Abstract
Melphalan flufenamide (melflufen), a first-in-class alkylating peptide-drug conjugate, plus dexamethasone was approved in Europe for use in patients with triple-class refractory relapsed/refractory multiple myeloma (RRMM) with ≥3 prior lines of therapy and without prior autologous stem cell transplantation (ASCT) or with a time to progression >36 months after prior ASCT. The randomized LIGHTHOUSE study (NCT04649060) assessed melflufen plus daratumumab and dexamethasone (melflufen group) versus daratumumab in patients with RRMM with disease refractory to an immunomodulatory agent and a proteasome inhibitor or who had received ≥3 prior lines of therapy including an immunomodulatory agent and a proteasome inhibitor. A partial clinical hold issued by the US Food and Drug Administration for all melflufen studies led to financial constraints and premature study closure on February 23rd 2022 (data cut-off date). In total, 54 of 240 planned patients were randomized (melflufen group, N=27; daratumumab group, N=27). Median progression-free survival (PFS) was not reached in the melflufen group versus 4.9 months in the daratumumab group (Hazard Ratio: 0.18 [95% Confidence Interval, 0.05-0.65]; P=0.0032) at a median follow-up time of 7.1 and 6.6 months, respectively. Overall response rate (ORR) was 59% in the melflufen group versus 30% in the daratumumab group (P=0.0300). The most common grade ≥3 treatment-emergent adverse events in the melflufen group versus daratumumab group were neutropenia (50% vs. 12%), thrombocytopenia (50% vs. 8%), and anemia (32% vs. 19%). Melflufen plus daratumumab and dexamethasone demonstrated superior PFS and ORR versus daratumumab in RRMM and a safety profile comparable to previously published melflufen studies.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy that accounts for approximately 10% of all hematologic malignancies.1,2 Despite the advent of new therapies and the improvement in response and survival rates, most patients will relapse with currently available standard-of-care therapies and have shorter remissions with each subsequent line of therapy, leaving an unmet need for the development of new and effective combinations that achieve deeper and more durable responses.1,3-5
Melphalan flufenamide (melflufen) is a first-in-class lipophilic alkylating peptide-drug conjugate that is rapidly distributed via passive transport to enter tumor cells due to its lipophilicity.6-10 Upon entering tumor cells, the peptide carrier functions as an enzymatic substrate utilizing the increased metabolic activity in cancer cells to release cytotoxic, hydrophilic alkylating metabolites (melphalan and desethyl-melflufen) leading to intracellular enrichment.9-11 In Europe, based on the phase II HORIZON and the phase III OCEAN studies, the doublet melflufen plus dexamethasone was approved for the treatment of adult patients with relapsed/refractory (RR)MM who have received ≥3 prior lines of therapy and whose disease is refractory to ≥1 proteasome inhibitor (PI), ≥1 immunomodulatory agent, and one anti-CD38 monoclonal antibody and have demonstrated disease progression on or after the last therapy; if a patient has received a prior autologous stem cell transplant (ASCT), time to progression (TTP) must be >36 months before initiating melflufen therapy.12-14 Daratumumab, an anti-CD38 monoclonal antibody with known efficacy and manageable safety that has a non-overlapping mechanism of action with melflufen, was first approved as a monotherapy in patients who have received ≥3 prior lines of therapy including a PI and an immunomodulatory agent or are double refractory to an immunomodulatory agent and a PI; more recently, daratumumab has been approved as first- and second-line treatment for MM.15-18 Real-word data show that daratumumab monotherapy is used substantially in RRMM but is more effective when given in earlier lines of therapy and as part of combination regimens.19
In the phase I/II ANCHOR study (N=33), the triplet combination of melflufen (30 or 40 mg) plus daratumumab and dexamethasone demonstrated clinical activity and manageable safety.20 The overall response rate (ORR) was 73%, and with a median follow-up of 18.9 months, the median progression-free survival (PFS) was 12.9 months (95% confidence interval [CI]: 7.7-15.4) and the median duration of response was 12.6 months (95% CI: 7.6-24.2). The overall safety profile of melflufen in triplet combination with daratumumab and dexamethasone was consistent with the known safety profile of melflufen as part of a doublet regimen, consisting primarily of clinically manageable hematologic adverse events (AE).13,14,20,21 Melflufen 30 mg was chosen as the recommended dose for future evaluation in combination with daratumumab and dexamethasone because drug exposure was similar at both dose levels of melflufen, but more dose modifications were reported with melflufen 40 mg. These results supported further evaluation of the efficacy and safety of melflufen plus daratumumab and dexamethasone in the phase III LIGHTHOUSE study.
