Abstract
Background/Aim
Biomaterials are essential in modern medicine, both for patients and research. Their ability to acquire and maintain functional vascularization is currently debated. The aim of this study was to evaluate the vascularization induced by two collagen-based scaffolds (with 2D and 3D structures) and one non-collagen scaffold implanted on the chick embryo chorioallantoic membrane (CAM).
Materials and Methods
Classical stereomicroscopic image vascular assessment was enhanced with the IKOSA software by using two applications: the CAM assay and the Network Formation Assay, evaluating the vessel branching potential, vascular area, as well as tube length and thickness.
Results
Both collagen-based scaffolds induced non-inflammatory angiogenesis, but the non-collagen scaffold induced a massive inflammation followed by inflammatory-related angiogenesis. Vessels branching points/Region of Interest (Px^2) and Vessel branching points/Vessel total area (Px^2), increased exponentially until day 5 of the experiment certifying a sustained and continuous angiogenic process induced by 3D collagen scaffolds.
Conclusion
Collagen-based scaffolds may be more suitable for neovascularization compared to non-collagen scaffolds. The present study demonstrates the potential of the CAM model in combination with AI-based software for the evaluation of vascularization in biomaterials. This approach could help to reduce and replace animal experimentation in the pre-screening of biomaterials.
Keywords: Polypropylene scaffolds, collagen scaffolds, chorioallantoic membrane, CAM, IKOSA, angiogenesis
Biomaterials assume a pivotal role in the design and development of an extensive array of biomedical products and devices, encompassing, among others, prosthetic heart valves, hip joint replacements, dental implants, and ocular contact lenses. Of particular note is a substantial subset of biomaterials characterized by inherent biodegradability, while others exhibit bio-absorbable attributes, ensuring their gradual resorption within the host organism upon the fulfillment of their designated functional roles (1-3).
Bioengineering scaffolds need to have some essential characteristics. The scaffold must enhance molecular and mechanical cues to promote appropriate sprouting behavior while preventing any negative local or systemic side effects. This means that the scaffold must be histocompatible, compatible with blood, and mechanically appropriate for the uses for which it is designed (1-4).
Scaffolds, both natural and synthetic, are offered in the scientific market as amniotic membranes, and extracellular matrix components like collagen and laminin known to promote cellular growth (4-5). Collagen-based scaffolds are the most used biomaterials in clinical practice and research due to their structural similarities with the main connective tissue components, but their different conformational structure may give heterogeneous results related to their ability to acquire blood vessels and to sustain a functional vascular network development.
One polymer and two collagen-based scaffolds were tested here by using the chick embryo chorioallantoic membrane model (CAM), regarding the ability to acquire new blood vessels and to develop a functional vascular network. Polymer and collagen induced angiogenesis on CAM was evaluated by the IKOSA method, able of automatic quantification of the different steps of the angiogenic process, such as the vessel branching point (criterium for active angiogenesis) but also vessel thickness as a criterium of vessel maturation (and subsequently of their proper functionality). This experiment was necessary because, despite their wide use in clinical practice, their angiogenic abilities are less studied and described. Here we tested the de novo angiogenic properties of collagen and non-collagen-based scaffolds without co-culturing with other cell types, as they have been previously described in other studies (6,7). By testing the biomaterials themselves without any other cell type interference, we are able to objectively evaluate their effects on the induction and support of the angiogenic process. A thorough examination of vascular formation is scientifically relevant since angiogenesis presents a major obstacle to the creation of hollow organ organoids and tissue engineering for hollow organs.
Materials and Methods
Materials used for implantation on the chick embryo CAM model. For this investigation, we chose one material that is not made of collagen and two scaffolds made of collagen that are frequently used in surgical procedures. Aran Biomedical produces the non-collagen scaffold called MotifMesh. The use of this mesh, which is composed of polypropylene, is common in hernia surgeries. However, its application has generated controversy due to the occurrence of side effects, most notably inflammation. In order to conduct a comparative analysis, we employed two distinct collagen-based scaffolds: Optimaix 2D and Optimaix 3D, both manufactured by Matricel GmbH in Herzogenrath, Germany. The reason for choosing these two scaffolds is based on their comprehensive collagen content. Due to their resemblance to the collagen found in human tissues, they seem appropriate for evaluating direct interactions with human or experimental tissues.
CAM experimental method. We used the in ovo transillumination approach to carefully choose 30 fertilized hen eggs for our CAM experimental model. The eggshells underwent a cleaning process using 70% concentrated alcohol, followed by a 72-hour incubation period at a temperature of 37˚C in an environment with 60% humidity. On the fourth day of incubation, we pierced the tapered end of each egg to extract roughly 3 ml of egg albumen. We subsequently sealed the puncture with Transpore Adhesive Medical Tape (Thermo Fisher Scientific, Waltham, MA, USA). After one day, we generated a shell window on every egg and selected only viable embryos. Prior to applying one of the three biomaterials onto the CAM, we evaluated the CAM’s integrity and vitality.
