Abstract
STUDY QUESTION
What is the efficacy and safety of long-term treatment (up to 2 years) with relugolix combination therapy (CT) in women with moderate to severe endometriosis-associated pain?
SUMMARY ANSWER
For up to 2 years, treatment with relugolix CT improved menstrual and non-menstrual pain, dyspareunia, and function in women with endometriosis; after an initial decline of <1%, the mean bone mineral density (BMD) remained stable with continued treatment.
WHAT IS KNOWN ALREADY
Endometriosis is a chronic condition characterized by symptoms of dysmenorrhea, non-menstrual pelvic pain (NMPP), and dyspareunia, which have a substantial impact on the lives of affected women, their partners, and families. SPIRIT 1 and 2 were phase 3, randomized, double-blind, placebo-controlled studies of once-daily relugolix CT (relugolix 40 mg, oestradiol 1 mg, norethisterone acetate 0.5 mg) in premenopausal women (age 18–50 years) with endometriosis and moderate-to-severe dysmenorrhea and NMPP. These trials demonstrated a significant improvement of dysmenorrhea, NMPP, and dyspareunia in women treated with relugolix CT, with minimal decline (<1%) in BMD versus placebo at 24 weeks.
STUDY DESIGN, SIZE, DURATION
Patients participating in this open-label, single-arm, long-term extension (LTE) study of the 24-week SPIRIT pivotal studies (SPIRIT 1 and 2) received up to an additional 80 weeks of once-daily oral relugolix CT treatment between May 2018 and January 2023.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Premenopausal women with confirmed endometriosis and moderate to severe dysmenorrhea and NMPP who completed the 24-week pivotal studies (SPIRIT 1 and 2 trials; Giudice et al., 2022) and who met all entry criteria were eligible to enrol. Two-year results were analysed by treatment group based on original randomization in pivotal studies: relugolix CT, delayed relugolix CT (relugolix 40 mg monotherapy for 12 weeks, followed by relugolix CT), or placebo→relugolix CT (placebo for 24 weeks followed by relugolix CT). The primary endpoints of the LTE study were the proportion of dysmenorrhea and NMPP responders at Week 52 and Week 104/end-of-treatment (EOT). A responder was a participant who achieved a predefined, clinically meaningful reduction from baseline in Numerical Rating Scale (NRS) scores (0 = no pain, 10 = worst pain imaginable) for the specific pain type with no increase in analgesic use. The predefined clinically meaningful threshold for dysmenorrhea was 2.8 points and for NMPP was 2.1 points. Secondary efficacy endpoints included change from baseline in Endometriosis Health Profile-30 (EHP-30) pain domain scores, a measure of the effects of endometriosis-associated pain on daily activities (function), NRS scores for dysmenorrhea, NMPP, dyspareunia, and overall pelvic pain, and analgesic/opioid use. Safety endpoints included adverse events and changes in BMD.
MAIN RESULTS AND THE ROLE OF CHANCE
Of 1261 randomized patients, 1044 completed the pivotal studies, 802 enrolled in the LTE, 681 completed 52 weeks of treatment, and 501 completed 104 weeks of treatment. Demographics and baseline characteristics of the extension population were consistent with those of the original randomized population. Among patients randomized to relugolix CT at pivotal study baseline who continued in the LTE (N = 277), sustained improvements in endometriosis-associated pain were demonstrated through 104 weeks. The proportion of responders at Week 104/EOT for dysmenorrhea and NMPP was 84.8% and 75.8%, respectively. Decreases in dyspareunia and improvement in function assessed by EHP-30 pain domain were also sustained over 2 years. At Week 104/EOT, 91% of patients were opioid-free and 75% of patients were analgesic-free. Relugolix CT over 104 weeks was well tolerated with a safety profile consistent with that observed over the first 24 weeks. After initial least squares mean BMD loss <1% at Week 24, BMD plateaued at Week 36 and was sustained for the duration of 104 weeks of treatment. Efficacy and safety results were generally consistent in women in the placebo→relugolix CT and delayed relugolix CT groups.
