The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see https://www.journals.elsevier.com/academic-pathology/news/pathology-competencies-for-medical-education-pcme.1
Primary
Objective HB3.1: Causes of hepatocellular carcinoma. Compare and contrast, in the context of geographic location, the epidemiological importance of the known etiologic agents associated with the development of hepatocellular carcinoma and suggest public health measures that might decrease its incidence.
Competency 2: Organ system pathology; Topic: HB: Hepatobiliary; Learning goal 3: Hepatic neoplasms.
Secondary objective
Objective HB3.2: Morphology of hepatocellular carcinoma. Discuss the morphologic features that distinguish between hepatocellular carcinoma and benign neoplasms or nonneoplastic liver disease.
Competency 2: Organ system pathology; Topic: HB: Hepatobiliary; Learning goal 3: Hepatic neoplasms.
Patient presentation
A 53-year-old farmer presents to the clinic with concerns of yellowish skin discoloration and abdominal pain. The patient states that over the past 3 months he experienced dull abdominal pain that he describes being mainly in his right upper belly. During this period, he also noticed progressive yellowing of both his skin and eyes. After further discussion, he also mentions unintentionally losing 22 pounds (lb) of weight and feeling fatigued. His past medical history is remarkable for chronic liver disease, diagnosed three years earlier, and elevated cholesterol for the past five years, managed with high dose niacin therapy. The patient states he is not on any medications, has no allergies, prior surgeries or hospitalizations. He states that he exercises occasionally by engaging in brisk walks about twice a week and admits to consuming 4–6 cans of beer with dinner for the past two decades and smoking a pack of cigarettes every day for the past three decades. The patient states that he has no recent travel or illness, or sudden changes in lifestyle. His occupation puts him in contact with several grains that were contaminated with mold leading to loss of his most recent harvest.
Diagnostic findings, Part 1
Physical examination is remarkable for a well-nourished individual in no acute distress. Vital signs are blood pressure of 119/75 mmHg, heart rate of 67 beats per minute, respiratory rate of 15 breaths per minute, temperature of 98.2 °F, oxygen saturation of 99 %, and body mass index of 33.47 kg/m2. The sclera are icteric. The patient is noticeably jaundiced. Cardiac examination reveals a normal S1 and S2 with regular rate and rhythm without murmurs, heaves, or gallops. Lungs are clear to auscultation. The abdomen is distended. Bowel sounds are present. A positive fluid wave is appreciated on abdominal examination. A palpable mass is present in the in the right upper quadrant (RUQ).
Questions/discussion points, Part 1
What is jaundice?
Jaundice is defined as yellow discoloration of the skin. Yellow discoloration of the sclera is termed icterus. In adults, jaundice is attributed to a very broad differential that range from clinically insignificant to life-threatening conditions.2 Jaundice and icterus typically result from the accumulation of unconjugated or conjugated bilirubin that precipitates into mucosal membranes, skin, and sclera of the eyes.2, 3, 4 Disorders leading to 1) increased production of unconjugated bilirubin (prehepatic), 2) disorders due to impaired hepatic function (hepatic), and 3) disorders with conjugated hyperbilirubinemia due to extrahepatic cholestasis (posthepatic) may lead to jaundice. Examples of prehepatic disorders include hemolytic anemias, hemoglobinopathies, and ineffective erythropoiesis. Other considerations include mechanical heart valve, hypersplenism, and resorption of large hematomas. Hepatic disorders leading to hyperbilirubinemia include hepatitis, cirrhosis, autoimmune disorders, hereditary hyperbilirubinemias, drugs and alcohol-related disorders, neoplasms, and genetic disorders. Bile duct obstruction due to gallstones, pancreatic, periampullary and gallbladder cancer, choledochal cyst and cholangitis may lead to extrahepatic cholestasis.2, 3, 4
What disorders are in the differential diagnosis based on the clinical presentation?
