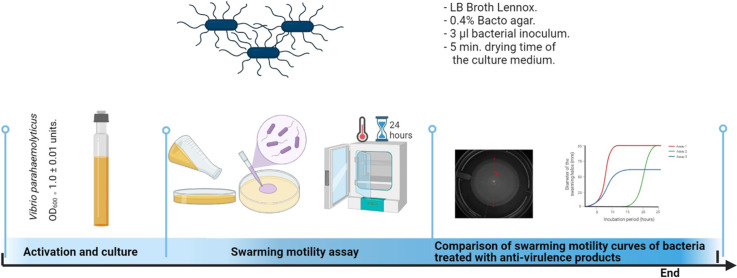
Abstract
Swarming motility is a type of movement used by pathogenic flagellated bacteria as virulence factor to colonize surfaces and cause damage to the host. Vibrio parahaemolyticus is a pathogenic flagellated bacterium that increases its virulence by switching from swimmer to swarming cells. The hosts of pathogenic V. parahaemolyticus include farmed shrimp. Therefore, methods to detect and quantify this movement are important to control shrimp diseases caused by pathogenic V. parahaemolyticus strains. We developed an optimized swarming motility assay by identifying the most optimal type of agar, and drying time of the culture medium, agar concentration and volume of the bacterial culture to achieve the fastest swarming motility during the migration of V. parahaemolyticus on Petri dishes during a 24-hour incubation period. The method includes data analysis that could be used as a tool to identify potential anti-virulence products by comparing the slopes of the linearized diameters of the swarming halos of bacteria treated with the products, as they migrate on Petri dishes over a 24-hour incubation period.
Here we report:
-
•
A simple method for detection and quantification of swarming motility halos of V. parahaemolyticus bacteria.
-
•
A method that could be used as a tool to identify potential anti-virulence products.
Keywords: Bacterial flagellum, Bacterial motility, Bacterial pathogenicity, Significant differences among slopes, Virulence factors, Penaeus (Litopenaeus) vannamei shrimp
Graphical abstract
Specification table
| Subject Area: | Veterinary Science and Veterinary Medicine |
| More specific subject area: | Shrimp health |
| Name of your method: | Swarming motility assay for Vibrio parahaemolyticus |
| Name and reference of original method: | Md. F. R. Mizan, I. K. Jahid, M. Kim, K. H. Lee, T. J. Kim, S.-D. Ha, Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation, Biofouling, vol. 32, pp. 497–509, 2016, doi:10.1080/08927014.2016.1149571[1]. |
| Resource availability: | All information is available in the manuscript |
Overview
Farmed shrimp production consists mainly of the species Penaeus (Litopenaeus) vannamei, with an annual production of 5.8 million tons [2] and a value of USD 26.7 billion [3], making it the world's number one aquaculture animal species [2]. With the high growth rate of human population, which is expected to increase by nearly 9 billion people by 2050 [4], shrimp aquaculture will increase its important role as source of global food in developed countries and for the economic development of several developing countries. However, the intensification of production systems due to global demand is increasing the risk of infectious diseases in shrimp, including bacterial diseases [5]. The major bacterial diseases in P. vannamei shrimp are caused by pathogenic Vibrio, the most important of which is acute hepatopancreatic necrosis disease (AHPND), with total losses estimated at 43 billion [6]. AHPND is caused by several Vibrio species, but mainly by the pathogenic Vibrio parahaemolyticus [7,8,9].
Vibrio parahaemolyticus is a gram-negative, halophilic, flagellated bacterium that is widespread in estuarine and coastal environments [10]. The bacterium is also the etiological agent of human gastrointestinal illness caused by the consumption of undercooked seafood [10,11]. V. parahaemolyticus increases its virulence by switching from swimmer to swarming cells when colonize solid or semi-solid surfaces [12]. Several methods have been proposed to assess the swarming motility of some pathogenic Vibrio species and other bacterial genera in Petri dishes [13,14]. However, there is a lack of information on swarming motility assays for V. parahaemolyticus. Therefore, there is a need for standardized in vitro assays to quantify the swarming motility of V. parahaemolyticus, which is crucial for the development of novel antibacterial agents and anti-virulence therapies to control AHPND and mitigate its economic impact.
The conditions required for bacteria to adopt the swarming motile life state are species dependent and influenced by surface moisture [15]. Different culture media can produce different levels of motility, as swarming of V. parahaemolyticus requires a nutrient-rich medium [16]. Swarming motility of V. parahaemolyticus also requires a smooth medium, but within a narrow range, as agar concentrations above 0.3% exclude swimming, but above 1% inhibit swarming [16]. The water content of the medium is another critical factor, as too little water content will result in poor swarming, while too much water may allow swimming [16]. Therefore, one of the most important factors influencing the swarming motility of V. parahaemolyticus in bacterial cultures on Petri dishes is the type of agar, the agar concentration (smoothness), the drying time of the culture medium (moisture content) and the bacterial inoculum volume. Considering that swarming migration of pathogenic V. parahaemolyticus requires specific conditions, we standardized a swarming motility assay by identifying the levels of these four key factors that promote their fastest swarming migration on Petri dishes during a 24-hour incubation period. The assay was standardized using a pathogenic strain of V. parahaemolyticus that causes AHPND in cultured shrimp [17].
Several treatments were tested (combinations of factor levels), with the best one being the one that promoted the fastest swarming motility migration of the pathogenic bacteria on Petri dishes (best treatment). Thus, the steeper the swarming motility curve over a 24-hour incubation period, the faster the swarming motility. Validation of the best treatment included estimation of the reproducibility (inter-assay variability) of five independent assays. Additional validation was performed by confirming developmental stages and morphology of V. parahaemolyticus during the swarm migration period under the best treatment conditions, and ability of the assay to detect anti-virulence activity. The method includes data analysis that could be used as a tool to identify potential anti-virulence products by comparing the slopes of the linearized diameters of swarming halo of bacteria treated with the products, as they migrate on Petri dishes over a 24-hour incubation period.
Method details
Materials
Biological material: Vibrio parahaemolyticus bacterial strain
Pipettes
Petri dishes
Microcentrifuge tubes (1.5 mL)
Permanent marker
Ruler or vernier caliper
Microsoft Excel and R software for data display and analysis
Reagents
Tryptic Soy Agar – TSA (Difco, Le Pont de Claix, France)
Sodium chloride – NaCl (VWR Chemicals BDH, United States)
Bacto agar (Difco, Le Pont de Claix, France)
LB Broth, Lennox (Difco, Le Pont de Claix, France)
Distilled water
Equipments
Ultra-Upright freezer (−80 °C) (Thermo Scientific, 931)
Standard incubator (Thermo Scientific, Heratherm IGS180)
Vertical laminar flow chamber (Air Tech Biological Security Camera, BGM-1002 W)
Digital shaking water bath (VWR International, 89032-226)
Microplate reader spectrophotometer (Thermo Scientific, Varioskan LUX)
Analytical balance (Sartorius, ENTRISS224–1S)
Autoclave (Yamato Scientific America, SQ500C)
Additional material and equipment used for the validation of the swarming motility test.
