Abstract
Induction of tumor cell senescence has become a promising strategy for anti-tumor immunotherapy, but fibrotic matrix severely blocks senescence inducers penetration and immune cells infiltration. Herein, we designed a cancer-associated fibroblasts (CAFs) triggered structure-transformable nano-assembly (HSD-P@V), which can directionally deliver valsartan (Val, CAFs regulator) and doxorubicin (DOX, senescence inducer) to the specific targets. In detail, DOX is conjugated with hyaluronic acid (HA) via diselenide bonds (Se-Se) to form HSD micelles, while CAFs-sensitive peptide is grafted onto the HSD to form a hydrophilic polymer, which is coated on Val nanocrystals (VNs) surface for improving the stability and achieving responsive release. Once arriving at tumor microenvironment and touching CAFs, HSD-P@V disintegrates into VNs and HSD micelles due to sensitive peptide detachment. VNs can degrade the extracellular matrix, leading to the enhanced penetration of HSD. HSD targets tumor cells, releases DOX to induce senescence, and recruits effector immune cells. Furthermore, senescent cells are cleared by the recruited immune cells to finish the integrated anti-tumor therapy. In vitro and in vivo results show that the nano-assembly remarkably inhibits tumor growth as well as lung metastasis, and extends tumor-bearing mice survival. This work provides a promising paradigm of programmed delivering multi-site nanomedicine for cancer immunotherapy.
Keywords: Cells senescence, Tumor stroma, Structure transformable, Programmed delivery, Anti-tumor immunotherapy
Graphical abstract
Cancer-associated fibroblasts (CAFs) triggered structure-transformable nano-assembly (HSD-P@V) was constructed for directionally delivering valsartan (Val, CAFs regulator) and doxorubicin (DOX, senescence inducer) to the specific targets, thus enhancing tumor immunotherapy.
1. Introduction
Senescence is the permanent cell cycle inhibition when cells are subjected to damage or stress signals [1,2], characterized by the secretion of senescence-associated secretory phenotype (SASP) [3,4]. Some SASP factors are inflammatory in nature and promote the recruitment and infiltration of immune cells [5], [6], [7], [8], [9]. Moreover, SASP can upregulate the expression of addressin in vascular endothelial cells to increase T cell adhesion and exosmosis, resulting in significantly enhancing T cell number [10]. In turn, it has been reported that SASP also drives the elimination of senescent cells via natural killer (NK) cells-mediated immunosurveillance [11,12], which can circumvent the negative effects of residual senescent tumor cells on tumor treatment [13,14]. Recently, it has been evident that some tumor cells undergo senescence in response to chemotherapeutic drugs, such as doxorubicin (DOX) [15], and thus not only inhibit the proliferation of tumor cells but also stimulate anti-tumor immune response [16].
Although induction of tumor cell senescence by non-lethal dose drug treatment has become a promising strategy, cancer-associated fibroblasts (CAFs) are still the barrier that impedes the infiltration of drugs and immune cells [17], [18], [19], [20]. In addition, transforming growth factor-β (TGF-β) secreted by CAFs promotes the formation of immunosuppressive microenvironment and inhibits the activity of immune cells [21], [22], [23]. Huang et al. used particles to modify CAFs as an effective strategy for treating desmoplastic cancers and remodeling the microenvironment [24]. Shen et al. designed a hybrid nanozyme bomb to accelerate tumor penetration of drugs through resting reversal of CAFs, thereby improving tumor suppression [25]. Therefore, combining tumor cell senescence induction and CAFs regulation has the potential to overcome the issues mentioned above and improve the anti-tumor efficacy.
It has been reported that valsartan (Val) can inactivate CAFs [26], reduce TGF-β secretion and downregulate collagen expression [27]. Accordingly, we propose the therapeutic regimen of DOX and Val combination. However, Val is poorly water-soluble [28], and common carriers, such as microemulsion [29] and nanoparticles [30], cannot totally solve the drug loading issues. Nanocrystals, known as “pure drug particles”, present a superior perspective to increase the solubility and loading efficiency [31]. But they are inherently unstable, and poly (ethylene glycol) (PEG), hyaluronic acid (HA) and polydopamine are commonly coated on the surface to improve stability [32,33]. Among them, HA with good biocompatibility, tumor targeting ability and easy modification capability attracts our attention [34]. Notably, Val mainly acts on CAFs, while DOX acts on tumor cells. The diverse location of targets requires a hierarchical drug co-delivery system to transport different drugs to specific sites. Some researchers have used cell microparticles [17] or hybrid bionanosystems [35] to target delivery of CAFs modulators and tumor therapeutics. However, cumbersome preparation procedures, low drug loading and uncontrolled drug release encouraged us to seek an intelligent system that can locally release enough Val in tumor microenvironment (TME), thus inhibiting CAFs activity, and then carry DOX to penetrate into the deep tumor cells, inducing senescence. Recently, the multi-drug co-assembly delivery system has attracted much attention because of easy synthesis, customizable modification and targeted release [36,37]. Nevertheless, achieving programmed and targeted delivery of multi-site drugs in one system remains a major challenge.
Fibroblast-activated protein-α (FAP-α), a protease specifically expressed on the surface of CAFs, has been shown to cleave the amino acid sequence GPA [38,39]. Therefore, FAP-α sensitive peptide (Ac-RQRQGPA-OH, Pep) was designed to achieve the CAFs-triggered release of Val. Furthermore, considering the aim of tumor cells targeting and the redox environment within tumor cells, we utilized the HA that coated Val nanocrystals (VNs) to encapsulate DOX via the linker of diselenide bond (Se-Se). As such, the hydrophobic SeSe-DOX was conjugated with the hydrophilic HA chains to form amphiphilic HA-SeSe-DOX (HSD) polymer micelles. To retain the stabilizer function of HA-based polymer for VNs, the hydrophilic Pep was also grafted onto the HSD to form the hydrophilic HSD/Pep (HSD-P) polymer again, which could wrap up VNs. Eventually, HSD-P@VNs (HSD-P@V) was constructed for the co-delivery of Val and DOX, and directionally distributed drugs to their specific targets. As mentioned above, we tailored the CAFs triggered structure-transformable nano-assembly HSD-P@V. Once arriving at TME and touching CAFs, HSD-P@V was quickly disassociated through the FAP-α cleavage effect on Pep and released VNs as well as self-assembled HSD polymer micelles. VNs regulated CAFs and depleted tumor stroma, leading to the enhanced penetration of HSD. Meanwhile, HSD targeted and entered tumor cells, releasing DOX under tumor intracellular microenvironment, inducing senescence and recruiting effector immune cells. Finally, senescent tumor cells were cleared to finish the integrated anti-tumor therapy (Scheme 1).
Scheme 1.
Preparation and anti-tumor mechanism of HSD-P@V. (A) Schematic illustration for the preparation and transformation of HSD-P@V. Hydrophilic HSD-P polymer was coated on VNs surface to obtain HSD-P@V nano-assembly. HSD-P@V was quickly disassociated via the FAP-α cleavage effect on Pep and released VNs as well as HSD polymer micelles. (B) Anti-tumor immune mechanism of HSD-P@V. In response to FAP-α expressed on CAFs, the released VNs acted on CAFs, thus inactivating CAFs and reducing collagen expression. Dissociated HSD micelles carrying DOX penetrated into deep tumor to induce tumor cell senescence and activate anti-tumor immune response.
