Abstract
The transcription factors E2A (E12/E47) and Pip are both required for normal B-cell development. Each protein binds to regulatory sequences within various immunoglobulin enhancer elements. Activity of E2A proteins can be regulated by interactions with other proteins which influence their DNA binding or activation potential. Similarly, Pip function can be influenced by interaction with the protein PU.1, which can recruit Pip to bind to DNA. We show here that a previously unidentified Pip binding site resides adjacent to the E2A binding site within the immunoglobulin κ 3′ enhancer. Both of these binding sites are crucial for high-level enhancer activity. We found that E47 and Pip can functionally interact to generate a very potent 100-fold transcriptional synergy. Through a series of mutagenesis experiments, we identified the Pip sequences necessary for transcriptional activation and for synergy with E47. Two synergy domains (residues 140 to 207 and 300 to 420) in addition to the Pip DNA binding domain (residues 1 to 134) are required for maximal synergy with E47. We also identified a Pip domain (residues 207 to 300) that appears to mask Pip transactivation potential. Part of the synergy mechanism between E47 and Pip appears to involve the ability of Pip to increase DNA binding by E47, perhaps by inducing a conformational change in the E47 protein. E47 may also induce a conformational change in Pip which unmasks sequences important for transcriptional activity. Based upon our results, we propose a model for E47-Pip transcriptional synergy.
B-cell development requires the activities of a variety of transcription factors, including E2A, PU.1, Ikaros, Pip, and BSAP (reviewed in references 10 and 35). The E2A gene encodes two highly related gene products, E12 and E47, generated by differential RNA processing. E2A products are members of the basic helix-loop-helix (bHLH) class of transcription factors and can form either homo- or heterodimers through the HLH domain (25, 27, 31, 38–40). This dimerization is responsible for the proper positioning of basic region sequences necessary for DNA binding. Another HLH protein, Id, which lacks the basic region, can dimerize with E2A proteins, but such heterodimers are incapable of binding to DNA (5, 9, 51, 60). Although E2A proteins are ubiquitously expressed, they are capable of heterodimerizing with tissue-specific bHLH factors and thereby can contribute to cellular differentiation (reviewed in references 43 and 61). The best-characterized case of this heterodimerization involves E2A and MyoD, which contribute to muscle differentiation. In B cells, E2A primarily binds to DNA as a homodimer and this dimerization process appears to be controlled by phosphorylation and/or redox potential (2, 4, 31, 57, 58). In addition to their ability to dimerize with bHLH factors, E2A proteins can also synergize with certain Ets domain transcription factors (Erg-3, Ets-1, and Fli-1) and with the LIM domain proteins Lmx1.1 and Lmx1.2 to stimulate transcription (28, 41, 52). In addition, E2A function can be augmented by interaction with the coactivator p300 (11).
Although E2A is ubiquitously expressed, deletion of the E2A gene by homologous recombination results in a severe defect in the B-cell lineage but, surprisingly, has little effect on other tissues (3, 65, 66). E2A-deficient animals fail to develop mature B cells. In these animals, B cells do not develop past the pro-B-cell stage. A similar defect in B-cell development is observed in transgenic mice overexpressing the Id protein (59), which inhibits E2A DNA binding. Therefore, E2A function is crucial for normal B-cell development.
Another transcription factor required for proper B-cell development, Pip (variously named LSIRF, IRF4, or ICSAT), is a member of the interferon response family of transcription factors (12, 20, 33, 63). Other IRF family members include IRF-1, IRF-2, ICSBP, ISGF3γ, and IRF-7 (14, 23, 30, 36, 42, 64). Pip was initially identified as a protein that binds to a sequence within the immunoglobulin κ [Ig(κ)] 3′ enhancer only in the presence of a second protein, PU.1 (48, 49). In other contexts, such as within interferon-responsive elements, Pip can bind to DNA in the absence of other proteins (33, 63). Unlike E2A, Pip is expressed almost exclusively in the lymphoid lineage (7, 12, 20, 33). Deletion of the Pip gene by homologous recombination causes a defect in late B-cell and T-cell functions (35). Pip knockout animals form surface immunoglobulin-positive B cells, but these cells do not mount antibody responses. In addition, Pip-deficient T cells cannot generate cytotoxic or antitumor responses. Therefore, Pip appears to be needed for activation of genes necessary for late-stage B-cell and T-cell functions.
The critical requirements for E2A and Pip in normal B-cell development indicate their importance for controlling genes necessary for this lineage. Early in B-cell development, Pip expression is very low (12, 33), whereas E2A products are expressed but are largely sequestered as inactive E2A-Id heterodimers (60, 62). At later stages of development (B-cell and plasma cell stages), Id expression ceases and Pip expression increases. This suggests that the functional activities of these proteins are likely to increase at later stages of B-cell development, leading to increased expression of genes regulated by E2A and Pip. The Ig(κ) light-chain gene contains two enhancers (the intron and 3′ enhancers), both of which contain E2A binding sites. These binding sites are necessary for high levels of enhancer activity (16, 17, 32, 34, 45, 46). In addition the Ig(κ) and Ig(λ) 3′ enhancers each contain Pip binding sites important for enhancer activity (13, 48). The Pip sites in the Ig(κ) and Ig(λ) 3′ enhancers lie adjacent to the binding site for the transcription factor PU.1, which serves to recruit Pip to bind DNA. As one might expect from the increased E2A and Pip activities late in B-cell development, the Ig(κ) intron and 3′ enhancers are more active in late-stage B cells (1, 46).
Given the expression pattern of E2A and Pip in B-cell development and their roles in controlling enhancer activity, it would be very interesting to determine whether these proteins influence the activities of one another. Currently, the only factors known to interact with E2A are other HLH family members, LIM domain proteins, and p300. Similarly, Pip is only known to interact with PU.1. Here, we identify a previously undetected Pip site directly adjacent to the E2A site in the Ig(κ) 3′ enhancer. Both sites are necessary for high-level enhancer activity. We show that E2A and Pip can functionally interact to produce a potent transcriptional synergy. This synergy requires the E2A and Pip transcriptional activation domains and involves a mechanism by which the Pip DNA binding domain greatly increases DNA binding by E2A.
MATERIALS AND METHODS
Plasmid constructions.
To prepare Pip carboxy-terminal deletion proteins 1-207, 1-182, 1-175, and 1-134, plasmid PIP/ATG (a gift from H. Singh, University of Chicago) was used as a template for PCR with various Pip reverse primers with terminal BamHI sites (see Table 1) and the T3 primer. For Pip carboxy-terminal deletion proteins 1-420, 1-300, and 1-240, PCR was performed with Pip reverse primers with terminal BamHI sites and the FEcoPipATG primer (Table 1). After PCR, the products were extracted with phenol-chloroform, precipitated with ethanol, digested with BamHI and HindIII (which cuts in the 5′ flanking sequence; Pip 1-207, 1-182, 1-175, and 1-134), or with BamHI and EcoRI (Pip 1-420, 1-300, 1-240), and purified by agarose gel electrophoresis. Digested products were cloned into plasmid pBluescript KS+ cut with either BamHI and HindIII or with BamHI and EcoRI or into cytomegalovirus (CMV) expression plasmid pCB6+ (44). Plasmids CMVPipΔ140-160, CMVPipΔ140-180, and CMVPipΔ140-207 were prepared by overlap extension PCR (26) by using PipKS+ and the primers listed in Table 1. Forward and reverse primers were used with the T7 and T3 primers, respectively. After the first round of PCR, the products were gel purified, mixed, and reamplified with the T7 and T3 primers. Final products were digested with HindIII and XbaI and cloned into the HindIII-XbaI sites of pCB6+. To prepare Pip 1-300 with residues 140 to 207 deleted (Pip 1-300Δ140-207), CMVPipΔ140-207 was used as a template for PCR with primers RPip1-300 and hcmv. Products were digested with EcoRI and BamHI and cloned into the EcoRI-BamHI sites of pCB6+. To produce CMVGAL:Pip1-450, plasmid PIP/ATG was linearized with EcoRI and then subjected to partial digestion with NcoI. The DNA product containing the full-length sequence was gel purified and ligated into the blunted EcoRI site of plasmid CMVGAL (8). To produce CMVGAL:Pip1-207, CMVGAL:Pip1-182, CMVGAL:Pip1-175, and CMVGAL:Pip1-134, the appropriate clones described above for pBluescript KS+ were linearized with BamHI and then subjected to either partial or complete (CMVGAL:Pip1-134) digestion with NcoI. DNA fragments were purified and ligated into the blunted EcoRI site of CMVGAL. To prepare CMVGAL:Pip1-420 and CMVGAL:Pip1-300, the CMV-based plasmids prepared as described above were cut with EcoRI and BamHI. The appropriate DNA fragments were isolated and cloned into EcoRI- and BamHI-cut CMVGAL plasmid. To prepare glutathione S-transferase (GST) fusion constructs, KS+ clones of Pip 1-207, 1-182, and 1-134 were cut with HindIII and BamHI, and the appropriate DNA fragments were gel purified, blunted with Klenow polymerase, and then ligated into the blunted XmaI site of GST plasmid GEX2TK. For full-length GST-Pip, PIP/ATG was cut with HindIII and EcoRI, gel purified, blunted with Klenow polymerase, and then ligated into the blunted XmaI site of vector GEX2TK. Pip amino-terminal deletions were prepared by PCR with various Pip forward primers (Table 1) containing EcoRI sites and the T7 primer by using KS+Pip1-207 as the template plasmid. Amplified products were extracted with phenol-chloroform, precipitated with ethanol, digested with EcoRI and XbaI, and purified by polyacrylamide gel electrophoresis. Purified products were ligated into the CMVGAL vector cut with EcoRI and XbaI. A two-step PCR method was used to prepare Pip 1-134–VP16. Pip residues 1 to 134 were amplified by PCR by using the CMVGAL:Pip 1-182 template plasmid and the GAL4 and RVP16Pip primers (Table 1). The VP16 sequences were amplified from a VP16 expression plasmid (a gift from T. Kadesch, University of Pennsylvania) with the FPipVP16 and RXbaVP16 primers (Table 1). Each amplified product was then mixed and subjected to a second PCR with the GAL4 and RXbaVP16 primers. The final product was extracted with phenol-chloroform, precipitated with ethanol, cut with EcoRI and XbaI, gel purified, and then ligated into CMVGAL cut with EcoRI and XbaI. All E47 expression plasmids were gifts from T. Kadesch. The 3′ enhancer core region in reporter plasmid LBKCAT was previously described (47). Core mutants m7.1 to m7.6 were produced by overlap extension PCR (26) with the primers listed in Table 2 and the appropriate pUC forward and reverse primers. Amplified products were cut with HindIII, blunted with Klenow polymerase, cut with BamHI, and then cloned into reporter LBKCAT at the BamHI and blunted BglII sites. Oligonucleotide m7.1 to m7.6 LBKCAT plasmids were prepared by synthesizing the appropriate oligonucleotide sequences (Table 2) with BamHI and BglII termini which were ligated into BamHI-BglII-cut LBKCAT. Multimers (four copies) were generated as previously described (46).