The differential diagnosis for RUQ abdominal pain is broad to include hepatic, gallbladder, gastrointestinal, renal, pancreatic, cardiac and pulmonary disorders.5 In this particular clinical situation, the patient's constellation of painless jaundice, unintentional weight loss, occupational status, and physical exam findings of abdominal fluid distension and a palpable RUQ mass suggest a focal process. That said, the differential for a palpable RUQ mass can be narrowed to include liver, gallbladder and biliary tract disorders. Benign hepatic disorders that may present as a RUQ mass in adults include hepatic adenoma, hemangioma, and focal nodular hyperplasia. Malignant hepatic disorders include hepatocellular carcinoma (HCC), cholangiocarcinoma and metastatic disease. Non-neoplastic hepatic disorders include hepatic cysts, infectious disorders, including hepatic abscesses, and hematomas. Gallbladder distension due to cholelithiasis, biliary tract obstruction, gallbladder cancer and cholangiocarcinoma are other entities.
Clinical presentation varies among patients with HCC. HCC, initially, is often asymptomatic. In advanced disease, it may be symptomatic. Findings, initially, are nonspecific. Presentation with fatigue, vague abdominal pain and weight loss are frequent findings. Approximately 90–95 % of HCC patients present with the triad of significant weight loss over a short time span, right upper quadrant pain, and palpable liver mass in the late stages.6 Jaundice may be present secondary to advanced cirrhosis, if present, or due to a combination of diffuse infiltration of the hepatic parenchyma by tumor cells, liver failure or bile duct obstruction. In patients with advanced disease, obstructive jaundice as the presenting feature is observed in 1–12 % of patients with HCC.6,7
The patient's clinical presentation and history of chronic liver disease in this case raises considerable suspicion for cirrhosis. The palpable RUQ mass in the setting of chronic liver disease (cirrhosis) raises the possibility of HCC.8 Physical exam findings of abdominal distension, palpable mass and jaundice are also consistent with HCC.6,7 Additionally, the patient's occupational history as a grain farmer and loss of his harvest due to mold raises the possibility of prolonged aflatoxin exposure, which is a known carcinogen for HCC. His social history of long-term alcohol consumption and tobacco use further increase his risk for HCC.7,9
What additional workup is needed to establish a definitive diagnosis?
For high-risk patients with chronic liver disease, the American College of Radiology recommends utilizing non-invasive imaging in the initial diagnostic approach.10,11 Imaging techniques, including ultrasound (US), magnetic resonance imaging (MRI), and contrast-enhanced computed tomography (CT) are utilized. Unlike many other malignancies, the need for a liver biopsy is not required if imaging provides demonstrable evidence of HCC given that HCC has characteristic radiographic features.2,10,11 In the event imaging is inconclusive, a liver biopsy should be obtained.
Aside from imaging, certain laboratory tests may be supportive of a HCC diagnosis. Alpha-fetoprotein (AFP), an oncofetal antigen, is elevated in many patients with HCC. It should be noted that AFP levels cannot alone establish a HCC diagnosis and need to be used in conjunction with other diagnostic measures.9,10,12,13 Other markers such as des-γ-carboxy prothrombin (DCP), γ-glutamyl transferase (GGT), and α-1-fucosidase (AFU) can be used in conjunction with AFP.6,8
Diagnostic findings, Part 2
As part of the initial workup, a CT scan is obtained (Fig. 1, Fig. 2, Fig. 3).
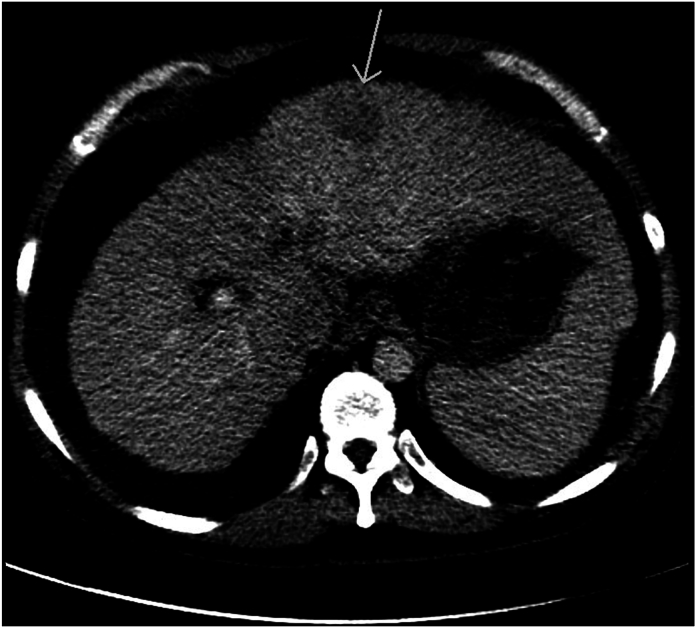
Fig. 1.
