Abstract
Spirochete colonization of the gastrointestinal tract is a poorly understood phenomenon presenting with varying signs and symptoms. Due to the lack of a unified approach and its varying presentations, the management decision for intestinal spirochetosis (IS) has always been challenging. While metronidazole is the commonly preferred antimicrobial treatment, it remains unclear if therapeutic intervention is indicated for everyone, especially asymptomatic patients. We present three patients, diagnosed with IS. They presented with varying demographics, clinical presentations, and past medical histories and underwent different clinical managements. Our decisions for treatment not only included presenting symptoms but also factors like history of pre-existing gastrointestinal diseases, age, and immune status.
Keywords: metronidazole, helicobacter pylori, ulcerative colitis, inflammatory bowel diseases, management, intestinal spirochetosis
Introduction
Spirochetes, tiny spiral-shaped bacteria sometimes found on the surface of epithelial cells, represents a well-debated conundrum in the gastroenterology and pathology world. Colonization of spirochetes in the lower gastrointestinal tract is a rare condition and can lead to a myriad of presentations, ranging from asymptomatic individuals to symptomatic diarrhea and abdominal pain [1,2]. The mechanism of diarrhea caused by spirochetes is believed to be related to the loss of microvilli, the absorptive surface of the colon, caused by bacterial colonization [3,4]. Due to limited literature availability on its pathogenicity and diverse response to antimicrobial treatment in this subset of patients, guidelines on managing this condition remain open-ended [5-8]. We report three patients, all diagnosed with IS, following colonoscopy and biopsy, all with different presentations, and past medical histories, resulting in different clinical managements.
Case presentation
Case 1
A 20-year-old male with a history of reflux esophagitis experienced six months of intermittent supraumbilical pain with anorexia and loose mucoid bowel movements. Family history included a parent with irritable bowel syndrome (IBS). His medications included bismuth or famotidine for reflux. Physical examination was significant only for audible borborygmi, but no tenderness or organ enlargement. Esophagogastroduodenoscopy (EGD) was unremarkable. Colonoscopy revealed a small aphthous ulcer in the terminal ileum. Random colonic biopsies were taken that on histopathological examination showed a hyperchromatic, fuzzy, colonic epithelial luminal brush border. A positive Warthin-Starry (WS) silver stain confirmed the diagnosis of IS (Figure 1).
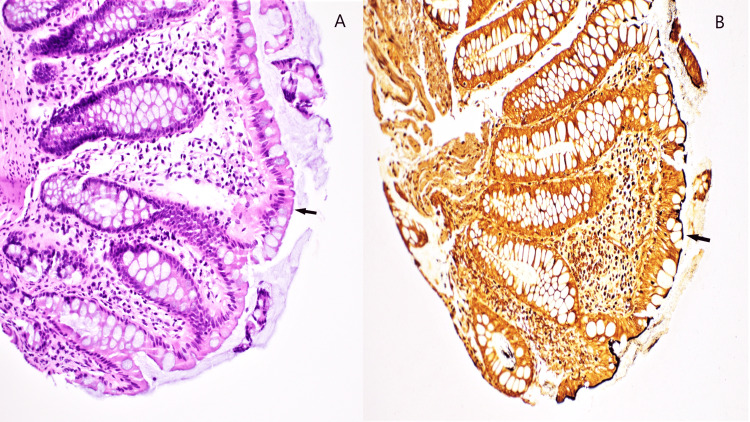
Figure 1. Intestinal spirochetosis as visualized on a colonic biopsy specimen.
A. Hematoxylin and eosin stain shows a purple, fuzzy "false-brush border" on luminal epithelial cells (arrows) in a colon biopsy specimen. B. Warthin-starry silver stain highlights the bacterial organisms as a black, fuzzy line covering the luminal epithelial cells (arrows).
The patient was not immunosuppressed and subsequent laboratory workup for all other infectious pathogens including HIV and inflammatory bowel disease (IBD) was negative. No specific treatment was prescribed initially; however, persistent symptoms prompted treatment with metronidazole. Follow-up colonoscopy with biopsies did not show spirochete colonization.