TABLE 1.
Oligonucleotides for Pip plasmid constructions
| Oligonucleotide | Sequence |
|---|---|
| RPip1-420 | GCGGGATCCGTTTTGTTGAGCAAAGTAATA |
| RPip1-300 | GCGGGATCCCTGTCCATTGTCGTCCGGGTA |
| RPip1-240 | GCGGGATCCCCTTATGCTTGGCTCAATGGG |
| RPip1-207 | GCGGGATCCTCAGCCTTGCCAGTGGTGGCC |
| RPip1-182 | GCGGGATCCTCAGGCATAATCCCTCCAGCT |
| RPip1-175 | GCGGGATCCTCAATAAGGTGCTGTCATGGG |
| RPip1-134 | GCGGGATCCTCATTTTTTGGCTCCCTCTGG |
| FPipΔ1-100 | GCGGAATTCCTGAACAAGAGCAATGAC |
| FPipΔ1-140 | GCGGAATTCTTGGATGACACACAGATG |
| FPipΔ1-160 | GCGGAATTCCTGCCAGCCCAGCAGGTT |
| FPipΔ1-180 | GCGGAATTCTATGCCCCTGACCAGTCA |
| FEcoPipATG | GCGGAATTCATGGCCATCGATATCGATCCC |
| FPipΔ140-160 | GGAGCAAAGCAGCTCCTGCCAGCCCAGCAGGTTCAT |
| RPipΔ140-160 | CTGCTGGGCTGGCAGGAGCTGCTTTGCTCCTTTTTT |
| FPipΔ140-180 | GGAGCAAAGCAGCTCTATGCCCCTGACCAGTCACAC |
| RPipΔ140-180 | TGACTGGTCAGGGGCGAGCTGCTTTGCTCCTTTTTT |
| FPipΔ140-207 | GGAGCAAAGCAGCTCCCATCTTGTGAAAATGGTTGC |
| RPipΔ140-207 | ATTTTCACAAGATGGGAGCTGCTTTGCTCCTTTTTT |
| FPipVP16 | GAGGGAGCCAAAAAAATGGCACCCAAGAAGAAG |
| RXbaVP16 | GCGTCTAGAAATTCGAGCTAGCTTCTA |
| RVP16Pip | CTTCTTGGGTGCCATTTTTTTGGCTCCCTCTG |
| GAL4 | CATCATCATCGGAAGAG |
| hcmv | GCAGAGCTCGTTTAGTG |
TABLE 2.
Oligonucleotides for reporter plasmid construction and for EMSA
| Oligonucleotide | Sequence |
|---|---|
| FCore m7.1 | AGAACCTTAGGCTACGCTGTTGCTTTCG |
| RCore m7.1 | CGAAAGCAACAGCGTAGCCTAAGGTTCT |
| FCore m7.2 | CCTTAGGCACATAGTGTGCTTTCGCTCC |
| RCore m7.2 | GGAGCGAAAGCACACTATGTGCCTAAGG |
| FCore m7.3 | AGGCACATCTGTGCAGTTCGCTCCCATC |
| RCore m7.3 | GATGGGAGCGAACTGCACAGATGTGCCT |
| FCore m7.4 | ACATCTGTTGCTGGATCTCCCATCCTCC |
| RCore m7.4 | GGAGGATGGGAGATCCAGCAACAGATGT |
| FCore m7.5 | CTGTTGCTTTCGAGAACATCCTCCTCCA |
| RCore m7.5 | TGGAGGAGGATGTTCTCGAAAGCAACAG |
| FCore m7.6 | TGCTTTCGCTCCACGACTCCTCCAACAG |
| RCore m7.6 | CTGTTGGAGGAGTCGTGGAGCGAAAGCA |
| Oligo 7 | GATCCACATCTGTTGCTTTCGCTCCCATCCAGTGTAGACAACGAAAGCGAGGGTAGGTCTAG |
| m7.1 | GATCCTACGCTGTTGCTTTCGCTCCCATCCAGATGCGACAACGAAAGCGAGGGTAGGTCTAG |
| m7.2 | GATCCACATAGTGTGCTTTCGCTCCCATCCAGTGTATCACACGAAAGCGAGGGTAGGTCTAG |
| m7.3 | GATCCACATCTGTGCAGTTCGCTCCCATCCAGTGTAGACACGTCAAGCGAGGGTAGGTCTAG |
| m7.4 | GATCCACATCTGTTGCTGGATCTCCCATCCAGTGTAGACAACGACCTAGAGGGTAGGTCTAG |
| m7.5 | GATCCACATCTGTTGCTTTCGAGAACATCCAGTGTAGACAACGAAAGCTCTTGTAGGTCTAG |
| m7.6 | GATCCACATCTGTTGCTTTCGCTCCACGACAGTGTAGACAACGAAAGCGAGGTGCTGTCTAG |
Cell culture and transfections.
S194 plasmacytoma cells were grown and transfected by the DEAE-dextran procedure as previously described (46). NIH 3T3 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and transfected by the calcium phosphate coprecipitation method (19). Calcium phosphate transfection mixtures generally contained 5 μg of reporter plasmid, 3 μg of effector plasmids, and 1 μg of a β-galactosidase expression plasmid to monitor transfection efficiency. The total amount of DNA was maintained constant by inclusion of the empty CMV expression plasmid pCB6+ (44). Cells were harvested 44 h posttransfection, and chloramphenicol acetyltransferase (CAT) assays and thin-layer chromatography were performed as described by Gorman et al. (18). Percent CAT activity was determined by excision of the substrate and acetylated products from the plate followed by liquid scintillation counting. Transfections were performed three to seven times, and representative data are shown. These determinations were necessary because in this study, synergy was defined as the percent acetylation observed with each Pip protein in the presence of E47 divided by the sum of the activities for each protein separately.
EMSA.
Electrophoretic mobility shift assays (EMSA) were performed with about 0.1 ng of labeled DNA probe (15,000 cpm) (Table 2) in a 20-μl reaction mixture containing 2 μg of poly(dI-dC), 10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 5% glycerol, and various proteins (see below). Mininuclear extracts of transfected cells were prepared by the method of Schreiber et al. (53) with minor modifications (8), and 8 μg was used for EMSA. Some extracts were treated with calf intestine alkaline phosphatase for 30 min at 37°C. Wild-type or mutant GST-Pip proteins were prepared as described by Kaelin et al. (29), and EMSA reactions contained 1 μg of each protein. For protease sensitivity studies, binding reaction mixtures were prepared as described above. After the reactions reached equilibrium (30 min), various amounts (1, 5, or 10 ng) of either proteinase K or trypsin were added to the mixtures, and they were incubated for an additional 5 min at room temperature. Digestions were stopped on ice and then subjected to electrophoresis.
RESULTS
Identification of a functional DNA sequence adjacent to the Ig(κ) 3′ enhancer E-box.
