Abstract
Advance care planning in decompensated cirrhosis is recommended but rarely performed.
INTRODUCTION:
To report outcomes of a 3-year quality improvement pilot study to improve advance directive (AD) completion.
METHODS:
The pilot consisted of champions, education, electronic health record templates, and workflow changes. We assessed changes, predictors, and effects of AD completion.
RESULTS:
The pilot led to greater (8.3%–36%) and earlier AD completion, particularly among those divorced, with alcohol-associated liver disease, and with higher Model of End-Stage Liver Disease–Sodium score. Decedents whose AD specified nonaggressive goals experienced lower hospital lengths of stay.
DISCUSSION:
Advance care planning initiatives are feasible and may reduce health care utilization among decedents requesting less aggressive care.
INTRODUCTION
Advance care planning (ACP) improves end-of-life (EOL) experience for seriously ill patients, but it is rarely performed for patients with decompensated cirrhosis (1,2). We previously reported 1-year results of a pilot quality improvement study, which improved advance directive (AD) completion rates over an 8-month period (3). In this study, we report final AD completion rates, predictors, and association with health care utilization among decedents, over a 3-year period.
METHODS
This pilot study was performed from November 1, 2018, to June 30, 2021, at a once-weekly hepatology clinic staffed by transplant hepatology fellows and attending hepatologists. The intervention consisted of a 1-hour education to clinic staff and fellows that included information about AD and ACP. Standardized electronic health record (EHR) templates were used to guide discussions. Processes were created to facilitate availability, completion, and uploading of AD during clinic. We included all patients with decompensated cirrhosis, defined as a patient with cirrhosis and a history of a portal hypertension-related complication. Chart abstraction occurred between June 2018 and July 2021.
Measures
Our primary outcome was the presence of a completed AD in the EHR. AD included health care proxy forms, a living will, or a medical order for life-sustaining treatment, which included do-not-resuscitate orders. Among decedents, we collected whether any limits to aggressive care (cardiopulmonary resuscitation, mechanical ventilation, dialysis, or artificial nutrition) were recorded. Other collected measures included age, gender, race/ethnicity, insurance status, primary language, cause of cirrhosis, and Model of End-Stage Liver Disease–Sodium (MELD-Na) score. Among patients who died during the study period, we collected dates of hospitalizations, overall length of stay (LOS), and intensive care unit LOS. Data were collected and managed using REDCap (4).