Methods
Study design and patients
LIGHTHOUSE (OP-108) was a randomized, controlled, open-label, phase III multicenter study investigating melflufen plus daratumumab and dexamethasone versus daratumumab in patients with RRMM (NCT04649060). Eligible patients aged ≥18 years must have been refractory to an immunomodulatory agent and a PI or had received ≥3 prior lines of therapy including an immunomodulatory agent and a PI. Prior treatment with, but not refractoriness to, an anti-CD38 monoclonal antibody was allowed (Online Supplementary Appendix). National regulatory authorities and independent ethics committees or institutional review boards approved the study, which was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation and Good Clinical Practice guidelines. All patients provided written informed consent.
Randomization and study treatment
Patients were randomized 1:1 to receive therapy in the melflufen group or daratumumab group until disease progression or unacceptable toxicity. In each 28-day cycle, patients in the melflufen group received melflufen 30 mg intravenously (IV) on day 1 and oral dexamethasone 40 mg (20 mg if aged ≥75 years) weekly; patients in both groups received daratumumab 1800 mg subcutaneously on days 1, 8, 15, and 22 in cycles 1-2, on days 1 and 15 in cycles 3-6, and on day 1 in cycle 7 and beyond. Patients in the daratumumab group received dexamethasone (or an equivalent corticosteroid) pre-dose (20 mg IV or orally; dose reduction to 12 mg following the second injection allowed) and post dose (4 mg orally for 2 days). Melflufen and dexamethasone dose modifications were permitted (Online Supplementary Appendix); patients with disease progression in the daratumumab group could opt to cross over to the melflufen group.
Endpoints and analyses
The primary endpoint was PFS, assessed by the investigator according to the International Myeloma Working Group uniform response criteria.22 Secondary endpoints included ORR, overall survival (OS), and frequency and grade of treatment-emergent AE (TEAE). TEAE were graded per the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0.23 Disease status was assessed at screening and pre-dose every cycle on day 1 (Online Supplementary Appendix).
Assuming a hazard ratio of 0.6 and a P value of 0.05, a total of 240 patients were planned for enrollment for an estimated 90% power to detect a significant result.
The intent-to-treat (ITT) population included all patients randomized and was the primary population for all efficacy data. Safety was evaluated in patients with ≥1 exposure to any study treatment. Based on observations from the OCEAN trial, additional subgroup analyses further divided patients with no prior ASCT or with TTP >36 months after a prior ASCT and patients with TTP <36 months after a prior ASCT.
PFS and OS were summarized using the Kaplan-Meier method and analyzed using log-rank test. Cox proportional hazard models were used to estimate Hazard Ratios (HR) and 95% CI. ORR was presented together with 95% CI based on the Clopper-Pearson method, and comparison between groups was performed using a Cochran-Maentel-Haenzel test. TEAE were recorded for each patient; all TEAE were reported, regardless of potential attribution to treatment or disease progression.
Results
Patients and treatment
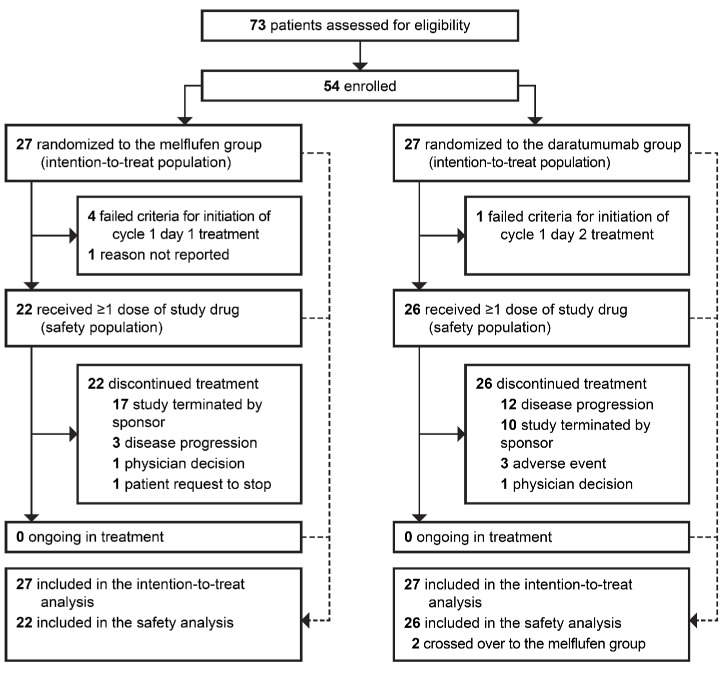
The LIGHTHOUSE study was initiated in December 2020, but enrollment stopped due to financial reasons after the US Food and Drug Administration requested a partial clinical hold for all melflufen studies. In total, 54 of the 240 patients planned for enrollment had been randomized to receive melflufen, daratumumab, and dexamethasone (melflufen group, N=27) or daratumumab (daratumumab group, N=27) by February 23rd 2022 (date of premature study closure and data cut-off). Two patients in the daratumumab group crossed over to receive melflufen, daratumumab, and dexamethasone after progression (Figure 1). These two patients were included in the daratumumab group for the OS analyses according to the ITT principle, but following crossover, were included in the melflufen group for safety analyses.