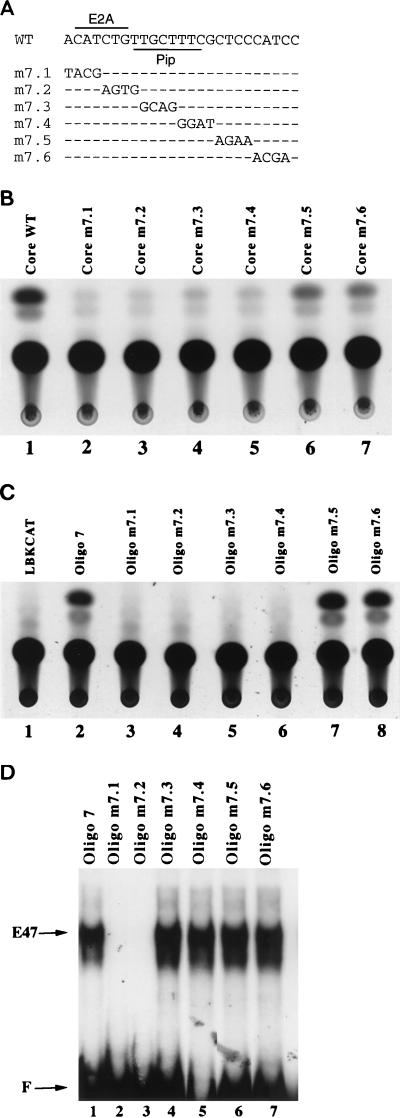
Previously, we used a linker scan approach to identify functional DNA sequences within the central 132-bp core region of the Ig(κ) 3′ enhancer (45). These studies confirmed previously identified functional sequences within the enhancer such as the PU.1 and Pip binding sites and an E-box (46, 48). In addition, a cyclic AMP response element (CRE)-like binding site and a region adjacent to the E-box appeared to be functionally important (45). However, since the linker scan mutants in that study were relatively large (10 bp), the limits of the functionally important sequences were not well defined. To better characterize the sequences adjacent to the enhancer E-box, we prepared six 4-bp mutants (m7.1 to m7.6) within the enhancer core region that span the E-box and the sequences immediately downstream of this sequence (Fig. 1A). These six mutant enhancer sequences or the wild-type enhancer core were inserted adjacent to the liver-bone-kidney alkaline phosphatase promoter driving expression of the CAT gene (LBKCAT). The activity of each mutant was compared to that of the unmutated enhancer core after transfection into S194 plasmacytoma cells.
FIG. 1.
Identification of a functional enhancer sequence adjacent to the E-box motif. (A) The Ig(κ) 3′ enhancer sequence that includes the E-box and adjacent sequences is shown at the top (WT). The sequences of mutants m7.1 to m7.6 are shown below it. Positions of E2A and Pip binding sites are indicated. (B) S194 plasmacytoma cells were transfected with the wild-type enhancer core reporter plasmid or mutant 7.1 to 7.6 in the context of the enhancer core. CAT assays of extracts from the transfected cells show that enhancer activity is greatly reduced in mutants m7.1 to m7.4. (C) S194 plasmacytoma cells were transfected with reporter plasmids containing 25-bp oligonucleotide multimers (four copies) of the wild-type enhancer sequence (oligonucleotide 7) or multimers of mutants m7.1 to m7.6. CAT assays of the transfected cell extracts show that enhancer activity is absent in mutants m7.1 to m7.4. (D) E47-DNA binding was assayed by EMSA with the multimerized wild-type 25-bp oligonucleotide enhancer probe (oligonucleotide 7) or with each mutant oligonucleotide probe (oligonucleotides m7.1 to m7.6). E47 binding to probes oligonucleotides m7.1 and m7.2 was abolished. The probes used are indicated above each lane, and the positions of free probe (F) and E47 bound to DNA are indicated by arrows.
In the context of the enhancer core, mutants m7.1 and m7.2, which destroy the E-box sequence, showed greatly reduced enhancer activity (10-fold reduction) compared to that of the unmutated enhancer (Fig. 1B, lanes 1 to 3). Interestingly, mutation of the 8 bp immediately adjacent to the E-box (mutants m7.3 and m7.4) resulted in the same loss of activity (Fig. 1B, lanes 4 and 5). On the contrary, the next two mutations (m7.5 and m7.6) resulted in much higher enhancer activity (Fig. 1B, lanes 6 and 7). A reporter plasmid containing a multimerized (four-copy) 25-bp oligonucleotide (oligonucleotide 7) which contains the E-box and adjacent functional sequence (Oligo7LBKCAT) can support enhancer activity in S194 cells (46). We therefore tested the six mutations described above for enhancer activity in the context of oligonucleotide 7. Similar to results obtained with the entire enhancer core, mutants m7.1 through m7.4 showed essentially no enhancer activity whereas activity was high with mutants m7.5 and m7.6 (Fig. 1C). These results indicate the existence of a functional 8-bp DNA sequence within the Ig(κ) 3′ enhancer directly adjacent to the enhancer E-box. This 8-bp sequence corresponds to enhancer nucleotides 483 to 490 (34).
E-box sequences are defined by a canonical CANNTG motif. This sequence motif (CATCTG) lies within 3′ enhancer nucleotides 476 to 481 and is mutated in mutants m7.1 and m7.2. Mutants m7.3 and m7.4, which show greatly reduced enhancer activity, do not disrupt the E-box sequence. However, it is possible that these sequences influence the ability of E2A (or another HLH protein) to bind to DNA. To test whether this was the case, multimers (four copies) of the wild-type oligonucleotide 7 and each 4-bp mutant were used as probes in EMSA with recombinant E47 protein. As expected, E47 bound to wild-type oligonucleotide 7 but not to mutants m7.1 and m7.2 (Fig. 1D, lanes 1 to 3). On the contrary, E47 bound efficiently to mutants m7.3 to m7.6 (lanes 4 to 7). Therefore, the low transcriptional activity of mutants m7.3 and m7.4 is not due to the inability of E47 to bind to DNA. Instead, another factor apparently binds to this sequence and cooperates with E47 to stimulate transcription.
E47 and Pip functionally synergize to simulate enhancer activity.
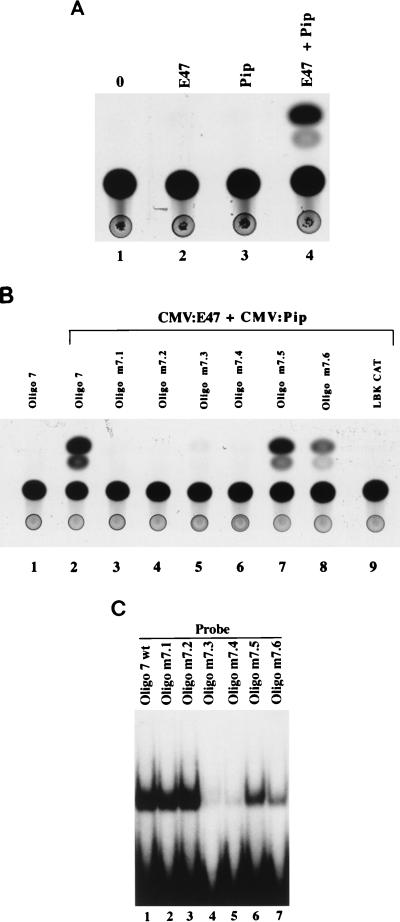
Inspection of the DNA sequence immediately adjacent to the E-box in oligonucleotide 7 revealed a potential binding site for the transcription factor Pip (Fig. 1A). Pip can physically and functionally interact with the transcription factor PU.1 to stimulate 3′ enhancer activity (49). However, Pip has never been shown to interact with, or synergize with, any other transcription factor. To determine whether Pip could functionally synergize with E47 to drive transcription, we transfected the oligonucleotide 7-dependent reporter plasmid (Oligo7LBKCAT) into NIH 3T3 cells in the presence of plasmids expressing either E47, Pip, or both. Neither E47 nor Pip alone stimulated enhancer activity (Fig. 2A, lanes 1 to 3). However, cotransfection of E47 and Pip led to a very dramatic increase in enhancer activity (Fig. 2A, lane 4). In numerous experiments, the synergy between these two factors ranged between 91- and 129-fold.
FIG. 2.
Pip can synergize with E47 to activate transcription. (A) NIH 3T3 cells were transfected with the Oligo7LBKCAT reporter plasmid alone (○) or with CMV:E47 (E47), CMV:Pip (Pip), or both (E47 + Pip). CAT assays of transfected cell extracts indicate that cotransfection of E47 and Pip results in a potent (100-fold) transcriptional synergy. The expression plasmids used in each transfection are indicated above each lane. (B) Transfections were performed with CMV:E47 plus CMV:Pip and either the wild-type reporter plasmid (oligonucleotide 7) or various mutant reporter plasmids (nucleotides m7.1 to m7.6). CAT assays of extracts isolated from transfected cells indicate that mutants m7.1 to m7.4 abolish synergy between E47 and Pip. The expression and reporter plasmids in each transfection are indicated above the lanes. (C) Pip can bind to a site adjacent to the E-box motif. Pip deletion protein GST–Pip 1-182 was used in EMSA with either the wild-type enhancer probe (oligo 7 wt) or with each enhancer mutant (oligonucleotides m7.1 to m7.6). Mutants m7.3 and m7.4 greatly reduce Pip DNA binding.
To be certain that Pip was functioning through the DNA sequences adjacent to the E-box in oligonucleotide 7, mutants m7.1 through m7.6 were tested for their ability to be activated by E47 and Pip. Mutants m7.1 through m7.4 were not activated by cotransfection with E47 and Pip (Fig. 2B, lanes 1 to 6), whereas mutants m7.5 and m7.6 supported enhancer activity (lanes 7 and 8). The parent reporter plasmid lacking the E2A and Pip binding sites (oligonucleotide 7) was not stimulated by E47 and Pip, confirming that these transcription factors were operating through the enhancer sequences (Fig. 2B, lane 9). The above results suggest that Pip can bind to the DNA sequences immediately adjacent to the E-box. Based upon our transfection data, one would predict that Pip can bind to the wild-type oligonucleotide 7 sequence and to mutants m7.1, m7.2, m7.5, and m7.6 but not to mutants m7.3 and m7.4. To determine whether this was the case, we used a Pip deletion protein (GST–Pip 1-182) in EMSA with each oligonucleotide 7 mutant sequence. The Pip deletion protein we used removes a carboxy-terminal domain that was shown by Brass et al. (7) to mask the Pip DNA binding domain. As expected, this Pip protein bound efficiently to wild-type oligonucleotide 7 and mutants m7.1, m7.2, m7.5, and m7.6 but very poorly to mutants m7.3 and m7.4 (Fig. 2C). Therefore, Pip can bind to the DNA sequence directly adjacent to the E-box in the Ig(κ) 3′ enhancer and can functionally synergize with E47 to stimulate transcription. The somewhat reduced Pip binding to mutants m7.5 and m7.6 correlates with slightly reduced transcriptional activity by these mutants in our transfection assays (Fig. 1B and 2B).
