Abstract
Background/Aim
In the literature, the studies about the role of matrix metalloproteinase-2 (MMP-2) in pterygium diagnosis are mainly based on its protein expression. The role of MMP-2 variants has never been examined. The aim of this study was to examine the association of MMP-2 genotypes with pterygium risk.
Materials and Methods
MMP-2 rs243865 and rs2285053 were genotyped in 140 pterygium cases and 280 non-pterygium controls by typical polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) genotyping technology.
Results
The genotypic frequency of MMP-2 rs243865 CC, CT and TT were 86.4%, 12.9% and 0.7% in the pterygium group and 81.1%, 17.1% and 1.8% in the non-pterygium group (p for trend=0.3389). The variant CT and TT carriers had a 0.70- and 0.38-fold pterygium risk (95%CI=0.39-1.26 and 0.04-3.25, p=0.2982 and 0.6686, respectively). As for MMP-2 rs2285053, the genotypic frequency of CC, CT and TT were 67.1%, 28.6% and 4.3% in the pterygium group, non-significantly different from those in non-pterygium group (p for trend=0.7081). The CT and TT carriers had a 0.88- and 0.71-fold pterygium risk (95%CI=0.56-1.38 and 0.27-1.88, p=0.6612 and 0.6456, respectively). The allelic analysis results showed that MMP-2 rs243865 variant T allele was not associated with pterygium risk (7.1% versus 10.4%, OR=0.67, 95%CI=0.39-1.13, p=0.1649). As for MMP-2 rs2285053, the T allele was not associated with pterygium risk either (18.6% versus 21.1%, OR=0.85, 95%CI=0.59-1.23, p=0.4136).
Conclusion
The genotypes at MMP-2 rs243865 or rs2285053 played minor role in determining individual susceptibility for pterygium among Taiwanese.
Keywords: Genotype, MMP-2, polymorphism, pterygium
Pterygium is a prevalent ocular surface condition characterized by aberrant epithelial and fibrovascular proliferation, invasion, and matrix remodeling (1,2). The development of fibrovascular tissues that excessively proliferate, resulting in an anomalous wing-shaped growth, resembles the overgrowth observed in tumors (3). The migration of these wedge-shaped abnormal tissues from the bulbar conjunctiva onto the cornea also shares certain tumorigenesis characteristics seen in solid cancers (4). Pterygium’s multifactorial nature, influenced by factors, such as heat, dust, atmospheric particles, immunological cytokines, extracellular matrix reorganization, UV radiation, and growth factors, contributes to the intricate etiology of this condition (5-13). Furthermore, a handful of studies have provided evidence indicating that genetic variations also play a pivotal role in determining individual susceptibility to pterygium (14-17). Nonetheless, a practical and easily accessible marker for pterygium is still conspicuously absent.
The equilibrium between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) plays a crucial role in regulating the extent of connective tissue degradation and remodeling. The extracellular matrix (ECM) is a network of interconnected macromolecules that form a dynamic scaffold outside of the cells (18-20). Numerous studies have reported up-regulated expression of various MMP types in pterygium samples. Elevated MMP levels have been linked to the dissolution of Bowman’s layer, leading to angiogenesis and the invasion and migration of pterygium tissues onto the cornea (21-28). Two known risk factors for pterygium, namely, UV radiation and inflammatory cytokines, have been shown to increase the expression levels of MMPs in both epithelial cells and fibroblasts (22,28,29).
MMP-2, also known as type IV collagenase or gelatinase A, is situated on chromosome 16q21 and encodes the MMP-2 enzyme (30,31). One of its main functions is the degradation of type IV collagen, contributing to the maintenance of balanced extracellular matrix components and concentrations (32,33). Prior research has predominantly concentrated on the roles of MMPs in pterygium etiology by comparing MMP expression levels in surgically excised pterygia with those from normal controls. However, studies examining the transcriptional or translational expression of MMP-2 in cultured pterygium fibroblasts have yielded conflicting results (21-23,25,27,34-36). The previous sample collection methods and the involvement of unpredictable confounding factors during primary culture have compelled us to explore alternative strategies for identifying clinically feasible predictive markers from a genomic perspective.