Reagents and others
Motility Test Medium (Difco, Le Pont de Claix, France)
Brain Heart Infusion Broth – BHI (Difco, Le Pont de Claix, France)
Biological material: microalgae Tetraselmis suecica
Marine agar 2216 (Difco, Le Pont de Claix, France)
Thiosulfate citrate bile salt sucrose agar - TCBS (Difco, Le Pont de Claix, France)
Glutamate starch phenol red agar – GSP (Sigma-Aldrich, St. Louis, Missouri, USA)
Sabouraud dextrose agar (Difco, Le Pont de Claix, France)
Guillard F2 (Sigma-Aldrich)
Ethyl acetate (JT Baker, Mexico)
Acetonitrile (Merck, Germany)
Tryptic Soy Broth – TSB (Difco, Le Pont de Claix, France)Eppendorf tubes
Sterile discs SD (diameter 6 mm, Biogram)
Florfenicol 99%
Cotton swabs
Forceps
Mueller Hinton Agar (Difco, 7 Loveton Circle)
Equipments
Centrifuge (EPPENDORF, 5804R)
Rotary evaporator (Rotavapor ® R-300, Büchi Labortechnik AG, Flawil, Switzerland)
Ultrasonic bath (Emerson /Branson Bransonic CPX3800H)
Neubauer chamber (Boeco, Germany)
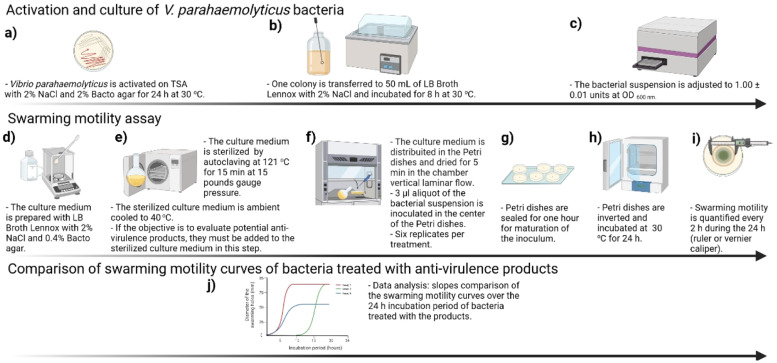
Activation and culture of V. parahaemolyticus bacteria
The protocol was standardized using the V. parahaemolyticus Ba94C2 strain (GenBank accession no. PRJNA335761), which causes AHPND and high mortality in farmed Penaeus (Litopenaeus) vannamei shrimp [17]. The bacterium stored at – 80 °C is activated on tryptic soy agar - TSA (Difco, Le Pont de Claix, France) supplemented with 2% NaCl (VWR Chemicals BDH, United States) and 2% of Bacto agar (Difco, Le Pont de Claix, France) for 24 h at 30 °C (Fig. 1a). Once the bacterium is activated, a colony is transferred to 50 mL of LB Broth, Lennox (Difco, Le Pont de Claix, France) supplemented with 2% NaCl (VWR Chemicals BDH, United States) and incubated for 8 h at 30 °C (Fig. 1b). The bacterial suspension is adjusted to an optical density of 1.00 ± 0.01 units at 600 nm (OD600), equivalent to 109 CFU/mL (Fig. 1c).
Fig. 1.
Diagram of the swarming motility assay to identify anti-virulence products against Vibrio parahaemolyticus. Created in BioRender.com (2023).
Swarming motility assay
Culture medium is prepared using LB Broth, Lennox (Difco, Le Pont de Claix, France) with 2% NaCl (VWR Chemicals BDH, United States) and 0.4% of Bacto agar (Difco, Le Pont de Claix, France) (Fig. 1d). The culture medium is sterilized by autoclaving at 121 °C for 15 min at 15 pounds gauge pressure (Fig. 1e). The sterilized culture medium is ambient cooled to 40 °C (Fig. 1e). The culture medium is distributed in the Petri dishes and allowed to dry for 5 min without lid in a vertical laminar flow chamber (six replicates), then a 3 µl aliquot of the bacterial suspension is inoculated in the center of the Petri dishes (Fig. 1f). The Petri dishes are sealed and kept in a sterile area for one hour to allow the bacterial cells of the inoculum to attach to the surface (Fig. 1g). The Petri dishes are inverted and incubated at 30 °C for 24 h (Fig. 1h). Swarming motility is quantified every two hours during the 24-hour incubation period by measuring the diameter of the swarming halos (ruler or vernier caliper) (Fig. 1i).
Comparison of swarming motility curves of bacteria treated with anti-virulence products
A simple evaluation of potential anti-virulence products could be made by comparing the slopes of the swarming motility curves over the 24-hour incubation period of bacteria treated with the products (Fig. 1j). In this case, the potential anti-virulence products are added to the sterilized culture medium at the concentration required for the study (anti-virulence product treatments, six replicates per treatment) (Fig. 1e). Positive (bacterial culture without potential anti-virulence products) and double negative (culture medium without bacterial inoculum or potential anti-virulence products) controls are included. Six replicates are also used for each control. For each replicate of anti-virulence product treatments and positive control, an XY curve showing the variability of the diameter of the swarming halos over the 24-hour incubation period is plotted. For each replicate, only the XY points falling on the exponential phase of the swarming motility S-shaped curve are used for data analysis. An F-test for lack of fit for regression with replicates is performed to test whether a simple linear regression function is a good fit for each anti-virulence product treatment and positive control [18]. The six replicates at each time level are the repeat observations needed to perform the test. Non-significant F-test results indicate that simple linear regression is appropriate. For each replicate of anti-virulence products and positive control, a linear regression of the time variation of the diameters of the swarming halos is performed. The slopes of the linear regressions are compared between anti-virulence products and control using the one-way ANOVA test or the Kruskal-Wallis non-parametric test. Significantly lower slopes of the swarming motility curves of the anti-virulence products compared with the positive control (bacterium culture without potential anti-virulence products) indicate lower swarming motility, and may indicate efficacy in controlling the pathogenic strain of V. parahaemolyticus being tested.
