Abstract
Background
Pseudomyxoma peritonei (PMP) is a clinical entity of subtle onset abdominal pain, ascites, and distention associated with characteristic imaging. In most cases, laparoscopic exploration will give the definitive diagnosis and histopathologic verification. However, usually there are difficulties in the diagnosis of this disease.
Case Report
Herein, we present a case of a 51-year-old female who developed ascites over 5 months. An investigational laparotomy established the diagnosis of PMP, after the discovery of a mucinous, grey-brown tumor that was CK20 positive and CK7 negative. Subsequently, chemotherapy with oxaliplatin combined with 5-FU (FOLFOX4 regimen), was initiated and the patient survived for 30 months. We also present a comprehensive review of the English literature concerning the different symptoms and radiological findings of this rare entity. According to the literature review, 35 cases of PMP with different clinical and radiological findings have been described. In the majority of the cases, ultrasound, computed tomography or magnetic resonance imaging was orientating towards a proper diagnosis before a diagnostic laparotomy.
Conclusion
The combination of a clinical picture with the characteristic imaging findings enables a prompt diagnosis of PMP, making prognosis more favorable.
Keywords: Pseudomyxoma peritonei (PMP), diagnosis, presenting symptoms, imaging findings, laparotomy
Pseudomyxoma peritonei (PMP) is a rare disorder within the abdomen clinically presented with abdominal pain and mass, fatigue, and weight loss. It is characterized by progressive accumulation of mucin in the peritoneal cavity and the presence of gelatinous ascites and peritoneal implants. Due to the fact that the symptoms are not specific, it is usually incidentally discovered during surgery for other reasons (1).
PMP is commonly associated with malignant tumors in the appendix, but there is a controversy regarding the epithelium from which it originates (2,3). Theories of ovaries being an alternative primary site of PMP origin are considered to be convincing, although not fully explained when it comes to immunochemistry. The term is still being applied for a wide range of diseases and some oncologists and pathologists apply PMP to any condition with gelatinous material in the abdomen and pelvis (4).
The disease is three to four times more common in females than males and its incidence estimated at 1-2 per million per year (5,6). This case report presents the challenging clinical diagnosis of a PMP and the intriguing effect that it had on the choice of treatment.
Case Report
A 51-year-old female presented to the emergency department with a five month history of gradually deteriorating abdominal pain, abdominal distension and ascites. The clinical examination did not reveal any palpable masses of the abdomen beyond marked ascites. The computed tomography (CT) scan of the upper and lower abdomen revealed an ovary enlargement and ascites. Serum tumor markers were also measured to be as follows: CEA=312.9 ng/ml, CA125=72.9 IU/ml, CA19-9=520.6 ng/ml.
The patient underwent an investigational laparotomy, and a tumor was found in the right iliac fossa, infiltrating the right ovary, right fallopian tube, and omentum. A second tumor was found in the left ovary as well.
An extrafascial total abdominal hysterectomy along with bilateral salpingo-oophorectomy and resection of the infracolic omentum was performed. Macroscopically, the right iliac tumor was described as having a mucinous, cystic form (5×5×3cm) with an irregular inner surface and a multilobar, mucinous, grey-brown outer surface. The second tumor sallied out from the left ovary, laterally, towards the fallopian interspace, infiltrating almost the entire left fallopian tube. It was a multilobar, mucinous, grey-brown tumor (3×3×2.5cm). The histopathology and immunohistochemistry report (CK20 positive and CK7 negative) confirmed the diagnosis of a mucinous adenocarcinoma, in the context of PMP.
Although the extensive sampling of the surgical specimen did not reveal histological evidence of appendix residue (CK20 positive rather than CK7) and elevated CEA, the patient was treated with oxaliplatin combined with 5-FU (FOLFOX4 regimen), as first line treatment for stage IV colorectal cancer. The re-evaluation after six cycles showed virtually stable disease and it was decided that she should continue with second line chemotherapy, using the combination of irinotecan and 5-FU (FOLFIRI regimen).
Three months later, the patient was stable and underwent plastic surgery for omphalocele and white line hernia. The pathology report showed infiltration with mucinous cystadenocarcinoma, with extracellular and intracellular mucin production (signet-ring cells). Palliative treatment was offered, however, the patient died 30 months after the primary diagnosis.
Review of the literature. We searched MEDLINE for papers using the following key words: “pseudomyxoma peritonei”, “cancer”, “malignancy”, “presenting symptoms” and “laparatomy”. Reference lists in the retrieved papers were checked to identify any other published data. Cases were included if there was both clinical and histological evidence of PMP. Only papers written in the English language and cases with patients older than 14 years were included in the analysis. Data regarding the primary tumor, age, sex, presenting symptoms, and the clinical and diagnostic work up were analyzed.
