Abstract
Background/Aim
The albumin-globulin ratio (AGR) is a useful biomarker for predicting postoperative complications and a poor prognosis in patients with various types of cancer and can be evaluated without invasive testing or surgery. In this study, we aimed to evaluate the usefulness of the AGR in predicting the short- and long-term prognoses of patients with gastric cancer who underwent radical resection at our institution.
Patients and Methods
This study is a retrospective cohort analysis in which eligible patients were selected from the medical records of patients who underwent radical resection for gastric cancer at Yokohama City University from 2000 to 2020 and their medical records were reviewed. A total of 240 patients with gastric cancer were classified into high-AGR (>1.57) and low-AGR (≤1.57) groups and their overall survival (OS), recurrence-free survival (RFS), and postoperative complication rates were compared.
Results
Of the total 240 patients, 87 were classified into the high AGR group and 153 were classified into the low AGR group; the incidence of postoperative complications in the two groups did not differ to a statistically significant extent (34.4% vs. 39.2%, p=0.491). The long-term findings showed that the 5-year OS and RFS rates were significantly better in the high AGR group [84.0% vs. 64.8% (p=0.005), 80.0% vs. 61.9% (p=0.015), respectively].
Conclusion
Preoperative low AGR is a risk factor for OS and DFS in patients with gastric cancer who undergo surgery. The AGR may be a useful biomarker that can be applied as a prognostic indicator for patients with gastric cancer.
Keywords: Gastric cancer, AGR, albumin, globulin
Gastric cancer is associated with high morbidity and mortality, which is responsible for more than 1 million new cases and 769,000 deaths annually worldwide (1,2). Local resection and systemic chemotherapy (postoperative chemoradiotherapy in the United States, perioperative chemoradiotherapy in Europe, and postoperative chemotherapy in Asia) are commonly used as standard treatments for gastric cancer that has not spread to other organs (3-5). However, postoperative recurrence of gastric cancer is not uncommon, and the prognosis after recurrence is poor (6,7). Therefore, it is important to evaluate the risk of recurrence and prognosis of patients with gastric cancer in order to make appropriate treatment choices for each patient and to accurately predict their prognosis.
Recently, many prognostic factors for cancer have been reported, among which the albumin-globulin ratio (AGR) may be a useful biomarker for predicting postoperative complications and a poor prognosis in patients with various types of cancer (8-10). The AGR, the ratio of albumin (an indicator of the nutritional status and the production of which is reduced by inflammatory cytokines) to globulin (which is elevated by increased acute phase proteins and may be associated with apoptosis and cancer progression) is an item that can be obtained from routine blood collection and evaluated without invasive testing or surgery. The prognostic value of the AGR for patients with gastric cancer has been increasingly reported in many countries (11,12).
Therefore, we aimed to investigate the usefulness of AGR for predicting the short-term and long-term prognoses of patients with gastric cancer who underwent radical resection at our institution.
Patients and Methods
Patients. Patients eligible for inclusion in the study were identified from the medical records of individuals who had undergone surgical radical resection for gastric cancer at Yokohama City University between the years 2000 and 2020. The inclusion criteria were as follows: 1) histologically confirmed gastric cancer, 2) clinical stage I-III according to the 8th edition of the Tumor-Node-Metastasis classification (published by the Union for International Cancer Control), and 3) complete resection of gastric cancer, defined as R0, in addition to radical lymph node dissection (13). Patients who did not meet these criteria, including those who received R1 or R2 resection, were excluded.
Surgery and adjuvant treatment. In all cases, laparoscopic or open, robot-assisted resection of ≥2/3 of the stomach and total gastrectomy, plus lymph node dissection was performed. Patients with clinical Stage IA disease received D1+ lymph node dissection, and patients with clinical Stage ≥IB disease received D2 lymph node dissection. Patients with pathological stage II/III disease underwent 1 year of postoperative adjuvant chemotherapy. Generally, S-1 monotherapy was administered to patients with pathological stage II disease, while S-1 with docetaxel or capecitabine plus oxaliplatin was administered to patients with pathological stage III disease.