At baseline, in the melflufen and daratumumab groups, respectively, the median age was 65 years (range, 43-80) and 68 years (range, 50-83), and the median number of prior lines of therapy was 3 (range, 1-9) and 4 (range, 1-7) (Table 1). All patients had previously been exposed to a PI and immunomodulatory agent, with 16 patients (59%) and 20 patients (74%) in the melflufen group and 23 patients (85%) and 26 patients (96%) in the daratumumab group having disease refractory to a PI and immunomodulatory agent, respectively. The proportion of double-refractory patients (i.e., refractory to a PI and an immunomodulatory agent) was substantially lower in the melflufen group (13 [48%]) compared with the daratumumab group (22 [81%]). No patient in either group had had prior exposure to an anti-CD38 monoclonal antibody. In the melflufen group, 16 patients (59%) had received a prior ASCT, of which 13 patients (48%) had a TTP <36 months and 3 patients (11%) had TTP >36 months after a prior ASCT. In the daratumumab group, 14 patients (52%) had received a prior ASCT, of which 12 patients (44%) had a TTP <36 months and 2 patients (7%) had a TTP >36 months after a prior ASCT.
Figure 1.
Patient disposition. Data cut-off date: February 23rd 2022.
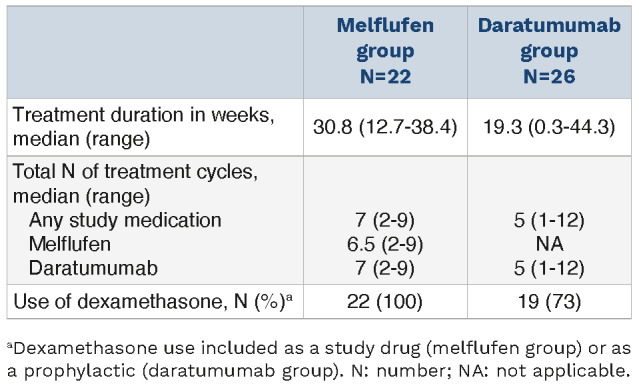
The median duration of treatment was 30.8 weeks (range, 12.7-38.4) and 19.3 weeks (range, 0.3-44.3) in the melflufen and daratumumab groups, respectively (Table 2). The median total number of cycles was 7 (range, 2-9) in the melflufen group and 5 (range, 1-12) in the daratumumab group.
Efficacy
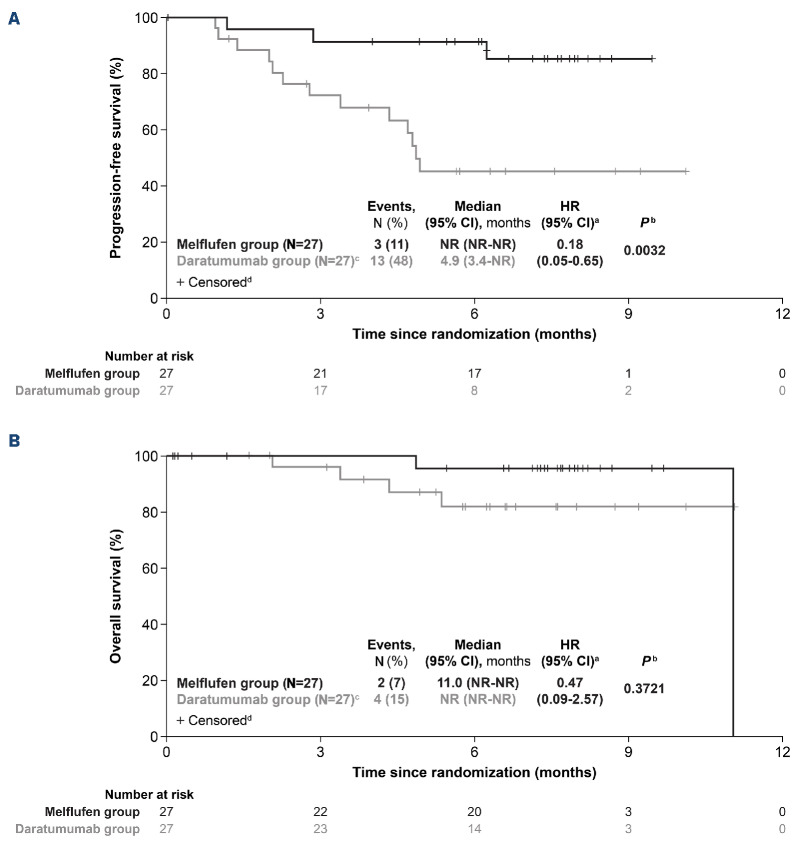
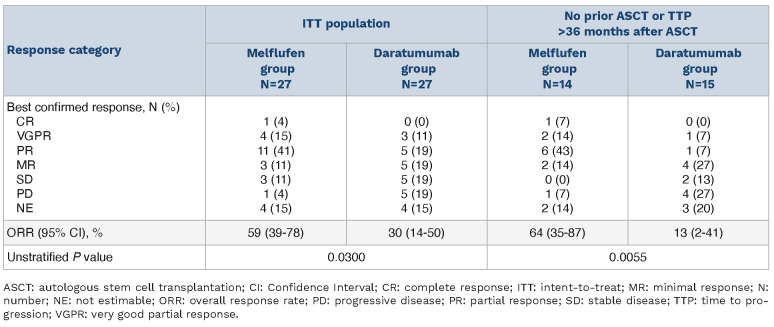