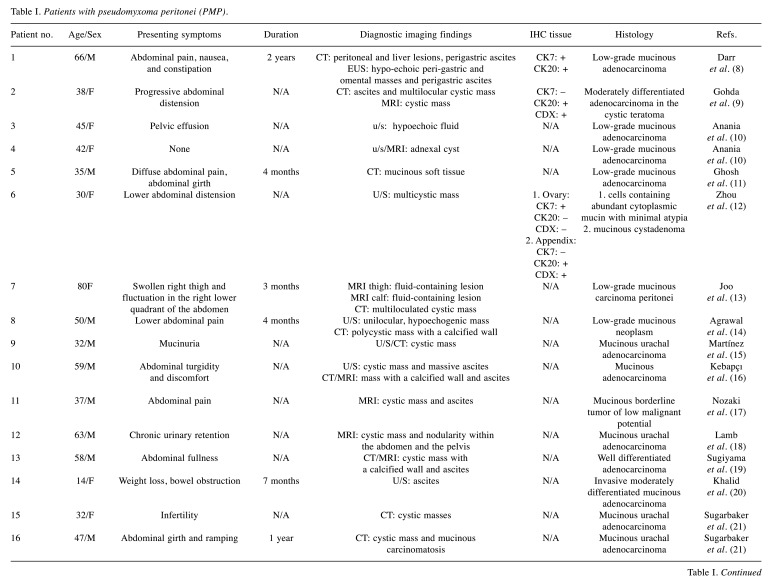
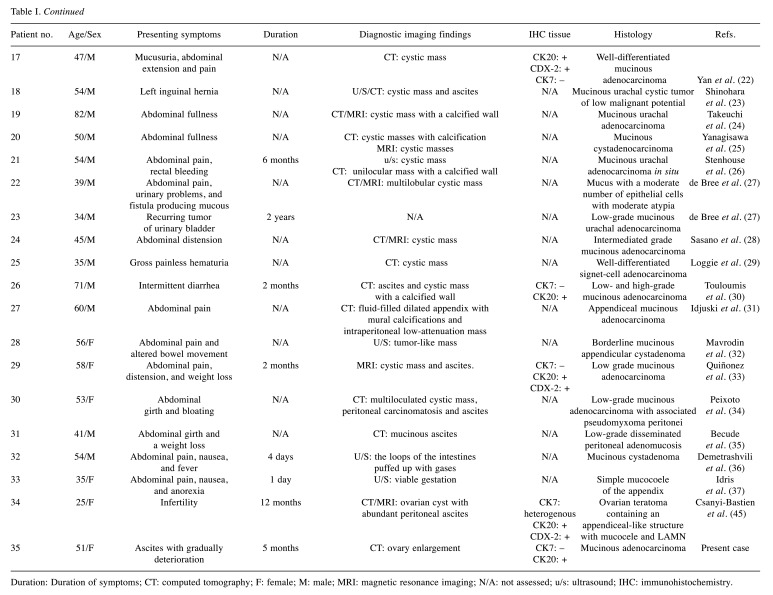
Table I summarizes the 35 cases, including ours, that were identified in the literature review for patients experiencing symptoms of “pseudomyxoma peritonei”, presented with a variety of clinical pictures, stages at time of surgery, and histologic types.
Table I. Patients with pseudomyxoma peritonei (PMP).
Duration: Duration of symptoms; CT: computed tomography; F: female; M: male; MRI: magnetic resonance imaging; N/A: not assessed; u/s: ultrasound; IHC: immunohistochemistry.
Discussion
This report describes the clinical experience of managing a rare PMP case. The correct diagnosis of the disease is considered of high importance since the discovery of the origin indicates the appropriate treatment regimen. It is emphasized that even with no histological evidence of appendix residue; we must consider the primary origin as colorectal cancer (7). In this case, the immunohistochemistry findings (positive CK20 and negative CK7) and elevated tumor markers (CA19-9 and CEA) strongly supported the colorectal origin of this PMP. What merits a particular mention is the classification that pathologists have compiled for PMP subtypes, with different prognosis for each one: disseminated peritoneal adenomucinosis (DPAM), peritoneal mucinous carcinomatosis (PMCA), and an intermediate PMCA type (PMCA-1) (7).
PMP is a slowly progressive disease that can be presented with a variety of symptoms. Usually, PMP exhibits the characteristic “jelly belly”, as the amount of mucoid fluid fills the peritoneal cavity. Symptoms that have been described are abdominal pain, nausea, vomiting, fatigue, and urinary abnormalities. It can also be presented with local symptomatology, mirroring the location of the primary or metastatic tumor, like appendicitis (38).
