Abstract
Aim
This study examined sex-based differences in associations of vascular risk factors with incident cardiovascular events in the UK Biobank.
Methods
Baseline participant demographic, clinical, laboratory, anthropometric, and imaging characteristics were collected. Multivariable Cox regression was used to estimate independent associations of vascular risk factors with incident myocardial infarction (MI) and ischaemic stroke for men and women. Women-to-men ratios of hazard ratios (RHRs), and related 95% confidence intervals, represent the relative effect-size magnitude by sex.
Results
Among the 363 313 participants (53.5% women), 8470 experienced MI (29.9% women) and 7705 experienced stroke (40.1% women) over 12.66 [11.93, 13.38] years of prospective follow-up. Men had greater risk factor burden and higher arterial stiffness index at baseline. Women had greater age-related decline in aortic distensibility. Older age [RHR: 1.02 (1.01–1.03)], greater deprivation [RHR: 1.02 (1.00–1.03)], hypertension [RHR: 1.14 (1.02–1.27)], and current smoking [RHR: 1.45 (1.27–1.66)] were associated with a greater excess risk of MI in women than men. Low-density lipoprotein cholesterol was associated with excess MI risk in men [RHR: 0.90 (0.84–0.95)] and apolipoprotein A (ApoA) was less protective for MI in women [RHR: 1.65 (1.01–2.71)]. Older age was associated with excess risk of stroke [RHR: 1.01 (1.00–1.02)] and ApoA was less protective for stroke in women [RHR: 2.55 (1.58–4.14)].
Conclusion
Older age, hypertension, and smoking appeared stronger drivers of cardiovascular disease in women, whereas lipid metrics appeared stronger risk determinants for men. These findings highlight the importance of sex-specific preventive strategies and suggest priority targets for intervention in men and women.
Keywords: Risk factors, Sex differences, Myocardial infarction, Stroke
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide, claiming 18.6 million lives each year.1 Important differences in the incidence, patterns, and outcomes of CVD have been described between men and women but the reasons for these differences are not completely understood.
Traditionally, CVDs were considered to predominantly affect men,2 which in part was due to a higher prevalence of some traditional risk factors (e.g. smoking) in men, but also to a historical under-representation of in women in health research.3 Understanding differences in sex-specific markers such as obstetric history, and variation in traditional risk factors is essential to optimizing CVD preventive strategies in men and women.4
Despite the substantial improvement made in narrowing the gap between sexes in cardiovascular outcomes over the past decades, existing evidence remains limited by restricted cohort sizes, lack of granularity in assessment of exposures such as lipid profiles, and for some risk factors such as smoking, contradictory results have been identified across populations.5 Moreover, the sex-based differences of more recently identified cardiovascular risk markers, such as ApoA, remain poorly understood.
The present study characterizes differences in distribution of cardiovascular risk factors between men and women in the UK Biobank cohort. These include traditional cardiovascular risk factors such as diabetes and hypertension, more novel risk factors such as ApoA, and emerging surrogate markers of cardiovascular risk such as aortic distensibility. Secondly, independent associations of a comprehensive host of risk factors with incident stroke and myocardial infarction (MI) are examined with a focus on defining sex-based differences in magnitudes of these associations.
Methods
Study population
The UK Biobank is a large population-based cohort that recruited over 500 000 people aged 40–69 years from across the UK between 2006 and 2010. Baseline assessment was performed according to a predefined protocol and included a touchscreen questionnaire, face-to-face interviews, a series of physical measures, and blood sampling.6 Incident health events are longitudinally tracked for all participants through electronic health record linkages with hospital admission and death registration data, with outcomes documented according to International Classification of Disease codes.7,8 Analysis was conducted on the set of participants for whom complete case data were available across the predefined set of exposures, outcomes, and covariates.
Selection and ascertainment of covariates
Major risk factors were selected on basis of existing literature and biological knowledge of their role in risk of stroke and MI. The following factors were included: age, ethnicity, Townsend deprivation index,9 body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference, hip circumference, waist-hip ratio (WHR), glycated haemoglobin (HbA1c), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, ApoA, apolipoprotein B (ApoB), smoking status, and diagnosed diabetes, hypertension and hypercholesterolaemia. All variables were extracted from baseline visit and were defined by UK Biobank field ID as identified in Supplementary material online, Tables S1 and S2.
Arterial phenotyping
Arterial compliance has previously been reported to be an important marker for cardiovascular risk prediction.10 To obtain a unifying reflection of the dynamic trend in the cardiovascular risk profile across different ages and sexes, we evaluated two measures of arterial compliance: arterial stiffness index (ASI) and aortic distensibility (AoD). ASI provides an estimate of large artery stiffness and has been linked to incident cardiovascular events and mortality,11 whilst AoD provides an estimate of aortic compliance and is an indicator of local aortic bioelastic function and has been demonstrated to predict ischaemic events.10
Additional details regarding the extraction, outlier definition, and categorization of variables are provided in Supplementary Methods online.
Ascertainment of outcomes
The primary outcomes were incident MI and incident stroke. Outcomes were ascertained using linked hospital and mortality data, as outlined in Supplementary material online, Table S3, over a mean of 12.66 [11.93, 13.38] years of prospective follow-up.
Individuals who had already experienced the outcome of interest prior to recruitment were excluded from analysis for that outcome. Individuals who suffered an endpoint during follow-up were left censored at the time of the event. In both analyses, individuals were right censored at date of death, or at the date of the last event reported in the UK Biobank (MI: 2021–11-12, stroke: 2021–10-25).
Statistical analysis
This study is reported following the STROBE Statement guidelines.12 Baseline characteristics are presented for the whole cohort and stratified by sex as number (percentage) for categorical variables, mean (standard deviation) for normally distributed continuous variables, and median [interquartile range] for non-normally distributed continuous variables. Normality of distribution was ascertained by visual inspection of histograms.
The prevalence of prior MI and stroke at baseline visit, and subsequent incidence during follow-up were defined for the entire cohort and separately for each sex. Multivariable Cox proportional hazard regression models were used to obtain hazard ratios (HRs) and 95% confidence intervals (CIs) for each risk factor on the outcome. To calculate whether the HR differed between women and men, sex was added to the model as an interaction term to calculate RHRs, 95% CIs, and P-values.13 These are presented as women-to-men RHRs in all cases, a RHR > 1 demonstrates a greater proportional hazard increase in women, whilst a RHR < 1 indicates a greater proportional hazard increase in men.