2. Materials and methods
2.1. Materials
Valsartan (Val, purity>98%), N-hydroxysuccinimide (NHS), dicyclohexylcarbodiimide (DCC), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) and selenium powder were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Doxorubicin hydrochloride (DOX·HCl) (purity>98%) and IR780 were obtained from Macklin (shanghai, China). Ac-Arg-Gln-Arg-Gln-Gly-Pro-Ala-OH (Ac-RQRQGPA-OH) was purchased from Taopu Biotechnology Co., Ltd. (Shanghai, China). Recombinant mouse fibroblast activation protein-α (FAP-α) was purchased from R&D SYSTEM Biotechnology Co., Ltd. (USA). FITC conjugated anti-collagen I antibody, AF594 conjugated anti-α-SMA antibody, Alexa Fluor®488 conjugated anti-mouse VCAM-1 and FITC conjugated anti-mouse FAP-α antibodies were obtained from Bioss Biotechnology Co., Ltd. (Beijing, China). Senescence β-galactosidase staining kit was purchased from Beyotime (Jiangsu, China). Nile red (NR, purity>95%) was purchased from Dalian Meilun Biological Technology Co., Ltd. (Liaoning, China). Mouse IL-15, IL-6, TNF-α and IFN-γ ELISA kits were purchased from Dakewe Biotechnology Co., Ltd. (Beijing, China). Alexa Fluor®488 conjugated anti-mouse CD3 (100204), Aleax-Flour®700 conjugated anti-mouse CD4 (10430), PerCP/Cyanine5.5 conjugated anti-mouse CD8 (10418), Brilliant Violet 421™ conjugated anti-mouse Foxp3 (126419), Aleax-Flour®647 conjugated anti-mouse CD49b (103511), PerCP/Cyanine5.5 conjugated anti-mouse CD11b (101228), PE/Cyanine7 conjugated anti-mouse Gr-1 (108416) and PerCP/Cyanine5.5 conjugated anti-mouse CD326 (118220) were purchased from Biolegend.
2.2. Preparation of 3,3′-diselenodipropionic acid (DSeDPA)
DSeDPA was synthesized according to a previously reported method [40]. Briefly, selenium (790 mg) and NaBH4 (50 mg) were dissolved in 2 M and 1 M NaOH of ice water, respectively. NaBH4 solution was added dropwise to the selenium solution and stirred at low temperature under a nitrogen atmosphere until it completely faded. Then, the solution was heated to 90 °C until it turned reddish brown. Afterward, 3-chloropropionic acid solution (1.7 M) was prepared and the pH of the solution was adjusted to ∼8 by Na2CO3. The solution was added to the above solution and stirred overnight under a nitrogen atmosphere. The supernatant was collected, and the pH was adjusted to 3-4. The product was filtered, washed with H2O, recrystallized with ethyl acetate at 90 °C, and further dried under vacuum to yield DSeDPA as a light-yellow solid.
2.3. Preparation of DOX-SeSe-COOH
DOX·HCl (17.4 mg) was dissolved in DMSO (2 ml), and triethylamine was added to get the desalted DOX. DSeDPA (5.0 mg), DCC (3.7 mg), and NHS (2.1 mg) were dissolved in DMSO and stirred at room temperature for 2 h under a nitrogen atmosphere. Then, the desalted DOX was added dropwise to the above solution and stirred at room temperature for 24 h in dark. After the reaction, the product was filtered with pre-cooled diethyl ether, washed with H2O, and further dried under vacuum to yield DOX-SeSe-COOH.
2.4. Preparation of HA-NH2
HA (200 mg, 0.25 mM) was dissolved in N, N-dimethylformamide (DMF) (20 ml), then EDC (520 mg, 2.7 mM) and NHS (310 mg, 2.7 mM) were added into the solution, and stirred at room temperature for 2 h under nitrogen protection. The solution was added slowly and dropwise to DMF (15 ml) solution containing ethylenediamine (1 ml) under ice bath and stirred at room temperature for 12 h. The product was filtered, washed with acetone, followed by dialysis in a dialysis bag (MWCO: 3,500 Da) and further dried under vacuum to yield HA-NH2.
2.5. Preparation of HSD
DOX-SeSe-COOH (20 mg) was dissolved in DMSO (2 ml), and EDC and NHS were added. The mixture was stirred at room temperature for 2 h under a nitrogen atmosphere. HA-NH2 (20 mM) was added to the above solution and stirred at room temperature for 24 h. The product was filtered and washed with acetone, followed by dialysis in a dialysis bag (MWCO: 3,500 Da). The solution was freeze-dried to give HSD.
2.6. Preparation of HSD-P
Ac-RQRQGPA-OH (66 mg) was dissolved in DMF (5 ml). Then, EDC and NHS were added. The mixture was stirred at room temperature for 2 h under nitrogen protection. Then, HSD (50 mg) was dissolved in DMF (5 ml), then the solution was added to the above solution. The mixture was stirred at room temperature for 48 h. The product was filtered and washed with acetone, followed by dialysis in a dialysis bag (MWCO: 3,500 Da). The solution was freeze-dried to give HSD-P. The preparation of HSD-D was the same as above.
2.7. Preparation of VNs
Val (10 mg) was dissolved in ethanol (1 ml), and the solution was added dropwise to Tween-80 (0.46 mM) under ultrasonic (200 W, 10 min). The mixture solution was freeze-dried to obtain VNs.
2.8. Preparation of HSD-P@V
HSD-P was added dropwise to the VNs solution, followed by ultrasound and dialysis in a dialysis bag (MWCO: 100 kDa). The product was collected and freeze-dried to obtain HSD-P@V.
2.9. Characterization
1H NMR was measured using Bruker Advance 400. Fourier transform infrared (FTIR) spectra were obtained by Nicolet Is10 Infrared spectrometer (Thermo, USA). C, N and H content were analyzed by FlashSmart organic element analyzer (Thermo, USA). The size and zeta potential of nanoparticles were detected by Nano ZS Zetasizer (Zetasizer Nano ZS-90, Malvern, UK). Transmission electron microscope (TEM) images were obtained by Tecnai 20 (FEI, USA). The particle concentration was measured by nanoparticle tracking analyzer (NTA, NANO-ZS300, Malvern, UK).
2.10. In vitro evaluation for the FAP-α responsiveness of HSD-P
HSD-D (4.0 mg) and HSD-P (4.0 mg) were dispersed in phosphate buffer (pH 6.8) (8 ml) containing FAP-α (1.6 mg) and incubated at 37 °C for 40 min. The particle concentration was measured by NTA at 0 and 40 min.
2.11. The stability of HSD-P@V
HSD-P@V was dissolved in PBS (pH 7.4) with and without 10% serum and shaken at 100 rpm at 37 °C. Samples were taken at different times, then the particle size and zeta potential were measured.
2.12. Release properties of HSD-P@V
HSD-P@V containing Val (2 mg) and DOX (0.2 mg) was dispersed in different phosphate buffers (pH 7.4, pH 6.8, pH 5.0) with or without FAP-α in dialysis bag (MWCO: 3,500 Da) and incubated for different times. The release of Val and DOX were determined by UV spectrophotometer at 256 nm and 481 nm, respectively.
2.13. Cell lines and cell culture
Murine breast cancer line (4T1) was acquired from Shanghai Institute of Cells, Chinese Academy of Sciences. The cells were cultured in RPMI-1640 culture medium containing 10% fetal bovine serum (FBS) at 37 °C under 5% CO2. Mouse embryonic fibroblast cells line (NIH-3T3) was derived from Wuhan Punosai Life Technology Co., Ltd. The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FBS at 37 °C under 5% CO2.
2.14. Induction of CAFs
According to the method reported in the literature [41], TGF-β with a final concentration of 10 ng/ml was added into the medium of NIH-3T3 cells, and CAFs were obtained after culturing at 37 °C under 5% CO2 for 12 h.