In the majority of cases as we can see in Table I, the diagnosis was made only by CT (11 cases) with the assistance of ultrasound (u/s) or magnetic resonance imaging (MRI), followed by histopathologic verification of an extensively sampled tumor. In six cases, only u/s was performed (10,12,20,32,36,37), but in two of them (36,37), u/s misled the diagnosis, resulting in delayed treatment. Thus, CT or MRI would be probably the most appropriate imaging option, offering accurate and prompt diagnosis. Ultrasound usually describes cystic masses and mucinous substance as retroperitoneal fluid. CT and MRI will differentiate the fluid (watery vs. mucinous) and confirm the cystic nature of the masses.
Another important aspect for the diagnosis and follow-up of PMP patients is the assessment of serum tumor markers CEA and CA19-9. CEA has been reported to be increased in 56% to 75% of patients and CA19-9 in 58% to 67% respectively, whilst the baseline tumor marker values seem to be related to the extent of tumor and completeness of resection (39,40).
Analysis of immunohistochemical markers' expression, such as cytokeratin (CK) 7, 20, CEA, and CDX-2 is very useful in characterizing the origin of tumors. Positive expression of CK20, CEA, and CDX-2 imply primary colorectal or appendiceal origin while CK7 and CA125 indicate a primary ovarian origin (41).
There is currently no consensus regarding the optimal treatment for progressive PMP. Cytoreductive surgery, with or without hyperthermic intraperitoneal chemotherapy (HIPEC), despite possible concomitant morbidity is considered the proper treatment (42). Although several approaches have been described so far, the radical removal of all intra-abdominal and pelvic disease and the administration of intraperitoneal heated chemotherapy (mitomycin, 5-FU) have been adopted by most clinicians (43,44).
Conclusion
To conclude, the reported symptoms for PMP vary, the clinical course is vague, and there is no certain pathognomic signs that can lead to the prompt diagnosis and management of this clinical entity. Therefore, clinicians should demonstrate high index of clinical suspicion, as to discover this medical entity without delay.
Conflicts of Interest
No potential conflicts of interest exist in relation to this study.
Authors’ Contributions
All Authors equally contributed to this study regarding the conception and design of the study, literature review and analysis, drafting, critical revision, editing, and final approval of the final version.
References
- 1.Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44–50. doi: 10.4251/wjgo.v2.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakakura EK. Pseudomyxoma peritonei: more questions than answers. J Clin Oncol. 2012;30(20):2429–2430. doi: 10.1200/JCO.2012.42.3764. [DOI] [PubMed] [Google Scholar]
- 3.Esquivel J, Sugarbaker PH. Clinical presentation of the pseudomyxoma peritonei syndrome. Br J Surg. 2002;87(10):1414–1418. doi: 10.1046/j.1365-2168.2000.01553.x. [DOI] [PubMed] [Google Scholar]
- 4.Limber GK, King RE, Silverberg SG. Pseudomyxoma peritonaei: a report of ten cases. Ann Surg. 1973;178(5):587–593. doi: 10.1097/00000658-197311000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19(12):1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin North Am. 2003;12(3):585–603. doi: 10.1016/s1055-3207(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 7.Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur J Surg Oncol. 2008;34(2):196–201. doi: 10.1016/j.ejso.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Darr U, Renno A, Alkully T, Khan Z, Tiwari A, Zeb W, Purdy J, Nawras A. Diagnosis of Pseudomyxoma peritonei via endoscopic ultrasound guided fine needle aspiration: a case report and review of literature. Scand J Gastroenterol. 2017;52(5):609–612. doi: 10.1080/00365521.2017.1284896. [DOI] [PubMed] [Google Scholar]
