Abstract
Background:
Women with cardiomyopathies are at risk for pregnancy complications. Optimal mode of delivery in these patients is guided by expert opinion and limited small studies.
Objectives:
The objective of this study is to examine the association of delivery mode with severe maternal morbidity events during delivery hospitalization and readmissions among patients with cardiomyopathies.
Methods:
The Premier inpatient administrative database was used to conduct a retrospective cohort study of pregnant patients with a diagnosis of a cardiomyopathy. Utilizing a target trial emulation strategy, the primary analysis compared outcomes among patients exposed to intended vaginal delivery versus intended cesarean delivery (intention to treat). A secondary analysis compared outcomes among patients who delivered vaginally versus by cesarean (as-treated). Outcomes examined were non-transfusion severe maternal morbidity during the delivery hospitalization, blood transfusion and readmission.
Results:
The cohort consisted of 2,921 deliveries. In the primary analysis (intention to treat), there was no difference in non-transfusion morbidity (adjusted odds ratio [aOR], 1.17; 95% confidence interval [CI], 0.91–1.51), blood transfusion (aOR, 1.27; 95% CI, 0.81–1.98) or readmission (aOR, 1.03; 95% CI, .73–1.44) between intended vaginal delivery and intended cesarean delivery. In the as-treated analysis, cesarean delivery was associated with a 2-fold higher risk of non-transfusion morbidity (aOR, 2.44; 95% CI, 1.85–3.22) and blood transfusion (aOR, 2.26; 95% CI, 1.34–3.81) when compared to vaginal delivery.
Conclusions:
In patients with cardiomyopathies, a trial of labor does not confer a higher risk of maternal morbidity, blood transfusion or readmission compared with planned cesarean delivery.
Keywords: Pregnancy, cardiomyopathy, preeclampsia, heart failure, cardio-obstetrics
Tweet summarizing our paper:
In patients with cardiomyopathies, a trial of labor does not confer a higher risk of maternal morbidity, blood transfusion or readmission compared with planned cesarean delivery.
Three hashtags: #SOAPHQ #ACOG #CardioOB
INTRODUCTION
Individuals with cardiomyopathy are at high risk of pregnancy complications, and those with ejection fraction less than 30% are counselled to avoid becoming pregnant or terminate their pregnancy due to the high associated risks of morbidity (39%) and mortality during pregnancy, delivery and postpartum.(1,2) Despite the short and long-term risks, many women with cardiomyopathies or history of heart failure become pregnant. With close follow up and multidisciplinary Pregnancy Heart Team care, many patients can achieve successful deliveries.(3) As Pregnancy Heart Teams develop across the world, data specific to each cardiac condition and data examining management strategies are needed to guide tailored clinical management and mode of delivery recommendations in these high-risk patients.
Vaginal delivery is the recommended mode of delivery for most patients with cardiomyopathies as the hemodynamic stress, and the risk of bleeding, thrombosis, air embolism and infection are lower than with cesarean delivery.(1,4) Though this recommendation is generally accepted by experts, data quantifying these risks are scant and would be useful as teams and patients make decisions about delivery mode. Currently the available data examining morbidity and mortality and optimal mode of delivery in patients with cardiomyopathies are from small retrospective and prospective databases and case series.(2,5–7) A multicenter randomized trial comparing outcomes according to mode of delivery in patients with cardiomyopathy is unlikely to be feasible. Large, observational studies examining real world data can fill this gap and provide estimates of morbidity events specific to this population to inform delivery planning and postpartum care. The objective of this observational study is to examine the association of delivery mode with severe maternal morbidity (SMM) events during delivery hospitalization and readmissions among patients with cardiomyopathies in the United States. We hypothesize that a trial of labor does not confer higher risk of SMM compared with unlabored cesarean delivery.
METHODS
Study Design and Population
This study was deemed exempt from review by the Duke University Health System Institutional Review Board. We conducted a retrospective cohort study using a large administrative database (Premier Healthcare Database, Premier Inc., Charlotte, North Carolina). Details of the dataset and readmission data have been previously described.(8) This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.(9) The cohort for this study consisted of patients 12 to 55 years old, who had both an International Classification of Diseases, Tenth Edition (ICD-10) diagnosis code for delivery after 25 weeks’ gestation (Z3A.25-Z3A.42) and for cardiomyopathy (Supplemental table 1) (Figure 1). Planned (intended) vaginal delivery was defined as an ICD-10 code for vaginal delivery (O80 or Z37.x or procedure codes: 10E0XZZ, 10D07Z3, 10D07Z4, 10D07Z5, 10D07Z6, 10D07Z7, 10D07Z8) and cesarean delivery was defined by presence of an ICD-10 procedure codes (10D00Z0, 10D00Z1, 10D00Z2) from January 1, 2016, to September 30, 2020. Patients with ICD-10 codes consistent with a delivery (i.e., Z37.*, O80, 10E0XZZ, 0W8NXZZ) were also classified as vaginal deliveries if no ICD-10 codes for cesarean delivery were present. Intrapartum cesarean delivery was defined as previously described as the presence of a cesarean delivery code plus an ICD-10 code for fetal distress, failed operative delivery, cord prolapse, fetal-maternal disproportion, obstructed labor, abnormal forces of labor, long labor, and failed induction (Supplemental Table 2).(8) Operative deliveries (e.g., vacuum or forceps) were classified as vaginal deliveries unless a cesarean delivery code was also present.
Figure 1:
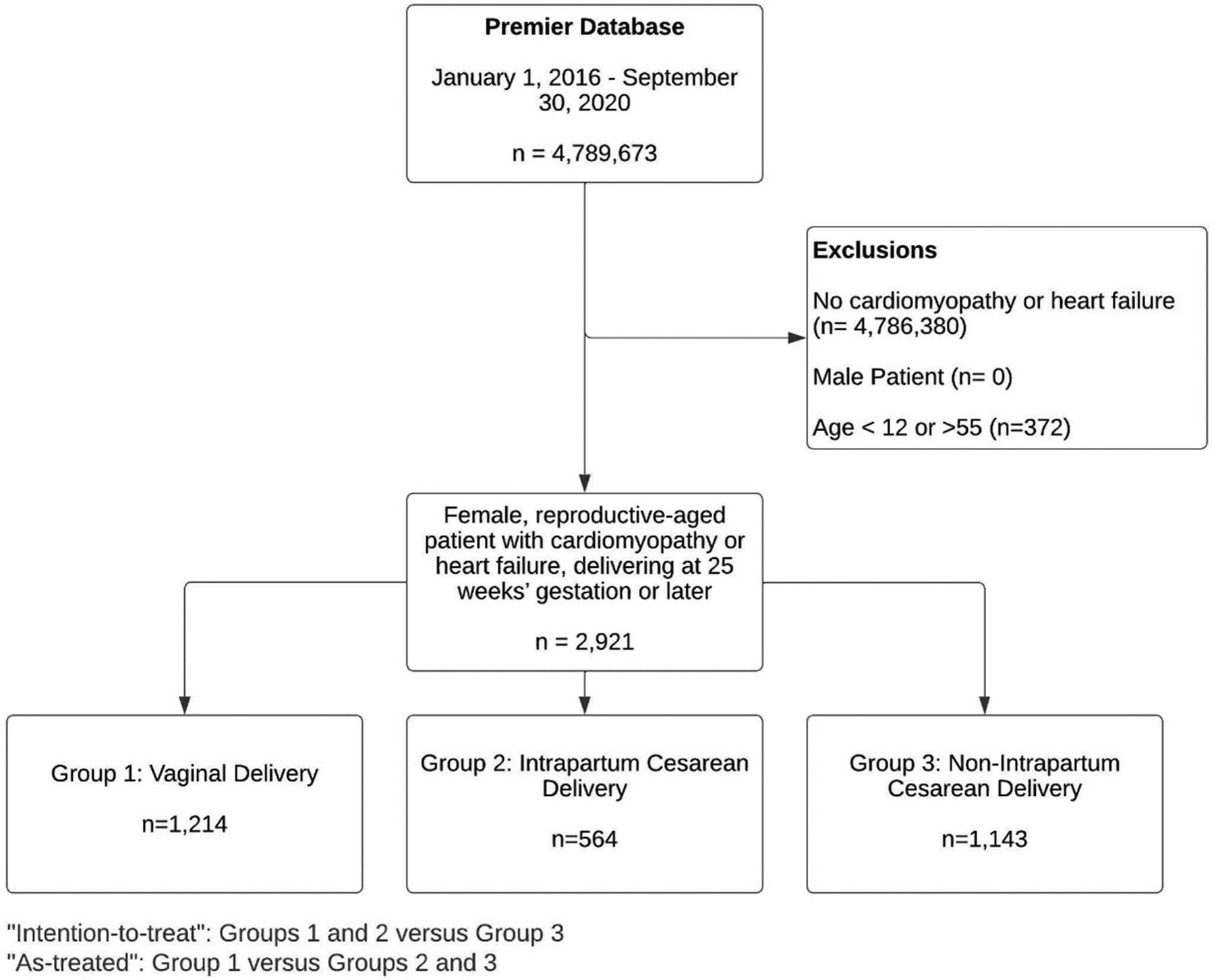
Derivation of study cohort. This flowchart demonstrates how the cohorts were created for the ‘Intention to Treat’ and ‘As Treated’ analyses.