Statistical analysis
To identify predictors of completion, we used a general linear model for binomials. To understand the association between our quality improvement initiative and completion rates, we conducted a survival analysis using the R library survival (5). Baseline AD completion rate was assessed from time of earliest completed AD (March 20, 2012) to start of the intervention (November 1, 2018). This rate was then compared with AD completion rate during the intervention period up until the completion of the last AD (November 1, 2018, to March 25, 2021). Curves were compared using a log-rank test. Between-participant, signed rank tests were used to investigate whether the presence of an AD was associated with hospital and intensive care unit (ICU) LOS for decedents.
RESULTS
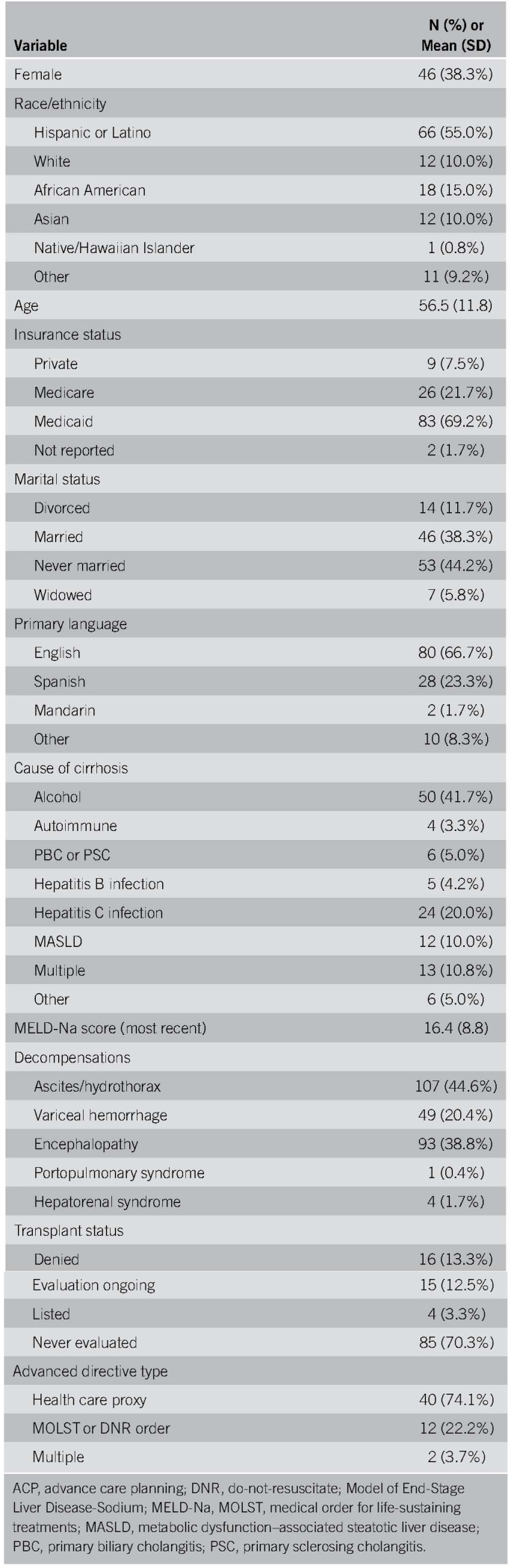
One hundred twenty patients were seen across 512 clinic visits, with an average of 4.3 visits per patient. Most of them were men, of Latino origin, English-speaking, and never married. The most common cause of cirrhosis was alcohol-associated liver disease (ALD) (41%), and the mean MELD-Na at first clinic visit was 16.4 (SD: 8.8). The most common decompensation was ascites and/or hepatic hydrothorax. Sixty-nine percent were never evaluated for liver transplantation (Table 1). AD completion increased from 8.3% to 36%. Most were health care proxy forms (N = 40/54, 74%).
Table 1.
Characteristics and advance directive completion rates for patients receiving ACP intervention (N = 120)
Patients who were divorced, compared with married (β = 3.33, P = .001), those with higher MELD-Na score, (β = 0.12, P = .004), and with diagnosis of ALD, compared with non-ALD (β = 1.72, P = .002) were more likely to complete an AD (Table 2). Patients seen during the intervention had less time from clinic visit to AD completion compared with those seen preintervention (χ2 = 18.4, P < 0.001). The median time to AD's filed preintervention (median [SD]: 1,028 [SD: 931] days) was much longer than postintervention (246 [SD: 251] days) (Figure 1).
Table 2.
Significant predictors of advance directive completion
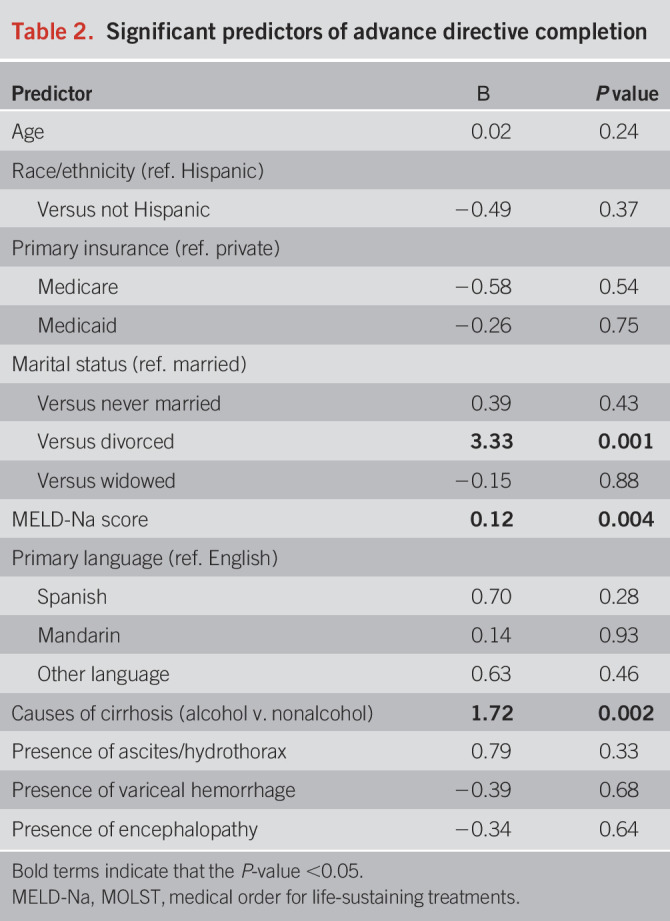
Figure 1.