Validation method
Effects of agar type and concentration on swarming motility
A first swarming motility assay (assay 1) was performed to determine the more appropriate agar type and concentration (smoothness). Three types of agars were evaluated: LB Broth, Lennox (Difco, Le Pont de Claix, France), Brain Heart Infusion Broth – BHI (Difco, Le Pont de Claix, France) and Motility Test Medium (Difco, Le Pont de Claix, France). Three agar concentrations were also evaluated: 0.4, 0.5 and 0.6% of Bacto agar (Difco, Le Pont de Claix, France). Only two of the three agar types (LB, Lennox and BHI broths) were included in the data analysis because small motility halos and no variation over time were observed when using the Motility Test Medium (mean ± standard deviation during the 24 h incubation period: 5.3 ± 0.0 mm, 5.7 ± 0.0 mm and 7.0 ± 0.2 mm, at 0.4, 0.5 and 0.6% of agar consistency, respectively). Therefore, a total of six treatments (2 levels of agar type x 3 levels of agar concentration) were evaluated in the assay 1. There were six replicates per treatment. The V. parahaemolyticus Ba94C2 strain was activated and cultured as described above. The culture media were prepared with 2% NaCl (VWR Chemicals BDH, United States) and 0.4, 0.5 or 0.6% of Bacto agar (Difco, Le Pont de Claix, France), depending on the correspondent treatment. The culture media were poured into the Petri dishes and left open for 5 min to allow some drying by evaporation of the medium. A 3 µl aliquot of the V. parahaemolyticus Ba94C2 bacterial suspension (OD600 = 1.0 ± 0.01 units) was inoculated into the centre of the Petri dishes. The Petri dishes were sealed and kept in a sterile area for one hour to allow the bacterial inoculum to mature and initiate the swarming motility. The Petri dishes were inverted and incubated at 30 °C for 24 h. Swarming motility halos were measured every two hours for a period of 24 h. For each replicate, an XY curve showing the variability of the diameters of the swarming halos over the 24-hour incubation period was plotted, and only the XY points falling on the exponential phase of the swarming motility S-shaped curve were used for data analysis. Based on this analysis, the XY points of the curves at 2 h, 4 h, 6 h and 8 h of all replicates were removed to avoid points that fell on the lag phase of the S-shaped curve. An F-test for lack of fit for regression with replicates was performed to test whether a simple linear regression function was a good fit for each of the six treatments. The replicates at each time level were the repeat observations needed to perform the test. Non-significant F-test results indicated that simple linear regression was appropriate. For each replicate, a linear regression of the time variation of the diameters of the swarming halos was performed, and the slopes of the linear regressions were compared between treatments through an one-way ANOVA model after the parametric assumptions of the ANOVA model were tested and met. Homogeneity of variance of all treatments was tested using the Levene test. The normality assumption was tested using the Shapiro-Wilk normality test. The Tukey's honestly significant difference post hoc test was used to compare treatment means. The effect of the treatments was considered significant at p < 0.05. Slopes were expressed as mean ± standard deviation. Data analysis was performed using the R software version 4.3.0 [19].
The slopes of the swarming motility of the V. parahaemolyticus strain were significantly higher (p < 0.05) with the LB Broth, Lennox agar compared to the Brain Heart Infusion Broth (Table 1). The 0.4% agar consistency promoted the highest significant (p < 0.05) swarming motility of the V. parahaemolyticus strain compared to the other two agar concentrations (Table 1). The treatment with the fastest swarming migration of V. parahaemolyticus on Petri dishes during the 24-hour observation period was obtained using the LB Broth, Lennox agar at 0.4% consistency, which was significantly higher (p < 0.05) compared to all other treatments (Table 1). Therefore, this combination was considered the most appropriate and was always used for the subsequent swarming motility assays performed in the validation method.
Table 1.
Results of the first swarming motility assay (assay 1): Effects of agar type and concentration (Percentage of Bacto agar) on swarming motility of V. parahaemolyticus on Petri dishes. Swarming motility was estimated from the slopes (mean ± standard deviation, 6 replicates per treatment) of the linearized exponential phase of the S-shaped curve.
| Agar type and concentration (Percentage of Bacto agar) |
Slopes (mm/hour) |
|---|---|
| LB Broth, Lennox - 0.4% | 4.00 ± 0.08 e |
| LB Broth, Lennox - 0.5% | 1.67 ± 0.10 c |
| LB Broth, Lennox - 0.6% | 0.20 ± 0.08 b |
| Brain Heart Infusion Broth - 0.4% | 1.82 ± 0.06 d |
| Brain Heart Infusion Broth - 0.5% | 0.16 ± 0.09 b |
| Brain Heart Infusion Broth - 0.6% | 0.00 ± 0.00 a |
Slopes indicated with different letters are significantly different at p < 0.05, based on one-way ANOVA model and Tukey's honestly significant difference post hoc test.
Effects of the drying time of culture medium and bacterial inoculum volume on swarming motility
After determining the most appropriate agar type and consistency, a second independent swarming motility assay (assay 2) was performed to evaluate the optimal drying time of the culture medium (moisture content) and the volume of bacterial inoculum to be used. Three drying times were evaluated: 5 min, 15 min and 30 min, and three bacterial inoculum volumes were also evaluated: 3, 4 and 5 µl, for a total of nine treatments (3 levels of drying time x 3 levels of bacterial inoculum volume). Each treatment had six replicates. The V. parahaemolyticus Ba94C2 strain was activated and cultured as described above. Briefly, the culture medium was prepared using LB Broth, Lennox with 2% NaCl and 0.4% Bacto agar. The bacterial suspension started at 1.0 ± 0.01 at OD600, and diameters of the motility halos were measured every two hours for 24 h for each replicate. The slopes of the motility halo curves for all replicates were obtained by the data analysis previously described in assay 1 of the validation method. The slopes of the linear regressions were compared between treatments through an one-way ANOVA model after the parametric assumptions of the ANOVA model were tested and met. Homogeneity of variance of all treatments was tested using the Levene test. The normality assumption was tested using the Shapiro-Wilk normality test. The Tukey's honestly significant difference post hoc test was used to compare treatment means. The effect of the treatments was considered significant at p < 0.05. Slopes were expressed as mean ± standard deviation. Data analysis was performed using the R software version 4.3.0 [19].
Results of assay 2 showed that in general the slopes of the swarming migration of V. parahaemolyticus were significantly higher (p < 0.05) when the medium was dried for 5 min than the other two treatments of 15 and 30 min of drying (Table 2). Slopes of the swarming migration of V. parahaemolyticus were non-significantly different between three volumes of the bacterial inoculum tested when the culture medium was dried for 5 min (Table 2). Therefore, 3 µl was considered an adequate volume of the culture medium to reduce the risk of inoculum dispersion due to movement of the Petri dish at the time of incubation, and was used for the subsequent swarming motility assays performed in the validation method.
Table 2.
Results of the second swarming motility assay (assay 2): Effects of the drying time of culture medium and bacterial inoculum volume on swarming motility of V. parahaemolyticus on Petri dishes. Swarming motility was estimated from the slopes (mean ± standard deviation, 6 replicates per treatment) of the linearized exponential phase of the S-shaped curve.
| Drying time of the culture medium (min) and volume of the bacterial inoculum (µL) | Slopes (mm/hour) |
|---|---|
| 5 min - 3 µL | 3.87 ± 0.19 e |
| 5 min - 4 µL | 3.39 ± 0.10 e |
| 5 min - 5 µL | 2.74 ± 0.37 de |
| 15 min - 3 µL | 3.84 ± 0.65 de |
| 15 min - 4 µL | 2.41 ± 0.29 b |
| 15 min - 5 µL | 1.78 ± 0.27 cd |
| 30 min - 3 µL | 3.40 ± 0.16 bc |
| 30 min - 4 µL | 3.20 ± 0.09 a |
| 30 min - 5 µL | 2.87 ± 0.22 bcd |
Slopes indicated with different letters are significantly different at p < 0.05, based on one-way ANOVA model and Tukey's honestly significant difference post hoc test.