HCC CT1
Axial post contrast CT demonstrates a hypodense left hepatic lobe mass (arrow) as well as cirrhotic liver morphology with a micronodular capsular contour.
Fig. 2.
HCC delay T1 dynamic post contrast delayed phase imaging demonstrates non-peripheral washout and an enhancing capsule of a left hepatic lobe mass.
Fig. 3.
HCC T2FS T2FS imaging demonstrates a heterogeneous mildly T2 hyperintense left hepatic lobe mass.
Questions/discussion points, Part 2
Describe what is seen in the CT scan
The CT scan demonstrates a hypodense left hepatic lobe mass within a cirrhotic liver with a micronodular capsular contour. Given the prevalence of hepatocellular carcinoma in the cirrhotic patient population, this mass should be viewed as suspicious for HCC until proven otherwise.
What are some imaging characteristics that are unique to HCC?
HCC, excluding the fibrolamellar variant, can have a wide array of imaging features that can include abnormal large focal masses, multiple nodules, and diffuse infiltrative patterns similar to that of cirrhosis.13 On ultrasound, HCC appearance will largely be determined by several factors including the echogenicity of the liver and the size and location of the lesion within the liver. Smaller focal HCC lesions on US may appear hypoechoic when compared with healthy liver tissue while larger HCC lesions are likely to demonstrate varying degrees of echogenicity attributed to a combination of fatty changes, necrosis, and calcifications caused by the malignancy.11,13, 14, 15 HCC on CT scan is typically characterized by ring-enhancement patterns seen surrounding the lesion that are described in radiology as “washout effects” whereby HCC masses become hypoattenuating during the portal venous stage of CT scanning.13, 14, 15 For the scope of this discussion, features of HCC on MRI imaging will display similar features of ring-enhancement patterns and “washout” effects as seen in CT imaging.13, 14, 15
Diagnostic findings, Part 3
Imaging is consistent with HCC. As the patient is being evaluated for several treatment protocols, he expires. Permission to perform an autopsy from the legal next of kin is granted. Images of the 2010 g (normal 1500–1800 g) liver are shown in Fig. 4, Fig. 5, Fig. 6, Fig. 7.
Fig. 4.
Section of liver with large mass (arrows) within the left lobe that has extended into and expanded the hepatic vein (∗). The non-neoplastic liver has a nodular appearance consistent with cirrhosis.
Fig. 5.
Section of well-differentiated HCC with trabecular pattern. No portal tracts are present. (H&E, low magnification).
Fig. 6.
Hepatocellular carcinoma. The malignant cells are arranged in a trabecular pattern. The malignant cells are larger than non-neoplastic hepatocytes and show pleomorphism. (H&E, intermediate magnification).
Fig. 7.
This HCC image demonstrates a pseudoglandular growth pattern characterized by tumor cells surrounding a dilated space. (H&E, intermediate magnification).
Questions/discussion points, Part 3
Describe the gross and histological features observed in the liver at autopsy?
The section of liver shows a large distinct green mass in the left lobe that extends into the hepatic vein (Fig. 4). Histologic examination shows a prominent trabecular pattern with pleomorphic hepatocytes arranged in trabeculae of varying thickness. Portal areas are absent. (Fig. 5, Fig. 6). Islands of neoplastic hepatocytes with prominent necrosis are seen in the background of the cirrhotic liver. Areas with a pseudoglandular appearance are also present (Fig. 7).
What gross and histological features are unique to HCC?
HCC is a malignant tumor of hepatocellular origin that displays considerable heterogeneity.16 On gross examination, HCC varies in color based on the amount of fat and bile within the tumor. Most tumors are brown or tan; however, there is variation from yellow to green. Four distinct growth patterns are recognized: 1) single distinct tumor nodule, 2) multiple distinct tumor nodules associated with a large single dominant nodule, 3) multiple distinct tumor nodules randomly distributed not associated with a dominant nodule, and 4) myriad small tumor nodules similar in size to cirrhotic nodules diffusely distributed.8,17, 18, 19, 20 Hemorrhage and necrosis are common.
