Abstract
Abnormalities in impulse generation and transmission are among the first signs of cardiac remodeling in cardiomyopathies. Accordingly, 12-lead electrocardiogram (ECG) of patients with cardiomyopathies may show multiple abnormalities. Some findings are suggestive of specific disorders, such as the discrepancy between QRS voltages and left ventricular (LV) mass for cardiac amyloidosis or the inverted T waves in the right precordial leads for arrhythmogenic cardiomyopathy. Other findings are less sensitive and/or specific, but may orient toward a specific diagnosis in a patient with a specific phenotype, such as an increased LV wall thickness or a dilated LV. A “cardiomyopathy-oriented” mindset to ECG reading is important to detect the possible signs of an underlying cardiomyopathy and to interpret correctly the meaning of these alterations, which differs in patients with cardiomyopathies or other conditions.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10741-023-10358-7.
Keywords: ECG, Electrocardiogram, Cardiomyopathy, Hypertrophic cardiomyopathy, Amyloidosis
Introduction
Abnormalities in impulse generation and transmission are among the first signs of cardiac remodeling in cardiomyopathies. These abnormalities manifest as changes on the 12-lead electrocardiogram (ECG). The ECG may be a source of red flags for diagnosis, may help to define the disease stage and predict patient outcomes, and sometimes even suggest specific genetic backgrounds. Herein, we will provide an overview of the ECG findings in patients with cardiomyopathies and their clinical significance. We will focus on ECG findings in adult patients, as cardiomyopathies in children would require a dedicated paper. The following “cardiomyopathy-oriented” approach to ECG will refer to specific diseases more than the large nosographic categories proposed by new Guidelines [1]. Most notably, the non-dilated left ventricular (LV) cardiomyopathy will not be considered as a clinical entity, to better characterize its various manifestations (e.g., laminopathies, neuromuscular disease, and sarcoidosis). Furthermore, LV non-compaction (LVNC) will be still regarded as a specific disorder rather than as a “hypertrabeculated” phenotype associated with other forms of cardiomyopathy, as recently proposed [1].
P wave and PR interval
P wave abnormalities
Structural atrial disease may alter P wave duration or morphology. Patients with hypertrophic cardiomyopathy (HCM) have longer P wave duration (92% of cases) and greater P wave dispersion than healthy subjects [2]. P wave duration was associated with a more severe HCM phenotype and left atrial (LA) electromechanical delay, while P wave dispersion with a more severe diastolic dysfunction and mitral regurgitation [2]. Among patients with dilated cardiomyopathy (DCM), 72% had signs of LA enlargement and 15% of right atrial (RA) enlargement [3].
Long PR interval and atrioventricular blocks
Expansion of extracellular spaces or the accumulation of intracellular material may lead to a prolongation of PR interval and atrioventricular (AV) blocks.
In cardiac amyloidosis (CA), the ECG can show first- (21%), second-, or third-degree AV block (3%) [4]. The incidence of symptomatic AV block is higher in amyloid transthyretin (ATTR)—than light-chain (AL) CA (first-degree AV block was present in 18% of those with AL-CA, but up to 33% of those with ATTRwt-CA and 25% for ATTRv [5], possibly because these patients are older and have a greater amyloid burden and longer survival.
The cardiac phenotype of neuromuscular diseases is highly variable. Cardiac involvement is found in 80% of patients with myotonic dystrophy (DM) type 1 and 10–20% of patients with DM type 2. Furthermore, up to 20% of patients with DM type 1 develop progressive AV or intraventricular conduction defects and ventricular or supraventricular arrhythmias [6]. Conduction defects in DM type 2 are usually limited to first-degree AV or bundle branch block (BBB), but life-threatening arrhythmias and sudden cardiac death (SCD) have been reported [7]. Conduction defects ranging from sinus bradycardia, prolongation of the PR interval, to complete heart block can also be detected in Emery-Dreifuss cardiomyopathy [8].
Laminopathies are caused by LMNA gene mutations. Up to 92% of patients > 30 years have arrhythmias, including ventricular ectopic beats, non-sustained ventricular tachycardia, and first-degree AV block (which may progress to more advanced AV blocks) [9]. AV block is due to fibrosis in the AV node. Even asymptomatic mutation carriers with preserved or only mildly decreased LV contractility or patients with minor conduction defects have a high risk of SCD [10].
In cardiac sarcoidosis (CS), AV blocks may develop because of granulomas affecting the conduction system; in a prospective study on middle-aged patients with unexplained AV block, 34% had CS [11]. Iron chelating therapy has substantially reduced the risk of AV blocks in patients with hemochromatosis [12]. First-degree and advanced AV block are found in 3–25% of patients with LVNC) [13].
Short PR interval
A PR interval < 120 ms may denote accelerated conduction through the AV node or accessory pathways. Disease progression may lead to the development of AV blocks.
Anderson-Fabry disease (AFD) is characterized by glycosphingolipid accumulation increasing conduction velocity. AFD should be suspected in patients with LV hypertrophy (LVH) and a short PR interval. Similarly, a short PR interval, prolonged QRS duration, right BBB (RBBB), R in aVL ≥ 1.1 mV, and ST depression in the inferior leads may help differentiate AFD from HCM [14]. Progressive glycosphingolipid deposition can ultimately lead to AV blocks. First-degree AV block disappearance following enzyme replacement therapy (ERT) was reported [15].
Several studies reported abnormalities within the AV node and His bundle in glycogen storage disorders, including the presence of fasciculoventricular pathways possibly due to disruption of the physiological conduction because of glycogen-filled myocytes [16]. Danon disease is due to deficient lysosome-associated membrane protein 2. A pattern of ventricular pre-excitation is found in over 70% of cases. These patients have also a high risk of atrial and ventricular arrhythmias and SCD even at a young age [17]. Pompe disease is an autosomal recessive disease caused by a deficiency in the lysosomal enzyme alpha-1, 4 glucosidase. LVH is found in 12% of patients and a short PR interval in 10% [18]. ERT leads to increased PR interval and decreased LV voltages in children [19], but not in adults [18]. PRKAG2 syndrome is caused by abnormalities in the Ras/MAPK pathway causing glycogen accumulation within the cardiomyocytes. The most common ECG findings are a short PR interval (in 68% of patients), a BBB (mainly RBBB), abnormal QRS morphology, intraventricular conduction delays > 120 ms, and, in the later disease stages, advanced AV or sinoatrial blocks [20]. These patients have a higher risk of SCD, probably due to high-degree AV block or fast conduction of supraventricular tachyarrhythmias through accessory pathways [20].
Mitochondrial diseases are due to mitochondrial or nuclear DNA mutations. In a small cohort, 68% had an abnormal ECG, and 22% of them presented a pre-excitation pattern [21]. AV block is common in Kearns-Sayre syndrome, possibly because of a higher mutation load in the AV node [22].
Atrial fibrillation
Atrial fibrillation (AF) often complicates the course of inherited cardiomyopathies and may be the presenting feature [23].
AF is found in 17 to 30% of patients with HCM, most commonly in elderly patients and those with LV outflow obstruction [24, 25]. Atrial enlargement and fibrosis are associated with a higher risk of AF [23, 26]. Patients with HCM and AF have higher risk of all-cause mortality and cardiac deaths compared to HCM controls without AF, with an increased incidence of SCD and HF- and stroke-related death [27].
The prevalence of AF in familial DCM ranges between 36 and 76% [23, 24, 28–30]. The prevalence of AF in familial DCM is close to the prevalence of non-familial form [31, 32].
Patients with right-sided arrhythmogenic cardiomyopathy (ACM) show AF in 9 to 30% of cases [33, 34]. AF is likely a consequence of atrial involvement due to desmosomal dysfunction, RA enlargement and dysfunction, or both. Atrial conduction abnormalities have been found in right-sided ACM, resulting in P wave alterations independent from RV morphological anomalies [35]. Furthermore, AF predicts worse outcomes in patients with right-sided ACM [36].
AF has been reported in 1 to 29% of patients with LVNC, with a lower incidence in children than in adults [23, 24, 37, 38]. AF is associated with LVNC severity and predicts survival [39, 40].
The prevalence of AF in patients with CA ranges between 15 and 44% and is higher in ATTR-than AL-CA. Cardiac amyloid infiltration causes ventricular wall thickening and diastolic dysfunction, leading to atrial dilation and predisposing to AF [41, 42]. Amyloid deposition promotes atrial fibrosis and remodeling, further increasing the risk of AF [43]. AF does not seem to predict a worse outcome [44, 45].
