Abstract
The toxin AaH-II, from the scorpion Androctonus australis Hector venom, is a 64 amino acid peptide that targets voltage-gated Na+ channels (VGNCs) and slows their inactivation. While at macroscopic cellular level AaH-II prolongs the action potential (AP), a functional analysis of the effect of the toxin in the axon initial segment (AIS), where VGNCs are highly expressed, was never performed so far. Here, we report an original analysis of the effect of AaH-II on the AP generation in the AIS of neocortical layer-5 pyramidal neurons from mouse brain slices. After determining that AaH-II does not discriminate between Nav1.2 and Nav1.6, i.e. between the two VGNC isoforms expressed in this neuron, we established that 7 nM was the smallest toxin concentration producing a minimal detectable deformation of the somatic AP after local delivery of the toxin. Using membrane potential imaging, we found that, at this minimal concentration, AaH-II substantially widened the AP in the AIS. Using ultrafast Na+ imaging, we found that local application of 7 nM AaH-II caused a large increase in the slower component of the Na+ influx in the AIS. Finally, using ultrafast Ca2+ imaging, we observed that 7 nM AaH-II produces a spurious slow Ca2+ influx via Ca2+-permeable VGNCs. Molecules targeting VGNCs, including peptides, are proposed as potential therapeutic tools. Thus, the present analysis in the AIS can be considered a general proof-of-principle on how high-resolution imaging techniques can disclose drug effects that cannot be observed when tested at the macroscopic level.
Keywords: AaH-II toxin, Voltage-gated sodium channels, Axon initial segment, Action potential, Neocortical layer-5 pyramidal neuron, Calcium
Subject terms: Biological techniques, Biophysics, Chemical biology, Neuroscience, Physiology
Introduction
Since its isolation from the venom of the scorpion Androctonus australis hector, the alpha-toxin AaH-II was found to bind with very high affinity to rat brain axolemma1. Specifically, AaH-II targets diverse voltage-gated Na+ channels (VGNCs)2 with nanomolar affinity3, and it prolongs their inactivation in different systems4,5 by trapping a deactivated state6. After a scorpion sting, the mixture of different molecules that forms the venom causes paralysis, cardiac arrhythmia and death in mammals7, but it may also exacerbate the systemic inflammatory response and promote the development of lung injury8,9.
The study of the effects of this toxin is important to unravel the specific role and function of VGNCs, but it may also suggest novel pathways for medical research. Initially, AaH-II was studied for its toxicity and research focused on means to neutralize the peptide. Indeed, low doses of the toxin can generate an immunogenic response leading to expression of antibodies and this response can be used to create protective therapies against scorpion envenoming by designing constructs either with fusion proteins10 or nanoparticles11. However, natural peptides targeting VGNCs have also been proposed as interesting pharmacological tools for understanding physiological processes or exploring novel therapeutic strategies. For example, a similar scorpion toxin, Amm VIII, was reported to have hyperalgesic effects triggered by gain-of-function of VGNCs12, while Hm1a, a selective activator of Nav1.1, the principal VGNC expressed in GABAergic interneurons, could rescue mouse models of epileptic Dravet syndrome from seizures and immature death13. Most of these effects are concentration-dependent and while low concentrations may have therapeutic effects, high concentrations can be detrimental. Thus, given the interest in designing novel peptides targeting VGNCs and inspired by animal toxins, the fine characterisation of the effects of these peptides, and AaH-II in particular, at minimal doses becomes important, in particular in the central nervous system.
In the brain, VGNCs are highly expressed in the axon initial segment (AIS)14,15 where they trigger the generation of the action potential (AP)16,17. It is therefore expected that the effect of a VGNC activator is stronger in this neuronal compartment where the channel density is higher with respect to the other sites. In neocortical pyramidal neurons, the VGNC isoforms that mediate AP generation are Nav1.2 and Nav1.618, which are distinguished by different biophysical properties19. Recently, using a cutting-edge technique to record fast Na+ currents in the AIS20, in combination with ultrafast membrane potential (Vm) imaging21 and Ca2+ imaging22,23 techniques, we were able to characterise the Na+ and Ca2+ currents mediated by Nav1.2 and the way in which its functional interaction with the BK Ca2+-activated K+ channel shapes AP generation in the AIS of mouse neocortical layer-5 (L5) pyramidal neurons24. This preparation is ideal to optically record Na+ currents because the AIS can be seen under transmitted light and it does not spatially overlap with other structures of the cells, such as basal dendrite, allowing a clear discrimination of fluorescence signals. Here, using the same approach in the identical preparation, we characterised the effect of the mutated toxin AaH-IIR62K (AaH-II) on the generation of the AP at a concentration that produces the minimal perturbation of the somatic AP. We show that, in contrast to the VGNC inhibitor G1G4-huwentoxin-IV used in the previous study, AaH-II does not distinguish between Nav1.2 and Nav1.6. Yet, we found an effect that can be attributed to the exquisite permeability to Ca2+ of Nav1.225.
Results
The present study was motivated by the interest in understanding the effect of a strong VGNC modulator at low doses and, in long terms, in using similar molecules as potential therapeutic tools. The first set of experiments was a dose–response analysis of the effect of AaH-II in HEK293 cells, expressing either human Nav1.2 or human Nav1.6, using automated patch-clamp recordings, similarly to what it was done in previous studies24,26. This analysis was necessary to establish the efficacy of the toxin on the two VGNC isoforms expressed in L5-pyramidal neurons18. Na+ currents were induced in voltage clamp by 50-ms pulses from − 100 to 0 mV. The examples reported in Fig. 1A show that bath perfusion of AaH-II, at the concentrations of 0.3 nM or 10 nM, prolonged the duration of both Nav1.2-mediated or Nav1.6-mediated Na+ currents by delaying their inactivation. The test was performed in distinct groups of cells at concentrations ranging from 0.01 to 10 nM. While the effects of the toxin at different concentrations on the current amplitudes are reported in Table 1, to quantify the effect of the toxin, we calculated the half inactivation time (T50) defined as the time at which the Na+ current reaches 50% of the peak current (see red arrows in Fig. 1A). As shown in the plots of Fig. 1B, AaH-II targeted both Nav1.2 and Nav1.6 VGNCs, poorly discriminating between them, and it produced a consistent effect on both channels in the concentration range 0.3–10 nM. The effect of the toxin was already maximal at 3 nM concentration.