HCC has three distinct architectural characteristics that distinguish tumor from non-tumor. They are 1) absence of normal portal tracts, 2) abnormal arteriolization of the hepatic lobule, and 3) abnormal thickness and organization of hepatocellular plates.20 These features do not distinguish between malignant and benign hepatic tumors.
Four characteristic histological patterns are observed in H&E sections: trabecular, solid, pseudoglandular (pseudoacinar) and macrotrabecular.8,17,20 The trabecular pattern is the most common and shows tumor cells in trabeculae of various thickness separated by sinusoids.8,16, 17, 18, 19, 20 Additional cytological features may include polygonal cells with significant nuclear atypia with highly irregular nuclear membranes, in addition to cytoplasmic aberrations such as the presence of Mallory-Denk bodies.16,20 In clinical practice, HCCs are routinely graded as well-differentiated, moderately differentiated and poorly- differentiated. More formal grading systems with prognostic significance exist.8,20 Staining for keratin, CD10, CD34, reticulin, and annexin-2 can be useful in the identification and diagnosis of HCC.18 Depending on the histological subtype, certain cytogenetic anomalies such as JAK, STAT, and TP53 mutations may be seen.16, 17, 18
A notable variant of HCC most common among young adults is the fibrolamellar variant. It accounts for 1 % of HCC cases.8,20 Cirrhosis is not an associated feature. Alpha-fetoprotein levels are normal. On gross examination, fibrolamellar carcinomas tend to be large, yellow to tan tumors frequently associated with a central scar in a noncirrhotic liver.8,20 Large polygonal tumor cells with prominent eosinophilic cytoplasm and collagen deposited in parallel stacks are most often seen (Fig. 8, Fig. 9).
Fig. 8.
Hepatocellular carcinoma, fibrolamellar variant. This section of liver shows an approximate 5.5 cm diameter yellow mass and a second 2 cm diameter yellow mass. The non-neoplastic liver has a homogenous consistency without nodularity.
Fig. 9.
The fibrolamellar variant is characterized by large eosinophilic cells with prominent nucleoli surrounded by collagen arranged in parallel, plate-like stacks. (H&E, intermediate magnification).
Distinguishing histological features between hepatic adenomas and well-differentiated HCC include whether cytological atypia is present and the presence or absence of cirrhosis. Hepatic adenomas, in general, occur in non-cirrhotic livers and lack cytological atypia. In situations where there is atypia, a diagnosis of “well-differentiated hepatocellular neoplasm” may be prudent.20
What is the most common primary hepatic malignant tumor in children?
In the pediatric population, hepatoblastoma is the most common malignant hepatic tumor. It affects approximately 150 children in the US annually and most often seen in children less than three years of age.19, 20, 21 Hepatoblastomas have been associated with the Beckwith-Wiedemann and familial adenomatous polyposis syndromes.19,20 Hepatoblastomas have a wide-array of histological features and are categorized by the International Pediatric Liver Tumor Consensus Classification as having epithelial or mesenchymal features or a combination of both. Approximately 56–67 % of hepatoblastomas are of epithelial origin 20,21.
What predisposing or risk factors contribute to the development of HCC?
Several etiologies put individuals at increased risk for HCC, including hepatitis B and C infections, cirrhosis, developmental disorders, and steatotic liver disease encompassing the subcategories of metabolic dysfunction-associated steatotic liver disease (MASLD) formerly termed nonalcoholic fatty liver disease (NAFLD), MASLD with 140–350 g/week in females and 210–420 g/week in males alcohol consumption (MetALD), metabolic dysfunction-associated steatohepatitis (MASH) formerly termed nonalcoholic steatohepatitis (NASH), alcohol-related liver disease (ALD), specific etiology SLD and cryptogenic SLD, cirrhosis, and developmental disorders.22 Patients with cirrhosis, or any condition that predisposes them to cirrhosis, such as metabolic, infectious, or environmental disorders, are at the greatest risk for developing HCC.16, 17, 18, 19,23
Viral hepatitis, especially those caused by hepatitis B and C infections, is globally the most frequent cause of HCC and other liver cancers.24,25 Both hepatitis B and C viruses are blood-borne pathogens that are contracted through horizontal transmission such as through exposure to infected blood. Adulthood hepatitis is estimated to progress to chronic hepatitis in less than 5 % of patients. In contrast, hepatitis acquired during early childhood progresses to chronic hepatitis in about 95 % of cases and thus emphasizes the importance of early childhood hepatitis B vaccinations.26 Hepatitis C, however, currently does not have a viable vaccine.
