Abstract
Aims
The aim of this systematic literature review was to provide updated information on human papillomavirus (HPV) prevalence in locally and regionally advanced (LA) and recurrent/metastatic (RM) head and neck cancer (HNC) worldwide.
Methods
Electronic searches were conducted on clinicaltrials.gov, MEDLINE/PubMed, Embase, and ASCO/ESMO journals of congresses for interventional studies (IS; Phase I–III trials) as well as MEDLINE and Embase for non‐interventional studies (NIS) of LA/RM HNC published between January 01, 2010 and December 31, 2020. Criteria for study selection included: availability of HPV prevalence data for LA/RM HNC patients, patient enrollment from January 01, 2010 onward, and oropharyngeal cancer (OPC) included among HNC types. HPV prevalence per study was calculated as proportion of HPV+ over total number of enrolled patients. For overall HPV prevalence across studies, mean of reported HPV prevalence rates across studies and pooled estimate (sum of all HPV+ patients over sum of all patients enrolled) were assessed.
Results
Eighty‐one studies (62 IS; 19 NIS) were included, representing 9607 LA/RM HNC cases, with an overall mean (pooled) HPV prevalence of 32.6% (25.1%). HPV prevalence was 44.7% (44.0%) in LA and 24.3% (18.6%) in RM. Among 2714 LA/RM OPC patients from 52 studies with available data, mean (pooled) value was 55.8% (50.7%). The majority of data were derived from Northern America and Europe, with overall HPV prevalence of 46.0% (42.1%) and 24.7% (25.3%) across studies conducted exclusively in these geographic regions, respectively (Northern Europe: 31.9% [63.1%]). A “p16‐based” assay was the most frequently reported HPV detection methodology (58.0%).
Conclusion
Over the last decade, at least one quarter of LA/RM HNC and half of OPC cases studied in IS and NIS were HPV+. This alarming burden is consistent with a potential implication of HPV in the pathogenesis of at least a subgroup of HNC, underscoring the relevance of HPV testing and prophylaxis to HNC prevention and management.
Keywords: cancer prevention, cancer risk factors, epidemiology and prevention, head and neck cancer, viral oncology
1. INTRODUCTION
Head and neck cancer (HNC) accounted for ~5% of new cancer cases and cancer‐related deaths worldwide in 2020, with an estimated annual burden of 931,931 incident cases and 467,125 deaths. 1 Most HNCs arise from the squamous epithelium of the oral cavity, oropharynx (OPX), larynx, and hypopharynx, collectively referred to as head and neck squamous cell carcinoma (HNSCC). 2 HNSCC incidence varies across regions, generally reflecting the diverse epidemiology of risk factors such as consumption of tobacco, alcohol, and areca nut. 2 Another cause of cancers developed in the head and neck (HN) is human papillomavirus (HPV) infection. 3 , 4 Among HNC subtypes, HPV has been most frequently associated with oropharyngeal squamous cell carcinoma (OPSCC) 5 , 6 , 7 and is considered to account to a great extent for the increasing incidence of oropharyngeal cancer (OPC) in several high‐income countries over the past decades. 8 , 9 , 10
Besides its etiological role, HPV is also recognized as a prognostic factor in OPC 11 , 12 , 13 , 14 , 15 , 16 and is utilized in the therapeutic algorithm of OPC patients. 17 , 18 This highlights the clinical utility of HPV testing in HNC, which, in the absence of diagnostic tests with regulatory approval for use in HNC, is performed by various methodologies. 19 Each of the available techniques has specific limitations; thus, a combined approach using multiple protein or nucleic acid‐based methods has been suggested for optimal detection of potentially causative HPV. 19 , 20 Testing of p16 is recommended by the European Head and Neck Society/European Society for Medical Oncology/European Society for Radiotherapy and Oncology, the National Comprehensive Cancer Network, the College of American Pathologists, and the American Society of Clinical Oncology guidelines as a surrogate HPV biomarker in OPSCC management. 12 , 13 , 21 , 22 , 23 Nevertheless, given than the prognostic impact of HPV in other HNC subsites is unclear, 24 , 25 , 26 routine examination for HPV presence for other HNC types is not warranted. 21
For patients with limited or early‐stage HNSCC, current treatment modalities are potentially curative, while for recurrent and/or metastatic (RM) HNSCC, treatment is complex, prognosis is poor, and the burden on quality of life and productivity can be substantial. 27 , 28 Importantly, more than 50% of HNSCC patients present with locally and regionally advanced (LA) or metastatic HNSCC at diagnosis, while recurrence rates for LA HNSCC are high. 28 , 29 , 30 The proportion of those patients who are HPV‐positive (HPV+) remains unknown, as available data are not only outdated, but also mainly refer to the totality of HNCs, not distinguishing the disease by stage. 5 , 31 , 32 , 33 , 34 , 35 , 36 , 37 This information is particularly relevant considering that available HPV prophylactic vaccines have shown preliminary efficacy against HN infections, opening an opportunity for primary prevention of the specific cancers, 38 , 39 , 40 , 41 , 42 with this potential being investigated in ongoing Phase III clinical trials. 43 , 44
Given the challenges in the management of LA and RM HNC, and the increasing incidence of HPV‐associated HNC, updated information on the HPV prevalence is essential, with possible implications for preventive interventions. This systematic literature review (SLR) primarily aimed to enhance understanding of HPV prevalence in LA and/or RM HNC based on evidence from the last decade (2010 to 2020). Additionally, HPV prevalence in LA and RM OPC, geographic distribution of HPV prevalence, and level of homogeneity between HPV testing methodologies were explored.
2. METHODS
This SLR was conducted and outcomes were reported in accordance with PRISMA guidelines (see Data S1). The study protocol was registered with PROSPERO, the international prospective register of systematic reviews (registration number: CRD42021256876) and is publicly available.
2.1. Information sources and search strategy
Identification of studies in LA and RM HNC was performed separately for IS (i.e., Phase I–III trials) and NIS. For IS, electronic searches were conducted on Clinicaltrials.gov using the keywords “Head and Neck” in combination with “Local”, “Regional”, “Advanced”, “Recurrent”, or “Metastatic” for Phase 1, 2, and 3 studies starting on or after January 01, 2010 until December 31, 2020. The corresponding National Clinical Trial (NCT) numbers were used to search PubMed and Embase databases as well as ASCO/ESMO journals of congresses for related articles and/or abstracts with available results. For NIS, MEDLINE via PubMed and Embase databases were searched for related publications using Medical subject heading terms and keywords developed for disease (HNC), outcome of interest (HPV), relevant cancer type (OPC) to expand search results, disease stage (local, regional, recurrent, metastatic, and advanced), and study design (epidemiology, real‐life, non‐interventional, and observational). The searches were restricted using embedded filters to publications from the last 10 years (January 01, 2010 to December 31, 2020). They were also restricted to articles published in English language and to studies conducted in “humans,” while congress abstracts and reviews were excluded. The detailed search strategy including search strings and resulting number of hits is provided in Data S2. Electronic searches for both IS and NIS were completed on March 19, 2021.
2.2. Study selection
Studies were selected based on prespecified eligibility criteria designed according to the PICOTS (population, intervention, comparisons, outcome, time, and study design) framework. Specifically, studies were selected if patients with RM and/or LA HNC had participated, OPX was included among the HN subsites, and HPV status of cancer was available, even if only the OPC subpopulation had been tested for HPV (population). There were no restrictions as to the intervention and comparator of the study, as long as they were intended for disease treatment and not management of safety events of previous therapies (intervention; comparator). Only studies with available results on HPV prevalence (i.e., prevalence of HPV‐related HNC), and/or on the number of HPV+ HNC patients, allowing the calculation of corresponding prevalence were selected (Outcome). Studies initiating enrollment of participants prior to January 01, 2010 were excluded (time). IS of any design were included as long as there was no prerequisite regarding the proportion of patients per HN subsite that needed to be enrolled and NIS of any design and direction of temporal observation (Study design). Articles published in a language other than English were excluded. Finally, only original, peer‐reviewed articles published in scientific journals were selected, with the exception of abstracts published in ASCO/ESMO congress abstract books for IS which were also included. Non‐original studies such as literature reviews were excluded. For IS for which full manuscripts were pending and corresponding abstracts in ASCO/ESMO congress abstract books were available, selection was based on information included in those abstracts. Study design and results captured in Clinicaltrials.gov were utilized cumulatively with manuscripts and/or abstracts available in ASCO/ESMO journals of congresses for selecting IS.
For study selection, an initial screening of titles/abstracts was performed against each eligibility criterion followed by examination of the full‐text article if a definite decision could not be made. Study review and selection was performed by two reviewers working independently (Athena Georgilis and Maria‐Filothei Lazaridou from Qualitis SA). The decisions of the reviewers were compared and any conflicts were resolved by a third reviewer (Charalampos Athanasopoulos for NIS and Georgios Trimis for IS).
2.3. Data extraction and analysis
Data from each of the studies that met the predefined eligibility criteria were extracted and cross‐checked by two independent reviewers with respect to the following variables: study design, country, study period, study population including disease stage, age, HPV status detection methodology, subsites where HPV status was assessed (any included site or only OPX), number of LA and/or RM HNC patients enrolled (“NHNC enrolled”), number of HPV+ LA and/or RM HNC patients (“NHNC HPV+”) and/or HPV prevalence (%) in LA and/or RM HNC as defined by the author, number of HPV− LA and/or RM HNC patients (“NHNC HPV−”) to reflect missing HPV status data, number of LA and/or RM OPC patients enrolled (“NOPC enrolled”), number of HPV+ LA and/or RM OPC patients (“NOPC HPV+”) and/or HPV prevalence (%) in LA and/or RM OPC as defined by the author.
The primary outcome of HPV prevalence in LA and RM HNC was calculated as the proportion (%) of “NHNC HPV+” over “NHNC enrolled.” Similarly, OPC fraction among LA and/or RM HNC patients was calculated as the proportion (%) of “NOPC enrolled” over “NHNC enrolled,” as available. For the secondary outcome of HPV prevalence in LA and/or RM OPC, HPV prevalence among selected HNC studies was calculated as the proportion (%) of “NOPC HPV+” over “NOPC enrolled,” as available. Hence, the estimated prevalence of HPV in HNC and OPC represented the minimum number of HPV+ patients in the pool of HNC or OPC patients enrolled in each study, respectively, as patients with no available data on HPV status were also included in the denominators (“NHNC enrolled” and “NOPC enrolled”).
Data extracted from selected studies was organized in summary tables and figures using standard Microsoft Excel® functions and descriptively analyzed. No inferential statistical analysis was conducted. Studies were categorized by design in the subgroups of IS or NIS and by HNC disease stage as either LA, RM, or Other, the latter of which included LA and/or RM stage as defined by the author with no further specification or both LA and RM. Studies were also grouped based on geographic region as defined by the International Agency for Research on Cancer. 45 In estimating the prevalence of HPV or OPC fraction across HNC studies overall and per the above‐described subgroups, mean and median proportion (%) of HPV+ or OPC patients across studies in each subgroup were calculated. HPV prevalence and OPC fraction overall and per subgroup were also estimated as pooled prevalence, that is, as proportion (%) of the sum of “NHNC or OPC HPV+” or “NOPC enrolled” across studies, respectively, over the sum of “NHNC or OPC enrolled” across studies.
2.4. Risk of bias
Taking into account the narrative nature of this SLR and that prevalence of HPV pertains to a baseline patient characteristic, study outcomes are not expected to be affected by the design, conduct, or the statistical power in the results of each included study. To reduce bias with respect to generalizability of HPV prevalence outcomes, during the study selection process, studies with a prespecified patient eligibility criterion regarding HPV status (e.g., HPV+ patients only) or associated with HPV status (e.g., OPC patients only) were excluded. No restrictions were applied with respect to HPV detection methodologies as distribution of different methodologies was an exploratory outcome of interest. Last, the effect of sample size on the primary outcome was examined by visual inspection of the distribution of studies around the overall prevalence of HPV (mean, median, pooled) in a plot of sample size (“NHNC enrolled”) against HPV prevalence.
3. RESULTS
3.1. Literature search results and characteristics of included studies
The search strategy identified a total of 2618 records, of which 855 corresponded to IS and 1763 to NIS. Following removal of duplicate records, records with lack of published articles and/or congress abstracts, and studies not fulfilling the PICOTS criteria, a total of 62 IS and 19 NIS were included in the evidence synthesis (Figure 1).
FIGURE 1.
PRISMA diagrams for selection of (A) interventional studies and (B) non‐interventional studies. HN, head and neck; HNC, head and neck cancer; HPV, human papilloma virus; n, number of studies; OPC, oropharyngeal cancer; OPX, oropharynx. †Number of excluded articles per reason does not add up to total number of excluded articles as many cases were excluded for more than one reason.
All included studies (N = 81) provided an HPV prevalence of LA and/or RM HNC captured between January 01, 2010 and December 31, 2020 and were used for addressing the primary study objective. Of the included studies, 43 IS and 9 NIS reported data on prevalence of HPV specifically for OPC, and were thus used to address the secondary outcome of interest.