Subsequently, we categorized the specimens into three distinct groups of 10 eggs each: MOME group implanted with tiny fragments of MotifMesh (MOME); OXMD 2D group using the OptiMaix 2D collagen scaffold fragments and OXMD 3D group, where OptiMaix 3D collagen-based scaffolds were implanted on the CAM surface.
The biomaterials were immediately administered to the CAM surface following a gentle, non-invasive scratching procedure. The experiments ended on the 13th day of incubation. The experimental protocols followed the ethical rules set by the European Union for the use of animals in research. Given that the CAM experimental approach does not entail the direct administration of traumatic procedures to chick embryos, ethical approval was not considered necessary. Furthermore, the absence of nerve fibers in the CAM guaranteed that the embryos did not suffer pain during the study.
After the experiment ended, we fixed the CAM in ovo by immersing it in a 10% buffered formalin solution for 30 minutes. After extraction, the CAM and its corresponding implants were buffered formalin-fixed for 24 hours. Subsequently, they underwent processing via paraffin embedding. Serial slices, with a thickness of 3 μm were produced and then stained using standard hematoxylin and eosin, as well as trichrome staining, for histopathologic assessment.
IKOSA CAM assay and network formation assay tests. To achieve precise mapping of the CAM vascularization resulting from various implanted biomaterials, we conducted an automated study utilizing the IKOSA software (KML Vision, Graz, Austria). This AI-powered software encompasses multiple applications for the swift, precise, yet intricate assessment of various experimental models. For our specific objective, we selected two IKOSA programs: the CAM assay (version 3.1.0) and the Network Formation Assay (version 2.1.0). These programs enabled us to quantify the total area, length, and mean thickness of vessels, as well as the number of branching points and vascular loops in vessel networks. The primary parameters used to evaluate angiogenesis are the branching points, loops, and total area of vessels. Ultimately, we divided the vessel number and branching point by the vessels’ total area. The calculation of ROI was based on pixel squares, similarly to the total area of vessels.
We determined the ratios by dividing the region of interest (ROI) into the total area of vessels and the number of branching sites. The parameters indicate the proportion of blood vessels occupying the ROI region, as well as the number of newly formed branching points. Then, we looked at the relationship between the number of branching points and the total vessel area to see if they were related. The total vessel area was used as a measure of neoangiogenesis.
Statistical analysis and interpretation of data. We collected the initial findings immediately through the IKOSA application and presented them in tabular format using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The XLSTAT statistical extension of the Office suite was utilized to generate visual representations, tables, and analytical data. Statistical analysis was based on correlation matrix and plots and was performed by using the JAMOVI software version 1.2.27.0. A p-value of <0.05 was considered statistically significant, while a p-value of <0.001 was considered highly statistically significant. Charts and correlation tables included as part of the results section in this study were automatically generated by the JAMOVI software (https://www.jamovi.org/).
Results
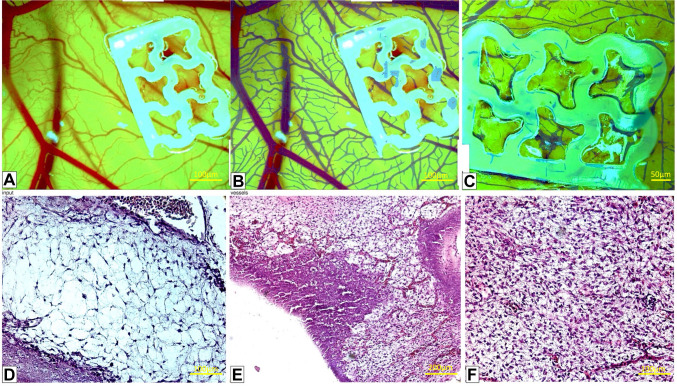
Motif Mesh (MOME) implant on CAM: stereomicroscopic, histological, and IKOSA/CAM assay evaluation. We found that Motif Mesh (MOME) did not adequately adhere to the chorioallantoic membrane. Specifically, MOME did not exhibit a continuous connection with the membrane at the site of implantation (Figure 1A). In addition, MOME triggered a significant inflammatory reaction within the CAM chorion (Figure 1E), which is atypical for the current stage of development. Furthermore, MOME caused necrosis of the CAM in addition to a significant inflammatory response. In this particular case, the angiogenic response was stimulated by the inflammatory reaction rather than by the substance itself, known as MOME (inflammatory-induced neovasculogenesis). Furthermore, in the observation field, there was a noticeable fibroblastic reaction, indicating the presence of myofibroblasts (as seen in Figure 1G). To summarize, the utilization of MOME resulted in inflammation and a fibroblastic response, making it inappropriate for future application as a potential biomaterial scaffold.
Figure 1. Stereomicroscopic (A), AI-enhanced IKOSA analysis (B, C) and histological evaluation (D-F) of the Motif Mesh (MOME) implant on the chick embryo chorioallantoic membrane (CAM). The IKOSA CAM Assay (B) and the Network Formation Assay (C) were used to calculate the number of branching points and vessel area.
