Abstract
Background: The prevalence of obesity has increased globally and is associated with many comorbidities such as type 2 diabetes and fatty liver and cardiovascular diseases. Bariatric surgery is considered an effective intervention for achieving weight loss and controlling lipidemia and glycemia.
Objectives: This Saudi retrospective observational study evaluates the clinical and biochemical benefits following bariatric surgery to obese diabetic patients.
Methodology: After gaining ethical committee approval, data was collected from the patients' medical records at a tertiary medical center (King Fahad General Hospital, Al-Madinah Al-Munawwarah, Saudi Arabia). The total sample size was 61 patients, of whom 78.33% (n=48) had a body mass index (BMI) of 40 or greater (obese class III).
Results: Following bariatric surgery, there were statistically significant reductions (p<0.001) in BMI and HbA1C (decreased from 45.53±7.791 kg/m2 and 7.9±1.82% to 33.42±6.18 kg/m2 and 6.06±1.35%, respectively, after surgery). Likewise, significant reductions (p<0.001) occurred to serum total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides that decreased from 234.4±26.7 mg/dl, 152.2±19.4 mg/dl, and 187.3±24.6 mg/dl to 158.4±17.3 mg/dl, 95.6±15.7 mg/dl, and 132.5±19.5 mg/dl, respectively. Interestingly, serum high-density lipoprotein (HDL) significantly increased (p<0.001) from 43.8±6.2 mg/dl to 52.3±4.6 mg/dl. Using the novel clinical therapeutic index, bariatric surgery decreased BMI by about 26.6%. Using the novel biochemical therapeutic index, bariatric surgery decreased HbA1C, serum total cholesterol, serum LDL cholesterol, and serum triglycerides by about 22.99%, 32.42%, 37.18%, and 29.26%, respectively, while serum HDL increased by about 19.4%.
Conclusion: Bariatric surgery is an effective intervention for obese diabetic patients resulting in weight loss, better control of diabetes and hyperlipidemia, and the metabolic profile. It is also recommended in Saudi Arabia for the high prevalence of obesity and diabetes mellitus.
Keywords: bmi, bariatric surgery, lipidemic control, glycemic control, diabetes mellitus
Introduction
Obesity is a globally epidemic disease that affects around 650 million people worldwide, and it is proven that longstanding obesity increases the risk of hepatic, metabolic, and cardiovascular morbidities and related mortality in addition to physical and psychological complications, including type 2 diabetes mellitus [1]. As type 2 diabetes and obesity are closely linked, patients with both diseases present a challenge to their clinicians [2]. Type 2 diabetes and liver disease are strongly correlated with obesity, and liver disease caused by obesity is a frequent reason for liver transplantation. In Saudi Arabia, the prevalence of obesity has risen in recent years. Compared to the global average of 11% for males and 15% for women in 2016, the national statistics for Saudi Arabia indicate that in 2013, 24.1% of Saudi men and 33.5% of Saudi women were obese. Type 2 diabetes is correspondingly prevalent and on the rise in Saudi Arabia; the risk factor for this condition is increased by more than seven times due to obesity. Additionally, non-alcoholic fatty liver disease (NAFLD), which affects over 30% of Middle Easterners and may affect 48% of Saudi Arabians by 2030, is also linked to obesity. Non-alcoholic steatohepatitis (NASH), a more severe form of NAFLD, has been connected to advanced liver fibrosis, cirrhosis, and liver cancer. In Saudi Arabia, NASH is now the most common reason for liver transplants and exceeds the hepatitis C virus. The financial and health costs of type 2 diabetes, liver disease, and liver cancer could be dramatically reduced with effective obesity control policies and lifestyle interventions [3].
In 2021, the global prevalence of diabetes is estimated to be 10.5% among people aged 21-80, with a suspected increase to 12.2% by 2045 [4]. Saudi Arabia has one of the highest rates of chronic diseases globally and is the highest in the Arabian Gulf region; the prevalence of diabetes was found to be 28% between 2016 and 2022 [5]. Epidemiological data indicate that chronic diseases are responsible for 70% of all deaths in Saudi Arabia [6].
Long-term management through anti-hyperglycemic medications and statins, together with lifestyle modifications, is essential to prevent diabetic complications and decrease the development of diabetes-associated sequelae [7]. This point was illustrated by a study conducted among the 50 largest metropolitan areas in the USA that reported that more than 44% of insured diabetic patients who received anti-hyperglycemic drugs were considered to be poorly controlled. In addition, patients with diabetes and obesity had more difficulties maintaining weight loss [8]. Indeed, there is a need for different therapeutic approaches in addition to the traditional tools. Bariatric surgery was found to be more effective in the management of obesity and can promote diabetic remission for patients with difficulty controlling their glycemic level. Bariatric surgery was found to be beneficial to glucose homeostasis through decreasing insulin resistance and increasing insulin secretion [9].
A meta-analysis study revealed that bariatric surgeries were associated with greater weight loss, a better lipid profile, increased remission rates of DM, and improved quality of life when compared with non-surgical management [10]. However, despite these good results, other studies reported that only 15% of treated surgical diabetic patients maintained remission for 10 years [11].