Exposures, Outcomes, and Covariates
In the primary analysis, the exposure variable of interest was intended route of delivery, to reflect the clinical decision faced when counseling pregnant patients - obstetric providers cannot know whether a trial of labor will result in a successful vaginal delivery or an intrapartum cesarean delivery (Supplemental Table 2). Utilizing a target trial emulation strategy, we refer to this approach as the ‘intention-to-treat’ approach (commonly utilized in randomized controlled trials), considering the primary comparison to be intended vaginal delivery, including both vaginal delivery and intrapartum cesarean delivery versus non-intrapartum cesarean delivery.(10) We performed a secondary analysis, an ‘as-treated analysis’, in which the ultimate mode of delivery was compared, that is, vaginal deliveries were compared to all cesarean deliveries including intrapartum cesarean deliveries.
The primary outcome was defined a priori as a diagnosis of any non-transfusion severe maternal morbidity, as defined by the Centers for Disease Control and Prevention (CDC), during the delivery hospitalization.(11) As utilized in prior studies, we adjusted the CDC definition to include acute events that were not included in the original definition, such as acute heart failure and aortic dissection codes, and removed codes deemed to reflect chronic illness not acute morbidity events (e.g., Moya Moya disease) (Supplemental Table 3).(8,11) A morbidity event had to be not present on admission in order to be included as an outcome. Secondary maternal outcomes included blood transfusion of 4 or more units of packed red blood cells and readmission to delivery hospital within 90 days. We present descriptively in-hospital mortality, intensive care unit admission, length of stay and the cardiovascular treatments provided at any point in the delivery hospitalization such as vasopressors, inotropes, pulmonary vasodilators and extracorporeal membrane oxygenator (ECMO) use.
Similar to our previous work,(8) the covariates in the models were selected a priori based on known maternal morbidity risk factors, prior literature, and subject-matter expertise: age, payor category, the comorbidities present in the expanded obstetric comorbidity index with some adjustments (Supplemental Table 4).(12) Cardiomyopathy codes present in the obstetric comorbidity index were not adjusted for in the analysis as they define the cohort.
Some heart failure ICD-10 codes signify chronic disease with an acute heart failure component (Supplemental table 1). These codes could therefore be used as both inclusion criteria and outcomes, thus these codes were not included as inclusion criteria in the primary analysis but as outcomes when not present on admission. We then conducted a sensitivity analysis where we added these acute on chronic codes as inclusion criteria.
Statistical Analysis
Demographic and clinical characteristics of the cohort are reported, stratified by delivery mode. Descriptive statistics were used to examine the study population with categorical variables reported as counts and frequencies, and continuous data reported as median with interquartile range. Patient characteristics were compared between groups defined by intended mode of delivery and by actual mode of delivery using Wilcoxon rank sum tests for continuous variables and using chi-squared or Fisher’s exact tests, as appropriate. Multivariable logistic regression models were used to compare outcomes (primary: any non-transfusion severe maternal morbidity, secondary: blood transfusion of four or more units of packed red blood cells and readmission to delivery hospital within 90 days) by intended (primary analysis), and actual mode of delivery (secondary analysis), and with acute on chronic codes as inclusion criteria (sensitivity analysis). Covariates included in the logistic regression models are as described earlier.(12) Treatments that occurred during the delivery hospitalization are reported descriptively, but were not included as covariates in the statistical models, as these variables were considered as mediators (occurring on the causal pathway from exposure to outcome) rather than confounders.
The results are reported as odds ratio with 95% confidence intervals. Statistical analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, North Carolina). A two-sided alpha level of 0.05 was pre-specified as statistically significant.
RESULTS
Demographic and Clinical Characteristics
The cohort consisted of 2,921 individual deliveries in people with a diagnosis of cardiomyopathy (Table 1 and Figure 1). Individuals whose intended mode of delivery was vaginal delivery were younger than whose intended mode of delivery was cesarean delivery (30 [26, 35] years versus 32 [28, 36] years). Individuals admitted with cesarean as the intended mode of delivery had more preexisting comorbidities than those whose intended mode of delivery was vaginal. The most common comorbidities (Table 1) were chronic hypertension (intended cesarean 32.3% versus intended vaginal 23.4%), preexisting anemia (intended cesarean 24.9% versus intended vaginal 21.9%), asthma (intended cesarean 22.4% versus intended vaginal 20.1%), mental health disorders (intended cesarean 20.9% versus intended vaginal 19.5%), substance use disorders (intended cesarean 17.3% versus intended vaginal 18.1%), gastrointestinal disease (intended cesarean 15.5% versus intended vaginal 11.8%), gestational diabetes (intended cesarean 12.6% versus intended vaginal 8.5%), preexisting diabetes (intended cesarean 11.0% versus intended vaginal 7.3%) and neuromuscular disease (intended cesarean 8.9% versus intended vaginal 8.3%). Preeclampsia or gestational hypertension complicated over 30% of deliveries (562 had codes for preeclampsia or gestational hypertension only, 327 had codes for preeclampsia with severe features or hemolysis elevated liver enzymes low platelet syndrome only, 81 had codes for both). Preterm delivery occurred in 25% of completed vaginal deliveries and 50% of all cesarean deliveries. Prior cesarean birth was more common among individuals whose intended mode of delivery was cesarean versus vaginal (53.5% versus 10.6%).