Exclusively relying on stereomicroscopy (Figure 1A), we inferred that MOME caused a moderate angiogenic response in the vicinity of the implant. Nevertheless, the study of the IKOSA CAM Assay (Figure 1B) demonstrated a distinct angiogenic response, characterized by the presence of many interconnected microvascular structures. This indicates that MOME has the capacity to promote the formation of new blood vessels, a process known as neovascularization. However, histological studies showed that the large number of blood vessels found by stereomicroscopy and the IKOSA CAM assay were generated by the MOME implant, causing a strong inflammatory response (Figure 1E).
On the final day of the experiment, there was a sudden decrease in the density of the vasculature (Figure 1C and F). An increase in the number of cells in the CAM stroma, likely resulting from a myofibroblastic reaction to MOME compared to the normal CAM (Figure 1D), probably caused this decrease.
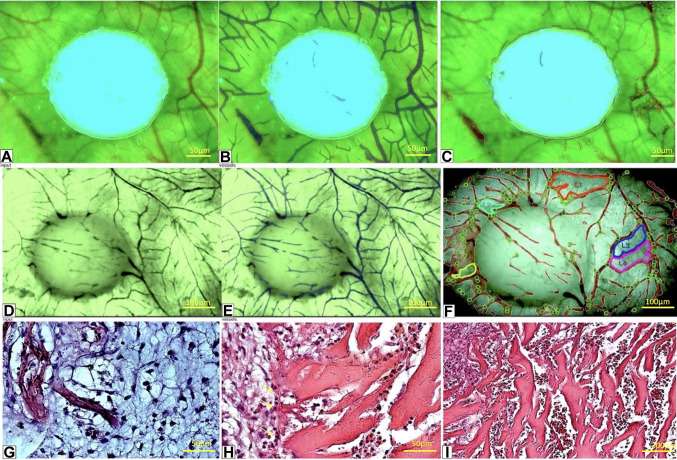
The OxiMaid 2D (OXMD 2D) collagen scaffold. The collagen scaffold did not elicit any inflammatory response upon implantation on the CAM. In addition, OXMD 2D exhibited strong adhesion to the CAM surface (Figure 2A), and, throughout the experiment, it seamlessly merged with the host tissues of the CAM as a whole entity. The acquisition of blood vessels commenced at an early stage of the experiment, specifically on day 1 after implantation, and gradually infiltrated the OXMD 2D implant (Figure 2A-F). Furthermore, there was also notable integration by the chorion along the outer edge of the implant (Figure 2G-H). Many vascular-like channels, lacking real endothelial cells, were present at a similar level (Figure 2H). However, some cells clung to the collagen fibers, forming a sparse layer that imitated potential future capillaries (Figure 2I). Along the edge of the sample, there were many channels that looked similar to blood vessels. This suggested that the channels were probably blood vessels entering the OXMD 2D implant. Overall, initial findings indicated that the OXMD 2D implant effectively adhered to the CAM without causing significant inflammation. It also demonstrates successful integration of the CAM within the collagen mesh, consistent cell adhesion to the collagen fibers, and the formation of pseudo-vascular channels filled with cells.
Figure 2. Stereomicroscopic view of the OxiMaid 2D collagen implant on day 1 (A-C) and day 5 (D-F) and corresponding microscopic images (GI). The IKOSA chorioallantoic membrane (CAM) assay (B, E) identified blood vessels around and inside the implant even from day 1 (B) while the Network Formation Assay detected and quantified the vessel branching points (C, F, green circles) and the vascular loops (L1 to L5 on F, as a sign of vessel remodeling) found on day 5 (F) but not present on day 1 (C).
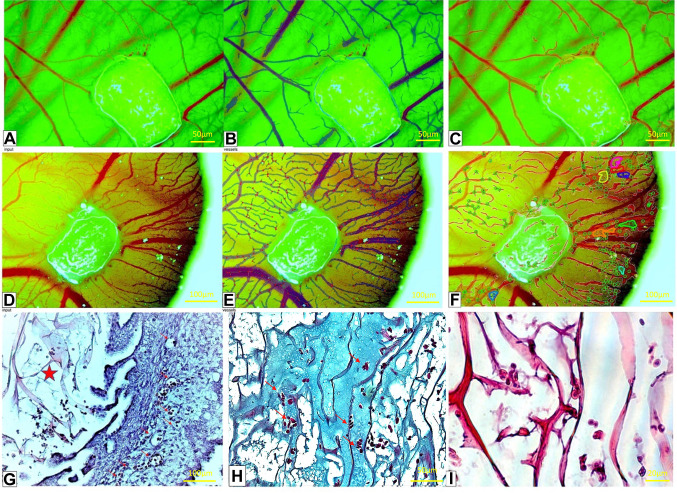
The OxiMaid 3D (OXMD 3D) collagen scaffold. In contrast to the OXMD 2D implant, the OXMD 3D implant resulted in a significant increase in the growth of blood vessels around the implant but also to penetrate inside it (Figure 3A-F). These blood vessels exhibited a small, varied size and shape and were largely filled with red blood cells (Figure 3G). There was no presence of an inflammatory response in the vicinity or inside the implant. The material exhibited excellent integration with the CAM chorion, comparable to that of the OXMD 2D implant. The strong vascular response surrounding the implant indicated that the implanted material effectively stimulated angiogenesis, as shown by the recruitment of blood vessels from the surrounding area. The collagen fibers of the material exhibited the capacity to self-organize into linear and tubular configurations (Figure 3I). In conclusion, there was no fibroblastic reaction and the cells attached well to the 3D structure. Additionally, there was enhanced formation of new blood vessels around the implant that are already carrying blood; the material was arranged in a three-dimensional manner; and it was feasible to identify cells from the CAM situated among the fibers. The trichrome staining (Figure 3H) showed that there were channel-like structures filled with red blood cells in the middle of the implant. This strongly suggested that the OXMD 3D implant had become vascularized.