Interestingly, studies reporting the impact of bariatric surgeries on glucose hemostasis are still more extensive and need to be elucidated. Therefore, this study was conducted to explore the association between bariatric surgery and glycemic control among patients with diabetes mellitus.
Materials and methods
Methodology
Ethical Committee Approval
Ethical approval was obtained from the Institutional Review Board (IRB) of the Faculty of Medicine, Al-Rayyan Medical Colleges, Al-Madinah Al-Munawwarah, Saudi Arabia (approval number: HA-03-M-122-008), according to the National Committee of Bioethics (NCBE). Prior to the study, the issue of confirming anonymity was addressed to participants. Confidentiality and privacy were maintained by encoding data and eliminating personally identifiable information.
A Retrospective Study
This retrospective observational study was conducted at King Fahad General Hospital in Al-Madinah Al-Munawwarah, Saudi Arabia. This study aimed at evaluating the clinical and biochemical effects of bariatric surgeries on patients with obesity and type 2 diabetes mellitus for achieving many objectives, e.g., to determine the improvement in body mass index (BMI) of the obese diabetic patients who underwent bariatric surgery and to investigate the association between bariatric surgery and both glycemic and lipidemic control among obese patients with diabetes mellitus. This study also aimed at assessing the changes in glycated hemoglobin among obese diabetic patients who have undergone bariatric surgery.
Data was collected from the patients' medical records at King Fahad General Hospital. Inclusion criteria were all of the following: patients >18 years old who were diagnosed with type 2 diabetes mellitus, having an HbA1C of >6.5% and a BMI of >30 (considered to be obese), and already underwent bariatric surgeries in the previous year. Exclusion criteria were any of the following: those having chronic cardiac disorders, older than 50 years, or having concurrent or recent participation in other studies.
Sample Size
The sample size included the patients who met the inclusion criteria with available clinical data. The sample was calculated, with a total of 61 patients (based also on data availability in light of the inclusion and exclusion criteria). We collected the data from the patients' medical records, including basic demographic characteristics such as age, gender, employment, and social status; BMI before and after surgery; past medical history and duration of diabetes mellitus; and laboratory investigations such as HbA1C before and after surgery.
Clinical Therapeutic Index (CTI)
It is a modification of our novel previously reported "clinical therapeutic index" [12]. CTI is the percentage improvement of a studied clinical parameter (such as BMI) following a medical procedure such as Al-hijamah (prophetic wet cupping therapy) or bariatric surgery. It is calculated using the following formula: CTI=(100×(the clinical parameter prior to bariatric surgery−same clinical parameter after surgery)/the clinical parameter prior to bariatric surgery). CTI is quite helpful to judge the surgical benefits and outcomes.
Biochemical Therapeutic Index (BTI)
It is also a modification of our novel previously reported "clinical therapeutic index" [12]. The novel BTI is the percentage reduction of an investigated biochemical parameter (such as serum total cholesterol, triglycerides, low-density lipoprotein (LDL), and others) or the percentage increase of a useful biochemical parameter (such as serum high-density lipoprotein (HDL)) following bariatric surgery. The formula to compute BTI is as follows: BTI=(100×biochemical parameter prior to bariatric surgery−same biochemical parameter after surgery)/biochemical parameter prior to bariatric surgery). BTI is quite helpful to judge the surgical benefits and outcomes.
Statistical Analysis
The IBM SPSS Statistics for Windows, Version 26.0 (Released 2019; IBM Corp., Armonk, New York, United States) was used in analyzing the data. The data was expressed in tables and graphs using different forms of figures. The qualitative variables were expressed as numbers and percentages, and the quantitative data (descriptive data) was expressed as the mean and standard error of the mean. Paired sample t-test was used to compare the data before and after treatment. The results will be considered highly significant when the significant probability is less than 0.001 (***p-value <0.001). Data was collected, analyzed, and presented as the mean±standard error of the mean using the SPSS software. *** denotes that p<0.001 (utilized as a significance indicator).
Results
A total of 61 patients were enrolled in this study. Regarding the demographic characteristics, the mean age of the studied participants was 35.639±7.882, and the age distribution was mostly in the range of 30-49 years, with 45.9% falling in the 30-39-year age group and 32.79% in the 40-50-year age group. The youngest age group (18-29 years) composed 21.31% of the participants in the study (Table 1).
Table 1. Basic characteristics of the studied patients (n=61).