Pip sequences necessary for synergy with E2A.
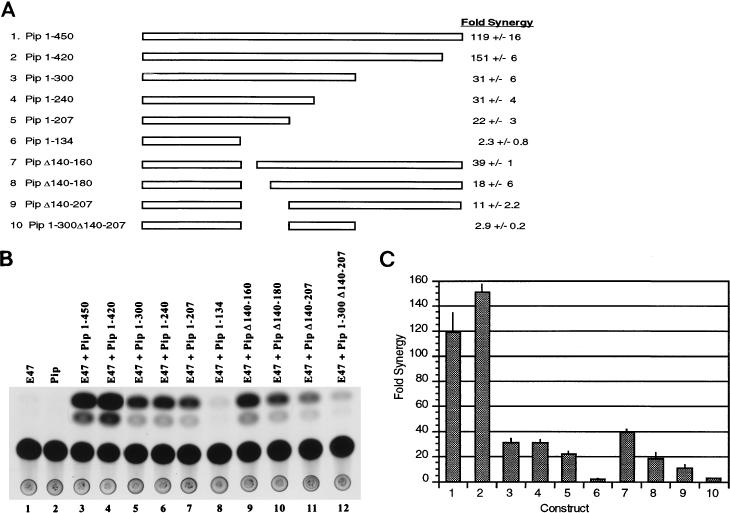
To identify the Pip sequences necessary for synergy with E47, we prepared a series of progressive C-terminal Pip deletions (Fig. 3A, constructs 1 to 6). These mutant constructs were assayed in the presence of E47 by using the E2A-Pip-dependent reporter plasmid Oligo7LBKCAT. In multiple experiments, full-length Pip (Pip 1-450) averaged a 119-fold synergy with E47 (Fig. 3B, lanes 1 to 3). Deletion protein Pip 1-420 showed the same ability to synergize with E47 (lane 4). However, a deletion to amino acid 300 (construct Pip 1-300) caused a reduction to 30-fold synergy (lane 5). Similar levels of synergy were observed for deletions to residues 240 and 207 (lanes 6 and 7). Further deletion to residue 134 nearly completely abolished synergy (lane 8). This construct contains only the Pip DNA binding domain (7). The above results suggest that at least two Pip domains contribute to synergy with E47. The first synergy domain lies between residues 134 and 207. The second synergy domain lies between residues 300 and 420. Deletion of this domain reduces synergy by three- to fourfold. Deletion of both domains abolishes synergy. To determine the strength of the second synergy domain in the absence of the first, we prepared three internal deletion mutants which abolish residues constituting synergy domain 1 (Fig. 3A, constructs 7 to 9). Progressive deletion of synergy domain 1 (Pip constructs Δ140-160, Δ140-180, and Δ140-207) caused progressive drops in synergy levels to 39-, 18-, and 11-fold, respectively (Fig. 3B, lanes 9 to 11). Thus, complete deletion of synergy domain 1 results in a 10-fold drop in synergy levels. However, this deletion mutant still exhibits an 11-fold synergy with E47, indicating that synergy domain 2 is capable of synergizing on its own with E47. A construct with both synergy domains deleted (Pip 1-300Δ140-207) (Fig. 3A, construct 10) reduced synergy to a very low level comparable to that of the Pip DNA binding domain alone (Fig. 3B, lane 12). In summary, synergy domain 1 supported 20- to 30-fold synergy with E47, synergy domain 2 supported 11-fold synergy, and both domains together supported over 100-fold synergy (Fig. 3C). Western blots with Pip antisera of mininuclear extracts isolated from transfected cells indicated comparable expression levels for each deletion protein (data not shown).
FIG. 3.
Identification of Pip sequences involved in synergy with E47. (A) Various Pip deletion mutant proteins are represented as rectangles. The Pip residues present in each construct are shown on the left. The fold synergy in NIH 3T3 cells for each protein in the presence of E47 is shown at the right. Error numbers represent standard deviations from three to five transfections. (B) Representative CAT assay. NIH 3T3 cells were cotransfected with plasmids expressing either wild-type Pip or various Pip mutants and wild-type E47. The plasmids used in each transfection are shown above the lanes. (C) Fold synergy for each construct (numbered as shown in panel A) is plotted. Synergy was defined as the percent acetylation observed with each Pip protein in the presence of E47 divided by the sum of the activities for each protein separately. Error bars represent standard deviations.
Definition of the Pip transactivation domain.
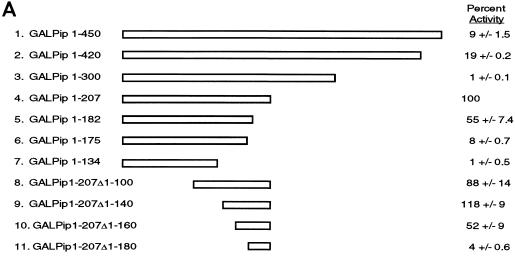
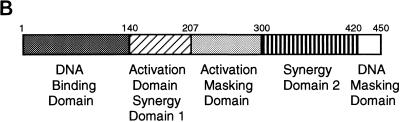
Little is known about the functional sequences within the Pip protein. Brass et al. (7) showed that the Pip DNA binding domain resides within the amino-terminal 134 amino acids and that residues 410 to 439 can inhibit DNA binding. The transcriptional activation domain was shown to reside within the carboxy-terminal two-thirds of the protein (7). To better characterize the Pip sequences necessary for transactivation, we prepared a variety of Pip mutants linked to the GAL4 DNA binding domain (GAL4 residues 1 to 147) (Fig. 4A). The activity of each mutant was then tested with a GAL4-responsive reporter plasmid (GALTKCAT). Full-length Pip linked to GAL4 showed weak transcriptional activation (about fivefold; 9% maximal activity) in this system (Fig. 4A, construct 1). The protein construct with deletion to residue 420 (construct 2) showed somewhat higher activation (ninefold; 19% maximal), but all activity was lost upon further deletion to residue 300 (construct 3). Interestingly, further deletion to amino acid 207 (construct 4) resulted in a dramatic increase in transactivation capacity (about 50-fold, defined as 100%). Deletion to amino acid 182 resulted in a somewhat modest drop in activity (to 55%; construct 5). However deletion of an additional 7 amino acids to position 175 resulted in a large drop in transactivation potential (8% of maximal; construct 6). Deletion to residue 134 abolished all transactivation (construct 7). The above results suggest that at least one and perhaps two transactivation domains reside within the Pip protein. The first transactivation domain lies between residues 140 and 207 and corresponds to synergy domain 1. Interestingly, the Pip sequence between residues 207 and 300 apparently can mask the activity of this transactivation domain. The second potential activation domain resides between amino acids 300 and 420. This corresponds to synergy domain 2. To date, all GAL-Pip fusion constructs that we have prepared that contain these sequences show low transactivation potential. A GAL-Pip fusion protein that contains Pip residues 300 to 420 showed very little activity, but this fusion protein was expressed at a very low level, making our conclusions equivocal (data not shown). Thus, it is uncertain whether this domain can function like a classical activation domain.
FIG. 4.
Identification of Pip activation domain sequences. (A) Various Pip sequences, represented as rectangles, were linked to the GAL4 DNA binding domain (residues 1 to 147). Pip residues present in each construct are listed on the left. Constructs were transfected into NIH 3T3 cells with the GALTKCAT reporter, and CAT activity was determined. The percent acetylation of the GAL–Pip 1-207 construct was defined as 100%, and all other levels are expressed relative to this value. Percent activity ± standard deviation is shown on the right. (B) Summary of the Pip functional domains. Pip residue positions are indicated. The DNA binding domain and the DNA masking domain were localized by Brass et al. (7).
To better define the amino-terminal boundary of the transactivation domain corresponding to synergy domain 1, we prepared progressive N-terminal deletions in the context of Pip residues 1 to 207. Deletion of Pip residues 1 to 100 or 1 to 140 had no effect on transactivation potential (Fig. 4A, constructs 8 and 9). Deletion of residues 1 to 160 lowered activation about twofold (construct 10), and deletion of sequences 1 to 180 abolished all transactivation (construct 11). Therefore, the amino-terminal boundary of the Pip transactivation domain lies between residues 140 and 160. Mininuclear extracts were prepared from transfected cells and assayed by EMSA with a GAL4 DNA binding site probe or by Western blotting with Pip antisera to assess the expression level of each protein. These studies showed equivalent levels of expression for all Pip mutant proteins (data not shown). In total, the above results indicate that the majority of the Pip transcriptional activation domain lies between residues 140 and 182 and that maximal activation requires the sequence from residues 140 to 207. A summary of the known Pip functional domains is shown in Fig. 4B.
The Pip and E47 activation domains can be replaced with a heterologous activation domain.