Figure 3. The OxiMaid 3D (OXMD 3D) implant on the chorioallantoic membrane (CAM) on day 1(A-C) and day 5 (D-F). A similar IKOSA CAM assay (B, E) and Network Formation assay (NFA; C, F) was applied. By using the NFA, vessels inside the OXMD 3D implant (CF) not visible by routine stereomicroscopy can be visualized (A, D). The OXMD 3D structure includes very fine collagen fibers (G, red star) and an extracellular matrix (H, blue) which may facilitate an angiogenic response initially observed by stereomicroscopy and histologically certified as highly organized vascular channels lined by collagen fibers (I).
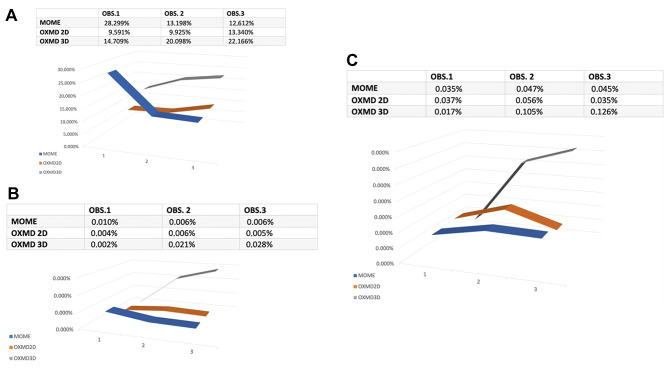
IKOSA CAM assay comparative analysis of MOME, OXDM 2D and OXDM 3D with emphasis on angiogenic status. The AI analyzed various characteristics that have been previously validated to affect the assessment of the angiogenic process, such as the total area of the vessels, the number of branching points, and the branching point density. Regarding absolute numbers, the MOME exhibited the highest total vessel area, followed by OXMD-3D and OXMD-2D. Nevertheless, OXMD-3D had the largest ratio of the overall vessel area to the region of interest (ROI), whereas OXMD-2D and MOME followed with lower ratios. Figure 4A indicates that OXMD-3D was the most effective biomaterial for stimulating the development of new blood vessels.
Figure 4. Comparative analysis on days 1 (OBS1), 3 (OBS2) and 5 (OBS5) among the three biomaterials used in the present study. Vessel total area (Px^2)/ Region of Interest (Px^2) parameter (A). Vessel branching points number/Region of Interest (Px^2) assessment highlighted that OXMD 3D had the highest increase of vessel branching points, suggesting an active angiogenesis which is still in progress on day 5 (B). Vessel branching points number/vessels total area (Px^2) shows the development of new blood vessels which are active in day 5 (C).
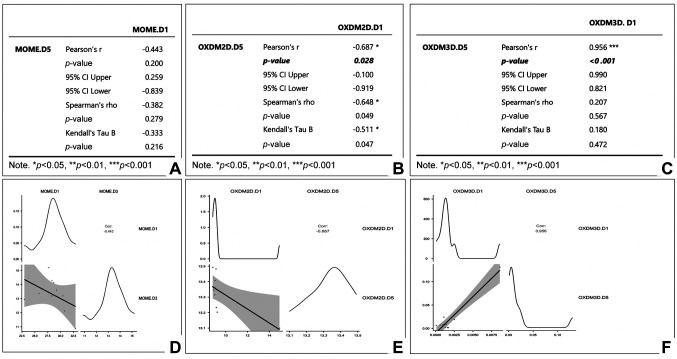
As shown in Figure 4B, it is evident that MOME exhibits a decrease of vessel branching points divided by the total area of vessels. Conversely, both OXMD-2D and OXMD-3D demonstrated a rise in this parameter. OXMD-3D exhibited the most significant rise, with an increase of 0.026% (p<0.001). These findings indicate that OXMD-3D is the most favorable biomaterial for enhancing the development of new branching sites, as it demonstrated a consistent and prolonged increase in this parameter throughout time. Although the increase reported in OXMD-2D was not as substantial as in OXMD-3D, it still exhibited a rise (p=0.028). Conversely, MOME demonstrated a decline in the parameter as time progresses but with no statistical significance. This implies that MOME may have a lower efficacy in stimulating angiogenesis compared to the other substances. Finally, as shown in Figure 4C, we observed that MOME experienced a minor rise in the parameter from day 1 to day 5 (p=0.200), while OXMD-2D and OXMD-3D demonstrate a substantial increase. The OXMD-3D exhibited the highest growth rate, amounting to an impressive +635.29% (p<0.001). Based on the examination of the third parameter, it can be concluded that OXMD-3D is the most favorable biomaterial for stimulating the growth of new blood vessels and the construction of a network for these vessels. Statistical analysis and significant correlations for each subgroup are presented in Figure 5.