Q waves
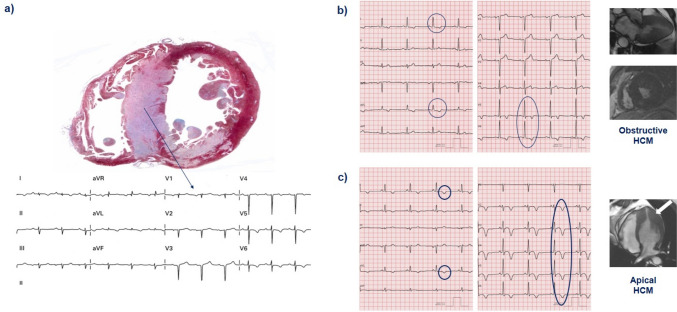
Q waves are found in 18 to 53% of patients with HCM [46]. The possible mechanisms are a loss of local electrical forces due to transmural fibrosis (Fig. 1a) or an initial displacement of the QRS vector due to a disproportionate thickening of the basal interventricular septum and/or basal LV free wall. Q waves may precede LV mass increase by several years and are less common in patients with biventricular hypertrophy [47].
Fig. 1.
Possible electrocardiographic findings in patients with left ventricular hypertrophy. a 72-year-old man with transthyretin amyloidosis experiencing sudden cardiac death. The explanted heart shows massive septal hypertrophy, explaining the poor R wave progression and QS complexes in V2–V3. Negative T waves in the antero-lateral leads: b 55-year-old man with obstructive hypertrophic cardiomyopathy (HCM) and c 49-year-old man with apical HCM
Q waves in DCM reflect vector displacement due to LV dilation and transmural fibrosis and were reported in 10 to 25% of patients [3, 48].
Q waves in CA are likely associated with the accumulation of amyloid and fibrosis. CA should be suspected when Q waves, particularly in the anterior leads, coexist with low QRS voltages or there is a discrepancy between QRS voltages and LV mass. Q waves seem to be more common in patients with AL-CA, being reported in 25 [49] to 47% [50], than in ATTR-CA (18%) [49].
QRS complex: high voltages
More than 30 ECG criteria to diagnose LVH have been developed, which are neither sensitive nor specific to detect LVH [51], particularly in cardiomyopathies, where the mechanism of increased LV mass is often different from the expansion of conductive tissues. ECG criteria for RV hypertrophy (RVH) have been proposed, but have poor sensitivity, likely because, for RVH to be manifested on the ECG, it must be severe enough to overcome the concealing effects of the larger LV forces [52]. ECG criteria for LVH are met by 41 [53] to 60% [46] of patients with HCM (Fig. 1b). Interestingly, LVH patterns such as increased voltages in the precordial leads and deep Q waves may precede LV mass increase in mutation carriers, possibly reflecting myocardial disarray, interstitial fibrosis, and microvascular remodeling [54]. In patients with established HCM, a single positive criterion for LVH is very rare. ECG criteria for LVH hold prognostic significance, and a score including QRS amplitude was proposed to predict SCD [55]. Anecdotal experiences suggest that a 6-week therapy with the myosin inhibitor mavacamten relieves ECG signs of LVH (Supplemental Fig. 1), possibly reflecting a restoration of the electrical properties of cardiomyocytes.
ECG criteria for LVH are met in 17 to 69% of patients with DCM [56, 57]. LVH is infrequent in ACM because of the progressive fibro-fatty replacement of the myocardium which often results in low QRS voltages on the 12-lead ECG.
Patients with CA have usually no ECG features of LVH, but normal or increased ECG voltages can be found in up to 25% of patients with ATTR-CA. The LV apex and the periapical segments are relatively spared by amyloid accumulation and may still generate sufficiently high voltages in leads V3 and V4 to meet ECG criteria for LVH [58].
ECG features of LVH are rare in some storage disorders (e.g., 6–10% in Pompe disease) [18, 19] and more common in others, such as Danon and Fabry disease, because of increased LV electrical mass due to glycosphingolipid accumulation driving cell enlargement [59]. Patients with Danon disease often exhibit particularly prominent QRS complexes [59]. ECG voltage criteria of left or biventricular hypertrophy are detected in > 40% of patients with LVNC [13].
QRS complex: low voltages
The most common definition of low QRS voltages is a nadir-to-zenith QRS amplitude in all peripheral leads ≤ 0.5 mV and ≤ 1 mV in all precordial leads. Low QRS voltages may be associated with cardiomyocyte loss and/or expansion of extracellular spaces by electrically inert tissue such as fibrosis, fat, or amyloid. Low QRS voltages, including cases where this feature is isolated, may be an expression of early-stage cardiomyopathy deserving further investigation [60]. Low QRS voltages may also be due to pericardial effusion or extra-cardiac conditions such as obesity or emphysema.
Low QRS voltages are rare in patients with HCM (< 3%) and can be seen in some patients with end-stage disease due to the extensive fibrosis [61]. Low QRS voltages predict SCD [61], but not a combination of all-cause death, major non-fatal arrhythmias, hospitalization for heart failure (HF), and stroke after adjustment for the main demographic and clinical variables [62].
Only 3–6% of patients with DCM have low QRS voltages and about 1.5% in both precordial and peripheral leads [3]. Loss of vital myocardium and diffuse LV fibrosis may reduce QRS amplitude, especially in precordial leads [3].
Low QRS voltages in the limb leads are found in 41% of patients with ACM and LV involvement and just in 17% of those without LV involvement [63]. Several studies showed a higher prevalence of low QRS voltages in ACM than in idiopathic RV outflow tract tachycardia [64], athlete’s heart [65], or DCM [63, 66]. The association between low QRS voltages and the extent of fibro-fatty replacement of the LV accounts for the prognostic significance of this finding [67].
Low QRS voltages in peripheral leads are found in 46 to 70% of patients with CA [68]. Low QRS voltages are found more often in patients with AL-CA than in ATTR-CA, but are a risk factor for cardiovascular death in both conditions [69]. As the ventricles are more affected, low QRS voltages may be associated with normal P wave voltages on peripheral leads. Low QRS voltage is also a common finding in non-dilated LV cardiomyopathy caused by DSP (15–44%) and PLN (15%) mutations [70–72].
QRS fragmentation and epsilon wave
QRS fragmentation (defined as the presence of various RSR′ patterns, R or S notching, and/or > 1 additional R wave in any non-aVR lead) is due to heterogenous action potential propagation different from BBB, due to focal alterations such as fibrosis or fibro-fatty replacement.
QRS fragmentation was detected in 75% of patients with HCM [73]. QRS fragmentation in ≥ 3 territories (inferior, lateral, septal, and/or anterior) had an incremental risk of ventricular tachyarrhythmias and SCD beyond conventional risk factors [73]. In DCM, QRS fragmentation has been reported in 23–26% of patients and predicts ventricular tachycardia and all-cause mortality [74], reasonably because of its relationship with the extent of LV fibrosis. In LVNC, the presence of fragmented QRS was associated with a significantly lower survival rate [75].
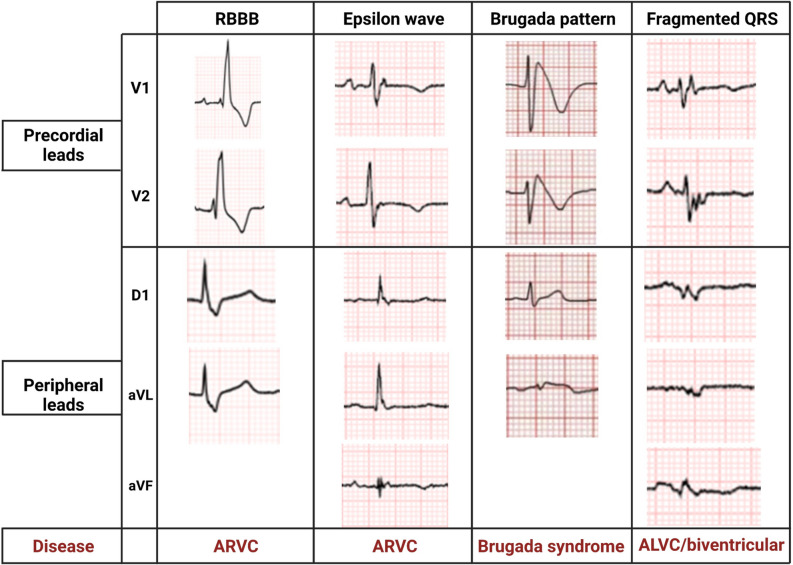
The epsilon wave is a low-voltage deflection between the QRS complex and the ST segment in the right precordial leads (V1–V3) (Supplemental Fig. 2). It is produced by a delayed RV free wall activation in the subepicardial regions due to fibro-fatty replacement. Epsilon waves in right precordial leads are currently classified as minor diagnostic criteria for ACM with RV involvement [66]. Epsilon waves in inferior or lateral peripheral leads may signal advanced stage or LV involvement. Diffuse epsilon waves, particularly if present in aVR, are related to low RV ejection fraction, high rate of HF hospitalization, HF-related death, SCD, and heart transplantation [76]. QRS fragmentation is more sensitive than the epsilon wave (present in about 10–35% of patients with ARVC) to diagnose ACM, but is less specific [77]. QRS fragmentation predicts a higher arrhythmic risk [78].
