Abstract
Background
Myocardial work (MW) is a new echocardiographic tool with a high sensitivity to detect early and subtle alterations of myocardial function. We aimed to evaluate the late effects of anthracyclines by assessing the global and segmental MW and intraventricular mechanical dispersion from speckle tracking echocardiography in childhood lymphoma survivors (CLS).
Methods
Thirty‐one young adults including CLS and age‐matched healthy controls were enrolled. All underwent echocardiography including an evaluation of left ventricular (LV) morphology and regional function. We assessed LV longitudinal (differentiating sub‐endocardial and sub‐epicardial layers), circumferential strains and twist, global and regional MW index (MWI). LV mechanical dispersion was assessed from the time dispersion of LV longitudinal strain, from myocardial wasted work (MWW) and myocardial work efficiency (MWE).
Results
The longitudinal strains both at the level of the sub‐endocardium and sub‐epicardium were reduced in CLS compared to controls. The global MWI was also decreased (1668 ± 266 vs 1870 ± 264%.mmHg in CLS patients and controls, respectively, p < 0.05), especially on the apical segments. An increase of LV intraventricular mechanical dispersion was observed in CLS. MWW and MWE remained unchanged compared to controls.
Conclusion
Our results strongly support that cardiac remodeling is observed in CLS, characterized by a decrease in MW and an increase in LV mechanical dispersion. The apex is specifically altered, but its clinical significance remains uncertain. MW as a complement to strain seems interesting in cancer survivors to detect myocardial dysfunction at early stage and adapt their follow‐up.
Keywords: anthracycline, childhood lymphoma survivors, intraventricular mechanical dispersion, myocardial work, regional myocardial function, speckle tracking echocardiography
Cardiac remodeling is observed in childhood lymphoma survivors, characterized by a modification in myocardial work and left ventricular mechanical dispersion. Myocardial work seems interesting in cancer survivors to detect myocardial dysfunction at an early stage and thus to adapt their follow‐up and management.

1. INTRODUCTION
Childhood cancer is a heterogeneous group. Among adolescents, the most common cancer group is lymphoma (27%). In most European and North American countries, survival exceeds 80% at 5 years, 1 , 2 especially with chemotherapy, which usually combines antineoplastic drugs whose anthracyclines. Current international protocols aim to reduce the intensity of chemotherapy without threatening survival, in order to limit its toxicity, particularly on the cardiovascular system. Indeed, cardiac adverse effects are one of the main causes of mortality among childhood cancer survivors, especially with anthracyclines which are associated with an increased risk for heart failure compared to their siblings, with an increasing incidence of prolonged survival. 3 , 4 , 5 The appearance of chemotherapeutic cardiotoxicity may indicate the interruption of treatment, 6 , 7 and therefore cardiotoxicity is an important prognostic factor.
Cardiology and oncology societies presented clinical guidelines on the prevention and treatment of anthracycline‐induced cardiotoxicity (AIC) focused on screening for left ventricular ejection fraction (LVEF) reduction throughout the treatment. 8 , 9 Recently, the American Society of Echocardiography and European societies recommended the Global Longitudinal Strain (GLS), from speckle tracking echocardiography (STE), as a marker of myocardial dysfunction. 8 , 9 , 10 , 11 Indeed, it may be associated with an early sign of evolving cardiomyopathy among childhood cancer survivors treated with anthracyclines despite their normal LVEF. 12 , 13 , 14 , 15 However, GLS represents only one component of LV strains. A comprehensive approach to assess LV systolic function needs to combine GLS with circumferential strains, 16 and also LV twisting mechanics. 17 During systole, due to the orientation of the myocardial fibers, the base and the apex rotate in opposing directions, inducing LV twists, maintaining an essential part of systolic function and could be reduced in childhood lymphoma survivors (CLS). 17 , 18 , 19 Another important feature is to propose a regional assessment of LV strain 16 and differentiate subendocardial and subepicardial layers that could provide additional information on the subtle myocardial remodeling in CLS.
Importantly, despite its capability to assess LV regional myocardial function, evaluation of LV strains remains limited by its dependence on loading conditions of the heart. 20 To overcome this limitation, a new echocardiographic tool based on the evaluation of myocardial work (MW) has been developed to detect subtle LV dysfunctions. 21 , 22 , 23 Initiated by the works of Russel et al., MW is obtained by integrating both cardiac deformation and noninvasive intra‐ventricular pressure. 24 , 25 In adult populations, MW has already been used in many diseases and previous work demonstrated its better sensitivity in predicting coronary artery disease and assessing early subclinical ischemic myocardial dysfunction compared to GLS. 26 Notably, MW can be assessed regionally, according to an 18‐segments model of the LV. However, both its global and regional assessment has never been explored in CLS and remains to be evaluated.
Myocardial performance can also be altered by an uncoordinated shortening of LV myocardial fibers, which could lead to LV dyssynchrony (LVD) characterized by an increased mechanical timing dispersion at end‐systole. 19 In childhood cancer survivors, structural myocardial abnormalities with the presence of diffuse interstitial fibrosis, and sarcomeric disruption are evidenced. 5 , 27 Myocardial fibrosis is usually associated with conduction disorders, so we questioned a potential increase of LV mechanical dispersion in CLS. 28 Segmental analysis of the timing of LV strains is one way to assess LV mechanical dispersion, and MW is another. Indeed, a study of MW, which includes myocardial constructive (MCW) and wasted work (MWW), could reveal an increase in mechanical dispersion via an increase in MWW. 19 , 29
In this context, the objective of the study was to compare the cardiac function of CLS, with healthy age‐matched controls, to determine the late effects of anthracyclines by assessing not only myocardial strain, but also MW and LV mechanical dispersion. We hypothesized that CLS treated with anthracyclines have adverse cardiac remodeling characterized by (1) a decrease of longitudinal strain, especially in the subendocardial layer, (2) a decrease of segmental and global MW, (3) increase of LV intraventricular mechanical dispersion associated with an increase of MWW.