Figure 5. Statistical analysis of data for three groups of biomaterials (A, B, C) and correlation plots (D, E, F) for each group. No significant correlations have been detected for the increase of new blood vessels development for non-collagen MOME group (p-value=0.200, A and D) compared to the angiogenic process induced by collagen-based scaffolds. A significant increase of newly formed blood vessels was induced by OXDM2D scaffold (p=0.028, B and E) but the strongest statistically significant angiogenesis induction was done by OXDM3D scaffold (p<0.001). D1: First observation on implant behavior; D3: day 3 of implantation; D5: last day of the experiment, day 5 of implantation.
According to the above, it can be inferred that OXMD-3D is the most favorable biomaterial for the purposes of tissue regeneration and healing. The data demonstrates a consistent rise in the "vessel total area/region of interest" parameter over time, with the largest rate of increase observed in the "vessel number branching points/vessel total area" parameter over the experiment. These findings indicate that OXMD-3D had the most potent effect on stimulating angiogenesis, leading to the development of a new network of blood vessels. Both parameters in OXMD-2D show an increase, although it is not as substantial as the increase reported in OXMD-3D. Conversely, MOME exhibits a decline in the "vessel total area/region of interest" metric with time, along with a minor increase in the "vessels number of branching points/vessels total area" statistic.
Discussion
Collagen-based scaffolds and polypropylene based meshes are widely used biomaterials in the field of medicine, but controversies arise from several side effects associated with their clinical use, particularly in surgical procedures (8,9). In the clinical use of polypropylene polymer-based meshes, inflammation has been found to be a prevalent adverse effect (10-12). Collagen-based scaffolds were also associated with inflammation, but to a lesser degree (13-15). Another contentious matter regarding the utilization of biomaterials is their capacity to stimulate angiogenesis and achieve vascularization (16-18).
Biomaterials serve dual purposes in both clinical and experimental contexts (19-22).
Although the chick embryo CAM model has some advantages as an experimental model, it is rarely used for testing polypropylene mesh (23) or collagen-based scaffolds (24,25). Furthermore, the evaluation of the CAM model through the utilization of artificial intelligence-driven techniques has been documented in only ten publications at present (26), with even fewer studies focusing on the implementation of polypropylene mesh and collagen scaffold implants (27-29).
Here, we conducted a comparative analysis of one non-collagen scaffold and two collagen-based scaffolds with distinct structures. The OPTIMAIX 2D scaffold consists mainly of pure porcine collagen with low levels of cysteine, tryptophan, and hexosamine. The ability to absorb medium is highly pronounced in this scaffold, as evaluated by Boztkurt et al. (28) using wet/dry ratio analysis. This scaffold is well-suited for conducting research both inside and outside of living organisms and has been shown to be compatible with several types of cells. When employed in a living organism, it undergoes natural degradation without inducing notable irritation at the site of implantation. The OPTIMAIX 2D material exhibits two clearly differentiated sides: one characterized by a compact fibrous arrangement, and the other by an exposed fibrous arrangement. Nevertheless, it is presently not advisable for human use (8).
Optimaix-3D collagen scaffolds are produced through a directional solidification process (9). To summarize, the production process starts by producing a consistent aqueous collagen dispersion. Subjecting the dispersion to controlled freezing forms ice crystals with a finger-like shape. These ice crystals then penetrate the dispersion, enabling collagen fibers to gather in the spaces between them, thus preventing the fibers from getting trapped inside the ice crystals. After freeze-drying, the ice turns directly into vapor, leaving a collagen structure that is open and porous. Chemically bonding the basic structure of this collagen scaffold using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) improves its resistance to deterioration caused by cultured cells (9).
A limited number of articles have reported on the evaluation of the CAM model using artificial intelligence-based techniques at present (26,27,29), with none specifically focusing on polypropylene polymers. Only a limited number of studies have documented the evaluation of collagen scaffold implants through the use of automated analysis software dedicated to the assessment of CAM derived stereomicroscopic images. Therefore, we conducted a comparative analysis of one non-collagen scaffold and two collagen-based scaffolds with distinct structures by applying IKOSA CAM Assay based evaluation.