Table 1:
Baseline characteristics of the cohort
| Vaginal Delivery (n=1214) | Intrapartum Cesarean Delivery (n=564) | Not Labored Cesarean Delivery (n=1143) | Intended VD (VD + Intrapartum CD) (n=1778) | All CD (Intrapartum + Not Labored CD) (n=1707) | p-value Intended VD vs. Not labored CD (Intention to treat) | p-value actual VD vs. intrapartum+ non labored CD (As-treated) | |
|---|---|---|---|---|---|---|---|
| Demographic Characteristics | |||||||
| Age, years (median, [Q1, Q3]) | 30 [25, 35] | 31 [26, 35] | 32 [28, 36] | 30 [26, 35] | 31 [27, 36] | <0.001 | <0.001 |
| Primary Payor | 0.020 | 0.043 | |||||
| Managed care | 341 (28.1%) | 182 (32.3%) | 309 (27.0%) | 523 (29.4%) | 491 (28.8%) | ||
| Medicaid | 668 (55.0%) | 288 (51.1%) | 617 (54.0%) | 956 (53.8%) | 905 (53.0%) | ||
| Medicare | 35 (2.9%) | 22 (3.9%) | 62 (5.4%) | 57 (3.2%) | 84 (4.9%) | ||
| Other | 170 (14.0%) | 72 (12.8%) | 155 (13.6%) | 242 (13.6%) | 227 (13.3%) | ||
| Race | 0.592 | 0.003 | |||||
| Asian | 43 (3.5%) | 17 (3.0%) | 33 (2.9%) | 60 (3.4%) | 50 (2.9%) | ||
| Black | 338 (27.8%) | 191 (33.9%) | 355 (31.1%) | 529 (29.8%) | 546 (32.0%) | ||
| Other | 127 (10.5%) | 70 (12.4%) | 111 (9.7%) | 197 (11.1%) | 181 (10.6%) | ||
| Unknown | 33 (2.7%) | 31 (5.5%) | 48 (4.2%) | 64 (3.6%) | 79 (4.6%) | ||
| White | 673 (55.4%) | 255 (45.2%) | 596 (52.1%) | 928 (52.2%) | 851 (49.9%) | ||
| Ethnicity | 0.282 | 0.023 | |||||
| Hispanic | 118 (9.7%) | 69 (12.2%) | 142 (12.4%) | 187 (10.5%) | 211 (12.4%) | ||
| Non-Hispanic | 873 (71.9%) | 370 (65.6%) | 782 (68.4%) | 1243 (69.9%) | 1152 (67.5%) | ||
| Unknown | 223 (18.4%) | 125 (22.2%) | 219 (19.2%) | 348 (19.6%) | 344 (20.2%) | ||
| Hospital Characteristics | |||||||
| Urban hospital | 1096 (90.3%) | 534 (94.7%) | 1059 (92.7%) | 1630 (91.7%) | 1593 (93.3%) | 0.342 | 0.003 |
| Teaching hospital | 815 (67.1%) | 391 (69.3%) | 724 (63.3%) | 1206 (67.8%) | 1115 (65.3%) | 0.012 | 0.307 |
| Bed size | 0.145 | 0.030 | |||||
| 000–099 | 38 (3.1%) | 7 (1.2%) | 26 (2.3%) | 45 (2.5%) | 33 (1.9%) | ||
| 100–199 | 78 (6.4%) | 38 (6.7%) | 65 (5.7%) | 116 (6.5%) | 103 (6.0%) | ||
| 200–299 | 142 (11.7%) | 58 (10.3%) | 116 (10.1%) | 200 (11.2%) | 174 (10.2%) | ||
| 300–399 | 177 (14.6%) | 78 (13.8%) | 177 (15.5%) | 255 (14.3%) | 255 (14.9%) | ||
| 400–499 | 184 (15.2%) | 80 (14.2%) | 138 (12.1%) | 264 (14.8%) | 218 (12.8%) | ||
| 500+ | 595 (49.0%) | 303 (53.7%) | 621 (54.3%) | 898 (50.5%) | 924 (54.1%) | ||
| Region of country | 0.125 | 0.022 | |||||
| Midwest | 322 (26.5%) | 133 (23.6%) | 259 (22.7%) | 455 (25.6%) | 392 (23.0%) | ||
| Northeast | 156 (12.9%) | 93 (16.5%) | 161 (14.1%) | 249 (14.0%) | 254 (14.9%) | ||
| South | 508 (41.8%) | 245 (43.4%) | 531 (46.5%) | 753 (42.4%) | 776 (45.5%) | ||
| West | 228 (18.8%) | 93 (16.5%) | 192 (16.8%) | 321 (18.1%) | 285 (16.7%) | ||
| Operative vaginal delivery | 125 (10.3%) | N/A | N/A | 125 (7.0%) | N/A | N/A | N/A |
| Comorbidities | |||||||
| Gestational Diabetes | 92 (7.6%) | 59 (10.5%) | 144 (12.6%) | 151 (8.5%) | 203 (11.9%) | <0.001 | <0.001 |
| HIV/AIDS | 1 (0.1%) | 1 (0.2%) | 9 (0.8%) | 2 (0.1%) | 10 (0.6%) | 0.004 | 0.029 |
| Preexisting Diabetes | 60 (4.9%) | 69 (12.2%) | 126 (11.0%) | 129 (7.3%) | 195 (11.4%) | <0.001 | <0.001 |
| Prior cesarean birth | 61 (5.0%) | 128 (22.7%) | 611 (53.5%) | 189 (10.6%) | 739 (43.3%) | <0.001 | <0.001 |
| Multiple gestation | 15 (1.2%) | 13 (2.3%) | 71 (6.2%) | 28 (1.6%) | 84 (4.9%) | <0.001 | <0.001 |
| Asthma | 217 (17.9%) | 140 (24.8%) | 256 (22.4%) | 357 (20.1%) | 396 (23.2%) | 0.133 | 0.001 |
| Bleeding disorder | 60 (4.9%) | 33 (5.9%) | 60 (5.2%) | 93 (5.2%) | 93 (5.4%) | 0.982 | 0.545 |
| BMI ≥ 40 kg/m2 | 84 (6.9%) | 67 (11.9%) | 137 (12.0%) | 151 (8.5%) | 204 (12.0%) | 0.002 | <0.001 |
| Chronic hypertension | 247 (20.3%) | 169 (30.0%) | 369 (32.3%) | 416 (23.4%) | 538 (31.5%) | <0.001 | <0.001 |
| Secondary hypertension | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0.753 | 0.809 |
| Hypertensive crisis | 8 (0.7%) | 7 (1.2%) | 30 (2.6%) | 15 (0.8%) | 37 (2.2%) | 0.000 | 0.001 |
| Chronic renal disease | 34 (2.8%) | 41 (7.3%) | 62 (5.4%) | 75 (4.2%) | 103 (6.0%) | 0.132 | <0.001 |
| Connective tissue or autoimmune disease | 20 (1.6%) | 9 (1.6%) | 19 (1.7%) | 29 (1.6%) | 28 (1.6%) | 0.948 | 0.988 |
| Substance use disorder | 229 (18.9%) | 92 (16.3%) | 198 (17.3%) | 321 (18.1%) | 290 (17.0%) | 0.614 | 0.192 |
| Advanced maternal age | 257 (21.2%) | 129 (22.9%) | 314 (27.5%) | 386 (21.7%) | 443 (26.0%) | <0.001 | 0.003 |
| Preexisting anemia | 247 (20.3%) | 142 (25.2%) | 285 (24.9%) | 389 (21.9%) | 427 (25.0%) | 0.056 | 0.003 |