Figure 1.
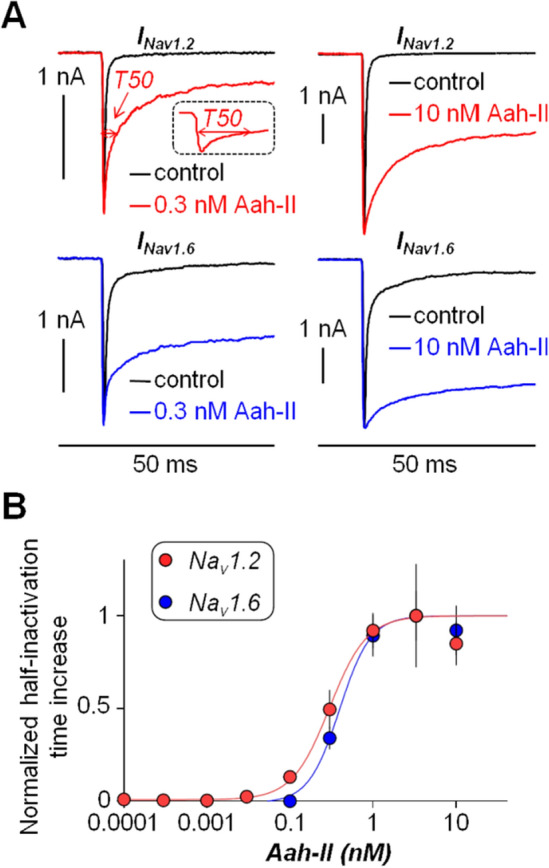
Dose–response analysis of the effect of AaH-II on Nav1.2 and Nav1.6 in HEK293 cells expressing either one or the other VGNC. (A) Nav1.2 or Nav1.6 currents, elicited by clamping the Vm from − 100 mV to 0 mV for 50 ms, in control solution and after addition of 0.3 nM or 10 nM of AaH-II. Red and blue traces are the Nav1.2 and Nav1.6 currents, respectively, in the presence of the toxin; on the top-left panel the current parameter T50 with 0.3 nM AaH-II is indicated with the red arrow also in the inset of the reported trace. (B) Mean ± SEM of the normalized half inactivation time increase following AaH-II addition at different concentrations for Nav1.2 and for Nav1.6 currents. Values reported in the plot were as follows. For Nav1.2 (red lines and symbols): 0.01 nM—0.004 ± 0.006 (N = 7); 0.03 nM—0.02 ± 0.01 (N = 6); 0.1 nM 0.13 ± 0.04 (N = 4); 0.3 nM 0.49 ± 0,11 (N = 5); 1 nM—0.92 ± 0.09 (N = 9); 3 nM—1 ± 0.28 (N = 4); 10 nM—0.85 ± 0.12 (N = 6). For Nav1.6 (blue lines and symbols): 0.01 nM—0.01 ± 0.005 (N = 7); 0.03 nM—0.007 ± 0.005 (N = 9); 0.1 nM—0.0009 ± 0.006 (N = 2); 0.3 nM—0.34 ± 0.06 (N = 20); 1 nM—0.89 ± 0.11 (N = 16); 3 nM—1 ± 0.13 (N = 20); 10 nM—0.92 ± 0.13 (N = 17). AaH-II does not discriminate between the two VGNC isoforms.
Table 1.
Mean ± SEM of the % change of the amplitude (N of cells) of the Nav1.2 and Nav1.6 current produced by AaH-II at different concentrations in HEK293 cells expressing either one or the other VGNC.
| 0.01 nM | 0.03 nM | 0.1 nM | 0.3 nM | 1 nM | 3.3 nM | 10 nM | |
|---|---|---|---|---|---|---|---|
| Nav1.2 | 8.2 ± 9.7 (N = 7) | 14.4 ± 3.2 (N = 6) | 6.9 ± 11.7 (N = 4) | 30.2 ± 5.7 (N = 5) | 31.8 ± 9.9 (N = 9) | 30.4 ± 9.4 (N = 4) | 56.5 ± 11.4 (N = 6) |
| Nav1.6 | 8.3 ± 2.4 (N = 7) | -1.8 ± 4.2 (N = 9) | -0.0 ± 3.3 (N = 2) | 4.5 ± 3.4 (N = 20) | 9.8 ± 3.3 (N = 16) | 20.7 ± 6.7 (N = 20) | 13.6 ± 5.0 (N = 17) |
With this acquired information, we next aimed at establishing the effective concentrations that change the shape of the AP of L5 pyramidal neurons in brain slices, recorded by using the patch clamp technique in current clamp mode. Specifically, we elicited APs by setting the initial Vm to − 80 mV and by injecting 2–3 nA current pulses of 3–4 ms duration, using a local delivery approach described in our previous report to apply the toxin24. Since in this study the equivalent concentration producing an effect in the brain slice was ~ 40 times larger with respect to the experiments in HEK293 cells, we initially tested the effect of local delivery of 40 nM AaH-II on the somatic AP of L5 pyramidal neurons, expecting at this concentration a maximal effect. As shown in the example of Fig. 2A, AaH-II delivery at 40 nM distorted dramatically the AP kinetics by increasing its amplitude and by widening its shape. The same effect was observed in all 5 cells tested at this concentration. Specifically, the change in AP width, quantified by the time at which Vm > − 30 mV during the AP, was 60.19 ± 28.82% and it was statistically significant (p < 0.01, paired t-test). Thus, in the example of Fig. 2B, AaH-II was tested at three lower concentrations (5 nM, 10 nM and 20 nM) that produced progressively larger effects evaluated by the change in AP amplitude and by the change in AP width. The same assessment was repeated in N = 8 cells (Fig. 2C). Whereas the change in amplitude was not significant at any concentration, the change in width was 12.83 ± 7.77% and 25.41 ± 12.75% for the AaH-II delivery at 10 nM and 20 nM respectively, in both cases statistically significant (p < 0.01, paired t-test). At 5 nM, a detectable change in the AP width was observed only in 50% of the cells.
Figure 2.
