Abstract
Cyclin E-cdk2 is a critical regulator of cell cycle progression from G1 into S phase in mammalian cells. Despite this important function little is known about the downstream targets of this cyclin-kinase complex. Here we have identified components of the pre-mRNA processing machinery as potential targets of cyclin E-cdk2. Cyclin E-specific antibodies coprecipitated a number of cyclin E-associated proteins from cell lysates, among which are the spliceosome-associated proteins, SAP 114, SAP 145, and SAP 155, as well as the snRNP core proteins B′ and B. The three SAPs are all subunits of the essential splicing factor SF3, a component of U2 snRNP. Cyclin E antibodies also specifically immunoprecipitated U2 snRNA and the spliceosome from splicing extracts. We demonstrate that SAP 155 serves as a substrate for cyclin E-cdk2 in vitro and that its phosphorylation in the cyclin E complex can be inhibited by the cdk-specific inhibitor p21. SAP 155 contains numerous cdk consensus phosphorylation sites in its N terminus and is phosphorylated prior to catalytic step II of the splicing pathway, suggesting a potential role for cdk regulation. These findings provide evidence that pre-mRNA splicing may be linked to the cell cycle machinery in mammalian cells.
Progression of a mammalian cell through the various phases of the cell cycle is strictly controlled by the regulated activities of the cyclin dependent kinases (cdks) and their regulatory subunits, the cyclins (for a review, see references 52 and 61). In mammalian cells 8 cdks and over 10 cyclins have so far been identified. The cyclin-kinase complexes are tightly regulated at the level of synthesis and destruction of the cyclin moiety through their association with inhibitors and by posttranslational modifications, including both stimulatory and inhibitory phosphorylation (38, 49, 62).
Entry of cells into G1 from G0 is dependent on the activities of the D-type cyclins in complexes with cdk4 and cdk6 (2, 53, 66). The major role of the cyclin D-cdk4 and -cdk6 complexes is likely to be the phosphorylation of the retinoblastoma gene product Rb (14, 28, 34). Upon phosphorylation, Rb has been shown to release members of the E2F family of transcription factors, which then act to induce the expression of their downstream target genes, including cyclin E, cyclin A, and DNA polymerase α (57, 73). Cyclin E complexed to cdk2 peaks in its activity shortly before S phase (11, 35) and is essential for the entry of cells into DNA synthesis (50, 51, 55, 56). Cyclin E expression is controlled by E2F (10, 12, 17), and the destruction of cyclin E is regulated by phosphorylation followed by ubiquitin-dependent proteolysis (6, 74). Overexpression of cyclin E leads to a shortening of the G1 phase of the cell cycle, and its activity contributes to the phosphorylation of Rb (56). However, while cells lacking a functional Rb molecule apparently no longer require the activity of D-type cyclin-cdk complexes (41, 43, 66), cyclin E-cdk2 activity remains indispensable (51). Ectopic overexpression of cyclin E can override a G1 arrest imposed by either p16INK4a or a phosphorylation-deficient pRb mutant (42), strongly suggesting the existence of other critical downstream targets for cyclin E-cdk2 for S-phase entry.
Such targets may include the Rb-related pocket proteins p107 and p130, which physically associate with cyclin E in a cell-cycle-dependent manner (7, 13, 15, 39, 40, 63, 78). The phosphorylation of p107 and p130 by cdks releases associated E2F transcription factor family members whose activities are required for S phase (4, 27, 44, 77). However, it appears that there are protein targets of cyclin E-cdk2 independent of pRb and its relatives that are essential for DNA synthesis. Studies with large T antigen to collectively inactivate Rb and its related pocket proteins showed that cyclin E-cdk2 activity is still required for S-phase entry (29). Additional cyclin E-cdk2 substrates likely include modulators of transcription factors (21), as well as components of the DNA replication complex such as the single-stranded DNA binding replication protein A and DNA polymerase α (71).
To identify novel cyclin E-cdk2 targets, we have characterized a number of cyclin E-associated proteins that are detected in anti-cyclin E immunoprecipitates of cell lysates. This analysis identified components of the pre-mRNA processing machinery as putative cyclin E-cdk2 targets. Several U2 snRNP proteins, as well as the spliceosome, are specifically immunoprecipitated by anti-cyclin E antibodies. One component of this complex, the spliceosome-associated protein SAP 155, can be efficiently phosphorylated by cdk2 in vitro and is also found phosphorylated in cyclin E complexes in vivo. These combined data provide evidence that some fraction of cyclin E in the cell is associated with the splicing machinery.
MATERIALS AND METHODS
Tissue culture cell lines.
C33A, 293, and SW13 cells were grown as monolayers and ML-1 cells were grown as suspension cultures in Dulbecco’s modified Eagle medium supplemented with nonessential amino acids (GIBCO-BRL) and 10% heat-inactivated fetal calf serum. All cell lines were obtained from the American Type Culture Collection (ATCC).
Immunological reagents.
For the generation of anti-SAP 155 immunoreagents, a cDNA plasmid library constructed in pSPORT (GIBCO-BRL) from RNA isolated from U937 cells (ATCC) as recently described (5) was used. Plating and screening of the library with a random primed probe derived as a PacI-EcoRI fragment from an expressed sequence tag (EST) clone encoding the N-terminal third of SAP 155 (GenBank accession number R96476) and isolation of positive clones were carried out following standard molecular biology procedures (58). A cDNA fragment containing the N-terminal 493 amino acid residues of SAP 155 (SAP155 N) isolated from the U937 cDNA library was cloned into pGEX-10N (Pharmacia) and transformed into Escherichia coli BL21 (GIBCO-BRL). Recombinant fusion protein was prepared as described after induction of fusion protein synthesis by the addition of 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at room temperature for 3 h (39). BALB/c mice and rabbits were immunized by standard protocols (24). Antisera were purified with protein A-Sepharose (Pharmacia) and cross-linked to protein A-Sepharose beads (Pharmacia) by dimethylpimelimidate (Pierce) as described previously (24). Cyclin E- specific monoclonal antibodies have been described (39). The cdk2-specific antisera used for Western analysis were purchased from Santa Cruz Biotechnology, Inc. The cdk2-specific antibodies used for immunoprecipitations were raised in rabbits against a C-terminal peptide covalently linked to keyhole limpet hemacyanin. Pab419 was as described previously (22). The monoclonal antibodies used to precipitate cyclin A and p107 have been previously described (15, 79). SAP 145-specific antisera were generated by the injection of recombinant glutathione-S-transferase (GST)–SAP 145 containing residues 279 through 575 (18) into rabbits.
Immunoprecipitations and Western analysis.
For all immunoprecipitations, cells were lysed in lysis buffer containing 150 mM NaCl, 50 mM HEPES (pH 7.0), 0.1% Nonidet P-40, and a cocktail of inhibitors (Pefabloc, 125 μg ml−1; aprotinin, 5 μg ml−1; leupeptin, 5 μg ml−1; pepstatin, 5 μg ml−1; 5 mM NaF; 0.1 mM sodium orthovanadate; 1 μM microcystin LR). Preclearing of lysates was performed by incubation with normal rabbit serum at 4°C for 30 min followed by absorption to Staphylococcus aureus cell pellets (Zysorbin; Zymed). Immunoprecipitation was carried out as described previously (23). For metabolic labeling, cells were starved for methionine by incubation in methionine-free Dulbecco modified Eagle medium for 30 min and subsequently labeled for 4 h with 500 μCi to 1 mCi of 35S-methionine (Amersham) per 10 ml of cells at medium density. Two washes with ice-cold phosphate-buffered saline buffer were applied before cell lysis. For metabolic labeling with [32P]orthophosphate, cells were incubated in phosphate free medium (ICN) in the presence of 32Pi (Amersham) for 2 h before harvesting and cell lysis. For immunoprecipitations and Western analyses, 500 μg of cell lysate was used per reaction. Antibodies were cross-linked to beads unless otherwise specified by standard procedures (24). Reimmunoprecipitations were carried out after disruption of complexes by the addition of 1% sodium dodecyl sulfate (SDS) and incubation at 95°C for 5 min. Samples were diluted 20-fold before the addition of reimmunoprecipitating antibody and incubation at 4°C on a rocker. For immunoprecipitations under denaturing conditions, lysates were boiled in the presence of 1% SDS, diluted, precleared with normal rabbit serum, and then incubated with specific antibodies as described above.