In the ITT population, with a median follow-up of 7.1 months in the melflufen group, the median PFS was not reached (NR) and with a median follow-up of 6.6 months in the daratumumab group, the median PFS was 4.9 months (95% CI: 3.4-NR; HR: 0.18 [95% CI: 0.05-0.65]; log-rank P=0.0032) (Figure 2A). OS was immature, with 2 events (7%) in the melflufen group and 4 events (15%) in the daratumumab group (HR: 0.47 [95% CI: 0.09-2.57]; log-rank P=0.3721) at a median follow-up of 7.6 months and 6.6 months, respectively (Figure 2B). The ORR was 59% (95% CI: 39-78) in the melflufen group and 30% (95% CI: 14-50) in the daratumumab group (P=0.0300) (Table 3). More patients in the melflufen group had a complete response (CR; 1 patient [4%] vs. 0 patients [0%]) and very good partial response (VGPR; 4 patients [15%] vs. 3 patients [11%]) compared with the daratumumab group.
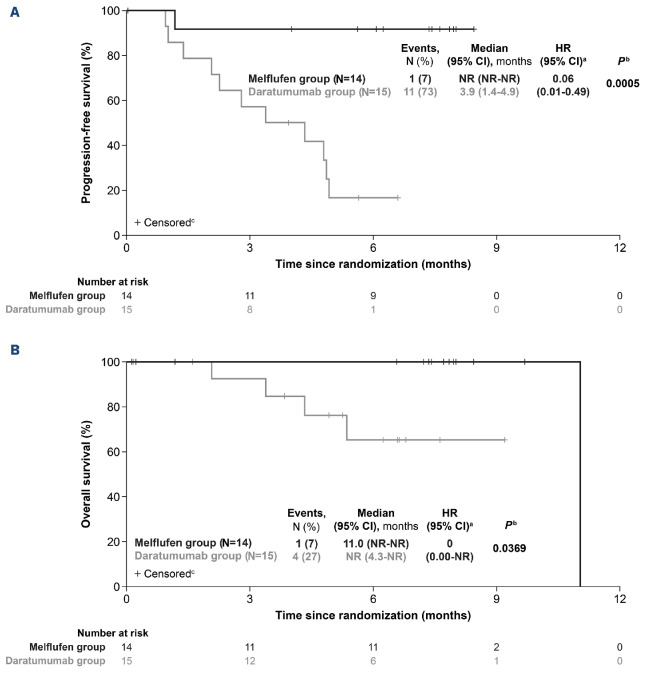
Efficacy endpoints were more pronounced in favor of the melflufen group compared with the daratumumab group among patients with no prior ASCT or TTP >36 months after a prior ASCT (melflufen group, N=14; daratumumab group, N=15). Median PFS was NR in the melflufen group and 3.9 months (95% CI: 1.4-4.9) in the daratumumab group (HR, 0.06 [95% CI: 0.01-0.49]; log-rank P=0.0005) (Figure 3A). Fewer OS events were reported in the melflufen group (1 event [7%] vs. 4 events [27%] in the daratumumab group; log-rank P=0.0369) (Figure 3B). The ORR was 64% (95% CI: 35-87) in the melflufen group and 13% (95% CI: 2-41) in the daratumumab group (P=0.0055) (Table 3). More patients in the melflufen group had a CR (1 patient [7%] vs. 0 patients [0%]) or VGPR (2 patients [14%] vs. 1 patient [7%]) compared with the daratumumab group.
Table 1.
Baseline patient characteristics.