SD: standard deviation; BMI: body mass index
| Characteristics | Category | Study group (n=61) | |
| No. | % | ||
| Gender | Female | 37 | 60.65 |
| Male | 24 | 39.35 | |
| Age | 18-29 | 13 | 21.31 |
| 30-39 | 28 | 45.9 | |
| 40-50 | 20 | 32.79 | |
| Nationality | Saudi | 51 | 83.6 |
| Non-Saudi | 10 | 16.4 | |
| Marital status | Single | 10 | 16.4 |
| Married | 45 | 73.77 | |
| Divorced | 6 | 9.83 | |
| Widowed | 0 | 0 | |
| Employment | Yes | 44 | 72.13 |
| No | 17 | 27.87 | |
| Special habits | Smoking | 15 | 24.59 |
| Alcohol | 0 | 0 | |
| Age (years) (mean±SD) | 35.639±7.882 | ||
| BMI (kg/m2) (mean±SD) | 45.528±7.791 | ||
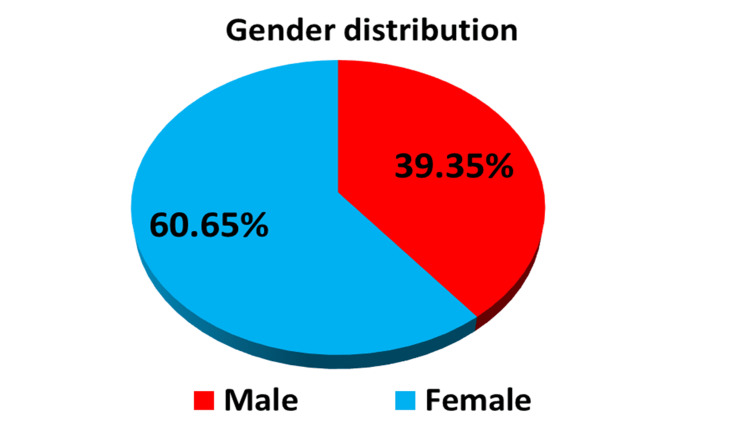
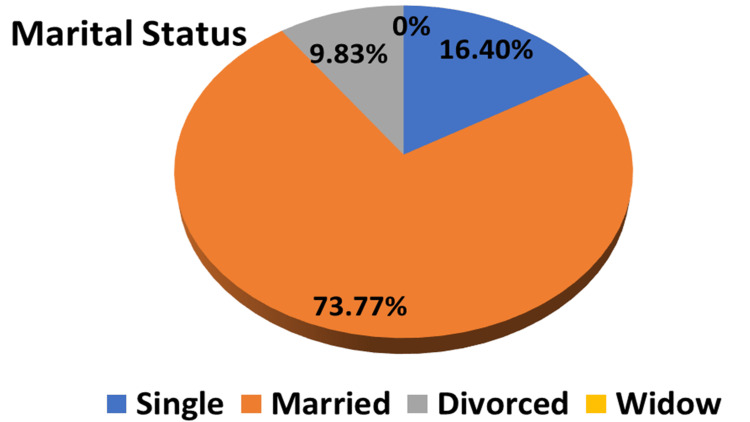
Gender distribution showed that most of the participants were females (60.65%) and 39.35% were males (Table 1 and Figure 1). The majority of participants were Saudis (83.6%) and 16.4% were non-Saudis (Table 1), while in terms of employment, about 72.13% were employed and 27.87% were non-employed (Table 1). Regarding marital status, most of them were married (73.77%), and only 9.83% (n=6) were divorced (Table 1 and Figure 2). 24.59% of the participants were smokers, and no one drank alcohol (Table 1).
Figure 1. A pie chart showing the gender distribution among the studied groups (n=61).
Figure 2. A pie chart showing the marital status distribution among the studied groups (n=61).
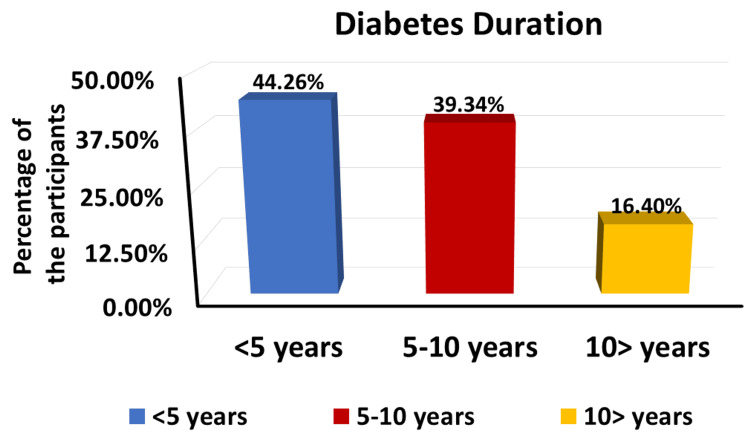
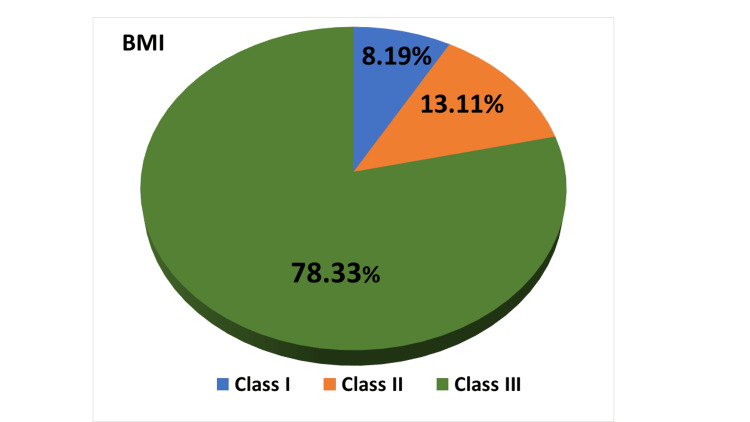
In terms of diabetes duration, the largest group among the participants (44.26%) had diabetes for less than five years, followed by 39.34% who had diabetes for 5-10 years and 16.4% who had diabetes for more than 10 years (Figure 3). The BMI distribution showed that almost half of the participants (78.33%) had a BMI of 40 or greater (obesity class III), followed by 13.11% with a BMI between 35 and 39.9 (obesity class II) and 8.19% with a BMI between 30 and 34.9 (obesity class I). None of the participants had a BMI below 30 (non-obese) (Figure 4).