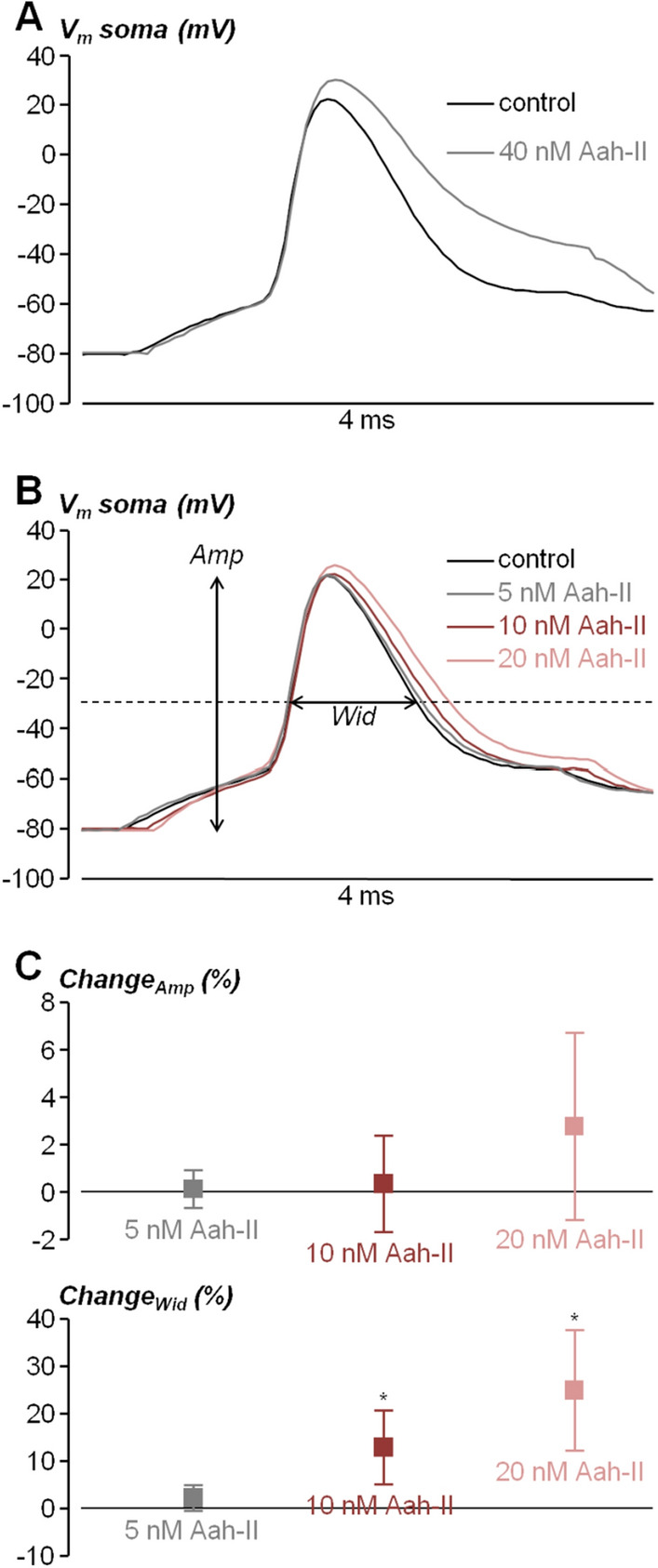
Dose–response analysis of the effect of AaH-II on the somatic AP from L5 pyramidal neurons in brain slices. (A) Somatic AP elicited by current pulse injection in a L5 pyramidal neuron in control condition (black trace) and after local delivery of 40 nM AaH-II (grey trace). (B) Somatic AP elicited by current pulse injection in another L5 pyramidal neuron in control condition (black trace) and after local delivery of 5 nM (grey trace), 10 nM (red trace) and 20 nM (pink trace) AaH-II. The amplitude (Amp) and width (Wid), used for the analysis, are indicated for the control trace. (C) Mean ± SD (N = 8 cells) of the Amp and Wid changes following AaH-II addition at 5 nM (grey lines and symbols), 10 nM (red lines and symbols) and 20 nM (pink lines and symbols). Values reported in the plot were the followings. For the Amp parameter: 5 nM—0.13 ± 0.81%; 10 nM—0.33 ± 2.02%; 20 nM 2.80 ± 3.94%. For the Wid parameter: 5 nM—2.34 ± 2.65%; 10 nM—12.83 ± 7.77%; 20 nM 25.41 ± 12.75%. “*” indicates that the change in the Wid parameter was significant at 10 nM (p = 0.0094, paired t-test) and at 20 nM (p = 0.0034, paired t-test).
Since 5 nM was too low to observe a consistent effect on the AP width, whereas at 10 nM the increase in AP was already substantial, we performed the following experiments by delivering AaH-II at the intermediate concentration of 7 nM, where the effect on the somatic AP width could be expected to be minimal but consistently detectable. In the next series of experiments, cells were also filled with the voltage-sensitive dye JPW1114, in order to simultaneously record the somatic AP with the patch clamp electrode and the AP in the AIS optically. Since the kinetics of the AP in the proximal part of the AIS is similar to that of the somatic AP (see24), to perform the analysis of the AP shape the AIS we averaged fluorescence over a 20 µm segment between ~ 10 and ~ 30 µm from the soma. As shown in the example of Fig. 3A, whereas local delivery of 7 nM AaH-II produced an increase in somatic AP width of ~ 7%, it produced in the same cell an increase in the corresponding axonal AP width of ~ 40%, calculated after normalising the optical AP in the AIS to the somatic AP. The combined analysis of the somatic and axonal AP following the delivery of 7 nM AaH-II was repeated in N = 9 cells (Fig. 3B). In control conditions (i.e. without the toxin), whereas the AP width ranged between 0.7 and 1.4 ms in the soma, it ranged between, 0.45 and 1 ms in the AIS. In the soma, a change in the AP width (11.00 ± 9.35%) was measured in 8/9 cells tested. Thus, we could refer to 7 nM as the minimal concentration producing a consistent effect on the somatic AP after local delivery. In the AIS, the change in AP width was instead 35.57 ± 15.89%. But most importantly, the effect on the axonal AP was significantly larger than the effect on the somatic AP in the cells tested (p < 0.01, paired t-test).
Figure 3.