Postoperative complications. In the present study, postoperative complications were defined as Clavien-Dindo (version 2.0) Grade ≥II complications, according to the definitions of the Japan Clinical Oncology Group (14).
Follow-up. All patients underwent postoperative follow-up examinations at 3-6 months, during which information on survival, disease progression, and time of death was documented for a minimum of 5 years where feasible. The patients’ serum tumor marker levels, including carcinoembryonic antigen and carbohydrate antigen 19-9, were assessed at intervals of at least 3-6 months, and computed tomography scans were conducted at least every 6-12 months.
Evaluation of the pathological response. The pathologic response to postoperative chemotherapy was characterized in accordance with international criteria, specifically the Response Evaluation Criteria in Solid Tumors (RECIST), as follows: 1) Complete response (CR), defined as complete tumor elimination; 2) Partial response (PR) defined as a ≥30% reduction in the sum of tumor sizes; 3) Stable disease (SD), defined as no change in tumor size, and 4) Progressive disease (PD) defined as a ≥20% increase in the sum of tumor sizes along with an absolute increase of ≥5 mm, or the emergence of a new lesion (15).
Determination of the albumin-globulin ratio. The AGR was determined by assessing a preoperative blood sample and dividing the serum albumin level by the serum globulin level, which is calculated as the difference between the serum total protein level and the serum albumin level.
Statistical analyses. Categorical variables are expressed as frequencies and percentages (%). Comparative analyses between groups were carried out utilizing the Chi-square test, Student’s t-test, and Mann-Whitney test. The Kaplan-Meier method was employed for the generation of overall survival (OS) and recurrence-free survival (RFS) curves. A log-rank test was used to compare the equality of survival curves. Univariate and multivariate hazard ratios were computed using the Cox proportional hazards model. All variables identified as significant in the univariate analysis were incorporated into the backward stepwise multivariate model. p-Values of <0.05 were considered to indicate statistical significance. All of the statistical analyses were conducted using SPSS (version 27.0, SPSS, Chicago, IL, USA).
Results
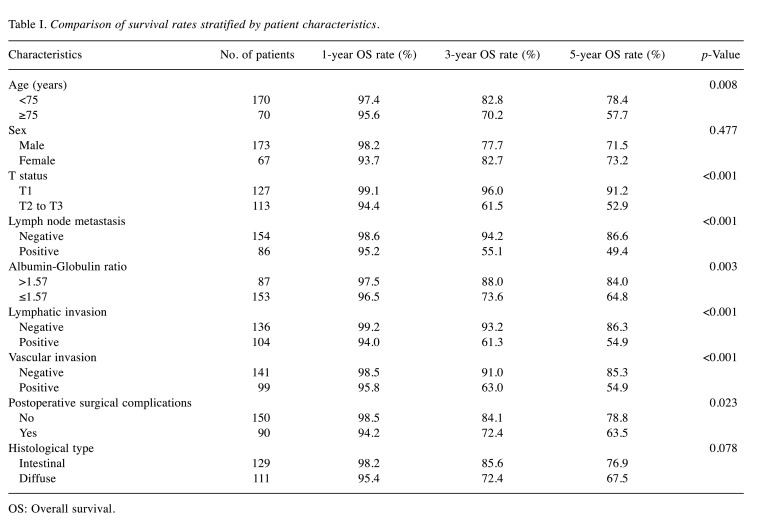
Patient background. A total of 240 patients with gastric cancer were selected for this study (Table I). Among the 240 patients, 173 were male and 67 were female. The surgical treatments included total gastrectomy (n=56), distal resection (n=166), proximal resection (n=5), and partial resection (n=1). D1 and D1+, D2, D3, sentinel lymph node dissection was performed in three, 114, 105, one, and one patient, respectively. Based on the patients’ 1-, 3-, and 5-year OS rates, and using a cutoff value of 1.57, 87 out of the 240 patients were classified into the high AGR (>1.57) patient group, while 153 were classified into the low AGR (≤1.57) patient group. When each clinicopathological factor was examined, patients in the low AGR group were significantly more likely to be older, have a high ASA-PS, a history of Chronic Obstructive Pulmonary Disease (COPD), a high T factor, venous infiltration, anticoagulant treatment, and drink alcohol.