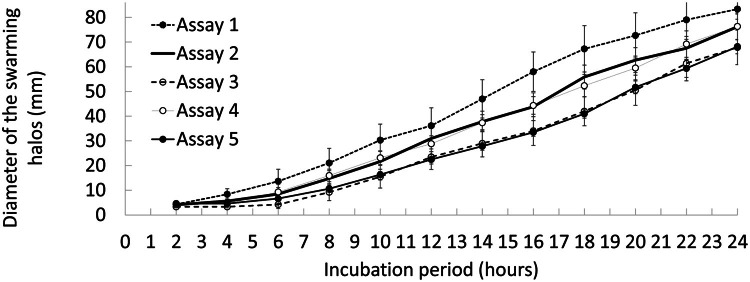
Inter-assay variability
As result of assays 1 and 2, the best treatment that promoted the fastest slopes of swarming motility halos of the pathogenic V. parahaemolyticus Ba94C2 strain on Petri dishes was: culture medium LB Broth, Lennox at a consistency of 0.4% agar, allowed to dry for 5 min and inoculating a 3 µL aliquot of the bacterial suspension in the center of the Petri dishes. Three further independent swarming motility assays (assays 3, 4 and 5) of the V. parahaemolyticus Ba94C2 strain were performed using such combination (best treatment). In this form, a single treatment was evaluated in each assay (six replicates per each assay), as the objectives of the assays 3, 4 and 5 were to obtain results of more independent tests to evaluate the reproducibility of the swarming motility assay. Assays 3, 4 and 5 were performed following the protocols described previously.
The slopes of the swarming motility curves obtained in the assays 3, 4 and 5 together with those obtained in assays 1 and 2 were analyzed through a one-factor (number of independent assays) ANOVA model. Five treatments (assays 1, 2, 3, 4 and 5) were compared, with six replicates per treatment. Results were considered significant at p < 0.05. Slopes were expressed as mean ± standard deviation. In addition, the reproducibility (inter-assays variability) of the swarming motility test for V. parahaemolyticus was also assessed by the concordance correlation [20]. Data analysis was performed in the R software version 4.3.0 [19].
No significant differences (p < 0.05) in slopes were found between the five independent trials (Table 3). In addition, the concordance correlation was between 0.80 and 1.00, showing an acceptable level of reproducibility of the method to estimate the swarming motility test for V. parahaemolyticus (Table 3).
Table 3.
Results of the one-factor (number of independent assay) ANOVA model and concordance correlation to assess the inter-assay variability of slopes (mean ± standard deviation, 6 replicates per assay) of the swarming motility assay of V. parahaemolyticus on Petri dishes cultured with LB Broth, Lennox, at a consistency of 0.4% Bacto agar, allowed to dry for 5 min and inoculating a 3 µL aliquot of the bacterial suspension in the center of the Petri dishes.
| ANOVA results | Concordance correlation |
||||
|---|---|---|---|---|---|
| Assay | Slopes (mm/hour) | Assay 1 | Assay 2 | Assay 3 | Assay 4 |
| Assay 1 | 4.00 ± 0.08 a | ||||
| Assay 2 | 3.87 ± 0.19 a | 0.95 | |||
| Assay 3 | 3.74 ± 0.17 a | 0.81 | 0.94 | ||
| Assay 4 | 3.86 ± 0.36 a | 0.94 | 1.00 | 0.95 | |
| Assay 5 | 3.73 ± 0.15 a | 0.80 | 0.94 | 1.00 | 0.95 |
Slopes indicated by the same letter a are not significantly different (p > 0.05), based on one-factor ANOVA model.
Fig. 2 shows the diameters of the swarming halos obtained in the five independent assays for the levels of the studied factors that promoted the fastest swarming motility halos of the pathogenic V. parahaemolyticus Ba94C2 strain on Petri dishes best treatment: LB Broth, Lennox culture medium, agar concentration of 0.4%, 5-min drying of the culture medium and a volume of 3 µL of bacterial inoculum. The time variation of the diameters of the swarming halo followed a logistic form (Fig. 2).
Fig. 2.
Evaluation of five independent swarming motility assays for estimation of its reproducibility. Vertical bars correspond to standard deviation.
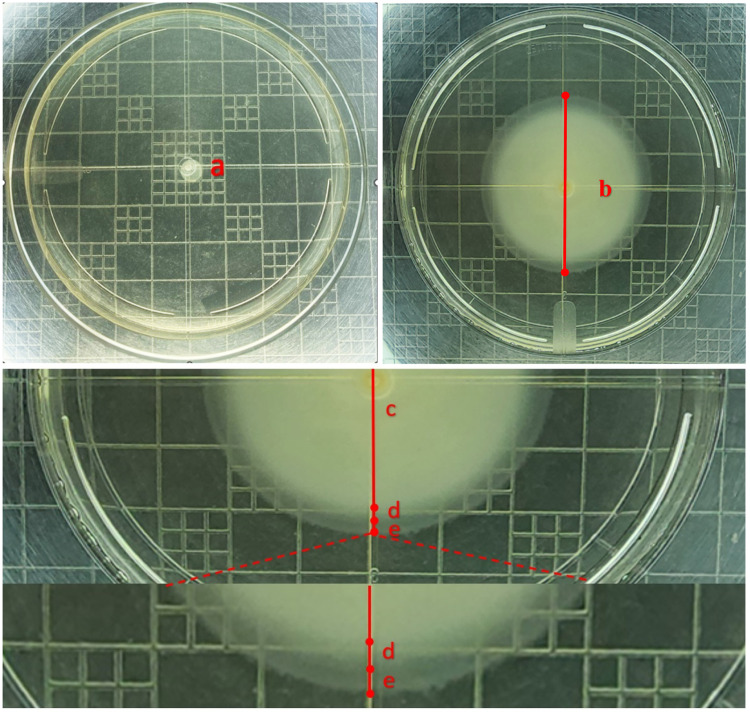
In addition, during the migration of the V. parahaemolyticus strain in the best treatment, it was possible to observe all the developmental stages and morphology of the motility swarming described by the literature [12]. The process started with the fixation of the microbial colony on the substrate in a non-swarming stage (Fig. 3a), to later form the swarm motility halo around the bacterial inoculum (Fig. 3b). This swarm motility halo is a complex microbial process that presented three different zones [12], which were evident during the swarming migration of the V. parahaemolyticus strain in the best treatment: a first zone called swarm colony center (Fig. 3c) formed by multiple non-swarming staked layers [12], a second zone called mature swarm colonies (Fig. 3d) which is the true swarm [12], and a third zone called swarm colony periphery (Fig. 3e) composed by the adventure cells which are ready to spread in a liquid environment [12].
Fig. 3.
Swarming motility process of bacterial suspension at the levels of incubation to promote the fastest swarming motility halos of V. parahaemolyticus strain Ba94C2 on Petri dishes: LB Broth, Lennox medium with 0.4% Bacto agar, 5 min of drying of the culture medium and 3 µl of bacterial inoculum. a) Fixation of the microbial colony on the substrate in a non-swarming stage (photo taken around 2-hours of the incubation period). b) Swarm motility halo around the bacterial inoculum (photo taken around 14-hours of the incubation period). c) Swarm colony center. d) Maturate swarm colonies. e) Swarm colony periphery. Areas marked b, c, d and e are parts of the same photo at different zoom levels.