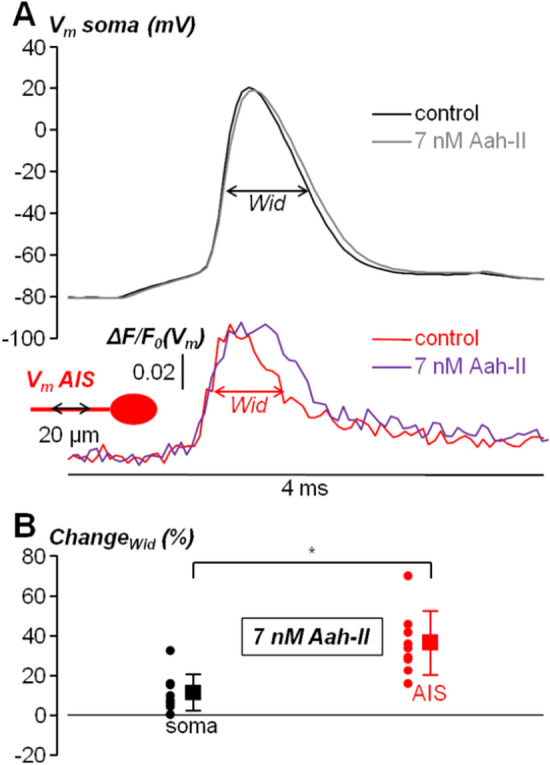
Effect of local delivery of 7 nM AaH-II on the somatic and AIS AP in L5 pyramidal neurons. (A) On the top, somatic AP elicited by current pulse injection in a L5 pyramidal neuron in control condition (black trace) and after local delivery of 7 nM AaH-II (grey trace). On the bottom, AIS (~ 10–30 µm from the soma) AP in the same cell in control condition (red trace) and after AaH-II delivery (purple trace). Wid parameters used for the analysis are indicated for the soma and AIS relative to the control traces. (B) Scatter plots and mean ± SD (N = 9 cells) of the soma (black lines and symbols) and AIS (red lines and symbols) Wid changes following AaH-II addition. “*” indicates that the change was significantly larger in the AIS (p = 3.4·10–4, paired t-test).
The result reported in Fig. 3 could be attributed to the fact that in the AIS, where the AP is generated, the density of VGNCs is higher than in the rest of the cell14. This means that the analysis of the effect of the toxin on the AIS is capable of disclosing major effects that cannot be revealed by analysing the somatic AP. In keeping with the result reported in Fig. 3B, we next assessed the effect of AaH-II delivery at minimal concentration on Na+ influx and Ca2+ influx in the AIS associated with the generating AP. In these two sets of experiments, cells were filled through the patch pipette either with the Na+ indicator ING-2, or with the Ca2+ indicator Oregon-Green BAPTA-5N (OG5N), and fluorescence transients associated with the AP, in the same AIS as in Fig. 3, were recorded at 10 k frames/s. As shown in the example of Fig. 4A, AaH-II delivery caused a large increase in the late Na+ influx, corresponding to an increase in the non-inactivating Na+ current. This phenomenon was consistently observed in N = 9 cells where this test was performed. In the example of Fig. 4B, AaH-II delivery caused instead a small increase in Ca2+ influx, corresponding to a widening of the Ca2+ current. Again, this phenomenon was consistently observed in N = 9 cells where this test was performed. We calculated mean ± SD of the change in the maximum fluorescence transients (Fig. 4C) for Na+ (85.2 ± 25.9%) and Ca2+ (11.1 ± 5.3%) and we established that for both signals the increase produced by the toxin was significant (p < 0.01, paired t-test).
Figure 4.
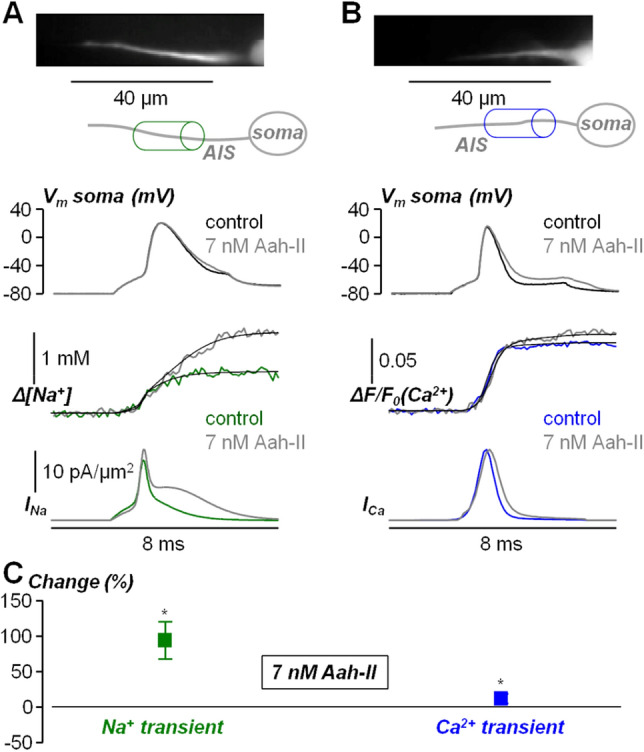
Effect of local delivery of 7 nM AaH-II on the Na+ and Ca2+ influx associated with the AP in the AIS. (A) Top, fluorescence image (Na+ indicator ING-2) of the AIS and its reconstruction with a region of interest indicated. Middle, somatic AP in control solution (black trace) and after delivering 7 nM of AaH-II (grey trace). Bottom, associated Na+ transients fitted with a model function to calculate the Na+ current (INa) in control solution (green trace) and after delivering 7 nM of AaH-II (grey trace). (B) Top, fluorescence image (Ca2+ indicator OG5N) of the AIS and its reconstruction with a region of interest indicated. Middle, somatic AP in control solution (black trace) and after delivering 7 nM of AaH-II (grey trace). Bottom, associated Ca2+ transients fitted with a model function to calculate the Ca2+ current (ICa) in control solution (blue trace) and after delivering 7 nM of AaH-II (grey trace). (C) Mean ± SD (N = 9 cells) of the Na+ (green lines and symbols) and Ca2+ (blue lines and symbols) changes following AaH-II addition. Values reported in the plot were 85.2 ± 25.9%for Na+ and 11.1 ± 5.3% Ca2+. “*” indicates that the change was significant for the Na+ (p = 1.07·10–6, paired t-test) and for Ca2+ (p = 1.58·10–5, paired t-test).