Aside from infectious etiologies, metabolic and genetic disorders, such as alpha-1-antitrypsin deficiency, hemochromatosis, and especially MASLD, and MASH have been associated with the development of HCC.24,25,27 Certain toxins and environmental substances, such as cigarette smoke, alcohol (ALD), aflatoxin produced by the Aspergillus flavus mold, and even oral contraceptives, have been shown to increase the risk of developing HCC.24,25,27 Though rare, developmental and genetic conditions, such as tyrosinemia type 1 and ataxia telangiectasia, have also been linked to increased risk of HCC.19,25,27
Describe the pathogenesis of HCC associated with hepatitis B and hepatitis C, chronic hepatitis, and cirrhosis
HCC is a malignancy that demonstrates significant heterogeneity and has numerous etiologies. HCC attributed to infectious etiologies are primarily associated with either hepatitis B or C infection. Hepatitis B infection is said to have “direct oncogenic” effects that can contribute to the eventual onset of HCC.17,28,29 During infection, the hepatitis B virus integrates its own DNA into the host genome which subsequently brings out host genetic instability.17,28,29 The genetic instabilities brought about by hepatitis B infection often include mutations in the vital TP53 tumor suppressor gene and malfunctioning in the Wnt/Beta-Catenin pathways that are believed to play roles in anticancer immune responses and can lead to the eventual development of HCC.28, 29, 30 In contrast, the carcinogenic effects of hepatitis C are attributed to oxidative stress placed on the host cell from hepatitis C core proteins which leads to altered activity of the MAPK and NFkB pathways that play a crucial role in cellular apoptosis and leads to eventual abnormal cellular proliferation.17,29, 30, 31, 32
HCC can additionally have metabolic etiologies. Several underlying metabolic disorders can lead to chronic hepatitis and liver cirrhosis, both of which can eventually progress to HCC. Several chronic medical conditions, especially diabetes mellitus and obesity, associated with MASLD have been shown to increase the risk of developing HCC. Diabetes mellitus is believed to alter a variety of insulin pathways that are responsible for anti-inflammatory and cellular proliferation cascades that play a role in cancer regulation.32,33 It has well been documented that obesity is an independent risk factor for the development of several hepatobiliary disorders including MASLD, MASH, and hepatic cirrhosis or fibrosis.32,33 Hemochromatosis, an iron-overload disorder, that causes excess iron deposition in the liver, increases the risk of cirrhosis and is a known risk factor for HCC.9,19
Lastly, ethanol consumption continues to remain a significant HCC risk factor.19,34,35 Excessive ethanol consumption for long-periods of time has shown strong correlation with increased risks of the development of HCC.19,33, 34, 35 Significant amounts of ethanol consumption for prolonged periods increases the risks of developing alcoholic steatosis and alcoholic hepatitis that can lead to eventual cirrhosis, which can then progress to HCC.31, 32, 33 Prolonged ethanol consumption is believed to impose chronic oxidative stress and inflammation, from an ethanol metabolite on the liver that in turn can lead to cirrhosis and eventual HCC.27 It is currently thought that chronic liver injury secondary to ethanol consumption and subsequent free-radical damage can cause cellular instability and defects in genetic repair pathways.25,27,33, 34, 35
What treatment options for HCC are available?