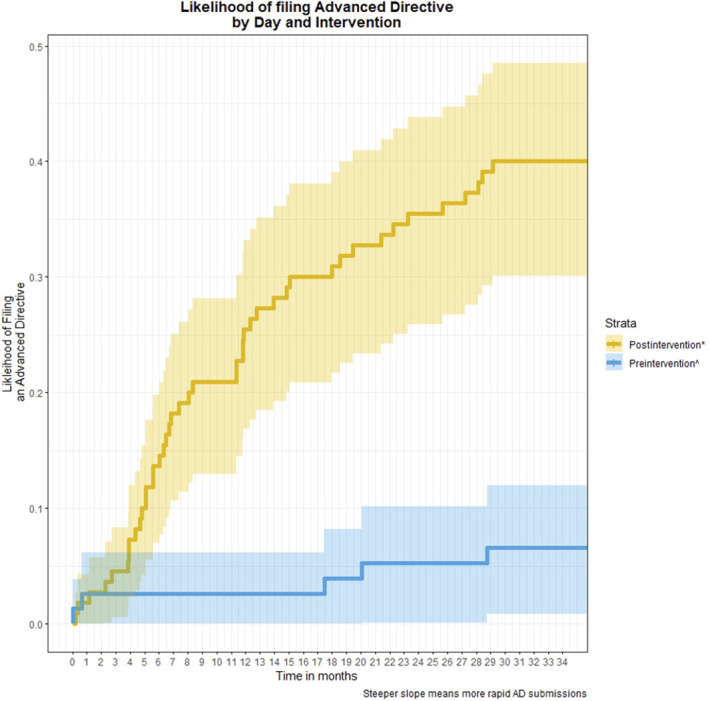
Trends in advance directive completion over intervention period. *Represents patients who did not previously have an advance directive and were eligible for the intervention. ^Represents patients who had an advance directive uploaded before intervention. Time 0 is assumed to be the date that the first advance directive was uploaded in the electronic health record for this cohort. AD, advance directive.
Of decedents (N = 20), 8 (40%) died in an acute care facility (see Figure, Supplementary Digital Content 1, http://links.lww.com/AJG/D105). Decedents had a median of 1 hospital visit lasting 11.5 days (SD = 26.2) and 0.5 ICU visits, lasting 6 days (SD = 7.0). Fifteen (75%) completed an AD, which were most often medical order for life-sustaining treatment (N = 10, 66.7%) forms. AD completion was associated with greater health care utilization at EOL, including higher hospital (median 13 vs 1 days, P < .001) and ICU (median 6.5 vs 0 days, P = .006) LOS. However, of decedents with AD (N = 15), those requesting limits to care (N = 9) experienced lower hospital (median 13 vs 16 days, P < .001) and ICU (median 6.5 vs 9 days, P < .001) LOS.
DISCUSSION
Our ACP intervention—featuring provider education, provider champions, EHR documentation templates, and workflow changes—led to earlier and greater completion of AD. AD completion was highest among patients with higher MELD-Na scores, those with ALD, and those who were divorced. Completion was associated with longer hospital LOS at EOL but lower LOS among those who preferred limits to life-sustaining treatments.
Most completed AD focused on identifying surrogate decision-makers, considered among the most important outcomes of ACP (6). That our intervention was more effective for groups that are likely to incur (high MELD-Na) and potentially require more support (divorced, patients with ALD) for complex medical decision-making suggests that future adaptations are needed.
The fact that AD completion was associated with longer hospital and ICU stays, but lower among decedents who requested less aggressive care, illustrates 2 important concepts. First, completion of AD, when specific, can be an important tool for ensuring goal-concordant care. Ideally, future ACP initiatives should focus on iterative discussions about goals, values, and preferences during clinic visits, which can promote and provide more helpful information in AD (7). This can be further promoted by formal, accessible communication skills training for trainees, and hepatology teams in ACP (8). Second, health care utilization as an outcome may not fully capture value. For instance, greater health care utilization may be aligned with a patient's wishes to receive aggressive care. Similarly, hospice utilization, which can avert costs, may not ultimately resonate with a patient's previous wishes. Future studies thus should include measures, such as quality of care and patient-reported outcomes (1,9).