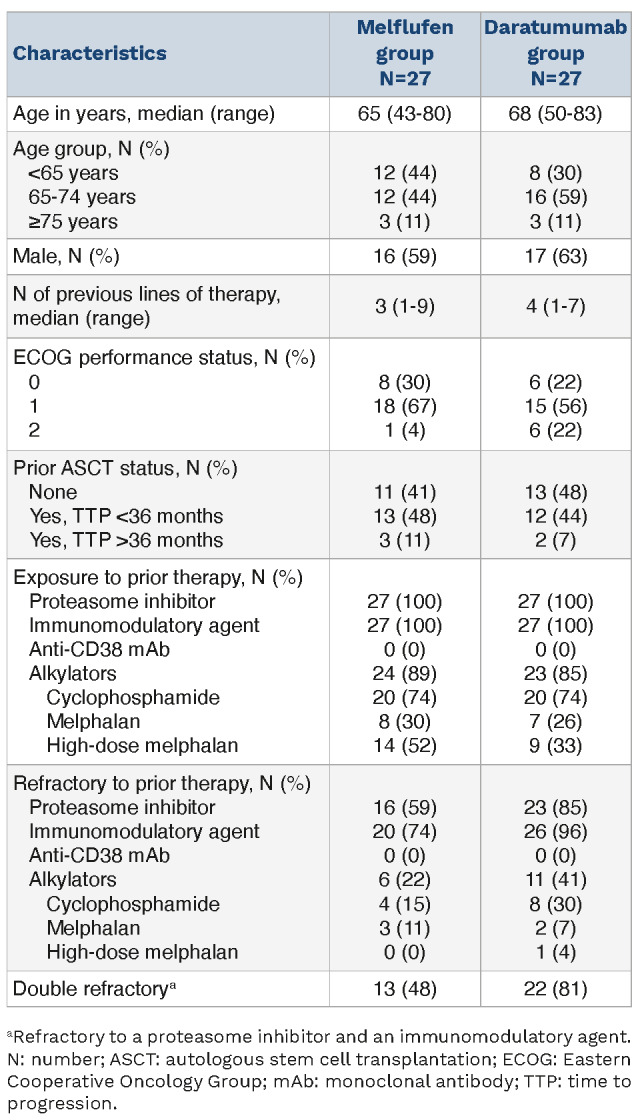
Table 2.
Treatment exposure.
Overall, no differences in efficacy between treatment groups were seen in patients with TTP <36 months after a prior ASCT (melflufen group, N=13; daratumumab group, N=12). Median PFS was NR in either arm (HR, 0.93 [95% CI: 0.13-6.59]; log-rank P=0.9391), with 2 events (15%) reported in the melflufen group and 2 events (17%) reported in the daratumumab group (Online Supplementary Table S1). For OS, 1 event (8%) was reported in the melflufen group and 0 events in the daratumumab group (P=0.3404). The ORR was 54% (95% CI: 25-81) in the melflufen group and 50% (95% CI: 21-79) in the daratumumab group (P=0.8505).
Safety
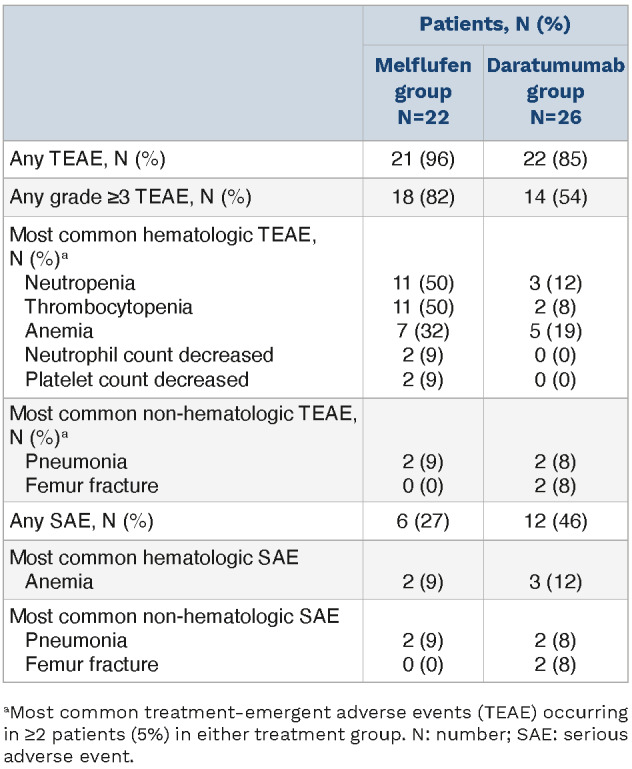
The safety population included patients who received ≥1 dose of melflufen, daratumumab, or dexamethasone (melflufen group) and 26 patients who received daratumumab monotherapy. In the safety population, ≥1 TEAE was reported in 21 patients (96%) with melflufen, daratumumab, and dexamethasone and 22 patients (85%) with daratumumab (Table 4). Overall, grade ≥3 TEAE occurred in 18 patients (82%) with melflufen, daratumumab, and dexamethasone and 14 patients (54%) with daratumumab. The most common hematologic grade ≥3 TEAE were neutropenia (melflufen group, 11 patients [50%]; daratumumab group, 3 patients [12%]), thrombocytopenia (melflufen group, 11 patients [50%]; daratumumab group, 2 patients [8%]), and anemia (melflufen group, 7 patients [32%]; daratumumab group, 5 patients [19%]). The most common non-hematologic grade ≥3 TEAE were pneumonia (melflufen group, 2 patients [9%]; daratumumab group, 2 patients [8%]) and femur fracture (melflufen group, 0 patients [0%]; daratumumab group, 2 patients [8%]); of these, 1 event (5%) of pneumonia in the melflufen group and 1 event (4%) of femur fracture in the daratumumab group were considered treatment-related TEAE by the investigator. Serious AE occurred in 6 patients (27%) with melflufen, daratumumab, and dexamethasone, and 12 patients (46%) with daratumumab. The most common serious AE (occurring in ≥4 patients overall) were anemia (melflufen group, 2 patients [9%]; daratumumab group, 3 patients [12%]) and pneumonia (melflufen group, 2 patients [9%]; daratumumab group, 2 patients [8%]). TEAE leading to treatment discontinuation occurred in 2 patients (9%) in the melflufen group (neutropenia and thrombocytopenia, N=1 each) and 4 patients (15%) in the daratumumab group (anemia, disease progression, hypercalcemia, and renal failure, N=1 each). Overall, 5 patients died on study before the crossover: 2 patients who received melflufen, daratumumab, and dexamethasone (1 due to disease progression and 1 due to unknown reasons, both >30 days after the last dose of study treatment) and 3 patients who received daratumumab (1 due to disease progression and 1 due to unknown reasons, both ≤30 days after the last dose of study treatment and 1 due to an AE [COVID-19 pneumonia], >30 days after the last dose of study treatment). In addition, one patient who crossed over to receive melflufen, daratumumab, and dexamethasone after progression on daratumumab died.
Figure 2.
Survival outcomes in the intention-to-treat population. Progression-free survival (A) and overall survival (B) in the intention-to-treat population with melflufen, daratumumab, and dexamethasone (melflufen group) or daratumumab monotherapy (daratumumab group). aUnstratified hazard ratio (HR). bLog-rank P value. cOverall, 2 patients randomized to the daratumumab group crossed over to the melflufen group following documented disease progression. dPatients alive at the time of study termination were censored. CI: Confidence Interval; N: number; NR: not reached.
Figure 3.
