/Dr checked you understood everything? | 71 | 81.6 | [71.9−89.1] | 135 | 85.4 | [79.0−90.5] | .547 | 40 | 80.0 | [66.3−90.0] | 177 | 83.9 | [78.2−88.6] | .653 |
| You understood what (if anything) would happen next, or what you needed to do next? | 69 | 79.3 | [69.3−87.3] | 131 | 82.9 | [76.1−88.4] | .600 | 37 | 74.0 | [59.7−85.4] | 174 | 82.5 | [76.6−87.3] | .243 |
| You could talk openly without feeling judged? | 65 | 75.6 | [65.1−84.2] | 136 | 86.1 | [79.7−91.1] | .060 | 37 | 75.5 | [61.1−86.7] | 176 | 83.8 | [78.1−88.5] | .246 |
| The nurse/Dr understood your needs? | 67 | 77.0 | [66.8−85.4] | 141 | 89.2 | [83.3−93.6] | .018 | 40 | 80.0 | [66.3−90.0] | 180 | 85.3 | [79.8−89.8] | .477 |
| You had enough time to talk about everything? | 60 | 69.0 | [58.1−78.5] | 116 | 73.4 | [65.8−80.1] | .553 | 29 | 58.0 | [43.2−71.8] | 158 | 74.9 | [68.5−80.6] | .027 |
Overall satisfaction with sexual healthcare received
When asked to rate overall satisfaction with sexual healthcare on a “happy face” slider scale, 81% of participants were happy overall (28.7% [86/300] were very happy, and 52.3% [157/300] were happy). Ten percent gave their experience a neutral rating (31/300), 4.7% were unhappy (14/300), and only one person was very unhappy. Eleven people did not answer the question. Respondents who were neutral or unhappy (n = 45) were more likely to have selected never in response to one or more of the items describing positive aspects of care (i.e. those in Figure 2 and Table 3). Proportionately fewer Māori (78.2%, 95% CI [68.0, 86.3]) than European (84.8%, 95% CI [78.2, 90.0]) gave very happy/happy ratings (but overlapping confidence intervals suggest these percentage differences did not reach statistical significance). Fewer participants in the NEET group (72%, 95% CI [57.5, 83.8]) than those in education/employment (84.3%, 95% CI [78.7, 89.0]) rated their overall experience(s) as happy/very happy.
Discussion
The majority of young people surveyed in the study region reported positive experience(s) of sexual healthcare in general practice. Just over half of all respondents were always able to get to services easily, with another third usually able to do so. Generally speaking, for the majority of participants, their experience aligned with their expectations of SRH care, but for the minority of young people who less often had positive experiences, Māori and young people NEET were overrepresented. Young Māori less often reported feeling welcomed by reception staff, having their name correctly pronounced, and feeling that the nurse/doctor understood their needs. Similarly, young people NEET reported fewer positive experiences than their peers who were in employment/education. Māori participants were more likely to place greater importance on factors related to ease of access than European, yet fewer reported being able to access sexual healthcare easily. Similarly, young people NEET were less often able to easily access sexual healthcare compared to those in employment/education.
A concerning finding was that proportionately fewer Māori reported feeling welcomed when attending healthcare services. Poor experience(s) with health services can impact on subsequent health-seeking behavior for any health issue: if a young person feels the staff were not friendly toward them, they may decide not to return (Tylee et al., 2007). It is therefore particularly important that all staff in the practice, including receptionists, are welcoming and approachable (with provision of appropriate training if necessary) (Turner et al., 2017). Although only a small number of participants were NEET, Māori were overrepresented in this category, highlighting the multiple forms of disadvantage and discrimination some young Māori face (Cormack et al., 2020). Unfortunately, findings related to differential experience of healthcare by Māori are not unexpected, as the literature shows that young people in more vulnerable groups including indigenous and youth NEET face more challenges accessing health services in general as well as for sexual healthcare (Clark et al., 2020; Peiris-John et al., 2020; Tipene & Green, 2017). Although we did not directly ask participants about their experience of discrimination or racism, the differential experiences reported by young Māori in this study are suggestive of systemic racism. Inadvertent perpetuation of systemic racism in the NZ health system is increasingly being recognized as negatively impacting on the way Māori are able to access services and the quality of care they receive, with disadvantage seen throughout their life course (Came et al., 2019; Cormack et al., 2018; Health Quality & Safety Commission, 2019; Houkamau, 2016).