Characteristics of the studies included in the evidence synthesis and outcomes of interest derived from each study are presented in Table 1 and Table S1. Of the IS, 42 (67.7%) were single‐arm and 20 (32.3%) were multi‐arm; of the latter 17 (27.5%) were randomized. Of the NIS, 13 (68.4%) were retrospective, 2 (10.5%) were prospective cohort studies, another 2 (10.5%) were cross‐sectional studies, and the remaining 2 were of a mixed cohort study design (10.5%). Sixty‐one (75.3%) of the included studies were single‐country studies conducted in Northern America, Europe, and Asia, 5 (6.2%) were multi‐country, single‐continent studies, and the remaining 15 (18.5%) were multi‐country, multi‐continent studies. Overall, the selected studies were conducted in 51 countries distributed in all continents (Table 1).
TABLE 1.
Patients' characteristics and outcomes of interest in studies included in the evidence synthesis.
| Study acronym or primary author (year) | Country | Median age (range) | Disease stage | HPV status detection methodology e | Subsites that HPV status was assessed | NHNC enrolled | NHNC HPV+ | NHNC HPV– | HPV prevalence (%) in HNC | NOPC enrolled | OPC fraction (%) in HNC | NOPC HPV+ | HPV prevalence (%) in OPC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interventional Studies | |||||||||||||
| NCT01045421 46 | CZ, FR, PL, USA | 58 (52–65) | RM | IHC | Any site | 55 | 14 | 16 | 25.5 | n/a | n/a | 10 | n/a |
| NCT01126216 47 | DE | 59 (36–80) | LA | p16 | Any site | 216 | 49 | 110 | 22.7 | 116 | 53.7 | 32 | 27.6 |
| NCT01133678 48 | USA | 57 (27–76) | LA | p16/ISH | OPX | 94 | 59 | n/a | 62.8 | 71 | 75.5 | 59 | 83.1 |
| NCT01172769 49 | DE | 61.5 (42–79) | RM | DNA | Any site | 40 | 4 | 20 | 10.0 | 15 | 37.5 | 3 | 20.0 |
| NCT01195922 50 | USA | 60 a (n/a) | LA | p16 | Any site | 16 | 8 | 8 | 50.0 | 8 | 50.0 | 6 | 75.0 |
| NCT01218048 51 | USA | 59 (31–93) | LA | n/a | Any site | 29 | 10 | 19 | 34.5 | 11 | 37.9 | 9 | 81.8 |
| NCT01255800 52 | USA | 57 (n/a) | RM | ISH | Any site | 9 | 5 | 3 | 55.6 | n/a | n/a | n/a | n/a |
| NCT01345682 53 , 54 | AR, AT, BE, BR, CH, CZ, DE, DK, ES, FR, GR, IL, IT, JP, MX, RU, SE, USA, ZA | n/a (n/a) | RM | p16 | Any site | 234 c | 35 | 199 | 15.0 | 80 | 34.2 | 23 | 28.8 |
| NCT01379339 55 | USA | n/a (n/a) | LA | p16 | OPX | 26 | 9 | 2 | 34.6 | 13 | 50.0 | 9 | 69.2 |
| NCT01412229 56 | USA | 57 a (39–70) | LA | p16 | OPX | 38 | 17 | 5 | 44.7 | 25 | 65.8 | 17 | 68.0 |
| NCT01417936 57 | BE, DE, FR | 60 (42–87) | RM | PCR | Any site | 26 | 1 | 20 | 3.8 | 13 | 50.0 | n/a | n/a |
| NCT01437449 58 | USA | 60 (24–80) | RM | p16 | OPX | 29 | 11 | n/a | 37.9 | 14 | 48.3 | 11 | 78.6 |
| NCT01449201 59 | KR | 60.5 (30–82) | RM | p16 | Any site | 48 | 8 | 26 | 16.7 | 11 | 22.9 | n/a | n/a |
| NCT01458392 60 | USA | 60.5 (45–78) | RM | n/a | Any site | 46 | 21 | 19 | 45.7 | 21 | 45.7 | n/a | n/a |
| NCT01468896 61 | USA | 60 a (41–82) | RM | n/a | Any site | 23 | 12 | 5 | 52.2 | 1 | 4.3 | n/a | n/a |
| NCT01472653 62 | SI | 57 (42–75) | LA | DNA/mRNA | OPX | 39 | 8 | 18 | 20.5 | 30 | 76.9 | 8 | 26.7 |
| NCT01566435 63 , 64 | USA | 57 (43–75) | LA | p16 | OPX | 30 | 17 | n/a | 56.7 | 18 | 60.0 | 17 | 94.4 |
| NCT01577173 65 | AU, BE, BG, DE, ES, FR, HU, IT, RO, UK, USA | 62.0 (28–84) | RM | qRT‐PCR | Any site | 121 | 25 | 85 | 20.7 | 36 | 29.8 | 14 | 38.9 |
| NCT01592721 66 | USA | 66.5 a (50–81) | LA | p16/ISH | Any site | 6 | 1 | 3 | 16.7 | 4 | 66.7 | 1 | 25.0 |
| NCT01612351 67 | USA | 57.5 a (41–77) | LA | p16 & PCR | OPX | 40 | 17 | n/a | 42.5 | 30 | 75.0 | 17 | 56.7 |
| NCT01696955 68 , 69 | USA | 60.5 & 63.6 (35–90) | RM | p16 | n/a | 78 | 31 | 47 | 39.7 | n/a | n/a | 31 | n/a |
| NCT01716416 70 | USA | 59 (38–72) | RM | p16 | OPX | 31 | 8 | 23 | 25.8 | 9 | 29.0 | 8 | 88.9 |
| NCT01737008 71 | CA | 53.5 a (48–66) | LA | p16 | Any site | 6 | 6 | n/a | 100.0 | 5 | 83.3 | 5 | 100.0 |
| NCT01816984 72 | USA | 61 a (47–73) | RM | p16 and/or PCR | n/a | 12 | 5 | 4 | 41.7 | 9 | 75.0 | 5 | 55.6 |
| NCT01836029 73 | USA | 58 (23–81) | RM | p16/ISH/PCR | OPX | 195 | 52 | 25 | 26.7 | 83 | 42.6 | 52 | 62.7 |
| NCT01848834 74 , 75 , 76 b | IL, JP, KR, TW, USA | 60 (20–84) | RM | p16 | OPX | 192 | 45 | 147 | 23.4 | 76 | 39.6 | 45 | 59.2 |
| NCT01856478 77 | CN, EG, HK, IN, KR, PH, TH, TW | 55.5 & 58.0 (27–83) | RM | p16 | Any site | 340 | 10 | 109 | 2.9 | 47 | 13.8 | n/a | n/a |
| NCT01911598 78 | BE, USA | 61 (29–82) | RM | qRT‐PCR | Any site | 24 | 5 | 19 | 20.8 | 9 | 37.5 | n/a | n/a |
| NCT01935921 79 | USA | n/a (n/a) | LA | n/a | OPX | 18 | 7 | 3 | 38.9 | 10 | 55.6 | 7 | 70.0 |
| NCT01946867 80 | ES, FR | 78 (65–91) | LA | p16 | OPX | 19 | 6 | 1 | 31.6 | 13 | 68.4 | 6 | 46.2 |
| NCT01969877 81 | SE | 61 (33–77) | LA | p16 | OPX | 291 | 221 | 25 | 75.9 | 248 | 85.2 | 221 | 89.1 |
| NCT02052960 82 , 83 | BE, DE, ES, FR, IT, PL, RO | n/a (n/a) | RM | p16 | n/a | 240 | 32 | n/a | 13.3 | n/a | n/a | n/a | n/a |
| NCT02105636 84 | AR, BR, CA, CH, DE, ES, FR, HK, IT, JP, KR, NL, TW, UK, USA | 60 (28–83) | RM | p16 | OPX | 361 | 93 | 93 | 25.8 | n/a | n/a | 93 | n/a |
| NCT02207530 85 | BE, CA, CZ, DE, ES, FR, GE, HU, KR, MY, TW, UK, USA | 60 (24–84) | RM | p16/ISH/PCR | Any site | 112 | 34 | 65 | 30.4 | 40 | 35.7 | 20 | 50.0 |
| NCT02252042 86 | AU, BE, CA, CH, DE, ES, FR, HU, IE, IT, KR, LT, MX, NL, PL, PT, RU, SE, UK, USA | 60 (54–66) | RM | p16 | OPX | 495 | 119 | 323 | 24.0 | n/a | n/a | 119 | n/a |
| NCT02255097 87 | DK, NO, USA | 61 (33–90) | RM | p16 | OPX | 171 | 37 | 131 | 21.6 | 100 | 58.5 | 37 | 37.0 |
| NCT02268695 88 | DE, ES, FR | 60 (55–64) | RM | ISH | OPX | 503 | 34 | 146 | 6.8 | 180 | 35.8 | 34 | 18.9 |
| NCT02274155 89 | USA | 60 a (n/a) | LA | p16 | OPX | 17 | 6 | 9 | 35.3 | 9 | 52.9 | 6 | 66.7 |
| NCT02277197 90 , 91 | USA | n/a (n/a) | RM | p16 | OPX | 12 | 1 | n/a | 8.3 | 3 | 25.0 | 1 | 33.3 |
| NCT02282371 92 | USA | 60 (36–73) | LA | n/a | Any site | 11 | 10 | n/a | 90.9 | 9 | 81.8 | n/a | n/a |
| NCT02308072 93 | UK | 60.81 (46.13–75.48) | LA | p16 | OPX | 16 | 5 | 2 | 31.3 | 7 | 43.8 | 5 | 71.4 |
| NCT02319044 94 | AU, BE, CA, CZ, DE, ES, FR, GE, HU, IL, KR, MY, TW, UK, USA | 61.0 (23–82) | RM | n/a | Any site | 267 | 75 | n/a | 28.1 | 107 | 40.1 | n/a | n/a |
| NCT02350712 95 | UK | 53 (31–65) | RM | n/a | OPX | 15 | 3 | 1 | 20.0 | 5 | 33.3 | 3 | 60.0 |
| NCT02358031 96 | AR, AT, AU, BR, CA, CH, CL, CO, CZ, DE, DK, EE, ES, FI, GR, HK, HU, IL, IT, JP, LV, MX, MY, NL, NO, PE, PH, PL, RU, SE, SG, TH, TR, TW, UK, USA, ZA d | 61.0 & 62.0 (54.5–68.0) | RM | p16 | OPX | 882 | 251 | n/a | 28.5 | 447 | 50.7 | 251 | 56.2 |
| NCT02369874 97 | AR, AU, BE, BR, BG, CL, CZ, DE, ES, FR, GE, HR, HU, IL, IT, JP, KR, PL, RO, RS, RU, TW, UA, USA | 60 (22–84) | RM | IHC/FISH/PCR [local standard procedures] | OPX | 736 | 91 | 178 | 12.4 | 274 | 37.2 | 91 | 33.2 |
| NCT02508389 98 | CA, USA | 57 (30–84) | LA | p16 | Any site | 223 | 160 | 63 | 71.7 | 172 | 77.1 | n/a | n/a |
| NCT02537223 99 | CA | 59 (52–70) | LA | p16 | OPX | 9 | 7 | n/a | 77.8 | 9 | 100.0 | 7 | 77.8 |
| NCT02538510 100 | USA | 61 (33–86) | RM | p16 | OPX | 25 | 13 | n/a | 52.0 | 17 | 68.0 | 13 | 76.5 |
| NCT02549742 101 | DK | 67.5 (47–87) | RM | p16 | Any site | 26 | 3 | 22 | 11.5 | 4 | 15.4 | 1 | 25.0 |
| NCT02573493 102 | USA | n/a (n/a) | LA | p16 | OPX | 80 | 46 | 34 | 57.5 | 57 | 71.3 | 46 | 80.7 |
| NCT02586207 103 | USA | 59.8 (36–81) | LA | ISH/p16 | Any site | 59 | 34 | 25 | 57.6 | 40 | 67.8 | 31 | 77.5 |
| NCT02609503 104 | USA | 63.1 a (39–86) | LA | p16 | OPX | 29 | 14 | n/a | 48.3 | 20 | 69.0 | 14 | 70.0 |
| NCT02626000 105 | AT, AU, BE, CA, CH, ES, FR, GR, IT, UK, USA | 62 (35–77) | RM | n/a | OPX | 36 | 5 | 4 | 13.9 | 9 | 25.0 | 5 | 55.6 |
| NCT02643056 106 | CH | 60 (42–87) | RM | n/a | n/a | 33 | 10 | 18 | 30.3 | n/a | n/a | n/a | n/a |
| NCT02707588 107 , 108 | FR | 65 (n/a) | LA | p16 | n/a | 133 | 37 | n/a | 27.8 | 80 | 60.2 | n/a | n/a |
| NCT02718820 109 | AT | 63 (44–77) | RM | p16 | OPX | 22 | 4 | n/a | 18.2 | 9 | 40.9 | 4 | 44.4 |
| NCT02764593 110 | USA | 62 (n/a) | LA | p16 | OPX | 39 | 24 | n/a | 62.0 | n/a | n/a | 24 | n/a |
| NCT02938273 111 | NL | 69 (61–77) | Other | n/a | OPX | 10 | 4 | 5 | 40.0 | 8 | 80.0 | 4 | 50.0 |
| NCT02999087 112 | CH | 61 a (40–78) | LA | p16 | OPX | 82 | 28 | n/a | 34.1 | 60 | 73.2 | 28 | 46.7 |
| NCT03003637 113 | NL | n/a (n/a) | Other | n/a | n/a | 32 | 1 | 31 | 3.1 | n/a | n/a | n/a | n/a |
| NCT03370276 114 | USA | n/a (n/a) | RM | p16 | OPX | 45 | 22 | 4 | 48.9 | 26 | 57.8 | 22 | 84.6 |
| NCT04397341 115 | CN | 53 (28–69) | LA | p16 | OPX | 58 | 6 | 19 | 10.3 | 25 | 43.1 | 6 | 24.0 |
| Non‐interventional Studies | |||||||||||||
| Bossi (2016) 116 | IT | n/a (n/a) | LA | n/a | OPX | 55 | 18 | 7 | 32.7 | 25 | 45.5 | 18 | 72.0 |
| Bossi (2019) 117 | IT | 60 (52–68) | LA | n/a | OPX | 129 | 47 | 12 | 36.4 | 59 | 45.7 | 47 | 79.7 |
| Botticelli (2020) 118 | IT | 67 (30–82) | RM | n/a | Any site | 61 | 2 | 11 | 3.3 | 14 | 23.0 | 2 | 14.3 |
| Byrne (2019) 119 | CA | 63 (57–68) | Other | p16 | Any site | 109 | 33 | 37 | 30.3 | 52 | 47.7 | n/a | n/a |
| Castelli (2019) 120 | FR | 59 & 62 a (n/a) | LA | p16 | Any site | 237 | 38 | 102 | 16.0 | 163 | 68.8 | n/a | n/a |