For Western analysis, cell lysates or immune complexes were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to polyvinylidene difluoride membrane (Millipore), and blocked and probed with the primary antibody as described previously (39). Polyclonal antisera were used at a 1:1,000 dilution, and monoclonal antibodies were used at a 1:2 dilution. Secondary antibodies were peroxidase-conjugated goat anti-rabbit or anti-mouse antibodies (Amersham) and were used according to the supplier’s recommendations. Protein bands were visualized by using chemiluminescence (ECL; Amersham).
For purification and sequencing of peptides associated with cyclin E, immunoprecipitations were carried out with lysates from 2 × 109 ML-1 cells with monoclonal antibody HE172 (39) immobilized to protein G-Sepharose beads (Pharmacia; see below). Proteins were separated on SDS–6% polyacrylamide gels and copper stained according to the manufacturer’s recommendations (Bio-Rad). Bands of interest were excised and sent for sequencing at Harvard Microchem (Cambridge, Mass.).
Epitope mapping of cyclin E monoclonal antibodies.
By deletion analysis all cyclin E monoclonal antibodies used were found to recognize the C-terminal 80 amino acids of cyclin E. For a fine mapping of this region, overlapping peptides encoding portions of the cyclin E C terminus synthesized onto paper (Research Genetics) were incubated with the respective monoclonal antibodies, and the signals were detected by Western analysis (see above).
In vitro binding assays.
For the in vitro binding assays, 2.5 μg of GST fusion proteins was incubated with 5 μl of freshly in vitro-translated proteins (TNT system 35S-methionine; Promega) in a small volume (10 to 20 μl) at room temperature for 10 min. After dilution to 200 μl in 250 mM NaCl–50 mM HEPES (pH 7.0)–0.1% Nonidet P-40–2% bovine serum albumin, samples were incubated for 1 h at 4°C in the presence of glutathione-agarose (Sigma) while being rocked. After washing, proteins bound to glutathione-agarose were separated by SDS-PAGE, and 35S-labeled proteins were visualized by autoradiography of dried, fixed, and amplified (Amplify; Amersham) gels.
Yeast two-hybrid analysis.
An N-terminal portion of SAP 155 encoding residues 1 through 493 was fused to the Gal4 DNA activation domain in pGBT8 (5a). Full-length cyclin E was integrated into pGAD-GH to yield a cyclin E-Gal4 activation domain fusion protein. After cotransfection into yeast strain CG1945 (Clontech), yeast cells were plated onto tryptophan and leucine-deficient medium by using the EZ-yeast transformation kit (Bio 101). Colonies were subsequently patched onto plates with or without histidine to assay for protein-protein interaction. For control experiments, full-length cdk2 was expressed as Gal4 DNA binding domain fusion protein, while p21 and stathmin (p18) were expressed as Gal4 activation domain fusion proteins.
Kinase assays.
For kinase assays, 300 μg of cell lysate was immunoprecipitated as described above. After collection of the immune complexes on protein A- or protein G-Sepharose (Pharmacia), beads were washed three times with lysis buffer followed by one wash in kinase buffer (100 mM Tris-HCl, pH 7.4; 10 mM MgCl; 1 mM dithiothreitol). The beads were resuspended in 25 μl of kinase buffer supplemented with 1 μM ATP, 2 μCi of [γ-32P]ATP (Amersham), and either 2.5 μg of histone H1 (Boehringer) or recombinant GST-SAP 155 N, followed by incubation at 30°C for 30 min. Reactions were stopped by the addition of 2× SDS-PAGE sample buffer. In the case of endogenous kinase assays, no substrate was added. Where specified, quantification of incorporated 32P was performed on a Molecular Dynamics Phosphorimager by using Imagequant software.
V8 proteolytic digest.
Proteolytic mapping with Staphylococcus aureus V8 protease was performed as described previously (24).
Immunoprecipitation of snRNA and pre-mRNA from spliceosome.
For immunoprecipitations of snRNA from nuclear extracts, RNA was extracted from immune complexes and resolved by electrophoresis on 8% denaturing polyacrylamide gels. For immunoprecipitations of pre-mRNA, spliceosomal complexes were assembled on biotinylated, 32P-labeled AdML pre-mRNA and isolated by gel filtration (54). Immunoprecipitated counts were detected by scintillation counting, and the percentage recovery of total input radioactivity was determined.
RESULTS
U2 snRNP proteins are detected in cyclin E immunoprecipitations.
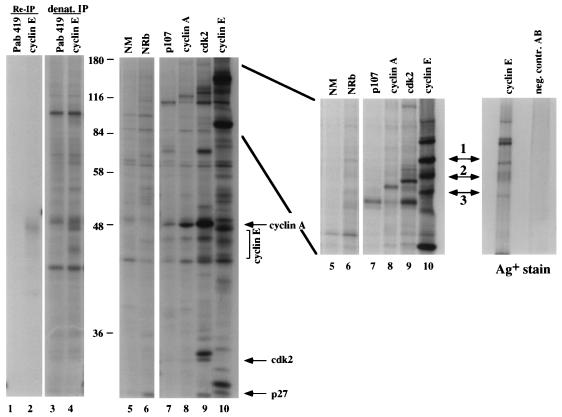
To identify novel cyclin E-associated molecules, immunoprecipitations were performed from 35S-methionine-labeled ML-1 cell lysates with monoclonal antibodies raised against cyclin E. Proteins coprecipitating with cyclin E from this cell line are shown in Fig. 1 (left panel, lane 10). Parallel immunoprecipitations with antibodies against p107, cyclin A, and cdk2 (Fig. 1, left panel, lanes 7, 8, and 9, respectively) allowed us to discriminate between known cyclin E-associated molecules (e.g., p27 and cdk2) and novel cyclin E-interacting polypeptides. A number of unique cyclin E-associated proteins in the range of 110 to 180 kDa were observed (Fig. 1, middle panel, lane 10), including p107 and p130, which can be seen most clearly precipitating with cyclin A (lane 8). Three unique proteins (Fig. 1, middle panel, arrows 1 to 3) were selected for further analysis. Immunoprecipitations performed under denaturing conditions (Fig. 1, left panel, lane 4), reimmunoprecipitations of disrupted cyclin E-protein complexes (Fig. 1, left panel, lane 2), and control immunoprecipitations with normal mouse and normal rabbit serum (Fig. 1, left panel, lanes 5 and 6, respectively) were applied to verify that these polypeptides were specifically precipitated through their association with cyclin E.
FIG. 1.
Identification of novel cyclin E-associated proteins. Cyclin E was immunoprecipitated from thymidine-blocked ML-1 cells by using monoclonal antibody HE172 (left and middle panels, lane 10), and coprecipitating protein bands were visualized by silver staining (Bio-Rad) on SDS–6% PAGE (right panel, left lane). In parallel, immunoprecipitations from [35S]methionine-labeled ML-1 cells with either control antibody (NM, normal mouse serum, lane 1; NRb, normal rabbit serum, lane 2) or antiserum against cyclin A (lane 4), cdk2 (lane 5), or p107 (lane 3) were performed, and the bands were visualized by autoradiography after SDS-PAGE. Reimmunoprecipitations and immunoprecipitations under denaturing conditions were carried out with HE172 (lanes 2 and 4, respectively) and Pab419 as a negative control antibody (lanes 1 and 3). Panels: left, SDS–12% PAGE; middle, SDS–6% PAGE; right, silver-stained gel of SDS–6% PAGE. Novel cyclin E-associated proteins are labeled 1 through 3. Protein bands were excised and microsequenced.
The three protein bands were isolated from the polyacrylamide gel, and peptide sequences were obtained from tryptic digestion products (Fig. 1, middle panel, arrows 1 to 3; Table 1). Based on these sequences, bands 2 and 3 were identified as SAP 145 and SAP 114 (SF3a120), respectively (18, 36), with their migration on SDS-PAGE corresponding well with their predicted molecular weights. Peptides from a third protein (protein band 1) matched a novel cDNA independently isolated as SAP 155, a component of the spliceosomal complex A (59a, 72). A fourth cyclin E-associated protein yielded a peptide sequence homologous to the 28-kDa B/B′ snRNP-associated core protein (59; data not shown).