2.15. In vitro construction of multicellular spheroids (MCSs)
100 µl of 1% sterilized agarose solution was added to the bottom of the 96-well plates. 4T1 and CAFs were mixed in a ratio of 2:1 and inoculated into 96-well plates covered with agarose at a density of 1 × 104 cells/well. After shaking at 200 rpm for 30 min, cells were incubated for 7 d to form MCSs.
2.16. In vitro regulation of extracellular matrix
To investigate the ability of extracellular matrix regulation, MCSs were collected and incubated with the medium containing PBS, VNs, HSD, HSD-D@V, HSD-P@V (containing 5 µg/ml of Val and 0.5 µg/ml of DOX) for 72 h. Then, MCSs were fixed with 4% paraformaldehyde for 20 min and stained with FITC conjugated anti-collagen I and AF594 conjugated anti-α-SMA overnight at 4 °C. Finally, Hoechst33342 was added and incubated for 15 min in the dark. MCSs were observed by confocal laser scanning microscopy (CLSM, Leica TCS SP8, Germany).
2.17. In vitro evaluation of drug penetration
MCSs were collected and incubated with the medium containing PBS, VNs, HSD, HSD-D@V, HSD-P@V (containing 5 µg/ml of Val and 0.5 µg/ml of DOX) for 72 h. After washing with PBS, MCSs were incubated with NR, VNs/NR, HSD/NR, HSD-D@V/NR and HSD-P@V/NR for 12 h and fixed with 4% paraformaldehyde for 20 min. The images were scanned and recorded using CLSM.
2.18. Cells senescence assay
4T1 cells (2 × 104/well) were seeded into 24-well plates and incubated overnight. Subsequently, cells were incubated with the medium containing PBS, DOX, VNs, HSD, HSD-D@V, and HSD-P@V (containing 5 µg/ml of Val and 0.5 µg/ml of DOX) for 72 h and detected by Senescence β-galactosidase staining kit.
2.19. Cells migration and proliferation assay
To evaluate the migration of 4T1 cells, the wound-healing assay was constructed. 4T1 cells (3 × 105/well) were seeded into 6-well plates and cultured overnight. After incubating with the medium containing PBS, DOX, VNs, HSD, HSD-D@V, and HSD-P@V (containing 5 µg/ml of Val and 0.5 µg/ml of DOX) for 72 h, 4T1 cells were scratched with 200 µl pipette tips and treated with fresh medium. The cells were observed and recorded at 0, 24 and 48 h by microscope.
The cell proliferation of HSD-P@V was measured using MTT assay. 4T1 cells (5 × 103/well) were seeded in 96-well plates and cultured for 24 h. Then, cells were incubated with mediums containing different preparations for 72 h. Finally, cells were incubated with 10 µl MTT (5 mg/ml) and measured at 490 nm.
2.20. Animal
Female BALB/c mice (6–8 weeks, 18-20 g) were purchased from SPF Biotechnology Co., Ltd. (Beijing, China). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhengzhou University. The animal laboratory's accreditation number is SCXK (YU) 2019-0004.
2.21. In vivo biodistribution of HSD-P@V
4T1 cells (1 × 106) suspended in PBS (100 µl) were subcutaneously injected into the right flank of BALB/c mice. Heparin (HP) was substituted for HA to synthesize PSD-P@V. When the tumor volumes reached ∼100 mm3, 4T1 tumor-bearing mice were intravenously injected with IR780, PSD-P@V/IR780, HSD-P@V/IR780 (IR780, 0.3 mg/kg). The mice were imaged by FX PRO animal living imaging instrument at different time points. After 48 h post-injection, the mice were sacrificed, and main organs and tumor tissues were collected to observe the drug distribution.
2.22. In vivo cellular targetability
4T1 tumor-bearing mice were intravenously injected with PBS, HSD-D@V, HSD-P@V. Two d later, the 4T1 tumor-bearing mice were injected with DOX/DiD, HSD-D@V/DiD and HSD-P@V/DiD. Then, they were sacrificed 12 h after injection. Tumor tissues were collected to prepare single-cell suspension. Then, cell suspension was stained with PerCP/Cyanine5.5 conjugated anti-mouse CD326 and FITC conjugated anti-mouse FAP-α in the dark and detected by flow cytometer (FACS Calibur, BD co., USA).
2.23. In vivo permeability
To evaluate the permeability of different preparations, 4T1 tumor-bearing mice were treated with DOX, HSD-D@V and HZD-P@V. After 8 h, mice were sacrificed and tumor tissues were collected. Then, frozen sections were prepared and stained with AF594 conjugated anti-CD31 (Biolegend co., 100712) overnight at 4 °C. After staining with DAPI for 15 min, images were captured by fluorescence microscopy and quantitative analysis was performed via ImageJ software.
2.24. In vivo anti-tumor effect
When the tumor volume reached ∼100 mm3, tumor-bearing mice were randomly divided into five groups and intravenously injected with PBS, VNs, HSD, HSD-D@V, HSD-P@V at an equivalent dose of DOX (1.5 mg/kg) and Val (40 mg/kg). The body weight and tumor volume of the mice were monitored every d. After the administration, tumor tissues were collected for subsequent detection.
2.25. Western blot analysis
Tumor tissue fragments were prepared by homogenization and lysed with RIPA buffer containing 1 mM phenylmethanesulfonyl fluoride (PMSF) and protease inhibitor mixture (1×) at 4 °C for 40 min. The protein samples of tumor tissues were obtained by centrifugation (4 °C, 12,000 rpm, 15 min) and separated by 12% sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE). Then, the protein on polyvinylidene difluoride (PVDF) membranes were incubated with anti-TGF-β (Bioss Co., bs-0086R) and anti-α-SMA primary antibody (Bioss Co., bs-10196R) at 4 °C overnight and then incubated with secondary antibody at room temperature for 1 h. Finally, the protein bands were observed by ECL reagent.
2.26. Immunofluorescence staining
Tumor tissues were fixed in 4% paraformaldehyde for 24 h and paraffin sections were prepared. Tumor slices were incubated with collagen I, α-SMA primary antibody and Alexa Fluor®488 anti-mouse VCAM-1 overnight at 4 °C, and the corresponding secondary antibodies were added at 37 °C for 1 h. Finally, the slices were stained with DAPI working solution and observed under CLSM.
2.27. ELISA analysis of cytokine level
The protein of tumor tissues was prepared according to the above steps. The concentrations of IL-15, IL-6, TNF-α and IFN-γ in tumor samples were measured by ELISA kits.
2.28. Flow cytometry analysis of immune cells
The tumor tissues were cut into small slices and incubated with RPMI-1640 culture medium (3 ml) lysate (containing 1.5 mg collagenase IV, 15 µl DNAase, and 300 µl trypsin-EDTA solution) at 37 °C for 40 min to prepare single-cell suspension. The cells were stained with live/dead staining and incubated on ice for 30 min. For charactering CD8+T cells, NK cells and MDSCs, cells were stained with Aleax-Flour 488 conjugated anti-CD3, Aleax-Flour 700 conjugated anti-CD4, Percp/Cy5.5 conjugated anti-CD8a, Aleax-Flour 647 conjugated anti-CD49b, Percp/Cy5.5 conjugated anti-CD11b and PE/Cy7 conjugated anti-Gr-1. For characterizing Tregs, cells were permeabilized by True-Nuclear™ Transcription Factor Buffer Set (Biolegend co., 424401) and stained with BV421 conjugated anti-Foxp3. Finally, all samples were analyzed by flow cytometry.
