Summary
Although the impact of sleep loss on social behaviors has been widely observed in recent years, the mechanisms underpinning these impacts remain unclear. In this study, we explored the detrimental effects of sleep deprivation on reciprocity behavior as well as its underlying psychological and neuroimaging mechanisms by combining sleep manipulation, an interpersonal interactive game, computational modeling and neuroimaging. Our results suggested that after sleep deprivation, individuals showed reduced reciprocity behavior, mainly due to their reduced weights on communal concern when making social decisions. At neural level, we demonstrated that sleep deprivation’s effects were observed in the precuneus (hyperactivity) and temporoparietal junction, dorsal lateral prefrontal cortex (DLPFC) (both hypoactivity), and reduced reciprocity was also accounted for by increased precuneus-thalamus connectivity and DLPFC-thalamus connectivity. Our findings contributed to the understanding of the psychological and neuroimaging bases underlying the deleterious impact of sleep deprivation on social behaviors.
Subject areas: Cognitive neuroscience, Techniques in neuroscience
Graphical abstract
Highlights
-
•
This study sheds new light on how sleep loss affects social function
-
•
Using interactive games, we investigated how sleep loss affected social reciprocity
-
•
Social emotions mediated the detrimental effects of sleep loss on reciprocity
-
•
Sleep reduced reciprocity involving in the precuneus, TPJ, DLPFC, and thalamus
Cognitive neuroscience; Techniques in neuroscience
Introduction
A good night’s sleep is beneficial to one’s physical and mental well-being.1,2 However, people are rarely able to ensure proper sleep every night due to a variety of causes, such as poor sleeping habits, work pressure, the use of medications, medical illnesses and mental illnesses, which cause sleep deprivation.3 Sleep deprivation has lately been considered as a “public health problem” by the Centers for Disease Control and Prevention in the United States.4 Total sleep deprivation (TSD) not only affects individual cognitive functions, such as attention, vigilance, and memory,5 but also raises the risk of physical diseases and mental illnesses.3,6 Recently, emerging research has demonstrated the detrimental impacts of TSD on social functions,7 such as causing lower empathy,8 decreased desire to engage in social interactions, reduced gratitude,9 social withdrawal,10 decreased helping behavior,11 increased immoral behaviors,12,13 and decreased trust and cooperation in social dilemmas.14,15
Reciprocity is an ubiquitous social behavior in human society, which can be broadly defined as a social norm of responding to a positive (or negative) action with another positive (or negative) action,16 and is essential to promote prosocial behaviors, mental health, and absorption into society.17,18,19 Reciprocity has been used in social psychology to explain a wide range of phenomena, such as altruism,20 interpersonal helping21 and gift giving,22 and has received extensive attention from psychology, sociology, economics and neuroscience.23,24,25 Yet, to date, only a few studies have investigated the influence of TSD on reciprocity. Two previous studies14,26 have explored this question using the trust game, in which participants received the investment of an investor and decided how much to reciprocate. One study showed that TSD did not significantly reduce participants’ reciprocity behavior (i.e., the amount given back to the investor) when participants were not informed of the specific amount that the investor invested.26 The other one suggested that sleep restriction reduced participants’ reciprocity behavior when the amount of investment was clear.14 However, these tasks could not dissociate the multiple motivations underlying the participants’ reciprocity behaviors or identify the specific motivation(s) that sleep loss impacts.
According to appraisal theory,27,28,29,30,31 receiving unsolicited favor may elicit different social emotions, which may ultimately affect the recipient’s reciprocal behavior. Previous research has shown that human reciprocity may involve multiple psychological concerns.32,33,34 Studies from the perspective of economics have suggested that when making decisions of how to reciprocate others’ investments or giving, people consider not only the internal preferences for the welfare of others (the intrinsic utility of repaying others’ kindness) induced by others’ kind intentions, but also external factors such as the pressure or obligation to reciprocate given the social norm of reciprocity.32 Extending this notion, evidence from social psychology and social neuroscience have revealed two lines of social emotions that support the internal and external motivations behind reciprocity. On the one hand, upon receiving altruistic favors, individuals commonly experience the feelings of communal concern, i.e., gratitude and guilt,35,36,37,38 which motivates reciprocity that derived from the care for the benefactor.39,40 These feelings of communal concern support the internal motivation of reciprocity. On the other hand, when receiving favors with strategic intentions (e.g., expecting repayment), individuals commonly experience the sense of obligation,41,42,43,44,45 which motivates reciprocity driven by the external pressure of social norms.46,47,48,49 Which psychological concern(s) of reciprocity does sleep deprivation impact? What are the neuroimaging mechanisms that contribute to these impacts? These questions remain largely unclear.
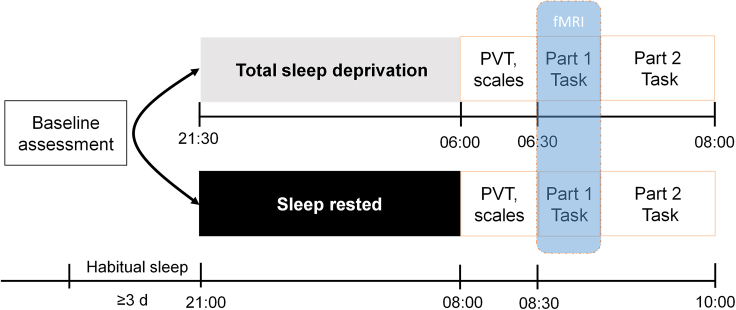
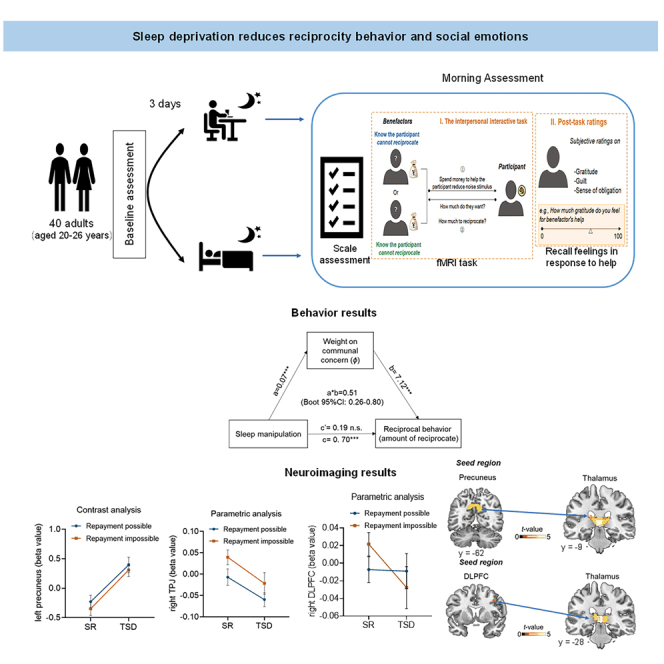
In this study, we combined TSD manipulation, an interpersonal interactive task, a computational modeling and neuroimaging to address above questions. Specifically, after either of two sleep manipulations (TSD or sleep-rested (SR)), 40 participants finished measuring levels of vigilance, subjective sleepiness and affective state, followed by the first part of the interpersonal interactive task within the scanner and other parts of tasks outside the scanner (Figure 1). In the interactive game, participants received favors from benefactors with altruistic or strategic intentions, and decided how much to reciprocate (Figure 2). Individuals made trade-off between two emotional motivations, i.e., the feelings of communal concern (gratitude and guilt) and the sense of obligation, to determine how much to reciprocate after receiving favors. First, we examined the effects of one night of sleep deprivation on the reciprocity behavior and underlying psychological mechanisms. We hypothesized that participants would be more likely to return less money to the benefactor and are less likely to be motivated by social emotions (i.e., communal concern and obligation) in response to benefactors’ help following TSD. Second, the current study explored the underlying neuroimaging mechanisms by which one night of experimental sleep loss reduced reciprocity behavior. We hypothesized that TSD would adversely impact the activation and functional connectivity of reciprocity-related regions.
Figure 1.
Experimental design
The present study was a repeated-measure counterbalanced design with 40 participants. After either of two sleep sessions, levels of vigilance, subjective sleepiness and affective state were measured, followed by a computerized task within the scanner and other computerized tasks outside scanner. PVT, psychomotor vigilance test.
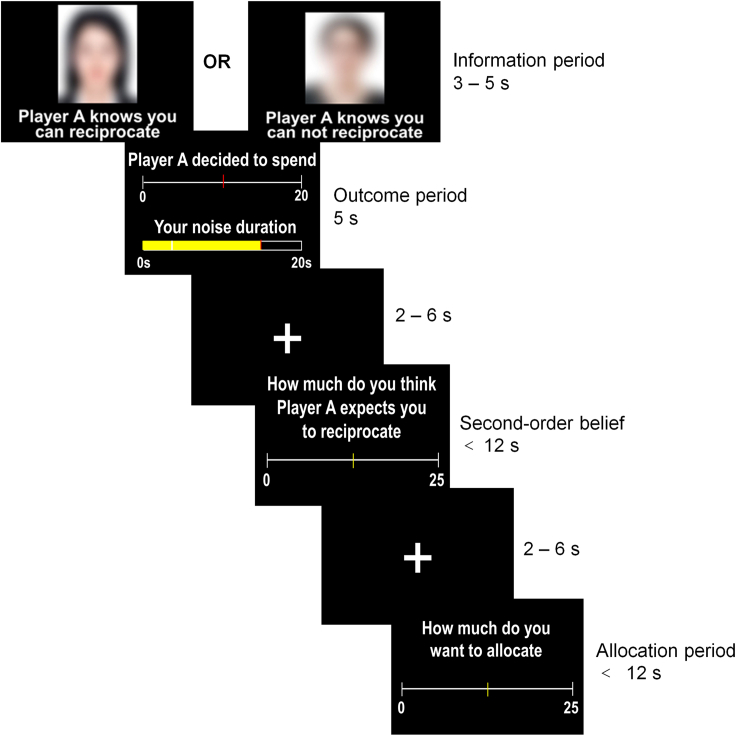
Figure 2.
Detailed procedure of the interactive task
In each trial, the benefactor decided how much of their endowment to spend (i.e., benefactor’s cost) to reduce the participant’s noise duration. The more the benefactor spent, the shorter the duration of noise stimulus. Participants indicated how much they thought the benefactor expected them to reciprocate (i.e., second-order belief of the benefactor’s expectation for repayment). Participants accepted their help and could reciprocate by allocating monetary points to the benefactor. Unbeknownst to participants, benefactors’ decisions were pre-determined by the computer program. We manipulated the perception of the benefactor’s intentions by providing extra information about whether the benefactor knew that the participant could (i.e., Repayment possible condition) or could not (i.e., Repayment impossible condition) reciprocate after receiving help.