Aspects of care deemed most important to young people surveyed included confidentiality and staff being welcoming of different genders, sexualities and cultures. It was encouraging to see that nearly three quarters of respondents reported always feeling that they trusted their consultation was treated confidentially. This finding reinforces the need for general practice teams to reassure young people that it is clinic policy to maintain confidentiality. Signs at reception, in the waiting room and consultation room, together with verbal reassurance in the consultation (and ideally wider promotion via sexuality education and advertising) would help to acknowledge this important aspect of care (Hargreaves, 2011; Patton et al., 2016). We saw that most survey participants felt the nurse or doctor explained everything well and understood what would happen next. This positive finding suggests most young people experienced good communication from their health providers.
Although not addressed in the current study, research in Australia showed that young people want their nurse or doctor to ask about sexual health even in consultations for other health issues (Turner et al., 2017). This reflects an understandable lack of confidence many young people experience in raising the issue when in need of sexual healthcare, and highlights the importance of clinicians having good communication skills that are youth-appropriate, and being proactive in prompting discussion of sexual health and wellbeing. Participants in the recently reported NZ Youth19 survey (a periodic national survey of school-aged young people; Clark et al., 2020; Peiris-John et al., 2020) placed importance on knowing the clinician when accessing general healthcare, something that wasn’t ranked as highly by participants in our survey. This difference may be due to differences in age (our survey included fewer people younger than 18, most of whom did not yet have experience of sexual healthcare), or different preferences for receipt of sexual and general health needs.
Strengths of the study include the diverse demographic characteristics of participants. Although not a representative sample, we achieved good participation by young Māori and other groups known to face challenges in accessing sexual healthcare (e.g., LGBTQ+ and NEET; Tipene & Green, 2017). We surveyed young people in a region that has high proportions of Māori, higher proportions of people living in greater deprivation, city, small town and rural dwellers and few choices for SRH care. Our study findings are likely to be at least partially generalizable to the experience of young people in other regions of NZ. Our recruitment method was both a strength and weakness of the study. Use of social media advertising allowed us to successfully engage with diverse groups of young people as has been shown in past research (Pedersen & Kurz, 2016). However, we acknowledge those not engaged with the social media platforms used to recruit participants, and people without access to a device, data or WIFI would have been excluded from participation (groups for whom accessing healthcare may also be more difficult).
Other limitations of the study also relate to the study sample. Despite attempts to increase the proportion of males in the sample (by targeting recruitment only to males in the final two weeks), participation by males was lower than that of females. This is a common limitation of health research, and research using social media recruitment (Whitaker et al., 2017). The sample was self-selected so reflects the views of young people willing to participate in research, and perhaps who have more positive experiences to share than those choosing not to participate. Some respondents may have shared the survey details with peer/friend groups leading to in-group responder bias. This could mean the responses are not as divergent as they would otherwise have been.
Measures taken to assess the legitimacy of responses revealed no indication of fraudulent responders, however we had no way to truly verify whether or not participants answered truthfully. The study sample was not sufficiently large to detect as statistically significant small differences between the responses of demographic subgroups. This may account for the lack of significant differences in the reported experiences of LGBTQ+ young people that might have been expected based on past research. Alternatively, it could be that the young people in this group had generally received acceptable care when being seen for their sexual health. The survey was kept brief to maximize completion rates, so we did not comprehensively cover all relevant aspects of care, nor ask for specific information about the nature of participant’s sexual healthcare needs. Qualitative research with young people, particularly young Māori, would provide further insights into their expectations and experiences of SRH care that could help to shape future service delivery improvements.