2.29. Lung metastasis ananlysis
On Day 30, the lungs of tumor-bearing mice were collected, fixed with 4% paraformaldehyde for 24 h and immersed in Bouin's Fluid for 24 h. The number of metastatic nodules was counted, and lung tissues were embedded into paraffin for hematoxylin and eosin (H&E) staining.
2.30. Safety evaluation
After administration, major organs of tumor-bearing mice were collected and stained with H&E to evaluate systemic toxicity. In addition, blood samples were collected and evaluated for changes in liver and kidney functions.
2.31. Statistical analysis
All data were obtained from at least three independent measurements (n ≥ 3) and expressed as mean ± standard deviation (SD). The P value was calculated by one-tailed Student's t-test with GraphPad Prism 8 Software. Statistical significance was indicated as *P < 0.05, **P < 0.01, ***P < 0.001.
3. Results and discussion
3.1. Synthesis and characterization of HSD-P
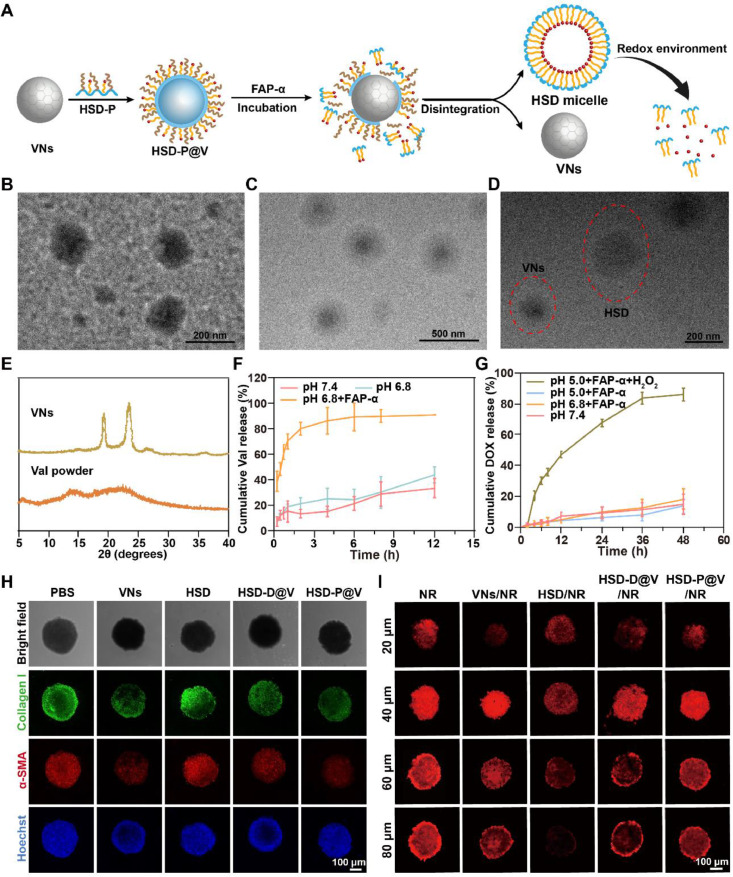
HSD-P was prepared by a multi-step amidation reaction, and the synthesis route was illustrated in Fig. 1A. HA was aminated with ethylenediamine to obtain HA-NH2. Fourier transform infrared (FTIR) spectroscopy (Fig. S1A) showed the characteristic peak of C=O-NH (1664.46 cm−1) that belonged to HA-NH2. 1H NMR (Fig. S1B-S1C) also indicated the successful synthesis of HA-NH2. To achieve responsive release of DOX in tumor cells, Se-Se bond containing carboxyl (3,3′-diselanediyldipropanoic acid, DSeDPA) was introduced into the structure of DOX. Then, DOX-SeSe was conjugated with HA-NH2 to obtain HSD. The successful preparation of DSeDPA, DOX-SeSe, and HSD were confirmed by 1H NMR (Fig. S2). Furthermore, TEM image (Fig. 1D) revealed that amphiphilic HSD could form uniformly spherical micelles of approximately 200 nm in size.
Fig. 1.
Synthesis and characterization of HSD-P. (A) Synthetic scheme of HSD-P. (B) 1H NMR spectrum of HSD-P. (C) 1H NMR spectrum of HSD-P after incubation PBS (pH 6.8) containing FAP-α. (D-F) TEM images of HSD (D), HSD-P (E) and HSD-P after incubation in PBS (pH 6.8) containing FAP-α (F).
Pep was grafted with HSD to obtain hydrophilic HSD-P. 1H NMR result presented the characteristic peak of Pep (Fig. 1B). After HSD-P was incubated in PBS (pH 6.8) containing FAP-α (simulating TME), the characteristic peak of Pep disappeared (Fig. 1C), and the contents of nitrogen, carbon and hydrogen were significantly reduced compared with those of HSD-P (Table S1). No nanoparticles were observed in TEM image for HSD-P (Fig. 1E), because the presence of Pep increased the proportion of hydrophilic chains in the HSD, resulting in the property changes from amphiphilic HSD micelles to hydrophilic HSD-P polymer. However, nanoparticles with size of ∼200 nm appeared again after incubation of HSD-P with PBS (pH 6.8) containing FAP-α (Fig. 1F), which had a similar morphology and size to HSD. The size and zeta potential of HSD-P in the presence of FAP-α were also consistent with those of HSD (Fig. S3). In addition, HSD/DP (HSD-D) (DP, Dragendo∼Positive) (Fig. S4) without FAP-α responsiveness was synthesized as a control.
Nanoparticle tracking analysis (NTA) (Fig. S5) was utilized to further analyze the presence of particles. HSD-D was hardly detected, even with or without FAP-α treatment. On the contrary, the particle number of HSD-P after FAP-α treatment was significantly increased, which was consistent with TEM result. These results indicated that the cleavage of Pep by FAP-α led to the structural recovery of amphiphilic HSD and self-assembled into polymer micelles.
3.2. Synthesis and characterization of HSD-P@V
The synthesis and structure transformation of HSD-P@V was shown in Fig. 2A. VNs was firstly synthesized according to the reported method [42], and displayed uniform spherical morphology with an average diameter of ∼150 nm (Fig. 2B). X-ray diffraction (XRD) result (Fig. 2E) showed that compared to Val powder, more single crystalline signal appeared in VNs due to the regular crystal form. The crystal structure of VNs was also demonstrated by a high-resolution TEM (HR-TEM) image (Fig. S6). Subsequently, HSD-P was coated on the surface of VNs by the nano-precipitation method [31], and finally, the regular core-shell nanosystem (HSD-P@V) with size of ∼200 nm was formed (Fig. 2C). The loading efficiency of Val and DOX was ∼57.28% and ∼2.14%, respectively. The encapsulation rate of Val and DOX in HSD-P@V was ∼68.56% and ∼41.2%, respectively. In addition, the prepared HSD-P@V maintained high stability in PBS (pH 7.4) with and without 10% serum (Fig. S7). Moreover, the FAP-α responsiveness of HSD-P@V was investigated through TEM and dynamic light scattering (DLS) analysis. As shown in Fig. 2D, both HSD micelles and VNs were observed in TEM image after co-incubation of HSD-P@V with FAP-α. DLS result (Fig. S8) showed that the particle size and zeta potential of HSD-P@V decreased after FAP-α incubation, which might be because there were both HSD micelles and VNs in the solution after Pep response to FAP-α. In addition, the particle size and zeta potential of the two kinds of nanoparticles were similar to that of HSD and VNs.
Fig. 2.
