Abstract
Background/Aim
Single-agent chemotherapy typically has curative outcomes in patients with low-risk gestational trophoblastic neoplasia (GTN). Although surgical intervention is a potential alternative, its efficacy in these patients remains unclear. This report describes a case in which surgical excision of a uterine polypoid lesion resolved chemotherapy-resistant low-risk GTN.
Case Report
A 43-year-old patient received pulse actinomycin D treatment for post-molar low-risk GTN without extrauterine metastasis. However, the patient showed resistance to the chemotherapy regimen. There was no initial evidence of protrusion of GTN into the uterine cavity; however, a polypoid lesion grew into the uterine cavity during therapy. This growth was successfully excised via a transvaginal approach using forceps with minimal blood loss. There was a postoperative decrease in human chorionic gonadotropin levels, which ultimately reached the predetermined threshold without the need for changing the therapeutic protocol.
Conclusion
Surgical resection should be considered a viable therapeutic strategy for uterine polypoid growth in chemotherapy-resistant low-risk GTN.
Keywords: Chemotherapy, drug resistance, gestational trophoblastic neoplasia, surgery
Gestational trophoblastic neoplasia (GTN) is characterized by neoplasms that present trophoblastic proliferation. Moreover, it comprises a spectrum of pathological status, including invasive moles, choriocarcinomas, placental site trophoblastic tumors, and epithelioid trophoblastic tumors. Notably, approximately 15% of complete hydatidiform moles and 1-4% of partial hydatidiform moles may progress to GTN (1,2). Most post-molar GTNs are categorized as low-risk GTN based on the International Federation of Gynecology and Obstetrics (FIGO) 2000 scoring system (3).
Low-risk GTNs generally exhibit a high degree of chemosensitivity. Monotherapy with chemotherapeutic agents, including methotrexate or actinomycin D, is the primary intervention for low-risk GTN (4,5). However, 10-40% of these patients may require changes in the treatment protocol due to the emergence of drug resistance or adverse reactions (6-8). Nonetheless, almost all patients with low-risk GTN can be successfully treated with chemotherapy even though 5-30% of these patients require a combination of chemotherapeutic agents to achieve remission (9-12).
Surgical intervention is another effective therapeutic modality for GTN. Osborne and colleagues reported that secondary curettage alone yielded a cure rate of 40% in patients with nonmetastatic low-risk GTN (13). Additionally, hysterectomy has been shown to yield an 82% cure rate in patients with nonmetastatic low-risk GTN without the need for adjunctive chemotherapy (14). However, hysterectomy may not be preferred by individuals who want to preserve their fertility.
This report describes a case in which surgical excision of a uterine polypoid lesion was effective in treating chemotherapy-resistant low-risk GTN.
Case Report
A 43-year-old woman presented to our hospital with a suspected molar pregnancy. Her preoperative serum human chorionic gonadotropin (hCG) level was extremely high (662,869 mIU/ml). She underwent dilation and evacuation through vacuum aspiration (Figure 1) (15). Histopathological evaluation revealed a complete hydatidiform mole with negative p57KIP2 staining. Molecular genotyping via short tandem repeat polymorphism analysis indicated an androgenetic diploid genotype (2), which was consistent with the histopathological diagnosis.
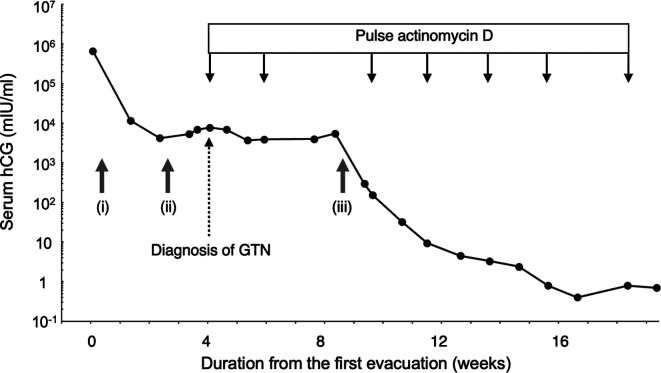
Figure 1. Clinical course. The clinical course showed a trend in serum Human chorionic gonadotropin (hCG) levels. (i) First evacuation, (ii) second evacuation, and (iii) third evacuation. GTN: Gestational trophoblastic neoplasia.