Polypropylene polymer meshes and scaffolds have been commonly used in the past, primarily for the surgical treatment of hernias but continue to be a subject of debate due to several reported adverse effects. Many studies in the literature frequently mention inflammation as the most prevalent concern (10,12). In our investigation, the presence of inflammation was the prevailing factor in the interaction between the MOME implant and the CAM. From the first day after implantation, there was a noticeable increase in vascular response, but by the fifth day, it significantly decreased. Most importantly, the development of blood vessels was not directly caused by the scaffold itself but was a response to inflammation. The decreased number of blood vessels on day 5 can be attributed to a strong response from myofibroblasts caused by inflammation, accompanied by a drop in all measures of blood vessel formation as determined by the IKOSA CAM test and Network Formation Assay (NFA). The difference was particularly clear when measuring the number of branching sites, the overall vascular area, and the results of tube formation experiments.
The OPTIMAIX 2D is well-suited for both laboratory-based investigations and tests conducted in living organisms and has been shown to be compatible with several types of cells (30,31). The current study examined the microscopic structure of the subject, which consists primarily of dense collagen fibers arranged in fascicles that form channel-like patterns. Researchers commonly employ it as a framework for seeding cells in both in vitro and in vivo experimental models, but there is limited data about its application as a scaffold for the development of blood vessels. We have demonstrated that OXDM 2D has angiogenic capabilities and the ability to facilitate the formation of new blood vessels. Nevertheless, our examination of branching points and total vascular area has revealed that OXDM 2D could serve as a framework for the growth of blood vessels. However, it is important to note that it does have certain limitations in terms of the development of the vascular network, which are likely attributed to the thickness and density of collagen fibers within its structure.
Although Optimaix 3D has not been previously studied for its vasculogenic potential, Optimaix 3D has been identified as a biomaterial that significantly promotes angiogenesis. In a study conducted by Woloszyk et al. (32), the researchers assessed the angiogenic capacity of two scaffold materials, Optimaix 3D and DegraPol, which are biomaterials often employed in bone regeneration. The authors found no significant changes between the two implants through macroscopic CAM examination. The authors utilized the multimodal approach of Optimaix 3D angiogenesis, which involved employing advanced techniques, such as microcomputed tomography (microCT) and magnetic resonance imaging (MRI). They showed that Optimaix 3D had a much higher level of vascularization than DegraPol by looking closely at branch points, junctions, vessel length, and vascular area values. The results we obtained are consistent with the prior findings reported by Woloszyk et al. (32), but we used a different method for quantification. The IKOSA CAM assay and NFA are AI programs that can measure angiogenesis using criteria similar to those used by Woloszyk et al. These criteria include where vessels branch off, how long they are, how much area they cover, and how thick they are. The consistent results support the IKOSA application as a reliable alternative way to measure angiogenesis in collagen-based scaffolds that are implanted on CAM. Prior studies have described the implantation of scaffolds made from collagen on the CAM (7); however, the use of the IKOSA CAM test was found to be highly restricted (33).
Conclusion
The present study reported that both collagen scaffolds induced a significant vascularization compared to the scaffold that did not contain collagen. Optimaix 3D resulted in the greatest angiogenic potential, followed by Optimaix 2D. These findings indicate that collagen-based scaffolds may be better suited for applications that require angiogenesis, such as tissue engineering and regenerative medicine. The CAM model confidently asserts its invaluable role in assessing vascularization in biomaterials. Therefore, it is highly advisable to combine the CAM assay with AI-enhanced evaluation to assess the compatibility of various materials, especially bio-inked printed scaffolds, which are a promising innovation in the expansive realm of tissue engineering.
Conflicts of Interest
The Authors declared no conflicts of interests.
Authors’ Contributions
ERGS, AVP and TAP designed the study and wrote the initial draft. AMC, AAC, ACB and MPF reviewed the slides and analyzed the microscopic findings. AMC, MCS, FRD and BDC applied IKOSA image analysis and performed statistical analysis. AS and ESB provided biomaterials and the idea of the study. AMC, AVP and ESB reviewed the final draft and made final corrections according to the reviewers’ suggestions.