TABLE 1.
Peptide sequence information from protein bands 1 through 3 associated with cyclin E in anti-cyclin E immunoprecipitationsa
| Protein band no. | Peptide sequence (N–C) | Identified protein | Reference |
|---|---|---|---|
| 1 | LLVDVDESTLSPEKQK | SAP 155 | 72 |
| VGAAEIISR | |||
| 2 | QTGIVLNRPVLR | SAP 145 | 18 |
| AAVLLEQER | |||
| 3 | IHEATGMPAGK | SAP 114 and SF3a120 | 36 |
For the numbering of protein bands, see Fig. 1. Peptide sequences from two tryptic peptides each of SAP 145 and SAP 155 are listed.
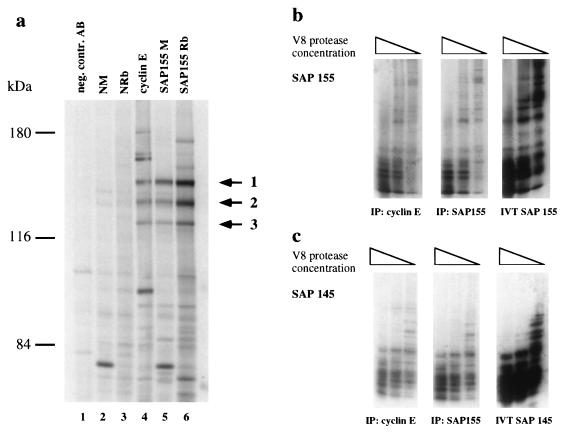
Polyclonal mouse and rabbit antibodies raised against SAP 155 were used to immunoprecipitate SAP 155 and associated proteins from 35S-methionine-labeled ML-1 cell lysates in order to compare the generated protein band pattern to the one obtained from cyclin E immune complexes (Fig. 2a). Significantly, both SAP 155-specific antisera (Fig. 2a, lanes 5 and 6) precipitated the same three protein bands (Fig. 2a, arrows) that were detected as cyclin E-associated proteins in cyclin E immunoprecipitates (Fig. 1, lane 6; Fig. 2, lane 4). These three protein bands were absent in the three negative control immunoprecipitates with an isotype control antibody, normal mouse serum, or normal rabbit serum (Fig. 2a, lanes 1, 2, 3, respectively). The comigration of the three proteins detected in the individual immunoprecipitates suggested that they are the same set of proteins. To confirm this hypothesis and our initial microsequencing results, cDNAs encoding SAPs 145 and 155 were in vitro translated in rabbit reticulocyte lysates to produce proteins that could then be analyzed by V8 partial proteolytic digests generating a characteristic protein fingerprint (Fig. 2b and c, right panels). V8 partial proteolytic mapping of corresponding polypeptides from cyclin E (Fig. 2a, lane 4) and SAP 155 (Fig. 2a, lane 6) immunoprecipitates generated a pattern identical to the one derived from the respective in vitro-translated proteins (Fig. 2b and c, left and middle panels). The SAP 155 cDNA carries an internal deletion that yields a protein lacking 82 amino acid residues; this accounts for the slight differences observed in the V8 pattern (72). These analyses showed that protein bands 1 and 2 (Fig. 2a) detected in both cyclin E and SAP 155 immunoprecipitates correspond to SAP 155 and SAP 145, a finding consistent with the peptide sequence information obtained in our initial characterization. A similar analysis confirmed the identity of band 3 in Fig. 2a as SAP 114 (data not shown). These SAPs are components of U2 snRNP (3, 64) and both SAP 145 and SAP 114 can be coprecipitated with antiserum specific to SAP 155 (Fig. 2a, lanes 5 and 6). We thus conclude that the three proteins detected in cyclin E immunoprecipitations are components of the pre-mRNA splicing machinery.
FIG. 2.
SAP 114, SAP 145, and SAP 155 are present in anti-cyclin E immune complexes. (a) Mouse (SAP 155 M, lane 6) and rabbit (SAP 155 Rb, lane 7) polyclonal antisera were used in immunoprecipitations from [35S]methionine-labeled ML-1 cells. NM, normal mouse serum, lane 2; NRb, normal rabbit serum, lane 3; negative control antibody, Pab419, lane 1. Proteins were visualized after SDS-PAGE (6%) and autoradiography. 35S-labeled protein bands 1, 2, and 3 were cut from the gel and used for V8 proteolytic digest. (b) V8 proteolytic digest of SAP 155 detected in cyclin E complexes (left panel) and SAP 155 precipitates (middle panel) and from in vitro-translated SAP 155 (right panel). (c) V8 analysis of SAP 145 as described for panel b.
Characterization of the cyclin E-SAP 155 complex.
To provide further proof for the existence of the cyclin E-SAP 155 association in cells, we prepared immunoprecipitates from ML-1 cell lysates using a number of cyclin E monoclonal antibodies that recognize different epitopes (Fig. 3a) but which are all capable of precipitating cdk2 from cells (data not shown). By Western blot analysis we found that all of the tested cyclin E antibodies (except HE67, which recognizes a distinct epitope in the cyclin E C terminus) coprecipitate SAP 155 (Fig. 3a, lanes 2 to 6), showing that the association between cyclin E and SAP 155 can be detected in an epitope-independent fashion. To show that the observed association between cyclin E and SAP 155 depends on the presence of cyclin E, cell lysates were immunodepleted of cyclin E by preclearing with cyclin E-specific antibodies (Fig. 3b). This treatment effectively removed all of the detectable cyclin E while only slightly reducing total SAP 155 levels as shown by Western analysis (Fig. 3b, lanes 3 and 4). Cyclin E immunoprecipitations from these precleared lysates failed to precipitate SAP 155 (Fig. 3b, lane 2), whereas SAP 155 was still present in cyclin E complexes from extracts precleared with a control antibody (Fig. 3b, lane 1), demonstrating that the presence of cyclin E is essential to detect this association.
FIG. 3.
Specific association of cyclin E-cdk2 with SAP 155. Immunoprecipitation and Western analysis of SAP 155 association with cyclin E-cdk2 in ML-1 lysates. (a) In the left panel, an immunoprecipitation with the cyclin E-specific monoclonal antibodies HE67, 111, 135, 170, and 172 (lanes 2 through 6) is shown. Control immunoprecipitations were performed with an equivalent amount of coupled monoclonal antibody Pab419 (lane 1). All lysates were from ML-1 cells. Lysate control lanes contained 100 μg of cellular protein (lane 7). IP, immunoprecipitation. In the right panel is shown an epitope mapping of cyclin E-specific monoclonal antibodies. Peptides of the indicated overlapping sequences were synthesized onto paper (Research Genetics) and probed with the respective antibodies listed on the righthand side. (b) Detection of SAP 155 in HE172 immune complexes from ML-1 lysates depends on the presence of cyclin E protein in lysates. Cell lysates were precleared with the indicated antibodies three times. SAP 155 (upper panel) and cyclin E (lower panel) were detected in precleared lysates (lanes 3 and 4) or in cyclin E, and control antibody immune complexes were prepared from precleared lysates (lanes 2 and 1, respectively). (c) SAP 155 complexes were precipitated from lysates by using rabbit polyclonal SAP 155-specific antibody (lane 2). Coprecipitating cyclin E was detected with the cyclin E-specific monoclonal antibody HE12. The arrow indicates the top band of cyclin E detected in SAP 155 immune complexes. NRb, normal rabbit serum (lane 1). (d) Detection of SAP 155 associated with cdk2 (lane 2). NRb, normal rabbit serum (lane 1). (e) cdk2 associated with SAP 155 (lane 2) was detected by using antibody Sc-163 (Santa Cruz Biotechnology). NRb, normal rabbit serum (lane 1). (f) Absence of SAP 155 from cyclin A-specific immune complexes. Cyclin E (lane 3), cyclin A (lane 2), and control antibody immune complexes were tested for the presence of SAP 155 (top panel) and cdk2 (lower panel) by Western analysis. (g) SAP 155 associated with cyclin E complexes in different cell lines. Negative control, Pab419 (lanes 1, 2, 5, and 7); cyclin E-specific antibody, HE172 (lanes 2, 4, 6, and 8). SAP 155 detection was assessed by Western analysis by using polyclonal rabbit SAP 155 serum.