Results
Validation of sleep deprivation manipulation
Results showed that participants reported significantly higher scores of Karolinska Sleepiness Scale (KSS) (Z = 5.35, p < 0.001) and visual analog scale (VAS) (Z = 5.15, p < 0.001) (Table S1) in TSD session than that in sleep-rested (SR) session, indicating a significantly higher level of sleepiness after TSD. Participants missed significantly more targets (Z = −5.21, p < 0.001) and responded more slowly in Psychomotor Vigilance Test (PVT) (t39 = 7.88, p < 0.001) (Table S1), reflecting significantly lower vigilance in TSD session than in SR session. These results validated our manipulation of sleep deprivation.
From the perspective of basic cognition, consistent with previous studies,12,13,15,50 participants reported decreased positive affect (t39 = −9.62, p < 0.001) and increased negative affect (Z = 4.10, p < 0.001) in Positive and Negative Affect Schedule (PANAS) (Figure S1).
Effects of total sleep deprivation on reciprocity behavior and psychological bases in favor-receiving context
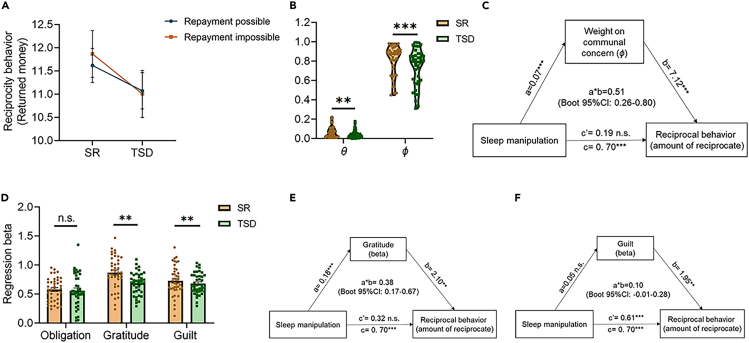
We tested the effect of TSD on reciprocal behavior by conducting 2 (Sleep manipulation: TSD vs. SR) × 2 (Condition: Repayment impossible vs. Repayment possible) two-way repeated measures ANOVA using the amount of reciprocity in each trial. Results revealed a significant main effect of Sleep manipulation on the amount of reciprocity (F1,39 = 9.40, p = 0.004), with participants following TSD reciprocating less money to benefactor after receiving help (mean difference = −0.70 ± 0.23, 95% confidence interval (CI) = [−1.17, −0.24], p = 0.004). There was no main effect of Condition (F1,39 = 0.09, p = 0.766) or interaction effects (F1,39 = 1.02, p = 0.319) (Figure 3A).
Figure 3.
Effects of total sleep deprivation on reciprocity behavior and emotional responses after receiving help
(A) Effects of total sleep deprivation on the participant’s reciprocity behavior (i.e., amount of reciprocate) (Two-way repeated measures ANOVA).
(B) Effect of total sleep deprivation on different concerns underlying reciprocity behavior using computational model (two-tailed paired t-test/Wilcoxon test).
(C) Mediation analysis to assess the relationship between Sleep manipulation and reciprocity behavior, using parameter φ as the mediator (Mediation analysis).
(D) Effect of total sleep deprivation on the contributions of self-reported sense of obligation, gratitude and guilt to reciprocity behavior, respectively (Linear mixed model).
(E) Mediation analysis to assess the relationship between Sleep manipulation and reciprocity behavior, using gratitude (regression beta) as the mediator (Mediation analysis).
(F) Mediation analysis to assess the relationship between Sleep manipulation and reciprocity behavior, using guilt (regression beta) as the mediator (mediation analysis). Error bars indicate standard error of the mean. ∗∗p < 0.01, ∗∗∗p < 0.001, n.s. = p > 0.05. SR, sleep-rested; TSD, total sleep deprivation.
Moreover, in order to test whether the reduction in reciprocity behavior caused by sleep deprivation was related to sleepiness and affective state, we performed correlation analysis. The change in reciprocity behavior was not significantly related to change in sleepiness (r = −0.05, p = 0.784, Figure S2A) or affective states (positive affect: r = 0.17, p = 0. 305, Figure S2B; negative affect: r = −0.07, p = 0. 674; Figure S2C). Thus, the adverse effects of TSD on reciprocity behavior do not appear to be adequately explained by changes in sleepiness or affective state.
To quantitatively capture the effect of TSD on different emotional motivations behind reciprocity and reciprocal behavior, a computational modeling approach was applied (Equation 1).34 The utility of each behavior U(DB) was modeled based on the competing latent motivations of self-interest, communal concern (guilt and gratitude), and obligation using Equation 1 (see STAR Methods).
| Equation 1 |
The central idea of this model is that upon receiving a favor DA from a benefactor A, the beneficiary B chooses an action DB that maximizes his/her overall utility U. This utility is comprised of a mixture of values arising from self-interest π weighted by a greed parameter θ, and feelings of communal concern UCommunal and obligation UObligation, which are weighted by the parameter φ between 0 and 1. Larger φ values reflect the participant’s higher sensitivity to feelings of communal concern relative to the obligation.
To further reveal the psychological bases underlying the effect of TSD on reciprocity, we examined how TSD influenced (1) participants’ self-reported ratings of social emotions, including gratitude, guilt and the sense of obligation after receiving help; (2) trade-off between feelings of communal concern and obligation basing on parameter φ and (3) the contributions of these social emotions to reciprocal behavior.
First, (Sleep manipulation: TSD vs. SR) × 2 (Condition: Repayment impossible vs. Repayment possible) two-way repeated measures ANOVAs were conducted on participants’ self-reported social emotions. There were significant main effects of Condition on the obligation (F1,39 = 16.82, p < 0.001), gratitude (F1,39 = 90.08, p < 0.001) and guilt (F1,39 = 88.34, p < 0.001) (Figure S3). No significant main effect of Sleep manipulation (obligation: F1,39 = 0.77, p = 0.385; gratitude: F1,39 = 0.07, p = 0.789; guilt: F1,39 = 0.18, p = 0.677) or interaction effect (obligation: F1,39 = 1.10, p = 0.302; gratitude: F1,39 = 0.10, p = 0.755; guilt: F1,39 = 0.86, p = 0.361) was observed. These results indicated that TSD did not significantly impact self-reported social emotion ratings after receiving help.
Second, from the perspective of individual difference, we applied computational modeling approach to quantitatively capture the effect of TSD on different emotional motivations behind reciprocity.34 By estimating and comparing these two model parameters (θ and φ) for each participant in the two Sleep manipulations, we found that compared to SR session, in TSD session participants showed lower sensitivity to feelings of communal concern relative to obligation, as reflected by the decreased parameter φ (t39 = −3.54, p < 0.001; Figure 3B). The relative sensitivity to feelings of communal concern (parameter φ) mediated the effect of Sleep manipulation on the amount of reciprocity (mediating effect estimate = 0.51, SE = 0.14, Boot 95% CI = [0.26, 0.80]; Figure 3C). To our surprise, participants became less greedy (reflected by decreased parameter θ) in the TSD session compared with SR session (Z = −2.78, p = 0.005). Consistent previous studies,34 we found that most participants had low θ values (i.e., greed), but showed a wide range of individual differences in parameter φ. Therefore, our subsequent analysis mainly focused on φ. These findings suggested that after TSD, individuals may consider less on the feelings of communal concern relative to obligation, which resulted in the reduced amount of reciprocity.
To be noted, here the parameter φ reflected the participant’s tradeoff between feelings of communal concern and obligation. A decreased parameter φ indicated a decreased weight on communal concern (i.e., gratitude and guilt), or an increased weight on obligation, or both. To verify whether the decreased parameter φ represented decreased weight on communal concern and to further dissociate the effects of these three social emotions (gratitude, guilt and sense of obligation), we estimated the contributions of self-reported gratitude, guilt and the sense of obligation to reciprocity behavior respectively for each participant in each Sleep session using linear mixed models (LMMs). Results showed a significant interaction effect between Sleep manipulation and gratitude ratings on the amount of reciprocity (β = 0.10 ± 0.04, t = 2.76, p = 0.006), and a significant interaction effect between Sleep manipulation and guilt ratings on the amount of reciprocity (β = 0.11 ± 0.04, t = 2.93, p = 0.003) (Figure 3D; Table S2). Specifically, compared with SR session, participants were less likely to reciprocate due to changes in the feelings of gratitude and guilt (that is communal concern) in TSD session, which may contribute to their reduced amount of reciprocity. No significant interaction effect between obligation ratings and Sleep manipulation was observed (β = 0.04 ± 0.04, t = 0.90, p = 0.371; Figure 3D; Table S2). These results indicated that TSD decreased weight on communal concern (i.e., gratitude and guilt), rather than increased weight on obligation.
To examine whether emotional motivations mediated the effects of TSD on reciprocal behavior, simple mediation model analyses and multiple mediation model analyses were performed. Mediation analyses showed that the motivation role of gratitude to reciprocity (regression beta representing the contribution of gratitude to reciprocity) mediated the effect of Sleep manipulation on reciprocity (mediating effect estimate = 0.38, SE = 0.13, Boot 95% CI = [0.17, 0.67]; Figure 3E), while the motivation role of guilt in reciprocity (regression beta representing the contribution of guilt to reciprocity) did not mediate the effect of Sleep manipulation on reciprocity (mediating effect estimate = 0.10, SE = 0.07, Boot95% CI = [-0.01, 0.28]; Figure 3F). These results indicated that the feelings of communal concern (i.e., gratitude and guilt), but not obligation, decreased after TSD, which resulted in the reduced reciprocity to the benefactor.
Neuroimaging bases underlying the effect of sleep deprivation on reciprocity
To examine the neuroimaging bases underlying the reduction in the motivation role of feelings of communal concern (i.e., gratitude and guilt) and the resultant decreased reciprocity behavior triggered by TSD, we focused on the Outcome period in which participants learned about benefactor’s decision to help and would thus infer benefactor’s intentions, generate emotional responses and decide how much to reciprocate (Figure 2). We fit two general linear models (GLMs) to each voxel’s timeseries to identify brain regions that showed differential responses to benefactor’s help in TSD and SR sessions.