These study findings reinforce the need to ensure SRH care is delivered in a more equitable way to young people, in ways that align with WHO recommendations for youth-friendly care (World Health Organization, 2012). Services that have been specifically designed to meet the needs of young people including Youth One Stop Shops, student health and school clinics, as well as those that specialize in SRH care (Family Planning) are likely to perform better on many of the youth-friendly aspects of care (Ministry of Health New Zealand, 2009). Yet, there are only 11 Youth One Stop Shops and 23 Family Planning services in NZ (one Youth One Stop Shop but no Family Planning in our study region). Furthermore, not all schools have health services, so the majority of young people receive SRH care in general practice. It is critical that general practices are aware of their key role in SRH service provision, and that they have the skills, knowledge and youth-friendly attitudes to foster engagement with young people in healthcare – for all types of consultation, including sexual health (Patton et al., 2016). This may require review of youth-friendly policies and practices, and training in the provision of youth-appropriate and culturally safe healthcare to ensure they are effectively meeting the needs of young Māori and other vulnerable groups. Adaptations and improvements in services would ideally involve direct input from young Māori to help shape the delivery of positive healthcare experiences that are currently being enjoyed by their more privileged peers. This would be a positive step toward addressing current inequities in the SRH and wellbeing of young people in New Zealand.
Acknowledgements
This work was supported by the Hawkes Bay Medical Research Foundation (grant-in-aid 2019) and a University of Otago Dean’s grant (grant-in-aid, 2019). The funding bodies played no part in the study design, collection, analysis or interpretation of the data, writing of the report or decision to submit the manuscript for publication. We acknowledge the young people who took part in focus groups during survey development and those who completed a survey. We thank staff at Te Taiwhenua o Heretaunga for their support of the survey development and organization of focus group participants.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Data availability statement
Data are not publicly available. Due to the nature of this research, participants of this study were not asked for permission for their data to be shared publicly, so supporting data are not available.
References
- Atkinson, J., Salmond, C., Crampton, P. (2019). NZDep2018 Index of Deprivation. Interim research report. https://www.otago.ac.nz/wellington/otago730394.pdf.
- Bender, S. S., & Fulbright, Y. K. (2013). Content analysis: A review of perceived barriers to sexual and reproductive health services by young people. The European Journal of Contraception & Reproductive Health Care, 18(3), 159–167. 10.3109/13625187.2013.776672 [DOI] [PubMed] [Google Scholar]
- Came, H., McCreanor, T., & Manson, L. (2019). Upholding Te Tiriti, ending institutional racism and Crown inaction on health equity. New Zealand Medical Journal, 132(1492), 61–66. [PubMed] [Google Scholar]
- Clark, T. C., Lambert, M., Fenaughty, J., Tiatia-Seath, J., Bavin, L., Peiris-John, R., Sutcliffe, K., Crengle, S., & Fleming, T. (2020). Youth19 Rangatahi smart survey, initial findings: Sexual and reproductive health of new zealand secondary school students. The Youth19 Research Group, The University of Auckland and Victoria University of Wellington. Retrieved December 09, 2020 from https://www.youth19.ac.nz/publications [Google Scholar]
- Cormack, D., Harris, R., & Stanley, J. (2020). Māori experiences of multiple forms of discrimination: Findings from Te Kupenga 2013. Kōtuitui: New Zealand Journal of Social Sciences Online, 15(1), 106–122. 10.1080/1177083X.2019.1657472 [DOI] [Google Scholar]
- Cormack, D., Stanley, J., & Harris, R. (2018). Multiple forms of discrimination and relationships with health and wellbeing: Findings from national cross-sectional surveys in Aotearoa/New Zealand. International Journal for Equity in Health, 17(1), 26. 10.1186/s12939-018-0735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, S. D., Tschann, J., Gurvey, J. E., Fortenberry, J. D., & Ellen, J. M. (2002). Attitudes about sexual disclosure and perceptions of stigma and shame. Sexually Transmitted Infections, 78(5), 334–338. 10.1136/sti.78.5.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, E., Jones, R., Tipene-Leach, D., Walker, C., Loring, B., Paine, S.-J., & Reid, P. (2019). Why cultural safety rather than cultural competency is required to achieve health equity: A literature review and recommended definition. International Journal for Equity in Health, 18(1), 174. 10.1186/s12939-019-1082-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison, H. J., Bromhead, C., Grainger, R., Dennison, E. M., & Jutel, A. (2017). Barriers to sexually transmitted infection testing in New Zealand: A qualitative study. Australian and New Zealand Journal of Public Health, 41(4), 432–437. 10.1111/1753-6405.12680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison, H. J., Woods, L., Bromhead, C., Kennedy, J., Grainger, R., Jutel, A., & Dennison, E. M. (2018). Healthcare-seeking behaviour of people with sexually transmitted infection symptoms attending a Sexual Health Clinic in New Zealand. The New Zealand Medical Journal, 131(1481), 40–49. https://pubmed.ncbi.nlm.nih.gov/30161111 [PMC free article] [PubMed] [Google Scholar]
- Environmental Science and Research Ltd. (2021). New Zealand Sexually Transmitted Infection (STI) Surveillance Dashboard. Retrieved January 29, 2021 from https://www.esr.cri.nz/our-services/consultancy/public-health/sti/.