We next performed immunoprecipitations with antibodies to SAP 155 and were able to clearly demonstrate the presence of cyclin E associated with SAP 155 (Fig. 3c, lane 2) and its absence from control immunoprecipitations (Fig. 3c, lane 1). Thus, we can demonstrate the existence of a cyclin E-SAP 155 association in cells through both components.
To further analyze the association between cyclin E and U2 snRNP, we wished to establish whether cdk2, the catalytic partner of cyclin E, was also present in cyclin E-SAP 155 complexes. cdk2 immunoprecipitations were performed and tested for the presence of SAP 155. Indeed, SAP 155 was found to coprecipitate with cdk2 (Fig. 3d, lane 2). Likewise, we could clearly detect the presence of cdk2 in SAP 155 immune complexes (Fig. 3e, lane 2). Since cdk2 is also known to bind to and be activated by cyclin A, we analyzed whether this cyclin subunit can also be detected in complexes with SAP 155. In contrast to the cyclin E complexes, the cyclin A immunocomplexes from asynchronously growing HeLa cells did not contain detectable amounts of SAP 155, whereas the presence of cdk2 was readily detected (Fig. 3f, lane 2), indicating a specificity for cyclin E with regard to SAP 155 interaction (see also Discussion). Together these data provide strong evidence for the existence of a complex containing SAP 155, SAP 145, SAP 114, and cyclin E, a complex we have designated the U2/E/k2 complex.
Since most of our analysis has been performed in either ML-1 cells or HeLa cells, we also analyzed several other cell lines for the existence of the U2/E/k2 complex. Cyclin E immunoprecipitates were prepared from four different cell lines, and in all of them SAP 155 was clearly detected specifically in the anti-cyclin E immunoprecipitations (Fig. 3a, lanes 2, 4, 6, and 8) but not in control antibody precipitates (Fig. 3a; lanes 1, 3, 5, and 7). We therefore conclude that the U2/E/k2 complex is found in a variety of different cell types.
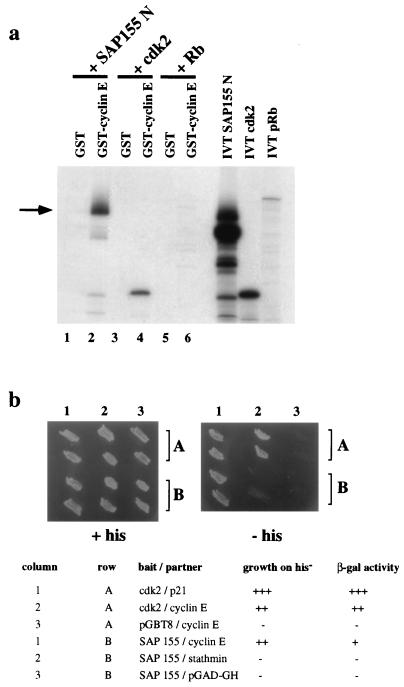
As an antibody-independent approach to test the binding of cyclin E to SAP 155, the N-terminal portion of SAP 155 (SAP 155 N, residues 1 to 493) was in vitro translated and tested in in vitro binding assays for its association with GST-cyclin E produced in E. coli. As shown in Fig. 4a, a strong binding of SAP 155 N to GST-cyclin E was observed (lane 2), while no interaction with the GST moiety was detected (lane 1). As expected, in parallel control experiments, cdk2 was found to also associate efficiently with GST-cyclin E but not with GST alone (Fig. 4a, lanes 3 and 4), while in vitro-translated Rb did not form any complexes with GST-cyclin E or GST (lanes 5 and 6).
FIG. 4.
Antibody-independent proof for SAP 155 and cyclin E association. (a) In vitro binding of SAP 155 to GST-cyclin E. N-terminal portion of SAP 155 (residues 1 to 493) was in vitro translated and mixed with GST or GST-cyclin E. The arrow indicates the enriched top band after binding to GST-cyclin E. Full-length cdk2 and pRB were in vitro translated and used in binding experiments as positive and negative controls, respectively. (b) Yeast two-hybrid interaction of SAP 155 and cyclin E. Two yeast colonies per transformation harboring the expression constructs listed below the panels were patched onto selective medium with (left panel) or without histidine (right panel). β-Galactosidase activity levels were indicated by blue color formation within 10 min (+++), 30 min (++), and 2 h (+).
The interaction of SAP 155 with cyclin E could also be demonstrated with the yeast two-hybrid system (Fig. 4b). SAP 155 N was fused to the Gal4 DNA binding domain (Gal4 BD) and coexpressed in yeast cells with full-length cyclin E fused to the Gal4 transactivation domain (Gal4 TA). Interaction of SAP 155 N and cyclin E was indicated by growth of the transformed yeast cells on His− medium (Fig. 4, column 1, row B) and the resulting β-galactosidase activity. SAP 155 was ineffective in both assays on its own or when coexpressed with a nonspecific protein partner (Fig. 4b, lanes 2 and 3, row B). As control experiments, full-length cdk2 fused to Gal4 DB was shown to interact with cyclin E-Gal4 TA (lane 2, row A) and with p21-Gal4 TA (lane 1, row A), whereas cyclin E alone did not display transactivation (lane 3, row A). Taken together, these findings confirm our data obtained from the immunoprecipitation experiments (Fig. 3) indicating the existence of a complex between cyclin E and SAP 155.
Cyclin E associates with the U2 snRNP and the spliceosome.
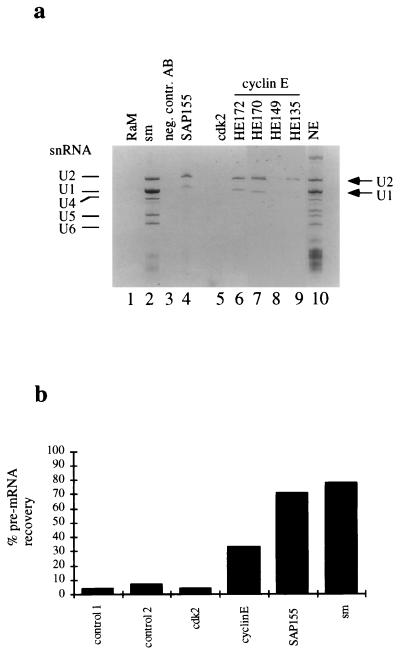
The observation that cyclin E associates with several U2 snRNP proteins suggests that cyclin E antibodies may precipitate the entire U2 snRNP. To test this possibility, cyclin E and SAP 155 immunoprecipitates were prepared from HeLa cell nuclear extracts by using cyclin E-specific antibodies and several control antibodies. RNA was isolated from the immunoprecipitations and resolved by electrophoresis on an 8% denaturing polyacrylamide gel. Both cyclin E and SAP 155 precipitated U2 snRNA (Figure 5a, lanes 4, 6, 7, and 9). The precipitation of U2 snRNA could be shown by using several different cyclin E monoclonal antibodies that recognize distinct epitopes in the C terminus of cyclin E (Fig. 5a, lanes 6, 7, and 9). U1 snRNA was also detected in these complexes because it coprecipitates with U2 snRNAs (30). Antibodies to Sm antigen containing proteins which immunoprecipitate all snRNPs were used as a positive control (Fig. 5a, lane 2). No immunoprecipitation of U2 snRNAs was detected with several negative control antibodies (Fig. 5a, lanes 1 and 3). Immunoprecipitations with a cdk2-specific antibody did not yield detectable levels of U2 snRNA (Fig. 5a, lane 5; see also below and Discussion). This is consistent with our finding that the amount of SAP 155 isolated in anti-cdk2 immunoprecipitates was repeatedly lower than the amount associated with cyclin E (compare Fig. 3a and d). The significance of this observation is currently unknown and may merely reflect the differential accessibility of the antibodies to the different proteins in the complex.