The literature contains reports indicating that MMP-2 polymorphic variants, specifically rs243865 and rs2285053, have the potential to influence mRNA and protein expression levels of MMP-2 in various cancer types, including oral cancer (37), esophageal cancer (38), breast cancer (39), colorectal cancer (40), and leukemia (41). However, the genetic variations of MMP-2 have not been explored in relation to pterygium, and the genotype-phenotype connection of MMP-2 remains uncharted. Given the above information, this study aimed to investigate whether MMP-2 rs243865 and rs2285053 genotypes contribute to the risk of pterygium in a representative Taiwanese population, which contains 140 pterygium cases and 280 non-pterygium controls.
Materials and Methods
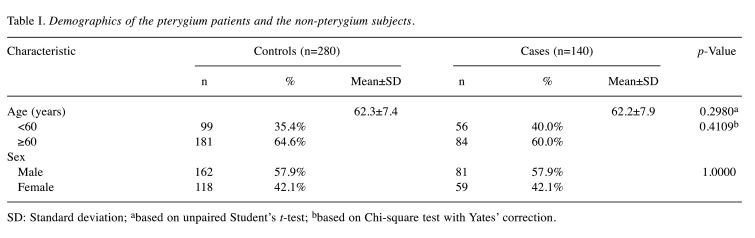
Recruited pterygium and non-pterygium population. The research concepts, association hypotheses, and experimental protocols of the present study have received approval from the Changhua Christian Hospital Institutional Review Board. Additionally, informed consent has been signed and obtained from all the subjects. A total of 140 individuals diagnosed with pterygium and 280 non-pterygium control subjects, were recruited for this study. All participants willingly completed a questionnaire and provided peripheral blood samples for genotyping. The non-pterygium control subjects were selected based on the absence of pterygium, endometriosis, myoma, or any type of cancer. The demographic characteristics of all participants are summarized in Table I.
Table I. Demographics of the pterygium patients and the non-pterygium subjects.
SD: Standard deviation; abased on unpaired Student’s t-test; bbased on Chi-square test with Yates’ correction.
MMP-2 genotyping methodologies. DNA was extracted from the peripheral blood of each subject as we routinely conduct (42-45). The patterns of MMP-2 rs243865 and rs2285053 genotypes among 140 pterygium cases and 280 non-pterygium controls were accessed via polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) genotyping methodology. The design of primer sequences was as we previously published (46,47). Also, the PCR conditions have been as we previously published (46,47). The PCR fragments were digested by restriction enzymes Xsp I and Hinf I overnight for MMP-2 rs243865 and rs2285053, respectively. After the enzyme digestion, the genotyping profiles of MMP-2 rs243865 and rs2285053 of each sample were identified by two independent researchers after 3% agarose gel electrophoresis.
MMP-2 statistical analysis methodologies. The ages between the pterygium patient and non-pterygium control groups are shown as the mean±standard deviation (SD), and unpaired Student’s t-test was applied for the statistical comparison. Pearson’s chi-square (when n≥5) or Fisher exact test (when n<5) was used for evaluating of the contributions of MMP-2 polymorphisms to pterygium risk. The associations were also checked using odds ratios (ORs) and individual corresponding 95% confidence intervals (CIs). Any consequence was taken as statistically significant when the outcome p-value was less than 0.05.
Results
Comparison of age and sex distributions between the pterygium patient and non-pterygium control groups. First, it is essential to examine the age distributions between the pterygium and non-pterygium groups. The mean age of the pterygium and non-pterygium groups was assessed, and the results indicated no significant difference between the two groups (p=0.2980). This observation remained consistent even after stratifying the data using a threshold age of 60 years (p=0.4109). Second, it’s worth noting that, as part of our recruitment process, we matched the pterygium and non-pterygium groups, ensuring there is no disparity in the distribution of men and women between these groups (p=1.0000).
