Abstract
Objectives
The Knee Injury and Osteoarthritis Outcome Score (KOOS) has been utilized to evaluate short- and long-term outcomes in individuals following knee injuries, such as those with anterior cruciate ligament reconstruction and knee osteoarthritis, but has not yet been applied to individuals undergoing total knee arthroplasty (TKA) in China. The aim of this study was to assess the psychometric properties of the Simplified Chinese version of the KOOS in Chinese individuals undergoing TKA.
Methods
This study distributed 170 questionnaires, and assessed the KOOS of the participants, along with requiring them to complete the Short Form 36 (SF-36) survey. There were 35 participants completed a test-retest reliability survey with a 24-h interval, 129 participants completed a pre - surgery survey, and 119 individuals completed a post - surgery survey 6 weeks after the surgery. The following tests were conducted: Cronbach's alpha (α) to assess internal consistency, intraclass correlation coefficient (ICC) to evaluate test-retest reliability, Spearman's correlation coefficient (ρ) to examine construct validity, effect size (ES) to detect measure responsiveness, minimal detectable change (MDC) to assess measurement errors. Floor and ceiling effects (<15%) were also asses evaluated.
Results
The simplified Chinese version of the KOOS showed good test-retest reliability in participants after TKA, with an ICC of 0.82–0.97 (95% CI). The internal consistency of the five subscales of the KOOS was good (Cronbach's α = 0.70–0.96). No floor or ceiling effects were found. Regarding construct validity, a strong positive correlation was found between each of the three KOOS subscales (activities of daily living, knee-related Quality of Life, and sport and recreation subscales) and the general health and bodily pain subscales of the SF-36 (0.53 < ρ < 0.61). The subscales of the simplified Chinese version of the KOOS showed responsiveness (ES: 0.68 to 0.86) before and after 6 weeks of physical treatment. The MDC ranged from 10.28 to 23.24.
Conclusions
The Chinese version of the KOOS showed good psychometric properties and was found to be valid, reliable, and simple as an assessment tool for symptoms, pain, activity of daily living, sports and recreational activity and quality of life for the Chinese population suffering from TKA.
Keywords: Total knee arthroplasty, Patient-reported outcomes, KOOS, Knee injury and osteoarthritis outcome score
1. Introduction
Total knee arthroplasty (TKA) is a highly successful end-stage operation used to alleviate chronic knee pain at a relatively low risk [1]. TKA can enhance the quality of life by reducing knee pain and improving long-term knee function [2]. Health professionals can subjectively and objectively evaluate the condition and progress of individuals who have undergone TKA [1,3]. Currently, these scoring scales are commonly used in clinical practice to assess the treatment outcomes and prognosis of TKA in individuals with knee osteoarthritis (OA). The scales include the Oxford Knee Score (OKS), OKS-Activity and Participation Questionnaire (OKS-APQ), Knee Injury and Osteoarthritis Outcome Score (KOOS), 12-item short form Knee Injury and Osteoarthritis Outcome Score (KOOS-12), KOOS Physical function Short form (KOOS-PS), Western Ontario and McMaster Universities Arthritis Index-Total Knee arthroplasty function short form (WOMAC-TKA), Lower Extremity Functional Scale (LEFS), Forgotten Joint Score (FJS), Patient's Knee Implant Performance (PKIP), and University of California Los Angeles (UCLA) activity score [2,4,5]. A previous systematic review emphasized that the KOOS is the most suitable knee-related patient-reported outcome measurement (PROM) tool for evaluating knee-related health issues, suitable for both clinical and research purposes [[6], [7], [8]].