| de Ridder (2020) 121 | NL | n/a (n/a) | RM | p16 | Any site | 198 | 23 | 171 | 11.6 | 89 | 44.9 | n/a | n/a |
| Galot (2020) 122 | BE | n/a (n/a) | RM | p16 | OPX | 39 | 5 | 17 | 12.8 | 22 | 56.4 | 5 | 22.7 |
| Grünwald (2020) 123 | AU, BR, CA, DE, ES, IT, KR, TW, UK | 60 (54–67) | RM | p16 | OPX | 733 | 35 | 21 | 4.8 | 221 | 30.2 | 35 | 15.8 |
| Hilke (2020) 124 | DE | n/a (n/a) | LA | p16 | Any site | 20 | 5 | 14 | 25.0 | 14 | 70.0 | 5 | 35.7 |
| Kim (2020) 125 | KR | 57.8 & 47.4 (16–74) | RM | p16 | Any site | 15 | 5 | 7 | 33.3 | n/a | n/a | n/a | n/a |
| Martens (2019) 126 | NL | 66.4 a (41.0–90.1) | RM | n/a | Any site | 28 | 8 | 16 | 28.6 | n/a | n/a | n/a | n/a |
| Martens (2020) 127 | NL | 62.3 & 63.3 a (57.3–69.3) | LA | n/a | Any site | 174 | 65 | 109 | 37.4 | 125 | 71.8 | n/a | n/a |
| Nadler (2019) 128 | USA | 62 (32–87) | RM | n/a | Any site | 325 | 70 | 56 | 21.5 | 219 | 67.4 | n/a | n/a |
| Noij (2018) 129 | NL | 59.2 (43–81) | Other | p16, DNA | Any site | 82 | 39 | 31 | 47.6 | 63 | 76.8 | n/a | n/a |
| Pitak‐Arnnop (2020) 130 | DE | 67 a (n/a) | RM | p16, ISH, DNA | Any site | 9 b | 4 | 5 | 44.4 | n/a | n/a | n/a | n/a |
| Porter (2020) 131 | USA | 63 (44–89) | RM | n/a | Any site | 60 | 15 | 9 | 25.0 | 21 | 35.0 | 15 | 71.4 |
| Smirk (2018) 132 | UK | 63 (n/a) | RM | n/a | Any site | 29 | 6 | 21 | 20.7 | 9 | 31.0 | n/a | n/a |
| Sridharan (2018) 133 | USA | 57 (20–89) | Other | p16, ISH, DNA | Any site | 100 | 42 | 58 | 42.0 | 51 | 51.0 | 42 | 82.4 |
| Velez (2018) 134 | USA | 58.5 (27.9–81.5) | RM | p16 | Any site | 54 | 16 | 7 | 29.6 | 14 | 25.9 | 3 | 21.4 |
Abbreviations: DNA, deoxyribonucleic acid; FISH; fluorescent in situ hybridization; HNC, head and neck cancer; HPV, human papilloma virus; IHC, immunohistochemistry; ISH, in situ hybridization; LA, locally and regionally advanced; mRNA, messenger ribonucleic acid; N, number of patients; n/a, not available; OPC, oropharyngeal cancer; OPX, oropharynx; PCR, polymerase chain reaction; qRT‐PCR, quantitative reverse transcription PCR; RM, recurrent and/or metastatic. Country codes: AR, Argentina; AT, Austria; AU, Australia; BE, Belgium; BG, Bulgaria; BR, Brazil; CA, Canada; CH, Switzerland; CL, Chile; CN, China; CO, Colombia; CZ, Czech Republic; DE, Germany; DK, Denmark; EE, Estonia; EG, Egypt; ES, Spain; FI, Finland; FR, France; GE, Georgia; GR, Greece; HK, Hong Kong; HR, Croatia; HU, Hungary; IE, Ireland; IL, Israel; IN, India; IT, Italy; JP, Japan; KR, Republic of Korea; LT, Lithuania; LV, Latvia; MX, Mexico; MY, Malaysia; NL, Netherlands; NO, Norway; PE, Peru; PH, Philippines; PL, Poland; PT, Portugal; RO, Romania; RS, Serbia; RU, Russian Federation; SE, Sweden; SG, Singapore; SI, Slovenia; TH, Thailand; TR, Turkey; TW, Taiwan; UA, Ukraine; UK, United Kingdom; USA, United States of America; ZA, South Africa.
Mean.
For multiple applicable cohorts but with potentially overlapping data, the largest cohort was included.
For HPV prevalence evidence synthesis, data from the subgroup of patients volunteering for tumor biomarker analysis were used, as available in Cohen et al. (2017). 54
This study included 17 sites that actively screened individuals but did not have any participants randomly allocated to study treatment.
In cases where marker or target was not specified (e.g., IHC rather than p16 by IHC), the detection methodology is recorded as reported in the source.
According to the disease stage of the included population, 31 (38.3%) studies were classified as LA HNC, 45 (55.6%) as RM HNC, and the remaining 5 (6.2%) as Other. The selected studies cumulatively included 9607 LA and/or RM HNC patients. Median patient age ranged from 47 to 78 years across studies (Table 1).
3.2. Prevalence of HPV in HNC
The proportion of HPV+ patients over HNC patients enrolled in each study, that is, HPV prevalence per study, and overall HPV prevalence are presented in Figure 2 and Table 2. The prevalence of HPV in HNC varied considerably across studies, ranging from 2.9% to 100.0%, with a mean value of 32.6%. To account for variations in sample size of each included study, the pooled HPV prevalence was also calculated across studies and was found to be 25.1%. In the IS (n = 62), the prevalence of HPV ranged from 2.9% to 100.0%, with a mean value of 34.5% and a pooled HPV prevalence of 27.1%; while in NIS (n = 19) the prevalence of HPV ranged from 3.3% to 47.6%, with a mean value of 26.5% and a pooled HPV prevalence of 19.4%. In a further analysis by disease stage and regardless of study design, prevalence of HPV was examined in the subgroups of patients with LA and RM, as these represent distinct disease phenotypes with different management approaches and survival outcomes. In LA HNC studies (n = 31) HPV prevalence ranged from 10.3% to 100.0% (mean 44.7%), with a pooled fraction of 44.0%, while in RM HNC studies (n = 45), HPV prevalence ranged from 2.9% to 55.6% (mean 24.3%), with a pooled fraction of 18.6%. Interestingly, the prevalence of HPV exceeded 50.0% in about one sixth of all studies included in the evidence synthesis.
FIGURE 2.
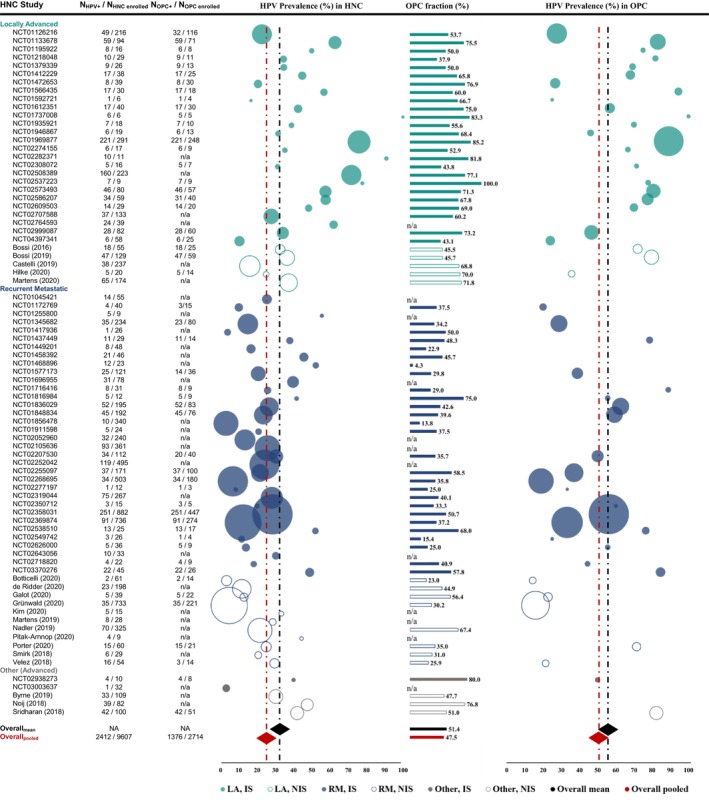
HPV prevalence in LA and RM HNC, OPC fraction, and HPV prevalence in LA and RM OPC. HNC, head and neck cancer; HPV, human papilloma virus; IS, interventional studies; LA, locally and regionally advanced; N, number of patients; NA, not applicable; n/a, not available; NIS, non‐interventional studies; OPC, oropharyngeal cancer; RM, recurrent and/or metastatic. Circle size corresponds to number of patients included in the study indicated, ranging from 6 to 882 patients across 81 studies in HNC, and from 3 to 447 patients across 52 studies in OPC. Overall HPV prevalence is provided as mean and pooled HPV prevalence across studies and depicted as a black and red diamond, respectively. Overall OPC fraction is provided as mean and pooled OPC fraction across studies as a black and red bar, respectively.
TABLE 2.
HPV prevalence in LA and RM HNC and OPC, and OPC fraction, per design and stage.
| HPV prevalence in HNC | |||||||
|---|---|---|---|---|---|---|---|
| n studies | Mean | Median | Range (min, max) | NHNC pts enrolled | NHPV+ HNC pts | Pooled | |
| Interventional Studies | |||||||
| LA | 26 | 47.6% | 43.6% | 10.3%–100.0% | 1624 | 812 | 50.0% |
| RM | 34 | 25.2% | 23.7% | 2.9%–55.6% | 5484 | 1119 | 20.4% |
| Other | 2 | NA | NA | 3.1%–40.0% | 42 | 5 | NA |
| Overall | 62 | 34.5% | 30.4% | 2.9%–100.0% | 7150 | 1936 | 27.1% |
| Non‐interventional Studies | |||||||
| LA | 5 | 29.5% | 32.7% | 16.0%–37.4% | 615 | 173 | 28.1% |
| RM | 11 | 21.4% | 21.5% | 3.3%–44.4% | 1551 | 189 | 12.2% |
| Other | 3 | NA | NA | 30.3%–47.6% | 291 | 114 | NA |
| Overall | 19 | 26.5% | 28.6% | 3.3%–47.6% | 2457 | 476 | 19.4% |
| Interventional and non‐interventional studies | |||||||
| LA | 31 | 44.7% | 37.4% | 10.3%–100.0% | 2239 | 985 | 44.0% |
| RM | 45 | 24.3% | 23.4% | 2.9%–55.6% | 7035 | 1308 | 18.6% |
| Other | 5 | NA | NA | 3.1%–47.6% | 333 | 119 | NA |
| Overall | 81 | 32.6% | 29.6% | 2.9%–100.0% | 9607 | 2412 | 25.1% |
| OPC fraction in HNC | |||||||
| n studies | Mean | Median | Range (min, max) | N HNC pts enrolled | N OPC pts enrolled | Pooled | |
| Interventional Studies | |||||||
| LA | 25 | 65.8% | 67.8% | 37.9%–100.0% | 1585 | 1090 | 68.8% |
| RM | 27 | 38.3% | 37.5% | 4.3%–75.0% | 4213 | 1645 | 39.0% |
| Other | 1 | NA | NA | NA | 10 | 8 | NA |
| Overall | 53 | 52.0% | 50.0% | 4.3%–100.0% | 5808 | 2743 | 47.2% |
| Non‐interventional Studies | |||||||
| LA | 5 | 60.4% | 68.8% | 45.5%–71.8% | 615 | 386 | 62.8% |
| RM | 8 | 39.2% | 33.0% | 23.0%–67.4% | 1499 | 609 | 40.6% |
| Other | 3 | NA | NA | NA | 291 | 166 | NA |
| Overall | 16 | 49.4% | 46.7% | 23.0%–76.8% | 2405 | 1161 | 48.3% |
| Interventional and Non‐interventional Studies | |||||||
| LA | 30 | 64.9% | 68.1% | 37.9%–100.0% | 2200 | 1476 | 67.1% |
| RM | 35 | 38.5% | 37.2% | 4.3%–75.0% | 5712 | 2254 | 39.5% |
| Other | 4 | NA | NA | NA | 301 | 174 | NA |
| Overall | 69 | 51.4% | 50.0% | 4.3%–100.0% | 8213 | 3904 | 47.5% |
| HPV Prevalence in OPC | |||||||
| n studies | Mean | Median | Range (min, max) | N OPC pts enrolled | N HPV+ OPC pts | Pooled | |
| Interventional Studies | |||||||
| LA | 22 | 64.9% | 70.0% | 24.0%–100.0% | 829 | 557 | 67.2% |
| RM | 20 | 50.4% | 52.8% | 18.9%–88.9% | 1436 | 643 | 44.8% |
| Other | 1 | ΝΑ | NA | ΝΑ | 8 | 4 | NA |
| Overall | 43 | 57.8% | 59.2% | 18.9%–100.0% | 2273 | 1204 | 53.0% |
| Non‐interventional Studies | |||||||
| LA | 3 | 62.5% | 72.0% | 35.7%–79.7% | 98 | 70 | 71.4% |
| RM | 5 | 29.1% | 21.4% | 14.3%–71.4% | 292 | 60 | 20.5% |
| Other | 1 | ΝΑ | NA | ΝΑ | 51 | 42 | NA |
| Overall | 9 | 46.2% | 35.7% | 14.3%–82.4% | 441 | 172 | 39.0% |
| Interventional and Non‐interventional Studies | |||||||
| LA | 25 | 64.6% | 70.0% | 24.0%–100.0% | 927 | 627 | 67.6% |
| RM | 25 | 46.1% | 44.4% | 14.3%–88.9% | 1728 | 703 | 40.7% |
| Other | 2 | ΝΑ | NA | ΝΑ | 59 | 46 | NA |
| Overall | 52 | 55.8% | 57.9% | 14.3%–100.0% | 2714 | 1376 | 50.7% |
Abbreviations: HNC, head and neck cancer; HPV, human papilloma virus; LA, locally and regionally advanced; N pts, number of patients; n, number of studies; NA, not applicable; OPC, oropharyngeal cancer; RM, recurrent/metastatic.