Ability of the optimized swarming motility assay to detect anti-virulence activity
The ability of the optimized swarming motility test to detect anti-virulence activity was evaluated with an experiment using an extract of the microalgae Tetraselmis suecica. T. suecica is a marine microalga used in aquaculture with quorum sensing (QS) inhibitory activity against Gram-negative bacteria [21]. QS is a communication mechanism of bacterial populations based on cell density in the population through which signaling molecules are produced, released, and detected to regulate gene expression and behavior [22]. One of the most studied QS signals is acylated homoserine lactones (AHLs) [23]. Since QS regulate the virulence of some aquaculture pathogens [24] and swarming is a QS-regulated phenotype [25], compounds that interfere with QS may in turn reduce the swarming motility of aquaculture pathogenic bacteria. The objective of this experiment was to test if extracts of T. suecica that interfere with QS can decrease the swarming motility of pathogenic V. parahaemolyticus. The strain of T. suecica was obtained from the algae collection of the National Center for Aquaculture and Marine Research (CENAIM). The microalgae production was carried out according to Guillard (1975) using Guillard F2 medium (Sigma-Aldrich). The culture started with 10 mL tubes (7 days) and ended with 400 mL Erlenmeyer flasks (3 days). The culture was maintained with continuous light (48 µmol/m2s) for 24 h at 18 ± 2 °C and salinity of 35 g/L. Microalgal density was estimated during culture using a Neubauer chamber (Boeco, Germany). Microalgae were harvested by centrifugation (4000 rpm for 5 min, Centrifuge (EPPENDORF, 5804R) when the culture reached the exponential phase (3 days after the start of the culture in the Erlenmeyer flasks). The absence of growth of total bacteria, vibrios, Pseudomonas/Aeromonas and fungi was confirmed by plating aliquots (100 μL) of the supernatant on Petri dishes with Marine agar 2216 (Difco, Le Pont de Claix, France), thiosulfate citrate bile salt sucrose agar – TCBS (Difco, Le Pont de Claix, France), glutamate starch phenol red agar – GSP (Sigma-Aldrich, St. Louís, Missouri, USA), and Sabouraud dextrose agar (Difco, Le Pont de Claix, France).
An extract of T. suecica for the QS-interfering activity of the V. parahaemolyticus Ba94C2 strain was prepared following the procedures described by [21], with slight modifications. Briefly, 9 mL of supernatant was extracted from the microalgae culture and mixed with the same volume of ethyl acetate (JT Baker, Mexico). The mixture was centrifuged at 3000 rpm for 10 min, and the ethyl acetate fractions were extracted. This extraction procedure was repeated twice. The samples were then evaporated at 30 °C using a rotary evaporator (Rotavapor ® R-300, Büchi Labortechnik AG, Flawil, Switzerland), redissolved in 1 mL of 100% acetonitrile (Merck, Germany) and diluted in 3 mL of distilled water. The sample adhering to the walls was resuspended by ultrasound for 15 min (Ultrasonic bath, Emerson /Branson Bransonic CPX3800H). The extract was transferred to Eppendorf tubes and stored at - 80 °C until further use. A total of three dilutions of this extract were prepared with distilled water (10−4, 10−5 and 10−6) and their anti-virulence activities against the V. parahaemolyticus strain were evaluated according to the protocol and the statistical analysis of the optimized motility assay.
Activation and culture of the V. parahaemolyticus bacteria and the swarming motility assay were performed according as described in the Method Details section. Since the objective was to evaluate the anti-virulence activity of the T. suecica extract at the three dilutions (treatments, six replicates per treatment), a total of 6 µl of each dilution was added to the sterilized culture medium (LB Broth, Lennox with 2% NaCl and 0.4% of Bacto agar) in the step shown in Fig. 1e of the swarming motility assay. Positive (bacterial culture without extracts) and double negative (culture medium without bacterial inoculum or extracts) controls were included. Six replicates were also used for each control.
For each replicate of treatments and positive control, an XY curve was plotted showing the variability in swarming halo diameter over the 24-hour incubation period. For each replicate, only the XY points falling on the exponential phase of the S-shaped swarming motility curve were used for data analysis by removing the XY points of the curves at 2 h, 4 h, 6 h and 8 h. A F-test for lack of fit for regression with replicates was performed to test whether a simple linear regression function was a good fit for each treatment and positive control. Non-significant F-test results indicated that simple linear regression was an appropriate analysis. For each replicate of treatments and positive control, linear regression was performed on the time variation of swarming halo diameters. The slopes of the linear regressions were compared between treatments and positive control using the one-way ANOVA test after the parametric assumptions of the ANOVA model were tested and met. Homogeneity of variance between treatments and positive control was tested using the Levene test. The normality assumption was tested using the Shapiro-Wilk normality test. Tukey's honestly significant difference post hoc test was used to compare treatment means. Treatment effects were considered significant at p < 0.05. Slopes were expressed as mean ± standard deviation. Data analysis was performed using the R software version 4.3.0.
To prove that the T. suecica extract at the three dilutions (10−4, 10−5 and 10−6) did not affect the growth of the pathogenic V. parahaemolyticus strain, antibiograms were performed by the in vitro agar disk-diffusion method [26]. Sterile discs SD (diameter 6 mm, Biogram) were impregnated with 40 µl of each of three different dilutions of the T. suecica extract. A sterile disc prepared with florfenicol 99% at 70 ppm was used as positive control. Another sterile disc prepared with a 10−4 dilution of the solution prepared with 1 mL of 100% acetonitrile and 3 mL of distilled water was used as negative control. The V. parahaemolyticus Ba94C2 strain was activated on TSA and incubated at 30 °C for 24 h. One colony was transferred to TSB (Difco, Le Pont de Claix, France) liquid medium containing 2% NaCl and incubated at 30 °C for 4 h. The bacterial suspension was adjusted to an optical density of 0.23 ± 0.01 units at 600 nm (OD600), equivalent to 108 CFU/mL and diluted to 106. The bacterial suspension (100 μL) was inoculated onto Mueller Hinton Agar (MH) (Difco, 7 Loveton Circle) and spread evenly over the agar surface using sterile cotton swabs. The extract-impregnated sterile discs and the positive and negative control discs were placed on the agar surface with sterile forceps and gently pressed to ensure contact with the agar surface. MH plates were incubated agar down for 24 and 48 h at 30 °C. Each treatment (10−4, 10−5 and 10−6 dilutions of the T. suecica extract and positive and negative controls) had six replicates. Thus, a total of six plates were prepared, and one replicate of each treatment was placed on each plate. Antibacterial activity was determined by measuring the diameter of the bacterial inhibition zone surrounding the disc.