2. METHODS
2.1. Study design and population
This study collected the data of our previous prospective cross sectional case–control study run from January 2022 to May 2022 in Clermont‐Ferrand University Hospital, France. 30 Sixteen CLS were included and compared with 15 healthy controls, recruited from the database of the Centre d'Investigation Clinique of Clermont‐Ferrand University Hospital, and matched for sex, age (± 5 years), and body mass index (BMI ± 5 kg/m2). None of the participants had cardiac medication at the time of evaluation and all had electrocardiogram (ECG) recording and were in sinus rhythm. CLS aged between 18 and 25 years old were followed for childhood lymphoma post‐therapeutic surveillance, and considered as recovered (i.e., free of any lymphoma medication or radiotherapy for at least 1 year), with no physical disability at the day of inclusion. To guarantee a late cardiac functional assessment and the absence of any progressive malignancy, only patients who have recovered from treatment for over 1 year were eligible for the study. Subjects were excluded if they had been clinically diagnosed with a running infection or undergone corticosteroid treatment for at least 1 week within the last month before the day of visit. 30 , 31
2.2. Anthropometrics
Height was determined using a mural stadiometer with the subject barefoot (graded in cm, precision of 0.5 cm), and weight using a digital weight scale (graduated in kg, with 0.1 kg precision), permitting calculation of BMI (body weight/body height2, with weight in kg and height in m). Blood pressure (BP) were recorded at rest, using an automatic device (General Electric Dynamap PRO 300 V2, Boston, MA, USA).
2.3. Biological data
Blood samples were collected after 12 h of fasting and at least 10 min resting. The two main cardiotoxicity biomarkers, troponin I and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), were assessed in all participants. 3 Standard blood test as hemoglobin, C‐reactive protein, thyroid hormones and albumin were also performed to identify irregularities, which could influence cardiovascular parameters. 16
2.4. Echographic conventional recordings
Echocardiography was carried out with the subject in the left lateral decubitus position, with Vivid ultrasound systems (GE Healthcare, Horten, Norway) using a 3.5 MHz transducer (M4S probe), by the same, experimented, cardiologist, blinded of the status of patients. 2D echocardiographic measurements were made in accordance with the American Society of Echocardiography guidelines. 32 Cine loops are stored in one R–R interval length to facilitate analysis. An ECG signal is recorded for R–R interval clip storage and no ambiguous ECG complexes or leading T‐waves were displayed. Thickness parameters were measured from the parasternal long axis view. LV volumes and LVEF were assessed using the Simpsons biplane method. Stroke volume and cardiac output were assessed from a 5‐chamber view and then indexed to body surface area. LV mass was estimated by the Lang formula and indexed to body surface area. Diastolic function was assessed by Doppler from peak early (E) and atrial (A) trans‐mitral flow velocities. Septal and lateral E' wave from tissue Doppler completes this evaluation. The LV filling pressures were evaluated from the E/E' ratio.
2.5. Two‐dimensional strain analysis from STE
Cine loops were recorded in parasternal long and short axis (basal and apical) and apical (5, 4, 2, and 3 chambers) views and saved for blinded off‐line analysis (EchoPAC, BT204 Version, GE Healthcare), with ECG recording. Time was expressed as a percentage of systolic duration avoiding therefore bias from differences in heart rate and cine loop frame rates. We used apical 4‐, 2‐, and 3‐chambers views to assess LV regional strains and time to peak (TTP) strains to obtain an18‐segments model, in accordance with the nomenclature of Lang et al. 32 A segment‐by‐segment validation must be done to continue the analysis. When analysis was not possible or unreliable, the segment or loop were excluded from the analysis. Time‐derivation of longitudinal strains results in both systolic and diastolic strain rates. Notably, the diastolic GLS‐rate was used as an indicator of LV relaxation. 33
LV twisting mechanics were assessed by STE using images from the apical and basal short‐axis views. Basal planes were assessed regarding the mitral valve and apical planes toward the apex with no visible papillary muscles. With LV contraction, the cardiac apex and the base rotates in counterclockwise and clockwise directions, respectively, when viewed from the apex. Twist represents the mathematical difference in the rotation of the apex and base, at each percentage of systolic duration. 13 Peak systolic twisting velocity was defined by the peak positive rate of torsional deformation, whereas the peak diastolic untwisting velocity was defined by the peak negative rate of torsional deformation. 34 Untwisting velocity is thought to be an important initial manifestation of active relaxation.
The LV mechanical dispersion was quantified by calculating the standard deviation (SD) of TTP systolic strain in the longitudinal direction in the 18‐segments model (SD18S), and the maximum delay between the segments with the earliest and the latest TTP (Delta1‐18). 19
2.6. MW
MW was estimated using the Automatic Function Imaging (AFI) of the EchoPAC software (Version 203; GE Healthcare). MW was assessed by the combination of LV strain data (recorded on the apical 4‐, 3‐, and 2‐chambers views) obtained by STE, as a function of time throughout the cardiac cycle, and a noninvasively estimated LV pressure curve as previously described. 24 The valvular markers of mitral and aortic opening and closure were taken first on the Doppler from peak early (E) and atrial (A) trans‐mitral flow and trans‐aortic, for reproducibility, and then imported into the MW analysis with a secondary modification on the 2D section for synchronization. All data are averaged on 2 cardiac cycles, synchronized with ECG, and we assumed that the high and low pressure measured was respectively equivalent to peak systolic and diastolic LV pressures and are uniform throughout the ventricle. Strain and MW evaluation was done by a single investigator. The global myocardial work efficiency (MWE) depends on the MCW and MWW, and was obtained as follows:
In systole, the “positive” work performed by the myocardium during shortening represents MCW, including lengthening in isovolumic relaxation, whereas the “negative” work performed by the myocardium during lengthening, including shortening in isovolumic relaxation, represents the energy loss, which is defined as MWW. 19 For both global and segmental analyses, we constructed LV pressure‐strains loops. The area of the loops represented the Myocardial work index (MWI).