LIMITATIONS, REASONS FOR CAUTION
The study was conducted as an open-label study without a control group over the 80 weeks of the extension period. Of the 802 patients who were enrolled in this LTE study, 681 patients (84.9%) and 501 patients (62.5%) of patients completed 52 and 104 weeks of treatment, respectively. In addition, there currently are no comparative data to other hormonal medications. Finally, a third (37.4%) of the study population terminated participation early.
WIDER IMPLICATIONS OF THE FINDINGS
In conclusion, relugolix CT offers an additional option to help address an important unmet clinical need for effective, safe, and well-tolerated medical treatments for endometriosis that can be used longer-term, reducing the need for opioids and improving quality of life. The findings from this study may help support the care of women with endometriosis seeking longer-term effective medical management of their symptoms.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by Myovant Sciences GmbH (now Sumitomo Pharma Switzerland GmbH). C.M.B. reports fees from Myovant, grants from Bayer Healthcare, fees from ObsEva, and Chair of ESHRE Endometriosis Guideline Group (all funds went to the University of Oxford); N.P.J. reports personal fees from Myovant Sciences, during the conduct of the study, personal fees from Guerbet, personal fees from Organon, personal fees from Roche Diagnostics; S.A.-S. reports personal fees from Myovant Sciences, personal fees from Bayer, personal fees from Abbvie, personal fees from UpToDate; J.S.P., and R.B.W. are employees and shareholders of Myovant Sciences; J.C.A.F. and S.J.I. are shareholders of Myovant Sciences (but at time of publicaion are no longer employess of Myovant Sciences); M.S.A. and K.W. have no conflicts to declare; V.M. is a consultant to Myovant; L.C.G. reports personal fees from Myovant Sciences, Inc and Bayer. The authors did not receive compensation for manuscript writing, review, and revision.
TRIAL REGISTRATION NUMBER
Keywords: endometriosis, pain, relugolix, long-term treatment, dysmenorrhea, non-menstrual pelvic pain, dyspareunia
Introduction
Endometriosis is a chronic inflammatory condition characterized by symptoms of menstrual and non-menstrual pain, infertility, and dyspareunia, affecting 10% of women in their reproductive years (Vercellini et al., 2011; Johnson and Hummelshoj, 2013; Dunselman et al., 2014; Kuznetsov et al., 2017; Zondervan et al., 2020). Pelvic pain, a common symptom of endometriosis, may occur with menses (dysmenorrhea), between or in the absence of menses (non-menstrual pelvic pain [NMPP]), and/or with sexual intercourse (dyspareunia).
Treatments for endometriosis include surgical removal and pharmacologic suppression of lesions (Becker et al., 2017; Soliman et al., 2018; Lamvu et al., 2019; Singh et al., 2020). While surgical resection and/or ablation are sometimes successful, symptoms may persist or recur despite appropriate surgery (Singh et al., 2020; Becker et al., 2022). Pharmacologic management of endometriosis includes analgesics and therapies to lower endogenous oestrogen, given that oestradiol is a key driver of endometrial growth and contributes to local inflammation and pain (Zondervan et al., 2018, 2020). Oral contraceptives, progestins and, less often, androgens are used with few approved specifically for management of pain associated with endometriosis (e.g. medroxyprogesterone, dienogest, and danazol). Approved therapies also include GnRH agonists and antagonists, which function by inducing a hypoestrogenic state, thus posing the risk of decline in bone mineral density (BMD). The GnRH antagonist, elagolix, is approved for reduction of endometriosis-associated pain in USA (Orilissa USPI, 2023). However, use of its high dose (200 mg twice daily) is limited to 6 months because of concerns of BMD loss. The lower dose (150 mg daily) is approved for use up to 2 years, though dysmenorrhea and NMPP responder rates are not qualitatively as high as in the group that received 200 mg twice daily, and its effects on dyspareunia and the use of rescue analgesics were not statistically different compared with placebo (Taylor et al., 2017; Orilissa USPI, 2023). Thus, the clinical need remained for an effective oral therapeutic agent that significantly improved moderate-to-severe pain symptoms and could be used for longer term because hypoestrogenic risk, particularly as manifested as BMD loss, had been mitigated.