- 9.Gohda Y, Noguchi R, Horie T, Igari T, Nakamura H, Ohta Y, Yamaguchi K, Ikenoue T, Hatakeyama S, Yusa N, Furukawa Y, Yano H. Pseudomyxoma peritonei of a mature ovarian teratoma caused by mismatch repair deficiency in a patient with Lynch syndrome: a case report. BMC Med Genet. 2016;17(1):94. doi: 10.1186/s12881-016-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anania G, Giaccari S, Solfrini G, Scagliarini L, Vedana L, Resta G. Appendicular mucocele: two case reports and literature review. G Chir. 2015;36(6):276–279. doi: 10.11138/gchir/2015.36.6.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh RK, Somasundaram M, Ravakhah K, Hassan C. Pseudomyxoma peritonei with intrathoracic extension: a rare disease with rarer presentation from low-grade mucinous adenocarcinoma of the appendix. BMJ Case Rep. 2016;2016:bcr2015211076. doi: 10.1136/bcr-2015-211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Chen X, Li Y, Huang L. Two independent primary mucinous tumors involving the appendix and ovary accompanied with acellular pseudomyxoma peritonei. Int J Clin Exp Pathol. 2015;8(9):11831–11834. [PMC free article] [PubMed] [Google Scholar]
- 13.Joo MW, Chung YG, Hur SY, Lee A, Jung CK, Jee WH, Kim JH. Pseudomyxoma peritonei extending to the lower extremity: a case report. World J Surg Oncol. 2015;13:221. doi: 10.1186/s12957-015-0639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal AK, Bobiński P, Grzebieniak Z, Rudnicki J, Marek G, Kobielak P, Kazanowski M, Agrawal S, Hałoń A. Pseudomyxoma peritonei originating from urachus-case report and review of the literature. Curr Oncol. 2014;21(1):e155–e165. doi: 10.3747/co.21.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez A, Ferron G, Mery E, Gladieff L, Delord JP, Querleu D. Peritoneal pseudomyxoma arising from the urachus. Surg Oncol. 2012;21(1):1–5. doi: 10.1016/j.suronc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Kebapçı M, Şaylısoy S, Can C, Dündar E. Radiologic findings of urachal mucinous cystadenocarcinoma causing pseudomyxoma peritonei. Jpn J Radiol. 2012;30(4):345–348. doi: 10.1007/s11604-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki T, Yasuda K, Watanabe A, Fuse H. Laparoscopic management of urachal mucinous borderline tumor associated with pseudomyxoma peritonei. Surg Laparosc Endosc Percutan Tech. 2011;21(3):e152–e155. doi: 10.1097/SLE.0b013e3182222bd4. [DOI] [PubMed] [Google Scholar]
- 18.Lamb BW, Vaidyanathan R, Laniado M, Karim O, Motiwala H. Mucinous adenocarcinoma of the urachal remnant with pseudomyxoma peritonei. Urol J. 2010;7(2):138–139. [PubMed] [Google Scholar]
- 19.Sugiyama K, Ito N. Mucinous cystadenocarcinoma of the urachus associated with pseudomyxoma peritonei with emphasis on MR findings. Magn Reson Med Sci. 2009;8(2):85–89. doi: 10.2463/mrms.8.85. [DOI] [PubMed] [Google Scholar]
- 20.Khalid K, Ahmed MS, Malik MS. Adenocarcinoma of urachal cyst associated with pseudomyxoma peritonei masquerading as abdominal tuberculosis: A case report and review of literature. Indian J Urol. 2008;24(2):258–260. doi: 10.4103/0970-1591.40626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugarbaker PH, Verghese M, Yan TD, Brun E. Management of mucinous urachal neoplasm presenting as pseudomyxoma peritonei. Tumori. 2008;94(5):732–736. doi: 10.1177/030089160809400515. [DOI] [PubMed] [Google Scholar]
- 22.Yan TD, Sugarbaker PH, Brun EA. Pseudomyxoma peritonei from mucinous adenocarcinoma of the urachus. J Clin Oncol. 2006;24(30):4944–4946. doi: 10.1200/JCO.2006.06.7223. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara T, Misawa K, Sano H, Okawa Y, Takada A. Pseudomyxoma peritonei due to mucinous cystadenocarcinoma in situ of the urachus presenting as an inguinal hernia. Int J Clin Oncol. 2006;11(5):416–419. doi: 10.1007/s10147-006-0594-1. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi M, Matsuzaki K, Yoshida S, Nishitani H, Uehara H. Imaging findings of urachal mucinous cystadenocarcinoma associated with pseudomyxoma peritonei. Acta Radiol. 2004;45(3):348–350. doi: 10.1080/02841850410004959. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa S, Fujinaga Y, Kadoya M. Urachal mucinous cystadenocarcinoma with a cystic ovarian metastasis. AJR Am J Roentgenol. 2003;180(4):1183–1184. doi: 10.2214/ajr.180.4.1801183. [DOI] [PubMed] [Google Scholar]