2.7. Statistical analysis
All values were expressed as mean ± standard deviation. Statistical analyses were performed using Medcalc (version 19.1, Medcalc Software). One‐way analysis of variance (ANOVA) was used to compare groups, after checking the normality of distribution of each variable by the Shapiro–Wilk test. In the absence of normal distribution, the nonparametric Kruskal‐Wallis test was used. Statistical significance for all analyses was assumed at p < 0.05.
3. RESULTS
3.1. Participants characteristics
Clinical data of the two populations are presented in Table 1. The mean time interval between the end of chemotherapy and patient enrolment was 6.3 years. CLS were treated by chemotherapy with a mean cumulative dose of anthracyclines of 160 mg/m2 with a maximal dose of 286 mg/m2 in one patient. Doxorubicin was mainly used. Mean cumulative corticosteroids dose was 2600 mg/m2. Alkylating‐agent and type II topo‐isomerase inhibitors were used for all patients. Four Hodgkin lymphoma survivors had a total of 19.6 Gy mediastinal and cervical targeted radiation therapy (depending on diagnosis stage or relapse). In the course of therapy, we noted only two relapses, respectively 15 and 24 months after the date of diagnosis. These two were Hodgkin disease and with the same extension (stade 2A) with no spreadings, and beneficiated of autologous hematopoietic stem cell transplantation. A heatmap for individual treatment exposure are presented in Figure 1.
TABLE 1.
Baseline Characteristics of Patients and Controls.
| Patients (n = 16) | Controls (n = 15) | |
|---|---|---|
| Age | 21 ± 2 | 23 ± 1,5* |
| Male (n) | 9 | 7 |
| Female (n) | 7 | 8 |
| BMI | 23,5 ± 3,2 | 22,3 ± 2,5 |
| Heart rate at rest (bpm) | 81 ± 13 | 70 ± 13* |
| Systolic BP (mmHg) | 118 ± 12 | 119 ± 16 |
| Diastolic BP (mmHg) | 76,6 ± 9,6 | 74,9 ± 8,8 |
| Mean BP (mmHg) | 89,5 ± 10,3 | 88,0 ± 7,8 |
| Hodgkin disease | 12 | |
| Non‐Hodgkin lymphomas # | 4 | |
| Age at diagnosis (years) | 14,7 ± 1,5 | |
| Cumulative dose of Anthracyclines (mg/m2) | 160,3 ± 37,4 | |
| Time since end of treatment (years) | 6,3 ± 2,4 | |
| Radiotherapy (n) | 4 | |
| Hematopoietic stem cells transplantation for relapse (n) | 2 | |
| Other chemotherapy | All |
Note: Quantitative variables are expressed as mean ± standard deviation. *: significantly different from Controls (*: p < 0.05). # Non Hodgkin lymphomas gather, one B and one T lymphoblastic lymphoma, one Gray zone lymphoma and one large B cells lymphoma.
Abbreviation: BMI, body mass index.
FIGURE 1.

Heatmap for treatment exposure. Cumulative doses in mg/m2. Corticosteroids (prednisone or dexamethasone) are recorded as prednisone‐equivalent. Vinkaalcaloids: vinblastin, vincristin, vinorelbin. Anthracyclines: daunorubicine or doxorubicine. Type II topoisomerase inhibitor: etoposide. Alkylating agents: bendamustin, carmustin, cyclophosphamide, dacarbazin, ifosfamide, melphalan or procarbazin. Anti‐metabolites: cytarabin, gemcitabin, 6‐mercaptopurin, methotrexate or 6‐thioguanin. Monoclonal antibodies (mAb): brentuximab or rituximab. # Non Hodgkin lymphomas.
There was no significant difference between CLS and healthy controls in term of BP. No patient had symptoms of heart failure. Heart rate was significantly higher in CLS compared to controls. No statistical difference was shown on troponin I and NT‐proBNP. A mean NT‐proBNP value of 51 ± 33 ng/L was found in CLS. Hemoglobin was higher in CLS. None had anemia. Nonsignificative difference was found on CRP, normal for all, thyroid hormone nor on nutritional status estimated by albumin levels.
3.2. Left ventricular morphology and function
Data of LV morphology and function are presented in Table 2. LV posterior wall thickness, LV septum thickness, LV end‐diastolic and end‐systolic volume, LV mass and LV mass indexed to body surface area were comparable in both populations. LVEF was significantly decreased in CLS but no LVEF below 60% was detected. There was no significant difference in the early to late diastolic filling ratio (E/A ratio) and E/E' ratio.
TABLE 2.
Left ventricular morphology and function.
| Patients (n = 16) | Controls (n = 15) | |
|---|---|---|
| LV Morphology | ||
| LV septum thickness (cm) | 0.84 ± 0.12 | 0.85 ± 0.17 |
| LV posterior wall thickness (cm) | 0.85 ± 0.16 | 0.91 ± 0.11 |
| LV end‐diastolic volume (mL) | 114 ± 32 | 106 ± 27 |
| LV end‐systolic volume (mL) | 33 ± 9 | 32 ± 8 |
| LV mass (g) | 145 ± 48 | 143 ± 38 |
| LV mass/BSA | 80 ± 11.5 | 83 ± 9.7 |
| LV systolic function | ||
| Ejection fraction (%) | 65.2 ± 5.3 * | 69.6 ± 6.3 |
| LV diastolic function | ||
| E wave (m/s) | 0.86 ± 0.14 | 0.89 ± 0.15 |
| A wave (m/s) | 0.51 ± 0.08 | 0.57 ± 0.13 |
| E/A | 1.72 ± 0.33 | 1.59 ± 0.29 |
| E/E' lat | 4.5 ± 0.7 | 4.5 ± 0.9 |
Note: Values are mean ± standard deviation. *: significantly different from Controls (*: p < 0.05).