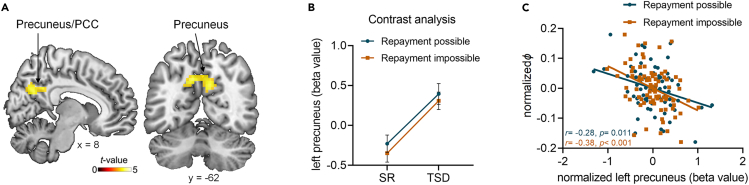
In the GLM1, we modeled Repayment possible condition and Repayment impossible condition as separate task-related regressors, and defined two contrasts corresponding to the effect of these two conditions in first-level analysis (for details, see whole brain analysis in STAR Methods). In second-level analysis, paired-t tests were conducted to reveal the differences between TSD and SR sessions. Whole-brain analysis revealed that compared with the SR session, participants exhibited increased activity in the precuneus/posterior cingulate cortex (PCC) in the Repayment impossible session in TSD session (Figure 4A; Table 1; Clusters survive p < 0.001 at voxel level and pFWE < 0.05 at cluster level). No other region was detected in the reverse contrast (i.e., SR > TSD) or in the Repayment possible condition under the same threshold. To further examine the role of precuneus, we extracted the averaged neural activity in this region (MNI coordinate: −9, −64, 35) in four conditions of 2 (Sleep manipulation: TSD vs. SR) × 2 (Condition: Repayment impossible vs. Repayment possible) and conducted ANOVA. The main effect of Sleep manipulation was significant (F1,39 = 19.70, p < 0.001): the activation in precuneus was significantly higher in the TSD session compared with SR session (mean difference = 0.40 ± 0.09, 95% CI = [0.22, 0.58], p < 0.001, Figure 4B). There was no significant interaction effect between the two experimental factors (F1,39 = 0.34, p = 0.566). These results suggested that TSD caused a general increase in precuneus activity when participants faced with others’ help.
Figure 4.
Effects of total sleep deprivation on the brain activation related to reciprocity in Repayment possible and Repayment impossible conditions
(A) Whole-brain contrast of total sleep deprivation vs. sleep rested in the Repayment impossible condition.
(B) Parameter estimates (beta values) corresponding to the two conditions in two sleep sessions were extracted from precuneus (MNI coordinate: −9, −64, 35) for illustrative purposes (Two-way repeated measures ANOVA).
(C) Correlation analysis between activation in left precuneus and parameter φ (Pearson’s correlation analyses). Error bars indicate standard error of the mean. PCC, posterior cingulate cortex; SR, sleep-rested; TSD, total sleep deprivation.
Table 1.
Whole brain analysis
| Regions | Hemisphere | t | Cluster size |
MNI coordinates |
||
|---|---|---|---|---|---|---|
| (voxels) | x | y | z | |||
| Repayment impossible (TSD>SR) | ||||||
| Precuneus/PCC | L | 4.55 | 186 | −9 | −64 | 35 |
| Precuneus | R | 4.15 | 18 | −55 | 32 | |
| R | 4.02 | 9 | −61 | 29 | ||
PCC, posterior cingulate cortex; SR, sleep-rested; TSD, total sleep deprivation.
To test whether the precuneus activity was related to behavioral changes, we examined the relationships between parameter φ during reciprocity generated from computational modeling and the activities in precuneus in Repayment possible and Repayment impossible conditions, respectively. We observed significant negative correlations in both conditions (Repayment possible condition, r = - 0.28, p = 0.011; Repayment impossible condition, r = - 0.38, p < 0.001; Figure 4C). The higher the activation in precuneus, the lower parameter φ during reciprocity, and thus resulted in the lower amount of reciprocity. These results identified precuneus, a critical area in default mode network (DMN) that has been linked to social cognition51 and moral cognition,52 played an important role in the effect of TSD on reciprocity.
We then applied GLM2 to explore brain regions in which activation varies as a function of participants’ amount of reciprocity in Repayment possible and Repayment impossible conditions. In the GLM2, we appended trial-by-trial amount of reciprocity as a parametric modulator to the regressors of Repayment impossible and Repayment possible conditions, respectively, and compared the differences between TSD and SR sessions. At whole-brain level, no regions survived the threshold. As further exploratory analyses, we extracted and analyzed the parametric beta values of regions of interest (ROIs) selected from previous studies on reciprocity in favor-receiving context34,53 and reciprocity-related emotions, e.g., gratitude,53 guilt54 and obligation34 (for details, see STAR Methods).
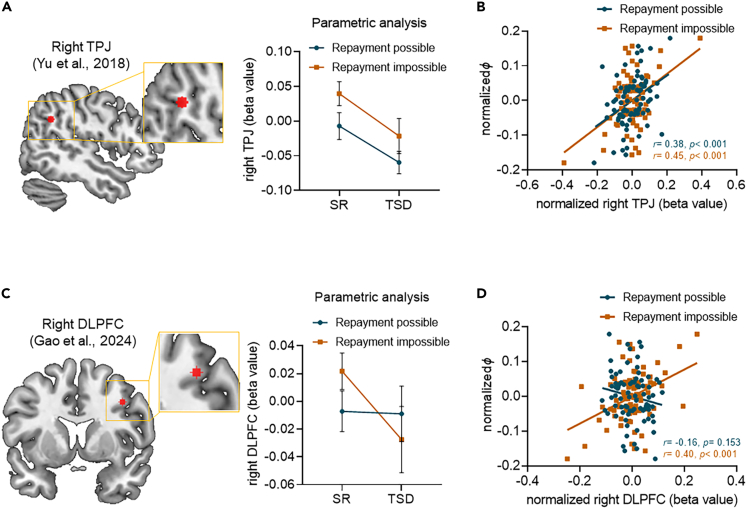
On the one hand, we found that the main effect of Sleep manipulation on the neural response to reciprocity in the right TPJ (MNI coordinate: 48, −52, 31)53 was significant (F1,39 = 9.62, p = 0.004): the neural response to reciprocity in the right TPJ was significantly decreased no matter whether the benefactor knew that participants would reciprocate or not following TSD (mean difference = −0.06 ± 0.02, 95% CI = [−0.09, −0.02], p = 0.004; Figure 5A). There was no significant interaction effect between the two experimental factors (F1,39 = 0.07, p = 0.793). To test the impact of a relative neural response change in the right TPJ within an individual on the parameter φ, Pearson’s correlation analyses were performed. In both Repayment possible and Repayment impossible conditions, we observed significant TPJ activation-behavior associations, with the higher the neural responses to reciprocity in the TPJ, the higher the participant’s sensitivity to feelings of communal concern (parameter φ) during reciprocity (Repayment possible condition, r = 0.38, p < 0.001; Repayment impossible condition, r = 0.45, p < 0.001; Figure 5B). These findings indicated the important role of right TPJ in supporting reciprocity that derived from feelings of communal concern in Repayment possible and impossible conditions, which was significantly inhibited by TSD.
Figure 5.
Effects of total sleep deprivation on the TPJ and DLPFC activations related to reciprocity behavior
(A and C) Regions of interest of total sleep deprivation vs. sleep rested in the Repayment possible and Repayment impossible conditions. Parameter estimates (beta values) corresponding to the two conditions in two sleep manipulation sessions were extracted from TPJ (MNI coordinate: 48, −52, 31) and DLPFC (MNI coordinate: 39, 8, 38) for illustrative purposes (Two-way repeated measures ANOVA in right).
(B and D) Correlation analysis between activation in right TPJ, DLPFC and parameter φ, respectively (Pearson’s correlation analyses). Error bars indicate standard error of the mean. DLPFC, dorsal lateral prefrontal cortex; SR, sleep-rested; TPJ, temporoparietal junction; TSD, total sleep deprivation.
On the other hand, we observed that the neural response to reciprocity in the right DLPFC (MNI coordinate: 39, 8, 38)34 was significantly reduced in Repayment impossible condition (t39 = −2.16, p = 0.037), but not in Repayment possible condition (t39 = −0.10, p = 0.920), following TSD (although the interaction effect did not reach significance, F1,39 = 2.46, p = 0.125) (Figure 5C). Similarly, the neural response to reciprocity in the right DLPFC was analyzed within individuals, considering person’s deviation from their average on a particular sleep session. Consistently, we found that the neural response to reciprocity in the right DLPFC was positively correlated with the participant’s parameter φ during reciprocity in Repayment impossible condition (r = 0.40, p < 0.001), but not in Repayment possible condition (r = -0.16, p = 0.153) (Figure 5D). These results indicated that the activation in right DLPFC might support reciprocity that derived from feelings of communal concern in Repayment impossible condition, and sleep deprivation significantly inhibited these activities.
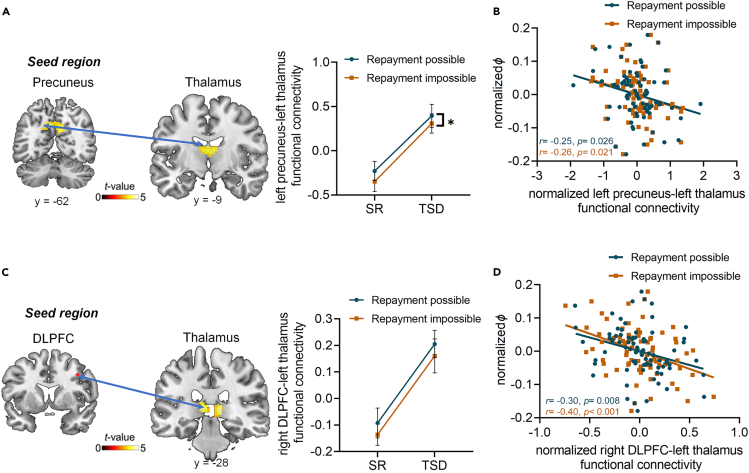
We further performed psychophysiological interaction analyses (PPI)55 using precuneus, TPJ and DLPFC as seed regions which were related to reciprocity behavior in the above results (see details in STAR Methods). There was a significant main effect of Sleep manipulation on precuneus-thalamus connectivity (F1,39 = 19.64, p < 0.001) and DLPFC-thalamus connectivity (F1,39 = 20.30, p < 0.001) (Table S3): compared with SR session, participants exhibited higher functional connectivity between left precuneus and left thalamus (mean difference = 0.64 ± 0.14, 95% CI = [0.35, 0.93], p < 0.001; Figure 6A) as well as higher functional connectivity between right DLPFC and left thalamus (mean difference = 0.30 ± 0.07, 95% CI = [0.16,0.43], p < 0.001; Figure 6C) in TSD session. No significant main effect of Condition on the functional connectivity between DLPFC and thalamus (F1,39 = 2.84, p = 0.504) or interaction between the two experimental variables (left precuneus-left thalamus: F1,39 = 0.11, p = 0.746; right DLPFC-left thalamus: F1,39 = 0.00, p = 0.984) (Table S3) was observed on these connectivities. Here, these functional connectivities were normalized. Moreover, the strength of these connectivities were negatively correlated with the parameter φ during reciprocity (left precuneus-left thalamus: Repayment possible: r = −0.25, p = 0.026, Repayment impossible: r = −0.26, p = 0.021; Figure 6B; right DLPFC-left thalamus: Repayment possible: r = −0.30, p = 0.008, Repayment impossible: r = −0.40, p < 0.001; Figure 6D). PPI analyses with TPJ as the seed region failed to survive the whole-brain cluster-level threshold. These findings suggested the functional connectivities between precuneus and thalamus and between DLPFC and thalamus as the crucial neuroimaging bases underlying the TSD effect on reciprocity, indicating that thalamus might play the role of the hub during this process.
Figure 6.
Effects of total sleep deprivation on the reciprocity-related coupling of precuneus-thalamus and DLPFC-thalamus
(A and C) Higher functional connectivity between precuneus and thalamus, DLPFC and thalamus were found after receiving help following total sleep deprivation relative to the sleep rested session (Two-way repeated measures ANOVA in right).