3.3. Prevalence of HPV in OPC
In light of the increasing incidence of OPC reported in the literature, and the established role of HPV in OPC pathogenesis and prognosis, we assessed the proportion of HPV+ patients in the highly relevant subgroup of patients with LA and/or RM OPC. The proportion of patients with OPC among those with LA and/or RM HNC, referred to as the OPC fraction, is presented in Figure 2 and summarized in Table 2. The OPC fraction among studies with available HN subsite proportions (n = 69) ranged from 4.3% to 100.0%, with a mean of 51.4%. Based on pooled data, of the 8213 LA and/or RM HNC patients, 3904 had OPC, resulting in a pooled fraction of 47.5%. The mean (and pooled) fractions in IS (n = 53; range 4.3% to 100.0%) and NIS (n = 16; range 23.0% to 76.8%) were 52.0% (47.2%) and 49.4% (48.3%), respectively. Upon analysis by disease stage, the mean (and pooled) OPC fraction was 64.9% (67.1%) in LA HNC studies (n = 30; range 37.9% to 100.0%) and 38.5% (39.5%) in RM HNC studies (n = 35; ranging from 4.3% to 75.0%) (Table 2).
HPV prevalence in LA and/or RM OPC was available for 52 studies and ranged from 14.3% to 100.0%, with a mean value of 55.8% and a pooled fraction of 50.7%. HPV prevalence in LA and/or RM OPC ranged from 18.9% to 100.0% in IS, and from 14.3% to 82.4% in NIS with available data, with respective mean (and pooled) rates of 57.8% (53.0%) and 46.2% (39.0%). Upon analysis by disease stage, the mean (and pooled) HPV prevalence in LA OPC studies was 64.6% (67.6%), ranging from 24.0% to 100.0%, while in RM OPC studies it was 46.1% (40.7%), ranging from 14.3% to 88.9% (Table 2).
3.4. Geographic distribution of HPV prevalence
To gain insight into the availability of published data on the prevalence of HPV across geographical regions, as well as to qualitatively assess potential variations among countries or regions, the geographic distribution of HPV prevalence was addressed as an exploratory objective. Of the 54 countries where the studies included in the analysis of the present review were conducted, 29 were located in Europe, 13 in Asia (including Hong Kong as territory of China), 5 in Southern America, 2 countries each in Northern America and Africa, and 1 country each in Central America and Oceania. Τhe following countries were included in more than ten studies each: United States of America (USA) (44 studies), Germany (16), France (15), Spain (13), Italy (12), Belgium (11), Canada (11), and the United Kingdom (11) (Table 1). Thus, although studies with published data on HPV prevalence in LA and RM HNC through the last decade display a wide geographic distribution, several geographic regions are underrepresented in the literature and further studies would be needed to more accurately capture the global epidemiological picture.
The prevalence of HPV in LA and RM HNC and OPC is summarized per geographical region in Table 3 and Figure S1, while it is also presented per disease stage in Figure S2. Based on the geographic regions included, studies can be broadly divided into those conducted in a single continent and those conducted in multiple continents. In single‐continent HNC studies conducted in Northern America (n = 34), the prevalence of HPV ranged from 8.3% to 100.0%; in Europe (n = 29) from 3.1% to 75.9%; in Eastern Asia (n = 3) from 10.3% to 33.3%. The mean (and pooled) prevalence of HPV among single‐continent studies conducted in Northern America was 46.0% (42.1%), followed by 24.7% (25.3%) in Europe, and 20.1% (15.7%) in Eastern Asia. Studies conducted in Europe were also grouped into those conducted in Northern Europe, Southern Europe, Western Europe, or multiple European regions (including Western, Central/Eastern, and Southern Europe) based on data availability. The respective mean (and pooled) HPV prevalence was 31.9% (63.1%), 23.2% (26.4%), 24.3% (23.5%), and 17.2% (9.4%). In studies conducted in multiple continents (n = 15) the prevalence of HPV ranged from 2.9% to 30.4%, and the mean (and pooled) prevalence of HPV was 19.8% (18.4%).
TABLE 3.
HPV prevalence in LA and RM HNC and OPC, and OPC fraction, per geographic region.
| HPV Prevalence in HNC | |||||||
|---|---|---|---|---|---|---|---|
| n studies | Mean | Median | Range (min, max) | NHNC pts enrolled | NHPV+ HNC pts | Pooled | |
| Northern America | 34 | 46.0% | 43.6% | 8.3%–100.0% | 1923 | 809 | 42.1% |
| Europe | 29 | 24.7% | 22.7% | 3.1%–75.9% | 2804 | 710 | 25.3% |
| Northern Europe | 5 | 31.9% | 20.7% | 11.5%–75.9% | 377 | 238 | 63.1% |
| Southern Europe | 4 | 23.2% | 26.6% | 3.3%–36.4% | 284 | 75 | 26.4% |
| Western Europe | 17 | 24.3% | 25.0% | 3.1%–47.6% | 1381 | 325 | 23.5% |
| Multiple European regions a | 3 | 17.2% | 13.3% | 6.8%–31.6% | 762 | 72 | 9.4% |
| Eastern Asia | 3 | 20.1% | 16.7% | 10.3%–33.3% | 121 | 19 | 15.7% |
| Multiple continents | 15 | 19.8% | 21.6% | 2.9%–30.4% | 4759 | 874 | 18.4% |
| OPC Fraction in HNC | |||||||
| n studies | Mean | Median | Range (min, max) | N HNC pts enrolled | N OPC pts enrolled | Pooled | |
| Northern America | 31 | 56.9% | 57.8% | 4.3%–100.0% | 1797 | 1051 | 58.5% |
| Europe | 24 | 53.7% | 51.9% | 15.4%–85.2% | 2462 | 1371 | 55.7% |
| Northern Europe | 5 | 41.7% | 33.3% | 15.4%–85.2% | 377 | 273 | 72.4% |
| Southern Europe | 4 | 47.8% | 45.6% | 23.0%–76.9% | 284 | 128 | 45.1% |
| Western Europe | 13 | 60.3% | 60.2% | 37.5%–80.0% | 1279 | 777 | 60.8% |
| Multiple European regions a | 2 | 52.1% | 52.1% | 35.8%–68.4% | 522 | 193 | 37.0% |
| Eastern Asia | 2 | 33.0% | 33.0% | 22.9%–43.1% | 106 | 36 | 34.0% |
| Multiple continents | 12 | 36.0% | 36.5% | 13.8%–58.5% | 3848 | 1446 | 37.6% |
| HPV Prevalence in OPC | |||||||
| n studies | Mean | Median | Range (min, max) | N OPC pts enrolled | N HPV+ OPC pts | Pooled | |
| Northern America | 25 | 70.0% | 75.0% | 21.4%–100.0% | 577 | 423 | 73.3% |
| Europe | 17 | 44.1% | 44.4% | 14.3%–89.1% | 829 | 426 | 51.4% |
| Northern Europe | 4 | 61.4% | 65.7% | 25.0%–89.1% | 264 | 230 | 87.1% |
| Southern Europe | 4 | 48.2% | 49.3% | 14.3%–79.7% | 128 | 75 | 58.6% |
| Western Europe | 7 | 35.3% | 35.7% | 20.0%–50.0% | 244 | 81 | 33.2% |
| Multiple European regions a | 2 | 32.5% | 32.5% | 18.9%–46.2% | 193 | 40 | 20.7% |
| Eastern Asia | 1 | NA | NA | NA | 25 | 6 | 24.0% |
| Multiple continents | 9 | 41.6% | 38.9% | 15.8%–59.2% | 1283 | 521 | 40.6% |
Abbreviations: HPV, human papilloma virus; HNC, head and neck cancer; N pts, number of patients; n, number of studies; NA, not applicable; OPC, oropharyngeal cancer.
The category of multiple European regions includes multi‐country studies conducted in Europe. These studies were conducted in Western, Central/Eastern, and Southern Europe for HNC, and Southern and Western Europe for OPC.
Among studies with available HN subsite proportions (regardless of HPV status), mean (and pooled) OPC fraction was 56.9% (58.5%), 53.7% (55.7%), 33.0% (34.0%), and 36.0% (37.6%) in studies conducted in Northern America (n = 31), Europe (n = 24), Eastern Asia (n = 2), and multiple continents (n = 12), respectively (Table 3). Moreover, based on the proportion of HPV+ OPC patients in studies with available data, the mean (and pooled) prevalence of HPV in LA and RM OPC was 70.0% (73.3%) in studies conducted in Northern America (n = 25); 44.1% (51.4%) in studies conducted in Europe (n = 17), and 41.6% (40.6%) in studies conducted in multiple continents (n = 9). In the only single‐country study conducted in Eastern Asia, the prevalence of HPV in LA and RM OPC was 24.0% (Table 3). Within Europe, the mean (and pooled) prevalence of HPV in LA and RM OPC was 61.4% (87.1%) in Northern Europe, 48.2% (58.6%) in Southern Europe, 35.3% (33.2%) in Western Europe, and 32.5% (20.7%) in multiple European regions (including Southern and Western Europe). Taken together, the above data illustrate high rates of HPV prevalence in LA and RM HNC and OPC across different geographical regions.
3.5. HPV detection techniques
In the absence of HPV diagnostic tests with regulatory approval for HNC over the examined period, and given that HPV testing is generally recommended for all newly diagnosed OPSCC but is not warranted for the other HNC types, the present review aimed to capture HPV detection techniques utilized in the included studies. HPV detection techniques are retrieved and analyzed as reported by the authors in the publications. Information on reported HPV detection assays across the included HNC studies are presented in Figure S3. In total, HPV status was assessed in any HN anatomical site in 37 studies (45.7%), in OPX only in 38 studies (46.9%) while 6 studies (7.4%) did not provide information on the site examined. With respect to specific methodologies, of the 81 studies, 47 (58.0%) reported using a p16INK4a‐based method, 2 studies (2.5%) employed quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR), 2 studies (2.5%) employed in situ hybridization (ISH), while in 1 study each the detection method was referred to as DNA testing, PCR (qRT‐PCR), and immunohistochemistry (IHC). Eight studies (9.9%) reported using multiple detection techniques to determine HPV status at a cohort level, even though at a patient level HPV status could also have been derived solely based on a single technique. For the remaining 19 (23.5%) studies the authors did not provide any relevant information. HPV detection methods are also presented for IS and NIS, by disease stage, and site examined in Figure S3. Irrespective of grouping, “p16‐based” detection methodologies were the most frequently reported across studies.
3.6. HPV prevalence in OPC using solely a p16‐based method
Considering that p16 overexpression is generally used as a surrogate marker for the presence of HPV in OPSCC and the recommendation for p16 testing in OPSCC clinical management, 14 , 15 a supplementary analysis was performed by isolating the studies reporting solely a p16‐based method for HPV testing and having available results in OPC. In total, 30 studies were included in this analysis (26 IS and 4 NIS; 16 LA and 14 RM), with prevalence of HPV ranging from 15.8% to 100.0% and a mean (and pooled) HPV prevalence of 57.2% (52.6%) (Figure S4), further supporting the main outcomes of this evidence synthesis.