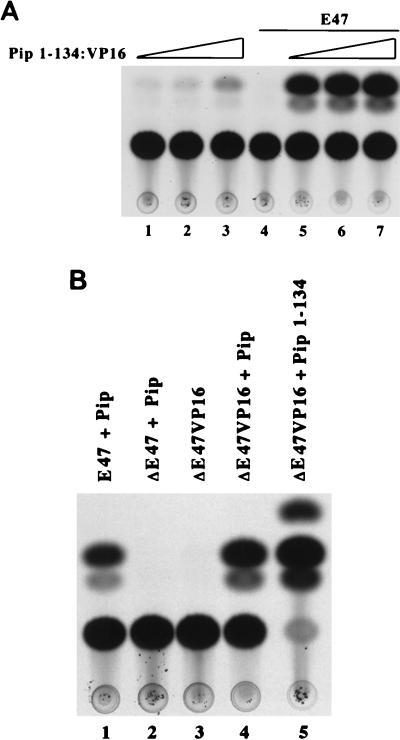
To determine whether the Pip activation domain is specifically required for synergy with E47, we fused the Pip minimal DNA binding domain (residues 1 to 134) with the potent transactivation domain from the herpesvirus VP16 protein. This protein was assayed for synergy by using the Oligo7LBKCAT reporter in the absence or presence of E47. High doses of Pip-VP16 activated the reporter plasmid in the absence of E47 (data not shown). Therefore, we titrated the amount of Pip-VP16 while holding the level of E47 constant. These studies showed that at low doses of Pip-VP16 (50 to 250 ng), very little activation was observed. However, addition of E47 resulted in a 40-fold level of synergy (Fig. 5A). Therefore, the Pip synergy domains can be replaced with a heterologous activation domain.
FIG. 5.
The Pip and E47 activation sequences can be replaced with a heterologous activation domain. (A) NIH 3T3 cells were transfected with GAL–Pip 1-134–VP16 either alone (lanes 1 to 3) or in the presence of 3 μg of E47 expression plasmid (lanes 5 to 7). Pip expression plasmid was included in quantities of 50 ng (lanes 1 and 5), 100 ng (lanes 2 and 6), or 250 ng (lanes 3 and 7). E47 alone (3 μg) is shown in lane 4. (B) NIH 3T3 cells were transfected with the various plasmids (3 μg) as indicated above the lanes. A representative CAT assay is shown.
We performed similar experiments to determine whether the E47 transactivation domain was specifically required for E47-Pip synergy. Deletion of the E47 activation domain (ΔE47) abolished synergy with Pip (Fig. 5B, lanes 1 and 2). A ΔE47-VP16 chimeric protein alone did not activate transcription (lane 3) but yielded a potent synergy in the presence of Pip (lane 4). Therefore, the E47 activation domain can also be replaced with a heterologous activation domain to yield a protein capable of synergy with Pip.
ICSBP can also synergize with E47.
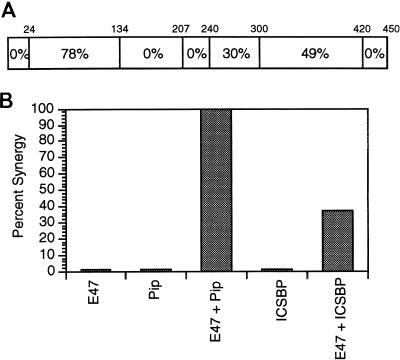
The IRF family member most closely related to Pip is ICSBP. This protein can repress transcription of a number of promoters and can abolish interferon induction. Pip and ICSBP show a modular arrangement of homology (Fig. 6A). The highest homology between these proteins (78%) lies within the DNA binding domain. Other regions of homology include synergy domain 2 (Pip residues 300 to 420; 49%) and the activation masking domain (residues 240 to 300; 30%). Interestingly, no homology exists within the Pip transcriptional activation domain which constitutes synergy domain 1. In light of these homologies, one might predict that ICSBP can synergize with E47 but less effectively than Pip due to the absence of synergy domain 1. Indeed, we found that ICSBP can synergize with E47 at a level that is 37% of that achieved by Pip (Fig. 6B). This is the first case of ICSBP being shown to function as a transcriptional activator rather than as a repressor.
FIG. 6.
ICSBP can synergize with E47. (A) Pip and ICSBP show a modular arrangement of identities. The rectangle represents the Pip protein sequence. The percent identity between Pip and ICSBP in the regions bounded by the Pip residues shown above the rectangle are indicated. (B) NIH 3T3 cells were transfected with E47, Pip, or ICSBP alone or with combinations of E47 plus Pip or E47 plus ICSBP. The level of synergy in the cotransfections was determined as described in the legend to Fig. 3. The level of synergy between E47 and Pip was defined as 100%, and the synergy between E47 and ICSBP is expressed relative to this value. The range in percent synergy for E47 plus ICSBP in two separate experiments was 1%.
Pip enhances DNA binding by E2A.
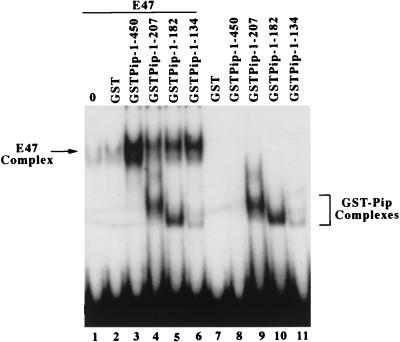
In light of the potent synergy between E47 and Pip, we performed EMSA experiments to explore their DNA binding properties. Bacterially produced E47 and GST-Pip were assayed for DNA binding ability either alone or when mixed together. E47 weakly bound to the oligonucleotide 7 sequence, while full-length GST–Pip 1-450 DNA binding was undetectable (Fig. 7, lanes 1 and 8). Addition of the GST protein alone did not influence E47 DNA binding (lane 2). However, addition of GST–Pip 1-450 resulted in a 10- to 15-fold enhancement in the ability of E47 to bind to DNA (lane 3). Interestingly, GST-Pip mutants 1-207, 1-182, and 1-134 were each capable of enhancing E47 DNA binding (lanes 4 to 6). As expected from the results of Brass et al. (7), these deletion proteins were capable of binding to DNA on their own (lanes 9 to 11). Curiously, an extra complex indicative of E47 and Pip simultaneously bound to DNA was not observed. Experiments with a variety of Pip deletions (1-207, 1-182, and 1-134) (Fig. 7) or Pip antibodies (data not shown) have failed to detect the presence of Pip in the enhanced protein-DNA complex. Possible explanations for this phenomenon are discussed below. In any case, the above results indicate that Pip can greatly enhance the ability of E47 to bind to DNA. The Pip sequences responsible for this enhancement lie within the amino-terminal 134 amino acids that comprise the Pip DNA binding domain.
FIG. 7.
Pip residues 1 to 134 can enhance E47 binding to DNA. EMSA was performed with the wild-type oligonucleotide 7 probe and E47 protein either alone (lane 1) or in the presence of various GST-Pip fusion proteins (lanes 3 to 6) or with GST protein alone (lane 2). Results for assays without E47 are also shown (lanes 7 to 11). The positions of the GST-Pip DNA and the E47-DNA complexes are indicated.
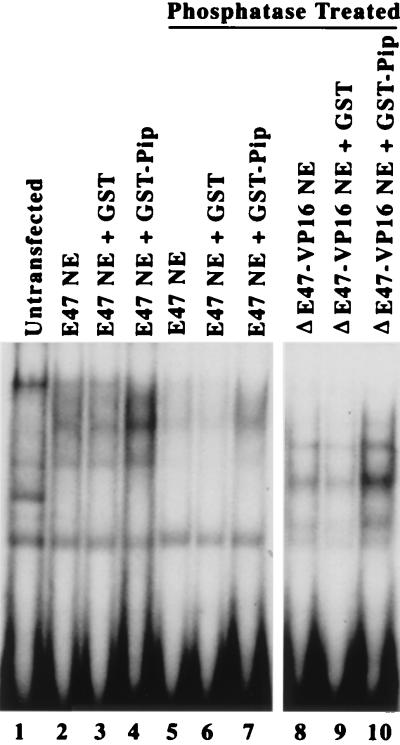
If Pip residues 1 to 134 contribute to Pip-E47 synergy by enhancing E47 DNA binding, this should be detectable in vivo by measuring the activity of the E47 DNA binding domain fused to the potent VP16 activation domain (ΔE47-VP16). Indeed, cotransfection of either wild-type Pip or the Pip DNA binding domain alone (residues 1 to 134) resulted in enhanced transcriptional activation by the ΔE47-VP16 chimera (Fig. 5B, lane 5). Nuclear extracts isolated from E47-plus-Pip-cotransfected cells did not show an increase in E47 binding compared to cells transfected with E47 alone. However, addition of exogenous GST-Pip protein led to an increase in E47 binding (Fig. 8, lanes 1 to 4). Since E47 dimerization can be controlled by phosphorylation (58), we treated extracts with phosphatase and tested their ability to respond to exogenous GST-Pip. Phosphatase treatment reduced E47 DNA binding, but the binding was enhanced upon addition of GST-Pip (Fig. 8, lanes 5 to 7). Similar results were obtained with ΔE47-VP16-transfected nuclear extracts (lanes 8 to 10).
FIG. 8.
GST-Pip can increase DNA binding by transfected E47. NIH 3T3 cells were transfected with plasmids expressing either E47 (lanes 2 to 7) or ΔE47-VP16 (lanes 8 to 10). Mininuclear extracts (NE) were prepared and subjected to EMSA with the wild-type oligonucleotide 7 probe. The proteins included in each sample are shown above the lanes. Samples in lanes 5 to 10 were treated with alkaline phosphatase prior to EMSA.
Mechanism of E47 recruitment by Pip.