In addition, growth curves of V. parahaemolyticus in LB Broth, Lennox (Difco, Le Pont de Claix, France) with and without T. suecica extract at the same concentrations as used in the motility assay were obtained and compared. V. parahaemolyticus growth was evaluated by measuring OD600 nm and quantifying viable cells (colony forming units – CFU/mL), at different time points. The V. parahaemolyticus Ba94C2 strain was activated on tryptic soy agar - TSA (Difco, Le Pont de Claix, France) supplemented with 2% NaCl (VWR Chemicals BDH, United States) and 2% of Bacto agar (Difco, Le Pont de Claix, France) for 24 h at 30 °C. Once the bacterium was activated, a colony was transferred to 50 mL of LB Broth, Lennox (Difco, Le Pont de Claix, France) supplemented with 2% NaCl (VWR Chemicals BDH, United States) and incubated for 4 h at 30 °C. The bacterial suspension was adjusted to an optical density of 0.23 ± 0.01 units at OD600, equivalent to 108 CFU/mL. A total of 6 µl of each dilution (10−4, 10−5 and 10−6) of T. suecica extract was added to the sterilized culture medium LB Broth, Lennox with 2% NaCl (treatments, six replicates per treatment). The bacterial suspension was then added to the treatments. Positive (bacterial culture without extracts) and negative (sterile culture medium without bacterial inoculum or extracts) controls were included. Six replicates were also used for each control. Cultures were incubated for 24 h at 30 °C in an orbital shaker (Orbital shaker-incubator). For V. parahaemolyticus growth, evaluated by optical density, consecutive readings (200 µL) for each replicate were made every 2 h during a 24-hour period (0 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h, 16 h, 18 h, 20 h, 22 h and 24 h) using a microplate reader spectrophotometer (Thermo Scientific, Varioskan LUX) at OD600 nm. At the same time, the bacterial growth curve was also evaluated by quantifying the viable cells by seeding in duplicate three biological replicates for each treatment and control every 4 h during a 24-hour period (0 h, 4 h, 8 h, 12 h, 16 h, 20 h and 24 h). For this, a total of 1 mL of each treatment (10−4, 10−5 and 10−6 dilutions of the T. suecica extract), positive (bacterial culture without extracts) and negative (sterile culture medium without bacterial inoculum or extracts) controls were plated on Petri dishes of LB Broth, Lennox (Difco, Le Pont de Claix, France) supplemented with 2% NaCl (VWR Chemicals BDH, United States) and 2% of Bacto agar (Difco, Le Pont de Claix, France), and incubated at 30 °C for 24 h.
At each time point, the effect of treatments on optical density and CFU measurements was analyzed by ANOVA after testing and satisfying the parametric assumptions of the ANOVA model. Homogeneity of variance of all treatments was tested using the Levene test. The normality assumption was tested with the Shapiro-Wilk normality test. Tukey's honestly significant difference post hoc test was used to compare treatment means. A transformation (1/X) was used to meet the parametric assumptions for optical density at 12 h, and the non-parametric Kruskal-Wallis test was used to evaluate differences of optical density at 0 h between treatments. Treatment effects were considered significant at p < 0.05. Optical densities and CFU measurements were expressed as mean ± standard deviation. Data analysis was performed using R software version 4.3.0 [19].
The slopes of the swarming motility curves for all three treatments were significantly lower (p < 0.001) compared to the positive control (bacterial culture not treated with the extracts) (Table 4), indicating that the T. suecica supernatant was able to reduce the swarming motility and thus the virulence of the V. parahaemolyticus strain at all three dilutions tested. The lowest swarming motility was observed at the intermediate dilution (10−5), which was even significantly lower (p < 0.05) than that reported at the 10−4 and 10−6 dilutions (Table 4).
Table 4.
Slope (mean ± standard deviation, 6 replicates per treatment) of the swarming motility curves of V. parahaemolyticus Ba94C2 strain treated with three dilutions of an extract of T. suecica supernatant. For each replicate, the slopes were obtained from the linearized exponential phase of the S-shaped curve.
| Treatment received by the V. parahaemolyticus strain | Slopes (mm/hour) |
|---|---|
| 10−4 dilution of T. suecica supernatant extract | 2.67 ± 0.15 b |
| 10−5 dilution of T. suecica supernatant extract | 2.04 ± 0.15 a |
| 10−6 dilution of T. suecica supernatant extract | 2.62 ± 0.10 b |
| Positive control (bacterial culture non treated with any extract) | 4.02 ± 0.33 c |
*Slopes indicated with different letters are significantly different at p < 0.05, based on one-way ANOVA model and Tukey's honestly significant difference post hoc test.
The mean slope of the swarming motility curves obtained in the positive control of this experiment (4.02 ± 0.33, Table 4) was not significantly different (p > 0.05) from the slopes of the swarming motility curves obtained in the other five independent assays (Assays 1, 2, 3, 4 and 5, Table 3) using a Kruskal-Wallis rank sum test. In addition, the concordance correlations between the bacterial swarming halo diameters reported in this experiment and those reported in Assays 1, 2, 3, 4, and 5 (0.99, 0.96, 0.84, 0.95, and 0.83, respectively) also indicated an acceptable level of reproducibility. These last two observations provided further evidence of the reproducibility of the proposed swarming motility assay for V. parahaemolyticus.
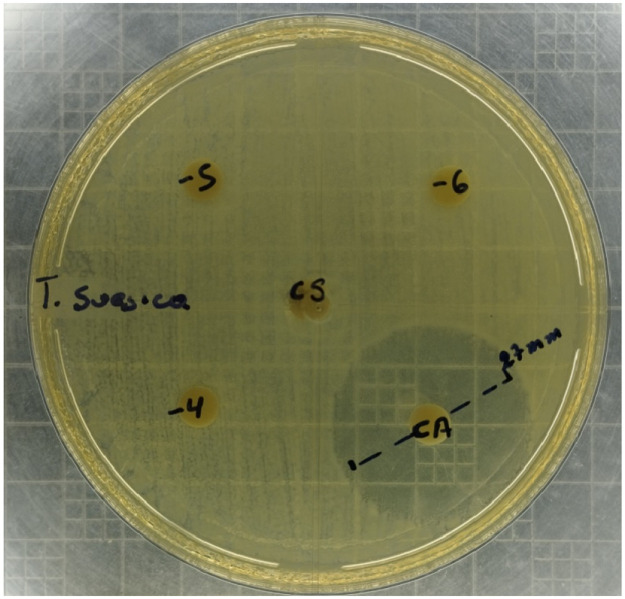
The positive control inhibited the bacterial growth with a 27 mm inhibition zone in all six replicates, demonstrating the sensitivity of the V. parahaemolyticus Ba94C2 strain to florfenicol (Fig. 4). No inhibition halo was observed in any replicate of the negative control and T. suecica extract dilutions (Fig. 4), indicating that the growth of the V. parahaemolyticus Ba94C2 strain was not affected at any of the T. suecica dilutions.
Fig. 4.
Antibiogram (agar disk-diffusion method) obtained with the 10−4, 10−5 and 10−6 dilutions of the T. suecica extract and positive (florfenicol at 70 ppm, CA) and Negative control (CS).