Relugolix, an oral non-peptide GnRH receptor antagonist, competitively binds to pituitary GnRH receptors, blocking binding of endogenous GnRH with reversible, dose-dependent suppression of LH and FSH, and ovarian oestradiol and progesterone production (Miwa et al., 2011; Nakata et al., 2014; Osuga et al., 2021). Relugolix combination therapy (relugolix CT; 40 mg relugolix, 1 mg oestradiol, and 0.5 mg norethisterone acetate) was developed as a once-daily oral treatment for endometriosis-associated pain or symptomatic uterine fibroids to provide a treatment option that could be used for a longer duration. In USA, relugolix CT is approved for management of heavy menstrual bleeding associated with uterine fibroids as well as for treatment of moderate-to-severe pain associated with endometriosis (MYFEMBREE PI, 2023). In the European Union and other regions, relugolix CT is approved for treatment of moderate to severe symptoms in women with uterine fibroids as well as for the symptomatic treatment of endometriosis in women with a history of previous medical or surgical treatment (Ryeqo SmPC, 2023). In two replicate, 24-week, multinational phase 3, double-blind, randomized, placebo-controlled studies (SPIRIT 1 and SPIRIT 2), once-daily relugolix CT significantly reduced dysmenorrhoea, NMPP, and dyspareunia, improved daily function, and was well tolerated with minimal (<1%) bone density loss in women with moderate-to-severe endometriosis-associated pain (Giudice et al., 2022). Herein, we report the long-term (2-year) efficacy and safety of relugolix CT in the treatment of endometriosis-associated pain from the SPIRIT long-term extension (LTE) study.
Materials and methods
Study design and patients
This was a multinational, open-label, single-arm, long-term efficacy, and safety extension study (ClinicalTrials.gov: NCT03204318; NCT03204331; NCT03654274) of the two pivotal SPIRIT 1 and SPIRIT 2 trials (Giudice et al., 2022). As previously reported, participants who enrolled in the replicate pivotal SPIRIT 1 or SPIRIT 2 studies were premenopausal women 18–50 years of age who had moderate to severe dysmenorrhea and NMPP, as defined by patient self-assessment by Numerical Rating Scale (NRS) scores at baseline, were eligible to participate (Giudice et al., 2022).
Patients who completed 24 weeks of treatment in one of the three study groups in the pivotal studies and were not expecting to undergo additional procedures for endometriosis within the study period were eligible to enrol in the SPIRIT LTE study. Study drug was to be initiated on Days 1–14 of the menstrual cycle and administered in the fasted state in the morning at the same time each day. A missed dose of study drug was to be taken as soon as possible and in a fasted state if possible (at least 1 h before or 2 h after a meal). A negative pregnancy test was required at baseline and at each study visit. As a precautionary measure, patients were required to use non-hormonal contraception. Patients with a Z-score for BMD <−2.0 or with a decrease of BMD ≥7% from pivotal study baseline to Week 24 at lumbar spine, total hip, or femoral neck were excluded. Full inclusion/exclusion criteria can be found in the Supplementary Materials and Methods. There was no wash-out period from investigational product in the pivotal studies, and all patients received their first dose of relugolix CT after being deemed eligible and providing informed consent (Supplementary Fig. S1). Patients remained blinded to their treatment allocation during the pivotal studies.
All patients received oral relugolix CT once daily for up to 80 weeks. The study objectives were to evaluate long-term efficacy and safety up to 104 weeks of treatment with relugolix CT, which included the antecedent 24 weeks of treatment during either pivotal study. Patient visits occurred every 4 weeks. The studies were conducted in accordance with International Conference on Harmonisation guidelines and ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Assessments
The dual primary endpoints were the proportion of dysmenorrhea and NMPP responders at Week 52 and at 104/end-of-treatment (EOT), based on daily patient-reported NRS scores (0 = no pain, 10 = worst pain imaginable) and no increase in analgesic use. Secondary efficacy endpoints included the change from baseline in NRS score for dysmenorrhea, NMPP, and dyspareunia; Endometriosis Health Profile 30 (EHP-30); and analgesic and opioid use, all of which were reported for the pivotal studies. Only study-specified Tier 1 (ibuprofen) and Tier 2 (opioid-containing) analgesics were allowed throughout the study, and these medications were to be taken for control of pain, not prophylactically. Pain level and analgesic use were collected daily in a patient electronic diary.