Bundle branch blocks
Figure 2 provides some examples of RBBB and its possible mimics (fragmented QRS, epsilon wave, and Brugada pattern). The RBBB was reported in 4–5% of patients with HCM [79] and in 7% of patients with DCM [80]. A complete or incomplete RBBB was found in 32% of patients with right-sided ACM (n = 100) [81], while a larger study (n = 374) reported a prevalence of 19% [82]. RBBB may conceal depolarization abnormalities such as epsilon wave in V1 and V2 and QRS fragmentation in V1 making the diagnosis of ACM more challenging.
Fig. 2.
Right bundle branch block and its mimics. ALVC/ARVC, arrhythmogenic left/right ventricular cardiomyopathy
Left BBB (LBBB) is an uncommon finding (detected in 2%) of patients with HCM [83], mainly caused by the contractive asynchrony due to degeneration or fibrotic infiltration of the conduction system, with higher prevalence (22%) in end-stage disease reflecting a severe impairment of LV conduction [84]. LBBB may develop in up to 40% of cases after septal myectomy following damage to LBBB, but has no clear impact on mortality [79].
LBBB is common in patients with DCM, with a reported prevalence of 25–30% [86, 87]. LBBB does not predict mortality in patients with DCM and LV ejection fraction between 36 and 50% [87], while LBBB development independently predicts mortality in patients with idiopathic DCM, suggesting the possible benefit of early cardiac resynchronization therapy [88].
ST segment and T wave
T wave inversion is frequent in HCM (Fig. 1b). Epicardial cardiomyocytes depolarize and repolarize later than endocardial cardiomyocytes, creating a ventricular repolarization vector in the opposite direction from the QRS. Symmetric negative T waves are common in inferolateral leads. Negative T waves > 0.1 mV in all antero-lateral precordial leads suggest apical hypertrophy [89] (Fig. 1c) and could precede the development of LVH detectable at echocardiography or cardiac magnetic resonance. Giant symmetric positive T waves in the precordial leads may be an early manifestation of HCM and could be associated with persistent ST-segment elevation. ST-segment elevation myocardial infarction patterns (ST-segment segment elevation without giant positive T waves, giant positive T waves without ST segment elevation, or both ST segment elevation and giant T waves) are all independently associated with the risk of SCD in HCM [61]. ST-segment depression > 0.2 mV plus T wave inversion in precordial leads and ST-segment depression in high lateral leads (DI-aVL) were also associated with SCD [61].
T wave inversion in the anterior and right precordial leads in individuals with complete pubertal development (in the absence of complete RBBB) are major diagnostic criteria for right-sided ACM, while T wave inversion in leads V1 and V2 only or inverted T waves in V1–V3 and V4 in adult individuals with complete RBBB are minor diagnostic criteria [66]. T wave inversion is caused by fibro-fatty infiltration beginning in the subepicardium, where depolarization and repolarization become more delayed compared to the subendocardium. The J-point preceding the anterior T wave inversion could differentiate between athlete’s heart physiological adaptation and cardiomyopathy [90]. The extent of T wave inversion is related to the amount of fibro-fatty infiltration. Negative T wave depth ≥ 0.2 mV in V1 is strongly related to disease presence with a high negative predictive value [91]. Downsloping ST segment elevation with negative T wave in V1–V2 is related to advanced transmural right ventricular involvement, and negative T waves in inferolateral leads are common in left-sided ACM [92].
AFD can present with asymmetrical negative T waves and ST-T segment depression or elevation in inferolateral leads, and their presence seems related to fibrosis. Finally, CA is often characterized by non-specific repolarization abnormalities such as flattened or shallow T waves. Corrected QT interval prolongation is common in patients with LVNC (> 440 ms in 38%), but its significance is unclear [13].
Importance of a “cardiomyopathy-oriented approach” to ECG reading
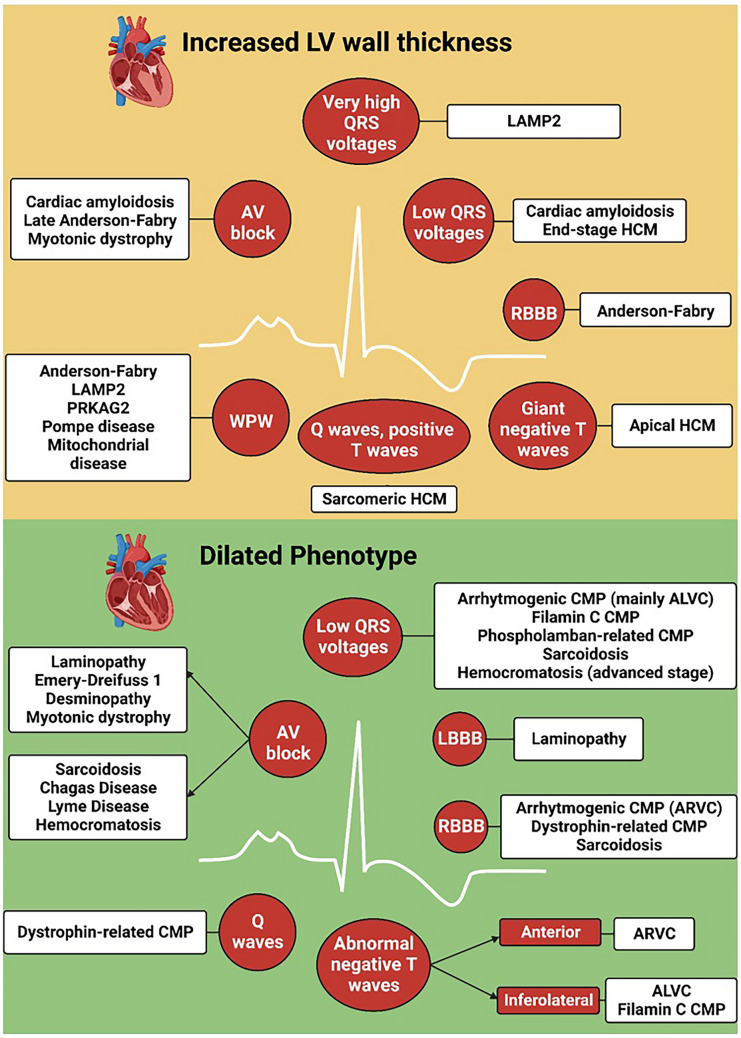
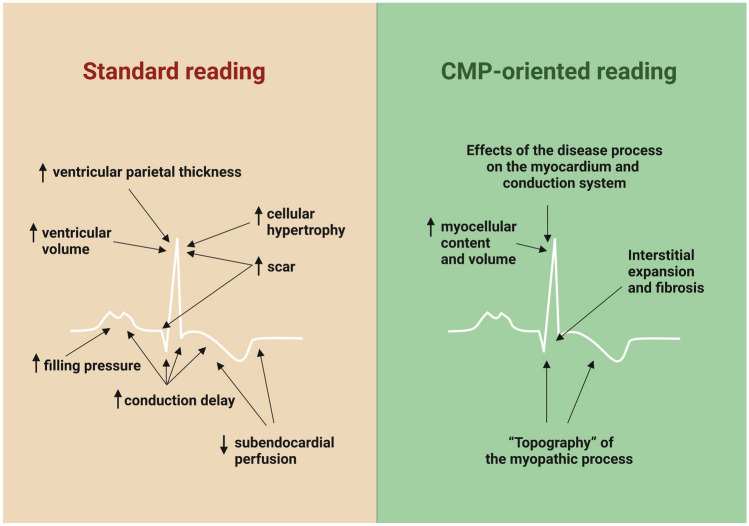
Patients with cardiomyopathies can display multiple ECG abnormalities (Table 1). A “cardiomyopathy-oriented” approach to ECG reading is important to detect the possible signs of an underlying cardiomyopathy and to interpret correctly these abnormalities (Fig. 3). Some findings are specific for certain disorders, such as the discrepancy between QRS voltages and LV mass for CA or the inverted T waves in the right precordial leads for ACM. Other findings are less specific, but may orient toward a specific diagnosis in patients with a hypertrophic or dilated phenotype (Central Illustration). Our understanding of the significance of ECG findings could be improved by studies aimed at correlating ECG with imaging or electrophysiological features, genetic background, and outcome. Another intriguing perspective is the automated interpretation of artificial intelligence (AI), which could assist clinicians in diagnosis, risk stratification, and follow-up. A great effort is needed to assemble large-scale datasets of digital ECG tracings. For diagnostic purposes, these datasets should include patients with a given cardiomyopathy and control individuals, who should be matched as closely as possible to patients (i.e., patients evaluated for a suspicion of the same cardiomyopathy, which was ultimately ruled out), rather than healthy individuals retrieved by institutional registries or patients with other cardiomyopathies. Properly trained AI algorithms could also capture subtle features that may inform on the response to treatment and disease evolution. Overall, the ECG still provides pivotal diagnostic and prognostic information and will probably acquire an even more important role in association with modern technologies such as AI.