FIG. 5.
Coimmunoprecipitation of U1 or U2 snRNA and AdML pre-mRNA with cyclin E. (a) snRNA was isolated from immunoprecipitations from HeLa nuclear extracts with the indicated antibodies and visualized on an 8% denaturing polyacrylamide gel. RαM, rabbit anti-mouse serum (lane 1); negative control AB, Pab419 (lane 3); HE172, 170, 149, and 135, monoclonal antibodies against cyclin E (lanes 6 through 9, respectively); NE, nuclear extract (lane 10). (b) Spliceosomal complexes were assembled on 32P-labeled AdML pre-mRNA before immunoprecipitation with the indicated antibodies (negative controls: 1, anti-SAP 130 antibody; 2, anti-GST antibody). Immunoprecipitated radioactivity was counted, and recovered counts were given as the percentage of total input.
To determine whether cyclin E antibodies also coimmunoprecipitate the spliceosome, spliceosomal complexes were assembled on 32P-labeled AdML pre-mRNA, isolated by gel filtration, and subsequently used for immunoprecipitation studies. As shown in Fig. 5b, cyclin E antibodies precipitated 33% of the spliceosomes compared to Sm antibodies that precipitated 78% of the spliceosomes. SAP 155 antibodies precipitated the spliceosome with almost the same efficiency as had the Sm antibodies (71%). Again, cdk2-specific antibodies were unable to precipitate detectable levels of the pre-mRNA substrate from the spliceosomal complex. We conclude that cyclin E associates with the U2 snRNP and the spliceosome, while cdk2 may only be detectable in SAP 155 immune complexes.
SAP 155 is a substrate for cyclin E-cdk2.
Of the U2 snRNP proteins, SAP 155 is a particularly attractive target for cyclin E-cdk2 since it contains 26 putative cdk phosphorylation sites (S/T-P-X-basic) clustered in the N-terminal portion of the protein (Fig. 6a, vertical bars above schematic SAP 155 sequence). SAP 155 is phosphorylated specifically in splicing complex C prior to catalytic step II of the splicing pathway by an unidentified protein kinase (72). The coprecipitation of cyclin E with SAP 155 makes cyclin E-cdk2 a likely candidate responsible for the phosphorylation of SAP 155.
FIG. 6.
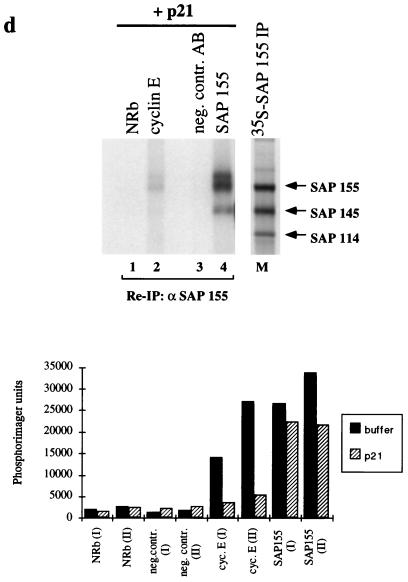
SAP 155 as a substrate for cyclin E-cdk2 both in vitro and in vivo. (a) Schematic representation of SAP 155 domain structure. Filled boxes indicate the TPGH repeats located near the N terminus. The hatched box covering the C-terminal two-thirds of the protein represents the presence of 22 protein phosphatase 2A regulatory subunit-like repeats (72). Vertical bars indicate putative TP and SP phosphorylation sites. (b) In vitro kinase assay. SAP 155 N (amino acid residues 1 through 493) fused to GST and SAP 155 N fused to histone H1 were used as substrates for recombinant cdk2 (lane 1) or cyclin E-cdk2 (lanes 2 through 5). Increasing amounts of cyclin E containing Sf9 lysate (0, 0.2, 0.5, 1, and 3 μl, lanes 2 through 5, respectively) were added to 3 μl of cdk2-containing Sf9 lysate before immunoprecipitation of the formed complexes through the HA-epitope (monoclonal antibody 12CA5; BABCO). Phosphorylated products were separated on SDS-PAGE and visualized by autoradiography for 15 min at room temperature. (c) Immunoprecipitation and kinase assays. Immunoprecipitates from lysates of asynchronously growing ML-1 cells with the indicated antibodies (lane 1, negative control AB [Pab419]; lane 3, normal rabbit antiserum; lane 2, HE172; lane 4, SAP 155) were incubated in the presence of [γ-32P]ATP. Phosphorylated SAP 155 was reprecipitated after the disruption of complexes with SAP 155-specific antibodies. Lane M, SAP 155 immunoprecipitate from 35S-methionine-labeled ML-1 cell lysate was loaded for molecular size comparison. The migration of SAP 155, SAP 145, and SAP 114 is indicated by arrows. (d) Immune complexes precipitated with the indicated antibodies were preincubated with 1 μg of recombinant p21 or an equivalent volume of buffer control (see Fig. 5c) before the addition of [γ-32P]ATP. Reimmunoprecipitated SAP 155 was separated on an SDS–6% polyacrylamide gel and visualized by autoradiography. Phosphorylated products were quantified with a Phosphorimager (lower panel). The units are arbitrary Phosphorimager counts. I and II denote different phosphorylated species of SAP 155 as marked. (e) Immunoprecipitation of SAP 155 and SAP 145 from [32P]orthophosphate-labeled ML-1 cell lysates. (Left panel) Lanes: 1, negative control AB, Pab419; 2, normal rabbit serum; 3, HE172, cyclin E; 4, SAP 155; marker lane (M), [35S]methionine-labeled SAP 155 immunoprecipitate as described for panel c. (Right panel) Disrupted immune complexes generated with the antibodies indicated above the lanes were reprecipitated with antiserum specific for either SAP 155 (lanes 1 through 4) or SAP 145 (lanes 5 through 7). The phosphorylated products were separated on SDS–6% PAGE.
We therefore investigated whether SAP 155 could serve as a cdk substrate by using recombinant baculovirus expressed cyclin E-cdk2 complexes. GST-SAP 155 N was used as the substrate, with histone H1 serving as a positive control (Fig. 6b). Increasing amounts of cyclin E (Fig. 6b, lanes 2 to 5) were preincubated with cdk2, immunoprecipitated through the epitope tag on the cdk2 molecule, and added to a kinase reaction containing recombinant GST-SAP 155 N (Fig. 6b, lower panel). Histone H1 was used as a positive control substrate (Fig. 6b, upper panel). No kinase activity was observed after the addition of cdk2 alone (lane 1), whereas addition of preformed cyclin E-cdk2 complexes led to the efficient phosphorylation of the GST-SAP 155 protein in a cyclin-dependent manner (Fig. 6b, lanes 2 through 5).
We next examined whether SAP 155 protein endogenously present in U2/E/k2 complexes could also serve as a cyclin E-cdk2 substrate. Immunoprecipitations from ML-1 lysates were performed as before, followed by an incubation of the precipitated proteins with [γ-32P]ATP. Phosphorylated SAP 155 was visualized by SDS-PAGE after reimmunoprecipitation of the disrupted complexes with antisera specific to SAP 155. As shown in Figure 6c, we detected phosphorylated SAP 155 in anti-cyclin E immunoprecipitates (lane 2) and in positive control SAP 155 immune complexes (lane 4), while no signal was obtained with normal rabbit serum or a monoclonal isotype control antibody (Fig. 6c, lanes 1 and 3, respectively). The phosphorylated SAP 155 migrated as two bands (Fig. 6c, I and II) shifted with respect to SAP 155 precipitated from a lysate of 35S-methionine-labeled cells (Fig. 6c, righthand side), possibly due to its phosphorylation. Importantly, these results indicate that in cyclin E complexes, SAP 155 can serve as a substrate for a kinase that is also present in these same complexes.