| Bariatric surgery | 10 (0.8%) | 11 (2.0%) | 19 (1.7%) | 21 (1.2%) | 30 (1.8%) | 0.275 | 0.032 |
| Gastrointestinal disease | 126 (10.4%) | 83 (14.7%) | 177 (15.5%) | 209 (11.8%) | 260 (15.2%) | 0.004 | 0.000 |
| Mental health disorder | 227 (18.7%) | 119 (21.1%) | 239 (20.9%) | 346 (19.5%) | 358 (21.0%) | 0.339 | 0.130 |
| Neuromuscular disease | 91 (7.5%) | 57 (10.1%) | 102 (8.9%) | 148 (8.3%) | 159 (9.3%) | 0.572 | 0.083 |
| Placental abruption | 25 (2.1%) | 30 (5.3%) | 24 (2.1%) | 55 (3.1%) | 54 (3.2%) | 0.106 | 0.070 |
| Placenta accreta spectrum | 2 (0.2%) | 3 (0.5%) | 7 (0.6%) | 5 (0.3%) | 10 (0.6%) | 0.172 | 0.080 |
| Preeclampsia/gestational hypertension | 217 (17.9%) | 150 (26.6%) | 276 (24.1%) | 367 (20.6%) | 426 (25.0%) | 0.026 | <0.001 |
| Preeclampsia with severe features/HELLP | 93 (7.7%) | 116 (20.6%) | 199 (17.4%) | 209 (11.8%) | 315 (18.5%) | <0.001 | <0.001 |
| Preterm delivery | 302 (24.9%) | 291 (51.6%) | 566 (49.5%) | 593 (33.4%) | 857 (50.2%) | <0.001 | <0.001 |
| Thyrotoxicosis | 17 (1.4%) | 8 (1.4%) | 19 (1.7%) | 25 (1.4%) | 27 (1.6%) | 0.579 | 0.692 |
| Congenital heart disease | 45 (3.7%) | 11 (2.0%) | 38 (3.3%) | 56 (3.1%) | 49 (2.9%) | 0.794 | 0.207 |
| Valvular heart disease | 140 (11.5%) | 67 (11.9%) | 160 (14.0%) | 207 (11.6%) | 227 (13.3%) | 0.061 | 0.156 |
| Pulmonary hypertension | 31 (2.6%) | 18 (3.2%) | 64 (5.6%) | 49 (2.8%) | 82 (4.8%) | <0.001 | 0.002 |
| Eisenmenger syndrome | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 0.423 | 0.236 |
| Arrythmias | 210 (17.3%) | 65 (11.5%) | 202 (17.7%) | 275 (15.5%) | 267 (15.6%) | 0.116 | 0.233 |
| Aortopathies | 5 (0.4%) | 5 (0.9%) | 6 (0.5%) | 10 (0.6%) | 11 (0.6%) | 0.893 | 0.401 |
| Coronary artery/ischemic/atherosclerotic | 68 (5.6%) | 40 (7.1%) | 76 (6.6%) | 108 (6.1%) | 116 (6.8%) | 0.533 | 0.190 |
| Other cardiac disease | 27 (2.2%) | 21 (3.7%) | 31 (2.7%) | 48 (2.7%) | 52 (3.0%) | 0.984 | 0.177 |
Outcomes
Among patients exposed to intended vaginal deliveries, 68% (n=1,214) were successful in achieving a vaginal birth. One third of the intended vaginal deliveries resulted in intrapartum cesarean deliveries (n=564). Operative vaginal delivery was performed in 10% of the vaginal deliveries (n=125). The primary outcome of non-transfusion severe maternal morbidity occurred in 14.8% of all deliveries (8.2% of completed vaginal deliveries, 17.1% of intended cesarean deliveries and 24.5% of intrapartum cesarean deliveries). The most common non-transfusion severe maternal morbidity events across the whole cohort were need for mechanical ventilation (5.6%), respiratory distress (5.6%), renal failure (3.4%), shock (2.7%), heart failure (3.3%) and pulmonary edema (1.3%) (Table 2). Red blood cell transfusion of more than 4 units occurred more often in completed cesarean deliveries, (planned 4.6% or intrapartum 6%) compared to successful vaginal deliveries, 1.9%. Readmission within 90 days of delivery hospitalization occurred in 6.7% of deliveries.
Table 2:
Mortality and Morbidity Events at delivery hospitalization by delivery mode
| Vaginal Delivery (n=1214) | Intrapartum Cesarean Delivery (n=564) | Not Labored Cesarean Delivery (n=1143) | Intended VD (VD + Intrapartum CD) (n=1778) | All CD (Intrapartum + Not Labored CD) (n=1707) | p-value Intended VD vs. Not labored CD (Intention to treat) | p-value actual VD vs. intrapartum+ non labored CD (As treated) | |
|---|---|---|---|---|---|---|---|
| In-hospital Mortality | 4 (0.3%) | 5 (0.9%) | 3 (0.3%) | 9 (0.5%) | 8 (0.5%) | 0.387 | 0.771 |
| Severe Maternal Morbidity Event (composite) | 100 (8.2%) | 138 (24.5%) | 195 (17.1%) | 238 (13.4%) | 333 (19.5%) | 0.006 | <0.001 |
| Individual SMM Elements | |||||||
| Acute myocardial infarction | 1 (0.1%) | 3 (0.5%) | 6 (0.5%) | 4 (0.2%) | 9 (0.5%) | 0.176 | 0.043 |
| Dissection (not aneurysm) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | N/A | N/A |
| Acute renal failure | 23 (1.9%) | 31 (5.5%) | 46 (4.0%) | 54 (3.0%) | 77 (4.5%) | 0.152 | <0.001 |
| Acute respiratory distress syndrome | 30 (2.5%) | 59 (10.5%) | 76 (6.6%) | 89 (5.0%) | 135 (7.9%) | 0.060 | <0.001 |
| Amniotic fluid embolism | 0 (0.0%) | 1 (0.2%) | 1 (0.1%) | 1 (0.1%) | 2 (0.1%) | >0.999 | 0.514 |
| Cardiac arrest | 4 (0.3%) | 10 (1.8%) | 14 (1.2%) | 14 (0.8%) | 24 (1.4%) | 0.236 | 0.003 |
| Ventricular fibrillation | 1 (0.1%) | 4 (0.7%) | 1 (0.1%) | 5 (0.3%) | 5 (0.3%) | 0.414 | 0.411 |
| Conversion of cardiac rhythm | 6 (0.5%) | 15 (2.7%) | 18 (1.6%) | 21 (1.2%) | 33 (1.9%) | 0.366 | 0.001 |