Our study has several strengths. It was conducted at an urban center with a racially and ethnically diverse, economically under-resourced population, which is critical given the significant disparities experienced in ACP by systemically disadvantaged and minoritized groups (10). It also incorporated the role of trainees to help guide improvement efforts. Our limitations include less potential to account for residual confounding from unobserved variables, presence of missing data outside our health system, assumption that clinic enrollment rates were steady over the study period, and lower generalizability to transplant centers. Notwithstanding, this is the first study to report sustainable improvement in AD documentation for the population with cirrhosis by addressing provider and system barriers.
CONFLICTS OF INTEREST
Guarantor of the article: Arpan Patel, MD, PhD.
Specific author contributions: A.P.: conceptualization, methodology, data curation, writing-original draft, writing—review and editing. C.A.B.: data curation, writing-review and editing. N.P.: formal analysis, writing-original draft, writing-review and editing. S.M.K.: data curation, writing-review and editing. A.N.: data curation, writing-review and editing. N.K.: data curation, writing-review and editing. C.C.: data curation, writing-review and editing. L.C.: data curation, writing-review and editing. A.H.: data curation, writing-review and editing. P.P.: conceptualization, methodology, writing-review and editing. D.D.: data curation, writing-review and editing. C.W.: conceptualization, methodology, writing-review and editing. T.S.: conceptualization, methodology, writing-review and editing. R.A.: conceptualization, methodology, data curation, writing-review and editing.
Financial support: AASLD Transplant Hepatology Fellowship Award (AASLDF 50035) to A.P.
Potential competing interests: No conflicts of interest are declared.
Institutional review board: The Mount Sinai Department of Medicine Quality Improvement committee approved this study as exempt for IRB Review.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/D105
Contributor Information
Chip A. Bowman, Email: chipbowman@gmail.com.
Nicole Prause, Email: NPrause@mednet.ucla.edu.
Saikiran M. Kilaru, Email: saikiran.kilaru@nyulangone.org.
Andrew Nguyen, Email: andrewavn@gmail.com.
Nina Kogekar, Email: nina.kogekar@gmail.com.
Cynthia Cohen, Email: ccohen531@gmail.com.
Lindsay Channen, Email: lindseychannen@gmail.com.
Alyson Harty, Email: alyson.harty@mountsinai.org.
Ponni Perumalswami, Email: pperumal@med.umich.edu.
Douglas Dietrich, Email: douglas.dieterich@mssm.edu.
Thomas Schiano, Email: thomas.schiano@mountsinai.org.
Ritu Agarwal, Email: ritu.agarwal@mssm.edu.
REFERENCES
- 1.McMahan RD, Tellez I, Sudore RL. Deconstructing the complexities of advance care planning outcomes: What do we know and where do we go? A scoping review. J Am Geriatr Soc 2021;69(1):234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel AA, Ryan GW, Tisnado D, et al. Deficits in advance care planning for patients with decompensated cirrhosis at liver transplant centers. JAMA Intern Med 2021;181(5):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel A, Kogekar N, Agarwal R, et al. Improving advance care planning in outpatients with decompensated cirrhosis: A pilot study. J Pain Symptom Manage 2020;59(4):864–70. [DOI] [PubMed] [Google Scholar]
- 4.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terry M, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer: New York, NY, 2000. ISBN 0-387-98784-3. [Google Scholar]
- 6.Sudore RL, Heyland DK, Lum HD, et al. Outcomes that define successful advance care planning: A Delphi panel consensus. J Pain Symptom Manage 2018;55(2):245–55.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers J, Steinberg L, Seow H. Controversies about advance care planning. JAMA 2022;327(7):684–5. [DOI] [PubMed] [Google Scholar]
- 8.Back AL, Fromme EK, Meier DE. Training clinicians with communication skills needed to match medical treatments to patient values. J Am Geriatr Soc 2019;67(S2):S435–41. [DOI] [PubMed] [Google Scholar]
- 9.Ernecoff NC, Wessell KL, Bennett AV, et al. Measuring goal-concordant care in palliative care research. J Pain Symptom Manage 2021;62(3):e305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao JK, Anderson LA, Lin FC, et al. Completion of advance directives among U.S. consumers. Am J Prev Med 2014;46(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