Characterization of HSD-P@V and in vitro evaluation on HSD and VNs. (A) Schematic illustration for the preparation and transformation of HSD-P@V. (B-D) TEM images of VNs (B), HSD-P@V (C) and HSD-P@V after incubation in PBS (pH 6.8) containing FAP-α (D). (E) XRD analysis of VNs and Val powder. (F) Val and (G) DOX release profiles under different conditions. Data are shown as the mean values ± SD (n = 3). (H) Fluorescence distribution of collagen I and α-SMA in MCSs after incubation with different formulations for 72 h. (I) Fluorescence distribution of MCSs after incubation with NR, VNs/NR, HSD/NR, HSD-D@V/NR and HSD-P@V/NR for 12 h (MCSs in each group was pretreated with corresponding non-fluorescent labeling formulations for 72 h before co-incubation with NR labeling formulation).
Then, the responsive release behavior of HSD-P@V in vitro was evaluated. At 2 h, ∼ 80% of Val was released from HSD-P@V in PBS (pH 6.8) containing FAP-α (Fig. 2F). In contrast, less than 20% of Val was released at pH 7.4 or pH 6.8 without FAP-α. For DOX (Fig. 2G), the cumulative release rate reached 90% at 48 h in PBS (pH 5.0) containing FAP-α and H2O2 (simulating the redox environment in tumor cells), while there was rare liberation under other conditions. These results suggested that HSD-P@V could rapidly respond to FAP-α to release Val in TME, and then release DOX in tumor cells, thus achieving programmed drug release.
3.3. Matrix regulation and penetration of HSD-P@V
As the major component of TME, CAFs secreted collagen Ⅰ, which constituted the dense extracellular matrix (ECM) barrier and hindered the penetration of drugs as well as immune cells. Therefore, we constructed MCSs to investigate the matrix regulation ability of different preparations. As can be seen in Fig. 2H, compared with PBS and HSD, VNs could significantly reduce the expression of collagen Ⅰ and α-SMA. Furthermore, HSD-P@V showed a similar ability to VNs in regulating ECM components, but HSD-D@V exhibited a weaker capacity of suppressing collagen Ⅰ and α-SMA compared to VNs and HSD-P@V. It was attributed to the responsiveness of Pep to FAP-α on CAFs, thereby releasing VNs to degrade the ECM. Considering that degradation of ECM can promote drug penetration, we used nile red (NR) to label preparation and evaluated their properties on MCSs. As expected, the permeability was positively correlated with the ability of matrix regulation. In Fig. 2I, VNs and HSD-P@V still showed strong red fluorescence signals when the MCSs depth reached ∼80 µm, while the red fluorescence of other groups became weaker with the increase of MCSs depth due to the presence of collagen. It indicated that collagen overexpressed in TME seriously impeded drug penetration. Fortunately, VNs were verified to effectively regulate CAFs, degrade collagen and break down the dense physical barrier, thus promoting drug penetration into the deep tumor cells.
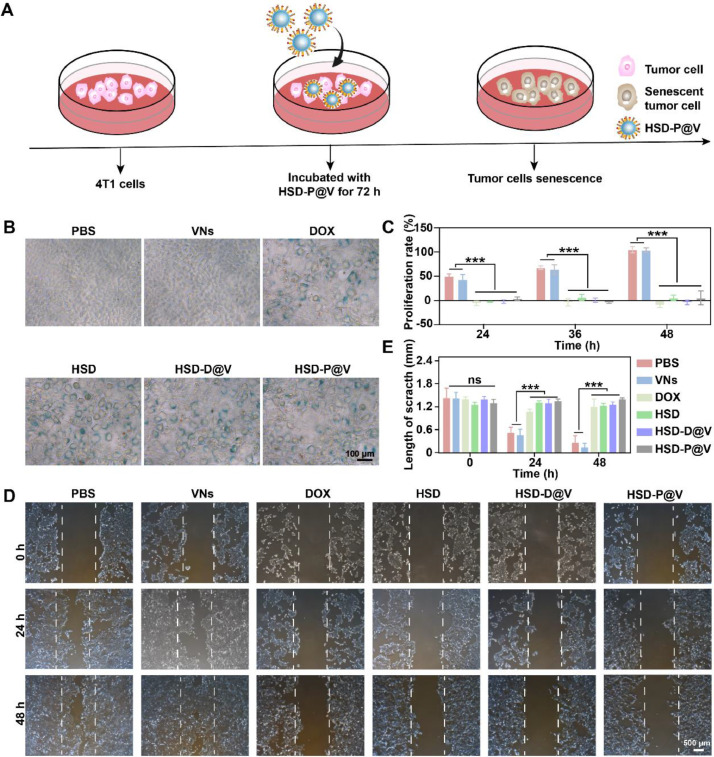
3.4. Cells senescence induction and proliferation inhibition in vitro
Once reaching TME, HSD-P@V was expected to transform into HSD micelles, which can target tumor cells and release DOX intracellularly to induce tumor cell senescence. The scheme of inducing tumor cells senescence in vitro was described in Fig. 3A. Since senescence-associated β-galactosidase (SA-β-gal) is a classic marker of senescent cells [43,44], 4T1 cells senescence was investigated by β-galactosidase staining kit. As shown in Fig. 3B and S9, compared to PBS and VNs groups, tumor cells treated with the preparations containing DOX overexpressed SA-β-gal. In addition, other typical characteristics of senescence, such as cell enlargement and nucleus depression [45], were also observed. These results verified that HSD-P@V possessed perfect ability to induce tumor cell senescence.
Fig. 3.
Cells senescence induction and proliferation inhibition of HSD-P@V in vitro. (A) The scheme for inducing tumor cell senescence. (B) Senescence analysis of 4T1 tumor cells treated with different formulations for 72 h by β-galactosidase staining. (C) Proliferation of 4T1 tumor cells treated with different formulations for different time. (D) Migration ability of 4T1 tumor cells after treatment with different preparations for 72 h and (E) the corresponding semi-quantitative analysis by ImageJ software. Data are shown as the mean ± SD (n = 3). ***P < 0.001; ns, not significant.
It has been reported that the stagnant cell cycle of senescent tumor cells can prevent cell proliferation and migration [46]. Accordingly, the proliferation rate of tumor cells treated with different preparations was preliminarily estimated by methyl thiazolyl tetrazolium (MTT) assay. As shown in Fig. 3C, tumor cells had nearly no proliferative capacity within 24 h after inducing tumor cell senescence, and there was negligible change even at 48 h. The migration ability of tumor cells was also investigated by cell scratch assay. As shown in Fig. 3D-E, HSD-P@V significantly inhibited tumor cell migration at 48 h, which was consistent with the phenomenon of DOX group. Taken together, these results suggested the strong potential of HSD-P@V in anti-tumor therapy.