- FitzGerald, C., & Hurst, S. (2017). Implicit bias in healthcare professionals: A systematic review. BMC Medical Ethics, 18(1), 19–19. 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves, D. S. (2011). Revised You're Welcome criteria and future developments in adolescent healthcare. Journal of Clinical Research in Pediatric Endocrinology, 3(2), 43–50. 10.4274/jcrpe.v3i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathorn, E., Land, L., & Ross, J. D. (2011). How to assess quality in your sexual health service. Sexually Transmitted Infections, 87(6), 508–510. 10.1136/sextrans-2011-050107 [DOI] [PubMed] [Google Scholar]
- Health Quality & Safety Commission . (2019). A window on the quality of Aotearoa New Zealand’s health care 2019 – A view on Māori health equity | He matapihi ki te kounga o ngā manaakitanga ā-hauora o Aotearoa 2019 – He tirohanga ki te ōritenga hauora o te Māori. https://www.hqsc.govt.nz/assets/Health-Quality-Evaluation/PR/Window_2019_web_final.pdf.
- Health Quality and Safety Commission New Zealand. (2019). Primary care patient experience survey. https://www.hqsc.govt.nz/our-programmes/health-quality-evaluation/projects/patient-experience/.
- Houkamau, C. (2016). What you can’t see can hurt you. How do stereotyping, implicit bias and stereotype threat affect Mäori health? MAI Journal: A New Zealand Journal of Indigenous Scholarship, 5(2), 124–136. 10.20507/MAIJournal.2016.5.2.3 [DOI] [Google Scholar]
- Howarth, A. R., Day, S., Greene, L., & Ward, H. (2017). They made me feel comfortable: A comparison of methods to measure patient experience in a sexual health clinic. BMC Health Services Research, 17(1), 325. 10.1186/s12913-017-2264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, E. C., Bulu, S., Harris, J., Humphreys, D., Malverus, J., & Gray, N. J. (2013). “Be kind to young people so they feel at home”: A qualitative study of adolescents’ and service providers’ perceptions of youth-friendly sexual and reproductive health services in Vanuatu. BMC Health Services Research, 13, 455. 10.1186/1472-6963-13-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean, R. (2018). Resources to address stigma related to sexuality, substance use and sexually transmitted and blood-borne infections. Canada Communicable Disease Report, 44(2), 62–67. 10.14745/ccdr.v44i02a05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matich, P., Harvey, C., Page, P., Johnston, K., Jukka, C., Hollins, J., & Larkins, S. (2015). Young people’s perceptions of sexual and reproductive health in regional and rural Queensland: Capturing the views of adolescents through reference groups and a user-friendly electronic survey. Sexual Health, 12(3), 231–239. 10.1071/SH13131 [DOI] [PubMed] [Google Scholar]
- Mazur, A., Brindis, C. D., & Decker, M. J. (2018). Assessing youth-friendly sexual and reproductive health services: A systematic review. BMC Health Services Research, 18(1), 216–216. 10.1186/s12913-018-2982-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health New Zealand . (2009). Evaluation of youth one stop shops. Version 1.1 Wellington: Ministry of Health. https://www.health.govt.nz/system/files/documents/publications/youth-one-stop-shop-evaluation-synopsis-v1.1.pdf
- Ministry of Health . (2017). HISO 10001:2017 ethnicity data protocols. Wellington: Ministry of Health, https://www.health.govt.nz/publication/hiso-100012017-ethnicity-data-protocols.