Unadjusted models including only sex and age were conducted (Supplementary material online, Table S4). Multivariate models additionally included BMI, WHR, HbA1c, SBP, DBP, total cholesterol, HDL-C, LDL-C, triglycerides, ApoA, ApoB, and Townsend deprivation index as continuous variables. Abdominal obesity, smoking status, ethnicity, diabetes, high cholesterol, and hypertension were also included as categorical variables.
Arterial stiffness measures were not included as covariates. This is because these measures act as ‘proxy’ measures capturing the downstream vascular consequence of a range of adverse cardiometabolic factors which, in this study, is described by the host of cardiometabolic covariates that are already included in the model. The additional inclusion of arterial stiffness measures in the main models is therefore likely to be problematic from a causal perspective as it is likely to attenuate significant (and biologically causal) associations due to adjustment for a mediator.
Each model was assessed for multicollinearity to ensure variance inflation factors (VIF) for all covariates were less than 10. Each model was assessed for a violation of the proportional hazard assumption by visual assessment of scaled Schoenfeld residuals.
Poisson regression was used to obtain unadjusted incidence rates of stroke and MI per 1000 person years by sex. All analyses were performed using R version 4.2.1.14
Results
Baseline characteristics
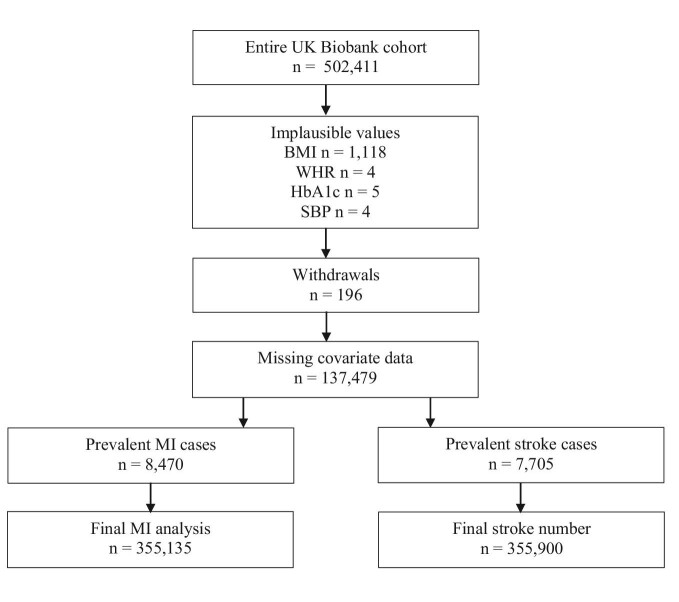
The baseline characteristics of the 363 605 participants (53.8% female) included in the study are reported in Table 1. Missingness and reasons for exclusion are shown in Figure 1. At baseline visit, 8830 (19.6% female) participants reported a prior MI, and 6377 reported a previous stroke (40.9% female). In both men and women, the median age at enrolment was 58 years, 94.9% were of white ethnicity, and median Townsend deprivation index was –2.18 in women and –2.17 in men (i.e. more affluent that the UK national average).
Table 1.
Characteristics of study cohort at baseline stratified by sex
| Overall | Female | Male | |
|---|---|---|---|
| No (%) | 363 605 | 195 542 (53.8) | 168 063 (46.2) |
| Age (years) | 58.00 [50.00, 63.00] | 58.00 [50.00, 63.00] | 58.00 [50.00, 64.00] |
| Ethnic group | |||
| All other ethnic groups combined | 18 574 (5.1) | 9946 (5.1) | 8628 (5.1) |
| White | 345 031 (94.9) | 185 596 (94.9) | 159 435 (94.9) |
| Townsend deprivation index | –2.18 [–3.66, 0.43] | –2.18 [–3.65, 0.37] | –2.17 [–3.67, 0.50] |
| Smoking status | |||
| Never | 198 656 (54.6) | 116 470 (59.6) | 82 186 (48.9) |
| Previous | 126 944 (34.9) | 61 832 (31.6) | 65 112 (38.7) |
| Current | 38 005 (10.5) | 17 240 (8.8) | 20 765 (12.4) |
| Hypertension | 107 814 (29.7) | 50 301 (25.7) | 57 513 (34.2) |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 136.00 [124.50, 149.50] | 133.00 [121.00, 147.00] | 139.00 [128.50, 151.50] |
| Diastolic blood pressure | 82.23 (10.10) | 80.65 (9.93) | 84.07 (9.98) |
| Diabetes | 19 455 (5.4) | 7472 (3.8) | 11 983 (7.1) |
| HbA1c (mmol/mol) | 35.20 [32.80, 37.90] | 35.20 [32.70, 37.70] | 35.30 [32.80, 38.10] |
| BMI (kg/m2) | 26.72 [24.13, 29.85] | 26.08 [23.43, 29.64] | 27.29 [24.98, 30.03] |
| Waist-to-hip ratio | 0.87 (0.09) | 0.82 (0.07) | 0.94 (0.06) |
| Abdominal obesity | |||
| Normal | 182 633 (50.2) | 134 536 (68.8) | 48 097 (28.6) |
| Abdominal obesity | 180 972 (49.8) | 61 006 (31.2) | 119 966 (71.4) |
| High cholesterol | 72 296 (19.9) | 29 209 (14.9) | 43 087 (25.6) |
| Lipids | |||
| Cholesterol (mmol/L) | 5.70 (1.13) | 5.88 (1.11) | 5.49 (1.12) |
| HDL cholesterol (mmol/L) | 1.40 [1.17, 1.68] | 1.56 [1.33, 1.82] | 1.24 [1.06, 1.45] |