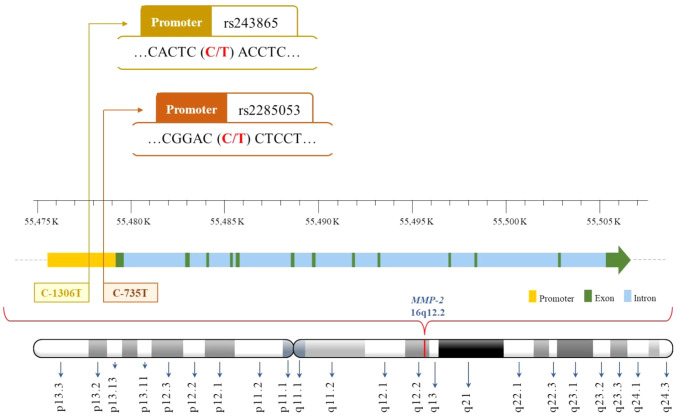
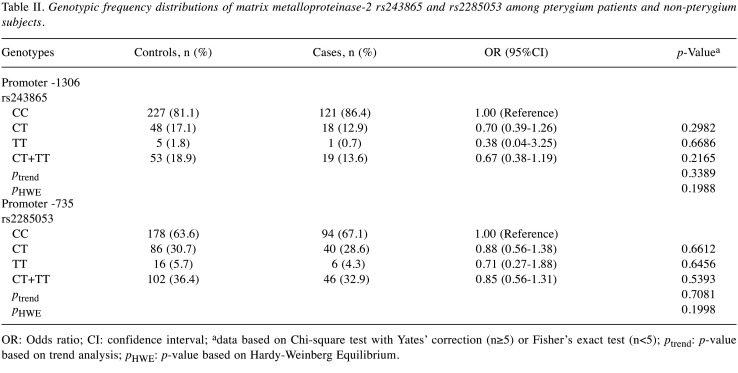
Association of MMP-2 genotypes and pterygium risk. The physical map illustrating the locations of MMP-2 polymorphisms is presented in Figure 1. First, the genotypic frequencies of MMP-2 polymorphisms of the control group fit the Hardy-Weinberg equilibrium hypothesis (p=0.1988 and 0.1998, respectively) (Table II). Second, the genotypic frequencies for MMP-2 rs243865 (CC, CT, and TT) in the pterygium group were 86.4%, 12.9%, and 0.7%, respectively. When compared to the non-pterygium group with frequencies of 81.1%, 17.1%, and 1.8%, no significant difference was observed (p for trend=0.3389). In specific terms, carriers of the CT and TT variants exhibited a 0.70- and 0.38-fold risk for developing pterygium (95%CI=0.39-1.26 and 0.04-3.25, p=0.2982 and 0.6686, respectively). CT+TT carriers showed a 0.67-fold risk for pterygium (95%CI=0.38-1.19, p=0.2165) (Table II, top section). Third, concerning MMP-2 rs2285053, the genotypic frequencies of CC, CT, and TT in the pterygium group were 67.1%, 28.6%, and 4.3%, respectively. There was no significant difference compared to the non-pterygium group (p for trend=0.7081). In detail, carriers of the CT and TT variants had a 0.88- and 0.71-fold risk for developing pterygium (95%CI=0.56-1.38 and 0.27-1.88, p=0.6612 and 0.6456, respectively). CT+TT carriers exhibited a 0.85-fold risk for pterygium (95%CI=0.56-1.31, p=0.5393) (Table II, bottom section).
Figure 1. Physical map of matrix metalloproteinase-2 rs243865 and rs2285053 polymorphic sites.
Table II. Genotypic frequency distributions of matrix metalloproteinase-2 rs243865 and rs2285053 among pterygium patients and non-pterygium subjects.
OR: Odds ratio; CI: confidence interval; adata based on Chi-square test with Yates’ correction (n≥5) or Fisher’s exact test (n<5); ptrend: p-value based on trend analysis; pHWE: p-value based on Hardy-Weinberg Equilibrium.