In a parallel set of experiments, cyclin E immunoprecipitations were preincubated with purified p21, a known inhibitor of cdk2 (25, 26, 76), prior to the kinase assay (Fig. 6d). Figure 6c represents the respective control experiment with preincubation of the complexes in an equal volume of buffer. Quantitation of the phosphorylated SAP 155 obtained by inclusion of the p21 preincubation step showed that over 80% of the SAP 155 phosphorylation in cyclin E complexes was inhibited by p21 (Fig. 6d, lane 2, both species, I and II; for quantitation, see Fig. 6d, lower panel) when compared to the buffer control experiment (Fig. 6c, lane 2), suggesting that the major kinase activity associated with SAP 155 in cyclin E immune complexes is sensitive to p21 and likely consists of cyclin E-cdk2. Similar results were obtained using the closely related inhibitor molecule p27 (data not shown). When SAP 155 immune complexes were tested for their inherent kinase activity by incubation with [γ-32P]ATP, SAP 155 was again found to be efficiently phosphorylated and appeared as two differently migrating species on SDS-PAGE (Fig. 6c, I and II). Treatment of SAP 155 immune complexes with p21 reduced the level of SAP 155 phosphorylation by up to 30%, demonstrating the presence of cdk activity in those complexes. The existence of other kinases in the SAP 155 complex is suggested by the residual activity remaining after p21 treatment (Fig. 6d, lane 4 and lower panel). The identity of these p21-insensitive, SAP 155-associated kinases is currently unknown. They are likely to be distinct from the other cell-cycle-dependent kinases, since neither cdc2 nor the D-type cyclin-associated kinases cdk4 and cdk6 were detected in SAP 155 immunoprecipitates (data not shown). Taken together, these findings indicate the existence of at least two distinct SAP 155-containing complexes in the cell, both of which display kinase activity, albeit with different sensitivities to the universal cdk inhibitor p21.
Since SAP 155 is efficiently phosphorylated by cyclin E-cdk2 in vitro and also serves as an endogenous substrate in cyclin E complexes from cells, we attempted to detect phosphorylated SAP 155 in asynchronously growing cells metabolically labeled with 32Pi. Immunoprecipitations from 32P-labeled ML-1 lysates showed the presence of a strongly phosphorylated band in both cyclin E and SAP 155 complexes (Fig. 6e, left panel, lanes 3 and 4, respectively) comigrating with 35S-methionine-labeled SAP 145 (Fig. 6e, marker lane M). No phosphorylated polypeptides were detected in control immunoprecipitations (Fig. 6e, left panel, lanes 1 and 2). By using antisera raised against SAP 145, we confirmed the identity of the strongly phosphorylated band as SAP 145 by reimmunoprecipitation of disrupted cyclin E complexes (Fig. 6e, right panel, lane 6). The reprecipitated protein is recognized by the SAP 145-specific antiserum and comigrates with the 32P-labeled SAP 145 protein after SDS-PAGE (Fig. 6e, right panel, compare lanes 6 and 7). SAP 145 contains 10 Ser and Thr residues followed by proline to serve as putative cdk phosphorylation sites (18).
Reimmunoprecipitation of disrupted cyclin E and SAP 155 immune complexes with SAP 155-specific antibodies confirmed the presence of phosphorylated SAP 155 molecules in cyclin E complexes in the cell (Fig. 6e, right panel, lane 3). The phosphorylated SAP 155 present in cyclin E immunoprecipitates comigrated with SAP 155 reprecipitated from SAP 155 immunoprecipitates (Fig. 6e, compare lanes 3 and 4) and was again shifted in its mobility on SDS-polyacrylamide gels with regard to 35S-methionine-labeled SAP 155 (Fig. 6e, compare lanes 2 and 3 to marker lane M). Since SAP 155 is phosphorylated during splicing catalysis (72), we conclude that cyclin E is present in spliceosomes. This finding raises the possibility for a regulatory function for cyclin E in pre-mRNA processing.
DISCUSSION
Our experiments demonstrate the existence of a complex containing both cyclin E and several components of the U2 snRNP in vivo, in particular SAP 155, SAP 145, and SAP 114. We were able to detect the association between both cyclin E and SAP 155, as well as cdk2 and SAP 155, through either of the components in cellular lysates. The cyclin E-SAP 155 association can also be shown in the yeast two-hybrid system and can be reconstituted in vitro by using recombinant components. Furthermore, we found that cyclin E-specific antibodies were able to precipitate U1 and U2 snRNAs, as well as the pre-mRNA substrate from preassembled spliceosomes.
We find that one component of U2 snRNP, SAP 155, serves as an excellent substrate for cyclin E-cdk2 both in the U2/E/k2 complex precipitated from cells and as a recombinant protein in vitro. Phosphorylation of SAP 155 in the U2/E/k2 complex can be inhibited by preincubation of these complexes with p21, a known cyclin-kinase inhibitor. Taken together, these data indicate that the likely kinase activity responsible for SAP 155 phosphorylation in cyclin E precipitates is cdk2. We were unable to detect the presence of any other cyclin-kinase complex in SAP 155 immune complexes, especially one involving cyclin A, which is also capable of binding and activating cdk2. Collectively, these data suggest a role for cyclin E-cdk2 in the phosphorylation and hence the regulation of SAP 155 activity in vivo. SAP 155 is the first non-RS domain-containing spliceosomal protein shown to be phosphorylated, and its phosphorylation is only observed in assembled spliceosomes prior to catalytic step II (72). This strictly controlled regulation of SAP 155 phosphorylation is consistent with our finding that, as determined by [32P]orthophosphate labeling of asynchronously growing cells, only a small pool of SAP 155 was phosphorylated. The presence of phosphorylated SAP 155 in cyclin E complexes therefore suggests that cyclin E is present in actively working spliceosomes and could contribute to the tight regulation of SAP 155 phosphorylation in the cell.
Even though cdk2 was present at low levels in SAP 155 immunoprecipitates and antibodies specific to cdk2 were able to coprecipitate SAP 155, we failed to detect snRNA or pre-mRNA substrates in the respective cdk2 immunoprecipitates. This finding could be explained by a masking of the cdk2 epitope once the spliceosome is fully assembled on a pre-mRNA substrate. Alternatively, only a fraction of the cyclin E-containing spliceosomes might also contain its kinase partner, which is consistent with the idea that cyclin E binds to its target in the spliceosomal complex and by binding to cdk2 brings the kinase into close proximity to its substrate at a specific time during splicing catalysis. Such a scenario could explain the observation that at least in vitro SAP 155 is only phosphorylated in fully assembled, active spliceosomes (72). When analyzing the association of SAP 155 with cyclin E across the cell cycle in fractions of elutriated cells, we did not observe a prominent cell cycle dependence of this association (data not shown). We are currently investigating the binding of cyclin E to SAP 155 in cells exiting quiescence and entering the G1 phase of the cell cycle.
While the cdk-specific inhibitor p21 almost completely blocked phosphorylation of SAP 155 in cyclin E complexes, the majority of the kinase activity precipitated with SAP 155-specific antibodies was insensitive to p21. We conclude that SAP 155 is associated with both p21 sensitive and p21 insensitive kinases, the former one most likely consisting of cyclin E-cdk2. In both our in vitro kinase assays and in immunoprecipitations from [32P]orthophosphate-labeled cells, we detect multiple species of phosphorylated SAP 155 (Fig. 5c and d). SAP 155 could be phosphorylated on multiple sites by different kinases, which might be integrating signals from different pathways to affect splicing catalysis. Phosphorylated SAP 155 was detectable throughout the cell cycle when analyzing elutriated fractions of asynchronously growing ML-1 cells (data not shown). The lack of cell cycle dependence of SAP 155 phosphorylation in cyclin E complexes could be explained by the action of other non-cell-cycle-dependent kinases. Phosphopeptide mapping and two-dimensional gel analysis will be required to address this issue.
To address the question of functionality, we investigated the effects of inhibition of cyclin E-cdk2 activity on in vitro splicing reactions. Using a variety of p21 peptides, recombinant CKI proteins, and control mutants (e.g., of p21 [1]), as well as chemical kinase inhibitors, we were unable to detect an inhibitory effect on the splicing of an adenovirus major late-derived pre-mRNA substrate. Two possible explanations could reconcile the apparent discrepancy between these results and a putative role for cyclin E-cdk2 in the regulation of mRNA processing. First, it could be possible that in this in vitro system we have lost the control exerted by cyclin E-cdk2 in vivo under physiological conditions and in the appropriate environment of the cell nucleus. A second explanation could be that cyclin E-cdk2 displays a pre-mRNA substrate specificity to regulate only a subset of selected pre-mRNA transcripts whose products might be involved in cell cycle progression and growth regulation. Unless these respective pre-mRNA substrates are identified and established for their use in in vitro splicing reactions, the inhibition of cyclin E-cdk2 will be without apparent effect in such assays.