3.7. Distribution of HPV prevalence by number of enrolled HNC patients
As a means to evaluate the potential effect of variations across individual sample sizes on the primary outcome of overall prevalence of HPV in LA and RM HNC, the prevalence of HPV reported for each included study was plotted against the respective sample size (Figure 3). No obvious asymmetry was observed around the calculated overall mean HPV prevalence. Based on this distribution, no apparent bias in the estimation of the study primary outcome arising from sample size can be inferred.
FIGURE 3.
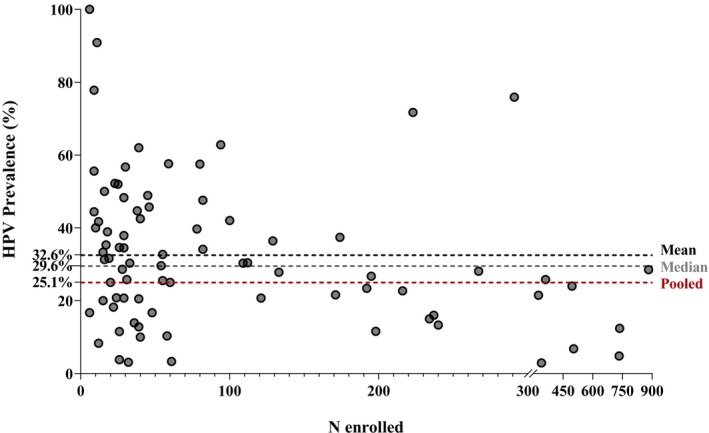
HPV prevalence by number of HNC cases in each study. HPV, human papilloma virus; N, number of enrolled HNC patients. HPV prevalence from studies included in the evidence synthesis is plotted against each study's size. To visualize distribution of studies around the overall HPV prevalence and potential effect of sample size, estimated mean, median, and pooled HPV prevalence across studies are provided in dotted lines.
4. DISCUSSION
Contemporary data on HPV burden in advanced HNC are needed considering that available published reviews are not only outdated but also lack disease stage‐specific estimates. Thus, the present SLR aimed to fill this gap through a systematic review of published data on the prevalence of HPV in LA and RM HNC captured over the last decade. Using specific selection criteria, 81 studies were identified, reporting data from numerous countries and covering available literature from 2010 to 2020. The results revealed a considerable HPV prevalence in LA and RM HNC and OPC across different regions. Numerically highest rates were reported in the LA setting, as well as in the regions of Northern America and Northern Europe. In addition, the data uncover substantial variation in HPV prevalence among studies, as well as in HPV testing methodologies.
The primary outcome of “ALARM,” prevalence of HPV in LA and RM HNC, was first retrieved from each study as the minimum proportion of HPV+ cases, unadjusted for HPV status data availability. In this manner, selection bias which would otherwise arise from increased testing of a certain type of cases as per the investigator's clinical judgment was minimized to the extent possible. The average HPV prevalence in the LA and RM setting of HNC across studies was estimated at 32.6%, while the pooled HPV prevalence, derived from the sum of HPV+ patients over the sum of enrolled HNC patients in all studies was 25.1%. Other reviews have estimated HPV prevalence in HNC (any stage) at 22%–35%, though based on literature data preceding 2008. 31 , 32 , 33 , 34 A more recent review of studies published between 2003 and 2014 estimated the mean percentage of HPV+ HNSCC patients at 32.9%, 5 which was however derived from patients with available HPV status data only, regardless of disease stage. When we plotted reported HPV prevalence against the size of the population in each study, an apparently even distribution around the mean was observed, suggesting there was no significant bias due to outliers representing very small or very large sample sizes. On the other hand, data are slightly skewed in relation to the pooled estimate, which could derive from the large proportion of patients with unknown HPV status in some studies. Indeed, the top 10 studies with highest sample size contributed data for 51% of the total number of patients included in this SLR (4933 out of 9607) but more than half of those patients had unknown HPV status. This indicates that the estimated pooled prevalence of HPV is most likely underestimated, and also explains why the pooled estimate is smaller than the mean. As the latter does not account for missing data either, and given that previous literature suggests that more than 70% of HPV+ HNC patients have LA and/or RM HNC, 5 , 31 the mean HPV prevalence of 32.6% estimated here is probably lower than the actual proportion of HPV+ HNC fraction, further highlighting the important contributing role of HPV in LA and RM HNC.
Evidence to date suggests that HPV prevalence is higher among OPC than other HN subsites. 5 , 6 , 7 , 36 , 135 , 136 Importantly, though the prognostic impact of HPV status in non‐OPC HNC is unclear, 24 , 25 , 26 HPV infection is an established prognostic indicator of treatment outcome in OPC. 14 , 15 , 16 Hence, findings of the overall HPV prevalence herein should be interpreted in the context of the contribution of OPC, while special attention should also be drawn to HPV+ estimates within this highly relevant HNC subgroup.
Irrespective of HPV status, almost half of the overall population in “ALARM” had OPC. The etiological role of HPV in oropharyngeal carcinogenesis is widely recognized 4 and p16 testing is recommended for OPSCC management, 12 , 14 , 21 hence HPV testing is expected to be more frequent among OPC patients. Indeed, in “ALARM” in almost half of the studies (47%), HPV status was assessed in OPX only, reflecting current clinical practice. Though the study has not been designed to make any such comparisons, the prevalence of HPV is numerically higher in OPC than in not site‐specific HNC, consistent with the published literature. 5 , 6 , 7 Specifically in LA and RM OPC, our data report the mean HPV prevalence at 55.8%, with a pooled prevalence of 50.7%, which is almost double than the overall rate in LA and RM HNC. The prevalence of HPV in LA and RM OPC is close to that reported in a previous review, which showed a mean of 49.9% HPV‐positive OPSCC, most of which comprised of stage III/IV disease (85.7%). 5 Furthermore, in our supplementary analysis of HPV status in OPC tested using solely a p16‐based method which is the guideline recommended method, 12 , 13 , 21 , 22 , 23 the mean prevalence of HPV was 57.2%, further supporting the robustness of the main study outcomes. As OPSCC has been increasing worldwide over the past years, 9 , 35 , 136 , 137 , 138 our results reinforce the substantial contribution of HPV+ OPC to the overall burden especially in LA and RM HNC.
In “ALARM,” HPV prevalence was also investigated by disease stage. Estimates of HPV prevalence in LA HNC were numerically higher than in RM HNC (mean: 44.7% vs. 24.3%; pooled: 44.0% vs. 18.6%). This should also be interpreted taking into account the OPC fraction and HPV status availability, both of which were higher among LA than in RM patients (65% vs. 39% and at least 70% vs. 48%, respectively). Nevertheless, a numerically higher HPV prevalence was also noted in LA than in RM OPC patients (mean: 64.6% vs. 46.1%; pooled: 67.6% vs. 40.7%), which might be worth investigating further, especially considering that a large proportion of HNC cases are either diagnosed at LA stage or experience disease recurrence from LA to RM stage, and that HPV+ cancers are considered to have better prognosis. 28 , 29 , 30
Previous literature has shown that the incidence of HNC anatomical subsites classified as a proxy for HPV infection, including the oropharynx, has been rising and an increased OPSCC HPV prevalence has been observed over the years especially in Northern America and Northern Europe. 9 , 35 , 136 , 137 , 138 Moreover, HPV prevalence in OPC was higher in more developed regions than in developing countries. 6 , 34 , 138 This is also reflected by the outcomes of the present review in terms of the heterogeneous geographic distribution of HPV prevalence being highest in studies conducted in Northern America and Northern Europe. The observed patterns could be attributable to several factors, such as HPV epidemiology which shows variation by ethnicity and gender, and is linked to lifestyle behaviors. 136 , 139 , 140 , 141 , 142 , 143 , 144 Nevertheless, regardless of the regional variations in HPV prevalence and the factors that could contribute to the observed patterns, the results of the present study demonstrate that, though ranking lower in terms of prevalence than Northern America and Northern Europe, other parts of Europe and the globe, in general, have substantial rates of HPV+ HNC. These findings suggest that the need to implement preventive measures against HPV is imperative worldwide, and not only in the countries with the highest HPV burden.
The results of “ALARM” reveal a considerable inconsistency in the availability of HPV prevalence data across countries and continents, with many parts of the world being underrepresented. In particular, countries in continents other than Northern America or Europe were mainly represented by the group of multi‐continent studies, which could not be stratified further as relevant publications did not contain the required level of detail. Thus, there is a dearth of information on the HPV burden in those countries. This is in line with previous literature on specific ethnic groups which seems to be lacking in terms of population‐based studies. 145 Altogether these observations suggest a need for further investigation, in order to represent all geographical regions in the literature and better assess the burden of this disease.
Another factor that could be contributing to the variation in reported HPV prevalence across studies is the heterogeneity in HPV detection assays. Many of the studies included in the present SLR reported p16‐based detection as the main assay (63% of studies, including four studies which used multiple techniques) but differences in the exact methodology, including specimen storage methods, p16‐positivity threshold used to define HPV status, and source of result (e.g., medical records archived or freshly collected samples) cannot be excluded. Furthermore, methodology was not specified for one fourth of the studies of the evidence synthesis, uncovering significant literature gaps. As depicted in the present review, clinical practice usually relies on a single technique for HPV status assessment, even though each technique has its limitations. In OPC, p16 testing is generally the preferred method of HPV detection, yet for other HNC sites there is no clear guidance on the HPV testing methodology. 12 , 18 , 146 Novel diagnostic algorithms for the detection of HPV‐driven HNC are being examined, with the combined use of HPV‐DNA testing followed by p16 IHC having shown high concordance rates with E6*I mRNA detection and proposed to be helpful in OPC and oral cavity cancers. 147 To improve the precision of HPV burden estimates, standardization of HPV detection is necessary.
Methodological limitations are presumably also a source of bias in the present analysis, as in non‐OPC HNC cases where HPV prevalence has been derived solely based on p16 overexpression, the estimates may not be accurate. Along this line, in the context of the primary outcome, the HPV prevalence, is possibly underestimated, as a result of the large proportion of patients with unknown HPV status. In any case, such limitations of the present review mainly derive from limitations of the individual studies included. In addition, certain limitations are due to the selection criteria applied in the present literature search, such as the exclusion of studies written in languages other than English, studies published in report format, for example, on government websites or studies that did not specify any study period or cancer stage. The above criteria may impact on the representativeness of the outcomes, but were employed as a method to ensure quality of included data. It should also be noted this SLR was designed to provide descriptive insight into the relevant literature from a qualitative point of view, including all studies that met a minimum set of criteria, with no restrictions in geographic location or patient eligibility (i.e., target indication, line of therapy, histology, or HN subsite) which increase the generalizability of the present findings. The latter is further enhanced by the fact that overlapping data have been avoided to the extent feasible based on geographic location, site, period of enrollment, and eligibility criteria in order to represent unique cases of HNC.
The results of the present literature search indicate a substantial proportion of HPV+ patients among LA and RM HNC patients in the last decade, which merits consideration particularly in light of increased awareness campaigns and preventive measures availability. HPV vaccines are effective in protecting against high‐risk HPV types in women and men. 135 , 148 , 149 , 150 , 151 , 152 In Europe, most countries recommend HPV vaccination, with many of them having introduced gender‐neutral HPV vaccination. 153 , 154 , 155 HPV prevalence estimates can inform policy decisions and justify strategies to aim for higher levels of HPV vaccination coverage as well as ensure gender neutral vaccination for adolescents, timely catch‐up programs, and the possibility to vaccinate adults. Such measures are anticipated to prevent a significant proportion of LA and/or RM HNC especially in regions with a very high burden of HPV‐attributable HNC.
5. CONCLUSIONS
This SLR is the first review on HPV burden, which focused on LA and RM HNC and reported results from the last decade (2010–2020). More than 80 studies provided information on HPV status demonstrating that a substantial HPV burden exists with at least one in four HNC cases being HPV+ and at least half of OPC cases contributing to this proportion. The proportion of HPV+ cases was considerable in most regions examined, and highest in Northern America and Northern Europe, with at least one in three LA and/or RM HNC cases being HPV+. More quality data are however needed for a better representation of geographic diversity, and implementation of homogeneous HPV detection methodologies is necessary to allow for more precise HPV burden estimation. Nevertheless, the results of this evidence synthesis come to reinforce the significant role of HPV in LA and RM HNC disease with a considerable proportion of LA and RM HNC cases being potentially preventable, highlighting the potential benefit from increasing HPV immunization coverage.
AUTHOR CONTRIBUTIONS
Sofia Agelaki: Conceptualization (equal); writing – review and editing (lead). Ioannis Boukovinas: Conceptualization (supporting); writing – review and editing (equal). Ilias Athanasiadis: Conceptualization (supporting); writing – review and editing (equal). Georgios Trimis: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); visualization (equal); writing – review and editing (equal). Ioannis Dimitriadis: Conceptualization (equal); writing – review and editing (equal). Lazaros Poughias: Conceptualization (supporting); writing – review and editing (equal). Edith Morais: Conceptualization (supporting); writing – review and editing (equal). Ugne Sabale: Conceptualization (supporting); writing – review and editing (equal). Goran Bencina: Conceptualization (equal); writing – review and editing (equal). Charalampos Athanasopoulos: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); supervision (lead); visualization (lead); writing – original draft (lead).
FUNDING INFORMATION
This work was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc., Rahway, NJ, USA.