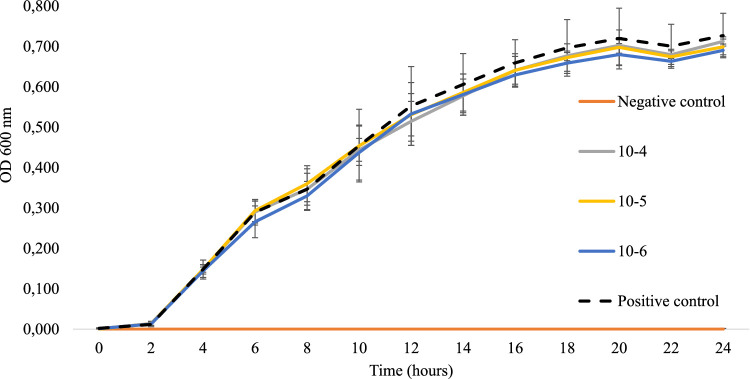
At all times of measurements, optical densities were not significantly different (p > 0.05) between treatments (10−4, 10−5 and 10−6 dilutions of T. suecica extract) and positive control (Fig. 5).
Fig. 5.
Growth curve of the pathogenic V. parahaemolyticus strain evaluated by measuring OD600 nm every 2 h during a 24-hour period with (10−4, 10−5 and 10−6 dilutions) and without (positive control) T. suecica extract. No significant differences (p > 0.05) of optical density were found between treatments at any time point.
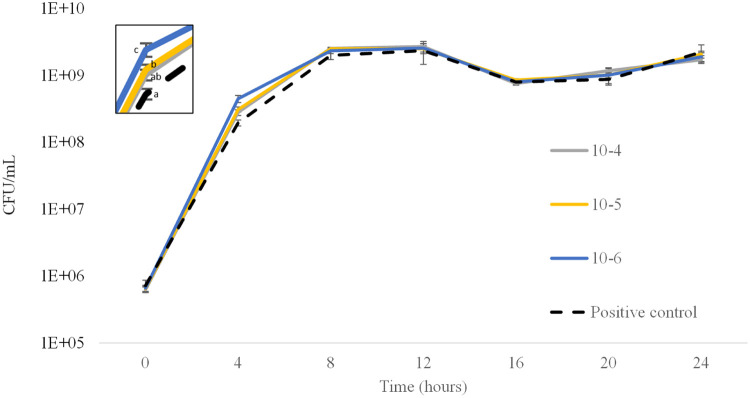
Similarly, the number of viable cells was not significantly different (p > 0.05) between treatments and positive control at all times of measurements (Fig. 6), except at time 4 h when a significantly higher (p < 0.05) growth of the V. parahaemolyticus strain treated with 10−5 and 10−6 dilutions of T. suecica extract compared with the control was observed. At this time, even the growth of the V. parahaemolyticus strain treated with 10−6 dilution of T. suecica extract was significantly higher (p < 0.05) than the 10−5 dilutionbrk (Fig. 6). At this time, non-significant differences (p > 0.05) of V. parahaemolyticus growth were found between control and 10−4 dilution (Fig. 6). Therefore, the growth of V. parahaemolyticus was not inhibited by the T. suecica extracts.
Fig. 6.
Growth curve of the pathogenic V. parahaemolyticus strain evaluated by quantifying number of viable cells every 4 h during a 24-hour period with (10−4, 10−5 and 10−6 dilutions) and without (positive control) T. suecica extract. No significant differences were found between treatments at any time point, except for the number of viable cells at 4 h (upper left box).
The decreased swarming motility in the presence of the extracts seems be caused by anti-virulence activity rather than antibacterial activity since the extract did not affect the growth of V. parahaemolyticus. These results provide evidence that the proposed method and data analysis can detect anti-virulence activity against pathogenic V. parahaemolyticus of a compound previously shown to inhibit QS activity against Gram-negative bacteria.
Additional information
The growth of Vibrio depends on the specific nutrient requirements of the culture media [27]. There are several methods of swarm motility assays for V. parahaemolyticus in the literature, but the different culture media used make it difficult to compare results between studies [1,13,28,29]. Therefore, firstly it is important to establish the correct environmental conditions of cultivation (culture medium) to start the bacterial quorum, achieve the swarming motility and display the pathogenesis mechanisms to overcome the host's defenses. In our study, the LB Broth, Lennox supplemented with NaCl (final concentration 2%) promoted the best bacterial growth. The main advantage over similar methods is the ease of controlled preparation of commercial LB broth, Lennox medium, supplemented with NaCl (final concentration 2%) and agar (0.4%), which allows observation and quantification of the swarming motility during a 24-hour incubation period.
The onset of swarming is related to the ability of the bacterium to change its motility behavior from a liquid medium to a semi-solid medium and drying time of the culture medium (moisture content) [15]. The consistency of the culture medium is adjusted by adding gelling agent (Bacto agar), which facilitates the swarming motility [15,30]. Here we have observed that the V. parahaemolyticus Ba94C2 strain achieves adequate swarming motility with a culture medium consistency of 0.4% agar, which provided enough smoothness to allows the swarming motility and resulting in a defined halo of motility. We observed that drying the culture medium (open plate) for 5 min was enough to eliminate the humidity excess of the culture medium. This probably promoted a correct colonization and maturation of the colony (adaptation from liquid to semi-solid medium) and allowed bacteria to activate the swarming physiology.
Bacteria are inoculated on swarming agar plates at different inoculum volume [15,28]. In our study, the pattern observed with the volume of the bacterial inoculum was not as evident as we observed with the other three studied variables (type of agar, agar consistency, and culture medium drying time). The swarming motilities were not significantly different at all three volumes of the bacterial inoculum when the medium was dried for 5 min. The use of a volume of 3 µl was considered adequate to reduce the risk of dispersion of the inoculum due to movement of the Petri dish at the time of incubation.
We have developed the standardization of a simple method that allows reliable detection and quantification of swarming motility using LB broth supplemented with NaCl (final concentration 2%). The results of this study were highly reproducible using a V. parahaemolyticus pathogenic strain of shrimp cultured in marine and brackish water. Therefore, it will be necessary to standardize the swarming assay for other V. parahaemolyticus strains, taking into account their specific growth requirements. For example, V. parahaemolyticus strains that affect seafood require growth media with higher NaCl content than those used in this study [30]. However, the steps described in the validation method could be used a guide to develop effective swarming motility assays for other V. parahaemolyticus strains. The method is also useful for identifying potential anti-virulence products by comparing the slopes of the swarming motility migration on Petri dishes estimated during an observation period of the bacteria treated with different products.
In conclusion, the main aspects of the method are the simplicity to quantify the swarming motility of V. parahaemolyticus bacteria and the data analysis methodology proposed to evaluate the efficacy of potential anti-virulence products. The performance of both aspects was evaluated. Thus, the swarming motility assay showed high reproducibility and the assay was able to detect anti-virulence against pathogenic V. parahaemolyticus of a compound previously shown to inhibit QS activity against Gram-negative bacteria. Additional validations were performed by confirming the developmental stages, and morphology of V. parahaemolyticus during the swarming migration period under the best treatment conditions. The validation method can be used to standardize the swarming assay for other V. parahaemolyticus strains and bacteria of other genera. The optimized swarming motility assay proposed in this study may be useful for the discovery of effective products that act as quorum sensing inhibitors and protect shrimp by controlling the virulence of pathogenic V. parahaemolyticus causing AHPND without killing them. The assay will be useful not only in aquaculture, but also in agriculture and even in human medicine, where the identification of products that inhibit QS may help overcome the public health threat of multidrug-resistant bacteria.