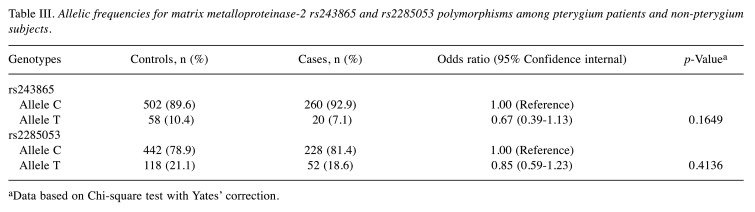
Association of MMP-2 allelic frequencies and pterygium risk. The results of allelic frequency analysis indicated that the variant T allele at MMP-2 rs243865 does not appear to be associated with pterygium risk (7.1% versus 10.4%, OR=0.67, 95%CI=0.39-1.13, p=0.1649) (Table III). Similarly, in the case of MMP-2 rs2285053, the results suggest that the variant T allele does not seem to be associated with an increased risk for pterygium either (18.6% versus 21.1%, OR=0.85, 95%CI=0.59-1.23, p=0.4136) (Table III).
Table III. Allelic frequencies for matrix metalloproteinase-2 rs243865 and rs2285053 polymorphisms among pterygium patients and non-pterygium subjects.
aData based on Chi-square test with Yates’ correction.
Discussion
There is a lack of consensus among ophthalmologists regarding the optimal understanding of pterygium etiology and its management. This is partly attributed to the multitude of risk factors involved, and the absence of a reliable marker for personalized therapeutic strategies. Early investigations into protein and mRNA expression patterns by translational scientists revealed an over-expression of MMPs, particularly MMP-1 (22,23,29,48,49), MMP-2 (29,34) and MMP-3 (22,23,29). However, the genomic roles of MMPs have rarely, if ever, been investigated. In the current study, we investigated the potential contribution of MMP-2 rs243865 and rs2285053 genotypes to pterygium susceptibility in a representative Taiwanese population (control:case=280:140). As far as we know, there is significant potential in identifying a genetic target within MMP-2 for the development of therapeutic strategies for pterygium.
In the current dataset, it is evident that the T allele of MMP-2 rs243865 does not appear to be a significant contributor to individual pterygium susceptibility (Table II and Table III). However, it’s noteworthy that both MMP-2 rs243865 variant CT and TT genotypes were less prevalent in the pterygium group compared to the non-pterygium group (12.9% versus 17.1% and 0.7% versus 1.8%). This finding raises considerable interest, and a larger pterygium population could help validate the results for MMP-2 rs243865. Furthermore, our current study is the first to demonstrate the potential contribution of MMP-2 rs243865 genotypes to pterygium susceptibility on a global scale. As for MMP-2 rs2285053, no association has been found between MMP-2 rs2285053 genotypes and pterygium (Table II and Table III). The minor allelic frequencies of MMP-2 rs243865 along with rs2285053 in our study are 10.4% and 21.1%, respectively (Table III). These frequencies closely resemble those reported on the NCBI website for East Asian populations, with minor allelic frequencies of 10.9% among 1,712 subjects and 23.7% among 1,170 subjects (50,51). This information, coupled with the adherence of the two polymorphic sites to Hardy-Weinberg Equilibrium, suggests that our collection of pterygium samples can be considered representative of the broader Taiwanese population.