CONFLICT OF INTEREST STATEMENT
Sofia Agelaki is a member of the executive board of the Hellenic Society of Medical Oncology, has served as an investigator in clinical studies for MSD Greece, has received consulting fees for participating in Expert Input Forums for MSD Greece, and has received lecture honoraria by MSD Greece. Ioannis Boukovinas, former president of the Hellenic Society of Medical Oncology, has served as an investigator in clinical studies for MSD Greece, has received consulting fees for participating in Advisory Boards for MSD Greece, has received lecture honoraria by MSD Greece, and has received support for attending international congress from MSD Greece. Ilias Athanasiadis has served as an investigator in clinical studies for MSD Greece, has received consulting fees for participating in Advisory Boards for MSD Greece, has received lecture honoraria by MSD Greece, and has received support for attending international congress from MSD Greece. Georgios Trimis, Ioannis Dimitriadis, Lazaros Poughias, and Charalampos Athanasopoulos are employees of MSD Greece and own stock in Merck & Co., Inc., Rahway, NJ, US. Edith Morais is an employee of MSD France and owns stock in Merck & Co., Inc., Rahway, NJ, US. Sabale Ugne is an employee of MSD Sweden and owns stock in Merck & Co., Inc., Rahway, NJ, US. Goran Bencina is an employee of MSD Spain and owns stock in Merck & Co., Inc., Rahway, NJ, US.
Supporting information
Data S1: Supporting information.
ACKNOWLEDGMENTS
The authors wish to thank Qualitis SA, a member of Optimapharm Group, for their support in data acquisition, data analysis, and medical writing undertaken as part of the project work performed for MSD Greece.
Agelaki S, Boukovinas I, Athanasiadis I, et al. A systematic literature review of the human papillomavirus prevalence in locally and regionally advanced and recurrent/metastatic head and neck cancers through the last decade: The “ALARM” study. Cancer Med. 2024;13:e6916. doi: 10.1002/cam4.6916
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are included in the article/supplementary material. Data sharing is not applicable as no new data were created or analyzed in this study.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. doi: 10.1038/s41572-020-00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418‐424. doi: 10.1016/s0300-9785(83)80033-7 [DOI] [PubMed] [Google Scholar]
- 4. International Agency for Research on Cancer . Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1‐636. [PMC free article] [PubMed] [Google Scholar]
- 5. Götz C, Bischof C, Wolff KD, Kolk A. Detection of HPV infection in head and neck cancers: promise and pitfalls in the last ten years: a meta‐analysis. Mol Clini Oncol. 2019;10(1):17‐28. doi: 10.3892/mco.2018.1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta‐analysis. Lancet Oncol. 2014;15(12):1319‐1331. doi: 10.1016/s1470-2045(14)70471-1 [DOI] [PubMed] [Google Scholar]
- 7. Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. Natl Cancer Instit. 2016;108(6):djv403. doi: 10.1093/jnci/djv403 [DOI] [PubMed] [Google Scholar]
- 8. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294‐4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta‐analysis of trends by time and region. Head Neck. 2013;35(5):747‐755. doi: 10.1002/hed.22015 [DOI] [PubMed] [Google Scholar]
- 10. Chaturvedi AK, Anderson WF, Lortet‐Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550‐4559. doi: 10.1200/jco.2013.50.3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spence T, Bruce J, Yip KW, Liu FF. HPV associated head and neck cancer. Cancer. 2016;8(8):75. doi: 10.3390/cancers8080075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS‐ESMO‐ESTRO clinical practice guidelines for diagnosis, treatment and follow‐up. Annal Oncol. 2020;31(11):1462‐1475. doi: 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 13. National Comprehensive Cancer Network (NCCN) . Head and Neck Cancers Version 3. 2021.
- 14. Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American joint committee on cancer (AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep. 2019;21(6):52. doi: 10.1007/s11912-019-0799-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nauta IH, Rietbergen MM, van Bokhoven A, et al. Evaluation of the eighth TNM classification on p16‐positive oropharyngeal squamous cell carcinomas in The Netherlands and the importance of additional HPV DNA testing. Annal Oncol. 2018;29(5):1273‐1279. doi: 10.1093/annonc/mdy060 [DOI] [PubMed] [Google Scholar]
- 16. Zakeri K, Dunn L, Lee N. HPV‐associated oropharyngeal cancer de‐escalation strategies and trials: past failures and future promise. J Surg Oncol. 2021;124(6):962‐966. doi: 10.1002/jso.26696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caudell JJ, Gillison ML, Maghami E, et al. NCCN guidelines® insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20(3):224‐234. doi: 10.6004/jnccn.2022.0016 [DOI] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network . NCCN Guidelines Version 2. 2022. Accessed September 27, 2022 https://wwwnccnorg/guidelines/guidelines‐detail?category=1&id=1437
- 19. Kim KY, Lewis JS Jr, Chen Z. Current status of clinical testing for human papillomavirus in oropharyngeal squamous cell carcinoma. J Pathol Clin Res. 2018;4(4):213‐226. doi: 10.1002/cjp2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute state of the science meeting, November 9‐10, 2008, Washington, D.C. Head Neck. 2009;31(11):1393‐1422. doi: 10.1002/hed.21269 [DOI] [PubMed] [Google Scholar]
- 21. Economopoulou P, Kotsantis I, Psyrri A. Special issue about head and neck cancers: HPV positive cancers. Int J Mol Sci. 2020;21(9):3388. doi: 10.3390/ijms21093388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fakhry C, Lacchetti C, Rooper LM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the College of American Pathologists Guideline. J Clin Oncol. 2018;36(31):3152‐3161. doi: 10.1200/jco.18.00684 [DOI] [PubMed] [Google Scholar]
- 23. Lewis JS Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Labor Med. 2018;142(5):559‐597. doi: 10.5858/arpa.2017-0286-CP [DOI] [PubMed] [Google Scholar]
- 24. Petrelli F, Cin ED, Ghidini A, et al. Human papillomavirus infection and non‐oropharyngeal head and neck cancers: an umbrella review of meta‐analysis. Europ Arch Oto‐Rhino‐Laryngol. 2023;280(9):3921–3930. doi: 10.1007/s00405-023-08027-4 [DOI] [PubMed] [Google Scholar]
- 25. Nauta IH, Heideman DAM, Brink A, et al. The unveiled reality of human papillomavirus as risk factor for oral cavity squamous cell carcinoma. Int J Cancer. 2021;149(2):420‐430. doi: 10.1002/ijc.33514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gallus R, Gheit T, Holzinger D, et al. Prevalence of HPV infection and p16(INK4a) overexpression in surgically treated laryngeal squamous cell carcinoma. Vaccine. 2022;10(2):204. doi: 10.3390/vaccines10020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh P, Bennett B, Bailey T, et al. Real‐world study of the impact of recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) on quality of life and productivity in Europe. BMC Cancer. 2021;21(1):854. doi: 10.1186/s12885-021-08557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Argiris A, Harrington KJ, Tahara M, et al. Evidence‐based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Fronti Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proceed. 2016;91(3):386‐396. doi: 10.1016/j.mayocp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 30. Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(6):761‐770. doi: 10.6004/jnccn.2017.0101 [DOI] [PubMed] [Google Scholar]
- 31. Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta‐analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Termine N, Panzarella V, Falaschini S, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta‐analysis (1988–2007). Ann Oncology. 2008;19(10):1681‐1690. doi: 10.1093/annonc/mdn372 [DOI] [PubMed] [Google Scholar]
- 33. Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta‐analysis. Int J Cancer. 2007;121(8):1813‐1820. doi: 10.1002/ijc.22851 [DOI] [PubMed] [Google Scholar]
- 34. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol, Biomark Prev. 2005;14(2):467‐475. doi: 10.1158/1055-9965.epi-04-0551 [DOI] [PubMed] [Google Scholar]
- 35. Reuschenbach M, Tinhofer I, Wittekindt C, Wagner S, Klussmann JP. A systematic review of the HPV‐attributable fraction of oropharyngeal squamous cell carcinomas in Germany. Cancer Med. 2019;8(4):1908‐1918. doi: 10.1002/cam4.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Sanjosé S, Serrano B, Tous S, et al. Burden of human papillomavirus (HPV)‐related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI cancer. Spectrum. 2019;2(4):pky045. doi: 10.1093/jncics/pky045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinkiewicz M, Dorobisz K, Zatoński T. Human papillomavirus‐associated head and neck cancers. Where Are we now? A systematic review. Cancer Manag Res. 2022;14:3313‐3324. doi: 10.2147/cmar.s379173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Timbang MR, Sim MW, Bewley AF, Farwell DG, Mantravadi A, Moore MG. HPV‐related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15(7–8):1920‐1928. doi: 10.1080/21645515.2019.1600985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diana G, Corica C. Human papilloma virus vaccine and prevention of head and neck cancer, what is the current evidence? Oral Oncol. 2021;115:105168. doi: 10.1016/j.oraloncology.2020.105168 [DOI] [PubMed] [Google Scholar]
- 40. Tumban E. A current update on human papillomavirus‐associated head and neck cancers. Viruses. 2019;11(10):922. doi: 10.3390/v11100922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on Oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262‐267. doi: 10.1200/jco.2017.75.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grün N, Ährlund‐Richter A, Franzén J, et al. Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up‐study 2013‐2014 after gradual introduction of public HPV vaccination. Infect Dis. 2015;47(1):57‐61. doi: 10.3109/00365548.2014.964764 [DOI] [PubMed] [Google Scholar]
- 43. Giuliano AR, Wilkin T, Bautista OM, et al. Design of a phase III efficacy, immunogenicity, and safety study of 9‐valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemp Clin Trials. 2022;115:106592. doi: 10.1016/j.cct.2021.106592 [DOI] [PubMed] [Google Scholar]
- 44. ClinicalTrials.gov . Bethesda (MD): National Library of Medicine (US). Identifier: NCT04255849. Accessed September 27, 2022. https://clinicaltrialsgov/ct2/show/NCT04255849
- 45. International Agency for Research on Cancer . Cancer Today. Data & Methods. Population dictionary. Accessed November 29, 2021. https://gcoiarcfr/today/data‐sources‐methods#population‐dictionary
- 46. Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase a inhibitor, in patients with breast cancer, small‐cell lung cancer, non‐small‐cell lung cancer, head and neck squamous‐cell carcinoma, and gastro‐oesophageal adenocarcinoma: a five‐arm phase 2 study. Lancet Oncol. 2015;16(4):395‐405. doi: 10.1016/s1470-2045(15)70051-3 [DOI] [PubMed] [Google Scholar]
- 47. Fietkau R, Hecht M, Hofner B, et al. Randomized phase‐III‐trial of concurrent chemoradiation for locally advanced head and neck cancer comparing dose reduced radiotherapy with paclitaxel/cisplatin to standard radiotherapy with fluorouracil/cisplatin: the PacCis‐trial. Radiothera Oncol. 2020;144:209‐217. doi: 10.1016/j.radonc.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 48. Villaflor VM, Melotek JM, Karrison TG, et al. Response‐adapted volume de‐escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27(5):908‐913. doi: 10.1093/annonc/mdw051 [DOI] [PubMed] [Google Scholar]
- 49. Grünwald V, Keilholz U, Boehm A, et al. TEMHEAD: a single‐arm multicentre phase II study of temsirolimus in platin‐ and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN group (AIO). Ann Oncol. 2015;26(3):561‐567. doi: 10.1093/annonc/mdu571 [DOI] [PubMed] [Google Scholar]
- 50. Day TA, Shirai K, O'Brien PE, et al. Inhibition of mTOR signaling and clinical activity of rapamycin in head and neck cancer in a window of opportunity trial. Clin Cancer Res. 2019;25(4):1156‐1164. doi: 10.1158/1078-0432.ccr-18-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Srivastava RM, Ettyreddy A, Ferris RL. Cetuximab ameliorates suppressive phenotypes of myeloid antigen presenting cells in head and neck cancer patients. J Immunother Cancer. 2015;3:54. doi: 10.1186/s40425-015-0097-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bowles DW, Keysar SB, Eagles JR, et al. A pilot study of cetuximab and the hedgehog inhibitor IPI‐926 in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016;53:74‐79. doi: 10.1016/j.oraloncology.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second‐line treatment in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck progressing on or after platinum‐based therapy (LUX‐Head & Neck 1): an open‐label, randomised phase 3 trial. Lancet Oncol. 2015;16(5):583‐594. doi: 10.1016/s1470-2045(15)70124-5 [DOI] [PubMed] [Google Scholar]
- 54. Cohen EEW, Licitra LF, Burtness B, et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second‐line recurrent and/or metastatic head and neck cancer. Ann Oncol. 2017;28(10):2526‐2532. doi: 10.1093/annonc/mdx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Camille N, Babu R, Bakst RL, et al. Phase I study of cabazitaxel‐PF induction chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol. 2014;32(15_suppl):e17009. doi: 10.1200/jco.2014.32.15_suppl.e17009 [DOI] [Google Scholar]