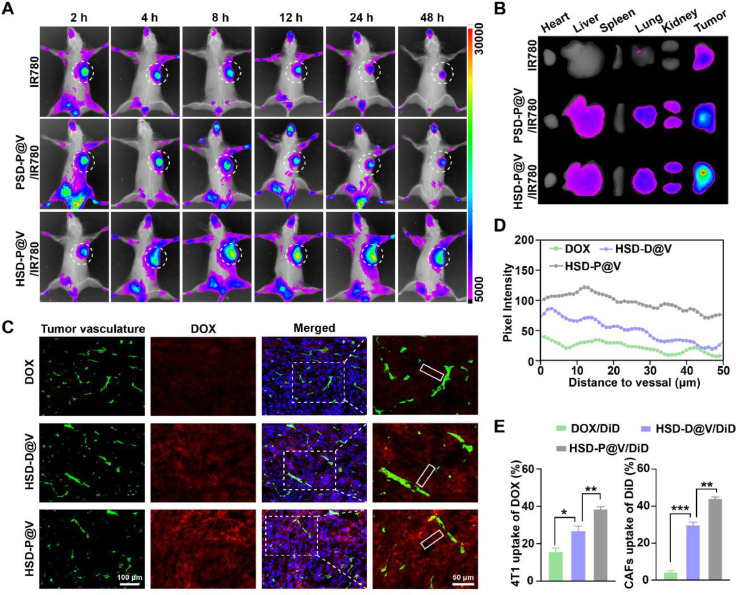
3.5. In vivo distribution and penetration of HSD-P@V
To evaluate the targeting ability of HSD-P@V in 4T1 tumor-bearing mice, HSD-P@V was labeled with near-infrared fluorescent dye IR780 to visualize biodistribution. Meanwhile, heparin (HP)-SeSe-DOX/Pep@VNs (PSD-P@V) labeled with IR780 (PSD-P@V/IR780) was synthesized as control. As shown in Fig. 4A, the fluorescence intensity of free IR780 rapidly decreased with the extension of time. Although PSD-P@V/IR780 displayed a fluorescence signal even at 48 h, it was significantly weaker than that of HSD-P@V/IR780. This was because HSD-P@V/IR780 possessed stronger targeting ability via HA-CD44 mediated tumor cell endocytosis and retention effect in tumor tissue. Ex vivo fluorescence imaging results (Fig. 4B) further confirmed that HSD-P@V/IR780 mainly remained in tumor sites rather than in other organs. Next, the penetration effect of HSD-P@V in tumor tissues was exploited for immunofluorescence analysis. It can be seen that free DOX presented the weakest fluorescence signal due to its non-targetability and fast clearance property (Fig. 4C-4D). The fluorescent intensity of HSD-D@V was stronger compared with that of free DOX but decreased remarkably with increasing distance from blood vessels. It was worth noting that HSD-P@V still presented strong fluorescence at ∼50 µm away from the blood vessels. This was probably because VNs, that were released quickly after Pep responded to FAP-α, could degrade the ECM, leading to the deep penetration of HSD micelles.
Fig. 4.
In vivo distribution and penetration of HSD-P@V. (A) In vivo fluorescence intensity of 4T1 tumor-bearing mice at different time points after intravenous injection of different formulations. (B) Ex vivo imaging of different formulations in major organs at 48 h post-injection. (C) Immunofluorescence analysis of DOX penetration: green fluorescence, tumor vessel; red fluorescence, DOX. (D) DOX fluorescence intensity profile with the distance from blood vessels increased in representative region (white rectangle). (E) Cellular uptake analysis of DOX and Val by 4T1 and CAFs after treatment with DOX/DiD, HSD-D@V/DiD and HSD-P@V/DiD for 12 h by flow cytometry. Data are shown as the mean ± SD (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001.
Since VNs and DOX respectively acted on CAFs and 4T1 cells, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocya-nine,4-chlorobenzenesulfonate salt (DiD) was used to label VNs and the accumulation of HSD-P@V in these cells was further evaluated by flow cytometry. As shown in Fig. 4E, ∼39% and ∼42% of HSD-P@V was absorbed by 4T1 and CAFs, respectively, which were significantly higher than those for DOX and HSD-D@V groups. These results indicated that HSD-P@V could effectively promote the uptake of drugs by CAFs and tumor cells, laying the foundation for excellent anti-tumor effects.
3.6. In vivo anti-tumor efficiency of HSD-P@V
Next, the anti-tumor effect was evaluated on 4T1 tumor-bearing mice. When the tumor size reached ∼100 mm3, mice were randomly divided into 5 groups and treated according to the scheme (Fig. 5A). As shown in Fig. 5B, HSD-P@V obviously inhibited the tumor growth, and tumor inhibition rate was up to ∼62%. Although HSD-D@V also showed better inhibitory effect than HSD, its tumor inhibition rate of ∼45% was still much lower than that of HSD-P@V. It indicated that the structure transformation triggered by TME played a crucial role in anti-tumor potency of HSD-P@V. Ex vivo tumor images (Fig. 5C) and tumor weight (Fig. 5D) results further confirmed excellent anti-tumor efficacy of HSD-P@V. In addition, H&E (Fig. 5F) result presented a large area of nuclear pyknosis and tumor cell necrosis after HSD-P@V treatment. The terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) (Fig. 5G) staining demonstrated the highest tumor cells apoptosis rate of 45.43 ± 2.58% in HSD-P@V group. Moreover, the survival rate of 4T1-bearing mice after treatment was investigated. HSD-P@V treatment extended mice survival (median survival 45 d) compared to other groups, including PBS (21 d), VNs (23 d), HSD (27 d), and HSD-D@V (35 d) (Fig. 5E).
Fig. 5.
In vivo anti-tumor efficiency of HSD-P@V. (A) Schematic diagram of tumor inoculation and administration. (B) Relative tumor volume of 4T1 tumor-bearing mice after intravenous administration. Data are shown as the mean ± SD (n = 6). (C) Images of tumor tissues and (D) tumor weight at the end of treatment. Data are shown as the mean ± SD (n = 6). (E) The survival curves of 4T1 tumor-bearing mice treated by different formulations. Data are shown as the mean ± SD (n = 9). (F) H&E and (G) TUNEL staining images of tumor tissues after the end of treatment. *P < 0.05, **P < 0.01 and ***P < 0.001.
Biosafety is essential for the clinical application of formulations. As can be seen in Fig. S10, no obvious body weight changes in the mice were observed. H&E staining (Fig. S11) showed no visible histological damage to major organs in each group. The liver and kidney functions analysis (Fig. S12) showed that all indexes were within the normal range. These results suggested that HSD-P@V possessed good biological safety.
3.7. In vivo anti-tumor mechanism of HSD-P@V
To elucidate the underlying mechanism of HSD-P@V-mediated superior anti-tumor efficacy, western blotting was performed to analyze the expression of TGF-β and α-SMA in tumor tissues. As shown in Fig. 6A, HSD-P@V downregulated TGF-β and α-SMA most significantly because it could efficiently accumulate in the tumor site and release more VNs. Meanwhile, immunofluorescence staining results showed that the expression of collagen Ⅰ and α-SMA decreased by ∼7-fold and ∼9-fold after HSD-P@V treatment, respectively (Fig. 6B). These results suggested that HSD-P@V treatment could remodel ECM by regulating CAFs.
Fig. 6.
In vivo anti-tumor mechanism of HSD-P@V. (A) Expression of TGF-β and α-SMA in tumor tissues by western blotting and semi-quantitative analysis. (B) The expression of collagen I and α-SMA in tumor tissues by immunofluorescence. (C, D) Senescence β-galactosidase staining images of tumor tissues (C) after the second dose and (D) at the end of treatment. (E) The expression of VCAM-1 in tumor tissues by immunofluorescence. (F) ELISA analysis of IL-15, IL-6, TNF-α and IFN-γ after treatment. All data are shown as the mean ± SD (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001.