| Disseminated intravascular coagulation | 7 (0.6%) | 13 (2.3%) | 12 (1.0%) | 20 (1.1%) | 25 (1.5%) | 0.849 | 0.023 |
| Heart failure or arrest during surgery or procedure | 0 (0.0%) | 3 (0.5%) | 0 (0.0%) | 3 (0.2%) | 3 (0.2%) | 0.285 | 0.271 |
| Puerperal cerebrovascular disorders | 1 (0.1%) | 1 (0.2%) | 4 (0.3%) | 2 (0.1%) | 5 (0.3%) | 0.218 | 0.411 |
| Pulmonary edema | 9 (0.7%) | 19 (3.4%) | 9 (0.8%) | 28 (1.6%) | 28 (1.6%) | 0.063 | 0.032 |
| Acute heart failure | 28 (2.3%) | 22 (3.9%) | 46 (4.0%) | 50 (2.8%) | 68 (4.0%) | 0.073 | 0.012 |
| Severe anesthesia complications | 0 (0.0%) | 3 (0.5%) | 2 (0.2%) | 3 (0.2%) | 5 (0.3%) | >0.999 | 0.080 |
| Sepsis | 14 (1.2%) | 13 (2.3%) | 14 (1.2%) | 27 (1.5%) | 27 (1.6%) | 0.510 | 0.332 |
| Shock | 18 (1.5%) | 27 (4.8%) | 35 (3.1%) | 45 (2.5%) | 62 (3.6%) | 0.391 | 0.001 |
| Sickle cell disease with crisis | 0 (0.0%) | 2 (0.4%) | 1 (0.1%) | 2 (0.1%) | 3 (0.2%) | >0.999 | 0.271 |
| Air and thrombotic embolism | 4 (0.3%) | 6 (1.1%) | 13 (1.1%) | 10 (0.6%) | 19 (1.1%) | 0.086 | 0.018 |
| Temporary tracheostomy | 0 (0.0%) | 4 (0.7%) | 5 (0.4%) | 4 (0.2%) | 9 (0.5%) | 0.326 | 0.013 |
| Ventilation | 25 (2.1%) | 67 (11.9%) | 71 (6.2%) | 92 (5.2%) | 138 (8.1%) | 0.233 | <0.001 |
| Eclampsia | 4 (0.3%) | 11 (2.0%) | 22 (1.9%) | 15 (0.8%) | 33 (1.9%) | 0.011 | <0.001 |
| Hysterectomy | 4 (0.3%) | 6 (1.1%) | 10 (0.9%) | 10 (0.6%) | 16 (0.9%) | 0.318 | 0.050 |
| Intensive care unit admission | 163 (13.4%) | 167 (29.6%) | 336 (29.4%) | 330 (18.6%) | 503 (29.5%) | <0.001 | <0.001 |
| Length of stay after delivery (days) | 1 [1, 2] | 3 [2, 4] | 2 [2, 4] | 1 [1, 3] | 2 [2, 4] | <0.001 | <0.001 |
| Hospital Readmission (n,%) | |||||||
| 90-day Readmission | 69 (5.7%) | 47 (8.3%) | 80 (7.0%) | 116 (6.5%) | 127 (7.4%) | 0.617 | 0.062 |
| Vasopressor use (any) | 16 (1.3%) | 42 (7.4%) | 70 (6.1%) | 58 (3.3%) | 112 (6.6%) | <0.001 | <0.001 |
| Norepinephrine | 12 (1.0%) | 32 (5.7%) | 52 (4.5%) | 44 (2.5%) | 84 (4.9%) | 0.002 | <0.001 |
| Vasopressin | 6 (0.5%) | 22 (3.9%) | 29 (2.5%) | 28 (1.6%) | 51 (3.0%) | 0.075 | <0.001 |
| Both | 2 (0.2%) | 12 (2.1%) | 11 (1.0%) | 14 (0.8%) | 23 (1.3%) | 0.682 | <0.001 |
| Inotrope use (any) | 13 (1.1%) | 47 (8.3%) | 76 (6.6%) | 60 (3.4%) | 123 (7.2%) | <0.001 | <0.001 |
| Epinephrine | 3 (0.2%) | 22 (3.9%) | 36 (3.1%) | 25 (1.4%) | 58 (3.4%) | 0.002 | <0.001 |
| Dobutamine | 7 (0.6%) | 17 (3.0%) | 35 (3.1%) | 24 (1.3%) | 52 (3.0%) | 0.002 | <0.001 |
| Dopamine | 2 (0.2%) | 1 (0.2%) | 3 (0.3%) | 3 (0.2%) | 4 (0.2%) | 0.684 | >0.999 |
| Milrinone | 3 (0.2%) | 16 (2.8%) | 21 (1.8%) | 19 (1.1%) | 37 (2.2%) | 0.102 | <0.001 |
| More than one | 2 (0.2%) | 8 (1.4%) | 15 (1.3%) | 10 (0.6%) | 23 (1.3%) | 0.039 | <0.001 |
| ECMO use | 0 (0.0%) | 8 (1.4%) | 1 (0.1%) | 8 (0.4%) | 9 (0.5%) | 0.099 | 0.013 |
| Any pulmonary vasodilator | 140 (11.5%) | 154 (27.3%) | 253 (22.1%) | 294 (16.5%) | 407 (23.8%) | <0.001 | <0.001 |
| Calcium Channel Blockers | 140 (11.5%) | 152 (27.0%) | 244 (21.3%) | 292 (16.4%) | 396 (23.2%) | 0.001 | <0.001 |
| Prostacyclin Analogues | 0 (0.0%) | 1 (0.2%) | 7 (0.6%) | 1 (0.1%) | 8 (0.5%) | 0.007 | 0.024 |
| PDE-5 Inhibitors | 0 (0.0%) | 2 (0.4%) | 7 (0.6%) | 2 (0.1%) | 9 (0.5%) | 0.017 | 0.011 |
| Obstetric morbidity | |||||||
| Blood transfusion 4 or more units pRBC | 23 (1.9%) | 34 (6.0%) | 53 (4.6%) | 57 (3.2%) | 87 (5.1%) | 0.012 | <0.001 |
In the primary ‘intention to treat’ analysis there was no difference in non-transfusion morbidity between intended vaginal delivery (including intrapartum cesarean deliveries) and intended cesarean delivery groups (adjusted odds ratio [aOR], 1.17; 95% confidence interval [CI], 0.91–1.51), blood transfusion (aOR, 1.27; 95% CI, 0.81–1.98) or readmission (aOR, 1.03; 95% CI, .73–1.44). In the secondary ‘as treated’ analysis, cesarean delivery (including intrapartum cesarean deliveries) was associated with a 2-fold increase of non-transfusion morbidity (aOR, 2.44; 95% CI, 1.85–3.22) and blood transfusion (aOR, 2.26; 95% CI, 1.34–3.81) compared to vaginal delivery, but no difference in 90-day readmission (aOR, 1.26; 95% CI, 0.89–1.79). Model results are presented in Supplemental Table 5 and Figure 2. The cumulative incidence of readmissions by mode of delivery is presented in Figure 3. Eleven of the 12 patients who died had at least one code for a morbidity event.
Figure 2:
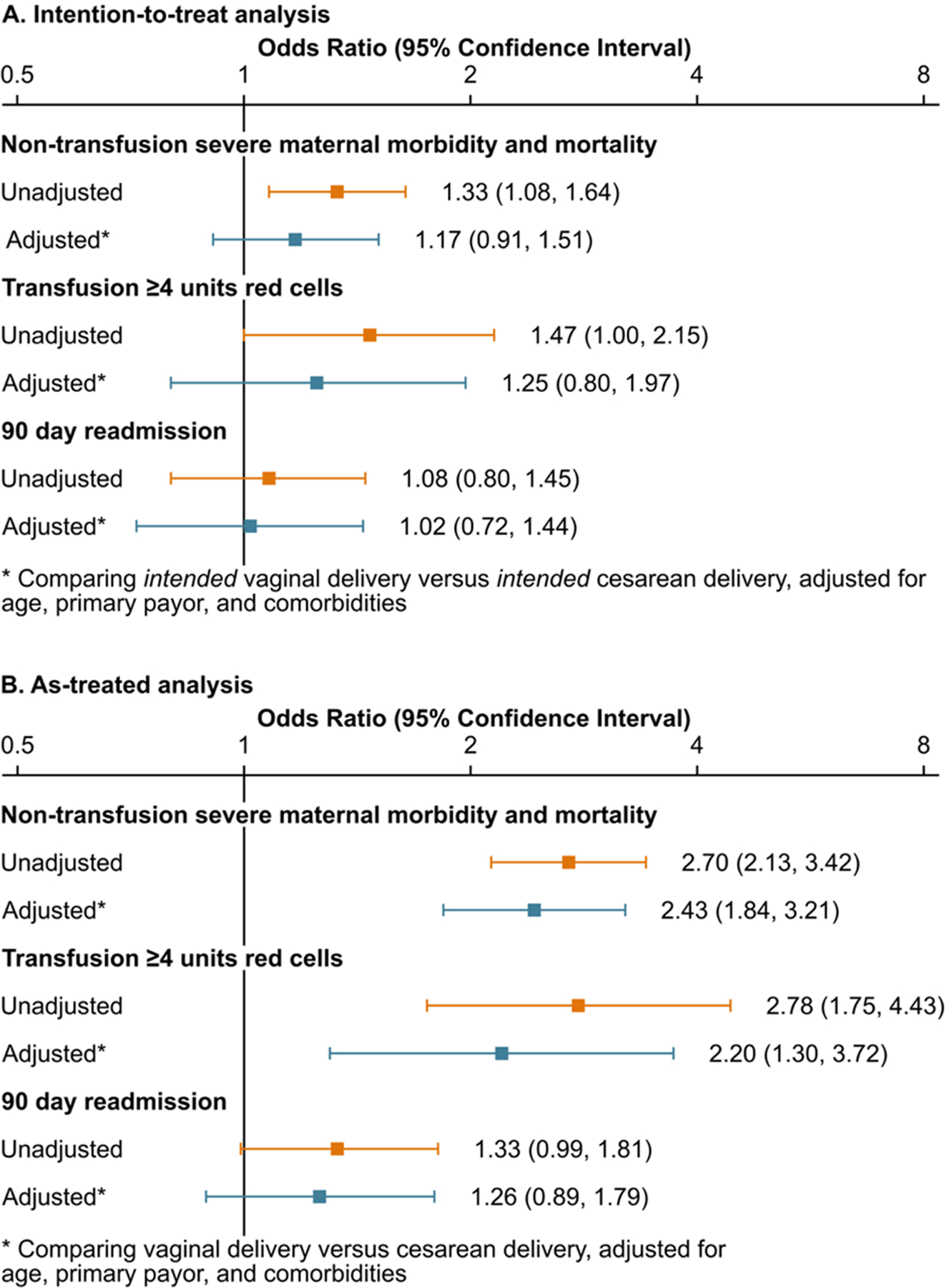
Main study analysis and sensitivity analysis, Unadjusted and adjusted outcomes among women with CM/HF by mode of delivery, primary analysis by intended mode of delivery and secondary analysis by actual mode of delivery.