Abbreviations: BSA, body surface area; LV, left ventricular.
3.3. Left ventricular regional strains and MW
LV strains are presented in Table 3 and Figure 2. GLS was diminished in CLS patients compared to controls, whereas no difference was observed on circumferential strains. We note a significant and similar difference in both sub‐endocardium and sub‐epicardium in CLS compared to controls. In CLS, the global strain rate values were significantly inferior and we note a significant difference in Diastolic GLS‐rate. LV twisting mechanics are presented in Table 3 and Figure 3. LV rotations and twist were similar between groups despite their patterns during the cardiac cycle appears slightly different. Peak untwisting rate, which occurred in very early diastole, was significantly lower in CLS compared to controls.
TABLE 3.
Left ventricular strains.
| Patients (n = 16) | Controls (n = 15) | |
|---|---|---|
| LV longitudinal strains | ||
| GLS (%) | ||
| Myocardium | −14.2 ± 2.5 *** | −17.1 ± 1.6 |
| Sub‐endocardium layer | −16.6 ± 2.7 *** | −19.8 ± 2.4 |
| Sub‐epicardium layer | −11.5 ± 2.6 ** | −14.1 ± 1.9 |
| Systolic GLS‐rate (.s−1) | −0.75 ± 0.11 ** | −0.86 ± 0.06 |
| Diastolic GLS‐rate (.s−1) | 1.26 ± 0.33 ** | 1.54 ± 0.24 |
| LV circumferential strains | ||
| CS (%) | ||
| Basal level | −11.3 ± 1.4 | −11.8 ± 3.8 |
| Apical level | −19.4 ± 6.4 | −19.3 ± 5.6 |
| Systolic CS‐rate (.s−1) | ||
| Basal level | −0.79 ± 0.11 | −0.82 ± 0.23 |
| Apical level | −1.22 ± 0.45 | −1.23 ± 0.29 |
| Diastolic CS‐rate (.s−1) | ||
| Basal level | 1.15 ± 0.31 | 1.32 ± 0.42 |
| Apical level | 1.62 ± 0.39 | 1.82 ± 0.63 |
| LV Twisting mechanics | ||
| Rotations (deg) | ||
| Basal level | −4.5 ± 2.6 | −3.6 ± 1.9 |
| Apical level | 6.1 ± 2.6 | 6.6 ± 2.3 |
| Twist (deg) | 7.7 ± 3.2 | 8.1 ± 3.7 |
| Untwisting rate (°/s−1) | ‐ 61.2 ± 23.3 * | −82.5 ± 30.4 |
Note: Values are mean ± standard deviation. *: significantly different from Controls (*: p < 0.05; **: p < 0.01; *** p < 0.001).
Abbreviations: CS, circumferential strain; GLS, global longitudinal strain.
FIGURE 2.

Longitudinal and circumferential strain. Longitudinal strain and circumferential strains (CS) in childhood lymphoma survivors (red curve) and controls (blue curve). Circumferential strain is displaced as basal and apical segments. Longitudinal strains are displaced as global longitudinal strain (GLS), sub‐endocardial and sub‐epicardial layers. Difference between groups: p < 0.05.
FIGURE 3.
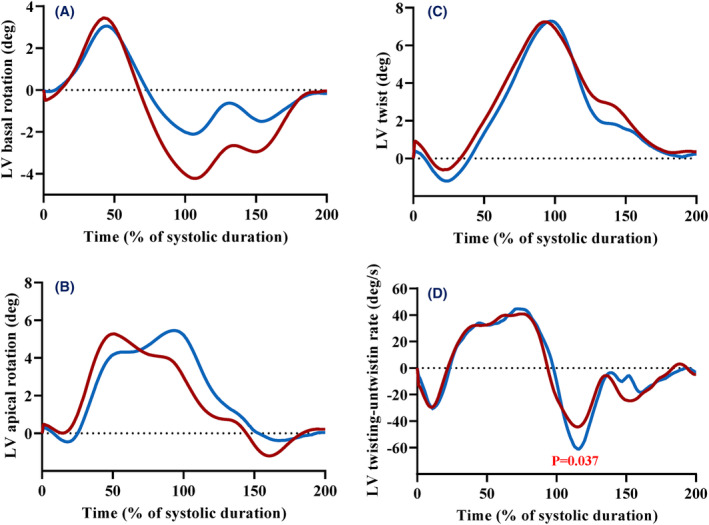
Left ventricular twisting mechanics. Twist and untwist mechanisms in childhood lymphoma survivors (red curves) and controls (blue curves). (A) Basal rotation, (B) apical rotation and (C) twist, function of time (% of systolic duration). (D) Twist –untwist rate, function of time (% of systolic duration). Difference between groups: p < 0.05.
MW data are presented in Table 4 and Figure 4. In CLS patients, the LV pressure‐strain loops (PSL) were shifted to the right, consequently to a decrease in their GLS. The MWI, calculated as the area of the PSL, was significantly different between groups (1668 ± 266 vs 1870 ± 264%. mmHg in CLS patients and controls, respectively). In CLS, segmental MWI values presented in Figure 5 were significantly lower in the apical segments, whereas no significant difference was shown in the mid and basal segments.
TABLE 4.