Table I. Comparison of survival rates stratified by patient characteristics.
OS: Overall survival.
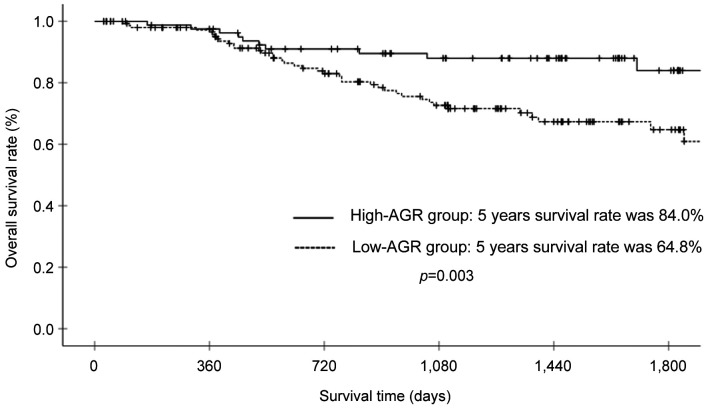
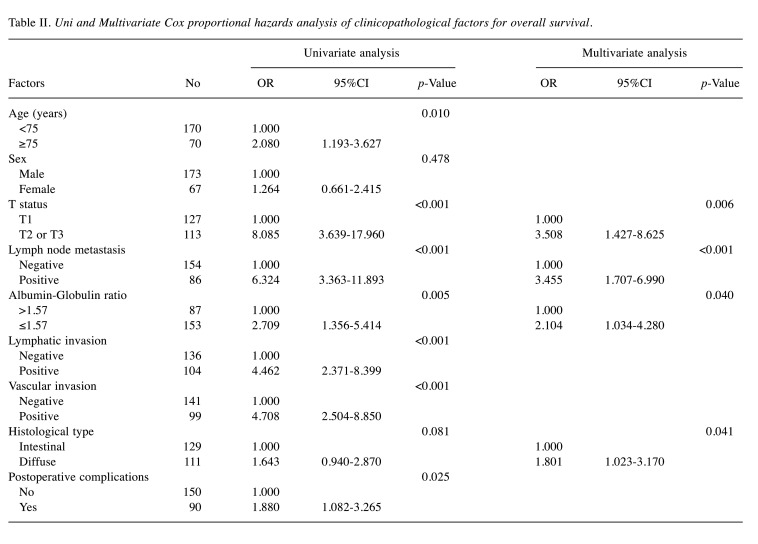
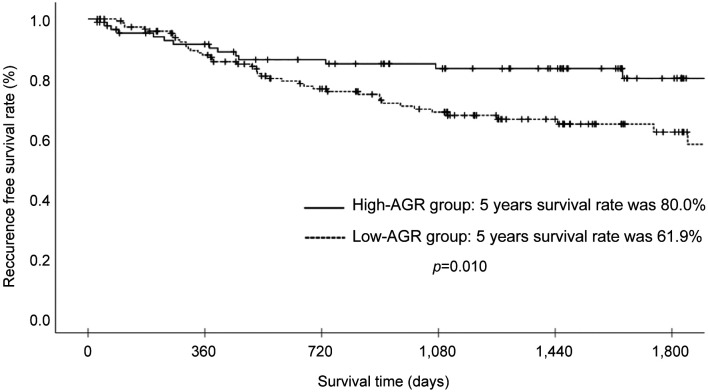
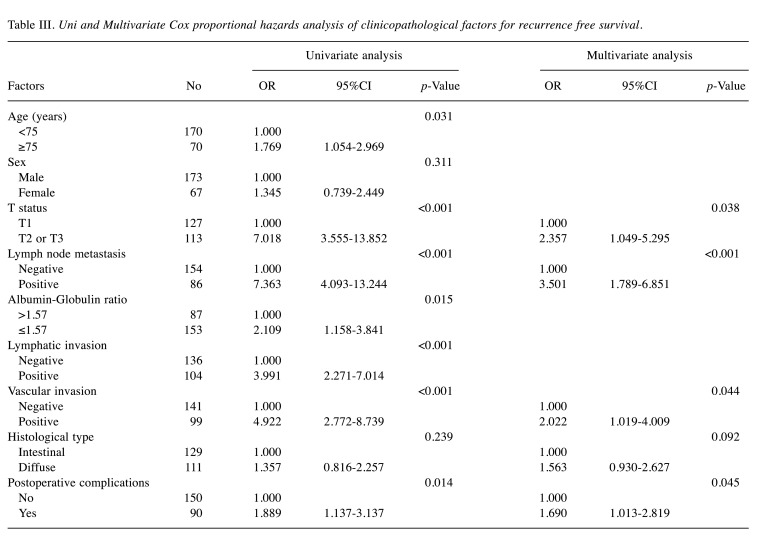
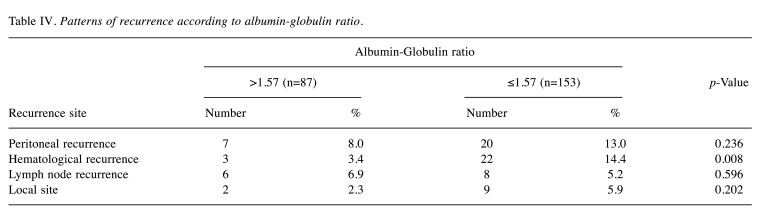
Survival analysis and recurrence pattern. The 5-year postoperative overall survival (OS) rate was 84.0% in the high AGR group and 64.8% in the low AGR group (p=0.003). The OS curves are shown in Figure 1. Each clinicopathological factor was analyzed as presented in Table I. OS was compared between groups using log-rank testing. There were significant differences in age (<75 vs. ≥75), UICC T status (T1 vs. T2-T3), lymph node metastasis, AGR (≤1.57 vs. >1.57), lymphatic invasion, vascular invasion, and postoperative surgical complications. The univariate analysis (Table II) of overall survival showed that age, T status, lymph node metastasis, AGR, lymphatic invasion, vascular invasion, and postoperative surgical complications were significantly associated with OS. In the multivariate analysis (Table II), AGR emerged as an independent predictor of OS [odds ratio (OR)=2.104, 95% confidence interval (CI)=1.034-4.280, p=0.040]. The 5-year postoperative RFS rate was 80.0% in the high AGR group and 61.9% in the low AGR group (p=0.010). The RFS curves are shown in Figure 2. The univariate analysis of RFS demonstrated that the AGR was a significant prognostic factor (Table III). A comparison of recurrence patterns between the high and low AGR groups revealed a significant difference in hematological recurrence (3.4% vs. 14.3%, p=0.008) (Table IV).
Figure 1. Overall survival of patients with gastric cancer in the high albumin-globulin ratio (AGR) group (>1.57) and low AGR group (≤1.57).
Table II. Uni and Multivariate Cox proportional hazards analysis of clinicopathological factors for overall survival.
Figure 2. Recurrence-free survival of patients with gastric cancer in the high albumin-globulin ratio (AGR) group (>1.57) and low AGR group (≤1.57).
Table III. Uni and Multivariate Cox proportional hazards analysis of clinicopathological factors for recurrence free survival.
Table IV. Patterns of recurrence according to albumin-globulin ratio.