Table 1.
ECG findings, their pathogenesis, and meaning in cardiomyopathies
| ECG findings | ||||
|---|---|---|---|---|
| Disease | Pathogenesis/electrogenesis | Prevalence | Disease stage | Prognostic/therapeutic implications |
| P wave | ||||
| HCM | LA and/or RA dilation | 92% [2] | P wave prolongation associated with the severity of HCM phenotype [2] | |
| DCM | LA and/or RA dilation |
72% (if LA dilatation) 15% (if RA dilatation) [3] |
||
| Long PR interval and AV blocks | ||||
| AFD | Accumulation of glycosphingolipids | 6–11% [93] | First-degree AV block may disappear following ERT [15] | |
| CA | Accumulation of amyloid and fibrous tissue |
21% (1st degree), 3% (2nd–3rd degree) [4] Higher prevalence in ATTR-CA (1st degree AV block in 18% patients with AL-CA but up to 33% of those with ATTRwt-CA and 25% for ATTRv) [5] |
Same indications to PM implantation than in other disease settings [94] | |
| KSS | Greater mutation load in AV node | 84% [95] | High risk of complete AV block and SCD [94] | |
|
Neuromuscular diseases: Emery-Dreifuss DM type 1 DM type 2 |
Up to 20% [96] |
‐ Emery-Dreifuss dystrophy: risk of AV block and SCD [97] ‐ DM type 1: risk of AV block and SCD [6] ‐ DM type 2 usually leads to mild AV or BBB, but arrhythmias and SCD have been reported [7] |
||
| Cardiac sarcoidosis | Granulomatous inflammation affecting the conduction system | 34% among middle-aged patients with unexplained AV block [11] | ||
| Laminopathy | Fibrosis in the AV node | Early | Higher risk of life-threatening arrhythmias [9] | |
| LVNC | 3–25% [13] | |||
| Short PR | ||||
| Danon disease | Accelerated nodal conduction, disruption of the annulus fibrosus by glycogen-filled myocytes | 70% in M [17] | Risk of lethal arrhythmias and SCD following atrial tachyarrhythmias [17] | |
| Pompe disease | 10% [18] | The short PR interval can normalize after ERT (in infantile disease) [18] | ||
| PRKAG2 syndrome | 68% [20] | Risk of lethal arrhythmias and SCD (high-degree AV block or fast conduction of atrial tachyarrhythmias) [20] | ||
| AFD | Stored glycosphingolipids increasing conduction velocity | 15–40% [98] | Early [93] | The short PR interval can normalize after ERT [15] |
| Mitochondrial disease | Proliferation of mitochondria altering cardiomyocyte functioning | 22% [21] | PM implantation may be considered in KSS | |
| Atrial fibrillation | ||||
| HCM | Atrial enlargement and fibrosis | 17–30% [24, 25] | Higher risk of all-cause mortality, cardiac death, SCD and stroke-related death [23, 26, 27] | |
| DCM | 36–76% [23, 24, 28–32] | |||
| ACM | Desmosomal dysfunction and RA enlargement or dysfunction | 9–30% [33, 34] | AF predicts worse outcomes in patients with right-sided ACM [36] | |
| LVNC | 1–29% [23, 24, 37, 38] | Lower incidence in children | AF is associated with LVNC severity and predicts survival [39] | |
| CA | Amyloid infiltration promoting atrial fibrosis and dilation |
Higher prevalence in ATTR- than AL-CA |
AF does not seem to predict a worse outcome [44, 45] | |
| Q waves | ||||
| HCM | Myocardial fibrosis and septal hypertrophy displacing the septal electrical vector | 18–53% [46, 53] | Early | May precede the increase in LV mass by several years, are less common in patients with biventricular hypertrophy, and help differentiate HCM from the athlete’s heart |
| CA | Accumulation of amyloid and fibrosis |
25–47% in AL-CA |
Independently predict death in patients with AL-CA [99] | |
| Idiopathic DCM | Vector displacement due to LV dilation and transmural fibrosis | 10–25% [3, 48] | ||
| Dystrophin cardiomyopathy | Scarring of the posterolateral region of the LV | 21% (DMD) [100], 42% (BMD) [101] | ||
| QRS complex: high voltages | ||||
| HCM | Cardiomyocyte hypertrophy | 41–60% [46, 53] |
Scores including QRS amplitude were proposed to predict SCD [55] Mavacamten may relieve ECG signs of LVH |
|
| DCM | LVH | 17–69% [56, 57] | Unclear prognostic relevance | |
| CA | LV apex spared by amyloid accumulation | Up to 25% in ATTR-CA [58] | No relationship with outcome [49] | |
| Pompe disease, Danon disease, FD | Accumulation of glycosphingolipids | 6–10% (less common in Pompe than in Danon and AFD) [59] | ||
| QRS complex: low voltages | ||||
| HCM | Extensive fibrosis | < 3% [61] | End-stage | Higher risk of SCD [61] |
| DCM | Loss of vital myocardium and diffuse LV fibrosis | 3–6% [3, 63] | Higher risk of death or heart transplantation, SCD, or life-threatening ventricular arrhythmias [3] | |
| ACM | Fibro-fatty replacement |
41% with LV involvement 17% without LV involvement [63] |
Advanced | Higher risk of life-threatening ventricular arrhythmias and SCD [67] |
| CA | Amyloid and fibrosis | 46–70% (higher in AL-CA) [68, 69] | Higher risk of CV death [69] | |
| QRS fragmentation and epsilon wave | ||||
| HCM | Myocardial disarray, interstitial fibrosis, conduction impairment | 75% [73] | Higher risk of ventricular tachyarrhythmias and SCD (++ in 3 coronary artery territories) [73] | |
| DCM | Myocardial scar | 23–26% [74] | Higher risk of ventricular tachycardias and all-cause death [74] | |
| Cardiac sarcoidosis | Myocardial granulomas | 50% [102] | Early | Association with LGE presence [102] |
| ACM | Fibro-fatty replacement in the subepicardial region of the RV free wall | 85% [103] | QRS fragmentation: higher risk of VT, VF, and appropriate ICD discharges [78]. Epsilon waves in aVR: higher risk of HF hospitalization, HF-related death, SCD, and heart transplantation [76] | |
| RBBB | ||||
| HCM | 4–5% [79] | One-third of patients develop complete heart block after myectomy [79] | ||
| AFD | 22% [14] | |||
| DCM | 7% [80] | Predictor of all-cause mortality [80] | ||
| ACM | 19 [82] to 32% [81] in ARVC | |||
| LBBB | ||||
| HCM | 2% [83], 22% in end-stage HCM [84], up to 40% after septal myectomy [85] | LBBB after surgery not associated with worse outcome [79] | ||
| DCM | Degeneration or fibrosis of the conduction system | 25–30% [87] |
No worse survival in patients with DCM and LVEF 36–50% [87] Shorter survival in patients with idiopathic DCM developing LBBB [88] |
|
| ST segment and T waves | ||||
| HCM |
- Epicardial cardiomyocytes depolarize and repolarize later than endocardial cardiomyocytes - ST-segment elevation may signal ventricular aneurysm |
Early |
Negative T waves in V3–V6 becoming positive may denote the development of an apical aneurysm STEMI patterns associated with higher risk of SCD [61] |
|
| DCM | T wave inversion in 62% of patients with FLNC mutations [104] | |||
| Right-side ACM | Fibro-fatty infiltration in the subepicardium |
- Negative T wave strongly related to disease presence - ST segment elevation with negative T wave in V1–V2 related to advanced transmural RV involvement [92] |
||
| CA | Amyloid infiltration | |||
| AFD | Asymmetrical negative T waves and ST-T segment depression or elevation in inferolateral leads related to fibrosis | |||
| Mitochondrial diseases | Asymmetrical negative T waves in 50% [21] | |||
ACM arrhythmogenic cardiomyopathy, AF atrial fibrillation, AFD Anderson-Fabry disease, AL light chain amyloidosis, ATTR transthyretin amyloidosis, AV atrioventricular, BBB bundle branch block, BMD Becker muscular dystrophy, CA cardiac amyloidosis, DCM dilated cardiomyopathy, DM myotonic dystrophy, DMD Duchenne muscular dystrophy, DMD dystrophin gene, ECG electrocardiogram, ERT enzyme replacement therapy, FLNC filamin-C gene, HCM hypertrophic cardiomyopathy, HF heart failure, ICD implantable cardioverter-defibrillator, KSS Kearns-Sayre syndrome, LA left atrium, LBBB left bundle branch block, LGE late gadolinium enhancement, LMNA lamin A/C, LV left ventricle, LVH left ventricular hypertrophy, LVNC left ventricular non-compaction cardiomyopathy, M male, PM pacemaker, PRKAG2 protein kinase AMP-activated non-catalytic subunit gamma 2, QTc corrected QT, RA right atrium, RBBB right bundle branch block, RV right ventricle, SCD sudden cardiac death, SCN5A sodium voltage-gated channel alpha subunit 5 gene, v variant, STEMI ST elevation myocardial infarction, VF ventricular fibrillation, VT ventricular tachycardia, wt wild-type
Fig. 3.