Case 2
A 66-year-old male presented to the hospital for an annual screening colonoscopy. He had a past medical history significant for long-standing ulcerative colitis (UC). The patient was not immunosuppressed, and his disease was well controlled on baseline mesalamine maintenance therapy. No active symptoms were reported, and the physical examination was within normal limits. The colonoscopy performed was significant for erythematous mucosa and diverticulosis in the sigmoid colon, two 4 mm polyps in the rectum, and non-bleeding internal hemorrhoids. Multiple biopsies were taken, and the characteristic false brush border was visualized on histology from the descending colon and rectal biopsies, confirmed to be IS by WS silver stain (Figure 2).
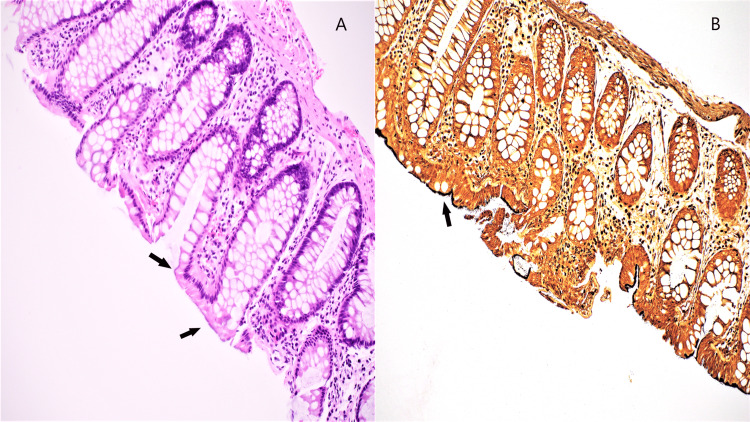
Figure 2. Intestinal spirochetosis in a rectal biopsy.
A. Hematoxylin and eosin stain with a false-brush border on luminal epithelial cells (arrow). B. Warthin-starry silver stain highlighting the bacterial organisms (arrow).
No active or chronic colitis was seen microscopically. In the absence of active symptoms, no further investigations for any other pathogens were undertaken, and no antimicrobial treatment was offered. On annual follow-up, the patient reported no new symptoms and health parameters were within normal limits.
Case 3
An 82-year-old female with a history of left-sided UC and acute rectal bleeding presented with generalized abdominal discomfort, chills without fevers, and two episodes of nausea and vomiting. A rectal exam was significant for blood mixed with stool and no abdominal tenderness. EGD with gastric biopsies was remarkable for a Helicobacter pylori (H. pylori) infection. Colonoscopy revealed inflammation characterized by altered vascularity, erosions, erythema, friability, and granularity in a continuous and circumferential pattern from the anus to the rectum, graded as Mayo score 2. Multiple diverticula were found in the sigmoid, descending, and transverse colon. On histopathology, there was moderately active chronic proctitis consistent with idiopathic IBD. Ascending and sigmoid colon biopsies were negative for active colitis, however, significant for the presence of IS, confirmed by WS silver stain (Figure 3).
Figure 3. Intestinal spirochetosis seen in a patient with ulcerative colitis.
A. Hematoxylin and eosin stain with faint, fuzzy appearance on the surface of luminal epithelial cells (arrows). B. Warthin-starry silver stain highlighting diffuse involvement by spirochetes (arrow).
The active IBD was treated with rectal and oral mesalamine and steroid enema and no other immunosuppressive therapy was offered to the patient. The patient was also treated with quadruple therapy for their H. pylori infection that included metronidazole, a treatment strategy also useful for IS. Follow-up was significant for the resolution of symptoms. Oral mesalamine maintenance therapy for IBD was continued.