Postoperative clinical course. A subgroup analysis was performed for the AGR, and clinicopathological factors, such as sex, age, medical history (diabetes, hypertension, COPD), ASA-PS, surgical technique, lymph node dissection area, vascular invasion, T factor, N factor, presence of blood transfusion, postoperative complications, anticoagulation medication, alcohol consumption, and smoking were examined. Postoperative complications were compared overall and for pancreatic fistula, anastomotic stenosis, intra-abdominal abscess, and suture failure. No significant differences were observed.
Discussion
The AGR is a potential prognostic factor in various types of cancer and has been increasingly reported in recent years. This study was conducted to investigate the usefulness of a low preoperative AGR as a prognostic factor in postoperative gastric cancer patients. The results showed that the preoperative AGR is a risk factor for OS and DFS after the surgical treatment of gastric cancer. Furthermore, patients with a low AGR who underwent surgery for gastric cancer had a worse prognosis than those with a high AGR. Therefore, the AGR will be applicable as a prognostic indicator in patients with gastric cancer in the future.
In the present study, we demonstrated that the AGR was one of the promising prognostic factors. Similar results have been reported in previous studies on the AGR; in a meta-analysis of 12 cohorts, Wei et al. compared 8305 patients with gastric cancer with low pretreatment AGRs to those with high AGRs (16). The cutoff values ranged from 1.14 to 1.93, and they found that OS (HR=1.531, 95%CI=1.300-1.803, p<0.001) and DFS/PFS (HR=2.008, 95%CI=1.162-3.470, p=0.012) were obviously shorter in GC patients with low pretreatment AGRs than in those with elevated AGRs, which indicated that a low pretreatment AGR could predict a poor prognosis in patients with GC. Among them, Toiyama et al. showed that in 384 patients with gastric cancer, the cutoff value of the preoperative AGR (determined using ROC curves) was 1.3793, indicating that the low preoperative AGR group had poorer DFS than the high AGR group. (HR=1.7264, 95%CI=1.0032-2.9709, p=0.0498) (17). Furthermore, a low AGR was associated with advanced cancer and early postoperative recurrence, indicating that it may be an independent predictor of recurrence in patients after radical gastric cancer surgery.
Although the mechanism underlying the association between the AGR and patient survival is unclear, there are some possible explanations. First, the AGR status might have some clinical impact on lymph node metastasis. For example, Jieshan et al., who analyzed 14 studies involving 4136 patients with various carcinomas revealed that cancer patients in the low AGR group exhibited a heightened risk of lymph node metastasis (HR=2.24, 95%CI=1.49-3.36, p<0.001) (18). In this study, the rate of lymph node metastasis in the high and low AGR groups did not differ to a statistically significant extent, but the T factor was more advanced in the low AGR group in comparison to the high AGR group. Thus, it can be inferred that gastric cancer was detected at a more advanced stage in the low AGR group, which would be related to the determinants of stage. Second, the AGR status might have some clinical impact on synchronous and/or metachronous other primary cancer occurrence. For example, Suh et al. investigated 26,974 healthy adults grouped by AGR values and found that those with a lower AGR were at a higher risk of developing various cancers, including liver and blood cancers (19). In the present study, complications of diabetes, hypertension, and COPD were compared in the high AGR and low AGR groups, but other primary cancers were not examined. Among the complications studied, the incidence of COPD was higher in the low AGR group.
Study limitations. First, it was a retrospective study that was conducted in a single center, which evaluated a single group of cases. Second, serum proteins, such as albumin and globulin may be affected by unknown substances. Third, the optimal cutoff value of AGR is unclear. Although there is no clear definition for the cutoff value of AGR, previous reports have used values of 1.14 to 1.93, and an AGR of 1.57 was used as the cutoff value in this study, which is similar to the values reported in other studies. Considering these limitations, a large, multicenter, prospective study is needed to conduct a precise evaluation.