Standard vs. cardiomyopathy (CMP)-oriented approach to electrocardiogram reading. Central Illustration Electrocardiographic findings in patients with a hypertrophic or dilated phenotype. ALVC/ARVC, arrhythmogenic left/right ventricular cardiomyopathy; AV, atrio-ventricular; CMP, cardiomyopathy; HCM, hypertrophic cardiomyopathy; LAMP2, lysosomal-associated membrane protein 2; LBBB, left bundle branch block; PRKAG2, protein kinase AMP-activated non-catalytic subunit gamma 2; RBBB, right bundle branch block; WPW, Wolff-Parkinson-White
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
A. A., A. M., P. M.: manuscript writing; A. B., A. P., G. V., M. M., A. A., I. O., M. E., G. F., G. S., P. E., and C. R.: critical revision.
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement.
Availability of data and materials
This is not applicable.
Declarations
Ethical approval
This is not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, De Winter T, Elliott PM, Flather M, Garcia-Pavia P, Haugaa KH, Ingles J, Jurcut RO, Klaassen S, Limongelli G, Loeys B, Mogensen J, Olivotto I, Pantazis A, Sharma S, Van Tintelen JP, Ware JS, Kaski JP; ESC Scientific Document Group (2023) ESC Guidelines for the management of cardiomyopathies. Eur Heart J 44(37):3503−3626. 10.1093/eurheartj/ehad194. PMID: 37622657
- 2.Mahfouz Badran H, Soltan G, Eltahan E, Yacoub MH, Faheem N (2021) Relation of atrial electromechanical delay to P-wave dispersion on surface ECG using vector velocity imaging in patients with hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol 26(1):e12801. 10.1111/anec.12801. Epub 2020 Sep 24. PMID: 32969115; PMCID: PMC7816806 [DOI] [PMC free article] [PubMed]
- 3.Merlo M, Zaffalon D, Stolfo D, Altinier A, Barbati G, Zecchin M, Bardari S, Sinagra G. ECG in dilated cardiomyopathy: specific findings and long-term prognostic significance. J Cardiovasc Med (Hagerstown) 2019;20:450–458. doi: 10.2459/JCM.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 4.Bejar D, Colombo PC, Latif F, Yuzefpolskaya M. Infiltrative cardiomyopathies. Clin Med Insights Cardiol. 2015;9:29–38. doi: 10.4137/CMC.S19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 6.Petri H, Vissing J, Witting N, Bundgaard H, Kober L. Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol. 2012;160:82–88. doi: 10.1016/j.ijcard.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Schoser BG, Ricker K, Schneider-Gold C, Hengstenberg C, Dürre J, Bültmann B, Kress W, Day JW, Ranum LP. Sudden cardiac death in myotonic dystrophy type 2. Neurology. 2004;63:2402–2404. doi: 10.1212/01.WNL.0000147335.10783.E4. [DOI] [PubMed] [Google Scholar]
- 8.Emery AE. Emery-Dreifuss muscular dystrophy - a 40 year retrospective. Neuromuscular disorders : NMD. 2000;10:228–232. doi: 10.1016/S0960-8966(00)00105-X. [DOI] [PubMed] [Google Scholar]
- 9.Otomo J, Kure S, Shiba T, Karibe A, Shinozaki T, Yagi T, Naganuma H, Tezuka F, Miura M, Ito M, Watanabe J, Matsubara Y, Shirato K. Electrophysiological and histopathological characteristics of progressive atrioventricular block accompanied by familial dilated cardiomyopathy caused by a novel mutation of lamin A/C gene. J Cardiovasc Electrophysiol. 2005;16:137–145. doi: 10.1046/j.1540-8167.2004.40096.x. [DOI] [PubMed] [Google Scholar]
- 10.Peretto G, Sala S, Benedetti S, Di Resta C, Gigli L, Ferrari M, Della BP. Updated clinical overview on cardiac laminopathies: an electrical and mechanical disease. Nucleus (Austin, Tex) 2018;9:380–391. doi: 10.1080/19491034.2018.1489195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, Davis D, Ohira H, Gollob MH, Leung E, Healey JS, Birnie DH. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. doi: 10.1111/jce.12401. [DOI] [PubMed] [Google Scholar]
- 12.Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac involvement in hemochromatosis. Cardiol Rev. 2014;22:56–68. doi: 10.1097/CRD.0b013e3182a67805. [DOI] [PubMed] [Google Scholar]
- 13.Sanna GD, Piga A, Parodi G, Sinagra G, Papadakis M, Pantazis A, Sharma S, Gati S, Finocchiaro G. The electrocardiogram in the diagnosis and management of patients with left ventricular non-compaction. Curr Heart Fail Rep. 2022;19:476–490. doi: 10.1007/s11897-022-00580-z. [DOI] [PubMed] [Google Scholar]
- 14.Vitale G, Ditaranto R, Graziani F, Tanini I, Camporeale A, Lillo R, Rubino M, Panaioli E, Di Nicola F, Ferrara V, Zanoni R, Caponetti AG, Pasquale F, Graziosi M, Berardini A, Ziacchi M, Biffi M, Santostefano M, Liguori R, Taglieri N, Nardi E, Linhart A, Olivotto I, Rapezzi C, Biagini E. Standard ECG for differential diagnosis between Anderson-Fabry disease and hypertrophic cardiomyopathy. Heart (British Cardiac Society) 2022;108:54–60. doi: 10.1136/heartjnl-2020-318271. [DOI] [PubMed] [Google Scholar]
- 15.Blum A, Podovitzky O, Sheiman J, Khasin M. Reversal of first-degree atrioventricular block in Fabry disease. Arch Intern Med. 2009;169:1925–1926. doi: 10.1001/archinternmed.2009.334. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Wang F, Chen X, Liang Y, Deng H, Liao H, Rao F, Wei W, Zhang Q, Zhang B, Zhan X, Fang X, Nair S, Shehata M, Wang X, Xue Y, Wu S. Fasciculoventricular pathways responsible for ventricular preexcitation in patients with Danon disease. Circ Arrhythm Electrophysiol. 2018;11:e006704. doi: 10.1161/CIRCEP.118.006704. [DOI] [PubMed] [Google Scholar]
- 17.Montañés ME, Granados MA, Valverde M, Palomino J, Fontenla A, Escribano L. Wolff Parkinson white pattern in Danon disease: when preexcitation is not what it seems. J Electrocardiol. 2020;62:161–164. doi: 10.1016/j.jelectrocard.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Forsha D, Li JS, Smith PB, van der Ploeg AT, Kishnani P, Pasquali SK. Cardiovascular abnormalities in late-onset Pompe disease and response to enzyme replacement therapy. Genetics in medicine : official journal of the American College of Medical Genetics. 2011;13:625–631. doi: 10.1097/GIM.0b013e3182142966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansong AK, Li JS, Nozik-Grayck E, Ing R, Kravitz RM, Idriss SF, Kanter RJ, Rice H, Chen YT, Kishnani PS. Electrocardiographic response to enzyme replacement therapy for Pompe disease. Genetics in medicine : official journal of the American College of Medical Genetics. 2006;8:297–301. doi: 10.1097/01.gim.0000195896.04069.5f. [DOI] [PubMed] [Google Scholar]
- 20.Porto AG, Brun F, Severini GM, Losurdo P, Fabris E, Taylor MRG, Mestroni L, Sinagra G. Clinical spectrum of PRKAG2 syndrome. Circ Arrhythm Electrophysiol. 2016;9:e003121. doi: 10.1161/CIRCEP.115.003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limongelli G, Tome-Esteban M, Dejthevaporn C, Rahman S, Hanna MG, Elliott PM. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur J Heart Fail. 2010;12:114–121. doi: 10.1093/eurjhf/hfp186. [DOI] [PubMed] [Google Scholar]
- 22.Bates MG, Bourke JP, Giordano C, d’Amati G, Turnbull DM, Taylor RW. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur Heart J. 2012;33:3023–3033. doi: 10.1093/eurheartj/ehs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung C, Enriquez A, Suarez-Fuster L, Baranchuk A. Atrial fibrillation in patients with inherited cardiomyopathies. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2019;21:22–32. doi: 10.1093/europace/euy064. [DOI] [PubMed] [Google Scholar]