CRediT authorship contribution statement
Francisco Pozo: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Martha Borbor: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – review & editing, Visualization, Supervision, Project administration. Ramiro Solórzano: Methodology, Investigation, Writing – review & editing. Stanislaus Sonnenholzner: Formal analysis, Data curation, Writing – review & editing. Bonny Bayot: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT) in the framework of the PIC-21-INE-ESPOL-004 Project “Biotecnología azul para el fortalecimiento de la industria acuícola ecuatoriana controlando Vibrios patógenos”. Francisco Pozo Miranda conducted the research as part of his Ph.D. thesis for the graduate program Doctoral en Biociencias Aplicadas con Mención en Biotecnología (ESPOL).
Method name: Swarming motility assay for Vibrio parahaemolyticus
Data availability
Data will be made available on request.
References
- 1.Mizan Md.F.R., Jahid I.K., Kim M., Lee K.H., Kim T.J., Ha S.-D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling. 2016;32:497–509. doi: 10.1080/08927014.2016.1149571. [DOI] [PubMed] [Google Scholar]
- 2.FAO. 2022. The state of world fisheries and aquaculture 2022. Towards blue transformation. Rome, FAO. 10.4060/cc0461en. [DOI]
- 3.J. Cai, X. Zhou, X. Yan, D. Lucentea, C. Lagana, Top 10 species groups in global aquaculture 2017, Written by FAO Fisheries and Aquaculture Technical Paper, 2019, [On line]. Available: https://www.researchgate.net/publication/334684427_Top_10_species_groups_in_global_aquaculture_2017_Written_by#fullTextFileContent.
- 4.Béne C., M Barangé, Subasinghe R.P., Pinstrup-Andersen P., Merino G., Hemre G.I., Williams M.J. Feeding 9 billion by 2050 – Putting fish back on the menu. Food Secur. 2015;7:261–274. doi: 10.1007/s12571-015-0427-z. [DOI] [Google Scholar]
- 5.Chandrakala N., Priya S. Vibriosis in Shrimp Aquaculture A review. Int. J. Sci. Res. Sci. Eng. Technol. 2017;3:27–33. doi: 10.32628/ijsrset17321. [DOI] [Google Scholar]
- 6.Kumar V., Roy S., Behera B.K., Bossier P., Das B.K. Acute hepatopancreatic necrosis disease (AHPND): virulence, pathogenesis and mitigation strategies in shrimp aquaculture. Toxins. (Basel) 2021;13:524. doi: 10.3390/toxins13080524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran L., Nunan L., Redman R., Mohney L., Pantoja C., Fitzsimmons K., et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- 8.Han J.E., Tang K.F.J., Tran L., Lightner D.V. Photorhabdus insect-related (PIR) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandran A., Priya P.S., Meenatchi R., Vaishnavi S., Pavithra V., Kumar T.T.A., Arockiaraj J. Insights into Molecular aspects of pathogenesis and disease management in Acute Hepatopancreatic Necrosis Disease (AHPND): an updated review. Fish Shellfish Immunol. 2023;142 doi: 10.1016/j.fsi.2023.109138. [DOI] [PubMed] [Google Scholar]
- 10.Letchumanan V., Chan K.-G., Lee L.-H. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X., Zang J., Yu W., Shi X., Wu Y. Occurrence and identification of pathogenic vibrio contaminants in common seafood available in a Chinese traditional market in Qingdao, Shandong province. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freitas C., Glatter T., Ringgaard S. The release of a distinct cell type from swarm colonies facilitates dissemination of Vibrio parahaemolyticus in the environment. ISMe J. 2019;14:230–244. doi: 10.1038/s41396-019-0521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaques S., McCarter L.L. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 2006;188:2625–2635. doi: 10.1128/jb.188.7.2625-2635.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai S., Tremblay J., Déziel É. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ. Microbiol. 2009;11:126–136. doi: 10.1111/j.1462-2920.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay J., Déziel É. Improving the reproducibility of Pseudomonas aeruginosa swarming motility assays. J. Basic Microbiol. 2008;48:509–515. doi: 10.1002/jobm.200800030. [DOI] [PubMed] [Google Scholar]
- 16.Kearns D.B. A field guide to Bacterial swarming motility. Nat. Rev. Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Restrepo L., Bayot B., Betancourt I., Pinzón A. Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain BA94C2, associated with acute hepatopancreatic necrosis disease isolate from South America. Genom. Data. 2016;9:143–144. doi: 10.1016/j.gdata.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neter J., Kutner M., Nachtsheim C., Wasserman W. 4th Edition. United States of America; The McGraw-Hill: 1996. Applied Linear Statistical Models; p. 1408. [Google Scholar]
- 19.R Core Team . R Foundation for Statistical Computing; Vienna: 2022. R: Language and Environment for Statistical Computing.https://www.R-project.org/ Available at. [Google Scholar]
- 20.Lin L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989:255–268. http://www.jstor.org/stable/2532051 [Internet]Available from: [PubMed] [Google Scholar]
- 21.Natrah F.M.I., Kenmegne M.M., Wiyoto W., Sorgeloos P., Bossier P., Defoirdt T. Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture. 2011;317:53–57. doi: 10.1016/j.aquaculture.2011.04.038. [DOI] [Google Scholar]
- 22.Jose R., Singh V. Swarming in bacteria: a tale of plasticity in motility behavior. J. Indian Inst. Sci. 2020;100:515–524. doi: 10.1007/s41745-020-00177-2. [DOI] [Google Scholar]
- 23.Boyer M., Wisniewski-Dyé F. Cell-cell signalling in bacteria: not simply a matter of quorum. FEMS Microbiol. Ecol. 2009;70:1–19. doi: 10.1111/j.1574-6941.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 24.Natrah F.M.I., Defoirdt T., Sorgeloos P., Bossier P. Disruption of bacterial Cell-to-Cell communication by marine organisms and its relevance to aquaculture. Mar. Biotechnol. 2011;13:109–126. doi: 10.1007/s10126-010-9346-3. [DOI] [PubMed] [Google Scholar]
- 25.Daniels R., Vanderleyden J., Michiels J. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 2004;28:261–289. doi: 10.1016/j.femsre.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.J.J. Farmer, J.M. Janda, F.W. Brenner, D.N. Cameron, y K.M. Birkhead, Vibrio, Bergey's manual of systematics of archaea and bacteria, pp. 1–79, 2015, doi:10.1002/9781118960608.gbm01078.
- 28.Ashrafudoulla Md., Mizan Md.F.R., Ha A.J.-W., Park S.H., Ha S.-D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020;91 doi: 10.1016/j.fm.2020.103500. [DOI] [PubMed] [Google Scholar]
- 29.Qiao Y., Jia R., Luo Y., Feng L. The inhibitory effect of Ulva fasciata on culturability, motility, and biofilm formation of Vibrio parahaemolyticus ATCC17802. Int. Microbiol. 2021;24:301–310. doi: 10.1007/s10123-021-00165-1. [DOI] [PubMed] [Google Scholar]
- 30.Sathiyamoorthi E., Lee J.-H., Tan Y., Lee J. Antimicrobial and antibiofilm activities of formylchromones against Vibrio parahaemolyticus and Vibrio harveyi. Front. Cell Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1234668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.