Survival outcomes in patients by autologous stem cell transplantation status. Progression-free survival (A) and overall survival (B) in patients with no prior autologous stem cell transplantation (ASCT) or with time to progression >36 months after a prior ASCT who received melflufen, daratumumab, and dexamethasone (melflufen group) or daratumumab monotherapy (daratumumab group). aUnstratified Hazard Ratio (HR). bLog-rank P value. cPatients alive at the time of study termination were censored. CI: Confidence Interval; N: number; NR: not reached.
Table 3.
Overall response rate.
Discussion
The LIGHTHOUSE study of melflufen plus daratumumab and dexamethasone in patients with RRMM refractory to an immunomodulatory agent and a PI or who had received ≥3 prior lines of therapy, including an immunomodulatory agent and a PI, builds upon the results of previous clinical studies of melflufen and dexamethasone in heavily pretreated patients with RRMM.13,14 The results of the present study were consistent with ANCHOR, which used the same triplet regimen, and demonstrated the efficacy and safety of melflufen as part of a triplet regimen.20 The premature termination of the LIGHTHOUSE study, which resulted in small numbers of patients enrolled, is a primary limitation of this study. Additionally, per protocol, patients discontinuing melflufen could continue with daratumumab and dexamethasone. However, how many patients chose to continue with daratumumab therapy was not captured after the study was terminated. While the interpretation of the results may be limited by the short follow-up time and small patient numbers, there were clear differences between patients treated with melflufen plus daratumumab and dexamethasone versus daratumumab. The addition of daratumumab to an alkylator-based regimen has previously been shown to provide significant clinical benefit in patients with newly diagnosed MM.17 In LIGHTHOUSE, PFS was significantly in favor of melflufen, daratumumab, and dexamethasone compared with daratumumab in patients who had received ≥3 prior lines of therapy including an immunomodulatory agent and a PI or had double-refractory disease. Results from LIGHTHOUSE were also comparable to other clinical trials evaluating triplet combinations in patients with RRMM who had received ≥3 prior lines of therapy including an immunomodulatory agent and a PI.24-26
Table 4.
Treatment-emergent adverse events: safety population.
Daratumumab monotherapy was first approved in 2015 for use in patients with RRMM who had received ≥3 prior lines of therapy, including an immunomodulatory agent and a PI, or who had disease that was double-refractory to these agents. Since then, daratumumab alone or in combination with other agents has become standard-of-care in the RRMM and newly diagnosed MM settings. Daratumumab has most commonly been used in second- or third-line RRMM in the real-world setting, similar to the patient population of the present study, with daratumumab monotherapy used in 12-26% patients treated in the United States and up to 48% of patients treated in Europe,19,27-30 which is why it was chosen as the comparator arm for the LIGHTHOUSE study. Although cross-trial evaluations can be confounded by differences in sample size and patient-, disease-, and treatment-related factors,31 results from the daratumumab group in LIGHTHOUSE were consistent with previously reported studies using daratumumab as a monotherapy in patients with RRMM who had received ≥3 prior lines of therapy.32-34 For example, in the phase III COLUMBA study of subcutaneous versus IV daratumumab, with a median follow-up of 29.3 months, the median PFS was 5.6 months for subcutaneous daratumumab and 6.1 months for IV daratumumab, which is generally comparable with the median PFS of 4.9 months in the daratumumab group demonstrated in the present study with a shorter follow up.31 Although the OS data are still too immature to draw conclusions, they trended in favor of melflufen, daratumumab, and dexamethasone therapy compared with daratumumab monotherapy.