- 24.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL and Group ESCSD ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;2021(42):373–498. doi: 10.1093/eurheartj/ehab648. [DOI] [PubMed] [Google Scholar]
- 25.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. doi: 10.1161/hc4601.097997. [DOI] [PubMed] [Google Scholar]
- 26.Palyam V, Azam AT, Odeyinka O, Alhashimi R, Thoota S, Ashok T, Sange I. Hypertrophic cardiomyopathy and atrial fibrillation: a review. Cureus. 2022;14:e21101. doi: 10.7759/cureus.21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masri A, Kanj M, Thamilarasan M, Wazni O, Smedira NG, Lever HM, Desai MY. Outcomes in hypertrophic cardiomyopathy patients with and without atrial fibrillation: a survival meta-analysis. Cardiovasc Diagn Ther. 2017;7:36–44. doi: 10.21037/cdt.2016.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finocchiaro G, Merlo M, Sheikh N, De Angelis G, Papadakis M, Olivotto I, Rapezzi C, Carr-White G, Sharma S, Mestroni L, Sinagra G. The electrocardiogram in the diagnosis and management of patients with dilated cardiomyopathy. Eur J Heart Fail. 2020;22:1097–1107. doi: 10.1002/ejhf.1815. [DOI] [PubMed] [Google Scholar]
- 29.Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, Mannarino S, Gambarin F, Favalli V, Grasso M, Agozzino M, Campana C, Gavazzi A, Febo O, Marini M, Landolina M, Mortara A, Piccolo G, Vigano M, Tavazzi L, Arbustini E. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52:1250–1260. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, Edvardsen T, Haugaa KH. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–860. doi: 10.1093/eurheartj/ehx596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:186–194. doi: 10.1016/S0735-1097(97)00434-8. [DOI] [PubMed] [Google Scholar]
- 32.Tayal U, Buchan Rachel J, Whiffin N, Newsome S, Mazzarotto F, Walsh R, Ware James S, Cook S, Prasad S. 143 clinical and genetic characteristics of familial dilated cardiomyopathy in a large UK prospective cohort. Heart. 2016;102:A103–A104. doi: 10.1136/heartjnl-2016-309890.143. [DOI] [Google Scholar]
- 33.Camm CF, James CA, Tichnell C, Murray B, Bhonsale A, te Riele AS, Judge DP, Tandri H, Calkins H. Prevalence of atrial arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2013;10:1661–1668. doi: 10.1016/j.hrthm.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Holmqvist F, Daubert JP. Arrhythmogenic right ventricular cardiomyopathy: arrhythmias upstream and downstream. Heart Rhythm. 2013;10:1669–1670. doi: 10.1016/j.hrthm.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 35.Platonov PG, Christensen AH, Holmqvist F, Carlson J, Haunso S, Svendsen JH. Abnormal atrial activation is common in patients with arrhythmogenic right ventricular cardiomyopathy. J Electrocardiol. 2011;44:237–241. doi: 10.1016/j.jelectrocard.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, Monteforte N, Memmi M, Gambelli P, Novelli V, Bloise R, Catalano O, Moro G, Tibollo V, Morini M, Bellazzi R, Napolitano C, Bagnardi V, Priori SG. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68:2540–2550. doi: 10.1016/j.jacc.2016.09.951. [DOI] [PubMed] [Google Scholar]
- 37.Brescia ST, Rossano JW, Pignatelli R, Jefferies JL, Price JF, Decker JA, Denfield SW, Dreyer WJ, Smith O, Towbin JA, Kim JJ. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127:2202–2208. doi: 10.1161/CIRCULATIONAHA.113.002511. [DOI] [PubMed] [Google Scholar]
- 38.Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, Papra E, Kiotsekoglou A, Tome MT, Pellerin D, McKenna WJ, Elliott PM. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J. 2005;26:187–192. doi: 10.1093/eurheartj/ehi025. [DOI] [PubMed] [Google Scholar]
- 39.Stollberger C, Blazek G, Gessner M, Bichler K, Wegner C, Finsterer J. Neuromuscular comorbidity, heart failure, and atrial fibrillation as prognostic factors in left ventricular hypertrabeculation/noncompaction. Herz. 2015;40:906–911. doi: 10.1007/s00059-015-4310-7. [DOI] [PubMed] [Google Scholar]
- 40.Stöllberger C, Blazek G, Wegner C, Finsterer J. Heart failure, atrial fibrillation and neuromuscular disorders influence mortality in left ventricular hypertrabeculation/noncompaction. Cardiology. 2011;119:176–182. doi: 10.1159/000331496. [DOI] [PubMed] [Google Scholar]
- 41.Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clinical electrophysiology. 2020;6:351–361. doi: 10.1016/j.jacep.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3:107–113. doi: 10.1016/S0735-1097(84)80436-2. [DOI] [PubMed] [Google Scholar]
- 43.Vergaro G, Aimo A, Rapezzi C, Castiglione V, Fabiani I, Pucci A, Buda G, Passino C, Lupón J, Bayes-Genis A, Emdin M, Braunwald E (2022) Atrial amyloidosis: mechanisms and clinical manifestations. Eur J Heart Fail 24(11):2019−2028. 10.1002/ejhf.2650. Epub 2022 Aug 21. PMID: 35920110; PMCID: PMC10087817 [DOI] [PMC free article] [PubMed]
- 44.Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC heart failure. 2018;5:772–779. doi: 10.1002/ehf2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchis K, Cariou E, Colombat M, Ribes D, Huart A, Cintas P, Fournier P, Rollin A, Carrié D, Galinier M, Maury P, Duparc A, Lairez O. Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2019;26:128–138. doi: 10.1080/13506129.2019.1620724. [DOI] [PubMed] [Google Scholar]
- 46.Delcrè SD, Di Donna P, Leuzzi S, Miceli S, Bisi M, Scaglione M, Caponi D, Conte MR, Cecchi F, Olivotto I, Gaita F. Relationship of ECG findings to phenotypic expression in patients with hypertrophic cardiomyopathy: a cardiac magnetic resonance study. Int J Cardiol. 2013;167:1038–1045. doi: 10.1016/j.ijcard.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 47.Lemery R, Kleinebenne A, Nihoyannopoulos P, Aber V, Alfonso F, McKenna WJ. Q waves in hypertrophic cardiomyopathy in relation to the distribution and severity of right and left ventricular hypertrophy. J Am Coll Cardiol. 1990;16:368–374. doi: 10.1016/0735-1097(90)90587-F. [DOI] [PubMed] [Google Scholar]
- 48.Pelto H, Owens D, Drezner J. Electrocardiographic findings suggestive of cardiomyopathy: what to look for and what to do next. Curr Sports Med Rep. 2013;12:77–85. doi: 10.1249/JSR.0b013e3182874abb. [DOI] [PubMed] [Google Scholar]
- 49.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114:1089–1093. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;69:1694–1703. doi: 10.1016/j.jacc.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 52.Dumont CA, Monserrat L, Soler R, Rodríguez E, Fernandez X, Peteiro J, Bouzas A, Bouzas B, Castro-Beiras A. Interpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance. Eur Heart J. 2006;27:1725–1731. doi: 10.1093/eurheartj/ehl101. [DOI] [PubMed] [Google Scholar]
- 53.Montgomery JV, Harris KM, Casey SA, Zenovich AG, Maron BJ. Relation of electrocardiographic patterns to phenotypic expression and clinical outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2005;96:270–275. doi: 10.1016/j.amjcard.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 54.Al-Mahdawi S, Chamberlain S, Chojnowska L, Michalak E, Nihoyannopoulos P, Ryan M, Kusnierczyk B, French JA, Gilligan DM, Cleland J et al (1994) The electrocardiogram is a more sensitive indicator than echocardiography of hypertrophic cardiomyopathy in families with a mutation in the MYH7 gene. Brit Heart J 72:105–111 [DOI] [PMC free article] [PubMed]