Since the architecture and the direct physical interactions in the U2/E/k2 complex are unknown, SAP 155 is only one of several possible substrates. SAP 145 is phosphorylated in vivo and also contains putative cdk phosphorylation sites. In independent studies with cdk2 as a bait molecule in a yeast two-hybrid screen, we isolated the putative RNA helicase U5-200kD as a candidate cdk2-interacting protein (37, 59b). The U5-200kD RNA helicase is known to be a component of the spliceosome, and we have demonstrated its presence in cyclin E immunoprecipitates from cells. Again, this molecule contains several proline-directed Ser or Thr residues that could serve as phosphorylation sites for regulation by cyclin-cdk complexes.
Previous work has suggested that pre-mRNA splicing is regulated by both phosphorylation and dephosphorylation events (45), perhaps as a function of the cell cycle (see below). Reversible phosphorylation of SR proteins is likely to serve as a regulatory mechanism of splicing in vitro (46, 47, 69, 70), suggesting that similar control mechanisms at the level of the SAP proteins might also exist. Our finding that cyclin E-cdk2 associates with SAPs and can use SAP 155 as a substrate and that SAP 155 seems to be phosphorylated prior to catalytic step II in vitro offers a first indication that such mechanisms might be in place and so provide a link to the cell cycle.
A connection between cell cycle progression and pre-mRNA processing has been implicated by several studies. For example, the dbf series of cell cycle mutants in Saccharomyces cerevisiae are grossly defective in DNA replication and arrest cells before or during S phase (31). Significantly, the dbf3-1 mutation resides in a gene that encodes a protein of the U5 snRNP (60). The USS1 gene encoding a U6 snRNA binding protein was isolated as a multicopy suppressor of dbf2 (9). With regard to mammalian cells, early studies reported a general repression of RNA and protein synthesis in cells entering mitosis (16, 33, 65). In terms of regulation, the phosphorylation status of SR proteins has been shown to be responsible for their subnuclear distribution in a cell-cycle-dependent fashion (8, 19, 48). Furthermore, a recent report by Jumaa et al. (32) shows cell-cycle- dependent differences in the levels of alternatively spliced transcripts from the srp20 splicing factor gene, with transcripts being induced in the late G1 and early S phases.
While the above-mentioned data support the notion that in eukaryotic organisms cell cycle progression and pre-mRNA processing are two biological processes that are not independent but linked to each other, the mechanism of this interplay remains largely obscure. A subset of all pre-mRNAs may be subject to a cell-cycle-dependent regulation of their processing, with the products of these mRNAs encoding components that are required for cell cycle progression. In this context it is interesting to note that indeed recent work has established the existence of a second spliceosome with a different composition of its snRNP components specific for a minor class (AT-AC) of introns (20, 67, 68). Among the genes that contain this type of intron are E2F, cdk5, and other growth regulatory genes (68, 75). The identification and characterization of proteins associated with both types of spliceosomes are still in progress, and it will be interesting to see whether pre-mRNA substrate-specific regulatory mechanisms exist.
In summary, cyclin E, a major cell cycle regulatory protein and an essential component of the G1-to-S transition in mammalian cells has been found to be stably associated with components of the splicing apparatus. It is tempting to speculate that this link between the cell cycle and pre-mRNA processing constitutes a way in which the cell cycle machinery exerts a growth-promoting effect by modulating the splicing activity in general. Alternatively, cyclin E-cdk2 could control the splicing of a particular pre-mRNA substrate whose protein product has an essential function during the G1-to-S transition of the mammalian cell cycle.
ACKNOWLEDGMENTS
We thank Michelle Garrett and Ali Fattaey for the generous gift of recombinant p21 and Bill Lane and the Harvard Microchemistry Department for peptide sequencing. We also thank M. McMahon, N. Solvason, D. Mahony, D. Parry, and P. Rickert for suggestions and critical review of the manuscript and Gary Burget and Maribel Andonian for assistance with graphics.
R.R. was supported by an NIH and Tobacco Research Council Grant. The DNAX Research Institute is supported by the Schering-Plough Corporation.
REFERENCES
- 1.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G. Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 3.Behrens S E, Tye K, Kastner B, Reichelt J, Lurhmann R. Small nuclear ribonucleoprotein (RNP) U2 contains numerous additional proteins and has a bipartite RNP structure under splicing conditions. Mol Cell Biol. 1993;13:307–319. doi: 10.1128/mcb.13.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijersbergen R L, Carlee L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 5.Bolin L M, McNeil T, Lucian L A, DeVaux B, Franz-Bacon K, Gorman D M, Zruawski S, Murray R, McClanahan T K. HNMP-1: a novel hematopoietic and neural membrane protein differentially regulated in neural development and injury. J Neurosci. 1997;17:5493–5502. doi: 10.1523/JNEUROSCI.17-14-05493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Chien C T, Bartel P L, Sternglanz R. R, Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 7.Cobrinik D, Whyte P, Jacks P D S, T, Weinberg R A. Cell cycle specific association of E2F with the p130 E1A binding protein. Genes Dev. 1993;7:2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- 8.Colwill K, Pawson T, Andrews B, Prasad J, Manley J L, Bell J C, Duncan P I. The Clk/Sty protein kinase phosphorylates splicing factors and regulates their intranuclear distribution. EMBO J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper M, Johnston L H, Beggs J D. Identification and characterization of Uss1p (Sdb23p): a novel U6 snRNA associated protein with significant similarity to core proteins of small nuclear ribonucleoproteins. EMBO J. 1995;14:2066–2075. doi: 10.1002/j.1460-2075.1995.tb07198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeGregorio J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulic V, Lees E, Reed S. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:158–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 12.Duronio R, O’Farrell P. Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev. 1995;9:1456–1468. doi: 10.1101/gad.9.12.1456. [DOI] [PubMed] [Google Scholar]
- 13.Ewen M, Faha B, Harlow E, Livingston D. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992;255:85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- 14.Ewen M, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 15.Faha B, Ewen M E, Tsai L H, Livingston D M, Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science. 1992;255:87–90. doi: 10.1126/science.1532458. [DOI] [PubMed] [Google Scholar]
- 16.Fan H, Penman S J. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970;50:665–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- 17.Geng Y, Eaton E, Picon M, Roberts J, Lundberg A, Gifford A, Sardet C, Weinberg R. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1179. [PubMed] [Google Scholar]
- 18.Gozani O, Feld R, Reed R. Evidence that sequence-independent binding of highly conserved U2 snRNP proteins upstream of the branch site is required for assembly of spliceosomal complex A. Genes Dev. 1996;10:233–243. doi: 10.1101/gad.10.2.233. [DOI] [PubMed] [Google Scholar]
- 19.Gui J-F, Lane W S, Fu X-D. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 20.Hall S L, Padgett R A. Conserved sequences in a class of rare eukaryotic nuclear introns with non-consensus splice sites. J Mol Biol. 1994;239:357–365. doi: 10.1006/jmbi.1994.1377. [DOI] [PubMed] [Google Scholar]
- 21.Hara E, Hall M, Peters G. Cdk2-dependent phosphorylation of Id2 modulates activity of E2A-related transcription factors. EMBO J. 1997;16:332–342. doi: 10.1093/emboj/16.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow E, Franza B J, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985;55:533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 25.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 26.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L-H, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, Fox M P, Wei N. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijmans E M, Voorhoeve P M, Beijersbergen R L, Bernards R. E2F5, a new E2F family member that interacts with p130 in vivo. Mol Cell Biol. 1995;15:3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann F, Livingston D. Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit. Genes Dev. 1996;10:851–861. doi: 10.1101/gad.10.7.851. [DOI] [PubMed] [Google Scholar]