After two weeks, a second dilation and curettage was needed due to persistent molar gestational tissue within the uterine cavity (Figure 2A). Small amount of tissue was retrieved. The pathological examination confirmed residual molar villi and trophoblasts.
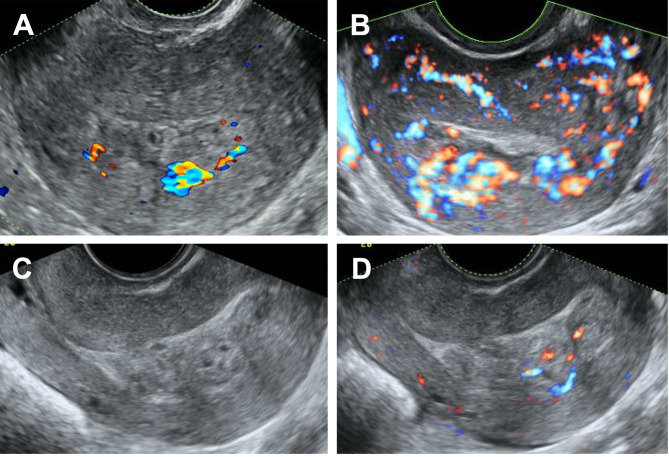
Figure 2. Transvaginal sonographic images. Images were obtained (A) before the second dilatation and evacuation, (B) upon diagnosis of gestational trophoblastic neoplasia, and (C) and (D) at the 4th week of actinomycin D administration (C: normal image, D: color Doppler image).
Her serum hCG levels continued to increase after two weeks of the second surgery to 7,759 mIU/ml. Transvaginal ultrasonography revealed a hypervascular myometrial lesion (Figure 2B). Accordingly, the patient was diagnosed with low-risk GTN with a risk score of 2 and stage I based on the FIGO 2000 system, which indicated the absence of extrauterine metastasis (3). The patient was started on a biweekly pulse actinomycin D regimen (1.25 mg/m²). However, serum hCG levels plateaued even after two chemotherapy cycles. At the time of GTN diagnosis, ultrasonography showed an empty uterine cavity (Figure 2B), which was subsequently occupied by an isoechoic, heterogeneous mass concurrent with the plateau in hCG levels (Figure 2C and D).
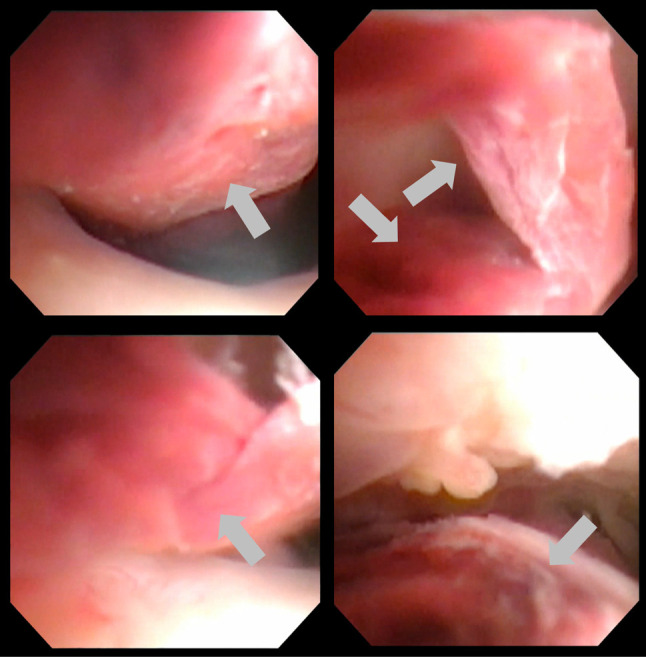
Hysteroscopic evaluation revealed a long polypoid tumor originating from the uterine fundus, which resembled an invasive mole with morphological changes (Figure 3). Given the pedunculated nature of the tumor and anticipated successful resection, we decided to attempt transvaginal excision before altering the treatment regimen.
Figure 3. Hysteroscopic images of the polypoid lesion. Grey arrows show the polypoid lesion.