Safety assessments included adverse events (AEs), mammograms in patients >40 years of age, and endometrial biopsy. AEs, including vasomotor symptoms, that participants reported at each study visit were evaluated and graded by the investigator according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Of note, the incidence of vasomotor symptoms was reported as a safety objective in the pivotal trials to determine the incidence with relugolix CT (Giudice et al., 2022). BMD by dual-energy X-ray absorptiometry was evaluated at the Week 36, Week 52, and Week 104/early termination visits. Treatment compliance and eDiary compliance also were evaluated.
Statistical analysis
Since this was an extension study, the sample size was determined by the number of patients who completed the two replicate pivotal SPIRIT studies (SPIRIT 1 and SPIRIT 2) who were eligible and willing to participate in the extension study. It was anticipated that this study would enrol approximately 800 patients (67% of the total number of patients enrolled in the pivotal studies). The pivotal study baseline (Giudice et al., 2022) was used as the reference point in the LTE study report for analysing all change from baseline-related endpoints unless otherwise specified. Efficacy and safety data were analysed using descriptive statistics for patients who enrolled in the study and who received at least one dose of study drug in the LTE period; they are described here by the randomized treatment group at pivotal study baseline: relugolix CT: relugolix CT for up to 104 weeks; delayed relugolix CT: 12 weeks of relugolix 40 mg monotherapy followed by relugolix CT for up to 92 weeks; and placebo → relugolix CT: 24 weeks of placebo followed by relugolix CT for up to 80 weeks.
There were no statistical comparisons among treatment groups performed for this extension study. The methods for analysing the efficacy endpoints in the LTE study, including missing data handling rules, were similar to those used in the two replicate SPIRIT 1 and SPIRIT 2 studies (Giudice et al., 2022).
The primary endpoints were the proportion of dysmenorrhea and NMPP responders at Week 52 and Week 104/EOT. A dysmenorrhea responder was pre-defined as having a decrease of at least 2.8 points in the dysmenorrhea NRS with no increase in analgesic use as recorded in a daily electronic diary (eDiary). An NMPP responder was pre-defined as having a decrease of at least 2.1 points in the NMPP NRS with no increase in analgesic use. The clinically meaningful change thresholds of 2.8 points decrease for dysmenorrhea and 2.1 points decrease for NMPP, as well as −20 points for the functional endpoint assessed by EHP-30 pain domain, established for the pivotal studies based on anchor-based analyses (Giudice et al., 2022), were applied to the LTE. The proportion of patients who had minimal to no pain (NRS ≤1) at Week 104 and the median time to minimal to no pain were assessed using the Kaplan–Meier method as post hoc analyses for both dysmenorrhea and NMPP performed on all patients enrolled on the pivotal and LTE studies.
The severity of all AEs was evaluated by the investigator based on CTCAE and were coded to preferred term and system organ class using MedDRA (version 22.0; MedDRA Maintenance and Support Services Organization (MSSO, Herndon, VA, USA)). Laboratory values were classified by toxicity grade based on the CTCAE.
Results
Patients
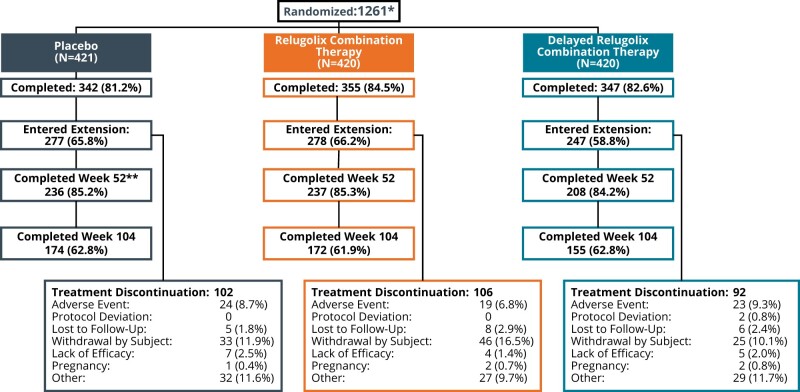
Patient disposition is shown in Fig. 1. Of 1261 women randomized, 1044 completed the primary SPIRIT studies (Giudice et al., 2022). A total of 802 (77%) women from 171 trial centres enrolled in the SPIRIT LTE study and 799 were analysed. Three patients (two in placebo group, one in relugolix CT group) from one study site were excluded from all efficacy and safety analyses owing to non-compliance with International Council for Harmonisation E6R2 Good Clinical Practice guidelines and identified data integrity issues. There were 681 (85%) and 501 (62%) patients who completed 52 and 104 weeks of treatment, respectively. The study drug compliance with relugolix CT cumulatively from pivotal baseline, and restricted to the LTE study period, was 98.7% and 98.8%, respectively.