HCC cases are overwhelmingly diagnosed during the late-stage of disease onset when patients are symptomatic emphasizing the importance of surveillance.36 With diagnostic delay, a legitimate reality, HCC during late-stage disease has a grave prognosis. No treatment modalities that are thought to improve prognosis or survival currently exist.36 However, in earlier stages of HCC, several treatment modalities exist: transplantation, surgery, and systemic therapies including chemotherapy and biologicals.25
Liver transplantation, more specifically, orthotopic liver transplantation can be used as a curative treatment for HCC and can be done with either living or deceased liver donor tissue.33 This treatment modality, due to costs and risks posed to living donors, is reserved for HCC patients with decompensated cirrhosis that meet certain criteria, such as the Milan criteria, which assess prognostic outcomes post-transplantation. Surgical resection remains a widely practiced treatment approach for HCC that involves non-cirrhotic liver tissue with an estimated 5-year survival rate from 41 % to 74 %.25,33 The viability and success of surgical resection depends on several factors such as tumor margins and whether organ function is preserved post-operation. Other minimally invasive procedures, such as percutaneous or microwave ablation and transarterial chemoembolization, can be used to treat cases in which surgical resection of HCC is contraindicated or not feasible. Radiation therapy methods can also be used, such as the utilization of transarterial radiation, whereby radioactive isotopes are directly delivered into tumor tissue through a catheter. Current guidelines recommend that patients with advanced HCC be treated with systemic therapy. In 2008, sorafenib, a class of kinase inhibitors, became the first FDA-approved systemic drug that demonstrated clinically significant improvement with respect to survival outcomes in patients with advanced HCC.25,27,33 It should be noted that there is ongoing research into new systemic therapies to treat HCC with lenvatinib being the latest FDA-approved systemic therapy.37
What is the global prevalence of HCC and how does the disease very across different regions in the world? Furthermore, what specific trends are seen within the United States?
Globally, HCC is the most common liver malignancy and one of the most prevalent neoplasms worldwide.8 It has been estimated to be the third-leading cause of cancer deaths with more than 500,000 annual cases.8,23,25,32,36,37 Generally speaking, individuals with underlying hepatic disease, such as cirrhosis and viral hepatitis, are at increased risk of developing HCC. Based on epidemiological data from the last six years, HCC appears to have the highest disease burden in Eastern Asia and Sub-Saharan Africa.25,27,32,38,39 Moreover, the World Health Organization estimates that more than 1 million people in 2030 will die from HCC alone.25
HCC has the highest prevalence in developing nations, with China having the highest prevalence of the disease.32 In the developing world, hepatitis B infection with hepatitis C infection as a close-runner up remains the most common etiology of HCC.38,39 In regions of high hepatitis B prevalence, hepatitis B is transmitted during the birthing process and is often undiagnosed until later in life.39 In developed nations, especially within the United States, alcohol consumption and MASLD are the most common etiologies believed to contribute to the development of HCC.27,32,38 Despite decreased incidence and prevalence of viral hepatitis in the United States, the annual incidence of HCC in adults has been slowly increasing across all racial groups over the last 15 years.39 Though a topic of ongoing investigation, some hypotheses have speculated that the increased incidence of obesity, metabolic syndrome, alcohol related liver disease and metabolic dysfunction-associated steatotic liver disease have contributed to the increase in HCC observed within the United States.32,38,39
What populations should be screened for HCC?
Considering how HCC is often diagnosed during late-stage disease, it is imperative that patients at risk for developing HCC be screened to ensure timely intervention if indicated. As mentioned, individuals with underlying chronic hepatic disease, such as viral hepatitis, cirrhosis, and MASLD, are at increased risks of developing HCC. Additionally, those with certain genetic disorders, such as alpha-1-antitrypsin deficiency, hereditary hemochromatosis, Wilson disease or those who have consistent environmental exposures, such as aflatoxin, are also at risk for HCC. Although there is some variation to current guidelines in the US, a majority of recommendations include screening patients with cirrhosis regardless of age, chronic viral hepatitis, and family history of HCC.25,27,38,39 Current screening guidelines in high risk patients usually begin with liver ultrasonography with further imaging studies, such as CT or MRI, performed if warranted.25, 26, 27,38,39
How can public health measures be improved to help reduce the incidence of HCC?
Considering how viral hepatitis is the leading cause of HCC, it is imperative to provide sufficient vaccine administration and education efforts to prevent further hepatitis B and C infections. The advent of the hepatitis B vaccine has considerably reduced both the incidence of hepatitis B and HCC on a global scale.33 There, however, does not yet exist a viable vaccine for the prevention of hepatitis C. Hepatitis B and C are both transmitted through infected blood and sexual contact. Education and efforts to ensure the use of clean needles and medical equipment are essential to prevent blood borne transmission of hepatitis B and C. Safe sexual practices using barrier prophylactics can also reduce the odds of contracting infection.