Discussion
IS is defined as the presence of filamentous non-treponemal spirochetes, Brachyspira aalborgi or Brachyspira pilosicoli on the surface epithelium of the gastrointestinal tract, commonly the large intestine. The prevalence of IS varies from 1.1 to 5% in developed countries, and the infection is thought to spread by the fecal-oral route [1]. The prevalence increases up to 54% in homosexual men and HIV-positive individuals, suggesting sexual transmission in some cases [7]. Clinical presentation varies significantly, and individuals may be asymptomatic or present with non-specific symptoms including abdominal pain, diarrhea, and rectal bleeding [1-3]. In our case series, IS was detected incidentally in two of three (Cases 2 and 3) patients, both of which had past medical history positive for IBD. None of the patients were immunosuppressed or had other infectious pathogens including HIV. The sexual orientations of the patients are not known.
The gold standard method for diagnosis of IS is histopathological assessment of the biopsy specimen with the organism carpeting the surface of the luminal epithelium. Differential diagnosis includes entero-adherent gram-negative Escherichia coli, a prominent glycocalyx, and artifacts from biopsies done on a poorly prepped colon. Spirochetes can be highlighted by silver stains like Steiner or WS stain [4] or immunohistochemical stains for Treponema pallidum which cross-reacts with Brachyspira species. Other newer methods of diagnosing IS include using polymerase chain reaction targeting genes specific to the Brachyspira genus, immunomagnetic separation, and fluorescent in situ hybridization with oligonucleotide probes against 16S rRNA gene of Brachyspira spirochetes [6-10]. However, none of these methods are currently used clinically [11].
The proposed mechanism of spirochete-mediated intestinal injury includes the erosion of the microvilli of the gut surface epithelium [3]. Pathogenic factors include bacterial sialidases, which cleave sialic acid residues in epithelial oligosaccharides, glycoproteins, and glycolipids to derive energy and evade the immune system. Spirochetes also have collagenase to facilitate bacterial colonization of the extracellular matrix components and a multidrug efflux pump system, conferring antibiotic resistance and survival in the gastrointestinal tract [4].
Metronidazole is commonly the preferred antimicrobial treatment of intestinal spirochetosis as reported in the literature; however, there are no formal treatment guidelines for IS [1]. Older studies have reported that metronidazole may be the drug of choice in eradicating IS [6,10]. However, based on newer research, a “wait-and-watch” approach with close follow-up is gaining popularity as an equally beneficial mode of management, especially for asymptomatic and IBS patients [7]. Alternatively, one study reported that metronidazole treatment in IBS patients paradoxically caused spirochetes to invade crypts and goblet cells, thereby evading antibiotic treatment and increasing disease severity [8].
In cases with concurrent IBD, antimicrobial treatment with metronidazole is preferred over active monitoring [12] as research suggests that patients with concurrent gastrointestinal disease (for example IBD, H. pylori-associated gastritis, polyps, etc.) may have an increased risk of spirochete infection due to elevated inflammatory mediators. For example, IBD increases baseline inflammation in the intestinal mucosa, predisposing to a greater immune response to spirochete colonization. These patients are also more likely to present with symptoms including abdominal pain, loss of appetite, nausea, and vomiting. IBD has also been associated with extra colonic lesions, increasing the severity of infection [9,13,14].
Further research is needed to understand the pathogenicity of IS and the risk factors that contribute to spirochete colonization. This understanding would aid in the development of more effective treatment guidelines and regimens. In the interim, healthcare providers could utilize algorithmic approaches to manage patients with IS [15], while considering factors such as age, history of pre-existing gastrointestinal diseases, and immune status. This would enable providers to make informed decisions about the necessity of metronidazole treatment further improving patient care.