The fact that the change in Na+ influx was larger is not surprising since Ca2+ influx is mostly mediated by voltage-gated Ca2+ channels in the AIS27. Yet, we have previously demonstrated that the VGCC-component of the Ca2+ influx decreases with the distance from the soma and that the Nav1.2-mediated Ca2+ component is dominant in the distal part of the AIS24. Thus, we hypothesised that the change in the AP waveform had negligible effect on the VGCC-component of the Ca2+ influx and that the Ca2+ transient increase produced by AaH-II delivery was due to an increase in the Nav1.2-mediated Ca2+ component. To assess this hypothesis, we reanalysed the Ca2+ imaging dataset distinguishing from the distal AIS (30–40 µm from the soma) and proximal AIS (5–15 µm from the soma). In the example of Fig. 5A, AaH-II local delivery caused an increase in Ca2+ influx in the distal AIS, but not in the proximal AIS. As shown in Fig. 5B, in control condition, the peak of the distal Ca2+ current occurred before the peak of the proximal Ca2+ current. Then, the distal Ca2+ current (but not the proximal Ca2+ current) was widened in shape by the delivery of the toxin. In the 9 cells tested, the mean ± SD of the change in the maximum Ca2+ transients (Fig. 4C) was 18.7 ± 10.3% in the distal AIS and 0.0 ± 3.0% in the proximal AIS. A significant increase in Ca2+ transient was measured only in the distal AIS (p < 0.01, paired t-test). The spatial distribution of the Ca2+ transient increase can be also visually appreciated in Supplemental Movie S1. The additional Ca2+ signal produced by the delayed inactivation of Nav1.2 is slower than the control Ca2+ signal and it can therefore be considered a spurious component. Overall, the results presented in this study show how an analysis of signalling in neuronal compartments of high VGNC density is necessary to unravel the effects of molecules targeting these channels, which are hidden in standard AP analysis from the cell bodies.
Figure 5.
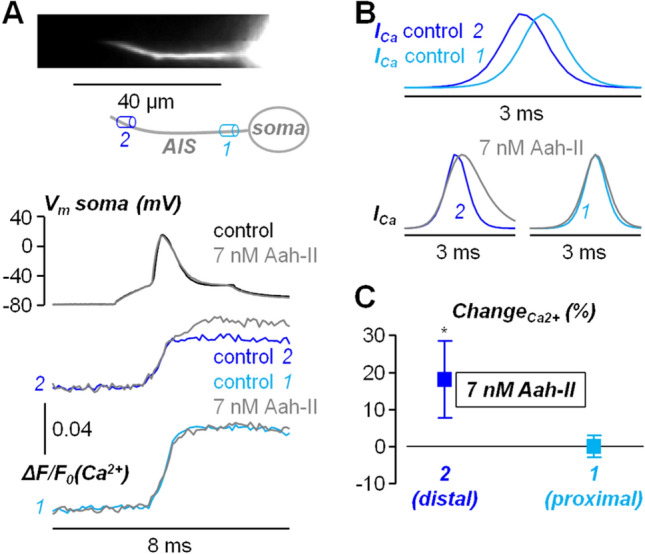
Spatial effect of AaH-II delivery on the Ca2+ influx associated with the AP in the AIS. (A) Top, Ca2+ fluorescence image of the AIS and its reconstruction with a proximal region (1) and a distal region (2) indicated. Middle, somatic AP in control solution (black trace) and after AaH-II delivery (grey trace). Bottom, associated Ca2+ transients in 2 and 1 in control solution (dark trace and light blue trace respectively) and after delivering 7 nM of AaH-II (grey traces). (B) Top, superimposed Ca2+ currents (ICa) in 2 and 1 showing the different kinetics. Bottom, ICa in 2 and 1 in control solution (dark trace and light blue trace respectively) and after AaH-II delivery (grey traces). (C) Mean ± SD (N = 9 cells) of the Ca2+ changes in distal AIS regions (30–40 µm from the soma, dark blue lines and symbols) and in proximal AIS regions (5–15 µm from the soma, light blue lines and symbols). Values reported in the plot were 18.7 ± 10.3% in the distal AIS and 0.0 ± 3.0% in the proximal AIS. “*” indicates that the change was significant in the distal AIS (p = 0.0011, paired t-test).
Discussion
Previous studies have analysed the effects of AaH-II by using patch clamp recordings from sites that do not necessarily correspond to where Na+ channels are highly expressed. The rationale of the present study was to optically analyse the effect of the toxin at sites with high VGNC expression at a concentration where the effect was just detectable in the soma. The first important finding that we report in this analysis is that local application of AaH-II produces a significant change in the shape of the somatic AP at a low 7 nM concentration, i.e. ~ 10 times less with respect to the VGNC inhibitor G1G4-huwentoxin-IV used in a previous study24. This AaH-II effect corresponds to a slight widening of the somatic AP, but when we analysed the corresponding AP in the AIS, using a Vm indicator, the widening was significantly larger. Consistently with this result, local delivery of AaH-II caused a significant increase in Na+ influx associated with firing activity, that can be recorded in the AIS28,29. Not surprisingly, since the primary effect of AaH-II was to prolong VGNC inactivation, the larger influx could be attributed to the slow (or persistent) component of the Na+ current30, which is normally larger for Nav1.6 in control condition31. When analysing Ca2+ influx associated with the AP, we found a significant increase after local delivery of AaH-II, that was however substantially smaller with respect to the increase in Na+ influx. It is known that a large component of Ca2+ influx in the AIS of L5 pyramidal neurons is mediated by voltage-gated Ca2+ channels27, but it is also known that Nav1.2 is permeable to Ca2+25. Thus, we hypothesised that the additional Ca2+ in the presence of the toxin could be mediated by Nav1.2. We have previously demonstrated that the Nav1.2-mediated Ca2+ current and the VGCC-mediated Ca2+ current can be clearly distinguished by the different kinetics, the first one peaking during the AP rising phase and the second one peaking in the AP falling phase24. In addition, we have demonstrated that the Nav1.2-mediated Ca2+ current is dominant in the distal part of the AIS where the VGCC-mediated Ca2+ current is weaker24. Consistently with this previous result and with the present hypothesis, we found that the increase in Ca2+ influx caused by AaH-II delivery occurred primarily in the distal part of the AIS. Yet, whereas in control condition the Nav1.2-mediated Ca2+ current is sharp and occurs during the rising phase of the generating AP24, the wider Ca2+ current in the presence of AaH-II produced a late abnormal Ca2+ transient that can perturb axonal homeostasis.