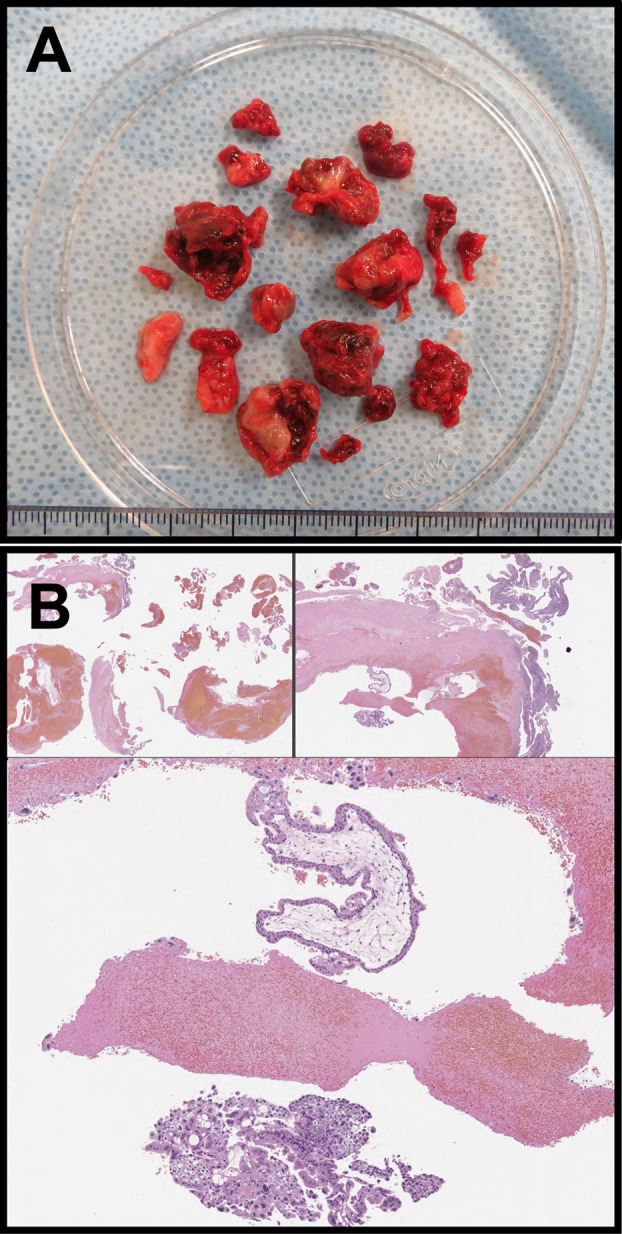
The patient was hospitalized for cervical dilation using three laminaria tents. Under intravenous anesthesia and abdominal sonographic guidance, the polypoid tumor was excised using forceps in a 16-min procedure with minimal blood loss. Subsequently, the patient was discharged on the same day. Macroscopic analysis of the excised tissue revealed a fragmented mass (Figure 4A). Microscopic evaluation revealed extravillous trophoblasts in the degenerated and necrotic tissues (Figure 4B).
Figure 4. Pathological findings of specimens removed during the third evacuation. (A) Macroscopic findings of the contents removed using forceps; (B) pathological findings of the polypoid products.
Following a third curettage, there was a significant decrease in serum hCG levels. Administration of intravenously pulse actinomycin D was continued, with serum hCG levels reaching the predetermined threshold of <1.0 mIU/ml at the start of the sixth treatment cycle. The treatment regimen was stopped after seven cycles (Figure 1). Subsequent follow-up hysteroscopy revealed no residual tumors or adhesions within the uterine cavity. The patient has remained disease-free for four years. Informed consent for the publication of this case report was obtained from the patient.
Discussion
Our findings show that excision of a intrauterine polypoid growth of low-risk GTN can be a viable treatment alternative for chemotherapy-resistant low-risk GTN. The excision would decrease the number of chemotherapy cycles or prevent having to switch to a multiple-agent regimen.
There have been few reports of polypoid lesions growing in patients with low-risk GTN undergoing chemotherapy. However, a similar case involving a placental polyp arising from an exaggerated implantation site has been reported (16). A placental polyp is a remnant of conception, which results in the development of pedunculated polypoid tissues within the uterine cavity (17). Its histological characteristics include villous, decidua, thrombotic masses, degenerative and regenerative tissues, and fibrous accretion. Our findings demonstrated that residual villous tissues or extravillous trophoblasts at the implantation site of hydatidiform moles may proliferate given the similar pathophysiology of placental polyps.