References
- 1.Parida P, Behera A, Mishra SC. Biomaterials used in medicine. IJAAS. 2012;1(3):31–35. [Google Scholar]
- 2.Marin E, Boschetto F, Pezzotti G. Biomaterials and biocompatibility: An historical overview. J Biomed Mater Res A. 2020;108(8):1617–1633. doi: 10.1002/jbm.a.36930. [DOI] [PubMed] [Google Scholar]
- 3.Vienken J. Biomaterials in medical devices: an interview with Jörg Vienken of Fresenius Medical Care, Germany. Biotechnol J. 2012;7(6):702–703. doi: 10.1002/biot.201200147. [DOI] [PubMed] [Google Scholar]
- 4.Saxena AK. Surgical perspectives regarding application of biomaterials for the management of large congenital diaphragmatic hernia defects. Pediatr Surg Int. 2018;34(5):475–489. doi: 10.1007/s00383-018-4253-1. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Yu JM, Gan YC, Qiu XZ, Gao ZC, Wang H, Chen SX, Xiong Y, Liu GH, Lin SE, McCarthy A, John JV, Wei DX, Hou HH. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):16. doi: 10.1186/s40779-023-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guasti L, Vagaska B, Bulstrode NW, Seifalian AM, Ferretti P. Chondrogenic differentiation of adipose tissue-derived stem cells within nanocaged POSS-PCU scaffolds: A new tool for nanomedicine. Nanomedicine. 2014;10(2):279–289. doi: 10.1016/j.nano.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Ribatti D. Two new applications in the study of angiogenesis the CAM assay: Acellular scaffolds and organoids. Microvasc Res. 2022;140:104304. doi: 10.1016/j.mvr.2021.104304. [DOI] [PubMed] [Google Scholar]
- 8.Seifalian A, Basma Z, Digesu A, Khullar V. Polypropylene pelvic mesh: What went wrong and what will be of the future. Biomedicines. 2023;11(3):741. doi: 10.3390/biomedicines11030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezvani Ghomi E, Nourbakhsh N, Akbari Kenari M, Zare M, Ramakrishna S. Collagen-based biomaterials for biomedical applications. J Biomed Mater Res. 2021;109(12):1986–1999. doi: 10.1002/jbm.b.34881. [DOI] [PubMed] [Google Scholar]
- 10.Heymann F, von Trotha KT, Preisinger C, Lynen-Jansen P, Roeth AA, Geiger M, Geisler LJ, Frank AK, Conze J, Luedde T, Trautwein C, Binnebösel M, Neumann UP, Tacke F. Polypropylene mesh implantation for hernia repair causes myeloid cell-driven persistent inflammation. JCI Insight. 2019;4(2):e123862. doi: 10.1172/jci.insight.123862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas D, Demetres M, Anger JT, Chughtai B. Histologic inflammatory response to transvaginal polypropylene mesh: a systematic review. Urology. 2018;111:11–22. doi: 10.1016/j.urology.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Klosterhalfen B, Klinge U, Müllen A, Jockenhoevel S. Influence of polypropylene mesh degradation on tissue inflammatory reaction. J Biomed Mater Res. 2023;111(8):1110–1119. doi: 10.1002/jbm.a.37494. [DOI] [PubMed] [Google Scholar]
- 13.Elango J, Zamora-Ledezma C, Ge B, Hou C, Pan Z, Bao B, Pérez Albacete Martínez C, Granero Marín JM, de Val JEMS, Bao C, Wu W. Paradoxical duel role of collagen in rheumatoid arthritis: Cause of inflammation and treatment. Bioengineering (Basel) 2022;9(7):321. doi: 10.3390/bioengineering9070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson FC, McTiernan CD, Islam MM, Buznyk O, Lewis PN, Meek KM, Haagdorens M, Audiger C, Lesage S, Gueriot FX, Brunette I, Robert MC, Olsen D, Koivusalo L, Liszka A, Fagerholm P, Gonzalez-Andrades M, Griffith M. Collagen analogs with phosphorylcholine are inflammation-suppressing scaffolds for corneal regeneration from alkali burns in mini-pigs. Commun Biol. 2021;4(1):608. doi: 10.1038/s42003-021-02108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowley AT, Nagalla RR, Wang SW, Liu WF. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv Healthc Mater. 2019;8(8):e1801578. doi: 10.1002/adhm.201801578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caballé-Serrano J, Zhang S, Sculean A, Staehli A, Bosshardt DD. Tissue integration and degradation of a porous collagen-based scaffold used for soft tissue augmentation. Materials (Basel) 2020;13(10):2420. doi: 10.3390/ma13102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katrilaka C, Karipidou N, Petrou N, Manglaris C, Katrilakas G, Tzavellas AN, Pitou M, Tsiridis EE, Choli-Papadopoulou T, Aggeli A. Freeze-drying process for the fabrication of collagen-based sponges as medical devices in biomedical engineering. Materials (Basel) 2023;16(12):4425. doi: 10.3390/ma16124425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu T, Hu H, Li Y, Jiang Q, Su J, Lin H, Xiao Y, Zhu X, Zhang X. Bioactive scaffolds based on collagen filaments with tunable physico-chemical and biological features. Soft Matter. 2020;16(18):4540–4548. doi: 10.1039/d0sm00233j. [DOI] [PubMed] [Google Scholar]