In 2000, Solomon and his research team initiated the development of standardized pterygium fibroblast culture systems, enabling investigations into mRNA and protein expression levels in pterygium (23). Their initial study focused on MMP-2 and cytokines, revealing that the transcriptional expression of MMP-2 was relatively high but showed no significant difference between primary pterygium body fibroblasts and normal human conjunctival fibroblasts (23). In 2001, Li et al. conducted a comprehensive analysis of transcriptional and translational MMP expression in cultured human pterygium head, body, and subconjunctival fibroblasts from 6 non-pterygium subjects and 14 pterygium cases (22). Their findings indicated that only MMP-1 and MMP-3 proteins and activity decreased progressively from pterygium head to body to subconjunctival fibroblasts. Notably, there was no significant difference in the transcript and protein expression of MMP-2 or other targets (22). In 2009, Yang and his colleagues provided evidence showing significantly higher MMP-2 and MMP-9 expression in pterygium fibroblasts compared to normal conjunctival specimens (34). However, their sample size remained limited, with only 15 cases collected and cultured. They further compared paired cultured fibroblasts from the same cases and tentatively proposed that the expression of MMP-2, along with MMP-9, might increase in conjunction with the progression of pterygium (34). In the near future, it is essential to investigate whether the mRNA and protein expression levels of MMP-2 correlate with the genotypes of MMP-2 rs243865 and/or rs2285053. Furthermore, exploring whether MMP-2 expression levels are up-regulated in line with the progression of pterygium stages is an area of interest.
In summary, this study investigated the genotypic variations of MMP-2 rs243865 and rs2285053 in the Taiwanese population and found that neither of them significantly contributes to an individual’s susceptibility to pterygium among Taiwanese. It is worthwhile to validate these findings in larger and more diverse populations.
Conflicts of Interest
All the Authors declare no conflicts of interest regarding this study.
Authors’ Contributions
Research design: Hu PS, Hsia NY, Wang ZH, Bau DT; patient and questionnaire summaries: Hu PS, Hsia NY, Chen HC, Hsia TC; experimental work: Wang ZH, Chang WS, Tsai CW, Wang YC; statistical analysis: Wang ZH, Lin ML, Wang YC; manuscript writing: Tsai CW, Bau DT; manuscript checking and discussing: Hu PS, Hsia NY, Wang ZH, Chen HC, Hsia TC, Lin ML, Wang YC, Chang WS, Bau DT, Tsai CW.
Acknowledgements
The Authors are grateful to Yu-Hsin Lin, Yu-Ting Chin and Hou-Yu Shih for their excellent technical assistance. All the participants in this study are appreciated. This study is supported by grants from Changhua Christian Hospital (110-CCH-IRP-018) and China Medical University and Asia University (CMU111-ASIA-06). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Batur M, Seven E, Tekin S, Ozer MD, Demir MS, Yaşar T. The role of anterior segment optical coherence tomography in the evaluation of the pterygium. Photodiagnosis Photodyn Ther. 2023;43:103704. doi: 10.1016/j.pdpdt.2023.103704. [DOI] [PubMed] [Google Scholar]
- 2.Baheran SS, Alany RG, Schwikkard S, Muen W, Salman LN, Freestone N, Al-Kinani AA. Pharmacological treatment strategies of pterygium: Drugs, biologics, and novel natural products. Drug Discov Today. 2023;28(1):103416. doi: 10.1016/j.drudis.2022.103416. [DOI] [PubMed] [Google Scholar]