- 55.Ostman-Smith I, Wisten A, Nylander E, Bratt EL, Granelli A, Oulhaj A, Ljungström E. Electrocardiographic amplitudes: a new risk factor for sudden death in hypertrophic cardiomyopathy. Eur Heart J. 2010;31:439–449. doi: 10.1093/eurheartj/ehp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Timonen P, Magga J, Risteli J, Punnonen K, Vanninen E, Turpeinen A, Tuomainen P, Kuusisto J, Vuolteenaho O, Peuhkurinen K. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300. doi: 10.1016/j.ijcard.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Momiyama Y, Mitamura H, Kimura M. ECG characteristics of dilated cardiomyopathy. J Electrocardiol. 1994;27:323–328. doi: 10.1016/S0022-0736(05)80270-5. [DOI] [PubMed] [Google Scholar]
- 58.Eötvös CA, Lazar RD, Zehan IG, Lévay-Hail EB, Pastiu G, Pop M, Bojan AS, Pop S, Blendea D (2021) Cardiac Amyloidosis with discordant QRS voltage between frontal and precordial leads. Medicina (Kaunas) 57(7):660. 10.3390/medicina57070660. PMID: 34199044; PMCID: PMC8306315 [DOI] [PMC free article] [PubMed]
- 59.Wang XY, Wang B, Zhu XL, Ma ZL, Liu Y, Lei CH, Yang QL, Hu D, Zhao XL, Liu ZR, Liu LW. Clinical and molecular characterization of seven patients with Danon disease. Exp Ther Med. 2021;21:395. doi: 10.3892/etm.2021.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valentini F, Anselmi F, Metra M, Cavigli L, Giacomin E, Focardi M, Cameli M, Mondillo S, D'Ascenzi F (2022) Diagnostic and prognostic value of low QRS voltages in cardiomyopathies: old but gold. Eur J Prev Cardiol 29(8):1177−1187. 10.1093/eurjpc/zwaa027. PMID: 33624098 [DOI] [PubMed]
- 61.Biagini E, Pazzi C, Olivotto I, Musumeci B, Limongelli G, Boriani G, Pacileo G, Mastromarino V, Bacchi Reggiani ML, Lorenzini M, Lai F, Berardini A, Mingardi F, Rosmini S, Resciniti E, Borghi C, Autore C, Cecchi F, Rapezzi C. Usefulness of electrocardiographic patterns at presentation to predict long-term risk of cardiac death in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2016;118:432–439. doi: 10.1016/j.amjcard.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Ledieu N, Larnier L, Auffret V, Marie C, Fargeau D, Donal E, Mirabel M, Jeunemaitre X, Puscas T, Marijon E, Reynaud A, Ritter P, Lafitte S, Mabo P, Réant P, Daubert C, Hagège AA. Prognostic value of the 12-lead surface electrocardiogram in sarcomeric hypertrophic cardiomyopathy: data from the REMY French register. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2020;22:139–148. doi: 10.1093/europace/euz272. [DOI] [PubMed] [Google Scholar]
- 63.Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin S, Pilichou K, Motta R, Aliberti C, Thiene G, McKenna WJ, Zorzi A, Iliceto S, Basso C, Perazzolo Marra M, Corrado D. Arrhythmogenic right ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc. 2020;9:e014628. doi: 10.1161/JAHA.119.014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli-Ducci C, Duru F, Elliott P, Hamilton RM, Haugaa KH, James CA, Judge D, Link MS, Marchlinski FE, Mazzanti A, Mestroni L, Pantazis A, Pelliccia A, Marra MP, Pilichou K, Platonov PGA, Protonotarios A, Rampazzo A, Saffitz JE, Saguner AM, Schmied C, Sharma S, Tandri H, Te Riele A, Thiene G, Tsatsopoulou A, Zareba W, Zorzi A, Wichter T, Marcus FI, Calkins H. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. doi: 10.1093/eurheartj/ehz669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brosnan MJ, Te Riele A, Bosman LP, Hoorntje ET, van den Berg MP, Hauer RNW, Flannery MD, Kalman JM, Prior DL, Tichnell C, Tandri H, Murray B, Calkins H, La Gerche A, James CA. Electrocardiographic features differentiating arrhythmogenic right ventricular cardiomyopathy from an athlete’s heart. JACC Clinical electrophysiology. 2018;4:1613–1625. doi: 10.1016/j.jacep.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, Migliore F, Pilichou K, Rampazzo A, Rigato I, Rizzo S, Thiene G, Anastasakis A, Asimaki A, Bucciarelli-Ducci C, Haugaa KH, Marchlinski FE, Mazzanti A, McKenna WJ, Pantazis A, Pelliccia A, Schmied C, Sharma S, Wichter T, Bauce B, Basso C. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–114. doi: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. doi: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng Z, Zhu K, Tian Z, Zhao D, Cui Q and Fang Q (2013) The findings of electrocardiography in patients with cardiac amyloidosis. Ann Noninvasive Electrocardiol 18:157–162 [DOI] [PMC free article] [PubMed]
- 69.Cipriani A, De Michieli L, Porcari A, Licchelli L, Sinigiani G, Tini G, Zampieri M, Sessarego E, Argirò A, Fumagalli C, De Gaspari M, Licordari R, Russo D, Di Bella G, Perfetto F, Autore C, Musumeci B, Canepa M, Merlo M, Sinagra G, Gregori D, Iliceto S, Perazzolo Marra M, Cappelli F, Rapezzi C (2022) Low QRS voltages in cardiac amyloidosis: Clinical correlates and prognostic value. JACC CardioOncol 4(4):458−470. 10.1016/j.jaccao.2022.08.007. PMID: 36444225; PMCID: PMC9700257 [DOI] [PMC free article] [PubMed]
- 70.Wang W, Murray B, Tichnell C, Gilotra NA, Zimmerman SL, Gasperetti A, Scheel P, Tandri H, Calkins H, James CA. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2022;24:268–277. doi: 10.1093/europace/euab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bariani R, Cason M, Rigato I, Cipriani A, Celeghin R, De Gaspari M, Bueno Marinas M, Mattesi G, Pergola V, Rizzo S, Zorzi A, Giorgi B, Rampazzo A, Thiene G, Iliceto S, Perazzolo Marra M, Corrado D, Basso C, Pilichou K, Bauce B. Clinical profile and long-term follow-up of a cohort of patients with desmoplakin cardiomyopathy. Heart Rhythm. 2022;19:1315–1324. doi: 10.1016/j.hrthm.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 72.Verstraelen TE, van Lint FHM, Bosman LP, de Brouwer R, Proost VM, Abeln BGS, Taha K, Zwinderman AH, Dickhoff C, Oomen T, Schoonderwoerd BA, Kimman GP, Houweling AC, Gimeno-Blanes JR, Asselbergs FW, van der Zwaag PA, de Boer RA, van den Berg MP, van Tintelen JP, Wilde AAM (2021) Prediction of ventricular arrhythmia in phospholamban p.Arg14del mutation carriers-reaching the frontiers of individual risk prediction. Eur Heart J 42:2842–2850 [DOI] [PMC free article] [PubMed]
- 73.Debonnaire P, Katsanos S, Joyce E, Van den Brink OV, Atsma DE, Schalij MJ, Bax JJ, Delgado V, Marsan NA (2015) QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 26:547–555 [DOI] [PubMed]
- 74.Basaran Y, Tigen K, Karaahmet T, Isiklar I, Cevik C, Gurel E, Dundar C, Pala S, Mahmutyazicioglu K, Basaran O. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography. 2011;28:62–68. doi: 10.1111/j.1540-8175.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- 75.Ning XH, Tang M, Chen KP, Hua W, Chen RH, Sha J, Liu ZM, Zhang S. The prognostic significance of fragmented QRS in patients with left ventricular noncompaction cardiomyopathy. Can J Cardiol. 2012;28:508–514. doi: 10.1016/j.cjca.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Gallo C, Blandino A, Giustetto C, Anselmino M, Castagno D, Richiardi E, Gaita F. Arrhythmogenic right ventricular cardiomyopathy: ECG progression over time and correlation with long-term follow-up. J Cardiovasc Med (Hagerstown) 2016;17:418–424. doi: 10.2459/JCM.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 77.Li GL, Saguner AM, Fontaine GH, Frank R (2018) Epsilon waves: Milestones in the discovery and progress. Ann Noninvasive Electrocardiol 23(6):e12571. 10.1111/anec.12571. Epub 2018 Jul 5. PMID: 29978588; PMCID: PMC6931672 [DOI] [PMC free article] [PubMed]