The KOOS is used to assess osteoarthritis and knee joint trauma [9]. Detailed information about the KOOS was first published in 1998 [10,11]. So far, KOOS has been translated into more than 50 languages to assess individuals with knee OA and other knee joint injuries, and its psychometric properties have been evaluated [9]. In a systematic review, the KOOS was evaluated and compared with other TKA outcome measures [9,12]. The KOOS, compared with other assessment tools for TKA prognosis, has been found to be more reliable, effective, and sensitive [2,13]. KOOS comprises pain, symptoms, activities of daily living (ADL), sport and recreation (Sport/Rec), and knee-related quality of life (QOL) subscales, which are are designed to be patient-friendly and provide a more accurate reflection of a patient's personal status [10,11]. The KOOS is increasingly becoming clinically accepted as a PROM after TKA [14]. It can significantly reduce the bias of clinical doctors, accurately measure a patient's subjective health status, better detect individual indicators of TKA, help address possible modifiable factors, and can promote clinical decision-making [14]. As a PROM, the KOOS is crucial for evaluating the impact of TKA on individuals [15]. To the best of our knowledge, the current simplified Chinese version of the KOOS has been translated and used for individuals with knee OA [16,17] and anterior cruciate ligament (ACL) injury [18], but it has not been used for individuals who have undergone TKA. This PROM tool needs to be revalidated for use in clinical or research settings [[19], [20], [21]]. The purpose of this study was to assess the psychometric properties of the KOOS in individuals following TKA in order to enhance its suitability for in a broader population.
2. Materials and methods
2.1. Participant recruitment
This study employed a convenient sampling method to select 170 TKA individuals who met the study's criteria for investigation between October 2022 and March 2023 in Yunnan, China. The institutional review board of the first affiliated hospital of Dali university approved this study, and each patient provided written informed consent, indicating their willingness to participate in the study. Individuals in the unit of inpatient orthopedic who were diagnosed by clinicians as requiring TKA surgery due to a knee joint issue were recruited. The diagnosis was based on clinical criteria and radiological confirmed by magnetic resonance imaging. The exclusion criteria were as follows: signs of anterior cruciate ligament and meniscal injury or systemic knee OA, physical therapy, or intra-articular drug injections (corticosteroids or hyaluronic acid) within 3 months prior to the study. The inclusion criteria were as follows: a diagnosis of knee OA necessitating TKA by an orthopedic physician, based on medical history, physical examination, and radiological assessment, an age of 18 years or older, and proficiency in simplified Chinese (Mandarin).
The participants received the same postoperative medical care and physical therapy. The study distributed a total of 170 questionnaires in two stages. In the first stage, 35 individuals completed two KOOS surveys with a 24-h interval to assess test-retest reliability [22]. 129 individuals completed the first KOOS and 36 item short form (SF-36) survey before surgery to assess the internal consistency and construct validity of KOOS. Six weeks after the surgery, 119 individuals completed the KOOS survey again to assess its responsiveness.
2.2. Knee function assessment scales (KOOS and SF-36)
We obtained the patient-friendly simplified Chinese version of the KOOS from Liying Yang, the developer. The KOOS is a self-report questionnaire that takes approximately 5–10 min to complete [10]. It is made up of 5 subscales: pain (9 items), symptoms (7 items), ADL (17 items), Sport/Rec (5 items), and knee-related QOL (4 items), totaling of 42 items [10,11]. Each item includes a five-point Likert scale response option, ranging from 0 (no problem) to 4 (extremely problematic). The response options were converted to a 0–100 scale scoring system, where 0 represents extreme problems and 100 represents no problems. According to the KOOS scoring rules, if the selection mark is placed outside the box, the nearest box is selected. If two boxes are marked, the box indicating a more serious question was selected. As long as at least 50% of each subscale is answered, the average score can be calculated. If more than 50% of the subscale items are left unanswered, the responses are considered invalid, and no subscale score is calculated. A user guide for the KOOS can be found on the internet (http://www.koos.nu).
The SF-36 questionnaire offers a concise method for assessing the health of the general population aged 14 years or older [23]. The SF-36 questionnaire can directly quantify an individual's health status, and due to its ease of use, it has become the most widely used QOL assessment tool worldwide [24]. It contains 36 items with 8 subscales, which including physical functioning (PF), role-physical (RP), body pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RM), and mental health (MH) subscales. Each subscale yielded a score of 0–100, with higher scores indicating better health status [25]. In this study, we obtained a permission license to use the simplified Chinese SF-36 questionnaire from Li et al. at Zhejiang University [26].
2.3. Psychometric properties and data analyses
Data was entered into a Microsoft Excel spreadsheet and analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). All tests were two-tailed and conducted at a 5% level of significance.
Internal consistency is evaluated by examining the correlations between different items within the same questionnaire or between the same subscales in a larger test [27]. Internal consistency is assessed using Cronbach's alpha (α), and α values equal to or greater than 0.7 indicate acceptable reliability [28].