Figure 3. Diabetes duration among the studied groups (n=61).
Figure 4. A pie chart showing the distribution of BMI among the studied groups (n=61).
BMI: body mass index
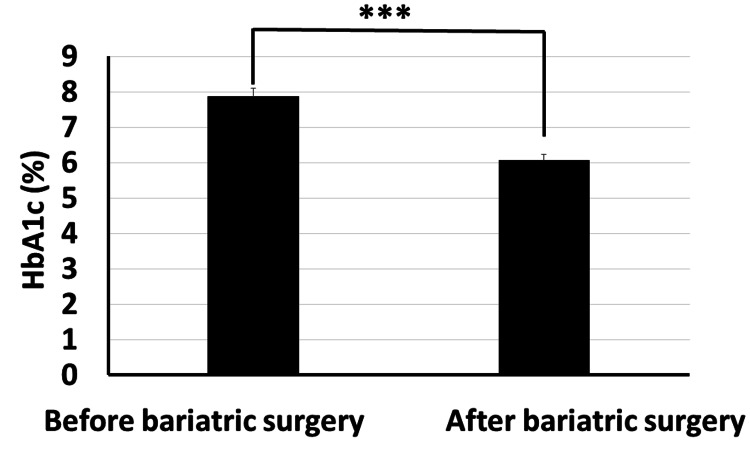
Regarding the metabolic parameters before and after surgery, they included glycemia (HbA1C) and lipidemia (lipid profile) parameters. The results showed statistically significant improvement in HbA1C in patients after surgery, as it significantly decreased from 7.874±1.824% before bariatric surgery to 6.061±1.350% after bariatric surgery with p<0.001 (Figure 5).
Figure 5. Bariatric surgery significantly decreased glycated hemoglobin in obese diabetic patients (p<0.001).
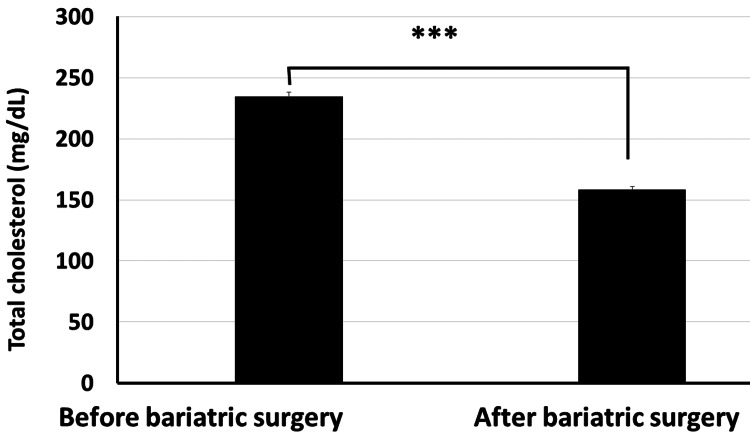
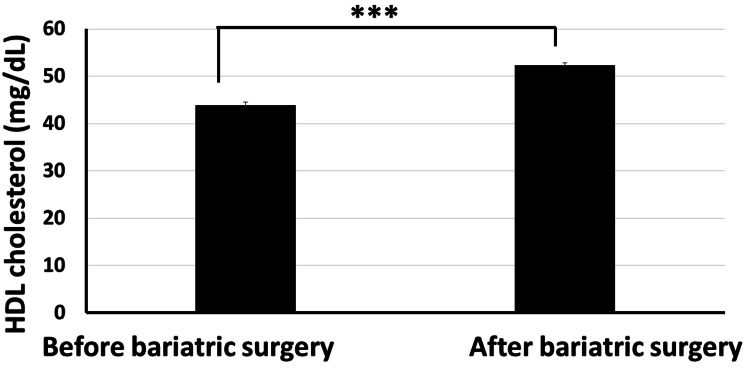
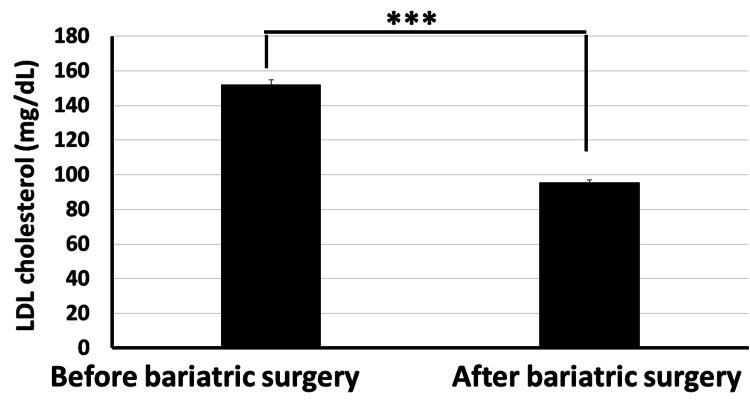
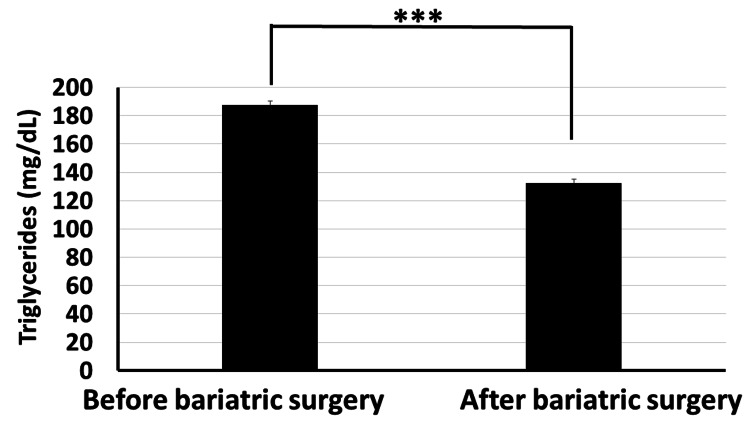
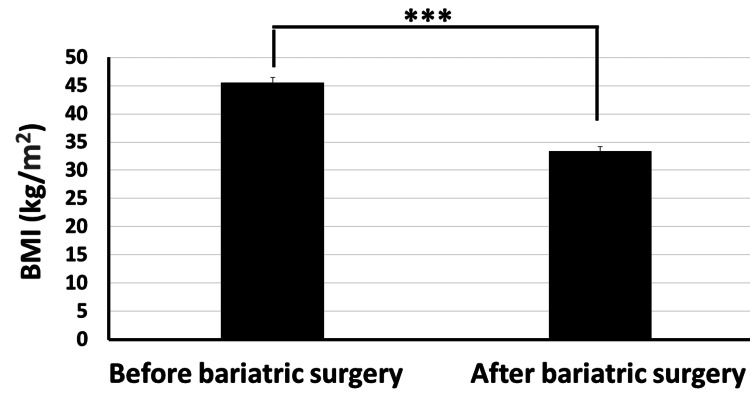
Moreover, our data revealed that the total cholesterol level significantly decreased from 234.4±26.7 mg/dl before bariatric surgery to 158.4±17.3 mg/dl post surgery (Figure 6). The HDL cholesterol level increased significantly from 43.8±6.2 mg/dl before surgery to 52.3±4.6 mg/dl post surgery (p<0.001) (Figure 7). The LDL cholesterol level also showed a significant reduction from 152.2±19.4 mg/dl before surgery to 95.6±15.7 mg/dl post surgery (p<0.001) (Figure 8). Triglyceride levels also significantly decreased, from 187.3±24.6 mg/dl before surgery to 132.5±19.5 mg/dl post surgery (p<0.001) (Figure 9). In addition to these metabolic parameters, BMI showed a statistically significant decrease after surgery, as it decreased from 45.528±7.791 kg/m2 before surgery to 33.416±6.184 kg/m2 post surgery (p<0.000) (Figure 10).
Figure 6. Bariatric surgery significantly decreased the serum total cholesterol in obese diabetic patients (p<0.001).
Figure 7. Bariatric surgery significantly increased the serum HDL cholesterol in obese diabetic patients (p<0.001).