Degradation of ECM promotes HSD micelles penetration, inducing more DOX accumulation in tumor cells. Accordingly, tumor cell senescence induced by DOX in vivo was investigated by β-galactosidase staining. In Fig. 6C, a large area of senescent tumor cells appeared in tumor tissue after two doses of HSD-P@V treatment, and senescence rate increased from ∼0.78% to ∼7.57%. Senescent tumor cells can secrete SASP factors [7,9], such as IL-15 and IL-6, to promote tumor inflammatory microenvironment, which recruits NK and T cells to secrete TNF-α and IFN-γ for enhancing anti-tumor effects [47], [48], [49], [50]. ELISA results showed (Fig. 6F) that the expression levels of IL-15, IL-6, TNF-α and IFN-γ in HSD-P@V group were markedly higher than those in other groups. In addition, SASP factors also facilitated addressin expression in vascular endothelial cells [51], such as VCAM-1, which boosted the recruitment and infiltration of immune cells. Based on this, immunofluorescence staining was performed to demonstrate the change of VCAM-1 after administration. As expected, VCAM-1 expression increased from ∼0.44% to ∼5.05% after HSD-P@V treatment (Fig. 6E). In addition, we found that after 5 doses, the area of senescent cells in the HSD-P@V group decreased from ∼7.57% to ∼1.17%. This phenomenon was because the recruited NK cells were able to help eliminate senescent tumor cells, which can effectively avoid the positive regulation of cell metastasis and angiogenesis by “stiff but no dead” senescent tumor cells (Fig. 6D). Taken together, transformable HSD-P@V can degrade tumor matrix, promote immune factors release by inducing tumor cells senescence, and also facilitate immune cells to clear the senescent tumor cells to exert more powerful anti-tumor effects.
3.8. Change of immune cells in TME and anti-metastasis effect of HSD-P@V
The changes of immune cells populations in TME were then investigated. The flow cytometry analysis (Fig. S13 and 7A) showed that the proportion of CD8+T cells increased from ∼10.6% to ∼39.8%, and NK cells increased from ∼1.01% to ∼5.76% after HSD-P@V treatment. On the contrary, the infiltration of immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSC) dramatically decreased especially in HSD-P@V group. Consistent with the results, the immunofluorescence staining images (Fig. 7B) also demonstrated that a large amount of CD8+T cells and NK cells infiltrated into tumor tissues in HSD-P@V group. In addition, lung metastasis was detected after different treatments. As shown in Fig. 7C-D, large amount of lung metastases foci was found in PBS, VNs, HSD and HSD-D@V groups, while the number of lung metastases in HSD-P@V group was negligible. These results suggested that HSD-P@V remodeled the immunosuppressive microenvironment and eliminated lung metastases.
Fig. 7.
Change of immune cells in TME and anti-metastasis effect of HSD-P@V. (A) Relative quantification of CD8+T cells, NK cells, Treg cells and MDSCs in tumor tissues. Data are shown as the mean ± SD (n = 6). (B) Immunofluorescence staining of CD8+T cells and NK cells in tumor tissues of each group. Data are shown as the mean values ± SD (n = 3). (C) Representative photos of the lung tissues of sacrificed mice (left) and the calculated lung metastasis nodules of mice after various treatments (right). The red circles indicate metastatic nodules. (D) The H&E staining of lung tissue sections after various treatments. *P < 0.05, **P < 0.01 and ***P < 0.001.
4. Conclusion
Herein, we constructed a transformable nano-assembly HSD-P@V to achieve the targeted and sequential release of CAFs regulator (Val) and senescence inducer (DOX) in CAFs and tumor cells, respectively. The FAP-α−sensitive peptide helped to accomplish HSD-P@V structure transformation into VNs and HSD micelles. Subsequently, Val inhibited CAFs activity, and promoted drug penetration as well as immune cells infiltration. Furthermore, HSD micelles that transformed from HSD-P@V penetrated into the tumor cells and induced senescence, thus recruiting immune cells and remodeling the immunosuppressive microenvironment. In summary, HSD-P@V can provoke a strong anti-tumor immune response, eradicate senescent tumor cells and inhibit tumor metastasis. This work provides a promising strategy for enhancing anti-tumor immunotherapy by regulating the extracellular matrix and inducing tumor cell senescence. This system for customized responsive release of loaded multi-target drugs to regulate the TME needs to be further explored.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by National Natural Science Foundation of China (81972893, 82172719), Natural Science Foundation of Henan (212300410071), Training program for young key teachers in Henan Province (2020GGJS019).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2024.100888.
Appendix. Supplementary materials
References
- 1.Wang L.Q., Lankhorst L., Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22(6):340–355. doi: 10.1038/s41568-022-00450-9. [DOI] [PubMed] [Google Scholar]
- 2.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Rao S.G., Jackson J.G. SASP: tumor suppressor or promoter? yes! Trends Cancer. 2016;2(11):676–687. doi: 10.1016/j.trecan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt C.A., Wang B., Demaria M. Senescence and cancer-role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19(10):619–636. doi: 10.1038/s41571-022-00668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19(8):439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 6.Kansara M., Leong H.S., Lin D.M., Popkiss S., Pang P., Garsed D.W., et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Investig. 2013;123(12):5351–5360. doi: 10.1172/JCI70559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H.X., Zhao H.F., Sun Y. Tumor microenvironment and cellular senescence: understanding therapeutic resistance and harnessing strategies. Semin Cancer Biol. 2022;86(Pt 3):769–781. doi: 10.1016/j.semcancer.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Eggert T., Wolter K., Ji J.L., Ma C., Yevsa T., Klotz S., et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30(4):533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrelli C., Ricci B., Vulpis E., Fionda C., Ricciardi M.R., Petrucci M.T., et al. Drug-induced senescent multiple myeloma cells elicit NK cell proliferation by direct or exosome-mediated IL15 trans-presentation. Cancer Immunol Res. 2018;6(7):860–869. doi: 10.1158/2326-6066.CIR-17-0604. [DOI] [PubMed] [Google Scholar]
- 10.Schmittnaegel M., Rigamonti N., Kadioglu E., Cassará A., Rmili C.W., Kiialainen A., et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9(385) doi: 10.1126/scitranslmed.aak9670. eaak9670. [DOI] [PubMed] [Google Scholar]
- 11.Ruscetti M., Leibold J., Bott M.J. NK cell–mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362(6421):1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyld L., Bellantuono I., Tchkonia T., Morgan J., Turner O., Foss F., et al. Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers. 2020;12(8):2134. doi: 10.3390/cancers12082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estepa-Fernández A., García-Fernández A., Lérida-Viso A., Morellá-Aucejo A., Esteve-Moreno J.J., Blandez J.F., et al. Engineering nanoparticle communication in living systems by stigmergy: an application to enhance antitumor therapy in triple-negative breast cancer. Nano Today. 2023;48 [Google Scholar]
- 14.Irene G., Beatriz L.T., Mónica S., María A., Andrea B., Viviana B., et al. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanosenolytic. J Control Release. 2020:323624–323634. doi: 10.1016/j.jconrel.2020.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C.H., Altschuler S.J., Wu L.F. Patterns of early p21 dynamics determine proliferation-senescence cell fate after chemotherapy. Cell. 2019;178(2) doi: 10.1016/j.cell.2019.05.041. 361–73 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B.H., Wu B., Feng N., Zhang X., Zhang X., Wei Y.P., et al. Aging microenvironment and antitumor immunity for geriatric oncology: the landscape and future implications. J Hematol Oncol. 2023;16(1):28. doi: 10.1186/s13045-023-01426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Yong T.Y., Wei Z.H., Bie N.N., Zhang X.Q., Zhan G.T., et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat Commun. 2022;13(1):2794. doi: 10.1038/s41467-022-30306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H.Q., Shi Y., Qian F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv Drug Deliv Rev. 2021:17237–17251. doi: 10.1016/j.addr.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Hou L., Chen D.D., Wang R.T., Wang R.B., Zhang H.J., Zhang Z.Z., et al. Transformable honeycomb-like nanoassemblies of carbon dots for regulated multisite delivery and enhanced antitumor chemoimmunotherapy. Angew Chem Int Ed Engl. 2021;60(12):6581–6592. doi: 10.1002/anie.202014397. [DOI] [PubMed] [Google Scholar]