Test-retest reliability refers to the extent to which the scores of the same patient remain consistent with repeated measurements. In this study, individuals who underwent TKA completed a second questionnaire 24 h after the procedure. Previous studies have assessed the test-retest reliability of KOOS within 24-h period [22]. The intraclass correction coefficient (ICC) was used to assess the test-retest reliability with 95% confidence intervals (95% CI). An ICC above 0.81 indicates excellent reliability, from 0.80 to 0.61 indicates good reliability, from 0.60 to 0.41 indicates moderate reliability, from 0.40 to 0.21 indicates fair reliability, and of 0.20 and below indicates low reliability [28,29].
Evidence of construct validity must be obtained before the study, assuming a pattern of association with other validation instruments designed to measure relatively similar constructs (positive correlation) [30,31]. During the development of the original [10,11] and other versions of the KOOS, the SF-36 was used to assess construct validity [9]. Therefore, this study also used the SF-36. The correlations between the KOOS and various SF-36 subscales were evaluated before the operation using Spearman's rank correlation coefficients (ρ), with values of 0.50 and above indicating strong correlation, between 0.35 and 0.50 indicating moderate correlation, and below 0.35 indicating weak correlation [[30], [31], [32]].
Responsiveness was defined as the ability to detect clinically important changes in effect size (ES) and standardized response mean (SRM) by comparing pre- and post-surgical outcomes [33]. ES is defined as the average difference between baseline and post-surgery scores, divided by the standard deviation (SD) of the pre-operative value. SRM is defined as the mean change between baseline and post-surgery scores divided by the SD of the mean change [34]. An ES < 0.5, from 0.5 to 0.8, and >0.8 was considered small, medium, and large, respectively [34,35].
Measurement error, which is not caused by actual changes in the structure to being evaluated, is a systematic and random error in patient scoring [36]. Standard error of measurement (SEM) was used to assess consistency at all levels. Test-retest reliability assesses the consistency of an individual's repeated measurements on the same instrument around the “true” measurement values. The formula for calculating SEM is as follows: SEM=SD √(1 − R), where SD represents the sample's SD and R is the test-retest reliability measure (ICC) [36]. The minimal detectable change (MDC) sets the threshold for clinical changes that exceed measurement error and is calculated using the following formula: MDC=SEM × 1.96 × √2, where 1.96 represents the constant associated with the 95% CI, and √2 accounts for the uncertainty in two measurements for evaluating changes [37,38].
Descriptive analysis was used to identify ceiling and floor effects, which were deemed to be present if more than 15% of the participants obtained the highest or lowest possible scores in each subscale [39].
3. Results
3.1. Participant characteristics
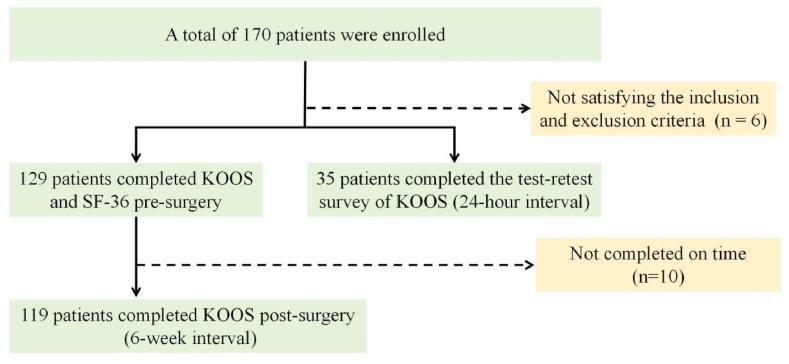
Of the 170 individuals, 129 completed the preoperative questionnaire survey, and 119 repeated the survey 6 weeks after surgery, 35 more individuals completed two KOOS surveys. Fig. 1 displays the process of individuals recruitment, Table 1 displays the characteristics of the participants.
Fig. 1.
The Flowchart of individuals Enrollment.
Table 1.