HDL: high-density lipoprotein
Figure 8. Bariatric surgery significantly decreased the serum LDL cholesterol in obese diabetic patients (p<0.001).
LDL: low-density lipoprotein
Figure 9. Bariatric surgery significantly decreased the serum triglycerides in obese diabetic patients (p<0.001).
Figure 10. Bariatric surgery significantly decreased the BMI in obese diabetic patients (p<0.001).
BMI: body mass index
BTI for parameters of both glycemia and lipidemia
As stated in the methodology section, BTI for the significant reductions is calculated as follows: BTI=(100×(biochemical parameter prior to bariatric surgery−same biochemical parameter after surgery)/biochemical parameter prior to bariatric surgery). BTI for HbA1C is calculated as 100×(7.87-6.06)/7.87=22.99%, i.e., glycated hemoglobin decreased by about 23% following bariatric surgery (Figure 5). BTI for serum total cholesterol is calculated as 100×(234.4-158.4)/234.4=32.42%, i.e., serum total cholesterol decreased by about 32.42% following bariatric surgery (Figure 6). BTI for serum HDL cholesterol is calculated as 100×(52.3-43.8)/43.8=19.4%, i.e., serum HDL cholesterol increased by about 19.4% following bariatric surgery (Figure 7). BTI for serum LDL cholesterol is calculated as 100×(152.2-95.6)/152.2=37.18%, i.e., serum LDL cholesterol decreased by about 37.18% following bariatric surgery (Figure 8). BTI for serum triglycerides is calculated as 100×(187.3-132.5)/187.3=29.26%, i.e., serum triglycerides decreased by about 29.26% following bariatric surgery (Figure 9).
CTI
As stated in the methodology section, CTI [12] is calculated as follows: CTI=(100×(the clinical parameter prior to bariatric surgery−same clinical parameter after surgery)/the clinical parameter prior to bariatric surgery). CTI for a reduction in BMI following bariatric surgery is calculated as 100×(45.52-33.41)=26.6%, i.e., BMI decreased by about 26.6% following bariatric surgery (Figure 10).