In the ITT population, melflufen, daratumumab, and dexamethasone therapy also demonstrated higher response rates with an ORR of 59% compared with the 30% observed with daratumumab monotherapy. Furthermore, responses in the melflufen group were deeper, with a numerically higher CR, VGPR, and partial response rates compared with the daratumumab group.
Both the ITT population and subgroup of patients with no prior ASCT or TTP >36 months after a prior ASCT, showed longer median PFS and OS with melflufen, daratumumab, and dexamethasone compared with daratumumab alone. Furthermore, treatment responses were strikingly different in this subgroup with an ORR of 64% in the melflufen group and 13% in the daratumumab group. Although these results should be interpreted with caution due to the small sample size of this target subgroup and immaturity of the data, they are in line with those from OCEAN showing a confirmed benefit for this patient population and with the approved indication for melflufen and dexamethasone in patients with RRMM for use in Europe.12,14 On the other hand, no differences in efficacy between treatment groups were seen in patients with TTP <36 months after a prior ASCT. This contrasts with what was observed in this subgroup of patients in the OCEAN study, where all efficacy endpoints favored pomalidomide and dexamethasone over melflufen and dexamethasone.14
In LIGHTHOUSE, the safety profile of melflufen was consistent with previous reports from the triplet of melflufen plus daratumumab and dexamethasone reported in ANCHOR and with the safety profile of melflufen as a doublet with dexamethasone.13,14,20 In LIGHTHOUSE, grade ≥3 TEAE with melflufen, daratumumab, and dexamethasone were most commonly hematologic, which is consistent with the known safety profile of melflufen. Despite a higher frequency of hematologic TEAE in the melflufen group compared with the daratumumab group, discontinuation rates due to hematologic TEAE, and TEAE in general, were generally similar in both treatment groups. Thus, the addition of melflufen to daratumumab did not increase toxicity beyond already known, and clinically manageable, hematologic TEAE.13,14,20 This is further supported by the data demonstrating that patients remained on therapy longer in the melflufen group (7 cycles) compared with the daratumumab group (5 cycles). Importantly, the safety profile of the daratumumab group was in line with previous reports of single-agent daratumumab in comparable patient populations, as well as in more advanced settings.32,34,36 Because of how TEAE and treatment-related TEAE are generally reported in clinical trials,23 and because safety data were not formally adjudicated by an independent committee, it cannot be ruled out that some TEAE reported in LIGHTHOUSE (e.g., femur fracture) were related to progression of disease rather than the study treatment.
Despite the small patient numbers and short follow up at the time of study termination, results from the LIGHTHOUSE study demonstrated a clinical benefit with melflufen plus daratumumab and dexamethasone compared with daratumumab in both the ITT population and in patients with no prior ASCT or with a TTP >36 months after a prior ASCT. The safety profile was consistent with previous reports of melflufen as a triplet and doublet, further confirming that melflufen has no safety signal affecting survival. These results further support the findings from OCEAN and the European label indication and confirm the clinical benefit of melflufen in patients with MM with no prior ASCT or a longer remission after a prior ASCT. The encouraging results of melflufen plus daratumumab and dexamethasone support the use of this combination in the real world and provide direction for future studies using combination of melflufen with other drugs in RRMM.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families for participating in this trial, the trial investigators and co-ordinators for their contributions to the trial, and Janssen Pharmaceuticals. We thank Jared D. Hoffman, MS, PhD, and Katherine Mills-Lujan, PhD, CMPP, of Team 9 Science for providing medical editorial assistance under the guidance of the authors, in accordance with Good Publications Practice (GPP) 2022 guidelines.
Funding Statement
Funding: Funding for the study (ClinicalTrials.gov identifier: NCT04649060) and for editorial assistance was provided by Oncopeptides AB.