Myocardial work and Left ventricular mechanical dispersion.
| Patients (n = 16) | Controls (n = 15) | |
|---|---|---|
| Myocardial work | ||
| Constructive work (%.mmHg) | 1834 ± 262 | 2042 ± 298 * |
| Wasted work (%.mmHg) | 123 ± 72 | 91 ± 50 |
| Global work efficiency (%) | 0.93 ± 0.03 | 0.95 ± 0.02 |
| LV mechanical dispersion | ||
| Delta 1–18 | 237 ± 149 | 145 ± 50 * |
| SD 18S (ms) | 88 ± 33 | 58 ± 26 * |
Note: Values are mean ± standard deviation. *: significantly different from Controls (*: p < 0.05).
FIGURE 4.

Myocardial work and its components. Noninvasive myocardial work estimation obtained from echocardiography. The left ventricle (LV) pressure–strain loop (PSL) is generated as a function of time of global longitudinal strain and systolic blood pressure, integrated to with the timing of aortic and mitral valve opening and closure. The area of the pressure–strain loop then provides the global myocardial work index (MWI) (at the left side). Global myocardial work efficiency (MWE) is then obtained by dividing the global constructive myocardial work (MCW) by the sum of both MCW and global myocardial wasted work (MWW). All global values are derived from averages of their segmental values.
FIGURE 5.

Segmental myocardial work index from the construction of left ventricular Pressure‐Strain loops. These 18 figures represent, for each line, the 3 (A3C), 2 (A2C), and 4 (A4C) chambers views, using the same coding with childhood lymphoma survivors (red curves) and controls (blue curves) on pressure strain loops (PSL). The area of the pressure–strain loops corresponds to the segmental myocardial work index (MWI). In each PSL segment, p < 0.05 indicates a significant difference with the corresponding segment of the control group.
4. DISCUSSION
Our study aimed to compare the cardiac function of CLS with age‐matched control subjects to determine the late anthracyclines cardiotoxicity using STE. In CLS, we observed: (1) a reduction of longitudinal strains in a similar manner in the sub‐endocardium and sub‐epicardium, without alteration of circumferential strains and twist, (2) a decrease of MW, mainly observed on apical segments, (3) an increase of LV intraventricular mechanical dispersion, but without significant alteration of MWW and global MWE.
4.1. Lower longitudinal strain without alteration of circumferential strains and twist in CLS
Our study confirmed that GLS, enabling a more sensitive analysis of systolic function than LVEF, was significantly lower in CLS than in controls although they received relatively low doses of anthracyclines. Of note, whereas GLS was decreased, LV CS, rotations and twist were unchanged in CLS. The LV twist plays an important role in promoting LV ejection during systole, therefore the maintenance of LV twist in CLS probably contributed to maintain their LVEF despite the decrease of the GLS. A similar pattern of impaired GLS without changes in CS was documented in young patients after chemotherapy, 13 , 16 , 18 , 35 but also in patients suffering of hypertrophic cardiomyopathy. 36 Since the sub‐endocardial layer is principally composed of fibers oriented longitudinally, it has been often hypothesized that sub‐endocardial layer was more sensitive to myocardial diseases such as fibrosis. For example, lower CS is found in transmural myocardial infarction, differentiating it from subendocardial infarction. 13 In adults treated with anthracyclines, previous studies observed that the late gadolinium cardiac magnetic resonance was enhanced specifically in the sub‐endocardium, suggesting the presence of fibrosis. 28 , 37 However, our results indicated that the decrease of LS was similar on both layers, that spoke in favor of an alteration of different zones of the myocardium wall rather than a specific alteration of the sub‐endocardial layer.
During systole, LV twist is induced by the contraction of the cardiomyocytes generating opposite rotations of the LV base and apex. This mechanism predominantly deforms the subendocardial fiber matrix, resulting in the storage of potential energy. Following untwist is associated with the release of these forces, which contributes to LV diastolic relaxation and early filling which onset and peak could be significantly delayed in diastolic heart failure. 38 In our study, we observed that the untwisting rate was lowered in CLS despite their normal LV twist. This result has been described by others 17 , 39 and supports that underlying mechanisms of diastolic function were altered in CLS patients with a potential decrease of LV intrinsic myocardial relaxation.
4.2. Lower MW in CLS, especially on apical segments
Noninvasive PSL have been previously validated as a surrogate of MW and overcome the load‐dependency of EF and LS. Indeed, MW takes into account deformation as well as afterload through interpretation of strain in relation to noninvasive LV pressure (estimated by measurement of cuff pressures). 19 , 40 , 41 , 42 Assessments of MW were already done in preclinical and adult cohorts of patients. 21 , 22 , 43 For example, MW has been assessed in ischemic cardiac disease, allowing the detection of subclinical coronary artery disease without any regional wall motion abnormalities, and has more sensitivity for myocardial oxygen consumption. 26 In pediatric population, Zhan et al. recently demonstrated that global MWI enhanced hypertensive heart disease evaluation and cardiac dysfunction during and after chemotherapy follow‐up. 42 , 44 In our study, while systolic BP were similar between groups, the MWI was reduced in CLS, linked in a large part to their lower GLS.