| LDL direct (mmol/L) | 3.56 (0.86) | 3.63 (0.86) | 3.49 (0.86) |
| Triglycerides (mmol/L) | 1.48 [1.05, 2.14] | 1.33 [0.96, 1.89] | 1.69 [1.18, 2.44] |
| Apolipoprotein A (g/L) | 1.51 [1.35, 1.70] | 1.61 [1.45, 1.79] | 1.41 [1.27, 1.56] |
| Apolipoprotein B (g/L) | 1.02 [0.86, 1.18] | 1.02 [0.87, 1.18] | 1.01 [0.86, 1.18] |
| Aortic distensibility ascending (×10−3, mmHg−1) (% missing = 92.93) | |||
| ≤53 years | 2.64 [1.88, 3.55] | 2.66 [1.84, 3.66] | 2.63 [1.92, 3.39] |
| 54 to 69 years | 1.27 [0.79, 1.99] | 1.15 [0.69, 1.89] | 1.42 [0.92, 2.08] |
| ≥70 years | 0.71 [0.48, 1.06] | 0.60 [0.42, 0.88] | 0.81 [0.56, 1.18] |
| Aortic distensibility descending (×10−3, mmHg−1) (% missing = 92.67) | |||
| ≤ 53 years | 3.40 [2.68, 4.28] | 3.42 [2.69, 4.31] | 3.37 [2.66, 4.24] |
| 54 to 69 years | 2.30 [1.70, 3.04] | 2.18 [1.58, 2.94] | 2.42 [1.84, 3.14] |
| ≥ 70 years | 1.56 [1.16, 2.05] | 1.40 [1.04, 1.83] | 1.71 [1.29, 2.19] |
| Arterial stiffness index (m/s) (% missing = 65.03) | 8.95 [6.86, 11.07] | 8.20 [6.30, 10.37] | 9.77 [7.72, 11.77] |
| Myocardial infarction | |||
| Prevalent cases | 8803 | 1728 (19.6) | 7075 (80.4) |
| Incident cases | 8470 | 2529 (29.9) | 5941 (70.1) |
| Stroke | |||
| Prevalent cases | 6377 | 2610 (40.9) | 3767 (59.1) |
| Incident cases | 7705 | 3089 (40.1) | 4616 (59.9) |
g/L, grams per litre; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; kg/m2, kilograms metres squared; LDL, low-density lipoprotein; mmHg, millimetres of mercury; mmol/mol, millimoles per mole; mmol/L, millimoles per litre; m/s, metres per second; N, number. Results are mean (standard deviation), number (percentage) or median [interquartile range].
Figure 1.
Flowchart of exclusion and inclusion. BMI: body mass index, WHR: waist-to-hip ratio, HbA1c: glycated haemoglobin, SBP: systolic blood pressure, MI: myocardial infarction.
Men were more likely to be current or previous smokers compared to women (12.4% vs 8.8% current, and 38.7% vs 31.6% previous). Women had lower rates of diagnosed hypertension compared to men (25.7% vs 34.2%), as well as lower SBP (133 mmHg vs 139 mmHg) and DBP (81 mmHg vs 84 mmHg, respectively). The prevalence of diabetes was higher in men than women (7.1% vs 3.8%) although levels of HbA1c were similar across the sexes. Compared to men, women had a lower BMI (26.08 kg/m2 vs 27.29 kg/m2), smaller WHR (0.82 vs 0.94), and lower prevalence of abdominal obesity (31.2% vs 71.4%).
Men had higher rates of diagnosed hypercholesterolaemia than women (25.6% vs 14.9%). Compared to men, women had higher total cholesterol (5.88 vs 5.49 mmol/L), HDL-C (1.56 vs 1.24 mmol/L), LDL-C (3.63 vs 3.49 mmol/L), ApoA (1.61 vs 1.41 g/L), and ApoB (1.02 s 1.01 g/L) but lower triglycerides (1.33 vs 1.69 mmol/L).
Women had lower ASI than men at baseline (8.20 m/s vs 9.77 m/s). At younger ages (≤ 53 year), women and men had similar AoD at the ascending (2.66 vs 2.63 10−3 mmHg−1) and descending (3.42 vs 3.37 10−3 mmHg−1) aorta. However, at older ages (≥70 years), women had lower AoD at both the ascending (0.60 vs 0.81 10−3 mmHg−1) and descending (1.40 vs 1.71 10−3 mmHg−1) aorta than men.
Myocardial infarction
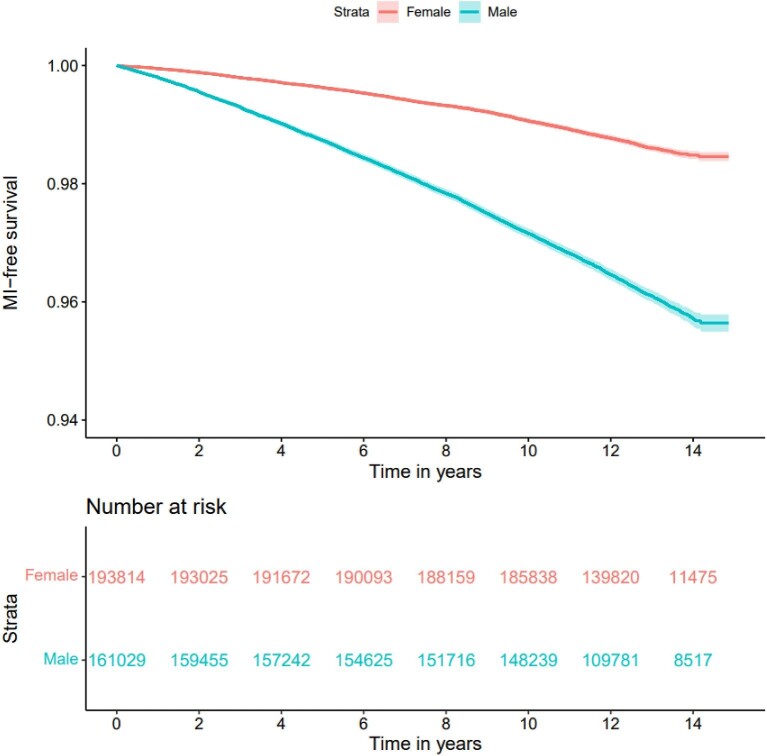
During the study period, 8470 incident cases of MI were recorded, of which 29% occurred in women (Figure 2). The crude unadjusted incidence rate of MI per 1000 person years was 1.06 (95% CI 1.02–1.10) in women and 3.04 (95% CI 2.99–3.11) in men (Supplementary material online, Table S5). Men had a 2.8 times greater unadjusted hazard of incident MI than women (HR 2.81, 95% CI: 2.70–2.92, P < 0.001).
Figure 2.
Unadjusted survival curve for myocardial infarction by sex.