- 19.Jing H, Gao B, Gao M, Yin H, Mo X, Zhang X, Luo K, Feng B, Fu W, Wang J, Zhang W, Yin M, Zhu Z, He X, Zheng J. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-β3-encapsulating electrospun poly(l-lactic acid-co-ε-caprolactone)/collagen scaffolds. Artif Cells Nanomed Biotechnol. 2018;46(sup1):985–995. doi: 10.1080/21691401.2018.1439844. [DOI] [PubMed] [Google Scholar]
- 20.Shojaati G, Khandaker I, Sylakowski K, Funderburgh ML, Du Y, Funderburgh JL. Compressed collagen enhances stem cell therapy for corneal scarring. Stem Cells Transl Med. 2018;7(6):487–494. doi: 10.1002/sctm.17-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreev AY, Osidak EO, Grigoriev TE, Krasheninnikov SV, Zaharov VD, Zaraitianc OV, Borzenok SA, Domogatsky SP. A new collagen scaffold for the improvement of corneal biomechanical properties in a rabbit model. Exp Eye Res. 2021;207:108580. doi: 10.1016/j.exer.2021.108580. [DOI] [PubMed] [Google Scholar]
- 22.O’Shaughnessy D, Grande D, El-Neemany D, Sajjan S, Pillalamarri N, Shalom D, Winkler H. Evaluation of the histological and biomechanical properties of poly-4-hydroxybutyrate scaffold for pelvic organ prolapse, compared with polypropylene mesh in a rabbit model. Int Urol J. 2022;33(8):2213–2220. doi: 10.1007/s00192-021-04851-6. [DOI] [PubMed] [Google Scholar]
- 23.Mangir N, Hillary CJ, Chapple CR, MacNeil S. Oestradiol-releasing biodegradable mesh stimulates collagen production and angiogenesis: An approach to improving biomaterial integration in pelvic floor repair. Eur Urol Focus. 2019;5(2):280–289. doi: 10.1016/j.euf.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Aleem AR, Shahzadi L, Tehseen S, Alvi F, Chaudhry AA, Rehman IU, Yar M. Amino acids loaded chitosan/collagen based new membranes stimulate angiogenesis in chorioallantoic membrane assay. Int J Biol Macromol. 2019;140:401–406. doi: 10.1016/j.ijbiomac.2019.08.095. [DOI] [PubMed] [Google Scholar]
- 25.Babrnáková J, Pavliňáková V, Brtníková J, Sedláček P, Prosecká E, Rampichová M, Filová E, Hearnden V, Vojtová L. Synergistic effect of bovine platelet lysate and various polysaccharides on the biological properties of collagen-based scaffolds for tissue engineering: Scaffold preparation, chemo-physical characterization, in vitro and ex ovo evaluation. Mater Sci Eng C. 2019;100:236–246. doi: 10.1016/j.msec.2019.02.092. [DOI] [PubMed] [Google Scholar]
- 26.Annese T, Tamma R, Ribatti D. IKOSA® CAM Assay Application to Quantify Blood Vessels on Chick Chorioallantoic Membrane (CAM) Methods Mol Biol. 2023;2572:129–139. doi: 10.1007/978-1-0716-2703-7_10. [DOI] [PubMed] [Google Scholar]
- 27.Schneider-Stock R, Flügen G. Editorial for special issue: The chorioallantoic membrane (CAM) model-traditional and state-of-the art applications: the 1st International CAM conference. Cancers (Basel) 2023;15(3):772. doi: 10.3390/cancers15030772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozkurt A, Deumens R, Beckmann C, Olde Damink L, Schügner F, Heschel I, Sellhaus B, Weis J, Jahnen-Dechent W, Brook GA, Pallua N. In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials. 2009;30(2):169–179. doi: 10.1016/j.biomaterials.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Faihs L, Firouz B, Slezak P, Slezak C, Weißensteiner M, Ebner T, Ghaffari Tabrizi-Wizsy N, Schicho K, Dungel P. A novel artificial intelligence-based approach for quantitative assessment of angiogenesis in the ex ovo CAM model. Cancers (Basel) 2022;14(17):4273. doi: 10.3390/cancers14174273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto L, Wolint P, Bopp A, Woloszyk A, Becker AS, Boss A, Böni R, Calcagni M, Giovanoli P, Hoerstrup SP, Emmert MY, Buschmann J. 3D-microtissue derived secretome as a cell-free approach for enhanced mineralization of scaffolds in the chorioallantoic membrane model. Sci Rep. 2021;11(1):5418. doi: 10.1038/s41598-021-84123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruoß M, Häussling V, Schügner F, Olde Damink LHH, Lee SML, Ge L, Ehnert S, Nussler AK. A standardized collagen-based scaffold improves human hepatocyte shipment and allows metabolic studies over 10 days. Bioengineering (Basel) 2018;5(4):86. doi: 10.3390/bioengineering5040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woloszyk A, Wolint P, Becker AS, Boss A, Fath W, Tian Y, Hoerstrup SP, Buschmann J, Emmert MY. Novel multimodal MRI and MicroCT imaging approach to quantify angiogenesis and 3D vascular architecture of biomaterials. Sci Rep. 2019;9(1):19474. doi: 10.1038/s41598-019-55411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribatti D, Annese T, Tamma R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc Res. 2020;131:104026. doi: 10.1016/j.mvr.2020.104026. [DOI] [PubMed] [Google Scholar]