Conclusions
IS poses a management conundrum because of limited understanding of the disease and diverse clinical presentation. There are no formal treatment guidelines available in the literature, both for symptomatic and asymptomatic patients. Where few older studies have reported metronidazole as the drug of choice in eradicating IS, newer research suggests that a “wait-and-watch” approach with close follow-up may also be equally beneficial, especially for asymptomatic patients. In conclusion, further research is warranted to understand the pathogenicity and predisposing risk factors of spirochete colonization, thus, improving and establishing treatment guidelines for IS.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Intestinal spirochetosis: a case series and review of the literature. Lemmens R, Devreker T, Hauser B, Degreef E, Goossens A, Vandenplas Y. Pediatr Gastroenterol Hepatol Nutr. 2019;22:193–200. doi: 10.5223/pghn.2019.22.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human intestinal spirochetosis--a review. Tsinganou E, Gebbers JO. Ger Med Sci. 2010;8:0. doi: 10.3205/000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intestinal spirochaetosis associated with hyperplastic and adenomatous colonic polyps. Calderaro A, Gorrini C, Montecchini S, Villanacci V, Bassotti G, Dettori G, Chezzi C. Pathol Res Pract. 2012;208:177–180. doi: 10.1016/j.prp.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Isolates from colonic spirochetosis in humans show high genomic divergence and potential pathogenic features but are not detected using standard primers for the human microbiota. Thorell K, Inganäs L, Backhans A, et al. J Bacteriol. 2019;201 doi: 10.1128/JB.00272-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Occurrence of human intestinal spirochetosis in comparison with infections by other enteropathogenic agents in an area of the Northern Italy. Calderaro A, Gorrini C, Peruzzi S, Piccolo G, Dettori G, Chezzi C. Diagn Microbiol Infect Dis. 2007;59:157–163. doi: 10.1016/j.diagmicrobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Human intestinal spirochetosis mimicking ulcerative colitis. Nishii S, Higashiyama M, Ogata S, et al. Clin J Gastroenterol. 2018;11:145–149. doi: 10.1007/s12328-017-0807-3. [DOI] [PubMed] [Google Scholar]
- 7.Human intestinal spirochetosis in Japan; its incidence, clinicopathologic features, and genotypic identification. Tanahashi J, Daa T, Gamachi A, Kashima K, Kondoh Y, Yada N, Yokoyama S. Mod Pathol. 2008;21:76–84. doi: 10.1038/modpathol.3800987. [DOI] [PubMed] [Google Scholar]
- 8.Association between Brachyspira and irritable bowel syndrome with diarrhoea. Jabbar KS, Dolan B, Eklund L, et al. Gut. 2021;70:1117–1129. doi: 10.1136/gutjnl-2020-321466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intestinal spirochetosis mimicking inflammatory bowel disease in children. Helbling R, Osterheld MC, Vaudaux B, Jaton K, Nydegger A. BMC Pediatr. 2012;12:163. doi: 10.1186/1471-2431-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Infective colitis associated with human intestinal spirochetosis. Calderaro A, Bommezzadri S, Gorrini C, et al. J Gastroenterol Hepatol. 2007;22:1772–1779. doi: 10.1111/j.1440-1746.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- 11.Human intestinal spirochetosis, irritable bowel syndrome, and colonic polyps: a systematic review and meta-analysis. Fan K, Eslick GD, Nair PM, et al. J Gastroenterol Hepatol. 2022;37:1222–1234. doi: 10.1111/jgh.15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Human intestinal spirochaetosis in two ulcerative colitis patients. Iwamoto J, Ogata S, Honda A, et al. Intern Med. 2014;53:2067–2071. doi: 10.2169/internalmedicine.53.2386. [DOI] [PubMed] [Google Scholar]
- 13.Risk of common infections in people with inflammatory bowel disease in primary care: a population-based cohort study. Irving PM, de Lusignan S, Tang D, Nijher M, Barrett K. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Human intestinal spirochaetosis in Parma: a focus on a selected population during 2002-2005. Peruzzi S, Gorrini C, Piccolo G, Calderaro A, Dettori G, Chezzi C. https://europepmc.org/article/med/17933280. Acta Biomed. 2007;78:128–132. [PubMed] [Google Scholar]
- 15.Intestinal spirochetosis: an enigmatic disease. Anthony NE, Blackwell J, Ahrens W, Lovell R, Scobey MW. Dig Dis Sci. 2013;58:202–208. doi: 10.1007/s10620-012-2305-2. [DOI] [PubMed] [Google Scholar]