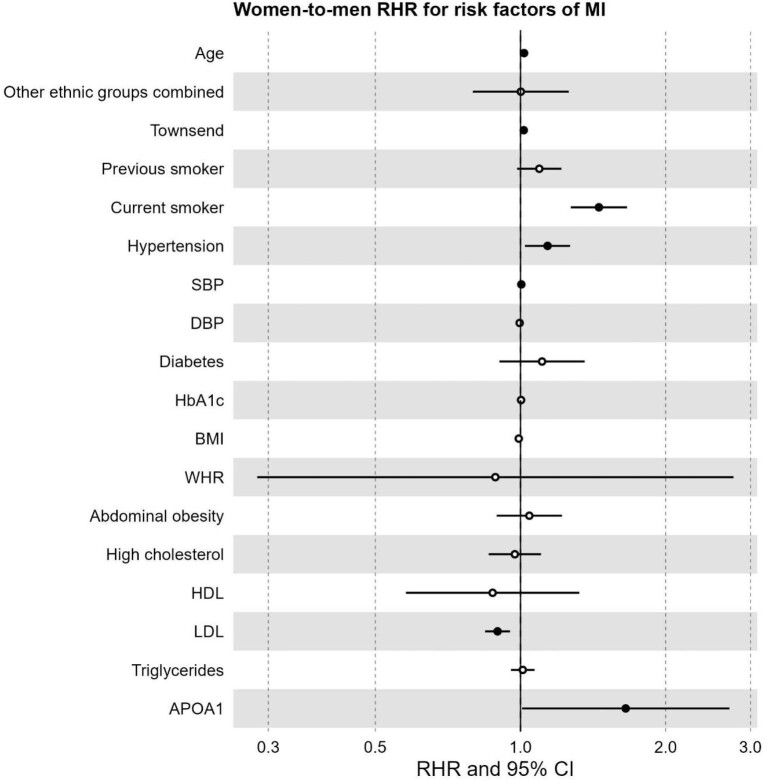
Older age was associated with a higher hazard of MI in both sexes but conferred a greater hazard in women compared to men (RHR 1.02, 95% CI 1.01–1.03, P < 0.001). Similarly, greater deprivation was associated with a proportionally greater hazard of MI in women (RHR 1.02, 95% CI 1.00–1.03, P = 0.045), as was current smoking when compared to never smoking (RHR 1.45, 95% CI 1.27–1.66, P < 0.001). The presence of clinical hypertension was associated with a proportionally greater hazard in women (RHR 1.14, 95% CI 1.02–1.27, P = 0.019), and so was higher SBP (RHR 1.00, 95% CI 1.00–1.01, P = 0.014). In contrast, the association between higher LDL-C and MI was more pronounced in men, with a 10% relative increase in the hazard of MI (RHR 0.90, 95% CI 0.84–0.95, P < 0.001). Finally, the inverse association between ApoA and MI was stronger in men compared to women (RHR 1.65, 95% CI 1.01–2.71, P = 0.047). There was no indication of differential impacts of other risk factors on MI. The results are reported in Table 2 and Figure 3.
Table 2.
Hazard ratios for risk factors of MI by women and men, including women-to-men ratio of hazard ratio
| Women | Men | Women-to-men RHR | |
|---|---|---|---|
| Covariates | HR (95% CI, P value) | HR (95% CI, P value) | RHR (95% CI, P value) |
| Age | 1.06 (1.06–1.07, P < 0.001) | 1.04 (1.04–1.05, P < 0.001) | 1.02 (1.01–1.03, P < 0.001) |
| Ethnicity | |||
| White | — | — | — |
| All other ethnic groups combined | 1.01 (0.83–1.23, P = 0.924) | 1.00 (0.89–1.13, P = 0.959) | 1.00 (0.80–1.26, P = 0.988) |
| Townsend Deprivation Index | 1.03 (1.02–1.04, P < 0.001) | 1.01 (1.00–1.02, P = 0.005) | 1.02 (1.00–1.03, P = 0.045) |
| Smoking status | |||
| Never | — | — | — |
| Previous | 1.22 (1.11–1.33, P < 0.001) | 1.11 (1.05–1.18, P < 0.001) | 1.09 (0.98–1.22, P = 0.096) |
| Current | 2.72 (2.43–3.04, P < 0.001) | 1.86 (1.73–2.00, P < 0.001) | 1.45 (1.27–1.66, P < 0.001) |
| Hypertension | 1.48 (1.35–1.62, P < 0.001) | 1.30 (1.22–1.38, P < 0.001) | 1.14 (1.02–1.27, P = 0.019) |
| Systolic blood pressure | 1.02 (1.01–1.02, P < 0.001) | 1.01 (1.01–1.01, P < 0.001) | 1.00 (1.00–1.01, P = 0.014) |
| Diastolic blood pressure | 0.99 (0.99–1.00*, P = 0.001) | 1.00 (0.99–1.00, P = 0.008) | 1.00 (0.99–1.00, P = 0.240) |
| Diabetes | 1.45 (1.22–1.73, P < 0.001) | 1.31 (1.18–1.45, P < 0.001) | 1.11 (0.90–1.36, P = 0.321) |
| HbA1c | 1.02 (1.01–1.02, P < 0.001) | 1.01 (1.01–1.02, P < 0.001) | 1.00 (1.00–1.01, P = 0.381) |
| BMI | 0.99 (0.98–1.00, P = 0.030) | 1.00 (0.99–1.01, P = 0.60 | 0.99 (0.98–1.00, P = 0.188) |
| Waist-to-hip ratio | 2.55 (0.98–6.65, P = 0.055) | 2.82 (1.53–5.22, P = 0.001) | 0.89 (0.28–2.77, P = 0.836) |
| Abdominal obesity | |||
| Normal | — | — | — |
| Abdominal obesity | 1.04 (0.92–1.19, P = 0.508) | 1.00 (0.92–1.09, P = 0.959) | 1.04 (0.89–1.22, P = 0.595) |
| High cholesterol | 1.31 (1.18–1.45, P < 0.001) | 1.34 (1.26–1.44, P < 0.001) | 0.97 (0.86–1.10, P = 0.673) |
| HDL cholesterol | 0.64 (0.46–0.88, P = 0.006) | 0.72 (0.56–0.93, P = 0.013) | 0.88 (0.58–1.32, P = 0.529) |
| LDL cholesterol | 1.30 (1.24–1.37, P < 0.001) | 1.45 (1.40–1.50, P < 0.001) | 0.90 (0.84–0.95, P < 0.001) |
| Triglycerides | 1.01 (0.96–1.07, P = 0.566) | 1.00 (0.98–1.03, P = 0.769) | 1.01 (0.96–1.07, P = 0.704) |
| Apolipoprotein A | 0.77 (0.52–1.14, P = 0.191) | 0.47 (0.35–0.64, P < 0.001) | 1.65 (1.01–2.71, P = 0.047) |
BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; RHR, ratio of hazard ratios.