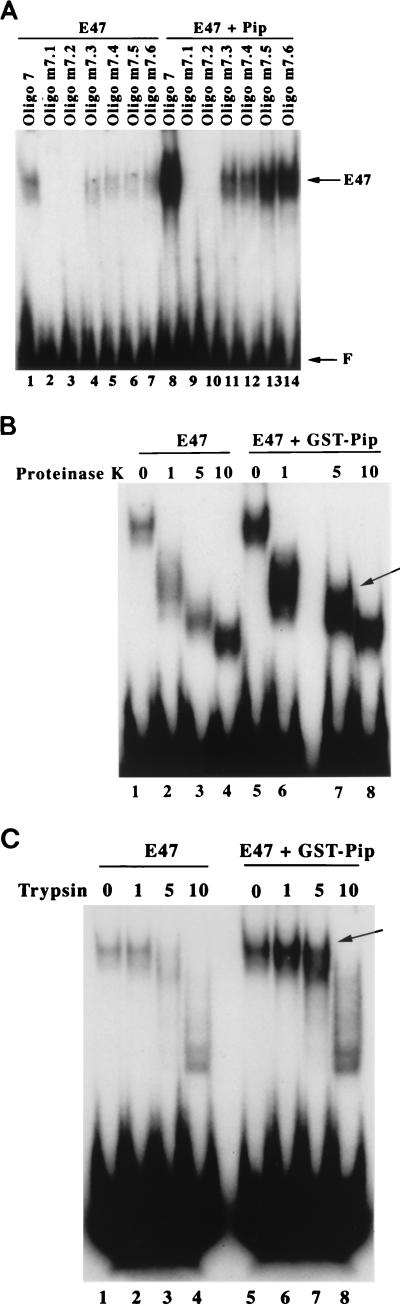
In an attempt to understand the mechanism of Pip enhancement of E47 DNA binding, we first performed EMSA with probes m7.1 to m7.6. As expected, Pip greatly increased DNA binding by E47 with the wild-type probe (Fig. 9A, lanes 1 and 8). Mutants m7.1 and m7.2 each abolish E47 DNA binding in either the absence or presence of Pip (Fig. 9A, lanes 2, 3, 9, and 10). Therefore, Pip cannot recruit E47 to DNA in the absence of an E2A DNA binding site. Mutants m7.5 and m7.6 which bind efficiently to both E47 and Pip showed enhanced binding by E47 (lanes 13 to 14). Mutants m7.3 and m7.4, which greatly reduce Pip DNA binding (Fig. 2C), resulted in much weaker E47 binding than with the unmutated probe (Fig. 9A, compare lane 8 with lanes 11 and 12). However, binding was greater in the presence of Pip than when E47 was assayed alone (lanes 4 and 5). This may indicate either that weak DNA binding by Pip to these probes can weakly enhance E47 DNA binding or possibly that Pip can weakly enhance E47 DNA binding by a mechanism that does not require Pip DNA binding. In either case, it is possible that Pip may induce a conformational change in E47.
FIG. 9.
(A) Maximal DNA binding by E47 requires E2A and Pip binding sites. EMSA was performed with wild-type or mutant oligonucleotide 7 probes and either E47 alone (lanes 1 to 7) or E47 plus Pip (lanes 8 to 14). The identity of each probe used in the EMSA is indicated above each lane. (B and C) The wild-type oligonucleotide 7 probe was incubated in an EMSA reaction mixture with either E47 alone or with E47 plus GST-Pip. After 30 min, various amounts of either proteinase K (B) or trypsin (C) were added to the reaction mixtures. Digestion was allowed to proceed for 5 min at room temperature. Samples were placed on ice and then subjected to electrophoresis. The amount, in nanograms, of each protease included in the reaction mixtures is shown above each lane. The position of complexes induced by GST-Pip are indicated with arrows.
To test this possibility, we performed partial-proteolysis EMSA studies with either E47 plus GST protein or E47 plus GST-Pip. In the presence of GST-Pip, an additional EMSA complex was observed with either 5 ng of proteinase K (Fig. 9B) or 5 ng of trypsin (Fig. 9C). Therefore, Pip may induce a conformational change in E47 which is more favorable for DNA binding. To determine whether enhanced DNA binding by E47 required the continual presence of Pip in the assay, E47 was preincubated with GST-Pip, which was then removed with glutathione agarose beads. The preincubated E47 did not bind to DNA more efficiently than E47 alone, indicating that Pip must be continually present to induce elevated E47 DNA binding (data not shown).
We next performed experiments to study some of the kinetic parameters of enhanced DNA binding by E47 in the presence of Pip. Off-rate experiments to measure the dissociation of E47 from the DNA showed very little difference in the half-life of the E47-DNA complex in the presence of Pip (data not shown). Competition with unlabeled oligonucleotide 7 sequence also revealed little difference in the affinity of E47 for its binding site in the presence of Pip (data not shown). Enhanced E47 DNA binding by Pip was observed at the earliest time point assayed (1 min) (data not shown), suggesting that Pip increases the rate at which E47 binds to DNA. An additional possibility is that Pip may increase E47 DNA binding by increasing the fraction of E47 molecules that can bind to DNA. This is reminiscent of the ability of HSP90 to increase the ability of MyoD to bind to DNA (54).
DISCUSSION
By mutagenesis, transient expression, and EMSA studies, we have identified a binding site for Pip directly adjacent to the E2A binding site in the Ig(κ) 3′ enhancer. Both the Pip and E2A sites are important for enhancer function since mutation of either sequence greatly reduced enhancer activity. Of considerable interest, we found that Pip and E47 can functionally interact to yield a synergy of over 100-fold. The only other known protein partner for Pip is the Ets domain transcription factor PU.1. PU.1 can induce the ability of Pip to bind to a site directly adjacent to the PU.1 site in the Ig(κ) 3′ enhancer (48, 49). Therefore, two Pip binding sites reside within this enhancer, one adjacent to the PU.1 site and one adjacent to the E2A site. While PU.1 can increase Pip DNA binding, we found that Pip can increase E47 DNA binding. E2A proteins can interact with a variety of other transcription factors with HLH domains (reviewed in reference 37), and these interactions can greatly influence E2A function by controlling DNA binding. In addition, E2A proteins can functionally synergize with the LIM domain proteins Lmx1.1 and Lmx1.2 and with p300 (11, 28). However, our results are the first observed interaction between E2A and an IRF family member.
Definition of Pip functional sequences.
Our deletion analyses identified three regions of Pip that contribute to synergy with E47. The first region, synergy domain 1 (residues 140 to 207), is rich in proline and glutamine residues and can function like a typical transactivation domain. Deletion of this sequence reduces synergy with E47 10-fold. Sequences immediately C-terminal to synergy domain 1 may control its function. Pip residues 1 to 300 show no transactivation activity when linked to a heterologous DNA binding domain (GAL4), whereas Pip residues 1 to 207 support very active transcription. This suggests that the sequence between residues 207 and 300 may mask the Pip activation domain (this masking domain is distinct from the domain between residues 410 and 439 found by Brass et al. (7) to mask Pip DNA binding). Within the masking domain is a segment rich in PEST sequences (residues 208 to 238). It is unclear whether the PEST sequences play a role in masking function. However, the masking function can be relieved in the presence of E47, since Pip 1-300 synergizes very strongly with E47.
The second synergy region, synergy domain 2 (residues 300 to 420), is also rich in glutamine residues, particularly between residues 354 and 420. However, we have been unable to definitively establish whether this sequence functions as a typical transactivation domain. GAL4 fusions with this sequence do not activate a GAL4-responsive promoter, but the GAL-Pip fusion protein did not accumulate to high levels. Therefore, the function of this domain remains uncertain. However, deletion of synergy domain 2 reduces synergy with E47 three- to fourfold, indicating its importance.
Finally, the Pip DNA binding domain (residues 1 to 134) is important for synergy with E47. This is evidenced by the observation that synergy requires Pip DNA binding since mutation of the Pip DNA binding site abolished activity. In addition, the Pip DNA binding domain can enhance DNA binding by E47. The specificity of the Pip DNA binding domain for synergy with E47 is suggested by domain swap experiments. Replacement of the Pip DNA binding domain with a heterologous DNA binding domain (GAL4) abolishes synergy with E47 when a GAL4-E2A responsive reporter is used (unpublished results). Thus, at least three regions of the Pip protein are involved in synergy with E47.
Although the Pip DNA binding domain is specific for synergy with E47, the same is not true of synergy domains 1 and 2. These Pip domains can be replaced with a heterologous activation domain to produce a protein yielding high levels of synergy with E47. Similarly, the E47 activation domain can be replaced with a heterologous activation domain. However, deletion of the E47 or the Pip activation domains abolishes synergy, indicating that each protein must contain functional sequences in addition to their DNA binding domains (i.e., each protein must contain an activation domain). The one exception to this rule is the ability of the Pip DNA binding domain alone to synergize with the ΔE47-VP16 chimera. Presumably, the very strong VP16 activation domain can obviate the need for the second activation domain normally supplied by Pip. This is supported by the ability of the Pip 1-134–VP16 protein to activate transcription on its own in the absence of E47 (data not shown). The inability of ΔE47-VP16 to activate transcription in the absence of Pip again reinforces the importance of the Pip DNA binding domain for increasing the ability of E47 to bind to DNA.
Mechanism of Pip and E47 synergy.