The strength of our study was to assess not only global MW, but also segmental MW. Based on regional assessments on an 18‐segments model of the LV, we observed that MW was specifically decreased at the apical region. To our knowledge, no previous studies assessed the segmental MWI in CLS. This result was in agreement with previous study, showing impaired LS specifically in apical segments, in a cohort of childhood‐cancer survivors. 16 , 45 , 46 , 47 We also noticed a specific and significant decrease of MW on the basal inferior in CLS compared to controls, whereas other basal segments remained strictly unchanged. The clinical significance and the underlying mechanisms of this regional alteration remain unclear. Specific segmental modifications of MW have already been showed in adult populations for precising diagnosis, as a predictor of mortality by evaluation of its apex‐to‐base ratio in cardiac amyloidosis, or as short‐term prognosis markers in ischemic diseases or post‐therapeutic prediction response in cardiac resynchronization therapy. 40 In our population and based on preclinical in‐vivo juvenile rat's study, our result could suggest starting fibrosis pattern in the apical regions in CLS. 48 Apical deterioration might be also explained by the extent of sensitivity toward the LV apical vortex, which is formed by the helical fibers that exist without any surrounding circumferential muscle. 49 Further investigations are needed to explore the role of the apex in myocardial mechanics and its specific implication in long‐term cardiac dysfunction in childhood‐cancer survivors. Our study underscored the ability of recent echocardiographic advancements, particularly those relying on regional left ventricular strains and MW, to detect subtle cardiac toxicity induced by anthracyclines in young patients with CLS. Despite the limited sample size in our study, these findings suggest that these novel assessments are particularly valuable and should be addressed in the follow‐up of such patients. This is supported by other recent studies that have highlighted MW as a highly sensitive evaluation of myocardial function during chemotherapy, aiding in the diagnosis of subclinical cardiotoxicity. 43 The assessment of GLS has become widely employed across various pathological conditions. The incorporation of MW assessment necessitates the inclusion of only arterial pressure, simplifying its integration into routine clinical practice.
4.3. Higher intraventricular mechanical dispersion in CLS
Interstitial fibrosis was detected in microscopic slides of heart tissue from patients who had cardiac transplants after AIC, without significant cardiac hypertrophy. 50 Myocardial fibrosis having adverse effects on excitability, sarcomeric organization, intracellular calcium homeostasis and myofilament Ca2+ sensitivity, 48 , 51 we hypothesized that these alterations could increase mechanical myocardial dispersion and decrease efficiency. Based on segmental STE analysis, we observed that SD18S and delta 1–18, both overall indexes of LV mechanical dispersion, were significantly increased in CLS compared to controls. Other studies also showed increased systolic intraventricular dyssynchrony index in childhood‐cancer survivors, with significant negative correlations with LVEF. 52 , 53 To complete the study of mechanical dispersion, we measured MCW and MWW to calculate MWE. In patients with increased mechanical dispersion, MWW is generally increased and consequently MWE is reduced. However, we did not find significant alteration of both MWW and MWE in CLS compared to controls. These findings were nevertheless concordant with Zhan et al. showing preserved MWE 42 but the discrepancies in our results between increased mechanical dispersion and unchanged MWE remained not fully understood.
4.4. Study limitations
Our study has several limitations. First, this is a single‐center study including a small group of patients. However, despite the small sample size, our data clearly showed a significant impact of anthracycline on LV regional myocardial function assessed by 2D‐strain echocardiography and MW. Analyzing differences in myocardial function among subtypes of NHL in young adults post‐lymphoma with AIC would be of interest. However, due to the limited size of the study population, it was not feasible to incorporate the NHL subtype in the statistical analysis. Moreover, we assessed only the late effects of anthracycline, known to develop even years after completion of treatment, and it would be interesting to assess their cardiac function before and during the treatment. Finally, it would be helpful to link the increase of the LV mechanical dispersion observed in CLS with a potential increase of myocardial fibrosis from cardiac magnetic resonance imaging with late gadolinium enhancement.
5. CONCLUSION
With more CLS now reaching adulthood, understanding the adverse effects of chemotherapy and particularly anthracyclines on the cardiovascular system are of major interest. Our results highlighted that the late cardiac effects of chemotherapy based on low to moderate doses of anthracyclines were characterized by a decrease of MW, a new echocardiographic tool that is useful to detect early and subtle dysfunction in various pathologies. Based on a regional analysis, our data strongly supported that MW was diminished specifically in apical segments. Moreover, our regional analysis enabled an assessment of the intraventricular mechanical dispersion, which was higher in CLS compared to controls. All‐in‐one, our study strongly supported a late effect of chemotherapy based on anthracyclines on regional myocardial function and LV mechanical dispersion in adolescents and young adults. However, further studies would be helpful to evaluate the clinical significance of these cardiac remodeling and their potential long‐term adverse cardiac events, to propose an adapted follow‐up to CLS and evaluate the benefit of cardio‐protective therapies.
AUTHOR CONTRIBUTIONS
Mohamed Jaber: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (lead); methodology (equal); writing – original draft (lead); writing – review and editing (equal). Alexandre Armand: Data curation (equal); methodology (equal). Emmanuelle Rochette: Methodology (equal); validation (equal). Severine Monzy: Investigation (equal). Victoria Greze: Project administration (supporting); writing – review and editing (supporting). Justyna Kanold: Supervision (supporting); writing – review and editing (supporting). Etienne Merlin: Supervision (supporting); writing – review and editing (supporting). Justine Paysal: Formal analysis (equal); investigation (equal); methodology (equal); supervision (lead); writing – review and editing (lead). Stéphane Nottin: Formal analysis (equal); investigation (equal); methodology (equal); supervision (lead); writing – review and editing (lead).
FUNDING INFORMATION
The authors have no relevant financial or non‐financial interests to disclose.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
ETHICS STATEMENT
Was granted by the Comité de Protection des Personnes Sud‐Est V (no. 21.02921.000036 cat 1).
ACKNOWLEDGMENTS
This project was part of a research program by the Health and Exercise Response in Children with Chronic and auto‐immUne pathoLogiEs (HERCCULE). The investigators were Alexandre Armand; Corinne Borderon; Aurélie Chausset; Eric Doré; Christian Dualé; Pascale Duché; Stéphane Echaubard; Solenne Fel; Pauline Girard‐Monin; Victoria Grèze; Florentina Isfan; Justyna Kanold; Emmanuelle Labraise; Etienne Merlin; Stéphane Nottin; Justine Paysal; Charline Pegon; Bruno Pereira; Ruddy Richard; Emmanuelle Rochette; Nadège Rouel; Oussama Saidi, Catherine Sarret and Daniel Terral.