(B and D) Correlation analysis between precuneus-thalamus connectivity, DLPFC-thalamus connectivity and parameter φ, respectively (Pearson’s correlation analyses). Error bars indicate standard error of the mean. ∗p < 0.05 indicates a significant main effect of Condition. DLPFC, dorsal lateral prefrontal cortex; SR, sleep-rested; TSD, total sleep deprivation.
Discussion
Although earlier studies have shown that TSD may result in decreased reciprocity behavior, the underlying mechanisms are unknown. In the study, we combined sleep manipulation, interactive task, computational modeling, and neuroimaging to explain this problem. Our findings established the following: (1) TSD reduced the participant’s reciprocity behavior by lowering the weight on communal concern; (2) TSD increased activity within precuneus/PCC, yet a blunting of reactivity within the right TPJ and right DLPFC, and these neural changes were related to the weight on communal concern; and (3) TSD increased the functional connectivity strength of precuneus-thalamus and DLPFC-thalamus after receiving help, and these connectivity alterations were similarly associated with the weight on communal concern.
Behaviorally, we found that sleep loss led to reduced reciprocity behavior in response to benefactor’s help, which is consistent with previous study.14 Meanwhile, we further explored the effects of TSD on the psychological bases underlying reciprocity behavior and found that the main reason for TSD-induced reduction in reciprocity was that participants felt less communal concern from benefactors. Our findings were in line with previous study34 on social behaviors and morality in two aspects. First, from the perspective of reciprocity behavior driven by internal factors, communal concern arises when individuals receiving altruistic favor. In response to others’ help, the communal concern is suggested to drive the participant’s compliance with the norm of reciprocity for proximity seeking and not burdening the benefactor in favor of self-interest.34,39,40,56,57 Therefore, we suggest that the lowing feelings of communal concern observed here reflects the impairment of moral internalization process following TSD. This notion is supported by evidence showing that communal concern reflects participant’s concern for perceiving care from benefactor and burdening the benefactor,34,36,40,57 while the sense of obligation reflects the participant’s self-regarding concern for the anticipatory cost of repaying the benefactor.34,43 Second, from the perspective of reciprocity behavior driven by external factors, the sense of obligation is suggested to drive the participant’s obey the norm of reciprocity due to external factors such as the external pressure of the benefactor’s expectation for repayment and reputation in response to others’ favor.43,58 Consistent with previous studies,9 there were no significant changes in sense of obligation following sleep loss. Considering that our findings suggest that there is no significant change in benefactor’s expectation of repayment following TSD, this may partly explain why sleep deprivation does not significantly alter the sense of obligation. As a result, the changes in reciprocity behavior observed following sleep loss were more likely to be explained by communal concern rather than obligation.
Our research not only confirmed the causative effect of TSD on individual reciprocity behavior, but also characterized the potential brain mechanism associated with this changed phenotype of diminished reciprocity behavior. Specifically, we found that the underlying neuroimaging mechanism of above TSD effect involves hyperactivity in the DMN (precuneus/PCC),59 yet hypoactivity in the theory of mind network (TPJ)10,11 and cognitive control network (DLPFC).60,61 Precuneus/PCC has been proved not only to link to social cognition such as emotions,62 self-referential thought63 and moral judgment,52 but also to involve in the regulation of social norms.64 When comparing the outcomes of decisions for self and others, the precuneus is active, especially for pro-self individuals who rely more on self-referencing to compute the best approach.65 Thus, our findings indicate that the activation of the precuneus, as a result of TSD, led to an increased emphasis on self-interest and a tendency toward more selfish decision-making (returned less tokens). These findings indicated that important role of precuneus in inhibiting reciprocity that derived from feelings of communal concern in Repayment possible and impossible conditions, with TSD significantly amplified these activities. The impairment of brain function caused by TSD not only directly affected reciprocity behavior, but also affected communal concern which motivated reciprocity behavior. The researchers found that higher guilt ratings were associated with decreased activation of the precuneus.66 Specifically, when individuals engaged in actions that resulted in harm to others compared to themselves, there was greater activation observed in the precuneus.67 Therefore, our study indicated that TSD-induced changes in precuneus were involved in both reciprocity and its emotion motivations.
As a reciprocity-related region,68 TPJ is not only linked to empathy69 and perspective taking,70 but also involved in social norm71,72 in a number of studies. Of note, our task needed to infer the intention of benefactor’s help before making a reciprocal decision.34 Previous studies have demonstrated that TSD-induced reduced activity in the TPJ is associated with impaired ability to infer the needs and perspectives of others.10,11 This deficit further leads to perceiving less care from benefactors’ assistance, consequently resulting in decreased reciprocal decision-making in response to their help under both repayment possible and repayment impossible conditions. Moreover, here we found that the participants’ communal concern (gratitude and guilt) also increased as a function of TPJ activation in different conditions.73,74 For example, TPJ has been shown to be correlated with gratitude.75 The TPJ is also activated in studies involving the guilt condition.73 Therefore, our study suggests that TSD-induced changes in TPJ play an important role in the reduction of reciprocity behavior and its emotional motivation.
As the brain region most vulnerable to TSD, DLPFC is involved in self-control,76 inhibition of self-interested impulse77 and reciprocity behavior.34 Consistent with previous studies,34 we speculated that the DLPFC regulates underslept participants’ behavior mainly by influencing communal concern processes. On the one hand, we found that the DLPFC was activated only in repayment impossible condition following TSD. On the other hand, consistent with previous studies, we found a positive correlation between DLPFC and reciprocity. Previous study indicated that the activation in right DLPFC increased as the gratitude increased when participants receiving help. The activation of the right DLPFC was observed when individuals experienced feelings of guilt compared to shame.78 We speculate that decreased DLPFC activity affected internal factors (gratitude and guilt)67,73,74 of reciprocal behavior by influencing cognitive control.76 Consistently, these findings indicated that important role of DLPFC in evoking reciprocity that derived from feelings of communal concern in repayment impossible condition, with TSD significantly inhibited these activities.
In addition to precuneus hyperactivity and TPJ, DLPFC hypoactivity, we found that precuneus-thalamus and DLPFC-thalamus functional connectivity correlated with the participant’s sensitivity to feelings of communal concern in reciprocity. The thalamus has been referred to as a relay station for converging sensory information from diverse systems and supporting multiple cognitive and affective functions through the connectivity between its subnuclei and cortical and subcortical regions (such as cognitive control network and the default network).79,80 Studies have found that decreased prosocial giving is associated with thalamic atrophy.81 Previous studies suggested that the thalamus could be an interacting node in this SD-affected network.82 Specifically, thalamus may affect the unstable reciprocal inhibition between task-related FPN activity and DMN activity in the sleep-deprived state.82 Previous research has shown that egocentric bias reduces as thalamic activation levels increase.83 Thalamic has been demonstrated to be activated by unpleasant conditions relative to the neutral condition.84 Therefore, we speculated that underslept participants felt less communal concern when the benefactor’s cost was lower, supported by thalamic. Similar to the previous study,85 participants exhibited stronger functional connectivity between the thalamus and prefrontal cortex following TSD. Moreover, recent studies indicated that increased functional connectivity between precuneus and thalamus was associated with the reward anticipation during the monetary incentive delay task86 and cognitive control.87 Consistently, our study suggested the stronger DLPFC-thalamus connections might be linked to emotional regulation88 and decision-making.89 Our results suggest that thalamus was recruiting precuneus and DLPFC for regulation social emotion and triggered less reciprocity behavior.
Limitations of the study
There are some limitations in this study. Firstly, our current study only proves the impact of TSD on reciprocity behavior, and cannot explain the impact of other phenotypes of sleep loss (sleep restriction, modest night-to-night variations in sleep) on reciprocal behavior. In order to effectively assess the detrimental effects of sleep loss on individual social function, chronic sleep restriction and other techniques with higher ecological validity are utilized in the future. Secondly, we used different test time schedules in SR (at 8:30 a.m.) and TSD (at 6:30 a.m.) sessions. This was designed to simulate the difference in performance obtained at the start of the workday and the effect of having to work through a night shift without sleep. Prior study has demonstrated that the homeostatic pressure induced by protracted wakefulness is adequate to minimize the influence of circadian clock dependent alerting upon cognitive performance.90 Lastly, in light of the limited sample size and lack of testing of the participants' personality traits (e.g., gratitude and obligation), future studies should take individual variability into account when examining how sleep deprivation affects reciprocity behavior, since interindividual variation in this impact has been found to be trait-like in a larger sample.91
Our findings expand the limited body of research on sleep and prosocial behavior by investigating the effects of TSD on reciprocity and emotional responses in real-life-like favor-receiving contexts. Our results suggest that sleep loss has a deleterious effect on reciprocal behavior mediated by communal concern, with the precuneus, TPJ, DLPFC and thalamus all playing a crucial role. Our findings will reveal the important role of sleep in prosocial behavior, help us understand the broader social consequences of sleep loss, and provide a theoretical basis for improving the reciprocity of individuals with sleep loss.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| MATLAB 2019a | MathWorks | https://www.mathworks.com/products/matlab.html |
| PsychToolBox 3.0.14 | Matlab | https://www.psychtoolbox.org |
Resource availability
Lead contact
Further information and request for resources should be directed to and will be fulfilled by the lead contact, Hongqiang Sun (sunhq@bjmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data reported in this paper will be shared by the lead contact upon request.
Original code in this paper will be shared by the lead contact upon request.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Forty-four healthy college students were recruited in the study through advertisements and posters. Four participants were excluded from the study due to (1) excessive head movements during fMRI scanning, (2) tattoo, (3) discomfort with sleep monitoring device, leaving 40 participants (12 males; age: 23.0 ± 1.5, 20–26 years). Participants met the following inclusion criteria: (1) college students between 18 and 26 years of age, (2) right-handed, (3) regular sleep habits (sleeping no less than 6.5 h and no more than 9 h per night, sleep onset no later than 1:00 a.m. and rising time before 9:00 a.m.), (4) scores no greater than 7 on the Pittsburgh Sleep Quality Index (PSQI), (5) scores between 8 and 21 on the Horne and Östberg Morningness-Eveningness Questionnaire (MEQ), (6) scores no greater than 10 on the Epworth Sleepiness Scale (ESS), (7) not on any long-term medications, (8) no symptoms/history of sleep disorders or psychiatric/neurologic disorders, (9) no traveling to a different time zone, shift work or irregular sleep-wake routines in the past 2 months, (10) < 3 caffeine-containing drinks daily, (11) nonsmokers, (12) no MRI contraindications, (13) normal or corrected-to-normal vision. Students majoring in psychology or economics were excluded from participation, because they might be familiar with social decision-making. Participants were instructed to abstain from medications, caffeine consumption and alcohol for 3 days before each study session to maintain a regular sleep-wake rhythm, verified by sleep diaries and wrist actiwatch (Actiwatch Spectrum PRO, Philips Respironics). All participants were provided written informed consent, and were paid for their involvement. The study was approved by the Research Ethics Review Board of Peking University Sixth Hospital. The trial was registered at chictr.org.cn prior to the start of the study (ChiCTR2100047062).