Figure 3:
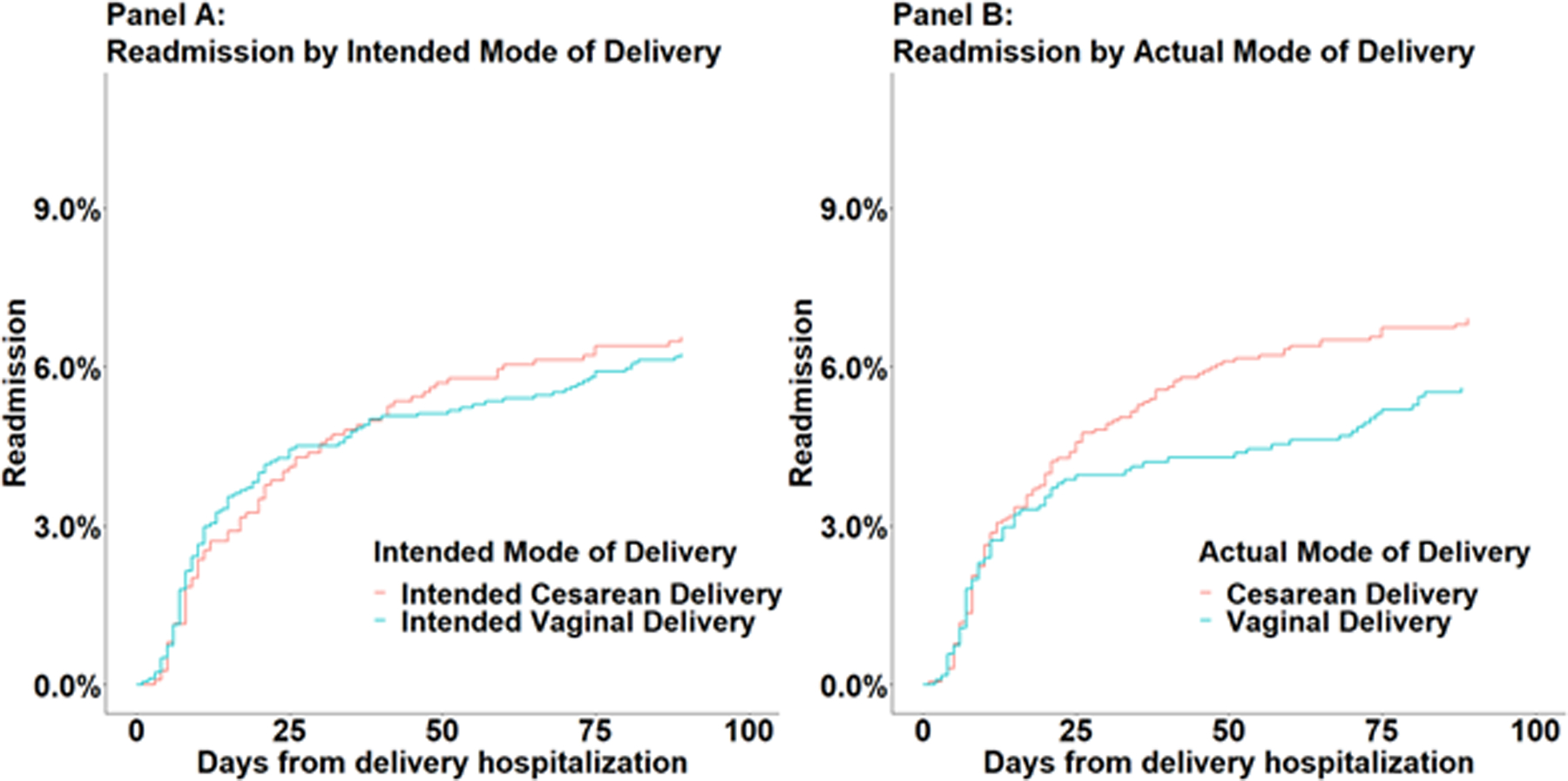
Cumulative incidence of readmissions, by mode of delivery.
Sensitivity Analysis
The sensitivity analysis which included people with acute on chronic heart failure (additional 112 individuals included) revealed similar results for both the ‘intention to treat’ analysis and the ‘as treated’ analysis with no difference in the risk of severe maternal morbidity between individuals who had a trial of labor and those who delivered via intended cesarean delivery but increased risk of non-transfusion morbidity and blood transfusion when intrapartum cesarean deliveries were analyzed in the cesarean delivery group. (Supplemental Tables 6–8 and Supplemental Figure 1). The cumulative incidence of readmissions by mode of delivery in the sensitivity analysis cohort is presented in Supplemental Figure 2.
Table 2 presents intensive care unit admission, length of stay and the cardiovascular treatments provided at any point in the delivery hospitalization. Patients who we delivered via intrapartum cesarean delivery or cesarean delivery were given vasopressors, inotropes, pulmonary vasodilators more often than patients who achieved vaginal deliveries. ECMO was used in less than 10 patients, all delivering via intrapartum cesarean delivery except one delivering via planned cesarean.
DISCUSSION
This large retrospective study of pregnant individuals with cardiomyopathies demonstrated that there may not be an association of intended delivery mode with severe maternal morbidity events during delivery hospitalization or 90-day readmission. A trial of labor in these patients did not confer a higher risk of SMM compared with unlabored cesarean delivery. However, the risk of morbidity is three-fold higher in the group of individuals that required an intrapartum cesarean delivery. It is not possible to predict which patients will need intrapartum deliveries, thus we performed our analyses to be clinically relevant to the usual decision-making process where providers and patients choose an intended mode of delivery rather than an actual mode of delivery. The comparison between the intention to treat primary analysis (trial of labor versus planned cesarean delivery) and the as-treated analysis (outcome of vaginal versus cesarean delivery) highlights the higher morbidity associated with cesarean deliveries that occur in the context of a trial of labor when compared to planned cesarean delivery, and suggests a high degree of vigilance is needed intraoperatively and postoperatively in these cases. Additionally, these data may be useful in informing delivery discussions in preconception and pregnancy in patients with cardiomyopathies.