- 30.Hong W, Bennett M, Xiao Y, Feld Kramer R, Wang C, Reed R. Association of U2 snRNP with the spliceosomal complex E. Nucleic Acids Res. 1997;25:354–361. doi: 10.1093/nar/25.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston L H, Thomas A P. The isolation of new DNA synthesis mutants in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1982;186:439–444. doi: 10.1007/BF00729466. [DOI] [PubMed] [Google Scholar]
- 32.Jumaa H, Guenet J-L, Nielsen P J. Regulated expression and RNA processing of transcripts from the Srp20 splicing factor gene during the cell cycle. Mol Cell Biol. 1997;17:3116–3124. doi: 10.1128/mcb.17.6.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanki J P, Newport J W. The cell cycle dependence of protein synthesis during Xenopus laevis development. Dev Biol. 1991;146:198–213. doi: 10.1016/0012-1606(91)90460-k. [DOI] [PubMed] [Google Scholar]
- 34.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 35.Koff A, Giordano A, Desai D, Yamashita K, Harper W, Elledge S, Nishimoto T, Morgan D, Franza R, Roberts J. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1693. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 36.Kramer A, Mulhauser F, Wersig C, Grnoning K, Bilbe G. Mammalian splicing factor SF3a120 represents a new member of the SURP family of proteins and is homologous to the essential splicing factor PRP21p of Saccharomyces cerevisiae. RNA. 1995;1:260–272. [PMC free article] [PubMed] [Google Scholar]
- 37.Lauber J, Fabrizio P, Teigelkamp S, Lane W S, Harmann E, Luehrmann R. The HeLa 200 kDa U5 snRNP-specific protein and its homologue in Saccharomyces cerevisiae are members of the DEXH-box family of putative RNA helicases. EMBO J. 1996;15:4001–4015. [PMC free article] [PubMed] [Google Scholar]
- 38.Lees E. Cyclin-dependent kinase regulation. Curr Opin Cell Biol. 1995;7:773–780. doi: 10.1016/0955-0674(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 39.Lees E, Faha B F, Dulic V, Reed S I, Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992;6:1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Graham C, Lacy S, Duncan A M V, Whyte P. The adenovirus E1A-associated 130kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993;7:2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- 41.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 43.Lukas J, Mueller H, Bartkova J, Sptikovsky D, Kjerulff A, Jansen-Durr P, Strauss M, Bartek J. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement for cyclin D1 function in G1. J Cell Biol. 1994;125:625–638. doi: 10.1083/jcb.125.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayol X, Garriga J, Grana X. Cell cycle dependent phosphorylation of the retinoblastoma-related protein p130. Oncogene. 1995;11:801–808. [PubMed] [Google Scholar]
- 45.Mermoud J E, Calvio C, Lamond A. Uncovering the role of Ser Thr protein phosphorylation in nuclear pre-mRNA processing. Adv Prot Phosphatases. 1994;8:99–118. [Google Scholar]
- 46.Mermoud J E, Cohen P, Lamond A. Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res. 1992;20:5263–5269. doi: 10.1093/nar/20.20.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mermoud J E, Cohen P T W, Lamond A. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 1994;13:5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misteli T, Spector D L. Serine/threonine phosphatase 1 modulates the subnuclear distribution of pre-mRNA splicing factors. Mol Biol Cell. 1996;7:1559–1572. doi: 10.1091/mbc.7.10.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 50.Ohtsubo M, Roberts J. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 51.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pines J. Cyclin from sea urchins to HeLas: making the human cell cycle. Biochem Soc Trans. 1996;24:15–33. doi: 10.1042/bst0240015. [DOI] [PubMed] [Google Scholar]
- 53.Quelle D, Ashmun R, Shurtleff S, Kato J, Bar-Sagi D, Roussel M, Sherr C. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 54.Reed R. Protein composition of mammalian spliceosomes assembled in vitro. Proc Natl Acad Sci USA. 1990;87:8031–8035. doi: 10.1073/pnas.87.20.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resnitzky D, Gossen M, Bujard H, Reed S. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley D J, Lee E Y, Lee W H. The retinoblastoma protein: more than a tumor suppressor. Annu Rev Cell Biol. 1994;10:1–29. doi: 10.1146/annurev.cb.10.110194.000245. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.Schmauss C, McAllister G, Ohosone Y, Hardin J A, Lerner M R. A comparison of snRNP-associated SM-autoantigens: human N, rat N and human B/B′. Nucleic Acids Res. 1989;17:1733–1743. doi: 10.1093/nar/17.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59a.Schmidt-Zachmann M S, Knecht S, Kraemer A. Molecular characterization of a novel, widespread nuclear protein that colocalizes with spliceosome components. Mol Biol Cell. 1998;9:143–160. doi: 10.1091/mbc.9.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59b.Seghezzi, W., and E. Lees. Unpublished data.
- 60.Shea J E, Toyn J H, Johnston L H. The budding yeast U5 snRNP Prp8 is a highly conserved protein which links RNA splicing with cell cycle progression. Nucleic Acids Res. 1994;22:5555–5564. doi: 10.1093/nar/22.25.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–556. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 62.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 63.Shirodkar S, Ewen M, DeCaprio J A, Morgan J, Livingston D M, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- 64.Staknis D, Reed R. Direct interactions between pre-mRNA and six U2 small nuclear ribonucleoproteins during spliceosome assembly. Mol Cell Biol. 1994;14:2994–3005. doi: 10.1128/mcb.14.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steward D L, Shaeffer J R, Humphrey R M. Breakdown and assembly of polyribosomes in synchronized Chinese hamster cells. Science. 1968;161:791–793. doi: 10.1126/science.161.3843.791. [DOI] [PubMed] [Google Scholar]
- 66.Tam S W, Theodoras A M, Shay J W, Draetta G F, Pagano M. Differential expression and regulation of cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- 67.Tarn W-Y, Steitz J A. A novel spliceosome containing U11, U12, and U5 snRNPs excise a minor class (AT-AC) intron in vitro. Cell. 1996;84:801–811. doi: 10.1016/s0092-8674(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 68.Tarn W-Y, Steitz J A. Pre-mRNA splicing: the discovery of a new spliceosome doubles the challenge. Trends Biol Sci. 1997;22:132–137. doi: 10.1016/s0968-0004(97)01018-9. [DOI] [PubMed] [Google Scholar]
- 69.Tazi J, Daugeron M C, Cathala G, Brunel C, Jeanteur P. Adenosine phosphorothioates (ATP alpha S and ATP tau S) differentially affect the two steps of mammalian pre-mRNA splicing. J Biol Chem. 1992;267:4322–4326. [PubMed] [Google Scholar]
- 70.Tazi J, Kornstaedt U, Rossi F, Jenteur P, Cathala G, Brunel C, Luehrmann R. Thiophosphorylation of U1-70K protein inhibits pre-mRNA splicing. Nature. 1993;363:283–286. doi: 10.1038/363283a0. [DOI] [PubMed] [Google Scholar]
- 71.Voitenleitner C, Fanning E, Nasheuer H P. Phosphorylation of DNA polymerase alpha-primase by cyclin A-dependent kinases regulates initiation of DNA replication in vitro. Oncogene. 1997;14:1611–1615. doi: 10.1038/sj.onc.1200975. [DOI] [PubMed] [Google Scholar]
- 72.Wang C, Seghezzi W, Chua K, Lees E, Gozani O, Reed R. Phosphorylation of the spliceosomal protein SAP155 is coupled with splicing catalysis. Genes Dev. 1998;12:1409–1414. doi: 10.1101/gad.12.10.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 74.Won K-A, Reed S I. Activation of cyclin E/cdk2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Krainer A R. U1-mediated exon definition interactions between AT-AC and GT-AG introns. Science. 1996;274:1005–1008. doi: 10.1126/science.274.5289.1005. [DOI] [PubMed] [Google Scholar]
- 76.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 77.Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Duerr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu L, Enders G, Lees J A, Beijersbergen R L, Bernards R, Harlow E. The pRB-related protein p107 contains two growth suppression domains: independent interactions with E2F and cyclin/cdk complexes. EMBO J. 1995;14:1904–1913. doi: 10.1002/j.1460-2075.1995.tb07182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu L, van den Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]