- 78.Peters S, Truemmel M, Koehler B. Prognostic value of QRS fragmentation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Med (Hagerstown) 2012;13:295–298. doi: 10.2459/JCM.0b013e32834bed0a. [DOI] [PubMed] [Google Scholar]
- 79.Cui H, Schaff HV, Nishimura RA, Geske JB, Dearani JA, Lahr BD, Ommen SR. Conduction abnormalities and long-term mortality following septal myectomy in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2019;74:645–655. doi: 10.1016/j.jacc.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 80.Lai L, Jiang R, Fang W, Yan C, Tang Y, Hua W, Fu M, Li X, Luo R. Prognostic impact of right bundle branch block in hospitalized patients with idiopathic dilated cardiomyopathy: a single-center cohort study. J Int Med Res. 2020;48:300060518801478. doi: 10.1177/0300060518801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jain R, Dalal D, Daly A, Tichnell C, James C, Evenson A, Jain R, Abraham T, Tan BY, Tandri H, Russell SD, Judge D, Calkins H. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation. 2009;120:477–487. doi: 10.1161/CIRCULATIONAHA.108.838821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peters S, Trümmel M, Koehler B. Special features of right bundle branch block in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Int J Cardiol. 2012;157:102–103. doi: 10.1016/j.ijcard.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 83.Lakdawala NK, Thune JJ, Maron BJ, Cirino AL, Havndrup O, Bundgaard H, Christiansen M, Carlsen CM, Dorval JF, Kwong RY, Colan SD, Køber LV, Ho CY. Electrocardiographic features of sarcomere mutation carriers with and without clinically overt hypertrophic cardiomyopathy. Am J Cardiol. 2011;108:1606–1613. doi: 10.1016/j.amjcard.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao Y, Yang KQ, Yang YK, Liu YX, Tian T, Song L, Jiang XJ, Zhou XL. Clinical characteristics and prognosis of end-stage hypertrophic cardiomyopathy. Chin Med J. 2015;128:1483–1489. doi: 10.4103/0366-6999.157656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui H, Schaff HV, Nishimura RA, Geske JB, Dearani JA, Lahr BD, Ommen SR (2019) Conduction abnormalities and long-term mortality following septal myectomy in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 74:645−655 [DOI] [PubMed]
- 86.Akhtar MM, Elliott P. Impact of left bundle branch block (LBBB) in dilated cardiomyopathy (DCM) with intermediate left ventricular systolic dysfunction (LVSD) Int J Cardiol. 2019;278:199–201. doi: 10.1016/j.ijcard.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 87.Gentile P, Paldino A, Cannatà A, Artico J, Barbati G, Ramani F, Fabris E, Aleksova A, Stolfo D, Zecchin M, Merlo M, Sinagra G. Left bundle branch block in dilated cardiomyopathy with intermediate left ventricular dysfunction: clinical phenotyping and outcome correlates. Int J Cardiol. 2019;278:180–185. doi: 10.1016/j.ijcard.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Aleksova A, Carriere C, Zecchin M, Barbati G, Vitrella G, Di Lenarda A, Sinagra G. New-onset left bundle branch block independently predicts long-term mortality in patients with idiopathic dilated cardiomyopathy: data from the Trieste Heart Muscle Disease Registry. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014;16:1450–1459. doi: 10.1093/europace/euu016. [DOI] [PubMed] [Google Scholar]
- 89.Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart (British Cardiac Society) 2004;90:645–649. doi: 10.1136/hrt.2003.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Finocchiaro G, Papadakis M, Dhutia H, Zaidi A, Malhotra A, Fabi E, Cappelletto C, Brook J, Papatheodorou E, Ensam B, Miles CJ, Bastiaenen R, Attard V, Homfray T, Sharma R, Tome M, Carr-White G, Merlo M, Behr ER, Sinagra G, Sharma S. Electrocardiographic differentiation between ‘benign T-wave inversion’ and arrhythmogenic right ventricular cardiomyopathy. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2019;21:332–338. doi: 10.1093/europace/euy179. [DOI] [PubMed] [Google Scholar]
- 91.Peters S. Electrocardiographic morphology in right precordial T waves in arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2016;214:228. doi: 10.1016/j.ijcard.2016.03.115. [DOI] [PubMed] [Google Scholar]
- 92.Kubala M, Pathak RK, Xie S, Casado Arroyo R, Tschabrunn CM, Hayashi T, Garcia FC, Supple GE, Santangeli P, Frankel DS, Zado ES, Callans DJ, Marchlinski FE. Electrocardiographic repolarization abnormalities and electroanatomic substrate in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2018;11:e005553. doi: 10.1161/CIRCEP.117.005553. [DOI] [PubMed] [Google Scholar]
- 93.Tassetti L, Fumagalli C, Argiro A, Zampieri M, Gori M, Verrillo F, Zocchi C, Cappelli F, Olivotto I. Prevalence and predictors of bradyarrhythmias requiring permanent pacing in patients with Anderson-Fabry disease. J Cardiovasc Electrophysiol. 2022;33:1072–1078. doi: 10.1111/jce.15409. [DOI] [PubMed] [Google Scholar]
- 94.Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. [DOI] [PubMed] [Google Scholar]
- 95.St-Pierre G, Steinberg C, Dubois M, Sénéchal M. What the cardiologist should know about mitochondrial cardiomyopathy? Can J Cardiol. 2019;35:221–224. doi: 10.1016/j.cjca.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 96.Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e128–e226. doi: 10.1016/j.hrthm.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 97.Puckelwartz M, McNally EM. Emery-Dreifuss muscular dystrophy. Handb Clin Neurol. 2011;101:155–166. doi: 10.1016/B978-0-08-045031-5.00012-8. [DOI] [PubMed] [Google Scholar]
- 98.Jastrzebski M, Bacior B, Dimitrow PP, Kawecka-Jaszcz K. Electrophysiological study in a patient with Fabry disease and a short PQ interval. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2006;8:1045–1047. doi: 10.1093/europace/eul121. [DOI] [PubMed] [Google Scholar]
- 99.Zhao L, Li J, Tian Z, Fang Q. Clinical correlates and prognostic values of pseudoinfarction in cardiac light-chain amyloidosis. J Cardiol. 2016;68:426–430. doi: 10.1016/j.jjcc.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Santos MA, Costa Fde A, Travessa AF, Bombig MT, Fonseca FH, Luna Filho B, Mussi A, Souza D. Oliveira A and Povoa R [Duchenne muscular dystrophy: electrocardiographic analysis of 131 patients] Arq Bras Cardiol. 2010;94:620–624. doi: 10.1590/S0066-782X2010005000024. [DOI] [PubMed] [Google Scholar]
- 101.Steare SE, Dubowitz V, Benatar A. Subclinical cardiomyopathy in Becker muscular dystrophy. Br Heart J. 1992;68:304–308. doi: 10.1136/hrt.68.9.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Homsi M, Alsayed L, Safadi B, Mahenthiran J, Das MK (2009) Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol 14:319–326 [DOI] [PMC free article] [PubMed]
- 103.Peters S, Trummel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008;5:1417–1421. doi: 10.1016/j.hrthm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 104.Gigli M, Merlo M, Graw SL, Barbati G, Rowland TJ, Slavov DB, Stolfo D, Haywood ME, Dal Ferro M, Altinier A, Ramani F, Brun F, Cocciolo A, Puggia I, Morea G, McKenna WJ, La Rosa FG, Taylor MRG, Sinagra G, Mestroni L. Genetic risk of arrhythmic phenotypes in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2019;74:1480–1490. doi: 10.1016/j.jacc.2019.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is not applicable.