Discussion
Lifestyle modifications, the use of medication, and using prophetic medicine remedies (alternate fasting, Nigella sativa, costus, natural honey, eating vinegar, and others) [13,14] are all effective ways to prevent and treat type 2 diabetes and other diseases including obesity, but it was shown that bariatric surgery may also reduce the risk of developing type 2 diabetes mellitus in obese people. The main findings of this study were promising in terms of the therapeutic benefits of bariatric surgery on the adequate control of the level of HbA1C and reducing the BMI among obese patients with type 2 diabetes mellitus in a Saudi tertiary health center (King Fahad General Hospital, Al-Madinah Al-Munawwarah, Saudi Arabia). Several types of bariatric surgeries were approved by the American Society for Metabolic and Bariatric Surgery (ASMBS) to maintain weight loss through various mechanisms [15]. Several clinical trials have revealed the efficacy of bariatric surgeries and their sustainability to maintain weight loss in addition to relieving many of the medical comorbidities associated with obesity when compared with other conventional methods [16].
The findings of this study have proved that bariatric surgery may be a vital treatment option for diabetic patients who are obese and who are unable to maintain adequate glycemic control with the use of more conventional techniques, as we observed statistically significant improvement in HbA1C in patients after surgery where it significantly decreased from 7.874±1.824 mg/dl before surgery to 6.061±1.350 mg/dl after surgery (p<0.001). Using the novel CTI, bariatric surgery decreased BMI by about 26.6%. Using the novel BTI, bariatric surgery decreased HbA1C, serum total cholesterol, serum LDL cholesterol, and serum triglycerides by about 22.99%, 32.42%, 37.18%, and 29.26%, respectively, while serum HDL increased by about 19.4%.
Our data is consistent with the report of Courcoulas et al. [17], who reported a 25% reduction in body weight and a remission of the use of antidiabetic drugs after two years of surgery and then no medication after three years. This glycemic control after surgery is attributed to an increased level of glucagon-like peptide-1, which has a role in stimulating insulin secretion and decreasing glucagon and gastric emptying [18]. Another randomized controlled trial in an Italian center showed diabetes remission after five years of follow-up among surgically treated patients compared to medically treated patients [19]. This is in agreement with other studies that reported the remission of type 2 diabetes mellitus and good glycemic control following both sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB) [11,20].
The timing of the application of bariatric surgery during the course of diabetes, which has a benefit-risk ratio, is still debated; however, the International Diabetes Federation suggested the indication for bariatric surgery for severe and less obese patients with poorly controlled type 2 diabetes mellitus not controlled by lifestyle or medical management [21], while other studies reported the efficacy of bariatric surgery in diabetic remission and preventing the progression of micro- and macrovascular complications when the duration of diabetes is short [22]. Other long-term controlled Swedish studies reported a decrease in the incidence of type 2 diabetes mellitus in severely obese patients after bariatric surgery [23].
This explains the significant remission of diabetes among the studied participants, as the largest group (44.26%, n=27) had diabetes for less than five years, followed by 39.34% (n=24) who had diabetes for 5-10 years, which is consistent with the evidence of the application of surgery during the early course of the disease [24].
Regarding the metabolic parameters and adiposity before and after surgery, in this study, BMI showed a statistically significant decrease after surgery, as it decreased from 45.528±7.791 kg/m2 before surgery to 33.416±6.184 kg/m2 post surgery with a p-value of <0.001. Moreover, our results reported a statistically significant reduction in the total cholesterol level, LDL, and triglycerides, in addition to a statistically significant improvement in the HDL level.
Mazidi et al. [25] found a marked effect of bariatric surgery on adiposity and metabolic profile, which was detected by a reduction in BMI and body fat after one year postoperatively, a marked improvement in HDL, and a reduction in cardiovascular risk factors such as total cholesterol level, triglycerides, and LDL level. Other studies reported 20% body weight loss after two and six years postoperatively [26]. This is consistent with Khorgami et al. [27] and Ding et al. [28], who reported a favorable effect of bariatric surgery on the metabolic profile of patients compared to medical management; they proved to be the most effective alternative management of diabetic patients with cardiometabolic disorders. The exact mechanism of bariatric surgery for improving the metabolic profile is still elusive; however, the hypothesis that bariatric surgery increases the intestinal peptides, which play a role in the regulation of energy expenditure and appetite, is considered to be the main cause of weight loss [29]. A systematic review of findings had provided evidence for a substantial and significant improvement in physical and mental health favoring the surgical group compared with controls spanning 5-25 years after surgery [30].
The outcome of this study indicates that bariatric surgery may be a vital therapeutic option for obese diabetic patients who are having difficulty achieving glycemic control with the use of standard techniques. Strength points in this study included the relatively young ages of the investigated sample (<50 years), the introduction of the novel indices (CTI and BTI), and the data on lipid profiles and glycemia in addition to the geographically studied region (western Saudi Arabia). We have to consider some limitations of this study, namely, small sample size, retrospective study design, absence of a control group, and short duration of follow-up, so we cannot correlate the short- and long-term overall effects of bariatric surgeries.