- 3.Han K, Ju MJ, Kim DH, Choi Y. Environmental exposures to lead, cadmium, and mercury and pterygium in Korean adults. Environ Sci Pollut Res Int. 2022;29(36):55058–55068. doi: 10.1007/s11356-022-19250-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Wu J, Geng J, Yuan Z, Huang D. Geographical prevalence and risk factors for pterygium: a systematic review and meta-analysis. BMJ Open. 2013;3(11):e003787. doi: 10.1136/bmjopen-2013-003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiasian L, Samavat B, Hadi Y, Arbab M, Abolfathzadeh N. Recurrent pterygium: a review. J Curr Ophthalmol. 2022;33(4):367–378. doi: 10.4103/joco.joco_153_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Acker SI, Van den Bogerd B, Haagdorens M, Siozopoulou V, Ní Dhubhghaill S, Pintelon I, Koppen C. Pterygium-The good, the bad, and the ugly. Cells. 2021;10(7):1567. doi: 10.3390/cells10071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021;13:25158414211020152. doi: 10.1177/25158414211020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezvan F, Khabazkhoob M, Hooshmand E, Yekta A, Saatchi M, Hashemi H. Prevalence and risk factors of pterygium: a systematic review and meta-analysis. Surv Ophthalmol. 2018;63(5):719–735. doi: 10.1016/j.survophthal.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Modenese A, Gobba F. Occupational exposure to solar radiation at different latitudes and pterygium: a systematic review of the last 10 years of scientific literature. Int J Environ Res Public Health. 2017;15(1):37. doi: 10.3390/ijerph15010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong SS, Peng Y, Liang YB, Cao D, Jhanji V. Does cigarette smoking alter the risk of pterygium? A systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55(10):6235. doi: 10.1167/iovs.14-15046. [DOI] [PubMed] [Google Scholar]
- 11.Anguria P, Kitinya J, Ntuli S, Carmichael T. The role of heredity in pterygium development. Int J Ophthalmol. 2014;7(3):563–573. doi: 10.3980/j.issn.2222-3959.2014.03.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu T, Liu Y, Xie L, He X, Bai J. Progress in the pathogenesis of pterygium. Curr Eye Res. 2013;38(12):1191–1197. doi: 10.3109/02713683.2013.823212. [DOI] [PubMed] [Google Scholar]
- 13.Saw S, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6(3):219–228. doi: 10.1076/opep.6.3.219.1504. [DOI] [PubMed] [Google Scholar]
- 14.Hu PS, Chang WS, Chou AK, Hsia NY, Hung YW, Lin CW, Wu CW, Huang CY, Wu MF, Liao CH, Tsai CW, Bau DT, Gong CL. The association of MMP-8 genotypes with pterygium. In Vivo. 2018;32(1):41–46. doi: 10.21873/invivo.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu PS, Wang YC, Liao CH, Hsia NY, Wu MF, Yang JS, Yu CC, Chang WS, Bau DT, Tsai CW. The association of MMP7 genotype with pterygium. In Vivo. 2020;34(1):51–56. doi: 10.21873/invivo.11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CB, Hsia NY, Wang YC, Wang ZH, Chin YT, Huang TL, Yu CC, Chang WS, Tsai CW, Yin MC, Bau DT. The significant association of MMP-1 genotypes with Taiwan pterygium. Anticancer Res. 2020;40(2):703–707. doi: 10.21873/anticanres.14000. [DOI] [PubMed] [Google Scholar]
- 17.Tsai CB, Hsia NY, Wang ZH, Yang JS, Hsu YM, Wang YC, Chang WS, Bau DT, Yin MC, Tsai CW. The contribution of MMP-9 genotypes to pterygium in Taiwan. Anticancer Res. 2020;40(8):4523–4527. doi: 10.21873/anticanres.14457. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Xu M, Lu F, He Y. Development of matrix metalloproteinases-mediated extracellular matrix remodeling in regenerative medicine: a mini review. Tissue Eng Regen Med. 2023;20(5):661–670. doi: 10.1007/s13770-023-00536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuault S, Ghossoub R, David G, Zimmermann P. A journey on extracellular vesicles for matrix metalloproteinases: a mechanistic perspective. Front Cell Dev Biol. 2022;10:886381. doi: 10.3389/fcell.2022.886381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabłońska-Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31(sup1):177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 21.