From the methodological point of view, the present results indicate that the effect of a molecule targeting VGNCs should be analysed at the sites where the channels are highly expressed because substantial changes of physiological signals can be hidden when the analysis is performed at the full cellular scale. In the case of neurons, these sites are the AIS and the nodes of Ranvier32,33. Mutations of VGNC genes are at the origin of a variety of channelopathies34,35 making VGNC potential interesting targets for future therapies36. In addition, VGNCs are the potential targets of novel treatments for cancer37 and inflammation-related diseases38. The present study represents a proof-of-principle on how our high-resolution imaging approach to explore AP generation in the AIS can be used to obtain relevant information on therapeutic candidates targeting VGNCs.
Methods
Ethical approval
Experiments in brain slices were performed at the Laboratory of Interdisciplinary Physics in Grenoble in accordance with European Directives 2010/63/UE on the care, welfare and treatment of animals. Procedures were reviewed by the ethics committee Cometh Grenoble n°12 (French Ministry of Research) affiliated to the animal facility of the University of Grenoble Alpes where experiments were carried out (accreditation #E3842110001). The same procedures and all methods are reported in accordance with ARRIVE guidelines.
Experiments in HEK293 cells expressing either Nav1.2 or Nav1.6.
The mutated toxin AaH-IIR62K was the one described in a previous report26 and it was produced by Smartox Biotechnology (Saint Egrève, France). Experiments on HEK293 cells were performed on stable lines expressing either human NaV1.2 or human NaV1.6 channels. Automated patch-clamp recordings in whole-cell configuration were performed using the SyncroPatch 384PE from Nanion (München, Germany) as previously described26. The intracellular solution contained (in mM): 10 CsCl, 110 CsF, 10 NaCl, 10 EGTA and 10 HEPES (pH 7.2, osmolarity 280 mOsm). The extracellular solution contained (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 glucose and 10 HEPES (pH 7.4, osmolarity 298 mOsm). Voltage clamp experiments were performed at a holding potential of − 100 mV at room temperature (18–22 °C) and currents, elicited by clamping the Vm at 0 mV for 50 ms, were sampled at 20 kHz. To quantitatively assess the effect of the toxin, we calculated the parameter T50 defined as the time of 50% of inactivation between the peak of the sodium current and the residual current at the end of the depolarisation. For each cell, T50 was obtained after 15 min of AaH-II incubation and the change in the T50 parameter was obtained from the value prior to AaH-II incubation.
Brain slices
The extracellular solution that we used contained (in mM): 125 NaCl, 26 NaHCO3, 1 MgSO4, 3 KCl, 1 NaH2PO4, 2 CaCl2 and 20 glucose, bubbled with 95% O2 and 5% CO2. Mice (C57BL/6j, 21–35 postnatal days old) were fed ad libitum until euthanasia. Animals were anesthetised by isoflurane inhalation and decapitated and the entire brain was quickly removed. Brain slices including the somato-sensory cortex (350 µm thick) were prepared using the procedure described in previous report20,24, using a Leica VT1200 vibratome (Wetzlar, Germany). Slices were incubated at 37 °C for 45 min and maintained at room temperature before use.
Electrophysiology, imaging and drug delivery in brain slices
Slices were transferred to the recording chamber and patch-clamp recordings were made at the temperature of 32–34 °C using a Multiclamp 700 A (Molecular Devices, Sannyvale, CA). Pipettes with a resistance of 4–6 MΩ were produced using a Sutter P-1000 puller (Sutter, Novato, CA). The intracellular solution contained (in mM): 125 KMeSO4, 5 KCl, 8 MgSO4, 5 Na2-ATP, 0.3 Tris-GTP, 12 Tris-Phosphocreatine, 20 HEPES, adjusted to pH 7.35 with KOH. For Vm recordings in the AIS, neuronal membranes were loaded with the voltage-sensitive dye D-2-ANEPEQ (JPW1114, 0.2 mg/mL, Thermo Fisher Scientific) for 30 min using a first patch clamp recording and then re-patched a second time with dye free solution. For Na+ or Ca2+ recordings in the AIS, either the Na+ indicator ING-2 (IonBiosciences, San Marcos, TX) or the Ca2+ indicator Oregon Green BAPTA-5N (Thermo Fisher Scientific) were added to the intracellular solution at the concentrations of 0.5 mM or 2 mM respectively. Somatic APs were elicited in current clamp mode by 2–3 ms current pulses of 2–3 nA through the patch pipette and electrical signals were acquired at 20 kHz. Notably, we used these pulses of strong current amplitude and very short duration to minimise the jitter in AP occurrence, allowing simple averaging of single trials in recordings lasting 8 ms. The bridge was corrected offline by using the recorded injected current and the measured Vm was corrected for − 11 mV junction potential. Vm imaging experiments were performed using an imaging system described in recent reports 39,40, which was based on an Olympus BX51 microscope equipped with a 60 × Nikon water immersion objective (NA = 1). Vm was excited using the 528 nm line of an LDI-7 laser (89 North, Williston, VT), band-pass filtered at 531 ± 40 nm and directed to the preparation using a 575 nm long-pass dichroic mirror. Fluorescence emission was long-pass filtered at > 610 nm and acquired with a NeuroCCD camera (Redshirt Imaging, Decatur, GA) at 20 kHz. Na+ and Ca2+ imaging experiments were performed using an imaging system described in recent reports20,24. This system was based on an upright Scientifica SliceScope microscope equipped with a motorised XY translation stage and PatchStar manipulators (Scientifica, Uckfield, UK), and a 60 × Olympus water immersion objective (NA = 1). Na+ or Ca2+ indicators were excited using the 520 nm line or the 470 nm line of a LaserBank (Cairn Research, Faversham, UK), band-pass filtered at 517 ± 10 nm or at 469 ± 17 nm and directed to the preparation using a 538 nm or a 495 nm long-pass dichroic mirror. Na+ or Ca2+ fluorescence was filtered at 559 ± 17 nm or at 525 ± 25 nm, demagnified by 0.5X and acquired at 10 kHz by a DaVinci 2 K CMOS camera (SciMeasure, Decatur, GA). To locally deliver AaH-II at a desired concentration in the proximity of the soma and AIS, we used a procedure described in a previous report24. Briefly, a pipette of 2–4 µm diameter was used to locally deliver AaH-II for 1 min, by gentle pressure application. The effect of the toxin delivery was stable for at least 10 min after application, allowing the reported measurements. Any possible long-lasting effect of our local drug delivery procedure on surrounding glial cells was not evaluated.