The therapeutic efficacy of dilation and evacuation for the management of low-risk GTN has been demonstrated. Osborne et al. observed a 40% remission rate in patients with nonmetastatic low-risk GTN after secondary curettage interventions (13). Unlike this previous study, curettage at GTN diagnosis in our case was not curative. The unfavorable hCG course could be attributed to the intrauterine residual molar tissues or trophoblasts, which had been removed by the second curettage in the previous effective cases. In our case, although the pathogenic trophoblasts did not protrude into the uterine cavity at the time of GTN diagnosis, they proliferated and formed a polypoid tumor during chemotherapy. It is difficult to distinguish between noninvasive and invasive residual molar tissues since pathological examination by hysterectomy is not usually performed to preserve fertility.
Notably, removal of polypoid lesions with myometrial hypervascularity involves a risk of massive bleeding. In our case, there was no significantly enhanced vascularity toward the tumor, and there was minimal intraoperative blood loss. However, trophoblasts can invade the uterine spiral artery to form physiological arteriovenous fistulas. Placental polyps often present myometrial hypervascularity with the accompanying risk of uterine bleeding. In the case of massive hemorrhage, some cases can be treated with invasive procedures such as hysterectomy or uterine artery embolization (18). Dilation and curettage can cause massive hemorrhage in cases of increased myofascial blood flow. We previously reported that cervical ripening can be safely performed using the maximal laminaria technique (19). A similar procedure was performed in this patient. Although the myometrial blood flow was not reduced, the polypoid tumor was safely removed using the transvaginal technique. The use of a Foley’s intrauterine catheter has been shown to effectively control bleeding after the removal of placental polyps (17).
The observed chemoresistance in our case could be attributed to resistance to actinomycin D, ancillary factors such as the pharmacokinetic parameters of drug delivery, or the tumorous condition encompassing hypoxia. In cases where resistance emanates from the first reason, tumor resection alone would be insufficient for a curative effect since the chemotherapy-resistant myometrial lesion would persist and thus require a change in chemotherapeutic regimen. However, remission was achieved in our patient, which indicates the involvement of the drug delivery apparatus or the tumorous condition such as hypoxia in the observed chemoresistance.
In conclusion, we report a case of post-molar low-risk GTN showing polypoid proliferation throughout the course of chemotherapy and plateau of hCG levels. The intracavitary region of the polypoid lesion was safely removed, which helped avoid alteration of the chemotherapeutic regimen. Therefore, the removal of a uterine polypoid tumor can be a viable treatment option for low-risk chemotherapy-resistant GTN.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Asuka Sato: Conceptualization; data curation; formal analysis; funding acquisition; writing – original draft; writing – review and editing. Hirokazu Usui: Conceptualization; data curation, formal analysis, funding acquisition, project administration, supervision; writing the original draft, writing the review, and editing. Natsuko Nakamura: Data curation; writing – review & editing. Eri Katayama: Data curation; writing – review & editing. Makio Shozu: Supervision; writing – review & editing. Kaori Koga: Conceptualization; supervision; writing - review & editing.
Acknowledgements
The Authors are grateful to the staff of the Department of Obstetrics and Gynecology, Chiba University Hospital, Chiba, Japan, for treating the patients.
Funding
This study was partially supported by JSPS KAKENHI (grant numbers JP17K16831 [AS], JP18K09281, JP21K19551, and JP22H03220 [HU]). The funding source had no role in the study design; collection, analysis, or interpretation of the data; writing of the report; or in the decision to submit the article for publication.