- 56. Weiss J, Gilbert J, Deal AM, et al. Induction chemotherapy with carboplatin, nab‐paclitaxel and cetuximab for at least N2b nodal status or surgically unresectable squamous cell carcinoma of the head and neck. Oral Oncol. 2018;84:46‐51. doi: 10.1016/j.oraloncology.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 57. Machiels JP, Specenier P, Krauß J, et al. A proof of concept trial of the anti‐EGFR antibody mixture Sym004 in patients with squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2015;76(1):13‐20. doi: 10.1007/s00280-015-2761-4 [DOI] [PubMed] [Google Scholar]
- 58. Trieu V, Pinto H, Riess JW, et al. Weekly docetaxel, cisplatin, and Cetuximab in palliative treatment of patients with squamous cell carcinoma of the head and neck. Oncologist. 2018;23(7):764.e86. doi: 10.1634/theoncologist.2017-0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim HS, Kwon HJ, Jung I, et al. Phase II clinical and exploratory biomarker study of dacomitinib in patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Clin Cancer Res. 2015;21(3):544‐552. doi: 10.1158/1078-0432.ccr-14-1756 [DOI] [PubMed] [Google Scholar]
- 60. Jimeno A, Posner MR, Wirth LJ, et al. A phase 2 study of dalantercept, an activin receptor‐like kinase‐1 ligand trap, in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Cancer. 2016;122(23):3641‐3649. doi: 10.1002/cncr.30317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McMichael EL, Benner B, Atwal LS, et al. A phase I/II trial of Cetuximab in combination with Interleukin‐12 administered to patients with Unresectable primary or recurrent head and neck squamous cell carcinoma. Clin Cancer Res. 2019;25(16):4955‐4965. doi: 10.1158/1078-0432.ccr-18-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strojan P, Zakotnik B, Žumer B, et al. Skin reaction to Cetuximab as a criterion for treatment selection in head and neck cancer. Anticancer Res. 2018;38(7):4213‐4220. doi: 10.21873/anticanres.12717 [DOI] [PubMed] [Google Scholar]
- 63. Adkins D, Ley J, Michel L, et al. Nab‐paclitaxel, cisplatin, and 5‐fluorouracil followed by concurrent cisplatin and radiation for head and neck squamous cell carcinoma. Oral Oncol. 2016;61:1‐7. doi: 10.1016/j.oraloncology.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adkins D, Ley J, Oppelt P, et al. Impact on health‐related quality of life of induction chemotherapy compared with concurrent cisplatin and radiation therapy in patients with head and neck. Cancer Clini Oncol. 2019;31(9):e123‐e131. doi: 10.1016/j.clon.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 65. Fayette J, Wirth L, Oprean C, et al. Randomized phase II study of Duligotuzumab (MEHD7945A) vs. Cetuximab in squamous cell carcinoma of the head and neck (MEHGAN study). Front Oncol. 2016;6:232. doi: 10.3389/fonc.2016.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bauman JE, Duvvuri U, Thomas S, et al. Phase 1 study of EGFR‐antisense DNA, cetuximab, and radiotherapy in head and neck cancer with preclinical correlatives. Cancer. 2018;124(19):3881‐3889. doi: 10.1002/cncr.31651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weiss JM, Grilley‐Olson JE, Deal AM, et al. Phase 2 trial of neoadjuvant chemotherapy and transoral endoscopic surgery with risk‐adapted adjuvant therapy for squamous cell carcinoma of the head and neck. Cancer. 2018;124(14):2986‐2992. doi: 10.1002/cncr.31526 [DOI] [PubMed] [Google Scholar]
- 68. Kochanny SE, Worden FP, Adkins DR, et al. A randomized phase 2 network trial of tivantinib plus cetuximab versus cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Cancer. 2020;126(10):2146‐2152. doi: 10.1002/cncr.32762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vokes EE, Worden FP, Adkins D, et al. A randomized phase II trial of the MET inhibitor tivantinib + cetuximab versus cetuximab alone in patients with recurrent/metastatic head and neck cancer. J Clin Oncol. 2015;33(15_suppl):6060. doi: 10.1200/jco.2015.33.15_suppl.6060 [DOI] [Google Scholar]
- 70. Adkins D, Mehan P, Ley J, et al. Pazopanib plus cetuximab in recurrent or metastatic head and neck squamous cell carcinoma: an open‐label, phase 1b and expansion study. Lancet Oncol. 2018;19(8):1082‐1093. doi: 10.1016/s1470-2045(18)30350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chiu JW, Chan K, Chen EX, Siu LL, Abdul Razak AR. Pharmacokinetic assessment of dacomitinib (pan‐HER tyrosine kinase inhibitor) in patients with locally advanced head and neck squamous cell carcinoma (LA SCCHN) following administration through a gastrostomy feeding tube (GT). Invest New Drugs. 2015;33(4):895‐900. doi: 10.1007/s10637-015-0245-3 [DOI] [PubMed] [Google Scholar]
- 72. Brisson RJ, Kochanny S, Arshad S, et al. A pilot study of the pan‐class I PI3K inhibitor buparlisib in combination with cetuximab in patients with recurrent or metastatic head and neck cancer. Head Neck. 2019;41(11):3842‐3849. doi: 10.1002/hed.25910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferris RL, Saba NF, Gitlitz BJ, et al. Effect of adding Motolimod to standard combination chemotherapy and Cetuximab treatment of patients with squamous cell carcinoma of the head and neck: the Active8 randomized clinical trial. JAMA Oncol. 2018;4(11):1583‐1588. doi: 10.1001/jamaoncol.2018.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of Pembrolizumab in biomarker‐unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE‐012 expansion cohort. J Clin Oncol. 2016;34(32):3838‐3845. doi: 10.1200/jco.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): an open‐label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956‐965. doi: 10.1016/s1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 76. Mehra R, Seiwert TY, Gupta S, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long‐term follow‐up in KEYNOTE‐012. Br J Cancer. 2018;119(2):153‐159. doi: 10.1038/s41416-018-0131-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guo Y, Ahn MJ, Chan A, et al. Afatinib versus methotrexate as second‐line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum‐based therapy (LUX‐Head & Neck 3): an open‐label, randomised phase III trial. Ann Oncol. 2019;30(11):1831‐1839. doi: 10.1093/annonc/mdz388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jimeno A, Machiels JP, Wirth L, et al. Phase Ib study of duligotuzumab (MEHD7945A) plus cisplatin/5‐fluorouracil or carboplatin/paclitaxel for first‐line treatment of recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer. 2016;122(24):3803‐3811. doi: 10.1002/cncr.30256 [DOI] [PubMed] [Google Scholar]
- 79. Ferris RL, Clump DA, Ohr J, et al. 1139 ‐ phase I trial of cetuximab, intensity modulated radiotherapy (IMRT), and ipilimumab in previously untreated, locally advanced head and neck squamous cell carcinoma (PULA HNSCC). Ann Oncol. 2017;28(suppl_5):v372‐v394. doi: 10.1093/annonc/mdx374.014 [DOI] [Google Scholar]
- 80. Hoffmann C, Calugaru V, Borcoman E, et al. Phase I dose‐escalation study of NBTXR3 activated by intensity‐modulated radiation therapy in elderly patients with locally advanced squamous cell carcinoma of the oral cavity or oropharynx. Eur J Cancer. 1990;2021(146):135‐144. doi: 10.1016/j.ejca.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 81. Gebre‐Medhin M, Brun E, Engström P, et al. ARTSCAN III: a randomized phase III study comparing Chemoradiotherapy with cisplatin versus Cetuximab in patients with Locoregionally advanced head and neck squamous cell cancer. J Clin Oncol. 2020;39(1):38‐47. doi: 10.1200/JCO.20.02072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baumeister H, Zurlo A, Fayette J, Dietrich B, Keilholz U. CetuGEX and cetuximab in recurrent/metastatic squamous cell carcinoma of the head and neck (RM‐HNSCC): PK/PD results from the phase II RESGEX study. J Clin Oncol. 2018;36(5_suppl):61. doi: 10.1200/JCO.2018.36.5_suppl.61 29116900 [DOI] [Google Scholar]
- 83. Keilholz U, Kawecki A, Dietz A, et al. Efficacy and safety of CetuGEX in recurrent/metastatic squamous cell carcinoma of the head and neck (RM‐HNSCC): results from the randomized phase II RESGEX study. J Clin Oncol. 2018;36(5_suppl):59. doi: 10.1200/JCO.2018.36.5_suppl.59 [DOI] [Google Scholar]
- 84. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2‐year long‐term survival update of CheckMate 141 with analyses by tumor PD‐L1 expression. Oral Oncol. 2018;81:45‐51. doi: 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zandberg DP, Algazi AP, Jimeno A, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single‐arm, phase II study in patients with ≥25% tumour cell PD‐L1 expression who have progressed on platinum‐based chemotherapy. Eur J Cancer. 1990;2019(107):142‐152. doi: 10.1016/j.ejca.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 86. Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): a randomised, open‐label, phase 3 study. Lancet. 2019;393(10167):156‐167. doi: 10.1016/s0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 87. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum‐ and Cetuximab‐refractory head and neck cancer: results from a single‐arm, Phase II Study. J Clin Oncol. 2017;35(14):1542‐1549. doi: 10.1200/jco.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guigay J, Aupérin A, Fayette J, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first‐line treatment in patients with recurrent or metastatic head and neck squamous‐cell carcinoma (GORTEC 2014‐01 TPExtreme): a multicentre, open‐label, randomised, phase 2 trial. Lancet Oncol. 2021;22(4):463‐475. doi: 10.1016/s1470-2045(20)30755-5 [DOI] [PubMed] [Google Scholar]
- 89. Duhen R, Ballesteros‐Merino C, Frye AK, et al. Neoadjuvant anti‐OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen‐specific tumor‐infiltrating T cells. Nat Commun. 2021;12(1):1047. doi: 10.1038/s41467-021-21383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bauman JE, Ohr J, Gooding WE, et al. Phase I study of Ficlatuzumab and Cetuximab in Cetuximab‐resistant, recurrent/metastatic head and neck cancer. Cancer. 2020;12(6):1537. doi: 10.3390/cancers12061537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bauman JE, Duvvuri U, Ferris RL, et al. Phase I study of the anti‐HGF monoclonal antibody (mAb), ficlatuzumab, and cetuximab in cetuximab‐resistant, recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. 2017;35(15_suppl):6038. doi: 10.1200/JCO.2017.35.15_suppl.6038 [DOI] [Google Scholar]
- 92. Dunn LA, Riaz N, Fury MG, et al. A phase 1b study of Cetuximab and BYL719 (Alpelisib) concurrent with intensity modulated radiation therapy in stage III‐IVB head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;106(3):564‐570. doi: 10.1016/j.ijrobp.2019.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Karam SD, Reddy K, Blatchford PJ, et al. Final report of a phase I trial of Olaparib with Cetuximab and radiation for heavy smoker patients with locally advanced head and neck cancer. Clin Cancer Res. 2018;24(20):4949‐4959. doi: 10.1158/1078-0432.ccr-18-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Siu LL, Even C, Mesía R, et al. Safety and efficacy of Durvalumab with or without Tremelimumab in patients with PD‐L1‐low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195‐203. doi: 10.1001/jamaoncol.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dillon MT, Grove L, Newbold KL, et al. Patritumab with Cetuximab plus platinum‐containing therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: an open‐label, phase Ib study. Clin Cancer Res. 2019;25(2):487‐495. doi: 10.1158/1078-0432.ccr-18-1539 [DOI] [PubMed] [Google Scholar]
- 96. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10212):1915‐1928. doi: 10.1016/s0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 97. Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open‐label phase III study. Ann Oncol. 2020;31(7):942‐950. doi: 10.1016/j.annonc.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 98. Anderson CM, Lee CM, Saunders DP, et al. Phase IIb, randomized, double‐blind trial of GC4419 versus placebo to reduce severe Oral Mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J Clin Oncol. 2019;37(34):3256‐3265. doi: 10.1200/jco.19.01507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Day D, Prawira A, Spreafico A, et al. Phase I trial of alpelisib in combination with concurrent cisplatin‐based chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Oral Oncol. 2020;108:104753. doi: 10.1016/j.oraloncology.2020.104753 [DOI] [PubMed] [Google Scholar]
- 100. Rodriguez CP, Wu Q, Voutsinas J, et al. A phase II trial of Pembrolizumab and Vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clini Cancer Res. 2020;26(4):837. doi: 10.1158/1078-0432.CCR-19-2214 [DOI] [PubMed] [Google Scholar]
- 101. Plaschke CC, Johannesen HH, Hansen RH, et al. The DAHANCA 32 study: Electrochemotherapy for recurrent mucosal head and neck cancer. Head Neck. 2019;41(2):329‐339. doi: 10.1002/hed.25454 [DOI] [PubMed] [Google Scholar]
- 102. Oppelt P, Ley J, Daly M, et al. Nab‐paclitaxel and cisplatin followed by cisplatin and radiation (arm 1) and nab‐paclitaxel followed by cetuximab and radiation (arm 2) for locally advanced head and neck squamous‐cell carcinoma: a multicenter, non‐randomized phase 2 trial. Med Oncol. 2021;38(4):35. doi: 10.1007/s12032-021-01479-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Powell SF, Gold KA, Gitau MM, et al. Safety and efficacy of Pembrolizumab with Chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: a phase IB study. J Clin Oncol. 2020;38(21):2427‐2437. doi: 10.1200/jco.19.03156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Weiss J, Sheth S, Deal AM, et al. Concurrent definitive Immunoradiotherapy for patients with stage III–IV head and neck cancer and cisplatin contraindication. Clini Cancer Res. 2020;26(16):4260. doi: 10.1158/1078-0432.CCR-20-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Harrington KJ, Kong A, Mach N, et al. Talimogene Laherparepvec and Pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY‐232): a multicenter, phase 1b study. Clini Cancer Res. 2020;26(19):5153‐5161. doi: 10.1158/1078-0432.ccr-20-1170 [DOI] [PubMed] [Google Scholar]