Data analysis
Data, from averages of 4–7 trials with identical somatic response, were analysed using custom-written code written in MATLAB. All optical recordings were corrected for photo-bleaching using multi-exponential fits of fluorescence transients in single trials without signal. The fractional change of fluorescence from the initial image (ΔF/F0) was calculated for each signal (Vm, Na+ and Ca2+) as previously described24. Only in Na+ imaging experiments the ΔF/F0 was calibrated into a Na+ transient as described in a previous report20. Since Vm optical transients were not calibrated with an absolute scale, but the size of the AP in the AIS is comparable so the somatic AP (see24), the baseline-to-peak Vm optical waveform was normalised to the somatic action AP to be able to measure the width of axonal AP consistently. Ion ΔF/F0 transients were fitted with model functions that were a multi-sigmoid sum for Na+20 and a 4-sigmoid product for Ca2+23. Na+ and Ca2+ currents were estimated by calculating the time-derivative of the fits. The currents were not however considered in the quantitative analysis of the effects of the toxin.
Statistics
The effects of AaH-II were quantified by calculating the percent change in the parameter analysed. To assess the consistency of an effect on groups of 8 or 9 cells, we performed a paired t-test and we considered 0.01 as the threshold to describe an effect as significant.
Supplementary Information
Acknowledgements
This work was supported by the Agence Nationale de la Recherche through two grants (ANR-21-CE18-0042—Nav12RESCUE and Labex Ion Channels Science and Therapeutics: program number ANR-11-LABX-0015). We are indebted to the National Infrastructure France Life Imaging and to the Fédération pour la Recherche sur le Cerveau (FRC—Grant Espoir en tête, Rotary France) for financing part of the research equipment. We are indebted to Fondation Leducq and the FEDER resources for financing the automated patch-clamp setup.
Author contributions
F.A., L.A.B. and M.C. designed the research and performed data analysis, F.A. and L.A.B. performed brain slice research, H.M., J.M. and M.D.W. designed and performed experiments in HEK293 cells, M.C. wrote the paper. All authors have given approval to the final version of the manuscript.
Data availability
The dataset used in the current study and metadata issued by the analysis are available in the public repository ZENODO at (https://zenodo.org/records/8316094).
Competing interests
M.D.W. is a founder of Smartox Biotechnology. All other authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fatima Abbas and Laila Ananda Blömer.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-55315-y.
References
- 1.DeVries GH, Lazdunski MJ. The binding of two classes of neurotoxins to axolemma of mammalian brain. Biol. Chem. 1982;257:11684–11688. doi: 10.1016/S0021-9258(18)33817-1. [DOI] [PubMed] [Google Scholar]
- 2.Billen B, Bosmans F, Tytgat J. Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Des. 2008;14:2492–2502. doi: 10.2174/138161208785777423. [DOI] [PubMed] [Google Scholar]
- 3.Alami M, Vacher H, Bosmans F, Devaux C, Rosso JP, Bougis PE, Tytgat J, Darbon H, Martin-Eauclaire MF. Characterization of Amm VIII from Androctonus mauretanicus mauretanicus: A new scorpion toxin that discriminates between neuronal and skeletal sodium channels. Biochem. J. 2003;375:551–560. doi: 10.1042/BJ20030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benoit E, Dubois JM. Properties of maintained sodium current induced by a toxin from Androctonus scorpion in frog node of Ranvier. J. Physiol. 1987;383:93–114. doi: 10.1113/jphysiol.1987.sp016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duval A, Malécot CO, Pelhate M, Rochat H. Changes in Na channel properties of frog and rat skeletal muscles induced by the AaH II toxin from the scorpion Androctonus australis. Pflugers Arch. 1989;415:361–371. doi: 10.1007/BF00370889. [DOI] [PubMed] [Google Scholar]
- 6.Clairfeuille T, Cloake A, Infield DT, Llongueras JP, Arthur CP, Li ZR, Jian Y, Martin-Eauclaire MF, Bougis PE, Ciferri C, Ahern CA, Bosmans F, Hackos DH, Rohou A, Payandeh J. Structural basis of α-scorpion toxin action on Nav channels. Science. 2019;363:8573. doi: 10.1126/science.aav8573. [DOI] [PubMed] [Google Scholar]
- 7.Bosmans F, Tytgat J. Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon. 2007;49:142–158. doi: 10.1016/j.toxicon.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sami-Merah S, Hammoudi-Triki D, Martin-Eauclaire MF, Laraba-Djebari F. Combination of two antibody fragments F(ab')(2)/Fab: An alternative for scorpion envenoming treatment. Int. Immunopharmacol. 2008;8:1386–1394. doi: 10.1016/j.intimp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Raouraoua-Boukari R, Sami-Merah S, Hammoudi-Triki D, Martin-Eauclaire MF, Laraba-Djebari F. Immunomodulation of the inflammatory response induced by Androctonus australis hector neurotoxins: biomarker interactions. Neuroimmunomodulation. 2012;19:103–110. doi: 10.1159/000330241. [DOI] [PubMed] [Google Scholar]
- 10.Legros C, Kaabi H, El Ayeb M, Céard B, Vacher H, Bougis PE, Martin-Eauclaire MF. Use of fusion protein constructs to generate potent immunotherapy and protection against scorpion toxins. Vaccine. 2001;20:934–942. doi: 10.1016/s0264-410x(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 11.Rebbouh F, Martin-Eauclaire MF, Laraba-Djebari F. Chitosan nanoparticles as a delivery platform for neurotoxin II from Androctonus australis hector scorpion venom: Assessment of toxicity and immunogenicity. Acta Trop. 2020;205:105353. doi: 10.1016/j.actatropica.2020.105353. [DOI] [PubMed] [Google Scholar]