- Ministry of Health . (2021). NZ Health System. Population profile. Population of Hawke’s Bay DHB, Ministry of Health. https://www.health.govt.nz/new-zealand-health-system/my-dhb/hawkes-bay-dhb/population-hawkes-bay-dhb. [Google Scholar]
- Morris, J. L., & Rushwan, H. (2015). Adolescent sexual and reproductive health: The global challenges. International Journal of Gynaecology and Obstetrics, 131(Suppl 1), S40–S42. 10.1016/j.ijgo.2015.02.006 [DOI] [PubMed] [Google Scholar]
- Patton, G. C., Sawyer, S. M., Santelli, J. S., Ross, D. A., Afifi, R., Allen, N. B., Arora, M., Azzopardi, P., Baldwin, W., Bonell, C., Kakuma, R., Kennedy, E., Mahon, J., McGovern, T., Mokdad, A. H., Patel, V., Petroni, S., Reavley, N., Taiwo, K., … Viner, R. M. (2016). Our future: A Lancet commission on adolescent health and wellbeing. The Lancet, 387(10036), 2423–2478. 10.1016/S0140-6736(16)00579-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, E. R., & Kurz, J. (2016). Using Facebook for health-related research study recruitment and program delivery. Current Opinion in Psychology, 9, 38–43. 10.1016/j.copsyc.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris-John, R., Farrant, B., Fleming, T., Bavin, L., Archer, D., Crengle, S., & Clark, T. (2020). Youth19 Rangatahi Smart Survey. Initial Findings: Access to Health Services. Youth19 Research Group, The University of Auckland and Victoria University of. Retrieved January 21, 2021 from https://www.youth19.ac.nz/publications. [Google Scholar]
- Polis, C. B., Hussain, R., & Berry, A. (2018). There might be blood: A scoping review on women’s responses to contraceptive-induced menstrual bleeding changes. Reproductive Health, 15(1), 114–114. 10.1186/s12978-018-0561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics New Zealand . (2021). Age and sex by ethnic group (grouped total responses), for census night population counts, 2006, 2013, and 2018 Censuses. http://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE8317#.
- Sutcliffe, L. J., Sadler, K. E., Low, N., & Cassell, J. A. (2011). Comparing expectations and experiences of care for sexually transmitted infections in general practice: A qualitative study. Sexually Transmitted Infections, 87(2), 131–135. 10.1136/sti.2010.043760 [DOI] [PubMed] [Google Scholar]
- Tipene, J., Green, A. (2017). He Pukenga Korero: Rangatahi and STIs in the Waikato. Online report. Retrieved December 18, 2020 from http://tewhariki.org.nz/assets/He-Pukenga-Korero-Final-Report-31-Aug-2017.pdf.
- Turner, L., Spencer, L., Strugnell, J., Di Tommaso, I., Tate, M., Allen, P., Cheek, C., Cooper, J., & Chang, J. (2017). Young people have their say: What makes a youth-friendly general practice? Australian Family Physician, 46(1), 70–74. [PubMed] [Google Scholar]
- Tylee, A., Haller, D. M., Graham, T., Churchill, R., & Sanci, L. A. (2007). Youth-friendly primary-care services: How are we doing and what more needs to be done? Lancet, 369(9572), 1565–1573. 10.1016/S0140-6736(07)60371-7 [DOI] [PubMed] [Google Scholar]
- Weston, R. L., Hopwood, B., Harding, J., Sizmur, S., & Ross, J. D. C. (2010). Development of a validated patient satisfaction survey for sexual health clinic attendees. International Journal of STD & AIDS, 21(8), 584–590. 10.1258/ijsa.2010.010159 [DOI] [PubMed] [Google Scholar]
- Whitaker, C., Stevelink, S., & Fear, N. (2017). The use of facebook in recruiting participants for health research purposes: A systematic review. Journal of Medical Internet Research, 19(8), e290 10.2196/jmir.7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . (2017). Fact sheets on sustainable development goals: Health targets. Sexual and Reproductive Health. http://www.euro.who.int/__data/assets/pdf_file/0005/348008/Fact-sheet-SDG-SRH-FINAL-04-09-2017.pdf?ua=1.
- World Health Organization . (2012). Making health services adolescent friendly: Developing national quality standards for adolescent-friendly health services. https://www.who.int/maternal_child_adolescent/documents/adolescent_friendly_services/en/
- World Health Organization . (2016). Global health sector strategy on sexually transmitted infections 2016–2021. Towards ending STI. http://apps.who.int/iris/bitstream/10665/246296/246291/WHO-RHR-246216.246209-eng.pdf?ua=246291.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available. Due to the nature of this research, participants of this study were not asked for permission for their data to be shared publicly, so supporting data are not available.