Method details
General procedure
The study was a repeated-measures cross-over design, with two sessions carried out in a counterbalanced order: one night of rest with portable sleep recording at their dormitory (i.e., sleep-rested, SR) and one night of sleep deprivation monitored by research assistants and by a wrist actiwatch (i.e., TSD).
Participants were briefed on the study’s protocol and requirements and given the informed consent during the first visit. Moreover, they completed a demographic questionnaire as well as completing the PSQI, ESS, MEQ, Beck Depression Inventory-II (BDI-II), and Self-Rating Anxiety Scale (SAS). At the end of the visit, participants were asked to maintain regular sleep habits in addition to keeping a sleep diary and wearing a wrist actiwatch. Three days later, participants returned to the lab and were randomly allocated to either an SR session (n = 24) or a TSD session (n = 16) (order counterbalanced). Participants attained 424 ± 38 min (s.d.) in bed across the three nights proceeding the SD and 425 ± 40 min (s.d.) preceding the SR condition according to actigraphy data, with similar means obtained from sleep diaries data (SD: 467 ± 30 min (s.d.); SR: 463 ± 44 min (s.d.))
In TSD session, participants arrived at the laboratory at 9:30 p.m. and were asked to stay awake for the whole night. Their subjective sleepiness was evaluated by a VAS and their vigilance was measured with the PVT during the first 10 min of every hour from 10:00 p.m. until 6:00 a.m. the next day. Participants were only allowed to engage in nonstrenuous activities such as the use of the internet, reading, watching movies (except for thriller movies and comedies), conversing and working on a computer, and so on. Participants were monitored by wrist actiwatch throughout the day as well as by the research assistants in the lab. Then they completed the assessment of immediate affective state (PANAS)92 and subjective sleepiness (KSS) at 6:00 a.m. in the following morning. Next, participants completed the fMRI scanning 6:30 a.m., which was close to the circadian nadir.93,94,95 The research assistant explained the experimental task to the participants. The experimental task consisted of three parts. Part 1 (the main task) was conducted in the fMRI scanner, while Part 2 and Part 3 were conducted outside the scanner after MRI scanning. Moreover, in order to ensure that participants understood the task requirements, they would practice 4 trials of the Part 1 of the task before scanning.
In SR session, participants arrived at the laboratory at 9:00 p.m. on the experimental day. They wore portable polysomnography (PSG) and then went to the dormitory to ensure they had more naturalistic sleep. During this session, participants obtained an average of 411.5 ± 68.5 min (s.d.) time asleep according to PSG. Participants returned to the lab at 8:00 a.m. the next day and removed electrodes. Then they entered into the scanner at 8:30 a.m., which was the start time of a regular workday, and completed the same assessment and experimental task as the TSD session.96 TSD and SR sessions were separated by at least 7 days apart (mostly 10 days).
Experimental task
In the interactive task (Figure 2), each participant came to the scanning room individually and was explained the task by research assistants. Participants were instructed that they would participate in an interactive game which consisted of two characters: Receiver and Benefactor. As Receivers, the participants were to receive noise punishments in the game, and the Benefactor decided whether and how much monetary endowments to spend to help the participant. Then, participants needed to estimate the number of monetary endowments the benefactor expected them to reciprocate, and then determined how much monetary endowments they wanted to reciprocate.
Noise calibration
The participant first underwent calibrations of noise-bearing.97 The intensity of the noise stimuli was calibrated individually so that it was unpleasant but bearable. Specifically, after the participant put on the earphones, we gradually increased the intensity of the noise stimuli until the participant reported ‘moderately unpleasant’ noise level. The intensity of noise stimulation was set to 6 on a 0–8 scale (0: "no feeling at all"; 8: "unbearable") after participant-specific noise thresholds were calibrated.
The main task
In Part 1 of the interactive task, each participant played multiple single-shot trials of the interpersonal game as a Receiver with same-sex anonymous Benefactors (the co-players). The participant was instructed that the co-player in each trial was distinct from the ones in any other trials and only interacted with the participant once during the experiment. In each trial, the participant was to receive a 20-s noise stimulation with the adverse level of 6. Each co-player was informed of the participant’s situation in the previous study and was endowed with 20 tokens (1 token represents 2 yuan). The co-player could decide whether to spend some of their tokens to reduce the duration of the participant’s noise (i.e., benefactor’s cost, DA)– more money resulted in shorter durations of noise. The maximum noise reduction was 16 s to ensure that participants felt some amount of noise stimulation on each trial.
Each trial began by informing the participant which benefactor from previous study was randomly selected as the co-player for the current trial with a blurred picture of the co-player and their subject ID (Information period, 3–5 s). The co-player’s decision on how much they chose to spend to help the participant was presented (Outcome period, 5 s). Next, the participant indicated how much he/she thought this co-player expected him/her to reciprocate (i.e., second-order belief of the co-player’s expectation for repayment; continuous rating scale from 0 to 25 using a two-button curved response box in the right hand. These buttons moved the slider on the screen left and right in increments of 1 token of the slider range, <12 s). At the end of each trial, the participant was endowed with 25 tokens and decided how much they wanted to allocate to the co-player as reciprocity from this endowment (DB, continuous choice from 0 to 25 using two-button, step of 1 token, <12 s). We focused on the tokens that the participant allocated to co-player after accepting his/her help (i.e., reciprocity behavior).
We manipulated the perceived intention of the co-player by providing participants with extra information regarding the co-player’s expectation of reciprocity (i.e., extra information about benefactor’s intention) below the co-player’s subject ID at the beginning of each trial. Each participant was instructed that, some co-players were informed that the participant would be endowed with 25 tokens and could decide whether to allocate some endowments to them as reciprocity (i.e., Repayment possible condition). The other co-players were informed that the participant had no chance to reciprocate after receiving help (i.e., Repayment impossible condition). In fact, participants could reciprocate in both conditions during the task. The endowment of the co-player (γA) was always 20 tokens, and the endowment of the participant (γB) in each trial was always 25 tokens. Before and after the second-order belief rating period, a fixation cross was presented for a variable interval ranging from 2 to 6 s, which was for the purpose of fMRI signal deconvolution. The scanning session consisted of three runs (in total 54 trials) and lasted for approximately 39 min. Each run lasted for 13 min and consisted of 18 trials, including the 9 levels of the benefactor’s cost (4, 6, 8, 10, 12, 14, 16, 18, 20) in Repayment possible condition and Repayment impossible condition, respectively, and trial order was pseudorandomized.
Post-task ratings
In Part 2, all of the decisions in Part 1 were displayed again in a random order. After being shown the co-player’s information and his/her decision, the participant was asked to recall how much they believed the benefactor cared about them as well as their feelings of gratitude, obligation and guilt when they received the help of the co-player. Ratings were conducted on a scale from 0 to 100, with 0 representing “not at all” and 100 representing “extremely intense”. The rating order was counter-balanced across trials.
-
(1)
“How much gratitude do you feel for this co-player’s decision?” (Gratitude)
-
(2)
“How much pressure did you feel for the decider’s expectation for repayment?” (Obligation)
-
(3)
“How much guilt do you feel for this co-player’s decision?” (Guilt)
-
(4)
“How much do you think this decider cares about you?” (Perceived care)
At the end of the experiment, two trials in Part 1 were randomly selected to be realized. The participant received the average noise stimulation in these two trials. The participant’s final payoff was the average amount of endowment the participant left for him/herself across the chosen trials. The participant was instructed that the final payoff of each co-player was the amount of endowment the co-player left plus the amount of endowment the participant allocated to him/her. Participants were informed of this arrangement before the experiment began. Moreover, the question “Do you think that the benefactor selected to participate in the trial with you are satisfied with the payment they received?” will be to test (dis)belief in they were actually playing with real other persons. No one were excluded from the analysis based on this question.
Image acquisition
Images were acquired using a 3T GE-MR750 scanner with an 8-channel head coil at Peking University Sixth Hospital (Beijing, China). T2-weighted echoplanar images (EPI) were obtained with blood oxygenation level-dependent (BOLD) contrast. Thirty-three transverse slices of 4.2 mm thickness that covered the whole brain except the cerebellum were acquired using multiband EPI sequence in an interleaved order (repetition time = 2000 ms, echo time = 30 ms, field of view = 224 × 224 mm2, flip angle = 90°). A high-resolution T1-weighted image was acquired using an MPRAGE sequence (192 sagittal slices; voxel size 1 × 1 × 1 mm).
fMRI preprocessing
The fMRI data preprocessing and univariate analyses were conducted using Statistical Parametric Mapping software SPM12 (Wellcome Trust Department of Cognitive Neurology, London). Images were slice-time corrected, motion corrected, resampled to 3 mm × 3 mm × 3 mm isotropic voxels, and normalized to MNI space using the EPInorm approach in which functional images are aligned to an EPI template, which is then nonlinearly warped to stereotactic space. Images were then spatially smoothed with an 8 mm FWHM Gaussian filter, and temporally filtered using a high-pass filter with a cutoff frequency of 1/128 Hz.
Quantification and statistical analysis
Behavior analyses
To investigate if participants’ sleepiness (KSS, VAS), vigilance (PVT) and affective state (PANAS) changed across the sleep-rested and TSD session, a two-tailed paired t-test/Wilcoxon test was used. To investigate the effects of TSD on reciprocity after receiving help, a 2 within-subject (Sleep manipulation: TSD vs. SR) × 2 within-subject (Condition: Repayment impossible vs. Repayment possible) two-way repeated measures analysis of variance (ANOVA) was used. In case of significance, post hoc Bonferroni analyses were performed. To test the relationships between sleep loss-related changes in reciprocity behavior sleepiness and affective state, Pearson’s correlation analyses was performed.
To further explore the psychological bases underlying the effect of TSD on reciprocity behavior, 2 within-subject (Sleep manipulation: TSD vs. SR) × 2 within-subject (Condition: Repayment impossible vs. Repayment possible) two-way repeated measures ANOVA was used for participants’ self-reported ratings of social emotions (gratitude, guilt and the sense of obligation).
Multilevel modeling
To quantitatively capture the effect of TSD on different emotional motivations behind reciprocity and reciprocal behavior, a computational modeling approach was applied (Equation 1).34 The utility of each behavior U(DB) was modeled based on the competing latent motivations of self-interest, communal concern (guilt and gratitude), and obligation using Equation 1. (see STAR Methods).