Figure 3.
Forest plot of women-to-men ratio of hazard ratios for risk factors of MI.RHR: ratio of hazard ratios, CI: confidence interval, SBP: systolic blood pressure, DBP: diastolic blood pressure, HbA1c: glycated haemoglobin, BMI: body mass index, WHR: waist-to-hip ratio, HDL: high-density lipoprotein, LDL: low-density lipoprotein, APOA1: Apolipoprotein A. Significant risk factors displayed as solid points (P-value < 0.05).
Stroke
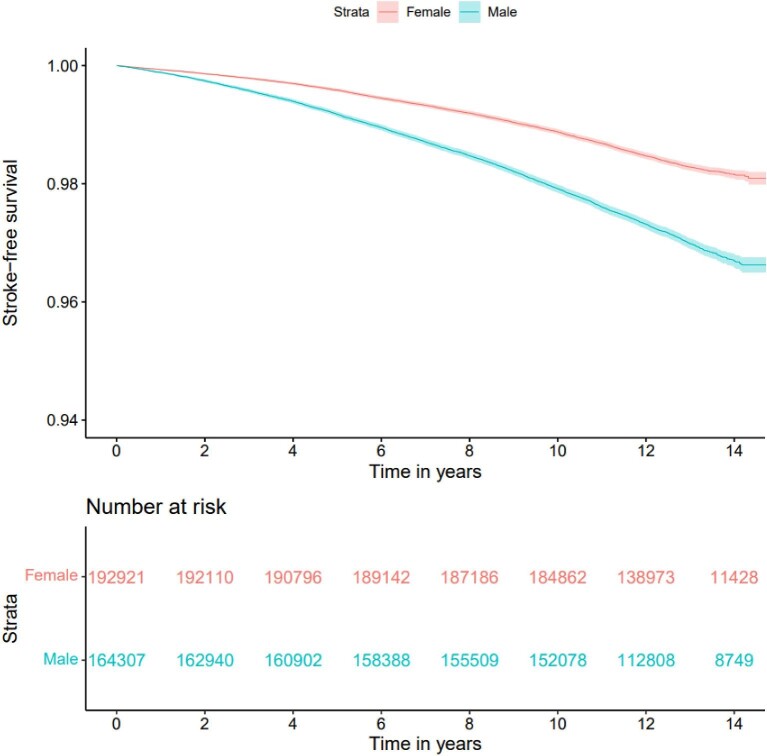
A total of 7705 incident cases of stroke were recorded. Among these 40.1% occurred in women (Figure 4). The crude unadjusted incidence rate per 1000 person years was 1.30 (95% CI 1.26–1.35) for women, and 2.30 (95% CI 2.24–2.37) in men (Supplementary material online, Table S5). Overall, men had an unadjusted 1.7 times greater hazard of incident stroke than women (HR 1.73, 95% CI 1.67–1.80, P < 0.001).
Figure 4.
Unadjusted survival curve for stroke by sex.
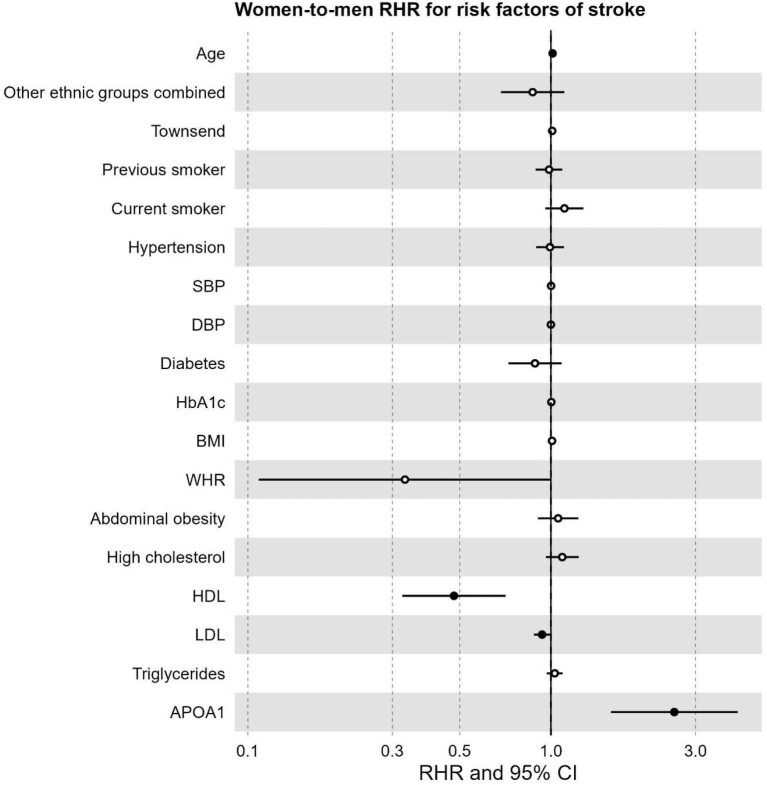
Older age was associated with proportionally greater hazard of stroke in women compared to men (RHR 1.01, 95% CI 1.00–1.02, P = 0.002). Conversely, higher HDL-C was more strongly associated with hazard of stroke in men, with a 52% proportionally greater hazard per unit HDL-C (RHR 0.48, 95% CI 0.32—0.71, P < 0.001). This was similar for LDL-C with a 6% proportionally greater hazard of stroke per unit LDL-C (RHR 0.94, 95% CI 0.88–1.00, P = 0.036). Finally, there was a large sex difference in ApoA (RHR 2.55, 95% CI 1.58–4.14, P < 0.001), suggesting a stronger protective effect in men compared to women. There was no indication of differential impacts of other risk factors on stroke. The results are reported in Table 3 and Figure 5.