Data from patients with all etiologies of cardiac disease from the International Registry of Cardiac Disease and Pregnancy (ROPAC), suggest that planned caesarean delivery confers no maternal benefit compared to trial of labor.(13) Expert opinions support the practice of trials of labor in the majority of patients with cardiac disease, reserving cesarean deliveries for obstetric or fetal indications and for patients on oral anticoagulants, severe aortic disease and in those with acute intractable heart failure.(1,4,14) Yet reports of outcomes by mode of delivery are limited in cohort size with most only reporting cardiomyopathies among all other etiologies of cardiac disease. Here we report the impact of delivery mode on morbidity, specifically in over 2,900 individuals with cardiomyopathy. This cohort had a slightly higher rate of planned, unlabored cesarean delivery (39%) compared to retrospective database (31%) and prospective hospital-based cohorts (24%), likely reflecting the increased likelihood of providers in specialized centers to follow expert guidance favoring vaginal deliveries.(13,14) The Premier hospitals taken together represent diversity in hospital types and Levels of Maternal Care such that it is expected that more cesarean deliveries are performed due to staffing and resource constraints.
One in seven patients, 15%, experienced a maternal morbidity event. The most common types of morbidity events in this cohort relate to cardiopulmonary failure (mechanical ventilation, respiratory distress, renal failure, shock, heart failure and pulmonary edema). Pregnant patients with cardiomyopathies should therefore be cared for in centers equipped to deliver advanced cardiopulmonary support peridelivery.(1,3,15,16) Beyond the high risk of morbidity among people who delivered via intrapartum cesarean, there is an high rate of intrapartum cesarean delivery in this cohort such that centers should also be prepared for urgent, high-risk intrapartum cesarean deliveries. The morbidity data here highlight that this population should deliver in centers where in-house consultation is available with critical care, cardiology, nephrology and cardiac surgery and centers where there are on-site medical or surgical intensive care units with expertise in managing critically ill obstetric patients (1,3,16). Factors which may be associated with being in the highest risk group, intrapartum cesarean delivery group, include those associated with a higher likelihood of induction failure, including a trial of labor after cesarean delivery, growth restricted fetus, morbid and super obesity, symptomatic heart failure, nulliparity, and extremes of maternal age; however, prediction of successful vaginal delivery remains notoriously challenging in obstetric practice (17).
The operative vaginal delivery rates reported from Premier are comparable to American academic centers of Pregnancy Heart Team excellence, approximately 10% of all vaginal deliveries among patients with cardiac disease.(14) The rate of operative vaginal deliveries was one-third of that reported in the ROPAC series of pregnant patients with all types of cardiac disease, likely reflecting that our cohort is specifically cardiomyopathy patients and reflecting the temporal trend of allowing more women with cardiac disease to have unassisted vaginal deliveries.(13) Over the last decade there has been a recognition that though there are select patients who should not push (large aortopathy, preload sensitive lesions, severe left ventricular outflow obstruction, hypertrophic cardiomyopathy, severe mitral or aortic stenosis) the Valsalva maneuver (maternal expulsive effort) is often well tolerated and not as harmful as previously thought and the bleeding and laceration complications associated with forceps delivery might be more detrimental.(18)
The high rates of 90-day readmission, preeclampsia and preterm delivery highlight the importance of thorough antenatal and postpartum cardiovascular follow-up as the physiologic changes of pregnancy take weeks to months to resolve and reaccumulation of fluid, heart failure and arrythmia events may occur in the interim. Enhanced preeclampsia surveillance is necessary in this population and research to identify overlapping mechanisms between preeclampsia and heart failure is necessary. These data about high rates of prematurity may aid in pre-pregnancy counseling and patient centered decision making.
There are a few notable strengths of this study. The size of our cohort allowed us to have the power to examine associations detect associations between mode of delivery and outcomes, overcoming limitations of previous studies.(13,14) The ‘Intention to treat’ analysis is clinically relevant and mirrors real-life decisions that patients and providers face. Additionally, we provide estimates of morbidity and readmissions among people with cardiomyopathies which may be useful in helping patients and providers decide if pregnancy risk is prohibitive but also in delivery center and postpartum care planning to ensure adequate resources and follow up are available or to ensure transfer of care is initiated when it can be anticipated that it may be necessary. The Premier dataset is not representative of all the United States, but disease burden and management are similar to previous reports such that we feel that these results are modestly generalizable, though caution is necessary when applying results to maternal centers with different levels of care. Not all centers may be able to mobilize care for a high-risk cesarean delivery at all hours, therefore, resulting morbidity after intrapartum cesarean delivery may differ by center. Centers without consistent comprehensive Pregnancy Heart Team care coverage may appropriately opt for more planned cesarean deliveries to ensure appropriate personnel and resources are available.
Several key limitations exist due to the confines of the dataset. Severity of cardiomyopathy and functional status are not captured in billing codes in administrative datasets, so we are unable to group patients by severity of disease or specifically control for these known risk factors for increased pregnancy morbidity in people with cardiomyopathy. As demonstrated by Grewal et al, moderate or severe left ventricular dysfunction or New York Heart Association Functional class III or IV are the main risk factors for adverse events in pregnancy among women with dilated cardiomyopathies.(2) Registries that can examine disease severity and functional status may have the ability to identify the exact factors contributing to the heightened morbidity risk among people who require intrapartum cesarean deliveries. We also included all forms of cardiomyopathy in a single category for study power, it would be useful to perform future comparisons by cardiomyopathy type. It is not possible to identify if a cesarean delivery was performed for maternal cardiac indications. Therefore, we cannot identify if any of the intrapartum cesarean deliveries were performed due to declining cardiopulmonary function such that the morbidity is not due to the cesarean delivery itself but rather the underlying condition that prompted the expedited delivery. Patients who are planned for an intended cesarean delivery differ from patients who are planned for an intended vaginal delivery in several important ways which can be observed and adjusted for in this analysis (e.g., higher rates of prior cesarean delivery), and potentially in ways that cannot be completely adjusted for in this analysis (e.g., higher rates of more severe cardiac disease). As with all observational studies, particularly those with administrative data, we cannot exclude completely the possibility of unmeasured confounding by indication. Though over 90% of the in-hospital mortalities are represented by co-existing morbidity codes, we are unable to account for patients who died outside of the delivery hospital. Only readmissions to delivery hospitals are available in this dataset, so there is underreporting of the readmission outcome. We used a vast array of covariables known to influence maternal outcomes; however, residual confounding is possible, with severity of disease and maternal functional status being the largest likely unmeasured variables. We are unable to examine fetal outcomes in this dataset. It has been previously demonstrated that planned cesarean delivery is associated with adverse fetal outcomes.(13)
Our large retrospective study of obstetric patients with cardiomyopathies supports current guidelines recommending avoidance of cesarean delivery in most patients with maternal cardiac disease unless there is an obstetric or fetal indication. Further studies examining outcomes based on management strategies are needed to advance the field of cardio-obstetrics. Randomized controlled trials examining management options are unfeasible; therefore, large observational studies examining real world data such as these can provide guidance on clinical management and inform individualized decisions about pregnancy continuation and care. These data should provide reassurance to patients and providers that a trial of labor is a safe option for most patients with cardiomyopathies.