For each trial, self-interest πB was defined as the percentage of tokens kept by the participant out of their endowment γB (Equation 2).
| (Equation 2) |
For each trial, we define UObligation as the appraisal of the amount of money that participant believes benefactor expect them to return (i.e., participant’s second-order beliefs EB’’) normalized by participant’s endowment size γB (Equation 3).
| (Equation 3) |
Moreover, we modeled the appraisals of second-order beliefs EB″ of the benefactor’s expectation for repayment (Equation 4), and used it to represent the feelings of obligation.34 For each trial, we modeled participant’s second-order belief EB″ of how much they believed the benefactor expected them to reciprocate based on how much the benefactor decided to spend to help DA and whether the benefactor knew repayment was possible. EB’’ is operationalized as DA in the Repayment possible condition and zero in the Repayment impossible condition.
| (Equation 4) |
In contrast, we define UCommunal in terms of the appraisal of how much participant believes benefactor cares about their welfare (i.e., perceived care ωB). UCommunal reflects a linear combination of both gratitude and guilt components.
| (Equation 5) |
We assume that participant infers perceived care ωB proportional to how much benefactor spent DA from his/her endowment γA and that this effect might be mitigated by the amount of money participant believes benefactor expects them to return (i.e., second-order belief EB’’).
| (Equation 6) |
κ reflects the degree to which the perceived strategic intention EB’’ reduces the perceived altruistic intention ωB. In each trial, the participant’s perceived care ωB was defined as a function of the benefactor’s cost DA and second-order belief EB'' (Equation 6). Specifically, we assumed that the perceived care from help increased as a linear function of how much the benefactor spent DA from his/her endowment γA. That is, when received a specific amount of benefactor’s cost, if the participant thought this benefactor expected more repayment, the less care the participant would perceive from the help. Here, the parameter κ ranges from [0, 1] and represents the degree to which the perceived strategic intention EB″ reduces the perceived altruistic intention ωB.
To investigate if participants’ emotional motivations (θ and φ) to reciprocal behavior changed across the SR and TSD session, a two-tailed paired t-test/Wilcoxon test was used.
To further dissociate the effects of TSD on the participants' emotional motivations (sense of obligation, gratitude and guilt), we used three LMMs using the lmerTest version 3.1–3 packages in R version 4.1.1. In the LMM for obligation, the amount of reciprocity was treated as a dependent variable, and ratings of obligation, Sleep manipulation and the interaction between these two factors were included as predictors, with participant as a random intercept and slope. In the LMM for gratitude, the amount of reciprocity was treated as a dependent variable, and ratings of gratitude, Sleep manipulation, and the interaction between these two factors were included as predictors, with participant as a random intercept and slope. In the LMM for guilt, the amount of reciprocity was treated as a dependent variable, and ratings of guilt, Sleep manipulation and the interaction between these two factors were included as predictors, with participant as a random intercept and slope.
To test whether the effect of TSD on reciprocity behavior could be mediated by participants’ emotion motivations, median analysis was performed. In the median analysis for communal concern, we set the reciprocity (the amount of reciprocity) as the independent variable, communal concern (φ) as mediating variable, and the Sleep manipulation as the dependent variable. Similar settings were applied to the median analysis for the motivation role of gratitude to reciprocal behavior (regression beta representing the contribution of gratitude to reciprocal behavior) and the motivation role of guilt to reciprocal behavior (regression beta representing the contribution of guilt to reciprocal behavior), respectively. To test these indirect pathways, we bootstrapped the indirect effect 5000 times using the SPSS version of process (http://www.processmacro.org/download.html).
fMRI data analyses
Whole-brain analysis
To examine the neuroimaging bases underlying the reduction in the motivation role of feelings of communal concern (i.e., gratitude and guilt) and the resultant decreased reciprocity behavior triggered by TSD, we focused on the Outcome period. In this period, participants learned about benefactor’s decision to help and generated emotional responses and decided how much to reciprocate. We conduced GLM1 and GLM2 to identify brain regions that showed differences between TSD and SR session from the perspective of general responses and the perspective of neural responses to reciprocity, respectively. In both the two GLMs, we included two key regressors for the two types of the trials in the task: Repayment possible condition and Repayment impossible condition of the Outcome period (5s). Other regressors for the GLM1 included: (a) Information period (onset of the presentation of the benefactor’s picture and extra information regarding intention, 3-5s), (b) Second-order belief rating period (starting from the time the rating screen presented and spanning to the time that the participant made choice, <12s), (c) Allocation period (starting from the time the rating screen presented and spanning to the time that the participant made choice,<12s), (d) Missed responses (the missing decision period for second-order belief or allocation, 12s), and (e) six head motion realignment parameters. In the GLM2, we add each participant’s trial-by-trial amount of reciprocity as parametric modulators on the two key regressors (Repayment possible condition and Repayment impossible condition). Other regressors were the same as GLM1. For both of the two GLMs, events in each regressor were convolved with a double gamma canonical hemodynamic response function. For the GLM1, contrasts were defined as the positive effects for the two key regressors of the two conditions. For the GLM2, contrasts were defined as the positive effects for the two parametric modulators of the two conditions. In the second-level analysis, we used a two-tailed paired t-test (TSD vs. SR) to assess group-level effects with resulting contrasts from the first-level models.
For whole brain analyses, all results were corrected for multiple comparisons using cluster correction p < 0.05 with a cluster-forming threshold of p < 0.001, which attempts to control for family wise error (FWE) using Gaussian Random Field Theory.98
ROI analysis
To further identify the specific activation pattern of each region identified in whole-brain analyses, in GLM1, we extracted and averaged the beta values of 27 voxels around the maximum activation peak in each region of interest (ROI) for each participant and each condition as the indicator of neural activity in region. These averaged beta values were then fed into 2 (Sleep manipulation: TSD vs. SR) × 2 (Condition: Repayment impossible vs. Repayment possible) ANOVAs. To examine brain-behavior associations, we analyzed the relationship between the beta (β) values of each ROI and the relative sensitivity to feelings of communal concern (parameter φ) during reciprocity generated from computational modeling using Pearson’s correlation analyses. All variables were normalized before Pearson’s correlation analyses. Normalized φ on a particular sleep session was calculated as φTSD/SR- (φTSD+ φSR)/2, normalized β of each ROI was calculated as βTSD/SR- (βTSD+ βSR)/2.
As further exploratory analyses, in GLM 2 we extracted and analyzed the parametric beta values of ROIs selected from previous studies on reciprocity in favor-receiving context34,53 and reciprocity-related emotions, e.g., gratitude,53 guilt54 and obligation,34 including left right dorsal lateral prefrontal cortex (DLPFC, peak MNI: −45, 5, 29), right DLPFC (peak MNI: 39, 8, 38); left inferior parietal lobule (IPL, peak MNI: −54, −40, 53), right IPL (peak MNI: 51, −28, 47), Right temporoparietal junction (TPJ, peak MNI: 48, −52, 31), left TPJ (−57, −61, 26), perigenual anterior cingulate cortex (pgACC, peak MNI: 9, 50, 1), ACC (peak MNI: −3, 20, 22), ACC (peak MNI: −5, 23, 28), anterior middle cingulate cortex (peak MNI: 0, 34, 16), dorsal medial prefrontal cortex (peak MNI: −9, 44, 41), left insula (peak MNI: −30, 16, 18), and right insula (peak MNI: 36, 30, −8). Following ANOVAs and correlation analyses were the same as GLM1.
Functional connectivity analysis
To examine the effects of TSD on reciprocity behavior depending not only on neural activities but also functional connectivities between brain regions, we performed PPI55 using precuneus (peak MNI: −9, −64, 35), TPJ (peak MNI: 48, −52, 31) and DLPFC (peak MNI: 39, 8, 38) that were shown related to reciprocity behavior in the above analysis results as seed regions. Each seed was defined as a sphere with a radius of 3 mm centered on the coordinates of the peak point. Functional linkage time series were extracted using GLM1 design matrix, which mainly included three regressors: (1) the BOLD signal time course from the seed region (physiological regressor); (2) task-related encoding activation (psychological regressor). Two psychological regressors were defined to access the strength of functional connectivity between the seed region and each voxel in the whole-brain in Repayment possible and Repayment impossible conditions respectively: Repayment possible condition vs. baseline activation (fixation screen), and Repayment impossible condition vs. baseline activation; (3) the PPI term, reflecting the product of the deconvolved time course in the seed regions with a vector representing the order of the psychological variables of interest (PPI regressor). The model also included the regressors of six movement-related covariates to minimize the influence of head movement on the results. These matrices were defined separately for each Sleep manipulation (TSD and SR sessions). At second-level analysis, the PPI contrast maps of the four conditions (2 Sleep manipulation × 2 Condition) were fed into a 2 × 2 flexible factorial analysis to identify regions that showed significant main effect of Sleep manipulation, main effect of Condition and interaction effect, respectively. All results were corrected for multiple comparisons using cluster correction p < 0.05 with a cluster-forming threshold of p < 0.001, which attempts to control for FWE using Gaussian Random Field Theory.98 To examine brain-behavior associations, we analyzed the relationship between the functional connectivity of each seed region and the relative sensitivity to feelings of communal concern (parameter φ) during reciprocity using Pearson’s correlation analyses. All variables were normalized before Pearson’s correlation analyses. Normalized functional connectivity (FC) on a particular sleep session was calculated as FCTSD/SR- (FCTSD+ FCSR)/2.
Acknowledgments
We thank Dr. Xiao Lin, Dr. Yundong Ma, Dr. Ran Zhu for their comments and suggestions on this article. We also thank the study participants. This work was supported by the National Key Research and Development Program of China (2021YFF0306500), the National Natural Science Foundation of China (81971235, 81771429, 82371491, and 32371094), the Young Elite Scientists Sponsorship Program by China Association for Science and Technology (YESS20210176 and 2021QNRC001), the Research Project of Shanghai Science and Technology Commission (20dz2260300) and the Fundamental Research Funds for the Central Universities.
Author contributions
H.S., W.Y., X.G., and J.C. designed the experiment. W.Y., J.C., and Z.K. prepared and conducted the experiment. W.Y. and X.G. analyzed the data. W.Y. wrote the paper. J.C., X.G., W.S., L.L., X.Z., and and H.S. reviewed and edited the manuscript. All authors contributed to and have approved the final manuscript.
Declaration of interests
The authors declare no conflicts of interest.
Published: February 7, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109155.
Contributor Information
Xiaoxue Gao, Email: xxgao@psy.ecnu.edu.cn.
Hongqiang Sun, Email: sunhq@bjmu.edu.cn.