The mechanism of the Pip and E47 activation domains in mediating synergy is uncertain. These domains could cooperate to form an interaction surface for a coactivator or for a component of the basal transcription apparatus. E2A has been shown to synergistically activate transcription with the coactivator protein p300 (11). However, we have no evidence that p300 contributes to the synergy between E47 and Pip (unpublished results). Alternatively, each protein could independently affect some component of the transcription process. However, the importance of the Pip DNA binding domain suggests a functional interaction between Pip and E47 rather than completely independent processes. Conformational changes in both proteins are likely to be involved in the synergy mechanism. Partial-proteolysis studies indicated that Pip can alter the protease sensitivity of E47. Interaction between Pip and E47 may, therefore, induce an E47 conformation which facilitates DNA binding. On the other hand, E47 may induce a conformational change in Pip. As mentioned above, in our GAL fusion studies, GAL–Pip 1-207 was found to be a very potent transcriptional activator on its own, while the GAL–Pip 1-300 protein was inactive. However, when assayed for synergy with E47, both proteins were equally active. Therefore, E47 may induce a conformational change which affects Pip residues 207 to 300, thereby exposing sequences necessary for transactivation and synergy with E47. These residues are deleted in Pip 1-207, enabling this protein to be transcriptionally active on its own.
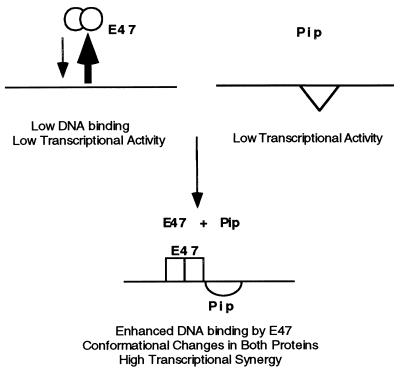
Based on all the above information, we propose a model for E47 and Pip synergy (Fig. 10). E47 binds to DNA poorly and by itself is a weak transcriptional activator. Similarly, Pip alone is a weak activator. This protein apparently can bind to DNA (as evidenced by the activity of the Pip-VP16 protein) but does so in a context which leaves the activation or synergy domains masked. We propose that Pip bound to DNA can induce a conformational change in E47 which enhances its ability to bind to DNA. The target of the conformational change appears to be the bHLH region because binding of ΔE47-VP16 (which contains the minimal E47 bHLH region) is also enhanced by Pip (Fig. 8). Subsequently (or simultaneously), E47 induces a change in Pip which exposes sequences necessary for activation and synergy with E47. The paired conformational changes which lead to enhanced E47 binding and exposure of Pip activation sequences results in potent transcriptional synergy between E47 and Pip.
FIG. 10.
Model of synergy between E47 and Pip. E47 dimers (empty circles) bind poorly to DNA. Pip (empty triangle) binds to DNA but in a transcriptionally inactive context. Pip can induce a conformational change in E47 (empty rectangles) which enhances E47 DNA binding. E47 induces a conformational change in Pip (empty semicircle) which exposes sequences necessary for transcription. These events result in high levels of synergy between E47 and Pip.
Interaction on DNA or in solution?
We believe that while the interaction between E47 and Pip can occur in solution, it occurs primarily on the DNA. GST-chromatography experiments showed very weak interactions between E47 and Pip, as did two hybrid studies (unpublished results). If the interaction is primarily on DNA, why then do we not observe a ternary complex of Pip and E47 bound to DNA? Several reasons are possible. Perhaps under our EMSA conditions, the complex of DNA-E47-Pip is unstable. This phenomenon is not unprecedented. For instance, the SRF-interacting protein Phox can increase SRF binding to DNA but a DNA-SRF-Phox ternary complex is not observed (22). Recently, an additional protein that can stabilize this ternary complex was isolated (21). Therefore, an additional protein which can stabilize DNA-E47-Pip ternary complexes may exist in vivo. Alternatively, Pip may assist DNA binding by E47 and then exit the complex. This would be analogous to the situation in which HSP90 induces a conformational change in MyoD leading to increased MyoD DNA binding, while HSP90 does not bind to DNA. Pip may also weakly interact with E47 in solution to induce E47 DNA binding. For instance, Pip can weakly increase E47 DNA binding to oligonucleotide mutants m7.3 and m7.4 (Fig. 9A) even though Pip DNA binding is disrupted. As expected, these mutants, which preclude Pip DNA binding, are transcriptionally inactive. Solution interaction between E47 and Pip appears to be a relatively minor mechanism for increased binding by E47 because we observed maximal increases in E47 binding when Pip could also bind to DNA. Additional experiments will be required to establish the mechanism of increased E47 DNA binding by Pip.
E47 synergy with other IRF proteins.
IRF family proteins carry a growing list of transcription factors. Some IRF proteins, such as IRF-1 and ISGF3, are known transcriptional activators, whereas others, such as ICSBP and IRF-2, can repress transcription (7, 15, 23, 24, 50, 55, 63). Because IRF proteins often bind to similar DNA sequences, the function of a particular promoter site can be altered by the cellular milieu of IRF proteins. Thus, various IRF proteins can compete for binding sites to mediate either transcriptional activation or repression. For instance, interferon-inducible transcription mediated by some IRF proteins can be repressed by other IRF family members (7, 41, 55, 63). In addition, ICSBP function can be altered by association with IRF-1 and IRF-2 (6, 55, 56). Finally, the function of some IRF proteins, such as ICSBP, can be influenced by phosphorylation (56). In this report, we show that the function of the IRF repressor ICSBP can be completely changed by interaction with another protein. Although ICSBP is known to repress transcription, we found that similar to Pip, ICSBP can functionally synergize with E47 to yield a potent transcriptional activation. This raises a new mechanism for regulation of transcription by IRF family members. Promoter activity can be changed by the particular IRF protein that binds to a site, as well as by the presence of flanking binding sites for other transcription factors. It will be interesting to determine whether other promoters that bind to ICSBP can be differentially regulated by proteins such as E47. It will also be interesting to determine whether other bHLH proteins can functionally synergize with IRF proteins.
Roles of E2A and Pip in Ig(κ) 3′ enhancer activity.
Functions of E2A proteins and Pip are required for normal B-cell development. Homozygous deletion of either gene results in B-cell defects. It is interesting that maximal Ig(κ) 3′ enhancer activity occurs when both proteins are maximally functional during B-cell development (late B-cell stages). Pip expression is very low in early B-cell stages (pro-B and pre-B-cell stages) and increases dramatically at later stages (plasma cell stage). Therefore, Pip activity is likely to be highest at the plasma cell stage. On the other hand, E2A expression levels do not change dramatically during B-cell development. However, expression of its inhibitory dimerization partner, Id, is regulated. Id levels are high at early stages of B-cell development, but expression ceases at the B-cell and plasma cell stages. Therefore, E2A activity is also highest in late B-cell stages. Together, these data suggest that activity of the Ig(κ) 3′ enhancer could be controlled, in part, by regulating the ability of E47 and Pip to functionally synergize during B-cell development.
ACKNOWLEDGMENTS
We thank T. Kadesch for plasmids expressing E47, ΔE47, ΔE47-VP16, and VP16; K. Ozato for the ICSBP-expressing plasmid; and H. Singh for plasmid ATG/Pip. We thank N. G. Avadhani, T. Kadesch, and S. Maitra for comments on the manuscript.
This work was supported by NIH grant GM42415 to M.L.A.