- 26.Stenhouse G, McRae D, Pollock AM. Urachal adenocarcinoma in situ with pseudomyxoma peritonei: a case report. J Clin Pathol. 2003;56(2):152–153. doi: 10.1136/jcp.56.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bree E, Witkamp A, Van De Vijver M, Zoetmulde F. Unusual origins of pseudomyxoma peritonei. J Surg Oncol. 2000;75(4):270–274. doi: 10.1002/1096-9098(200012)75:4<270::aid-jso9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Sasano H, Shlzawa S, Nagura H, Yamaki T. Mucinous adenocarcinoma arising in a giant urachal cyst associated with pseudomyxoma peritonei and stromal osseous metaplasia. Pathol Int. 1997;47(7):502–505. doi: 10.1111/j.1440-1827.1997.tb04531.x. [DOI] [PubMed] [Google Scholar]
- 29.Loggie BW, Fleming RA, Hosseinian AA. Peritoneal carcinomatosis with urachal signet-cell adenocarcinoma. Urology. 1997;50(3):446–448. doi: 10.1016/S0090-4295(97)00247-1. [DOI] [PubMed] [Google Scholar]
- 30.Touloumis Z, Galyfos G, Kavouras N, Menis M, Lavant L. Aggressive pseudomyxoma peritonei: a case report with an unusual clinical presentation. Case Rep Oncol Med. 2013;2013:926963. doi: 10.1155/2013/926963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idjuski S, Turkalj I, Petrovic K, Vanhoenacker FM. Pseudomyxoma peritonei due to mucinous adenocarcinoma of the appendix. JBR–BTR. 2013;96(3):184. doi: 10.5334/jbr-btr.266. [DOI] [PubMed] [Google Scholar]
- 32.Mavrodin C, Pariza G, Iordache V, Iorga P, Sajin M. Pseudomixoma peritonei, a rare entity difficult to diagnose and treat - case report. Chirurgia (Bucur) 2014;109(6):846–849. [PubMed] [Google Scholar]
- 33.Quiñonez E, Schuldt M, Retamero JA, Nogales FF. Ovarian strumal carcinoid containing appendiceal-type mucinous tumor patterns presenting as pseudomyxoma peritonei. Int J Gynecol Pathol. 2015;34(3):293–297. doi: 10.1097/PGP.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 34.Peixoto RD, Wilson S, Schaeffer DF, Lim HJ. Pseudomyxoma peritonei metastatic to the bone: case report and review of systemic management. Gastrointest Cancer Res. 2014;7(3-4):108–110. [PMC free article] [PubMed] [Google Scholar]
- 35.Becude L, Lugtenberg RT, Koster T. A 41-year-old man with an increased abdominal girth. Neth J Med. 2014;72(8):432–436. [PubMed] [Google Scholar]
- 36.Demetrashvili Z, Chkhaidze M, Khutsishvili K, Topchishvili G, Javakhishvili T, Pipia I, Qerqadze V. Mucocele of the appendix: case report and review of literature. Int Surg. 2012;97(3):266–269. doi: 10.9738/CC139.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Idris LO, Olaofe OO, Adejumobi OM, Kolawole AO, Jimoh AK. Giant mucocele of the appendix in pregnancy: A case report and review of literature. Int J Surg Case Rep. 2015;9:95–97. doi: 10.1016/j.ijscr.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA19-9 tumor markers in diagnosis and prognostic assessment of mucinous epithelial cancers of the appendix. J Surg Oncol. 2004;87(4):162–166. doi: 10.1002/jso.20107. [DOI] [PubMed] [Google Scholar]
- 39.van Ruth S, Hart AA, Bonfrer JM, Verwaal VJ, Zoetmulder FA. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2002;9(10):961–967. doi: 10.1007/BF02574513. [DOI] [PubMed] [Google Scholar]
- 40.Nonaka D, Kusamura S, Baratti D, Casali P, Younan R, Deraco M. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology. 2006;49(4):381–387. doi: 10.1111/j.1365-2559.2006.02512.x. [DOI] [PubMed] [Google Scholar]
- 41.Ioannidis O, Cheva A, Paraskevas G, Papadimitriou N, Konstantara A, Chatzopoulos S, Kotronis A, Makrantonakis A, Kakoutis E. Pseudomyxoma retroperitonei: report of 2 cases and review of the literature. Rev Esp Enferm Dig. 2012;104(5):268–275. doi: 10.4321/s1130-01082012000500009. [DOI] [PubMed] [Google Scholar]
- 42.Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 1. Patterns of dissemination and treatment options. Oncology (Williston Park) 2004;18(1):51. [PubMed] [Google Scholar]
- 43.Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Williston Park) 2004;18(2):207. [PubMed] [Google Scholar]
- 44.Csanyi-Bastien M, Blanchard F, Lamy A, Sabourin JC. A case of Pseudomyxoma Peritonei of an unexpected origin. Diagn Pathol. 2021;16(1):119. doi: 10.1186/s13000-021-01179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]