Supplemental information
References
- 1.Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol. 2019;19:702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- 2.Clement-Carbonell V., Portilla-Tamarit I., Rubio-Aparicio M., Madrid-Valero J.J. Sleep Quality, Mental and Physical Health: A Differential Relationship. Int. J. Environ. Res. Publ. Health. 2021;18:460. doi: 10.3390/ijerph18020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay A., Sigua N.L. What Is Sleep Deprivation? Am. J. Respir. Crit. Care Med. 2019;199:P11–P12. doi: 10.1164/rccm.1996P11. [DOI] [PubMed] [Google Scholar]
- 4.Hafner M., Stepanek M., Taylor J., Troxel W.M., van Stolk C. Why Sleep Matters-The Economic Costs of Insufficient Sleep: A Cross-Country Comparative Analysis. Rand Health Q. 2017;6:11. [PMC free article] [PubMed] [Google Scholar]
- 5.Waters F., Bucks R.S. Neuropsychological effects of sleep loss: implication for neuropsychologists. J. Int. Neuropsychol. Soc. 2011;17:571–586. doi: 10.1017/S1355617711000610. [DOI] [PubMed] [Google Scholar]
- 6.Abrams R.M. Sleep Deprivation. Obstet. Gynecol. Clin. N. Am. 2015;42:493–506. doi: 10.1016/j.ogc.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Ben Simon E., Vallat R., Barnes C.M., Walker M.P. Sleep Loss and the Socio-Emotional Brain. Trends Cognit. Sci. 2020;24:435–450. doi: 10.1016/j.tics.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Guadagni V., Burles F., Ferrara M., Iaria G. The effects of sleep deprivation on emotional empathy. J. Sleep Res. 2014;23:657–663. doi: 10.1111/jsr.12192. [DOI] [PubMed] [Google Scholar]
- 9.Palmer C.A., John-Henderson N.A., Bawden H., Massey A., Powell S.L., Hilton A., Carter J.R. Sleep Restriction Reduces Positive Social Emotions and Desire to Connect with Others. Sleep. 2023;46 doi: 10.1093/sleep/zsac265. [DOI] [PubMed] [Google Scholar]
- 10.Ben Simon E., Walker M.P. Sleep loss causes social withdrawal and loneliness. Nat. Commun. 2018;9:3146. doi: 10.1038/s41467-018-05377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Simon E., Vallat R., Rossi A., Walker M.P. Sleep loss leads to the withdrawal of human helping across individuals, groups, and large-scale societies. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes C.M., Schaubroeck J., Huth M., Ghumman S. Lack of sleep and unethical conduct. Organ. Behav. Hum. Decis. Process. 2011;115:169–180. doi: 10.1016/j.obhdp.2011.01.009. [DOI] [Google Scholar]
- 13.Christian M.S., Ellis A.P.J. Examining the effects of sleep deprivation on workplace deviance: a self-regulatory perspective. Acad. Manag. J. 2011;54:913–934. [Google Scholar]
- 14.Dickinson D.L., McElroy T. Sleep restriction and circadian effects on social decisions. Eur. Econ. Rev. 2017;97:57–71. doi: 10.1016/j.euroecorev.2017.05.002. [DOI] [Google Scholar]
- 15.Lin Y., Hu P., Mai Z., Jiang T., Mo L., Ma N. Sleep Deprivation Impairs Cooperative Behavior Selectively: Evidence from Prisoner’s and Chicken Dilemmas. Nat. Sci. Sleep. 2020;12:29–37. doi: 10.2147/NSS.S237402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gervasi D., Faldetta G., Pellegrini M.M., Maley J. Reciprocity in organizational behavior studies: A systematic literature review of contents, types, and directions. Eur. Manag. J. 2022;40:441–457. doi: 10.1016/j.emj.2021.07.008. [DOI] [Google Scholar]
- 17.Wang R., Chen H., Liu Y., Lu Y., Yao Y. Neighborhood social reciprocity and mental health among older adults in China: the mediating effects of physical activity, social interaction, and volunteering. BMC Publ. Health. 2019;19:1036. doi: 10.1186/s12889-019-7385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano A., Saral A.S., Wu J. Direct and indirect reciprocity among individuals and groups. Curr. Opin. Psychol. 2022;43:254–259. doi: 10.1016/j.copsyc.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Salazar M., Joel Shaw D., Czekóová K., Staněk R., Brázdil M. The role of generalised reciprocity and reciprocal tendencies in the emergence of cooperative group norms. J. Econ. Psychol. 2022;90 doi: 10.1016/j.joep.2022.102520. [DOI] [Google Scholar]
- 20.Krebs D. Empathy and altruism. J. Pers. Soc. Psychol. 1975;32:1134–1146. doi: 10.1037//0022-3514.32.6.1134. [DOI] [PubMed] [Google Scholar]
- 21.Molm L.D. The Structure of Reciprocity. Soc. Psychol. Q. 2010;73:119–131. [Google Scholar]
- 22.Cialdini R.B. Second edition. Scott Foresman; 1988. Influence: Science and Practice. [Google Scholar]
- 23.Flynn F.J., Yu A. Better to give than reciprocate? Status and reciprocity in prosocial exchange. J. Pers. Soc. Psychol. 2021;121:115–136. doi: 10.1037/pspi0000349. [DOI] [PubMed] [Google Scholar]
- 24.Sen S., Crawford C., Dees A., Nanda Kumar R., Hale J. Effects of parity, sympathy and reciprocity in increasing social welfare. Knowl. Eng. Rev. 2020;35:e31. doi: 10.1017/S0269888920000120. [DOI] [Google Scholar]
- 25.van Dijk E., De Dreu C.K.W. Experimental Games and Social Decision Making. Annu. Rev. Psychol. 2021;72:415–438. doi: 10.1146/annurev-psych-081420-110718. [DOI] [PubMed] [Google Scholar]
- 26.Anderson C., Dickinson D.L. Bargaining and trust: the effects of 36-h total sleep deprivation on socially interactive decisions. J. Sleep Res. 2010;19:54–63. doi: 10.1111/j.1365-2869.2009.00767.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellsworth P.C., Scherer K.R. Handbook of affective sciences. Series in affective science. Oxford University Press; 2003. Appraisal processes in emotion; pp. 572–595. [Google Scholar]
- 28.Frijda N.H. The place of appraisal in emotion. Cognit. Emot. 1993;7:357–387. doi: 10.1080/02699939308409193. [DOI] [Google Scholar]
- 29.Frijda N.H., Kuipers P., ter Schure E. Relations among emotion, appraisal, and emotional action readiness. Journal of Personality and Social Psychology. 1989;57:212–228. doi: 10.1037/0022-3514.57.2.212. [DOI] [Google Scholar]
- 30.Lazarus R.S., Smith C.A. Knowledge and appraisal in the cognition-emotion relationship. Cognit. Emot. 1988;2:281–300. doi: 10.1080/02699938808412701. [DOI] [Google Scholar]
- 31.Smith C.A., Ellsworth P.C. Patterns of cognitive appraisal in emotion. J. Pers. Soc. Psychol. 1985;48:813–838. doi: 10.1037/0022-3514.48.4.813. [DOI] [PubMed] [Google Scholar]
- 32.Malmendier U., te Velde V.L., Weber R.A. Rethinking Reciprocity. Annu. Rev. Econom. 2014;6:849–874. [Google Scholar]
- 33.van Baar J.M., Chang L.J., Sanfey A.G. The computational and neural substrates of moral strategies in social decision-making. Nat. Commun. 2019;10:1483. doi: 10.1038/s41467-019-09161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X., Jolly E., Yu H., Liu H., Zhou X., Chang L.J. The psychological, computational, and neural foundations of indebtedness. Nat. Commun. 2024;15:68. doi: 10.1038/s41467-023-44286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benedict R. Houghton Mifflin; 1946. The chrysanthemum and the Sword: Patterns of Japanese Culture. [Google Scholar]
- 36.Kotani M. Expressing Gratitude and Indebtedness: Japanese Speakers’ Use of “I’m Sorry” in English Conversation. Res. Lang. Soc. Interact. 2002;35:39–72. doi: 10.1207/S15327973RLSI35-1_2. [DOI] [Google Scholar]
- 37.Naito T., Washizu N. Note on Cultural Universals and Variations of Gratitude from an East Asian Point of View. International Journal of Behavioral Science. 2015;10:1–8. [Google Scholar]
- 38.Washizu N., Naito T. The emotions sumanai, gratitude, and indebtedness, and their relations to interpersonal orientation and psychological well-being among Japanese university students. Int. Perspect. Psychol. 2015;4:209–222. doi: 10.1037/ipp0000037. [DOI] [Google Scholar]
- 39.Le B.M., Impett E.A., Lemay E.P., Muise A., Tskhay K.O. Communal motivation and well-being in interpersonal relationships: An integrative review and meta-analysis. Psychol. Bull. 2018;144:1–25. doi: 10.1037/bul0000133. [DOI] [PubMed] [Google Scholar]
- 40.Baumeister R.F., Stillwell A.M., Heatherton T.F. Guilt: an interpersonal approach. Psychol. Bull. 1994;115:243–267. doi: 10.1037/0033-2909.115.2.243. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg M.S. In: Social Exchange: Advances in Theory and Research. Gergen K.J., Greenberg M.S., Willis R.H., editors. Springer US); 1980. A Theory of Indebtedness; pp. 3–26. [DOI] [Google Scholar]
- 42.Greenberg M.S., Westcott . New Directions in Helping. 1983. Indebtedness as a mediator of reactions to aid; pp. 85–112. [Google Scholar]
- 43.Watkins P., Scheer J., Ovnicek M., Kolts R. The debt of gratitude: Dissociating gratitude and indebtedness. Cognit. Emot. 2006;20:217–241. doi: 10.1080/02699930500172291. [DOI] [Google Scholar]
- 44.Naito T., Sakata Y. Gratitude, Indebtedness, and Regret on Receiving a Friend’s Favor in Japan. Psychologia. 2010;53:179–194. doi: 10.2117/psysoc.2010.179. [DOI] [Google Scholar]
- 45.Tsang J.A. The Effects of Helper Intention on Gratitude and Indebtedness. Motiv. Emot. 2006;30:198–204. doi: 10.1007/s11031-006-9031-z. [DOI] [Google Scholar]
- 46.Rotella A., Sparks A.M., Barclay P. Feelings of obligation are valuations of signaling-mediated social payoffs. Behav. Brain Sci. 2020;43:e85. doi: 10.1017/S0140525X19002322. [DOI] [PubMed] [Google Scholar]
- 47.Tomasello M. The moral psychology of obligation. Behav. Brain Sci. 2019;43:e56. doi: 10.1017/S0140525X19001742. [DOI] [PubMed] [Google Scholar]
- 48.Beeler-Duden S., Yucel M., Vaish A. The role of affect in feelings of obligation. Behav. Brain Sci. 2020;43:e60. doi: 10.1017/S0140525X19002449. [DOI] [PubMed] [Google Scholar]
- 49.Theriault J.E., Young L., Barrett L.F. The sense of should: A biologically-based framework for modeling social pressure. Phys. Life Rev. 2021;36:100–136. doi: 10.1016/j.plrev.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grèzes J., Erblang M., Vilarem E., Quiquempoix M., Van Beers P., Guillard M., Sauvet F., Mennella R., Rabat A. Impact of total sleep deprivation and related mood changes on approach-avoidance decisions to threat-related facial displays. Sleep. 2021;44:zsab186. doi: 10.1093/sleep/zsab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amft M., Bzdok D., Laird A.R., Fox P.T., Schilbach L., Eickhoff S.B. Definition and characterization of an extended social-affective default network. Brain Struct. Funct. 2015;220:1031–1049. doi: 10.1007/s00429-013-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bzdok D., Schilbach L., Vogeley K., Schneider K., Laird A.R., Langner R., Eickhoff S.B. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012;217:783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H., Gao X., Zhou Y., Zhou X. Decomposing Gratitude: Representation and Integration of Cognitive Antecedents of Gratitude in the Brain. J. Neurosci. 2018;38:4886–4898. doi: 10.1523/JNEUROSCI.2944-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H., Hu J., Hu L., Zhou X. The voice of conscience: neural bases of interpersonal guilt and compensation. Soc. Cognit. Affect Neurosci. 2014;9:1150–1158. doi: 10.1093/scan/nst090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 56.Peng C., Nelissen R.M.A., Zeelenberg M. Reconsidering the roles of gratitude and indebtedness in social exchange. Cognit. Emot. 2018;32:760–772. doi: 10.1080/02699931.2017.1353484. [DOI] [PubMed] [Google Scholar]