In conclusion, this study suggested that a low preoperative AGR is a risk factor for OS and DFS in postoperative patients with gastric cancer; the AGR may be a useful biomarker that can be applied as a prognostic indicator for gastric cancer patients.
Conflicts of Interest
The Authors declare no conflicts of interest in association with the present study.
Authors’ Contributions
MF, AK, and YM made substantial contributions to the concept and design. TA, KH, KK1(Keisuke Kazama), KK2 (Keisuke Komori), HT, AT, IH, OK, NK, and HC made substantial contributions to the acquisition of data and the analysis and interpretation of the data. TA, JM, KS, MI, TO, AS, NY, and YR were involved in drafting the article or revising it critically for important intellectual content. TA and YM gave their final approval of the version to be published.
Acknowledgements
This study was supported, in part, by the non-profit organization, the Yokoyama Surgical Research Group (YSRG).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition) Gastric Cancer. 2023;26(1):1–25. doi: 10.1007/s10120-022-01331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(2):167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 5.Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC, ESMO Guidelines Committee Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):1005–1020. doi: 10.1016/j.annonc.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama T, Kazama K, Maezawa Y, Hara K. Usefulness of nutrition and inflammation assessment tools in esophageal cancer treatment. In Vivo. 2023;37(1):22–35. doi: 10.21873/invivo.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoyama T, Hara K, Kazama K, Maezawa Y. Clinical impact of nutrition and inflammation assessment tools in gastric cancer treatment. Anticancer Res. 2022;42(11):5167–5180. doi: 10.21873/anticanres.16023. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama T, Maezawa Y, Hashimoto I, Rino Y, Oshima T. Clinical impact of nutrition and inflammation assessment tools in pancreatic cancer treatment. Anticancer Res: 2023;43(9):3849–3860. doi: 10.21873/anticanres.16572. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Kobayashi D, Takami H, Inokawa Y, Tanaka N, Kurimoto K, Nakanishi K, Umeda S, Shimizu D, Hattori N, Kanda M, Tanaka C, Nakayama G, Kodera Y. Albumin-globulin ratio indicates the survival outcome of pancreatic cancer cases who underwent preoperative treatment and curative surgical resection. Nutr Cancer. 2023;75(5):1330–1339. doi: 10.1080/01635581.2023.2191384. [DOI] [PubMed] [Google Scholar]
- 12.Atsumi Y, Kawahara S, Kakuta S, Onodera A, Hara K, Kazama K, Numata M, Aoyama T, Tamagawa A, Tamagawa H, Oshima T, Yukawa N, Rino Y. Low preoperative albumin-to-globulin ratio is a marker of poor prognosis in patients with esophageal cancer. In Vivo. 2021;35(6):3555–3561. doi: 10.21873/invivo.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14(2):101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Wei C, Yu Z, Wang G, Zhou Y, Tian L. Low pretreatment albumin-to-globulin ratio predicts poor prognosis in gastric cancer: insight from a meta-analysis. Front Oncol. 2021;10:623046. doi: 10.3389/fonc.2020.623046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toiyama Y, Yasuda H, Ohi M, Yoshiyama S, Araki T, Tanaka K, Inoue Y, Mohri Y, Kusunoki M. Clinical impact of preoperative albumin to globulin ratio in gastric cancer patients with curative intent. Am J Surg. 2017;213(1):120–126. doi: 10.1016/j.amjsurg.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Chi J, Xie Q, Jia J, Liu X, Sun J, Chen J, Yi L. Prognostic value of albumin/globulin ratio in survival and lymph node metastasis in patients with cancer: a systematic review and meta-analysis. J Cancer. 2018;9(13):2341–2348. doi: 10.7150/jca.24889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh B, Park S, Shin DW, Yun JM, Keam B, Yang HK, Ahn E, Lee H, Park JH, Cho B. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. 2014;25(11):2260–2266. doi: 10.1093/annonc/mdu274. [DOI] [PubMed] [Google Scholar]