Table 3.
Hazard ratios for risk factors of stroke by women and men, including women-to-men ratio of hazard ratio
| Women | Men | Women-to-men | |
|---|---|---|---|
| Covariates | HR (95% CI, P value) | HR (95% CI, P value) | RHR (95% CI, P value) |
| Age | 1.10 (1.10–1.11, P < 0.001) | 1.09 (1.08–1.09, P < 0.001) | 1.01 (1.00–1.02, P = 0.002) |
| Ethnicity | |||
| White | — | — | — |
| All other ethnic groups combined | 0.82 (0.68–1.00, P = 0.047) | 0.95 (0.82–1.09, P = 0.463) | 0.87 (0.68–1.11, P = 0.259) |
| Townsend Deprivation Index | 1.04 (1.02–1.05, P < 0.001) | 1.03 (1.02–1.04, P < 0.001) | 1.01 (0.99–1.03, P = 0.199) |
| Smoking status | |||
| Never | — | — | — |
| Previous | 1.07 (0.99–1.16, P = 0.088) | 1.09 (1.02–1.16, P = 0.013) | 0.99 (0.89–1.09, P = 0.786) |
| Current | 1.84 (1.64–2.07, P < 0.001) | 1.66 (1.52–1.81, P < 0.001) | 1.11 (0.96–1.28, P = 0.166) |
| Hypertension | 1.37 (1.26–1.49, P < 0.001) | 1.38 (1.29–1.48, P < 0.001) | 0.99 (0.89–1.10, P = 0.896) |
| Systolic blood pressure | 1.01 (1.01–1.01, P < 0.001) | 1.01 (1.01–1.01, P < 0.001) | 1.00 (1.00–1.00, P = 0.472) |
| Diastolic blood pressure | 1.00 (1.00–1.01, P = 0.220) | 1.00 (1.00–1.01, P = 0.126) | 1.00 (0.99–1.01, P = 0.964) |
| Diabetes | 1.16 (0.98–1.37, P = 0.088) | 1.30 (1.16–1.46, P < 0.001) | 0.89 (0.72–1.08, P = 0.241) |
| HbA1c | 1.02 (1.01–1.02, P < 0.001) | 1.02 (1.02–1.02, P < 0.001) | 1.00 (1.00–1.01, P = 0.357) |
| BMI | 1.00 (0.99–1.01, P = 0.650) | 0.99 (0.98–1.00, P = 0.141) | 1.01 (1.00–1.02, P = 0.165) |
| Waist-to-hip ratio | 1.85 (0.77–4.43, P = 0.168) | 5.62 (2.82–11.17, P < 0.001) | 0.33 (0.11–1.00, P = 0.051) |
| Abdominal obesity | |||
| Normal | — | — | — |
| Abdominal obesity | 0.98 (0.87–1.10, P = 0.746) | 0.93 (0.84–1.02, P = 0.134) | 1.06 (0.91–1.23, P = 0.483) |
| High cholesterol | 1.09 (0.99–1.20, P = 0.087) | 1.00 (0.93–1.08, P = 0.991) | 1.09 (0.96–1.23, P = 0.176) |
| HDL cholesterol | 0.85 (0.64–1.13, P = 0.274) | 1.78 (1.36–2.35, P < 0.001) | 0.48 (0.32–0.71, P < 0.001) |
| LDL direct | 0.96 (0.91–1.00, P = 0.065) | 1.02 (0.98–1.07, P = 0.280) | 0.94 (0.88–1.00, P = 0.036) |
| Triglycerides | 1.00 (0.95–1.05, P = 0.970) | 0.97 (0.94–1.00, P = 0.068) | 1.03 (0.97–1.09, P = 0.346) |
| Apolipoprotein A | 0.89 (0.63–1.26, P = 0.508) | 0.35 (0.25–0.49, P < 0.001) | 2.55 (1.58–4.14, P < 0.001) |
BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; RHR, ratio of hazard ratios.
Figure 5.
Forest plot of women-to-men ratio of hazard ratios for risk factors of stroke. RHR: ratio of hazard ratios, CI: confidence interval, SBP: systolic blood pressure, DBP: diastolic blood pressure, HbA1c: glycated haemoglobin, BMI: body mass index, WHR: waist-to-hip ratio, HDL: high-density lipoprotein, LDL: low-density lipoprotein, APOA1: Apolipoprotein A. Significant risk factors displayed as solid points (P-value < 0.05).
Missing data and multicollinearity assessment
ASI measurement was added to the UK Biobank protocol towards the end of recruitment (available for 35%). AoD is an image-derived metric and was available for the random subset of participants included in the UK Biobank Imaging Study (available for 7%). Given that these variables were not included in the main models, the impact of their missingness was not further assessed. For the variables included in the main model, a subanalysis comparing participants with complete data (72.37%) to those with missing data is reported in Supplementary material online, Table S6. The results of the analysis suggest that retained cases did not systematically differ from those with missing data.
VIF scores for variables scoring over 10 in the initial model are reported in Supplementary material online, Table S7. After removal of total cholesterol and ApoB from the model, all covariates had VIF score of less than 10.
Discussion
This study demonstrates sex differences in major risk factors for MI and stroke. First, emerging risk markers, including LDL-C and ApoA, were more strongly associated with cardiovascular outcomes in men compared to women. Second, current smoking, socioeconomic deprivation, hypertension, and older age were associated with disproportionately greater increases in hazards of cardiovascular outcomes in women compared to men. Third, examination of arterial compliance measures, which have been previously found to predict cardiovascular events, validated a baseline higher risk in men but a steeper age-related trajectory of increasing risk in women.
Lipid profiles
Previous studies have reported stronger associations between LDL-C and CVD in men. A prior Mendelian randomization study reported a 32% increased odds of CVD per 1 SD increase in genetically predicted LDL-C in women, with a corresponding 52% increased odds in men.15 Similarly, the observational PURE registry reported higher magnitudes of association of non-HDL-C traits with CVD in men.5
In this study, ApoA was associated with lower hazards of stroke and MI in men. Under causal assumptions, this suggests that ApoA might be less protective against CVD in women. This differential protective effect has been previously reported for MI,16 but not for stroke.17 This has important implications for the clinical investigation of interventions on ApoA aimed at reducing cardiovascular events and its use in risk prediction models.