Demographic characteristics of the participants in the pre-operative and post-operative stages and test-retest group.
| Pre-operative (n = 129) | Post-operative (n = 119) | Test-retest group (n = 35) | |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 64.81 ± 8.73 | 64.82 ± 8.56 | 66.71 ± 8.66 |
| Range | 25–83 | 25–82 | 32–83 |
| Sex (%) | |||
| Male | 33 (25.6%) | 31 (26.1%) | 10 (28.6%) |
| Female | 96 (74.4%) | 88 (73.9%) | 25 (71.4%) |
| Height (cm) | 158.67 ± 0.08 | 158.67 ± 7.95 | 157.29 ± 0.09 |
| Weight (kg) | 61.55 ± 9.87 | 61.63 ± 9.75 | 62.00 ± 11.02 |
| BMI (kg/m2) | 24.43 ± 3.41 | 24.16 ± 3.92 | 25.08 ± 4.25 |
| Limb affected | |||
| Left | 63 (48.8%) | 57 (47.9%) | 20 (57.1%) |
| Right | 58 (45.0%) | 55 (46.2%) | 12 (34.3%) |
| Both | 8 (6.2%) | 7 (5.9%) | 3(8.6%) |
SD, Standard deviation; BMI, Body Mass Index.
KOOS: Knee Injury and Osteoarthritis Outcome Score; SF-36:36 item short form.
3.2. Reliability
3.2.1. Internal consistency
The reliability test results for the five KOOS subscales are presented in Table 2. The Cronbach's α scores for pain was 0.91, symptoms was 0.70, ADL was 0.95, sport/recreation was 0.90, and QOL was 0.80, indicating acceptable internal consistency in all the subscales.
Table 2.
Internal consistency of the KOOS subscales (n = 129).
| KOOS subscales (number of items) | Subscale scores (Mean ± SD) | % Floor effect | % Ceiling effect | Cronbach's α |
|---|---|---|---|---|
| Pain (9) | 50.67 ± 15.99 | 0.8 | 0.8 | 0.91 |
| Symptoms (7) | 56.26 ± 18.26 | 0 | 0 | 0.70 |
| ADL (17) | 57.75 ± 18.17 | 0 | 0 | 0.95 |
| Sport/Rec (5) | 30.35 ± 19.49 | 7.8 | 0 | 0.90 |
| QOL (4) | 39.26 ± 17.88 | 3.1 | 0 | 0.80 |
KOOS, Knee injury and Osteoarthritis Outcome Score; ADL: Activities of daily living; Sport/Rec: Sports and recreation function; QOL, knee-related quality of life; SD, Standard deviation.
3.2.2. Test-retest reliability
Table 3 displays the test-retest reliability results for each of the five KOOS subscales. The ICC value for pain was 0.82, symptoms was 0.92, ADL was 0.97, sport/recreation was 0.93, and QOL was 0.85, indicating good reliability. The detectable change ranged from 10.28 to 23.24.
Table 3.
Test-retest reliability of the KOOS subscales (n = 35).
| KOOS Subscales | KOOS Score (Mean ± SD) |
Measurement error |
||||
|---|---|---|---|---|---|---|
| First assessment | Second assessment | ICC (95% CI) | P | SEM | MDC | |
| Pain | 49.80 ± 19.65 | 56.11 ± 17.02 | 0.82(0.72–0.90) | <0.001 | 8.25 | 22.89 |
| Symptoms | 56.69 ± 20.23 | 64.80 ± 18.36 | 0.92(0.88–0.95) | <0.001 | 5.67 | 15.70 |
| ADL | 57.31 ± 21.83 | 61.94 ± 19.63 | 0.97(0.95–0.98) | <0.001 | 3.71 | 10.28 |
| Sport/Rec | 26.86 ± 20.44 | 37.00 ± 23.83 | 0.93(0.88–0.96) | <0.001 | 5.31 | 14.74 |
| QOL | 43.31 ± 20.45 | 48.83 ± 20.80 | 0.85(0.77–0.92) | <0.001 | 8.38 | 23.24 |
KOOS, Knee injury and Osteoarthritis Outcome Score; ICC, intraclass correlation coefficient; CI, confidence interval; SD, Standard deviation; ADL, Activities of daily living; Sport/Rec, Sports and recreation function; QOL, knee-related quality of life; SEM, standard error of measurement; MDC, Minimum detectable change.
3.2.3. Ceiling/floor effect
There were no ceiling or floor effects in any of the simplified Chinese KOOS subscales (Table 2).