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis. Arch Ophthalmol. 2001;119(5):695. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 22.Li DQ, Lee SB, Gunja-Smith Z, Liu Y, Solomon A, Meller D, Tseng SC. Overexpression of collagenase (MMP-1) and stromelysin (MMP-3) by pterygium head fibroblasts. Arch Ophthalmol. 2001;119(1):71–80. [PubMed] [Google Scholar]
- 23.Solomon A, Li DQ, Lee SB, Tseng SC. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000;41(8):2154–2163. [PubMed] [Google Scholar]
- 24.Di Girolamo N, Coroneo MT, Wakefield D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44(11):4705. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- 25.Di Girolamo N, Wakefield D, Coroneo MT. Differential expression of matrix metalloproteinases and their tissue inhibitors at the advancing pterygium head. Invest Ophthalmol Vis Sci. 2000;41(13):4142–4149. [PubMed] [Google Scholar]
- 26.Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42(9):1963–1968. [PubMed] [Google Scholar]
- 27.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Di Girolamo N, Coroneo M, Wakefield D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am J Pathol. 2005;167(2):489–503. doi: 10.1016/S0002-9440(10)62992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siak JJ, Ng SL, Seet LF, Beuerman RW, Tong L. The nuclear-factor ĸB pathway is activated in pterygium. Invest Ophthalmol Vis Sci. 2011;52(1):230. doi: 10.1167/iovs.10-5735. [DOI] [PubMed] [Google Scholar]
- 30.Henriet P, Emonard H. Matrix metalloproteinase-2: Not (just) a “hero” of the past. Biochimie. 2019;166:223–232. doi: 10.1016/j.biochi.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Devarajan P, Johnston JJ, Ginsberg SS, Van Wart HE, Berliner N. Structure and expression of neutrophil gelatinase cDNA. Identity with type IV collagenase from HT1080 cells. J Biol Chem. 1992;267(35):25228–25232. [PubMed] [Google Scholar]
- 32.Yi YY, Chen H, Zhang SB, Xu HW, Fang XY, Wang SJ. Exogenous Klotho ameliorates extracellular matrix degradation and angiogenesis in intervertebral disc degeneration via inhibition of the Rac1/PAK1/MMP-2 signaling axis. Mech Ageing Dev. 2022;207:111715. doi: 10.1016/j.mad.2022.111715. [DOI] [PubMed] [Google Scholar]
- 33.Xie J, Wang CL, Yang W, Wang J, Chen C, Zheng L, Sung KLP, Zhou X. Modulation of MMP-2 and MMP-9 through connected pathways and growth factors is critical for extracellular matrix balance of intra-articular ligaments. J Tissue Eng Regen Med. 2018;12(1):e550–e565. doi: 10.1002/term.2325. [DOI] [PubMed] [Google Scholar]
- 34.Yang SF, Lin CY, Yang PY, Chao SC, Ye YZ, Hu DN. Increased expression of gelatinase (MMP-2 and MMP-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009;50(10):4588. doi: 10.1167/iovs.08-3147. [DOI] [PubMed] [Google Scholar]
- 35.Di Girolamo N, McCluskey P, Lloyd A, Coroneo MT, Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(3):671–679. [PubMed] [Google Scholar]
- 36.He MX, Zhang JF, Yang L, Qin B, Gu HW, Tang QY, Guan HJ, Shi HH. Doxycycline suppresses vasculogenic mimicry in human pterygium fibroblasts. Curr Eye Res. 2022;47(10):1381–1388. doi: 10.1080/02713683.2022.2108455. [DOI] [PubMed] [Google Scholar]
- 37.Patel BP, Shah PM, Rawal UM, Desai AA, Shah SV, Rawal RM, Patel PS. Activation of MMP-2 and MMP-9 in patients with oral squamous cell carcinoma. J Surg Oncol. 2005;90(2):81–88. doi: 10.1002/jso.20240. [DOI] [PubMed] [Google Scholar]