- 106. Siano M, Molinari F, Martin V, et al. Multicenter phase II study of Panitumumab in platinum pretreated, advanced head and neck squamous cell cancer. The Oncol. 2017;22(7):782.e70. doi: 10.1634/theoncologist.2017-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sun XS, Sire C, Tao Y, et al. A phase II randomized trial of pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN): first results of the GORTEC 2015‐01 “PembroRad” trial. J Clini Oncol. 2018;36(15_suppl):6018. doi: 10.1200/JCO.2018.36.15_suppl.6018 [DOI] [Google Scholar]
- 108. Bourhis J, Sire C, Tao Y, et al. LBA38 Pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA‐HNSCC): results of the GORTEC 2015‐01 “PembroRad” randomized trial. Ann Oncol. 2020;31:S1168. doi: 10.1016/j.annonc.2020.08.2268 [DOI] [PubMed] [Google Scholar]
- 109. Fuereder T, Minichsdorfer C, Mittlboeck M, et al. 921P Pembrolizumab plus docetaxel for the treatment of recurrent metastatic head and neck cancer: a prospective phase I/II study. Ann Oncol. 2020;31:S665. doi: 10.1016/j.annonc.2020.08.1036 [DOI] [PubMed] [Google Scholar]
- 110. Gillison ML, Ferris RL, Harris J, et al. Safety and disease control achieved with the addition of nivolumab (Nivo) to chemoradiotherapy (CRT) for intermediate (IR) and high‐risk (HR) local‐regionally advanced head and neck squamous cell carcinoma (HNSCC): RTOG foundation 3504. J Clini Oncol. 2019;37(15_suppl):6073. doi: 10.1200/JCO.2019.37.15_suppl.6073 [DOI] [Google Scholar]
- 111. Elbers JBW, Al‐Mamgani A, Tesseslaar MET, et al. Immuno‐radiotherapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: results from a phase‐I trial. Radiother Oncol. 2020;142:79‐84. doi: 10.1016/j.radonc.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 112. Tao Y, Aupérin A, Sun X, et al. Avelumab‐cetuximab‐radiotherapy versus standards of care in locally advanced squamous‐cell carcinoma of the head and neck: the safety phase of a randomised phase III trial GORTEC 2017‐01 (REACH). Eur J Cancer. 1990;141:21‐29. doi: 10.1016/j.ejca.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 113. Zuur L, Vos JL, Elbers JB, et al. LBA40 Neoadjuvant nivolumab and nivolumab plus ipilimumab induce (near‐) complete responses in patients with head and neck squamous cell carcinoma: the IMCISION trial. Ann Oncol. 2020;31:S1169. doi: 10.1016/j.annonc.2020.08.2270 [DOI] [Google Scholar]
- 114. Chung CH, Bonomi M, Steuer CE, et al. Concurrent Cetuximab and Nivolumab as a second‐line or beyond treatment of patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results of phase I/II study. Cancer. 2021;13(5):1180. doi: 10.3390/cancers13051180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hsieh C‐Y, Lein M‐Y, Yang S‐N, et al. Dose‐dense TPF induction chemotherapy for locally advanced head and neck cancer: a phase II study. BMC Cancer. 2020;20(1):832. doi: 10.1186/s12885-020-07347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bossi P, Bergamini C, Miceli R, et al. Salivary cytokine levels and Oral Mucositis in head and neck cancer patients treated with chemotherapy and radiation therapy. Int J Radiat Oncol Biol Physi. 2016;96(5):959‐966. doi: 10.1016/j.ijrobp.2016.08.047 [DOI] [PubMed] [Google Scholar]
- 117. Bossi P, Di Pede P, Guglielmo M, et al. Prevalence of fatigue in head and neck cancer survivors. Ann Otol Rhinol Laryngol. 2019;128(5):413‐419. doi: 10.1177/0003489419826138 [DOI] [PubMed] [Google Scholar]
- 118. Botticelli A, Mezi S, Pomati G, et al. The impact of Locoregional treatment on response to Nivolumab in advanced platinum refractory head and neck cancer: the need trial. Vaccine. 2020;8(2):191. doi: 10.3390/vaccines8020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Byrne K, Hallworth P, Monfared AAT, Moshyk A, Shaw JW. Real‐world systemic therapy treatment patterns for squamous cell carcinoma of the head and neck in Canada. Curr Oncol. 2019;26(2):167‐174. doi: 10.3747/co.26.3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Castelli J, Depeursinge A, Devillers A, et al. PET‐based prognostic survival model after radiotherapy for head and neck cancer. Euro J Nuclear Med Molec Imag. 2019;46(3):638‐649. doi: 10.1007/s00259-018-4134-9 [DOI] [PubMed] [Google Scholar]
- 121. de Ridder M, de Veij Mestdagh PD, Elbers JBW, et al. Disease course after the first recurrence of head and neck squamous cell carcinoma following (chemo)radiation. Euro Arch Oto‐Rhino‐Laryngol. 2020;277(1):261‐268. doi: 10.1007/s00405-019-05676-2 [DOI] [PubMed] [Google Scholar]
- 122. Galot R, van Marcke C, Helaers R, et al. Liquid biopsy for mutational profiling of locoregional recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020;104:104631. doi: 10.1016/j.oraloncology.2020.104631 [DOI] [PubMed] [Google Scholar]
- 123. Grünwald V, Chirovsky D, Cheung WY, et al. Global treatment patterns and outcomes among patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results of the GLANCE H&N study. Oral Oncol. 2020;102:104526. doi: 10.1016/j.oraloncology.2019.104526 [DOI] [PubMed] [Google Scholar]
- 124. Hilke FJ, Muyas F, Admard J, et al. Dynamics of cell‐free tumour DNA correlate with treatment response of head and neck cancer patients receiving radiochemotherapy. Radiother Oncol. 2020;151:182‐189. doi: 10.1016/j.radonc.2020.07.027 [DOI] [PubMed] [Google Scholar]
- 125. Kim H, Kwon M, Kim B, et al. Clinical outcomes of immune checkpoint inhibitors for patients with recurrent or metastatic head and neck cancer: real‐world data in Korea. BMC Cancer. 2020;20(1):727. doi: 10.1186/s12885-020-07214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Martens RM, Noij DP, Koopman T, et al. Predictive value of quantitative diffusion‐weighted imaging and 18‐F‐FDG‐PET in head and neck squamous cell carcinoma treated by (chemo)radiotherapy. Euro J Radiol. 2019;113:39‐50. doi: 10.1016/j.ejrad.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 127. Martens RM, Koopman T, Noij DP, et al. Predictive value of quantitative (18)F‐FDG‐PET radiomics analysis in patients with head and neck squamous cell carcinoma. EJNMMI Res. 2020;10(1):102. doi: 10.1186/s13550-020-00686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nadler E, Joo S, Boyd M, Black‐Shinn J, Chirovsky D. Treatment patterns and outcomes among patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Future Oncol. 2019;15(7):739‐751. doi: 10.2217/fon-2018-0572 [DOI] [PubMed] [Google Scholar]
- 129. Noij DP, Martens RM, Koopman T, et al. Use of diffusion‐weighted imaging and (18)F‐Fluorodeoxyglucose positron emission tomography combined with computed tomography in the response assessment for (chemo)radiotherapy in head and neck squamous cell carcinoma. Clini Oncol. 2018;30(12):780‐792. doi: 10.1016/j.clon.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 130. Pitak‐Arnnop P, Witohendro LK, Meningaud JP, et al. Which characteristics can be expected from p16(+)‐squamous cell carcinomas of the posterior oral cavity and oropharynx? ‐ distinctive results from Central Germany. J Stomatol Oral Maxillofac Surg. 2020;121(3):213‐218. doi: 10.1016/j.jormas.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 131. Porter A, Natsuhara M, Daniels GA, et al. Next generation sequencing of cell free circulating tumor DNA in blood samples of recurrent and metastatic head and neck cancer patients. Transl Cancer Res. 2020;9(1):203‐209. doi: 10.3747/co.26.3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Smirk R, Kyzas P. Outcome of salvage procedures for recurrent oral and oropharyngeal cancer. Br J Oral Maxillofac Surg. 2018;56(9):847‐853. doi: 10.1016/j.bjoms.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 133. Sridharan V, Rahman RM, Huang RY, et al. Radiologic predictors of immune checkpoint inhibitor response in advanced head and neck squamous cell carcinoma. Oral Oncol. 2018;85:29‐34. doi: 10.1016/j.oraloncology.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 134. Velez MA, Wang PC, Hsu S, et al. Prognostic significance of HPV status in the re‐irradiation of recurrent and second primary cancers of the head and neck. Am J Otolaryngol. 2018;39(3):257‐260. doi: 10.1016/j.amjoto.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 135. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664‐670. doi: 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br J Cancer. 2020;122(3):306‐314. doi: 10.1038/s41416-019-0602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus‐positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235‐3242. doi: 10.1200/jco.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Faraji F, Eisele DW, Fakhry C. Emerging insights into recurrent and metastatic human papillomavirus‐related oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2017;2(1):10‐18. doi: 10.1002/lio2.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shewale JB, Gillison ML. Dynamic factors affecting HPV‐attributable fraction for head and neck cancers. Curr Opin Virol. 2019;39:33‐40. doi: 10.1016/j.coviro.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 140. Gillison ML, Broutian T, Pickard RKL, et al. Prevalence of oral HPV infection in the United States, 2009‐2010. JAMA. 2012;307(7):693‐703. doi: 10.1001/jama.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009‐2012 findings: Association of Sexual Behaviors with higher prevalence of Oral oncogenic human papillomavirus infections in U.S. Men Cancer Res. 2015;75(12):2468‐2477. doi: 10.1158/0008-5472.CAN-14-2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tam S, Fu S, Xu L, et al. The epidemiology of oral human papillomavirus infection in healthy populations: a systematic review and meta‐analysis. Oral Oncol. 2018;82:91‐99. doi: 10.1016/j.oraloncology.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 143. Farahmand M, Monavari SH, Tavakoli A. Prevalence and genotype distribution of human papillomavirus infection in different anatomical sites among men who have sex with men: a systematic review and meta‐analysis. Rev Med Virol. 2021;31(6):e2219. doi: 10.1002/rmv.2219 [DOI] [PubMed] [Google Scholar]
- 144. Oliver SE, Gorbach PM, Gratzer B, et al. Risk factors for Oral human papillomavirus infection among young men who have sex with Men‐2 cities, United States, 2012‐2014. Sex Transm Dis. 2018;45(10):660‐665. doi: 10.1097/OLQ.0000000000000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Menezes FS, Fernandes GA, Antunes JLF, Villa LL, Toporcov TN. Global incidence trends in head and neck cancer for HPV‐related and ‐unrelated subsites: a systematic review of population‐based studies. Oral Oncol. 2021;115:105177. doi: 10.1016/j.oraloncology.2020.105177 [DOI] [PubMed] [Google Scholar]
- 146. Bussu F, Sali M, Gallus R, et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br J Cancer. 2013;108(5):1157‐1162. doi: 10.1038/bjc.2013.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mena M, Wang X, Tous S, et al. Concordance of p16(INK4a) and E6*I mRNA among HPV‐DNA‐positive oropharyngeal, laryngeal, and Oral cavity carcinomas from the ICO international study. Cancer. 2022;14(15):3787. doi: 10.3390/cancers14153787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. De Vincenzo R, Conte C, Ricci C, Scambia G, Capelli G. Long‐term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999‐1010. doi: 10.2147/IJWH.S50365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kjaer SK, Nygård M, Sundström K, et al. Final analysis of a 14‐year long‐term follow‐up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four nordic countries. E Clin Med. 2020;23:100401. doi: 10.1016/j.eclinm.2020.100401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Goldstone SE, Jessen H, Palefsky JM, et al. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non‐vaccine HPV types in males. Vaccine. 2013;31(37):3849‐3855. doi: 10.1016/j.vaccine.2013.06.057 [DOI] [PubMed] [Google Scholar]
- 151. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401‐411. doi: 10.1056/NEJMoa0909537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PloS One. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bonanni P, Faivre P, Lopalco PL, et al. The status of human papillomavirus vaccination recommendation, funding, and coverage in WHO Europe countries (2018‐2019). Expert Rev Vaccines. 2020;19(11):1073‐1083. doi: 10.1080/14760584.2020.1858057 [DOI] [PubMed] [Google Scholar]
- 154. Morais E, El Mouaddin N, Schuurman S, De A. Landscape assessment for gender neutral human papillomavirus vaccination recommendations including head and neck cancer burden data. Vaccine. 2021;39(39):5461‐5473. doi: 10.1016/j.vaccine.2021.08.043 [DOI] [PubMed] [Google Scholar]
- 155. European Cancer Organisation . ACTION AREA 1: HPV Prevention Via Gender Neutral Vaccination Programmes. Accessed June 2, 2023. https://www.europeancancer.org/2‐standard/107‐hpv‐action‐area‐1‐hpv‐prevention‐via‐gender‐neutral‐vaccination‐programmes
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information.
Data Availability Statement
The data that supports the findings of this study are included in the article/supplementary material. Data sharing is not applicable as no new data were created or analyzed in this study.