3.3. Validity
Spearman's correlations were used to assess the validity of the KOOS and SF-36 scales. The results, as shown in Table 4, revealed a moderately strong positive correlation (0.47–0.50) between two KOOS subscales (pain and symptoms) and two SF-36 subscales (GH and BP). A strong positive correlation (0.53–0.61) was found between three KOOS subscales (ADL, QOL, and Sport/Rec) and two SF-36 subscales (GH and BP).
Table 4.
Spearman's correlation coefficients (ρ) for the comparison of the five KOOS subscales with the eight SF-36 subscales.
| Scale | Subscales | SF-36 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GH | PF | RP | RE | BP | VT | MH | SF | ||
| KOOS | Pain | 0.27a | 0.47a | 0.16 | 0.24a | 0.50a | 0.29a | 0.11 | 0.23b |
| Symptoms | 0.37a | 0.50a | 0.14 | 0.29a | 0.48a | 0.39a | 0.28a | 0.31a | |
| ADL | 0.30a | 0.61a | 0.20b | 0.25a | 0.53a | 0.27a | 0.21b | 0.37a | |
| Sport/Rec | 0.36a | 0.61a | 0.25a | 0.26a | 0.55a | 0.32a | 0.15 | 0.25a | |
| QOL | 0.46a | 0.58a | 0.30a | 0.32a | 0.61a | 0.29a | 0.22b | 0.39a | |
KOOS, Knee injury and Osteoarthritis Outcome Score; ADL: Activities of daily living; Sport/Rec: Sports and recreation function; QOL, knee-related quality of life; SF-36, 36-item short form; GH, general health; PF, physical functioning; RP, role-physical; RE, role-emotional; BP, bodily pain; VT, vitality; MH, mental health; SF, social functioning.
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
3.4. Responsiveness
Out of the 129 participants, 119 underwent surgery and physical therapy. Table 5 displays the KOOS subscale scores before and after 6 weeks of physical therapy. The ES ranged from 0.68 to 0.86 and the SRM ranged from 0.76 to 1.09. The KOOS proved to be responsive for individuals who have undergone TKA. The treatment protocol elicited the most favorable response in the KOOS-QOL subscale showed the lowest response was in the KOOS-pain subscale.
Table 5.
Responsiveness: KOOS data at the first evaluation and 6-week follow-up.
| KOOS Subscales | KOOS Score (Mean ± SD) |
Responsiveness |
||
|---|---|---|---|---|
| Pre-operative (n = 129) | Post-operative(n = 119) | Effect size | SRM | |
| Pain | 50.67 ± 15.99 | 62.36 ± 18.21 | 0.68 | 0.80 |
| Symptoms | 56.26 ± 18.26 | 60.02 ± 19.86 | 0.72 | 0.76 |
| ADL | 57.75 ± 18.17 | 73.25 ± 19.74 | 0.82 | 0.77 |
| Sport/Rec | 30.35 ± 19.49 | 46.87 ± 27.75 | 0.69 | 0.80 |
| QOL | 39.26 ± 17.88 | 56.31 ± 21.67 | 0.86 | 1.09 |
KOOS, Knee injury and Osteoarthritis Outcome Score; ADL: Activities of daily living; ADL: Activities of daily living; Sport/Rec: Sports and recreation function; QOL, knee-related quality of life; SRM, standardized response mean; SD, Standard deviation.
4. Discussion
The KOOS was first developed for use with individuals undergoing TKA by Roos et al. [10,11]. The study demonstrated good internal consistency (Cronbach's α = 0.71–0.95) and test-retest reliability (ICC = 0.78–0.91). The study's results demonstrate similar strong psychometric properties to those of the original study, confirming that the simplified Chinese version of the KOOS is also individuals-relevancy, user-friendly for TKA individuals [10,11], and measure knee joint-related pain, symptoms, ADL, sport/Rec, and knee-related QOL.