Supplementary Material
Central Illustration:
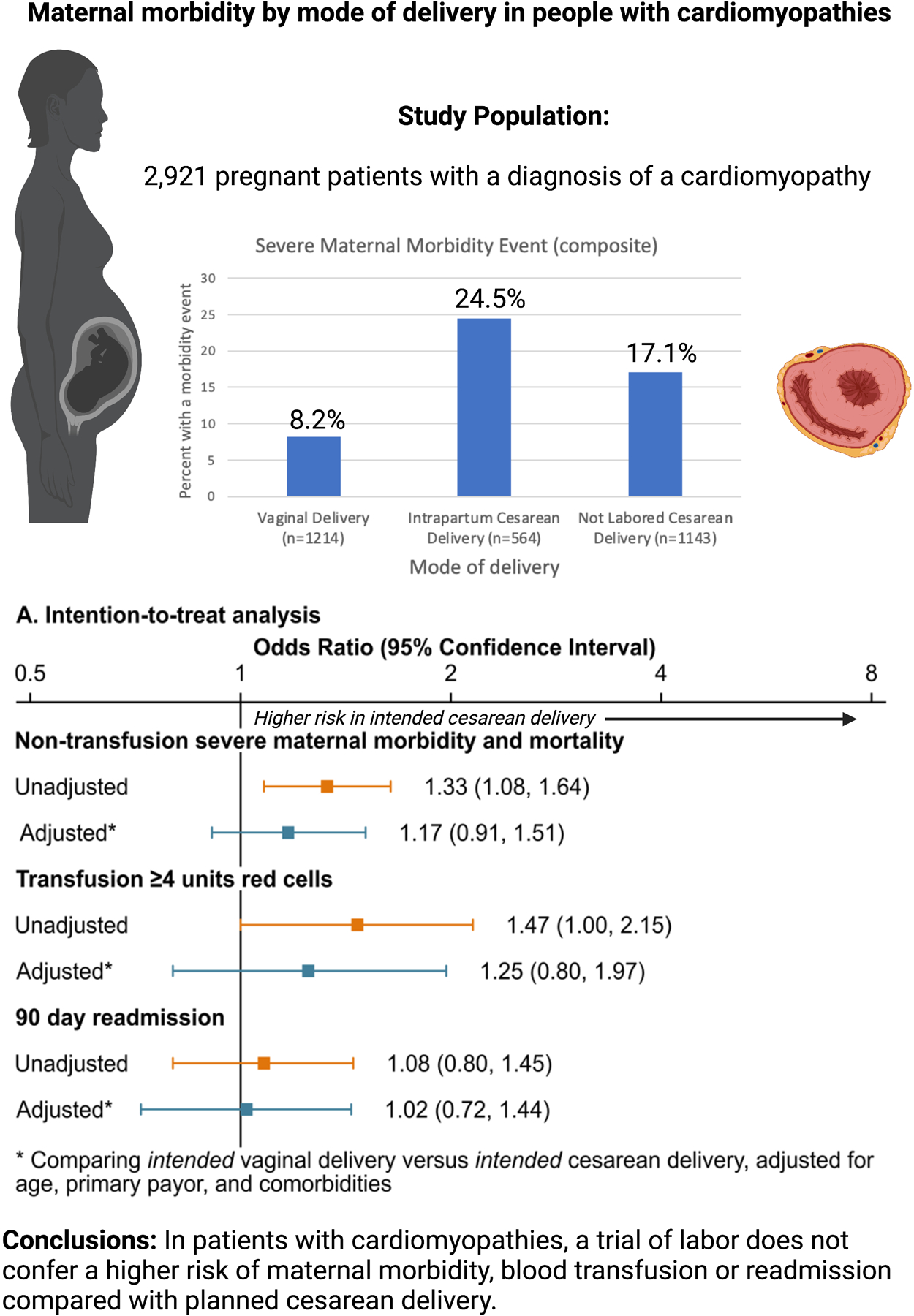
Maternal morbidity by mode of delivery (Created with BioRender.com)
COMPETENCY IN MEDICAL KNOWLEDGE:
In a cohort of pregnant patients with cardiomyopathies, a trial of labor does not confer a higher risk of maternal morbidity, blood transfusion or readmission compared with planned cesarean delivery.
TRANSLATIONAL OUTLOOK:
Our large observational studies examining real world data of obstetric patients with cardiomyopathies supports current guidelines recommending avoidance of cesarean delivery in most patients with maternal cardiac disease unless there is an obstetric or fetal indication. Further studies examining outcomes based on management strategies are needed to advance the field of cardio-obstetrics.
Acknowledgements:
MLM receives grant support from the Foundation for Anesthesia Education and Research. During the study period, JJF received support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) under award K12HD103083, and ME is supported by grant 1K01MH127309 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- aOR
adjusted odds ratio
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- ROPAC
International Registry of Cardiac Disease and Pregnancy
- SMM
severe maternal morbidity
Footnotes
Disclosures: Dr. Federspiel serves as a consultant for Hemosquid, SA. The other listed authors hold no disclosures or relevant conflicts of interest.
REFERENCES
- 1.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 2.Grewal J, Siu SC, Ross HJ et al. Pregnancy outcomes in women with dilated cardiomyopathy. J Am Coll Cardiol 2009;55:45–52. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians & Gynecologists’ Presidential Task Force on Pregnancy Heart Disease Committee on Practice Bulletins Obstetrics. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol 2019;133:e320–e356. [DOI] [PubMed] [Google Scholar]
- 4.Shotan A, Roos-Hesselink J, Baris L, Goland S, Yekel Y, Elkayam U. Cardiomyopathy and Pregnancy: Considerations for Women With Severely Reduced Left Ventricular Dysfunction. Can J Cardiol 2021;37:2067–2075. [DOI] [PubMed] [Google Scholar]
- 5.Goland S, van Hagen IM, Elbaz-Greener G et al. Pregnancy in women with hypertrophic cardiomyopathy: data from the European Society of Cardiology initiated Registry of Pregnancy and Cardiac disease (ROPAC). Eur Heart J 2017;38:2683–2690. [DOI] [PubMed] [Google Scholar]
- 6.Billebeau G, Etienne M, Cheikh-Khelifa R et al. Pregnancy in women with a cardiomyopathy: Outcomes and predictors from a retrospective cohort. Arch Cardiovasc Dis 2018;111:199–209. [DOI] [PubMed] [Google Scholar]
- 7.Yokouchi-Konishi T, Kamiya CA, Shionoiri T et al. Pregnancy outcomes in women with dilated cardiomyopathy: Peripartum cardiovascular events predict post delivery prognosis. J Cardiol 2021;77:217–223. [DOI] [PubMed] [Google Scholar]
- 8.Meng ML, Fuller M, Federspiel JJ et al. Maternal Morbidity According to Mode of Delivery Among Pregnant Patients With Pulmonary Hypertension. Anesth Analg 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 10.Hernan MA, Wang W, Leaf DE. Target Trial Emulation: A Framework for Causal Inference From Observational Data. JAMA 2022;328:2446–2447. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control, National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. Severe Maternal Morbidity in the United States. 2021.
- 12.Leonard SA, Kennedy CJ, Carmichael SL, Lyell DJ, Main EK. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol 2020;136:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruys TP, Roos-Hesselink JW, Pijuan-Domenech A et al. Is a planned caesarean section in women with cardiac disease beneficial? Heart 2015;101:530–6. [DOI] [PubMed] [Google Scholar]
- 14.Easter SR, Rouse CE, Duarte V et al. Planned vaginal delivery and cardiovascular morbidity in pregnant women with heart disease. Am J Obstet Gynecol 2020;222:77 e1–77 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MB, Arendt K, Bello NA et al. Team-Based Care of Women With Cardiovascular Disease From Pre-Conception Through Pregnancy and Postpartum: JACC Focus Seminar 1/5. J Am Coll Cardiol 2021;77:1763–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Obstetricians & Gynecologists. Levels of Maternal Care: Obstetric Care Consensus No, 9. Obstet Gynecol 2019;134:e41–e55. [DOI] [PubMed] [Google Scholar]
- 17.Gunatilake RP, Smrtka MP, Harris B et al. Predictors of failed trial of labor among women with an extremely obese body mass index. Am J Obstet Gynecol 2013;209:562 e1–5. [DOI] [PubMed] [Google Scholar]
- 18.Cauldwell M, Steer PJ, Swan L, Uebing A, Gatzoulis MA, Johnson MR. The management of the third stage of labour in women with heart disease. Heart 2017;103:945–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