REFERENCES
- 1.Atchison M L, Perry R P. The role of the κ enhancer and its binding factor NF-κB in the developmental regulation of κ gene transcription. Cell. 1987;48:121–127. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- 2.Bain G, Gruenwald S, Murre C. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol Cell Biol. 1993;13:3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain G, Maandag E C R, Izon D J, Amsen D, Kruisbeek A M, Weintraub B C, Krop I, Schlissel M S, Feeney A J, van Roon M, van der Valk M, te Riele H P J, Berns A, Murre C. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 4.Benezra R. An intermolecular disulfide bond stabilizes E2A homodimers and is required for DNA binding at physiological temperatures. Cell. 1994;79:1057–1067. doi: 10.1016/0092-8674(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 5.Benezra R, Davis R L, Lockshon D, Turner D L, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 6.Bovolenta C, Drigger P H, Marks M S, Medin J A, Politis A D, Vogel S N, Levy D E, Sakaguchi K, Appella E, Coligan J E, Ozato K. Molecular interactions between interferon consensus sequence binding protein and members of the interferon regulatory factor family. Proc Natl Acad Sci USA. 1994;91:5046–5050. doi: 10.1073/pnas.91.11.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brass A L, Kehrli E, Eisenbeis C F, Storb U, Singh H. Pip, a lymphoid-restricted IRF, contains a regulatory domain that is important for autoinhibition and ternary complex formation with the Ets factor PU.1. Genes Dev. 1996;10:2335–2347. doi: 10.1101/gad.10.18.2335. [DOI] [PubMed] [Google Scholar]
- 8.Bushmeyer S, Park K, Atchison M L. Characterization of functional domains within the multifunctional transcription factor, YY1. J Biol Chem. 1995;270:30213–30220. doi: 10.1074/jbc.270.50.30213. [DOI] [PubMed] [Google Scholar]
- 9.Christy B A, Sanders L K, Lau L F, Copeland N G, Jenkins N A, Nathans D. An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc Natl Acad Sci USA. 1991;88:1815–1819. doi: 10.1073/pnas.88.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desiderio S. Transcription factors controlling B-cell development. Curr Biol. 1995;5:605–608. doi: 10.1016/s0960-9822(95)00121-7. [DOI] [PubMed] [Google Scholar]
- 11.Eckner R, Yao T-P, Oldread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 12.Eisenbeis C F, Singh H, Storb U. Pip, a novel IRF family member, is a lymphoid-specific PU.1-dependent transcriptional activator. Genes Dev. 1995;9:1377–1387. doi: 10.1101/gad.9.11.1377. [DOI] [PubMed] [Google Scholar]
- 13.Eisenbeis C F, Singh H, Storb U. PU.1 is a component of a multiprotein complex which binds an essential site in the murine immunoglobulin λ2-4 enhancer. Mol Cell Biol. 1993;13:6452–6461. doi: 10.1128/mcb.13.10.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu X, Kessler D S, Veals S A, Levy D E, Darnell J E., Jr ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci USA. 1990;87:8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita T, Kimura Y, Miyamoto M, Barsoumian L, Taniguchi T. Induction of endogenous IFN-α and IFN-β genes by a regulatory transcription factor, IRF-1. Nature. 1989;337:270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 16.Fulton R, Van Ness B. Kappa immunoglobulin promoters and enhancers display developmentally controlled interactions. Nucleic Acids Res. 1993;21:4941–4947. doi: 10.1093/nar/21.21.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulton R, Van Ness B. Selective synergy of immunoglobulin enhancer elements in B-cell development: a characteristic of kappa light chain enhancers, but not heavy chain enhancers. Nucleic Acids Res. 1994;22:4216–4223. doi: 10.1093/nar/22.20.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham F L, Van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 20.Grossman A, Mittrucker H-W, Nicholl J, Suzuki A, Chung S, Antonio L, Suggs S, Sutherland G R, Siderovski D P, Mak T W. Cloning of human lymphocyte-specific interferon regulatory factor (hLSIRF/hIRF4) and mapping of the gene to 6p23-p25. Genomics. 1996;37:229–233. doi: 10.1006/geno.1996.0547. [DOI] [PubMed] [Google Scholar]
- 21.Grueneberg D A, Henry R W, Novina B A, C D, Cheriyath V, Roy A L, Gilman M. A multifunctional DNA-binding protein that promotes the formation of serum response factor/homeodomain complexes: identity to TFII-I. Genes Dev. 1997;11:2482–2493. doi: 10.1101/gad.11.19.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grueneberg D A, Natesan S, Alexandre C, Gilman M Z. Human and drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992;257:1089–1095. doi: 10.1126/science.257.5073.1089. [DOI] [PubMed] [Google Scholar]
- 23.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 24.Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Absence of the type 1 IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63:303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 25.Henthorn P, Kiledjian M, Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer μE5/κE2 motif. Science. 1990;247:467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- 26.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 27.Hu J-S, Olson E N, Kingston R E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992;12:1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson J D, Zhang W, Rudnick A, Rutter W J, German M S. Transcriptional synergy between LIM-homeodomain proteins and basic helix-loop-helix proteins: the LIMZ domain determines specificity. Mol Cell Biol. 1997;17:3488–3496. doi: 10.1128/mcb.17.7.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaelin W G J, Pallas D C, DeCaprio J A, Kaye F J, Livingston D M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 30.Kessler D S, Veals S A, Fu X-Y, Levy D E. IFN-α regulates nuclear translocation and DNA-binding activity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4:1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- 31.Lassar A B, Davis R L, Wright W E, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like protein in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- 32.Lenardo M, Pierce J W, Baltimore D. Protein binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987;236:1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama T, Grossman A, Mittrucker H-W, Siderovski D P, Kiefer F, Kawakami T, Richardson C D, Taniguchi T, Yoshinaga S K, Mak T W. Molecular cloning of LSIRF, a lymphoid-specific member of the interferon regulatory factor family that binds the interferon-stimulated response element (ISRE) Nucleic Acids Res. 1995;23:2127–2136. doi: 10.1093/nar/23.12.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer K B, Neuberger M S. The immunoglobulin κ locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989;8:1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittrucker H-W, Matsuyama T, Grossman A, Kundig T M, Potter J, Shahinian A, Wakeham A, Patterson B, Ohashi P S, Mak T W. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275:540–543. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 37.Murre C, Bain G, Dijk M A V, Engel I, Furnari B A, Massari M E, Matthews J R, Quong M W, Rivera R R, Stuiver M H. Structure and function of helix-loop-helix proteins. Biochim Biophys Acta. 1994;1218:129–135. doi: 10.1016/0167-4781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 38.Murre C, McCaw P S, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 39.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, Weintraub H, Baltimore D. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 40.Murre C, Voronova A, Baltimore D. B-cell- and myocyte-specific E2-box-binding factors contain E12/E47-like subunits. Mol Cell Biol. 1991;11:1156–1160. doi: 10.1128/mcb.11.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson B, Tian G, Erman B, Gregoire J, Maki R, Graves B, Sen R. Regulation of lymphoid-specific immunoglobulin mu heavy chain enhancer by ETS-domain proteins. Science. 1993;261:82–86. doi: 10.1126/science.8316859. [DOI] [PubMed] [Google Scholar]
- 42.Nelson N, Marks M, Driggers P, Ozato K. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription. Mol Cell Biol. 1993;13:588–599. doi: 10.1128/mcb.13.1.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson E N. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Patwardhan S, Gashler A, Siegel M G, Chang L C, Joseph L J, Shows T B, LeBeau M M, Sukhatme V P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991;6:917–928. [PubMed] [Google Scholar]
- 45.Pongubala J M R, Atchison M L. Activating transcription factor 1 and cyclic AMP response element modulator can modulate the activity of the immunoglobulin κ 3′ enhancer. J Biol Chem. 1995;270:10304–10313. doi: 10.1074/jbc.270.17.10304. [DOI] [PubMed] [Google Scholar]
- 46.Pongubala J M R, Atchison M L. Functional characterization of the developmentally controlled immunoglobulin kappa 3′ enhancer: regulation by Id, a repressor of helix-loop-helix transcription factors. Mol Cell Biol. 1991;11:1040–1047. doi: 10.1128/mcb.11.2.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pongubala J M R, Atchison M L. PU.1 can participate in an active enhancer complex without its transcriptional activation domain. Proc Natl Acad Sci USA. 1997;94:127–132. doi: 10.1073/pnas.94.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pongubala J M R, Nagulapalli S, Klemsz M J, McKercher S R, Maki R A, Atchison M L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin κ 3′ enhancer activity. Mol Cell Biol. 1992;12:368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pongubala J M R, Van Beveren C, Nagulapalli S, Klemsz M J, McKercher S R, Maki R A, Atchison M L. Effect of PU.1 phosphorylation on interaction with NF-EM5 and transcriptional activation. Science. 1993;259:1622–1625. doi: 10.1126/science.8456286. [DOI] [PubMed] [Google Scholar]
- 50.Reis L F L, Harada H, Wolchok J D, Taniguchi T, Vilcek J. Critical role of a common transcription factor IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992;11:185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riechmann V, van Cruchten I, Sablitsky F. The expression pattern of Id4, a novel dominant negative helix-loop-helix protein, is distinct from Id1, Id2, and Id3. Nucleic Acids Res. 1994;22:749–755. doi: 10.1093/nar/22.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera R R, Stuiver M H, Steenbergen R, Murre C. Ets proteins: new factors that regulate immunoglobulin heavy chain gene expression. Mol Cell Biol. 1993;13:7163–7169. doi: 10.1128/mcb.13.11.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts”, prepared from small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaknovich R, Shue G, Kohtz D S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine HSP90 (HSP84) Mol Cell Biol. 1992;12:5059–5068. doi: 10.1128/mcb.12.11.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharf R, Azriel A, Lejbkowitcz F, Winograd S S, Ehrlich R, Levi B-Z. Functional domain analysis of interferon consensus sequence binding protein (ICSBP) and its association with interferon regulatory factors. J Biol Chem. 1995;270:13063–13069. doi: 10.1074/jbc.270.22.13063. [DOI] [PubMed] [Google Scholar]
- 56.Sharf R, Meraro D, Azriel A, Thornton A M, Ozato K, Petricoin E F, Larner A D, Schaper F, Hauser H, Levi B-Z. Phosphorylation events modulate the ability of interferon consensus sequence binding protein to interact with interferon regulatory factors. J Biol Chem. 1997;272:9785–9792. doi: 10.1074/jbc.272.15.9785. [DOI] [PubMed] [Google Scholar]
- 57.Shen C-P, Kadesch T. B-cell-specific DNA binding by an E47 homodimer. Mol Cell Biol. 1995;15:4518–4524. doi: 10.1128/mcb.15.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sloan S R, Shen C-P, McCarrick-Walmsley R, Kadesch T. Phosphorylation of E47 as a potential determinant of B-cell-specific activity. Mol Cell Biol. 1996;16:6900–6908. doi: 10.1128/mcb.16.12.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X-H. Constitutive expression of the Id1 gene impairs mouse B cell development. Cell. 1994;79:893–900. doi: 10.1016/0092-8674(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 60.Sun X-H, Copeland N G, Jenkins N A, Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991;11:5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weintraub H. The myoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 62.Wilson R B, Kiledjian M, Shen C-P, Benezra R, Zwollo P, Dymecki S M, Desiderio S V, Kadesch T. Repression of immunoglobulin enhancers by the helix-loop-helix protein Id: implications for B-lymphoid-cell development. Mol Cell Biol. 1991;11:6185–6191. doi: 10.1128/mcb.11.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamagata T, Nishida J, Tanaka T, Sakai R, Mitani K, Yoshida M, Taniguchi T, Yazaki Y, Hirai H. A novel interferon regulatory factor family transcription factor, ICSAT/Pip/LSIRF, that negatively regulates the activity of interferon-regulated genes. Mol Cell Biol. 1996;16:1283–1294. doi: 10.1128/mcb.16.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]