For the authors, we recommend bariatric surgery for treating obese patients who have type 2 diabetes and consider it an alternative therapy for the management of cardiometabolic conditions. We do recommend bariatric surgery for treating obesity and obesity-related metabolic disturbances in Saudi Arabia as insulin-resistant diabetes mellitus.
The recommendations in our study are promising. The prevention of complications seen in obese diabetic patients can be reduced by losing weight through various weight loss models. It is necessary to have randomized cohort studies for the follow-up of obese patients with comorbidities such as diabetes mellitus and cardiovascular diseases, as most of the studies are observational. More studies are needed for long-term follow-up for the detection of the complications of bariatric surgeries regarding nutritional deficiencies and quality of life.
Conclusions
Following bariatric surgery, there were maximal and significant decreases (p<0.001) in BMI and HbA1C, serum triglycerides, serum LDL cholesterol, and serum total cholesterol. It's interesting to note that bariatric surgery significantly increased serum HDL (p<0.001). As bariatric surgery leads to weight loss, improved diabetes control, and an improvement in the metabolic profile, it is regarded as a successful intervention for obese diabetic patients. Given the high rates of diabetes and obesity in Saudi Arabia, surgery may be advised. Our data suggests that bariatric surgeries can achieve better HbA1C control and diabetic remission in obese patients who are having trouble achieving glycemic control through the use of traditional methods. Bariatric surgery is becoming an increasingly essential therapy option for obese patients who have type 2 diabetes and consider it an alternative therapy for the management of cardiometabolic conditions.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research in Taibah University and Al-Rayyan Medical Colleges, Al-Madinah Al-Munawwarah, Saudi Arabia, for kindly supporting the research facilities to achieve that project.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Salah Mohamed El Sayed, Ibrahim Abdel-Rahman, Abdulhamid Awadh Alharbi, Maryam Zain Alsaedi, Noof Mejzi Alamri Alharbi, Sajidah Basheer Al-Mughassil, Zainab Anwar Al-Bahar, Abdel-Raheem Donkol, Hussam Baghdadi, Mariam Eid Alanzi
Acquisition, analysis, or interpretation of data: Salah Mohamed El Sayed, Ibrahim Abdel-Rahman, Abdulhamid Awadh Alharbi, Maryam Zain Alsaedi, Noof Mejzi Alamri Alharbi, Sajidah Basheer Al-Mughassil, Zainab Anwar Al-Bahar, Abdel-Raheem Donkol, Hussam Baghdadi, Mariam Eid Alanzi
Drafting of the manuscript: Salah Mohamed El Sayed, Ibrahim Abdel-Rahman, Abdulhamid Awadh Alharbi, Maryam Zain Alsaedi, Noof Mejzi Alamri Alharbi, Sajidah Basheer Al-Mughassil, Zainab Anwar Al-Bahar, Abdel-Raheem Donkol, Hussam Baghdadi, Mariam Eid Alanzi
Critical review of the manuscript for important intellectual content: Salah Mohamed El Sayed, Ibrahim Abdel-Rahman, Abdulhamid Awadh Alharbi, Maryam Zain Alsaedi, Noof Mejzi Alamri Alharbi, Sajidah Basheer Al-Mughassil, Zainab Anwar Al-Bahar, Abdel-Raheem Donkol, Hussam Baghdadi, Mariam Eid Alanzi
Supervision: Salah Mohamed El Sayed, Ibrahim Abdel-Rahman, Abdulhamid Awadh Alharbi, Maryam Zain Alsaedi, Noof Mejzi Alamri Alharbi, Sajidah Basheer Al-Mughassil, Zainab Anwar Al-Bahar, Abdel-Raheem Donkol, Hussam Baghdadi, Mariam Eid Alanzi
Human Ethics
Consent was obtained or waived by all participants in this study. Institutional Review Board (IRB) of the Faculty of Medicine, Al-Rayyan Medical Colleges issued approval HA-03-M-122-008
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Obesity as a multisystem disease: trends in obesity rates and obesity-related complications. Sarma S, Sockalingam S, Dash S. Diabetes Obes Metab. 2021;23:3–16. doi: 10.1111/dom.14290. [DOI] [PubMed] [Google Scholar]
- 2.Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Obesity (Silver Spring) 2021;29:1950–1960. doi: 10.1002/oby.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.How could different obesity scenarios alter the burden of type 2 diabetes and liver disease in Saudi Arabia? Coker T, Saxton J, Retat L, et al. Obes Facts. 2023;16:559–566. doi: 10.1159/000533301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Sun H, Saeedi P, Karuranga S, et al. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevalence of type 2 diabetes mellitus and related factors among the general adult population in Saudi Arabia between 2016-2022: a systematic review and meta-analysis of the cross-sectional studies. Alwadeai KS, Alhammad SA. Medicine (Baltimore) 2023;102:0. doi: 10.1097/MD.0000000000034021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perioperative outcomes of Roux-en-Y gastric bypass and sleeve gastrectomy in patients with diabetes mellitus: an analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database. Leonard-Murali S, Nasser H, Ivanics T, Shakaroun D, Genaw J. Obes Surg. 2019;30:111–118. doi: 10.1007/s11695-019-04175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statin treatment-induced development of type 2 diabetes: from clinical evidence to mechanistic insights. Galicia-Garcia U, Jebari S, Larrea-Sebal A, et al. Int J Mol Sci. 2020;21:4725. doi: 10.3390/ijms21134725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes diagnosis and management among insured adults across metropolitan areas in the U.S. Yang W, Dall TM, Tan E, Byrne E, Iacobucci W, Chakrabarti R, Ellen Loh F. Prev Med Rep. 2018;10:227–233. doi: 10.1016/j.pmedr.