Previous studies have highlighted inverse associations between HDL-C and CVD.18–20 This was replicated in this study for MI, but paradoxically for stroke we identified a direct association with a 52% relative higher hazard per unit increase in men. This result is likely due to the inclusion of ApoA in this model, which is a known component of the HDL-C particle. In a post-hoc analysis excluding ApoA, the previously reported inverse association between HDL-C and stroke in men was replicated (HR 0.83, 95% CI 0.74–0.93, P = 0.001). In line with previous studies which did not demonstrate benefit of HDL-C augmentation on risk of cardiovascular events,21 this result suggests that previously described ‘protective’ signals of HDL-C on cardiovascular events may be conveyed predominantly by ApoA.
Age
Age was associated with an increased hazard of stroke and MI in women, compared with men. This is consistent with previous research that identified women experience their first stroke or MI event at older ages.22,23 This result was further validated by examining arterial compliance measures. Despite men having a higher baseline ASI, reflecting greater baseline cardiovascular risk, we observed a steeper age-related decline in AoD in women, which suggest a more rapid age-related increase in cardiovascular risk. Given the age demographic of this cohort, this increase in cardiovascular risk may occur after loss of the cardioprotective exposure to oestrogen. From a clinical perspective, this suggests that cardiovascular risk assessment and prevention strategies should be intensified with progressive age, particularly in women.
Smoking status
This study identified a proportionally greater association between current smoking status and MI in women, in line with previous findings.24,25 The mechanism behind the excess risk in women is likely multifactorial. It might relate to differences in smoking patterns, or it might be conferred by higher rates of smoking continuation: women are less likely to receive counselling, to stop smoking, and on average quitters stop at an older age than men.26–28 Overall, the results highlight the key importance of smoking cessation in women and call for further research exploring whether the heterogeneity in hazards relates to biological or structural differences in healthcare systems.
Hypertension
In this study, we identified a disproportionately higher hazard of MI associated with hypertension in women compared to men. The INTERHEART22 and PURE study5 have both previously reported similar findings. In this study, we did not identify any differences in hazards for stroke, though previous UK Biobank research found a higher risk in women only at higher stages of hypertension.29 There are multiple potential mechanisms behind this. Hypertension is known to be a major risk factor for hypertensive disorders of pregnancy30 and the development of the acute, severe cardiac, and endothelial dysfunction that ensues from these might act to heighten cardiovascular risk. Women with hypertension may also be treated differently to men, for example through avoidance of drug classes contraindicated in pregnancy.31 The results of this study provide evidence to support the growing view that sex-specific frameworks should be considered for screening, monitoring, and weighing of blood pressure as a component of global cardiovascular risk.32
Socioeconomic status
The results of this study identified an association of larger magnitude between Townsend deprivation index and MI in women compared to men. Lower socioeconomic status (SES) is known to be associated with CVD,33 although in a previous study on the UK Biobank no sex difference was found.34 However, a large meta-analysis of more than 22 million participants found that low SES was associated with 34% excess risk of developing coronary heart disease in women compared to men.35 The mechanism behind this is unclear. As the Townsend deprivation index is an area-based rather than individual-based measure, the excess risk might reflect a greater relative deprivation among women compared to men within a single area. The findings might also reflect important inequities in access to healthcare with lower SES that might disproportionately impact women.
Strengths and limitations
The key strength of this investigation lies in the inclusion of a broad set of risk factors including a detailed lipid profile of LDL-C, HDL-C, and ApoA, which highlighted the substantial differences in association across the sexes. Additionally, this study utilized a well-validated and intensely phenotyped population source, prospective outcome ascertainment with a substantial number of events ascertained through well-validated disease codes, and correlation of key findings with the novel cardiovascular risk marker of arterial compliance which further elucidate age-related cardiovascular risk trends.
Limitations of this study include the lack of diversity in ethnicity and SES in the UK Biobank, both of which may limit generalizability. The study population is also relatively healthy in comparison to the general public. The risk factor of smoking status was collected via self-report, which could lead to reporting bias. Finally, many of the lipid measures had more than 5% of data missing, though analysis of the characteristics of the individuals with missing data revealed no substantial systematic differences to the complete case cohort.
Conclusions
The results of this study identify that smoking, low SES, and hypertension were more strongly associated with MI in women, whereas lipid traits were more strongly associated with both MI and stroke in men. Considering the historically male predominance in health research providing the basis for decisions made in everyday clinical practice, these results encourage further elucidation of sex-specific treatment effects in order to better inform clinical decision-making and treatment prioritization.
Supplementary Material
Acknowledgment
This study was conducted using the UK Biobank resource under access application 2964.
Contributor Information
Elizabeth Remfry, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Maddalena Ardissino, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, London SW3 6LY, UK.
Celeste McCracken, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, National Institute for Health Research Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 9DU, UK.
Liliana Szabo, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Semmelweis University, Heart and Vascular Center, Hungary, Budapest 1122, Hungary; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield EC1A 7BE, UK.
Stefan Neubauer, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, National Institute for Health Research Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 9DU, UK.
Nicholas C Harvey, MRC Lifecourse Epidemiology Centre, University of Southampton, Southampton SO16 6YD, UK; NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton SO16 6YD, UK.
Mamas A Mamas, Keele Cardiovascular Research Group, Keele University, Keele ST5 5BG, UK; Institute of Population Health, University of Manchester, Manchester M13 9NT, UK.
John Robson, Wolfson Institute of Population Health Sciences, Queen Mary University of London, London E1 4NS, UK.
Steffen E Petersen, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield EC1A 7BE, UK; Health Data Research UK, London NW1 2BE, UK; Alan Turing Institute, London NW1 2DB, UK.
Zahra Raisi-Estabragh, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield EC1A 7BE, UK.