The psychometric properties of the simplified Chinese KOOS have been examined in and compared among different populations. The study by Zhang et al. on the use of KOOS for individuals who have undergone ACL reconstruction showed sufficient internal consistency (0.740 < Cronbach's α < 0.975), the retest reliability was significant (0.888 < ICC <0.941), and the construct validity was considered as good when it was compared with the SF-36 [18]. When the KOOS was used for individuals with knee OA, the Cronbach's α coefficient was greater than 0.70, the scale had good test-retest reliability (ICC = 0.89–0.92), and the relationship between the KOOS subscales and SF-36 domains was diverse (between KOOS-ADL and SF-36 RE [ρ = 0.27] and the KOOS-Sport/Rec correlated with SF-36 BF, BP, and VT domains [ρ = 0.34–0.63]) [16]. The study results revealed that in the TKA population, the simplified Chinese version of the KOOS had a Cronbachʼs α of 0.91 for pain, 0.70 for symptoms, 0.95 for ADL, 0.90 for Sport/Rec, and 0.80 for QOL. The ICC value for pain, symptoms, ADL, Sport/Rec and QOL were 0.82, 0.92, 0.97 and 0.85, respectively. Similar to the above studies, our result demonstrated that the KOOS has sufficient internal consistency and good test-retest reliability in individuals who have undergone TKA. The construct validity results showed low to high correlations between the subscales of the KOOS and SF-36 (0.11< ρ < 0.61), which is similar to the study findings among the knee OA population (between KOOS-ADL and SF-36 RE [ρ = 0.25] and KOOS-Sport/Rec correlated with SF-36 BF, BP, and VT domains [ρ = 0.32–0.61]) [16]. Among the studies on the KOOS in China, this study is the first to conduct a responsiveness test on the KOOS. Our results demonstrate that the KOOS has good responsiveness and can detect clinical changes in individuals after TKA.
The Polish and Greek versions of the KOOS have been administered to individuals who have undergone TKA. The results of the Polish language version of the KOOS showed good internal consistency (all Cronbach's α were between 0.90 and 0.92) and very good test-retest reliability (the ICCs of the KOOS subscales ranged between 0.81 and 0.86), with the smallest detectable change ranging from 18.2 to 24.3 [40]. The Greek version of the KOOS showed good internal consistency and retest reliability, with moderate effects noted in the KOOS pain and symptom subscales [41]. Compared to the two other language versions used for individuals after TKA, the simplified Chinese version of the KOOS demonstrates good internal consistency and test-retest reliability, with the smallest detectable change ranging from 10.28 to 23.24. All the subscales of the simplified Chinese version of the KOOS showed good responses, indicating their capacity to monitor clinical changes in individuals following TKA.
5. Conclusion
The Simplified Chinese version of the KOOS demonstrates satisfactory internal consistency, good test-retest reliability, and moderate construct validity. Therefore, it is a suitable objective tool for evaluating the TKA population in mainland China. This version of the KOOS can help physical therapists to quantify knee-related disability and provide useful guidance for future interventions.
6. Limitation
There are several limitations to this study. The subjects evaluated in this study may not fully represent the whole Chinese population. For test-retest reliability test, even following previous studies with a 24-h interval, it is not possible to determine totally whether the patient's condition remains unchanged.
Ethics statement
The research protocol was approved by the first affiliated hospital of Dali university (No. DFY202210140001). Before participating this research, an informed consent was obtained from all participants.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Yanfen Fu], upon reasonable request.
CRediT authorship contribution statement
Runlan Yao: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Liying Yang: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Conceptualization. Jianhua Wang: Validation, Investigation. Qiao Zhou: Validation, Investigation, Conceptualization. Xucheng Li: Supervision, Investigation, Data curation. Ziqing Yan: Visualization, Validation, Investigation. Yanfen Fu: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank all participants and physical therapists who participated in the study. Liying Yang and Runlan Yao should be considered joint first author.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e26786.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sattler L., Hing W., Vertullo C. Changes to rehabilitation after total knee replacement. Aust J Gen Pract. 2020;49(9):587–591. doi: 10.31128/AJGP-03-20-5297. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., et al. Patient-reported outcome measures used in individuals undergoing total knee arthroplasty. Bone Joint Res. 2021;10(3):203–217. doi: 10.1302/2046-3758.103.BJR-2020-0268.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meena A., et al. Knee Surg Sports Traumatol Arthrosc; 2022. Total Knee Arthroplasty Improves Sports Activity and the Patient-Reported Functional Outcome at Mid-term Follow-Up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Thaher Y., Alfuqaha O.A., Dweidari A. Health-related quality of life and outcome after total knee replacement: results from a Cross-sectional survey in Jordan. Adv Orthop. 2021;2021 doi: 10.1155/2021/5506809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitaloni M., et al. Global management of individuals with knee osteoarthritis begins with quality of life assessment: a systematic review. BMC Muscoskel. Disord. 2019;20(1):493. doi: 10.1186/s12891-019-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Graaf V.A., et al. Reliability and validity of the IKDC, KOOS, and WOMAC for individuals with meniscal injuries. Am. J. Sports Med. 2014;42(6):1408–1416. doi: 10.1177/0363546514524698. [DOI] [PubMed] [Google Scholar]
- 7.Collins N.J., et al. Measures of knee function: international knee documentation committee (IKDC) subjective knee evaluation form, knee injury and osteoarthritis outcome score (KOOS), knee injury and osteoarthritis outcome score physical function short form (KOOS-PS), knee outcome survey activities of daily living scale (KOS-ADL), Lysholm knee scoring scale, Oxford knee score (OKS), Western Ontario and McMaster Universities osteoarthritis Index (WOMAC), activity Rating scale (ARS), and Tegner activity score (TAS) Arthritis Care Res. 2011;63(Suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen C.F., et al. Knee Surg Sports Traumatol Arthrosc; 2021. Four of Five Frequently Used Orthopedic PROMs Possess Inadequate Content Validity: a COSMIN Evaluation of the mHHS, HAGOS, IKDC-SKF, KOOS and KNEES-ACL. [DOI] [PubMed] [Google Scholar]
- 9.Collins N.J., et al. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24(8):1317–1329. doi: 10.1016/j.joca.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Roos E.M., et al. Knee injury and osteoarthritis outcome score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 11.Roos E.M., et al. Knee injury and osteoarthritis outcome score (KOOS)--validation of a Swedish version. Scand. J. Med. Sci. Sports. 1998;8(6):439–448. doi: 10.1111/j.1600-0838.1998.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramlall Y., et al. Examining pain before and after primary total knee replacement (TKR): a retrospective chart review. Int J Orthop Trauma Nurs. 2019;34:43–47. doi: 10.1016/j.ijotn.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Collins N.J., Roos E.M. Patient-reported outcomes for total hip and knee arthroplasty: commonly used instruments and attributes of a "good" measure. Clin. Geriatr. Med. 2012;28(3):367–394. doi: 10.1016/j.cger.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Roos E.M., Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual. Life Outcome. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleeton G., et al. Self-reported knee instability before and after total knee replacement surgery. Arthritis Care Res. 2016;68(4):463–471. doi: 10.1002/acr.22692. [DOI] [PubMed] [Google Scholar]
- 16.Cheung R.T., Ngai S.P., Ho K.K. Chinese adaptation and validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) in individuals with knee osteoarthritis. Rheumatol. Int. 2016;36(10):1449–1454. doi: 10.1007/s00296-016-3539-7. [DOI] [PubMed] [Google Scholar]
- 17.Yang L., et al. The simplified Chinese version of the Knee Injury and Osteoarthritis Outcomes Score (KOOS) in individuals with knee osteoarthritis for mainland China: the study of reliability and validity. J Patient Rep Outcomes. 2023;7(1):80. doi: 10.1186/s41687-023-00619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q.H., et al. Reliability, validity, and responsiveness of the Chinese version of the knee injury and osteoarthritis outcome score (KOOS) in individuals with anterior cruciate ligament reconstruction in mainland China. Z. für Orthop. Unfallchirurgie. 2019;157(1):42–47. doi: 10.1055/a-0621-9504. [DOI] [PubMed] [Google Scholar]