- 20.Chhabra Y., Weeraratna A.T. Fibroblasts in cancer: unity in heterogeneity. Cell. 2023;186(8):1580–1609. doi: 10.1016/j.cell.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S.J., Ren J., Dijke P.T. Targeting TGF-β signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flavell R.A., Sanjabi S., Wrzesinski S.H., Licona-Limon P. The polarization of immune cells in the tumour environment by TGF-β. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiang L., Hoffman M.T., Ali L.R., Castillo J.I., Kageler L., Temesgen A., et al. Transforming growth factor-β blockade in pancreatic cancer enhances sensitivity to combination chemotherapy. Gastroenterology. 2023;165(4):874–890. doi: 10.1053/j.gastro.2023.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao L., Liu Q., Lin C.M., Luo C., Wang Y.H., Liu L.N., et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 2017;77(3):719–731. doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., He D.S., Hao T.J., Hu Y.M., Zhao Y., Li Z., et al. Gold mineralized “hybrid nanozyme bomb” for NIR-II triggered tumor effective permeation and cocktail therapy. Chin Chem Lett. 2023 [Google Scholar]
- 26.Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013:42516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallasch F.B., Schumacher U. Angiotensin inhibition, TGF-β and EMT in cancer. Cancers. 2020;12(10):2785. doi: 10.3390/cancers12102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sreeharsha N., Naveen N.R., Anitha P., Goudanavar P.S., Ramkanth S., Fattepur S., et al. Development of nanocrystal compressed minitablets for chronotherapeutic drug delivery. Pharmaceuticals. 2022;15(3):311. doi: 10.3390/ph15030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sood J., Sapra B., Tiwary A.K. Microemulsion transdermal formulation for simultaneous delivery of valsartan and nifedipine: formulation by design. AAPS PharmSciTech. 2017;18(6):1901–1916. doi: 10.1208/s12249-016-0658-0. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A., Ramisetty K.A., Bordignon S., Hodnett B.K., Davern P., Hudson S. Preparation, stabilisation, isolation and tableting of valsartan nanoparticles using a semi-continuous carrier particle mediated process. Int J Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120199. [DOI] [PubMed] [Google Scholar]
- 31.Ji P., Wang L., Chen Y.W., Wang S.Q., Wu Z.H., Qi X.L. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater Sci. 2020;8(1):462–472. doi: 10.1039/c9bm01605h. [DOI] [PubMed] [Google Scholar]
- 32.Park J.Y., Sun B., Yeo Y. Albumin-coated nanocrystals for carrier-free delivery of paclitaxel. J Control Release. 2017;263:90–101. doi: 10.1016/j.jconrel.2016.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuhrmann K., Pozomska A., Aeberli C., Castagner B., Gauthier M.A., Leroux J.C. Modular design of redox-responsive stabilizers for nanocrystals. ACS Nano. 2013;7(9):8243–8250. doi: 10.1021/nn4037317. [DOI] [PubMed] [Google Scholar]
- 34.Xu C.R., He W., Lv Y.Q., Qin C., Shen L.J., Yin L.F. Self-assembled nanoparticles from hyaluronic acid-paclitaxel prodrugs for direct cytosolic delivery and enhanced antitumor activity. Int J Pharm. 2015;493(1–2):172–181. doi: 10.1016/j.ijpharm.2015.07.069. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z.N., Ji P., Liu H.Z., Yu L.L., Zhang S.M., Liu P., et al. FNIII14 peptide-enriched membrane nanocarrier to disrupt stromal barriers through reversing CAFs for augmenting drug penetration in tumors. Nano Lett. 2023;23(21):9963–9971. doi: 10.1021/acs.nanolett.3c02983. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Yu H., Yang X.H., Zhang X.B., Wang Y.Q., Gu T.R., et al. Elaborately engineering of a dual-drug co-assembled nanomedicine for boosting immunogenic cell death and enhancing triple negative breast cancer treatment. Asian J Pharm Sci. 2022;17(3):412–424. doi: 10.1016/j.ajps.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y., Lin Y., Li B.W., Zhang F., Zhan C.Y., Xie X., et al. Combination therapy to overcome ferroptosis resistance by biomimetic self-assembly nano-prodrug. Asian J Pharm Sci. 2023;18(5):100844. doi: 10.1016/j.ajps.2023.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X.X., Li L.L., Zhao Y., An H.W., Cai Q., Lang J.Y., et al. In situ self-assembled nanofibers precisely target cancer-associated fibroblasts for improved tumor imaging. Angew Chem Int Ed Engl. 2019;58(43):15287–15294. doi: 10.1002/anie.201908185. [DOI] [PubMed] [Google Scholar]
- 39.Ji T.J., Zhao Y., Ding Y.P., Wang J., Zhao R.F., Lang J.Y., et al. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem Int Ed Engl. 2016;55(3):1050–1055. doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Y.N., Xia X.Y., Yu B., Tao L.J., Wang Q., Huang S.W., et al. Selenylsulfide bond-launched reduction-responsive superparamagnetic nanogel combined of acid-responsiveness for achievement of efficient therapy with low side effect. ACS Appl Mater Interfaces. 2017;9(36):30253–30257. doi: 10.1021/acsami.7b06818. [DOI] [PubMed] [Google Scholar]
- 41.Liu Q., Chen F.Q., Hou L., Shen L.M., Zhang X.Q., Wang D.G., et al. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano. 2018;12(8):7812–7825. doi: 10.1021/acsnano.8b01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao X.Q., Yang W.W., Feng T., Lin J., Huang P. Drug nanocrystals for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(3):e1499. doi: 10.1002/wnan.1499. [DOI] [PubMed] [Google Scholar]
- 43.Wang L.Q., Oliveira R.L.D., Wang C., Fernandes Neto J.M., Mainardi S., Evers B., et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 2017;21(3):773–783. doi: 10.1016/j.celrep.2017.09.085. [DOI] [PubMed] [Google Scholar]
- 44.Qu A.H., Wu X.L., Li S., Sun M.Z., Xu L.G., Kuang H., et al. An NIR-responsive DNA-mediated nanotetrahedron enhances the clearance of senescent cells. Adv Mater. 2020;32(14) doi: 10.1002/adma.202000184. [DOI] [PubMed] [Google Scholar]
- 45.Fecteau J.F., Messmer D., Zhang S., Cui B., Chen L., Kipps T.J. In vitro propagation of mesenchymal stromal cells from marrow aspirates of patients with chronic lymphocytic leukemia is dependent upon physiologic oxygen tension. Blood. 2011;118(21):2839. [Google Scholar]
- 46.Suski J.M., Braun M., Strmiska V., Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–778. doi: 10.1016/j.ccell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou P.C., Chuang T.F., Jan T.R., Gion H.C., Huang Y.C., Lei H.J., et al. Effects of immunotherapy of IL-6 and IL-15 plasmids on transmissible venereal tumor in beagles. Vet Immunol Immunopathol. 2009;130(1–2):25–34. doi: 10.1016/j.vetimm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Chibaya1 L., Murphy K.C., DeMarco K.D., Gopalan S., Liu H.B., Parikh C.N., et al. EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance. Nat Cancer. 2023;4(6):872–892. doi: 10.1038/s43018-023-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian L., Zhang Y., Pan X.Y., Ji M.C., Gong W.J., Tian F. IL-15, in synergy with RAE-1ɛ, stimulates TCR-independent proliferation and activation of CD8+ T cells. Oncol Lett. 2012;3(2):472–476. doi: 10.3892/ol.2011.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruscetti M., Morris J.P., Mezzadra R., Russell J., Leibold J., Romesser P.B., et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.03.008. 424–41 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martina S., Nicolò R., Ece K., Antonino C., Céline Wyser R., Anna K., et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9(385):eaak9670. doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.