- 57.Chang L.J., Smith A., Dufwenberg M., Sanfey A.G. Triangulating the neural, psychological, and economic bases of guilt aversion. Neuron. 2011;70:560–572. doi: 10.1016/j.neuron.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher J.D., Nadler A., DePaulo B.M. Academic Press; 1983. New Directions in Helping. [Google Scholar]
- 59.Gazes Y., Rakitin B.C., Steffener J., Habeck C., Lisanby S.H., Butterfield B., Basner R.C., Ghez C., Stern Y. Dual-tasking alleviated sleep deprivation disruption in visuomotor tracking: An fMRI study. Brain Cognit. 2012;78:248–256. doi: 10.1016/j.bandc.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishida M., Kikuchi S., Miwakeichi F., Suda S. Night duty and decreased brain activity of medical residents: a wearable optical topography study. Med. Educ. Online. 2017;22 doi: 10.1080/10872981.2017.1379345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harenski C.L., Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 63.Lambert B., Declerck C.H., Emonds G., Boone C. Trust as commodity: social value orientation affects the neural substrates of learning to cooperate. Soc. Cognit. Affect Neurosci. 2017;12:609–617. doi: 10.1093/scan/nsw170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng C., Deshpande G., Liu C., Gu R., Luo Y.-J., Krueger F. Diffusion of responsibility attenuates altruistic punishment: A functional magnetic resonance imaging effective connectivity study. Hum. Brain Mapp. 2016;37:663–677. doi: 10.1002/hbm.23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emonds G., Declerck C.H., Boone C., Vandervliet E.J.M., Parizel P.M. Comparing the neural basis of decision making in social dilemmas of people with different social value orientations, a fMRI study. Journal of Neuroscience, Psychology, and Economics. 2011;4:11–24. doi: 10.1037/a0020151. [DOI] [Google Scholar]
- 66.Bastin C., Rakesh D., Harrison B.J., Davey C.G., Allen N.B., Muller S., Whittle S. Feelings of shame and guilt are associated with distinct neural activation in youth. Biol. Psychol. 2021;159 doi: 10.1016/j.biopsycho.2021.108025. [DOI] [PubMed] [Google Scholar]
- 67.Morey R.A., McCarthy G., Selgrade E.S., Seth S., Nasser J.D., LaBar K.S. Neural systems for guilt from actions affecting self versus others. Neuroimage. 2012;60:683–692. doi: 10.1016/j.neuroimage.2011.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Bos W., van Dijk E., Westenberg M., Rombouts S.A.R.B., Crone E.A. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Soc. Cognit. Affect Neurosci. 2009;4:294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mai X., Zhang W., Hu X., Zhen Z., Xu Z., Zhang J., Liu C. Using tDCS to Explore the Role of the Right Temporo-Parietal Junction in Theory of Mind and Cognitive Empathy. Front. Psychol. 2016;7:380. doi: 10.3389/fpsyg.2016.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mano Y., Harada T., Sugiura M., Saito D.N., Sadato N. Perspective-taking as part of narrative comprehension: a functional MRI study. Neuropsychologia. 2009;47:813–824. doi: 10.1016/j.neuropsychologia.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Pegado F., Hendriks M.H.A., Amelynck S., Daniels N., Bulthé J., Masson H.L., Boets B., de Beeck H.O. Neural Representations Behind ‘Social Norm’ Inferences In Humans. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahmoodi A., Nili H., Bang D., Mehring C., Bahrami B. Distinct neurocomputational mechanisms support informational and socially normative conformity. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan L., Feng Q., Xu P. Using Functional Near-Infrared Spectroscopy to Assess Brain Activation Evoked by Guilt and Shame. Front. Hum. Neurosci. 2020;14:197. doi: 10.3389/fnhum.2020.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong W., Gao X., He Z., Yu H., Liu H., Zhou X. Affective evaluation of others’ altruistic decisions under risk and ambiguity. Neuroimage. 2020;218 doi: 10.1016/j.neuroimage.2020.116996. [DOI] [PubMed] [Google Scholar]
- 75.Liu G., Zeng G., Wang F., Rotshtein P., Peng K., Sui J. Praising others differently: neuroanatomical correlates to individual differences in trait gratitude and elevation. Soc. Cognit. Affect Neurosci. 2018;13:1225–1234. doi: 10.1093/scan/nsy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 77.Nihonsugi T., Ihara A., Haruno M. Selective increase of intention-based economic decisions by noninvasive brain stimulation to the dorsolateral prefrontal cortex. J. Neurosci. 2015;35:3412–3419. doi: 10.1523/JNEUROSCI.3885-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu R., Feng C., Zhang S., Mai X., Liu C. Differentiating guilt and shame in an interpersonal context with univariate activation and multivariate pattern analyses. Neuroimage. 2019;186:476–486. doi: 10.1016/j.neuroimage.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S., Li C.-S.R. Functional Connectivity Parcellation of the Human Thalamus by Independent Component Analysis. Brain Connect. 2017;7:602–616. doi: 10.1089/brain.2017.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pauly K., Seiferth N.Y., Kellermann T., Backes V., Vloet T.D., Shah N.J., Schneider F., Habel U., Kircher T.T. Cerebral dysfunctions of emotion-cognition interactions in adolescent-onset schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1299–1310. doi: 10.1097/CHI.0b013e318184ff16. [DOI] [PubMed] [Google Scholar]
- 81.Sturm V.E., Perry D.C., Wood K., Hua A.Y., Alcantar O., Datta S., Rankin K.P., Rosen H.J., Miller B.L., Kramer J.H. Prosocial deficits in behavioral variant frontotemporal dementia relate to reward network atrophy. Brain Behav. 2017;7 doi: 10.1002/brb3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krause A.J., Simon E.B., Mander B.A., Greer S.M., Saletin J.M., Goldstein-Piekarski A.N., Walker M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng C., Feng X., Wang L., Wang L., Gu R., Ni A., Deshpande G., Li Z., Luo Y.-J. The neural signatures of egocentric bias in normative decision-making. Brain Imaging Behav. 2019;13:685–698. doi: 10.1007/s11682-018-9893-1. [DOI] [PubMed] [Google Scholar]
- 84.Moll J., de Oliveira-Souza R., Eslinger P.J., Bramati I.E., Mourão-Miranda J., Andreiuolo P.A., Pessoa L. The Neural Correlates of Moral Sensitivity: A Functional Magnetic Resonance Imaging Investigation of Basic and Moral Emotions. J. Neurosci. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee Y.-J.G., Kim S., Kim N., Choi J.-W., Park J., Kim S.J., Gwak A.R., Lee Y.J. Changes in subcortical resting-state functional connectivity in patients with psychophysiological insomnia after cognitive-behavioral therapy: Changes in resting-state FC after CBT for insomnia patients. Neuroimage. Clin. 2018;17:115–123. doi: 10.1016/j.nicl.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Z., Bennett M., Orr C., Icke I., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Büchel C., Quinlan E.B., et al. Mapping adolescent reward anticipation, receipt, and prediction error during the monetary incentive delay task. Hum. Brain Mapp. 2019;40:262–283. doi: 10.1002/hbm.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao J., Tomasi D., Wiers C.E., Shokri-Kojori E., Demiral Ş.B., Zhang Y., Volkow N.D., Wang G.-J. Correlation between Traits of Emotion-Based Impulsivity and Intrinsic Default-Mode Network Activity. Neural Plast. 2017;2017 doi: 10.1155/2017/9297621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De la Peña-Arteaga V., Fernández-Rodríguez M., Silva Moreira P., Abreu T., Portugal-Nunes C., Soriano-Mas C., Picó-Pérez M., Sousa N., Ferreira S., Morgado P. An fMRI study of cognitive regulation of reward processing in generalized anxiety disorder (GAD) Psychiatr. Res. Neuroimaging. 2022;324 doi: 10.1016/j.pscychresns.2022.111493. [DOI] [PubMed] [Google Scholar]
- 89.Baird B., Smallwood J., Gorgolewski K.J., Margulies D.S. Medial and Lateral Networks in Anterior Prefrontal Cortex Support Metacognitive Ability for Memory and Perception. J. Neurosci. 2013;33:16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright K.P., Lowry C.A., Lebourgeois M.K. Circadian and wakefulness-sleep modulation of cognition in humans. Front. Mol. Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Dongen H.P.A., Baynard M.D., Maislin G., Dinges D.F. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 92.Ben Simon E., Rossi A., Harvey A.G., Walker M.P. Overanxious and underslept. Nat. Human Behav. 2020;4:100–110. doi: 10.1038/s41562-019-0754-8. [DOI] [PubMed] [Google Scholar]
- 93.Doran S.M., Van Dongen H.P., Dinges D.F. Sustained attention performance during sleep deprivation: evidence of state instability. Arch. Ital. Biol. 2001;139:253–267. doi: 10.4449/aib.v139i3.503. [DOI] [PubMed] [Google Scholar]
- 94.Akerstedt T., Gillberg M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 95.Comtet H., Geoffroy P.A., Kobayashi Frisk M., Hubbard J., Robin-Choteau L., Calvel L., Hugueny L., Viola A.U., Bourgin P. Light therapy with boxes or glasses to counteract effects of acute sleep deprivation. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-54311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J.C.J., Mulick D., Chee M.W.L. Odd one out: social ostracism affects self-reported needs in both sleep-deprived and well-rested persons. J. Sleep Res. 2014;23:448–457. doi: 10.1111/jsr.12141. [DOI] [PubMed] [Google Scholar]
- 97.Cao Y., Yu H., Wu Y., Zhou X. Can money heal all wounds? Social exchange norm modulates the preference for monetary versus social compensation. Front. Psychol. 2015;6:1411. doi: 10.3389/fpsyg.2015.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woo C.-W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this paper will be shared by the lead contact upon request.
Original code in this paper will be shared by the lead contact upon request.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.