- 19.Vogel N., et al. Patient-reported outcome measures (PROMs) following knee arthroplasty: a prospective cohort study protocol. BMJ Open. 2020;10(12) doi: 10.1136/bmjopen-2020-040811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freigang V., et al. Patient-reported outcome after patient-specific unicondylar knee arthroplasty for unicompartmental knee osteoarthritis. BMC Muscoskel. Disord. 2020;21(1):773. doi: 10.1186/s12891-020-03776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyman S., Yin K.L. Patient-reported outcome measurement for individuals with total knee arthroplasty. J. Am. Acad. Orthop. Surg. 2017;25(Suppl 1):S44–s47. doi: 10.5435/JAAOS-D-16-00637. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A.S.K., et al. Cross-Cultural adaptation and validation of the Hong Kong version of the knee injury and osteoarthritis outcome score (HK-KOOS) for individuals with knee osteoarthritis. Occup. Ther. Int. 2019;2019 doi: 10.1155/2019/8270637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren X.S., et al. Translation and psychometric evaluation of a Chinese version of the SF-36 Health Survey in the United States. J. Clin. Epidemiol. 1998;51(11):1129–1138. doi: 10.1016/s0895-4356(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 24.Frendl D.M., Ware J.E., Jr. Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med. Care. 2014;52(5):439–445. doi: 10.1097/MLR.000000000000010311. [DOI] [PubMed] [Google Scholar]
- 25.Lam C.L., et al. The SF-36 summary scales were valid, reliable, and equivalent in a Chinese population. J. Clin. Epidemiol. 2005;58(8):815–822. doi: 10.1016/j.jclinepi.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Li L., Wang H.M., Shen Y. Chinese SF-36 Health Survey: translation, cultural adaptation, validation, and normalisation. J. Epidemiol. Community Health. 2003;57(4):259–263. doi: 10.1136/jech.57.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed I., Ishtiaq S. Reliability and validity: importance in medical research. J. Pakistan Med. Assoc. 2021;71(10):2401–2406. doi: 10.47391/JPMA.06-861. [DOI] [PubMed] [Google Scholar]
- 28.Souza A.C., Alexandre N.M.C., Guirardello E.B. Psychometric properties in instruments evaluation of reliability and validity. Epidemiol Serv Saude. 2017;26(3):649–659. doi: 10.5123/S1679-49742017000300022. [DOI] [PubMed] [Google Scholar]
- 29.Terwee C.B., et al. The quality of systematic reviews of health-related outcome measurement instruments. Qual. Life Res. 2016;25(4):767–779. doi: 10.1007/s11136-015-1122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barks L., et al. Reliability and criterion-related validity of the seated posture scale. Rehabil. Nurs. 2019;44(4):213–220. doi: 10.1097/RNJ.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 31.Almanasreh E., Moles R., Chen T.F. Evaluation of methods used for estimating content validity. Res. Soc. Adm. Pharm. 2019;15(2):214–221. doi: 10.1016/j.sapharm.2018.03.066. [DOI] [PubMed] [Google Scholar]
- 32.Roberts P., Priest H. Reliability and validity in research. Nurs. Stand. 2006;20(44):41–45. doi: 10.7748/ns2006.07.20.44.41.c6560. [DOI] [PubMed] [Google Scholar]
- 33.Cumpston M.S., et al. Strengthening systematic reviews in public health: guidance in the Cochrane Handbook for Systematic Reviews of Interventions, 2nd edition. J. Public Health. 2022;44(4):e588–e592. doi: 10.1093/pubmed/fdac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husted J.A., et al. Methods for assessing responsiveness: a critical review and recommendations. J. Clin. Epidemiol. 2000;53(5):459–468. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 35.Deyo R.A., Diehr P., Patrick D.L. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Contr. Clin. Trials. 1991;12(4 Suppl):142s–158s. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 36.Ostelo R.W., et al. 24-item Roland-Morris Disability Questionnaire was preferred out of six functional status questionnaires for post-lumbar disc surgery. J. Clin. Epidemiol. 2004;57(3):268–276. doi: 10.1016/j.jclinepi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson G., Nevill A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26(4):217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 38.de Vet H.C., et al. Reproducibility and responsiveness of evaluative outcome measures. Theoretical considerations illustrated by an empirical example. Int. J. Technol. Assess. Health Care. 2001;17(4):479–487. [PubMed] [Google Scholar]
- 39.Terwee C.B., et al. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Paradowski P.T., Kęska R., Witoński D. Validation of the Polish version of the Knee injury and Osteoarthritis Outcome Score (KOOS) in individuals with osteoarthritis undergoing total knee replacement. BMJ Open. 2015;5(7) doi: 10.1136/bmjopen-2014-006947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moutzouri M., et al. Cross-cultural translation and validation of the Greek version of the Knee Injury and Osteoarthritis Outcome Score (KOOS) in individuals with total knee replacement. Disabil. Rehabil. 2015;37(16):1477–1483. doi: 10.3109/09638288.2014.972583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Yanfen Fu], upon reasonable request.