References
- 1.Albright BB, Shorter JM, Mastroyannis SA, Ko EM, Schreiber CA, Sonalkar S. Gestational trophoblastic neoplasia after human chorionic gonadotropin normalization following molar pregnancy: a systematic review and meta-analysis. Obstet Gynecol. 2020;135(1):12–23. doi: 10.1097/AOG.0000000000003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usui H, Qu J, Sato A, Pan Z, Mitsuhashi A, Matsui H, Shozu M. Gestational trophoblastic neoplasia from genetically confirmed hydatidiform moles: prospective observational cohort study. Int J Gynecol Cancer. 2018;28(9):1772–1780. doi: 10.1097/IGC.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 3.FIGO Oncology Committee FIGO staging for gestational trophoblastic neoplasia 2000. Int J Gynaecol Obstet. 2002;77(3):285–287. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie TA, Alazzam M, Tidy J, Hancock BW, Osborne R. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev. 2016;2016(6):CD007102. doi: 10.1002/14651858.CD007102.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok C, van Trommel N, Massuger L, Golfier F, Seckl M, Clinical Working Party of the EOTTD Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228–240. doi: 10.1016/j.ejca.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Yarandi F, Eftekhar Z, Shojaei H, Kanani S, Sharifi A, Hanjani P. Pulse methotrexate versus pulse actinomycin D in the treatment of low-risk gestational trophoblastic neoplasia. Int J Gynaecol Obstet. 2008;103(1):33–37. doi: 10.1016/j.ijgo.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Kizaki S, Hashimoto K, Matsui H, Usui H, Shozu M. Comparison of 5-day MTX and 5-day ETP treatment results and early predictors of drug resistance to 5-day MTX in patients with post-molar low-risk gestational trophoblastic neoplasia. Gynecol Oncol. 2015;139(3):429–432. doi: 10.1016/j.ygyno.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Maestá I, Nitecki R, Horowitz NS, Goldstein DP, de Freitas Segalla Moreira M, Elias KM, Berkowitz RS. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: The New England trophoblastic disease center experience. Gynecol Oncol. 2018;148(1):161–167. doi: 10.1016/j.ygyno.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 9.McNeish IA, Strickland S, Holden L, Rustin GJ, Foskett M, Seckl MJ, Newlands ES. Low-risk persistent gestational trophoblastic disease: Outcome after initial treatment with low-dose methotrexate and folinic acid from 1992 to 2000. J Clin Oncol. 2002;20(7):1838–1844. doi: 10.1200/JCO.2002.07.166. [DOI] [PubMed] [Google Scholar]
- 10.Sita-Lumsden A, Short D, Lindsay I, Sebire NJ, Adjogatse D, Seckl MJ, Savage PM. Treatment outcomes for 618 women with gestational trophoblastic tumours following a molar pregnancy at the Charing Cross Hospital, 2000-2009. Br J Cancer. 2012;107(11):1810–1814. doi: 10.1038/bjc.2012.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Qin J, Shen T, Fei W, Chen L, Xie X, Lu W. The 16-year experience in treating low-risk gestational trophoblastic neoplasia patients with failed primary methotrexate chemotherapy. J Gynecol Oncol. 2020;31(4):e36. doi: 10.3802/jgo.2020.31.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadducci A, Cosio S, Fanucchi A, Tana R, Manacorda S, Pistolesi S, Strigini FL. Prognosis of patients with gestational trophoblastic neoplasia and obstetric outcomes of those conceiving after chemotherapy. Anticancer Res. 2016;36(7):3477–3482. [PubMed] [Google Scholar]
- 13.Osborne RJ, Filiaci VL, Schink JC, Mannel RS, Behbakht K, Hoffman JS, Spirtos NM, Chan JK, Tidy JA, Miller DS. Second curettage for low-risk nonmetastatic gestational trophoblastic neoplasia. Obstet Gynecol. 2016;128(3):535–542. doi: 10.1097/AOG.0000000000001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolze PA, Mathe M, Hajri T, You B, Dabi Y, Schott AM, Patrier S, Massardier J, Golfier F. First-line hysterectomy for women with low-risk non-metastatic gestational trophoblastic neoplasia no longer wishing to conceive. Gynecol Oncol. 2018;150(2):282–287. doi: 10.1016/j.ygyno.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Sato A, Usui H, Shozu M. Comparison between vacuum aspiration and forceps plus blunt curettage for the evacuation of complete hydatidiform moles. Taiwan J Obstet Gynecol. 2019;58(5):650–655. doi: 10.1016/j.tjog.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Harada N, Nobuhara I, Haruta N, Kajimoto M. A placental polyp arising from an exaggerated placental site. J Obstet Gynaecol Res. 2011;37(8):1154–1157. doi: 10.1111/j.1447-0756.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 17.Alhussami R, Noorwali F, Ibrahim G. A rare medical dilemma: Presentation and management of placental polyp. Cureus. 2020;12(12):e12259. doi: 10.7759/cureus.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara T, Kanasaki H, Oride A, Hara T, Kyo S. Differential diagnosis and management of placental polyp and uterine arteriovenous malformation: Case reports and review of the literature. Womens Health (Lond) 2016;12(6):538–543. doi: 10.1177/1745505717692590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usui H, Sato A, Okayama J, Suzuki Y, Omoto A, Shozu M. Removal of retained products of conception showing marked vascularity without uterine artery embolization: Two case reports. J Obstet Gynaecol Res. 2018;44(8):1482–1486. doi: 10.1111/jog.13678. [DOI] [PubMed] [Google Scholar]