Teaching points
-
•
Hepatocellular carcinoma is most often diagnosed during late-stage disease and has a grave prognosis.
-
•
Jaundice is the accumulation of unconjugated or conjugated bilirubin. Diverse etiologies for hyperbilirubinemia include disorders associated with increased bilirubin production, disorders associated with impaired hepatocyte function and disorders associated with biliary tract obstruction.
-
•
Unlike other malignancies, a significant majority of HCC can be diagnosed with only clinical context and imaging, and thereby not requiring biopsy to establish a definitive diagnosis.
-
•
On CT and MRI imaging, HCC is said to have a characteristic hyper-attenuation or “washout” effect.
-
•
HCC, histologically, is classified into one of the following patterns: trabecular, solid, pseudoglandular (pseudoacinar) and macrotrabecular.
-
•
Hepatitis B and C are the most common etiologies of HCC on a global scale and disproportionately affect developing countries.
-
•
Cirrhosis is a significant risk factor for the development of HCC and thereby understanding the etiologies of cirrhosis can provide valuable insights into HCC surveillance. Cirrhosis is associated with many etiologies that include viral hepatitis, steatotic liver disorders (MASLD and ALD), and certain genetic conditions, such as alpha-1-antitrypsin deficiency and hemochromatosis.
-
•
In the U.S., an increasing number of HCC cases are believed to be attributed to the increased incidence of obesity, metabolic syndrome, hemochromatosis, and alcohol consumption.
-
•
On a global scale, hepatocellular carcinoma remains a significant public health concern especially amongst nations that have a high prevalence of hepatitis B and C infections.
Author's note
Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9 were obtained during the scope of US government employment for Dr. Conran.
Funding
The article processing fee for this article was funded by an Open Access Award given by the Society of ‘67, which supports the mission of the Association of Pathology Chairs to produce the next generation of outstanding investigators and educational scholars in the field of pathology. This award helps to promote the publication of high-quality original scholarship in Academic Pathology by authors at an early stage of academic development.
Declaration of competing interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article and that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- 1.Knollmann-Ritschel B.E.C., Huppmann A.R., Borowitz M.J., Conran R. Pathology competencies in medical education and educational cases: update 2023. Acad Pathol. 2023;10(3):100086. doi: 10.1016/j.acpath.2023.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fargo M.V., Grogan S.P., Saguil A. Evaluation of jaundice in adults. Am Fam Physician. 2017;95(3):164–168. [PubMed] [Google Scholar]
- 3.Patel J., Smith A. Evaluation of jaundice. BMJ best practice. April 2022. https://bestpractice.bmj.com/topics/en-us/511
- 4.Joseph A., Samant H. StatPearls Publishing; 2022. Jaundice. In StatPearls.https://europepmc.org/article/nbk/nbk544252#free-full-text [PubMed] [Google Scholar]
- 5.Ferri F.F. In: Ferri's Clinical Advisor 2023. Ferri F.F., editor. Elsevier; 2023. A-differential diagnosis. Abdominal pain, right upper quadrant.https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323755733010040?scrollTo=%23hl0001046 [Google Scholar]
- 6.Charach L., Zusmanovitch L., Charach G. Hepatocellular carcinoma. Part 2: clinical presentation and diagnosis. EMJ Hepatol. 2017;5(1):81–88. [Google Scholar]
- 7.Qin L.X., Tang Z.Y. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9(3):385–391. doi: 10.3748/wjg.v9.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torbenson M. In: Mills and Sternberg's Diagnostic Surgical Pathology. 7th ed. Longacre T.A., Greenson J.K., Hornick J.L., Reuter V.E., editors. Wolters Kluwer Health; 2022. Masses of the liver; pp. 1890–1955. [Google Scholar]
- 9.American Cancer Society Liver cancer risk factors. https://www.cancer.org/cancer/types/liver-cancer/causes-risks-prevention/risk-factors.html
- 10.Schwartz J.M., Carithers R.L., Sirlin C. In: Clinical Features and Diagnosis of Hepatocellular Carcinoma. Chopra S., Kressel H.Y., editors. UpToDate; Waltham, MA: 2022. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-hepatocellular-carcinoma [Google Scholar]
- 11.Parra N.S., Ross H.M., Khan A., et al. Advancements in the diagnosis of hepatocellular carcinoma. Int Journal Transl Med. 2023;3(1):51–65. doi: 10.3390/ijtm3010005. [DOI] [Google Scholar]
- 12.Attwa M.H., El-Etreby S.A. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(12):1632–1651. doi: 10.4254/wjh.v7.i12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard F, Yap J, Chang Z, et al. Hepatocellular carcinoma. Radiopaedia.org Accessed November 15, 2022. 10.53347/rID-1442. [DOI]
- 14.Jacobson DR. Hepatocellular Carcinoma Imaging. In: Karani J (Ed) emedicine.medscape.com. Accessed June 26, 2023 https://emedicine.medscape.com/article/369226-overview#showall.