Funding statement
E.R. is funded by the Wellcome Trust Health Data in Practice (HDiP) Programme (218584/Z/19/Z). M.A. recognizes the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports her Academic Clinical Fellowship post. C.M. and S.N. are supported by the Oxford NIHR Biomedical Research Centre ((IS-BRC-1215-20008). S.N. is also supported by the Oxford British Heart Foundation Centre of Research Excellence (RE/18/3/34214). L.S. received funding from the European Association of Cardiovascular Imaging (EACVI Research Grant App000076437). N.C.H. acknowledges support from MRC (MC_PC_21003; MC_PC_21001) and NIHR Southampton Biomedical Research Centre. This project was enabled through access to the MRC eMedLab Medical Bioinformatics infrastructure, supported by the Medical Research Council (www.mrc.ac.uk; MR/L016311/1). S.E.P. acknowledges support from the ‘SmartHeart’ EPSRC programme grant (www.nihr.ac.uk; EP/P001009/1) and also from the CAP-AI programme, London's first AI enabling programme focused on stimulating growth in the capital's AI Sector. CAP-AI is led by Capital Enterprise in partnership with Barts Health NHS Trust and Digital Catapult and is funded by the European Regional Development Fund and Barts Charity. S.E.P. has also received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 825903 (euCanSHare project). Z.R.-E. recognizes the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports her Academic Clinical Lectureship post.
Role of the funders
The funders provided support in the form of salaries for authors as detailed above but did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: S.E.P. provides consultancy to Cardiovascular Imaging Inc, Calgary, Alberta, Canada. The remaining authors have nothing to declare.
Data availability statement
This research was conducted using the UK Biobank resource under access application 2964. UK Biobank will make the data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.
Ethical approval
This study complies with the Declaration of Helsinki; the work was covered by the ethical approval for UK Biobank studies from the NHS National Research Ethics Service on 17th June 2011 (Ref 11/NW/0382) and extended on 18 June 2021 (Ref 21/NW/0157) with written informed consent obtained from all participants.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao M, Woodward M, Vaartjes I, Millett ERC, Klipstein-Grobusch K, Hyun K et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barc J, Erdmann J. Sex matters? Sex matters! Cardiovasc Res 2022;118:e1–e3. [DOI] [PubMed] [Google Scholar]
- 4. Bairey Merz CN, Ramineni T, Leong D. Sex-specific risk factors for cardiovascular disease in women-making cardiovascular disease real. Curr Opin Cardiol 2018;33:500–505. [DOI] [PubMed] [Google Scholar]
- 5. Walli-Attaei M, Joseph P, Rosengren A, Chow CK, Rangarajan S, Lear SA et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet North Am Ed 2020;396:97–109. [DOI] [PubMed] [Google Scholar]
- 6. UK Biobank . UK Biobank: protocol for a large-scale prospective epidemiological resource [cited 2022 Jun 7]. Available from: https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf. [Google Scholar]
- 7. UK Biobank . Hospital inpatient data [Internet]. 2020. Aug [cited 2022 Jun 7]. Available from: https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/HospitalEpisodeStatistics.pdf [Google Scholar]
- 8. UK Biobank . Mortality data: linkage to death registries [Internet]. 2020. Jun [cited 2022 Jun 7]. Available from: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/DeathLinkage.pdf [Google Scholar]
- 9. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Routledge; 1988. p. 240. [Google Scholar]
- 10. Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J Am Heart Assoc 2018;7:e007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pannier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring arterial compliance in humans. Am. J. Hypertens 2002;15:743–753. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 13. Woodward M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart 2019;105:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. R Core Team . R: A Language and Environment for Statistical Computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available from: https://www.R-project.org/. [Google Scholar]
- 15. Cupido AJ, Asselbergs FW, Schmidt AF, Hovingh GK. Low-density lipoprotein cholesterol attributable cardiovascular disease risk is sex specific. J Am Heart Assoc 2022;11:e024248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet North Am Ed 2001;358:2026–2033. [DOI] [PubMed] [Google Scholar]
- 17. O'Donnell MJ, McQueen M, Sniderman A, Pare G, Wang X, Hankey GJ et al. Association of Lipids, Lipoproteins, and Apolipoproteins with Stroke Subtypes in an International Case Control Study (INTERSTROKE). J Stroke 2022;24:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: The Northern Manhattan Stroke Study. JAMA 2001;285:2729–2735. [DOI] [PubMed] [Google Scholar]
- 19. Wannamethee SG, Shaper AG, Ebrahim S. HDL-cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke 2000;31:1882–1888. [DOI] [PubMed] [Google Scholar]
- 20. Sanossian N, Saver JL, Navab M, Ovbiagele B. High-density lipoprotein cholesterol. Stroke 2007;38:1104–1109. [DOI] [PubMed] [Google Scholar]
- 21. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJP, Komajda M et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 22. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 2008;29:932–940. [DOI] [PubMed] [Google Scholar]
- 23. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Y, Li SX, Liu Y, Rodriguez F, Watson KE, Dreyer RP et al. Sex-specific risk factors associated with first acute myocardial infarction in young adults. JAMA Netw Open 2022;5:e229953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet North Am Ed 2011;378:1297–1305. [DOI] [PubMed] [Google Scholar]
- 26. Peters SAE, Huxley RR, Woodward M. Do smoking habits differ between women and men in contemporary Western populations? Evidence from half a million people in the UK Biobank study. BMJ Open 2014;4:e005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dieleman LA, van Peet PG, Vos HMM. Gender differences within the barriers to smoking cessation and the preferences for interventions in primary care a qualitative study using focus groups in The Hague, The Netherlands. BMJ Open 2021;11:e042623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahmanian SD, Diaz PT, Wewers ME. Tobacco use and cessation among women: research and treatment-related issues. J Womens Health 2011;20:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong C, Zhou C, Fu C, Hao W, Ozaki A, Shrestha N et al. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK Biobank participants. Biol Sex Differ 2022;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ardissino M, Slob EAW, Millar O, Reddy RK, Lazzari L, Patel KHK et al. Maternal hypertension increases risk of preeclampsia and low fetal birthweight: genetic evidence from a Mendelian randomization study. Hypertension 2022;79:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalibala J, Pechère-Bertschi A, Desmeules J. Gender differences in cardiovascular pharmacotherapy—the example of hypertension: a mini review. Front Pharmacol 2020;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerdts E, Sudano I, Brouwers S, Borghi C, Bruno RM, Ceconi C et al. Sex differences in arterial hypertension. Eur Heart J 2022;43:4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 2018;363:k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Backholer K, Peters SAE, Bots SH, Peeters A, Huxley RR, Woodward M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health 2017;71:550–557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research was conducted using the UK Biobank resource under access application 2964. UK Biobank will make the data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.