- 38.Groblewska M, Mroczko B, Kozlowski M, Niklinski J, Laudanski J, Szmitkowski M. Serum matrix metalloproteinase 2 and tissue inhibitor of matrix metalloproteinases 2 in esophageal cancer patients. Folia Histochem Cytobiol. 2012;50(4):590–598. doi: 10.5603/20327. [DOI] [PubMed] [Google Scholar]
- 39.Dofara SG, Chang S, Diorio C. Gene polymorphisms and circulating levels of MMP-2 and MMP-9: a review of their role in breast cancer risk. Anticancer Res. 2020;40(7):3619–3631. doi: 10.21873/anticanres.14351. [DOI] [PubMed] [Google Scholar]
- 40.Kapral M, Wawszczyk J, Jurzak M, Dymitruk D, Weglarz L. Evaluation of the expression of metalloproteinases 2 and 9 and their tissue inhibitors in colon cancer cells treated with phytic acid. Acta Pol Pharm. 2010;67(6):625–629. [PubMed] [Google Scholar]
- 41.Lin CM, Zeng YL, Xiao M, Mei XQ, Shen LY, Guo MX, Lin ZY, Liu QF, Yang T. The relationship between MMP-2 -1306C>T and MMP-9 -1562C>T polymorphisms and the risk and prognosis of T-cell acute lymphoblastic leukemia in a Chinese population: a case-control study. Cell Physiol Biochem. 2017;42(4):1458–1468. doi: 10.1159/000479210. [DOI] [PubMed] [Google Scholar]
- 42.Yang MD, Lin KC, Lu MC, Jeng LB, Hsiao CL, Yueh TC, Fu CK, Li HT, Yen ST, Lin CW, Wu CW, Pang SY, Bau DT, Tsai FJ. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei) 2017;7(2):10. doi: 10.1051/bmdcn/2017070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Y, Ke TW, Wang YC, Chin YT, Yueh TC, Hung YC, Mong MC, Yang YC, Chang WS, Shen TC, Bau DT, Tsai CW. Impact of matrix metalloproteinase-8 genotypes on colorectal cancer risk in Taiwan. Anticancer Res. 2023;43(9):3979–3985. doi: 10.21873/anticanres.16585. [DOI] [PubMed] [Google Scholar]
- 44.Chang SM, Yang YC, Chen GL, Chen LH, Shen TC, Liu YF, Wang YC, Tsai CW, Hsia TC, Bau DT, Chang WS. The association of DNA ligase 1 Rs20579 polymorphism with lung cancer risk among Taiwanese. In Vivo. 2023;37(4):1504–1510. doi: 10.21873/invivo.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao CH, Chang WS, Hsu WL, Hu PS, Wu HC, Hsu SW, Wang BR, Yueh TC, Chen CH, Hsia TC, Huang WC, Bau DT, Tsai CW. Association of matrix metalloproteinase-7 genotypes with prostate cancer risk. Anticancer Res. 2023;43(1):381–387. doi: 10.21873/anticanres.16173. [DOI] [PubMed] [Google Scholar]
- 46.Yueh TC, Hung YC, Lee HT, Yang MD, Wang ZH, Yang YC, Ke TW, Pei JS, Tsai CW, Bau DT, Chang WS. Role of matrix metallopeptidase-2 genotypes in Taiwanese patients with colorectal cancer. Anticancer Res. 2022;42(11):5335–5342. doi: 10.21873/anticanres.16040. [DOI] [PubMed] [Google Scholar]
- 47.Fu CK, Mong MC, Yu CC, Yang MD, Wang ZH, Yang YC, Chen JC, Pei JS, Hsia NY, Tsai CW, Chang WS, Bau DT. Association of matrix metallopeptidase-2 genotypes with risk of gastric cancer in Taiwan. Anticancer Res. 2022;42(4):1749–1755. doi: 10.21873/anticanres.15651. [DOI] [PubMed] [Google Scholar]
- 48.An MX, Wu KL, Lin SC. Detection and comparison of matrix metalloproteinase in primary and recurrent pterygium fibroblasts. Int J Ophthalmol. 2011;4(4):353–356. doi: 10.3980/j.issn.2222-3959.2011.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan Q, Wan P, Liu W, Cheng Y, Gu S, Shi Q, Su Y, Wang X, Liu C, Wang Z. Tear film cytokines as prognostic indicators for predicting early recurrent pterygium. Exp Eye Res. 2022;222:109140. doi: 10.1016/j.exer.2022.109140. [DOI] [PubMed] [Google Scholar]
- 50.dbSNP rs243865. Available at: https://www.ncbi.nlm.nih.gov/snp/rs243865. [Last accessed on November 17, 2023]
- 51.dbSNP rs2285053. rs2285053. Available at: https://www.ncbi.nlm.nih.gov/snp/rs2285053. [Last accessed on November 17, 2023]