- 12.Abbas N, Gaudioso-Tyzra C, Bonnet C, Gabriac M, Amsalem M, Lonigro A, Padilla F, Crest M, Martin-Eauclaire MF, Delmas P. The scorpion toxin Amm VIII induces pain hypersensitivity through gain-of-function of TTX-sensitive Na+ channels. Pain. 2013;154:1204–1215. doi: 10.1016/j.pain.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Richards KL, Milligan CJ, Richardson RJ, Jancovski N, Grunnet M, Jacobson LH, Undheim EAB, Mobli M, Chow CY, Herzig V, Csoti A, Panyi G, Reid CA, King GF, Petrou S. Selective NaV1.1 activation rescues Dravet syndrome mice from seizures and premature death. Proc. Natl. Acad. Sci. USA. 2018;115:E8077–E8085. doi: 10.1073/pnas.1804764115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kole MH, Ilschner S, Kampa B, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 15.Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Leterrier C. The axon initial segment, 50 years later: A nexus for neuronal organization and function. Curr. Top. Membr. 2016;77:185–233. doi: 10.1016/bs.ctm.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Huang CY, Rasband MN. Axon initial segments: Structure, function, and disease. Acad. Sci. 2018;1420:46–61. doi: 10.1111/nyas.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 19.Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J. Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipis L, Canepari M. Optical measurement of physiological sodium currents in the axon initial segment. J. Physiol. 2021;599:49–66. doi: 10.1113/JP280554. [DOI] [PubMed] [Google Scholar]
- 21.Popovic M, Vogt K, Holthoff K, Konnerth A, Salzberg BM, Grinvald A, Antic SD, Canepari M, Zecevic D. Imaging submillisecond membrane potential changes from individual regions of single axons, dendrites and spines. Adv. Exp. Med. Biol. 2015;859:57–101. doi: 10.1007/978-3-319-17641-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaafari N, De Waard M, Canepari M. Imaging fast calcium currents beyond the limitations of electrode techniques. Biophys. J. 2015;107:1280–1288. doi: 10.1016/j.bpj.2014.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaafari N, Canepari M. Functional coupling of diverse voltage-gated Ca(2+) channels underlies high fidelity of fast dendritic Ca(2+) signals during burst firing. J. Physiol. 2016;594:967–983. doi: 10.1113/JP271830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipis L, Blömer LA, Montnach J, De Waard M, Canepari M. Nav1.2 and BK channels interaction shapes the action potential in the axon initial segment. J. Physiol. 2023;599:49–66. doi: 10.1101/2022.04.12.488116. [DOI] [PubMed] [Google Scholar]
- 25.Hanemaaijer NA, Popovic MA, Wilders X, Grasman S, PavónArocas O, Kole MH. Ca2+ entry through NaV channels generates submillisecond axonal Ca2+ signaling. Elife. 2020;9:e54566. doi: 10.7554/eLife.54566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montnach J, Blömer LA, Lopez L, Filipis L, Meudal H, Lafoux A, Nicolas S, Chu D, Caumes C, Béroud R, Jopling C, Bosmans F, Huchet C, Landon C, Canepari M, De Waard M. Synthetic analogues of huwentoxin-IV spider peptide with altered human Na V 1.7/Na V 1.6 selectivity ratios. Nat. Commun. 2022;13:417. doi: 10.1038/s41467-022-27974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipkin AM, Cunniff MM, Spratt PWE, Lemke SM, Bender KJ. Functional microstructure of CaV-mediated calcium signaling in the axon initial segment. J. Neurosci. 2021;41:3764–3776. doi: 10.1523/JNEUROSCI.2843-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleidervish IA, Lasser-Ross N, Gutnick MJ, Ross WN. Na+ imaging reveals little difference in action potential-evoked Na+ influx between axon and soma. Nat. Neurosci. 2010;13:852–860. doi: 10.1038/nn.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baranauskas G, David Y, Fleidervish IA. Spatial mismatch between the Na+ flux and spike initiation in axon initial segment. Proc. Natl. Acad. Sci. USA. 2013;110:4051–4056. doi: 10.1073/pnas.1215125110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J. Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz E, Stoler O, Scheller A, Khrapunsky Y, Goebbels S, Kirchhoff F, Gutnick MJ, Wolf F, Fleidervish IA. Role of sodium channel subtype in action potential generation by neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA. 2013;115:7184–7192. doi: 10.1073/pnas.1720493115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman SA, Desmazières A, Fricker D, Lubetzki C, Sol-Foulon N. Mechanisms of sodium channel clustering and its influence on axonal impulse conduction. Cell. Mol. Life Sci. 2016;73:723–735. doi: 10.1007/s00018-015-2081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson AD, Jenkins PM. Axonal membranes and their domains: assembly and function of the axon initial segment and node of Ranvier. Front. Cell. Neurosci. 2017;9:136. doi: 10.3389/fncel.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantegazza M, Cestèle S, Catterall WA. Sodium channelopathies of skeletal muscle and brain. Physiol. Rev. 2021;101:1633–1689. doi: 10.1152/physrev.00025.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisler MH, Hill SF, Yu W. Sodium channelopathies in neurodevelopmental disorders. Nat. Rev. Neurosci. 2021;22:152–166. doi: 10.1038/s41583-020-00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li ZM, Chen LX, Li H. Voltage-gated sodium channels and blockers: An overview and where will they go? Curr. Med. Sci. 2019;39:863–873. doi: 10.1007/s11596-019-2117-0. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Sandoval AL, Hernández-Plata E, Gomora JC. Voltage-gated sodium channels: From roles and mechanisms in the metastatic cell behavior to clinical potential as therapeutic targets. Front. Pharmacol. 2023;14:1206136. doi: 10.3389/fphar.2023.1206136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelkar S, Nailwal N, Bhatia NY, Doshi G, Sathaye S, Godad AP. An update on proficiency of voltage-gated ion channel blockers in the treatment of inflammation-associated diseases. Curr. Drug. Targets. 2022;23:1290–1303. doi: 10.2174/1389450123666220819141827. [DOI] [PubMed] [Google Scholar]
- 39.Ait Ouares K, Filipis L, Tzilivaki A, Poirazi P, Canepari M. Two distinct sets of Ca2+ and K+ channels are activated at different membrane potentials by the climbing fiber synaptic potential in purkinje neuron dendrites. J. Neurosci. 2019;39:1969–1981. doi: 10.1523/JNEUROSCI.2155-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ait Ouares K, Canepari M. The origin of physiological local mglur1 supralinear Ca2+ signals in cerebellar purkinje neurons. J. Neurosci. 2020;40:1795–1809. doi: 10.1523/JNEUROSCI.2406-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in the current study and metadata issued by the analysis are available in the public repository ZENODO at (https://zenodo.org/records/8316094).