- 15.Malhi H., Grant E.G., Duddalwar V. Contrast-enhanced ultrasound of the liver and kidney. Radiol Clin North Am. 2014;52(6):1177–1190. doi: 10.1016/j.rcl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Schlageter M., Terracciano L.M., D'Angelo S., Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20(43):15955–15964. doi: 10.3748/wjg.v20.i43.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen IY, Agostini-Vulaj D. Hepatocellular carcinoma overview. PathologyOutlines.com Accessed August 20, 2021 https://www.pathologyoutlines.com/topic/livertumorHCC.html.
- 18.Brunt E.M. Histopathologic features of hepatocellular carcinoma. Clin Liver Dis. 2013;1(6):194–199. doi: 10.1002/cld.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill R.M., Kakar S. In: Robbins and Cotran Pathologic Basis of Disease. 10th ed. Kumar V., Abbas A.K., Aster J.C., editors. Elsevier; 2021. Liver and gallbladder; pp. 823–871. [Google Scholar]
- 20.Torbenson M., Zen Y., Yeh M.M. 39–86. American Registry of Pathology; Washington, DC: 2018. Tumors of the Liver. AFIP Atlas of Tumor Pathology Series 4; pp. 117–131. Fascicle 27. 97-112. [Google Scholar]
- 21.Kremer N., Walther A.E., Tiao G.M. Management of hepatoblastoma: an update. Curr Opin Pediatr. 2014;26(3):362–369. doi: 10.1097/MOP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 22.Rinella M.E., Lazarus J.V., Ratziu V., et al. On behalf of the NAFLD nomenclature consensus group. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 Jun 24 doi: 10.1097/HEP.0000000000000520. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23.Marrero J.A., Kulik L.M., Sirlin C.B., et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 24.U.S Department of Health & Human Services Viral hepatitis in the United States: data and trends. https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/data-and-trends/index.html
- 25.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:∼:text=A safe and effective vaccine,chronic disease and liver cancer
- 27.Ghouri Y.A., Mian I., Rowe J.H. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanasekaran R., Bandoh S., Roberts L.R. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.6946.1. Faculty Rev-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levrero M., Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Pai S.G., Carneiro B.A., Mota J.M., et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arzumanyan A., Reis H.M., Feitelson M.A. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13(2):123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 32.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. Erratum in: JAMA. 2015 Oct 13;314(14):1521. doi:10.1001/jama.2015.12071. [DOI] [PubMed] [Google Scholar]
- 33.Balogh J., Victor D., 3rd, Asham E.H., et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization, International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 96. IARC; 2010. Alcohol consumption and ethyl carbamate; pp. 399–403. [Google Scholar]
- 35.Batey R.G., Burns T., Benson R.J., Byth K. Alcohol consumption and the risk of cirrhosis. Med J Aust. 1992;156(6):413–416. doi: 10.5694/j.1326-5377.1992.tb139846.x. [DOI] [PubMed] [Google Scholar]
- 36.Patel N., Yopp A.C., Singal A.G. Diagnostic delays are common among patients with hepatocellular carcinoma. J Natl Compr Cancer Netw. 2015;13(5):543–549. doi: 10.6004/jnccn.2015.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Center for Drug Evaluation and Research FDA approves lenvatinib for unresectable hepatocellular carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma
- 38.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2020;73(S1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal S., El-Serag H.B. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]