References
- 1.Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Dimopoulos MA, Kastritis E, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443-2448. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Otero P, Paiva B, San-Miguel JF. Roadmap to cure multiple myeloma. Cancer Treat Rev. 2021;100:102284. [DOI] [PubMed] [Google Scholar]
- 5.Jones JR, Weinhold N, Ashby C, et al. Clonal evolution in myeloma: the impact of maintenance lenalidomide and depth of response on the genetics and sub-clonal structure of relapsed disease in uniformly treated newly diagnosed patients. Haematologica. 2019;104(7):1440-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan D, Ray A, Viktorsson K, et al. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res. 2013;19(11):3019-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gullbo J, Tullberg M, Vabeno J, et al. Structure-activity relationship for alkylating dipeptide nitrogen mustard derivatives. Oncol Res. 2003;14(3):113-132. [DOI] [PubMed] [Google Scholar]
- 8.Ray A, Ravillah D, Das DS, et al. A novel alkylating agent melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br J Haematol. 2016;174(3):397-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickström M, Nygren P, Larsson R, et al. Melflufen - a peptidase-potentiated alkylating agent in clinical trials. Oncotarget. 2017;8(39):66641-66655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickström M, Viktorsson K, Lundholm L, et al. The alkylating prodrug J1 can be activated by aminopeptidase N, leading to a possible target directed release of melphalan. Biochem Pharmacol. 2010;79(9):1281-1290. [DOI] [PubMed] [Google Scholar]
- 11.Gullbo J, Wickstrom M, Tullberg M, et al. Activity of hydrolytic enzymes in tumour cells is a determinant for anti-tumour efficacy of the melphalan containing prodrug J1. J Drug Target. 2003;11(6):355-363. [DOI] [PubMed] [Google Scholar]
- 12.Pepaxti: Summary Of Product Characteristics - European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/pepaxti-epar-production-information_en.pdf Accessed April 12, 2023. [Google Scholar]
- 13.Richardson PG, Oriol A, Larocca A, et al. Melflufen and dexamethasone in heavily pretreated relapsed and refractory multiple myeloma. J Clin Oncol. 2021;39(7):757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schjesvold FH, Dimopoulos MA, Delimpasi S, et al. Melflufen or pomalidomide plus dexamethasone for patients with multiple myeloma refractory to lenalidomide (OCEAN): a randomised, head-to-head, open-label, phase 3 study. Lancet Haematol. 2022;9(2):e98-e110. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Terpos E, Boccadoro M, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(6):801-812. [DOI] [PubMed] [Google Scholar]
- 17.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518-528. [DOI] [PubMed] [Google Scholar]
- 18.DARZALEX [package insert]. Horsham, PA: Janssen Biotech, Inc; 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX-pi.pdf Accessed March 31, 2023. [Google Scholar]
- 19.Szabo AG, Klausen TW, Levring MB, et al. The real-world outcomes of multiple myeloma patients treated with daratumumab. PLoS One. 2021;16(10):e0258487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ocio EM, Efebere YA, Hajek R, et al. ANCHOR (OP-104): melflufen plus dexamethasone (dex) and daratumumab (dara) or bortezomib (BTZ) in relapsed/refractory multiple myeloma (RRMM) refractory to an IMiD and/or a proteasome inhibitor (PI) - updated efficacy and safety. Blood. 2020;136(s1):9-10. [Google Scholar]
- 21.Richardson PG, Bringhen S, Voorhees P, et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): a multicentre, international, open-label, phase 1-2 study. Lancet Haematol. 2020;7(5):e395-e407. [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). 2010. [June 14, 2010]. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/ CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf Accessed April 20, 2023. [Google Scholar]
- 24.Gasparetto C, Lentzsch S, Schiller G, et al. Selinexor, daratumumab, and dexamethasone in patients with relapsed or refractory multiple myeloma. EJHaem. 2021;2(1):56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096-2107. [DOI] [PubMed] [Google Scholar]
- 27.Atrash S, Thompson-Leduc P, Tai MH, et al. Treatment patterns and effectiveness of patients with multiple myeloma initiating daratumumab across different lines of therapy: a real-world chart review study. BMC Cancer. 2021;21(1):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cottini F, Huang Y, Williams N, et al. Real world experience of daratumumab: evaluating lymphopenia and adverse events in multiple myeloma patients. Front Oncol. 2020;10:575168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovas S, Varga G, Farkas P, et al. Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/ refractory multiple myeloma patients. Int J Hematol. 2019;110(5):559-565. [DOI] [PubMed] [Google Scholar]
- 30.Girvan A, Yu J, Emechebe N, Kamalakar R, Luo Y. Real-world treatment patterns and outcomes of daratumumab retreatment in multiple myeloma in the United States. Blood. 2022;140 (Suppl 1):5266-5267. [Google Scholar]
- 31.Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Usmani SZ, Nahi H, Legiec W, et al. Final analysis of the phase III non-inferiority COLUMBA study of subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma. Haematologica. 2022;107(10):2408-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SS, Min Byun J, Yoon SS, et al. Daratumumab monotherapy for relapsed/refractory multiple myeloma, focussed on clinical trial-unfit patients and subsequent therapy. Br J Haematol. 2021;193(1):101-112. [DOI] [PubMed] [Google Scholar]
- 34.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551-1560. [DOI] [PubMed] [Google Scholar]
- 35.Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207-1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.