Jaber M, Armand A, Rochette E, et al. Anthracycline‐induced cardiotoxicity on regional myocardial work and left ventricular mechanical dispersion in adolescents and young adults in post‐lymphoma remission. Cancer Med. 2024;13:e6857. doi: 10.1002/cam4.6857
Justine Paysal and Stéphane Nottin were two authors contributed equally.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Steliarova‐Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: a population‐based registry study. Lancet Oncol. 2017. Jun;18(6):719‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erdmann F, Frederiksen LE, Bonaventure A, et al. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021. Apr;1(71):101733. [DOI] [PubMed] [Google Scholar]
- 3. Franco VI, Lipshultz SE. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young. 2015;25(S2):107‐116. [DOI] [PubMed] [Google Scholar]
- 4. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood cancer survivor study cohort. BMJ. 2009;8(339):b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinherz LJ, Steinherz PG, Tan C. Cardiac failure and dysrhythmias 6–19 years after anthracycline therapy: a series of 15 patients. Med Pediatr Oncol. 1995;24(6):352‐361. [DOI] [PubMed] [Google Scholar]
- 6. Lima MAC, Brito HRA, Mitidieri GG, et al. Cardiotoxicity in cancer patients treated with chemotherapy: a systematic review. Int J Health Sci (Qassim). 2022;16(6):39‐46. [PMC free article] [PubMed] [Google Scholar]
- 7. Bloom MW, Hamo CE, Cardinale D, et al. Cancer therapy–related cardiac dysfunction and heart failure. Circulation: Heart Failure. 2016;9(1):e002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarantini L, Gulizia MM, Di Lenarda A, et al. ANMCO/AIOM/AICO consensus document on clinical and management pathways of cardio‐oncology: executive summary. Eur Heart J Suppl. 2017;19(Suppl D):D370‐D379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Čelutkienė J, Pudil R, López‐Fernández T, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the heart failure association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the cardio‐oncology Council of the European Society of cardiology (ESC). Eur J Heart Fail. 2020;22(9):1504‐1524. [DOI] [PubMed] [Google Scholar]
- 10. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911‐939. [DOI] [PubMed] [Google Scholar]
- 11. Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy. J Am Coll Cardiol. 2014;63(25):2751‐2768. [DOI] [PubMed] [Google Scholar]
- 12. Biswas M, Sudhakar S, Nanda NC, et al. Two‐ and three‐dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013;30(1):88‐105. [DOI] [PubMed] [Google Scholar]
- 13. Padmanabhan S, Singh S, Nanda CN. Speckle tracking echocardiography: clinical usefulness. Comprehensive Textbook of Echocardiography. 2014th ed. Jaypee Brothers Medical Publishers; 2014:360‐379. [Google Scholar]
- 14. Yu AF, Raikhelkar J, Zabor EC, et al. Two‐dimensional speckle tracking echocardiography detects subclinical left ventricular systolic dysfunction among adult survivors of childhood, adolescent, and young adult cancer. BioMed Res Int. 2016;2016:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merkx R, Leerink JM, de Baat EC, et al. Asymptomatic systolic dysfunction on contemporary echocardiography in anthracycline‐treated long‐term childhood cancer survivors: a systematic review. J Cancer Surviv. 2022;16(2):338‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amedro P, Vincenti M, Abassi H, et al. Use of speckle tracking echocardiography to detect late anthracycline‐induced cardiotoxicity in childhood cancer: a prospective controlled cross‐sectional study. Int J Cardiol. 2022;354:75‐83. [DOI] [PubMed] [Google Scholar]
- 17. Cheung Y, Li SN, Chan GC, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors: LV twist in childhood cancer survivors. Echocardiography. 2011. Aug;28(7):738‐745. [DOI] [PubMed] [Google Scholar]
- 18. Slieker MG, Fackoury C, Slorach C, et al. Echocardiographic assessment of cardiac function in pediatric survivors of anthracycline‐treated childhood cancer. Circ. Cardiovasc Imag. 2019;12(12):e008869. [DOI] [PubMed] [Google Scholar]
- 19. Paysal J, Merlin E, Terral D, et al. Left ventricular strains and myocardial work in adolescents with anorexia nervosa. Front Cardiovasc Med. 2022;8(9):798774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):260‐274. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Zhang P, Li M, Zhang M. Myocardial work: the analytical methodology and clinical utilities. Hellenic J Cardiol. 2022;68:46‐59. [DOI] [PubMed] [Google Scholar]
- 22. Ilardi F, D'Andrea A, D'Ascenzi F, et al. Myocardial work by echocardiography: principles and applications in clinical practice. JCM. 2021;10(19):4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Birat A, Ratel S, Dodu A, et al. A long‐duration race induces a decrease of left ventricular strains, twisting mechanics and myocardial work in trained adolescents. Euro J Sport Sci. 2023;23(7):1394‐1404. [DOI] [PubMed] [Google Scholar]
- 24. Russell K, Eriksen M, Aaberge L, et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: a non‐invasive index of myocardial work. European Heart J. 2012;33(6):724‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russell K, Eriksen M, Aaberge L, et al. Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J PhyHeart and Circ Physiol. 2013;305(7):H996‐H1003. [DOI] [PubMed] [Google Scholar]
- 26. Edwards NFA, Scalia GM, Shiino K, et al. Global myocardial work is superior to global longitudinal strain to predict significant coronary artery disease in patients with Normal left ventricular function and wall motion. J Am Society Echocardiography. 2019;32(8):947‐957. [DOI] [PubMed] [Google Scholar]