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Davies MJ, D'Alessio DA, Fradkin J, et al. Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Gloy VL, Briel M, Bhatt DL, et al. Br Med J. 2013;347:0. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Mingrone G, Panunzi S, De Gaetano A, et al. https://pubmed.ncbi.nlm.nih.gov/26369473/ Lancet. 2021;397:293–304. doi: 10.1016/S0140-6736(20)32649-0. [DOI] [PubMed] [Google Scholar]
- 12.Therapeutic benefits of Al-hijamah: in light of modern medicine and prophetic medicine. El Sayed SM, Al-quliti AS, Mahmoud HS, Baghdadi H, Maria RA, Nabo MM, Hefny A. https://pubs.sciepub.com/ajmbr/2/2/3/index.html Am J Med Biol Res. 2014;2:46–71. [Google Scholar]
- 13.Al-hijamah (prophetic wet cupping therapy) is a novel adjuvant treatment for viral hepatitis that excretes viral particles and excess ferritin percutaneously, synergizes pharmacotherapy, enhances antiviral immunity and helps better HCC prevention and treatment: a novel evidence-based combination with prophetic medicine remedies. El Sayed SM. J Hepatocell Carcinoma. 2023;10:1527–1546. doi: 10.2147/JHC.S409526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natural remedies of prophetic medicine are promising in the management of viral hepatitis: towards better preventive and therapeutic outcomes (a review article) El Sayed SM. Am J Clin Med. 2023;11:14–21. [Google Scholar]
- 15.Bariatric surgery for the management of type 2 diabetes mellitus-current trends and challenges: a review article. Alqunai MS, Alrashid FF. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8902546/ Am J Transl Res. 2022;14:1160–1171. [PMC free article] [PubMed] [Google Scholar]
- 16.The long-term effects of feeding honey compared with sucrose and a sugar-free diet on weight gain, lipid profiles, and DEXA measurements in rats. Chepulis L, Starkey N. J Food Sci. 2008;73:0–7. doi: 10.1111/j.1750-3841.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 17.Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. Courcoulas AP, Belle SH, Neiberg RH, et al. JAMA Surg. 2015;150:931–940. doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Christiansen CB, Trammell SA, Wewer Albrechtsen NJ, et al. Am J Physiol Gastrointest Liver Physiol. 2019;316:0–84. doi: 10.1152/ajpgi.00010.2019. [DOI] [PubMed] [Google Scholar]
- 19.Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Mingrone G, Panunzi S, De Gaetano A, et al. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 20.Long-term impact of bariatric surgery in diabetic nephropathy. Young L, Nor Hanipah Z, Brethauer SA, Schauer PR, Aminian A. Surg Endosc. 2019;33:1654–1660. doi: 10.1007/s00464-018-6458-8. [DOI] [PubMed] [Google Scholar]
- 21.Bariatric surgery: an IDF statement for obese type 2 diabetes. Dixon JB, Zimmet P, Alberti KG, Rubino F. Diabet Med. 2011;28:628–642. doi: 10.1111/j.1464-5491.2011.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bariatric surgery and microvascular complications of type 2 diabetes mellitus. Jackson S, le Roux CW, Docherty NG. Curr Atheroscler Rep. 2014;16:453. doi: 10.1007/s11883-014-0453-x. [DOI] [PubMed] [Google Scholar]
- 23.Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. Sjöström L. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 24.Timing of bariatric surgery in people with obesity and diabetes. Busetto L. Ann Transl Med. 2015;3:94. doi: 10.3978/j.issn.2305-5839.2015.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effect of bariatric surgery on adiposity and metabolic profiles: a prospective cohort study in Middle-Eastern patients. Mazidi M, Rezaie P, Jangjoo A, Tavassoli A, Rajabi MT, Kengne AP, Nematy M. World J Diabetes. 2017;8:374–380. doi: 10.4239/wjd.v8.i7.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Health benefits of gastric bypass surgery after 6 years. Adams TD, Davidson LE, Litwin SE, et al. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Outcomes of bariatric surgery versus medical management for type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Khorgami Z, Shoar S, Saber AA, Howard CA, Danaei G, Sclabas GM. Obes Surg. 2019;29:964–974. doi: 10.1007/s11695-018-3552-x. [DOI] [PubMed] [Google Scholar]
- 28.Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. Ding L, Fan Y, Li H, et al. Obes Rev. 2020;21:0. doi: 10.1111/obr.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechanisms for the metabolic success of bariatric surgery. Sandoval DA. J Neuroendocrinol. 2019;31:0. doi: 10.1111/jne.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mechanisms of weight loss and improved metabolism following bariatric surgery. Mulla CM, Middelbeek RJ, Patti ME. Ann N Y Acad Sci. 2018;1411:53–64. doi: 10.1111/nyas.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]