- 27. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardio‐protection? Progress in Cardiovascular Diseases. 2010;53(2):105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1‐mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harbo MB, Stokke MK, Sjaastad I, Espe EKS. One step closer to myocardial physiology: from PV loop analysis to state‐of‐the‐art myocardial imaging. Acta Physiologica. 2022;234(2):e13759. [DOI] [PubMed] [Google Scholar]
- 30. Armand A, Rochette E, Grèze V, et al. Fitness and metabolic response to exercise in young adult survivors of childhood lymphoma. Support Care Cancer. 2023;31(6):358. [DOI] [PubMed] [Google Scholar]
- 31. Grandperrin A, Schuster I, Moronval P, et al. Anabolic steroids use is associated with impairments in atrial and ventricular cardiac structure and performance in athletes. Med Sci Sports & Exer. 2022;54(5):780‐788. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Society Echocardiography. 2015;28(1):1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Khoury DS, Thohan V, Torre‐Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007;115(11):1376‐1383. [DOI] [PubMed] [Google Scholar]
- 34. Notomi Y, Lysyansky P, Setser RM, et al. Measurement of ventricular torsion by two‐dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45(12):2034‐2041. [DOI] [PubMed] [Google Scholar]
- 35. Çetin S, Babaoğlu K, Başar EZ, Deveci M, Çorapçıoğlu F. Subclinical anthracycline‐induced cardiotoxicity in long‐term follow‐up of asymptomatic childhood cancer survivors: assessment by speckle tracking echocardiography. Echocardiography. 2018;35(2):234‐240. [DOI] [PubMed] [Google Scholar]
- 36. Sun JP, Xu TY, Ni XD, et al. Echocardiographic strain in hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy. Echocardiography. 2019;36(2):257‐265. [DOI] [PubMed] [Google Scholar]
- 37. Ylänen K, Poutanen T, Savikurki‐Heikkilä P, Rinta‐Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among Long‐term survivors of childhood cancer. J Am College Cardiol. 2013;61(14):1539‐1547. [DOI] [PubMed] [Google Scholar]
- 38. Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1(3):366‐376. [DOI] [PubMed] [Google Scholar]
- 39. Yu W, Li SN, Chan GC, Ha SY, Wong SJ, Cheung YF. Transmural strain and rotation gradient in survivors of childhood cancers. Euro Heart J Cardiovasc Imag. 2013;14(2):175‐182. [DOI] [PubMed] [Google Scholar]
- 40. Abawi D, Rinaldi T, Faragli A, et al. The non‐invasive assessment of myocardial work by pressure‐strain analysis: clinical applications. Heart Fail Rev. 2022;27(4):1261‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao L, Wang Y, Gao M, Chen L. Clinical research progress of myocardial work in assessment and prediction of coronary artery disease in noninvasive pressure‐strain loop technique. J of Clinical Ultrasound. 2023;51(1):38‐45. [DOI] [PubMed] [Google Scholar]
- 42. Zhan J, Van den Eynde J, Cordrey K, et al. Deterioration in myocardial work indices precedes changes in global longitudinal strain following anthracycline chemotherapy. Int J Cardiol. 2022;15(363):171‐178. [DOI] [PubMed] [Google Scholar]
- 43. Mihalcea D, Memis H, Balinisteanu A, Vladareanu A, Mihaila S, Vinereanu D. Myocardial work—a new tool for early detection of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy induced‐cardiotoxicity in hematological patients. J of Clinical Ultrasound. 2022;51:377‐384. [DOI] [PubMed] [Google Scholar]
- 44. Zhan J, Van den Eynde J, Ozdemir E, et al. Left ventricular myocardial work indices in pediatric hypertension: correlations with conventional echocardiographic assessment and subphenotyping. Eur J Pediatr. 2022;181(7):2643‐2654. [DOI] [PubMed] [Google Scholar]
- 45. Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733‐740. [DOI] [PubMed] [Google Scholar]
- 46. Okuma H, Noto N, Tanikawa S, et al. Impact of persistent left ventricular regional wall motion abnormalities in childhood cancer survivors after anthracycline therapy: assessment of global left ventricular myocardial performance by 3D speckle‐tracking echocardiography. Journal of Cardiology. 2017;70(4):396‐401. [DOI] [PubMed] [Google Scholar]
- 47. Yu HK, Yu W, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three‐dimensional speckle‐tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr. 2013;26(8):846‐852. [DOI] [PubMed] [Google Scholar]
- 48. Chakouri N, Farah C, Matecki S, et al. Screening for in‐vivo regional contractile defaults to predict the delayed doxorubicin cardiotoxicity in juvenile rat. Theranostics. 2020;10(18):8130‐8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buckberg G, Nanda CN, Hoffman JI, Coghlan C. Newer aspects of structure/function to assess cardiac motion. Comprehensive Textbook of Echocardiography. 2014th ed. Jaypee Brothers Medical Publishers; 2014:1176‐1209. [Google Scholar]
- 50. Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late‐onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010;19(5):308‐311. [DOI] [PubMed] [Google Scholar]
- 51. Leerink JM, de Baat EC, Feijen EAM, et al. Cardiac disease in childhood cancer survivors. JACC: CardioOncology. 2020;2(3):363‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheung Y, Hong WJ, Chan GC, Wong SJ, Ha SY. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96(14):1137‐1141. [DOI] [PubMed] [Google Scholar]
- 53. Klitsie LM, Roest AAW, Van der Hulst AE, Stijnen T, Blom NA, Adj H. Assessment of intraventricular time differences in healthy children using two‐dimensional speckle‐tracking echocardiography. J Am Soc Echocardiogr. 2013;26(6):629‐639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