Figure 1.
Patient disposition in a 2-year efficacy and safety study of relugolix combination therapy in women with endometriosis-associated pain. *All patients will be described according to their baseline treatment assignment in SPIRIT Phase 3 studies. **One patient consented to Week 52 and completed at Week 52.
Twenty-six patients across both pivotal studies were not eligible for the LTE study because they met the BMD loss exclusion criteria of either percentage change ≥7% or Z-score <−2.0: most of them belonged to the delayed relugolix CT group (n = 17), seven women were in the relugolix CT group and two in the placebo group. A total of 300 (37.4%) patients discontinued participation in the LTE study, and reasons were similar across the three treatment groups. Withdrawal by the subject (104 patients [13.0%]) or for other reasons (88 patients [11%]) were the most common, which included personal decision (notably study fatigue); desire for pregnancy; and inability to meet study demands because of relocation, work, or childcare. AEs leading to discontinuation were reported in 66 patients (8.2%), most of which were reported for only one patient each, and the majority were non-serious. There were 16 patients (2.0%) who discontinued the study owing to lack of efficacy.
Baseline characteristics of those who completed the pivotal studies and enrolled in the LTE are summarized in Table 1. The mean age was 34 years and the majority of patients were White (92%). One-third had received a surgical diagnosis of endometriosis ≥5 years prior to the study. A high percentage (84%) had undergone prior surgical interventions for endometriosis, which was comparable to the pivotal studies. Demographics and baseline characteristics of the extension population also were generally similar to those of the pivotal studies population (Giudice et al., 2022).
Table 1.
Demographic and baseline characteristics of those who had completed the pivotal studies and enrolled in the long-term extension study.*
| Placebo → Relugolix CT | Relugolix CT | Delayed Relugolix CT | |
|---|---|---|---|
| (N = 275) | (N = 277) | (N = 247) | |
| Age—mean years (SD) | 34.3 (6.48) | 34.1 (6.55) | 35.1 (6.49) |
| BMI—kg/m2 mean (SD) | 26.3 (6.45) | 25.6 (6.03) | 26.0 (5.41) |
| Race—n (%)‡ | |||
| White | 248 (90.2%) | 254 (91.7%) | 236 (95.5%) |
| Black | 13 (4.7%) | 17 (6.1%) | 7 (2.8%) |
| Other | 14 (5.9%) | 6 (2.1%) | 4 (1.6%) |
| Geographic Region—n (%) | |||
| North America | 56 (20.4%) | 48 (17.3%) | 46 (18.6%) |
| Rest of world | 219 (79.6%) | 229 (82.7%) | 201 (81.4%) |
| Time since surgical diagnosis of endometriosis | |||
| Mean years (SD) | 3.9 (3.24) | 4.0 (3.52) | 4.7 (4.00) |
| <5—n (%) | 188 (68.4%) | 188 (67.9%) | 149 (60.3%) |
| 5–10—n (%) | 87 (31.6%) | 89 (32.1%) | 98 (39.7%) |
| Bone mineral density (BMD) | |||
| BMD (g/cm2) lumbar spine L1–L4—mean (SD) | 1.14 (0.15) | 1.14 (0.16) | 1.14 (0.14) |
| z-score§ | |||
| Lumbar spine, mean (SD) | 0.30 (1.01) | 0.18 (1.09) | 0.20 (1.02) |
| Total hip, mean (SD) | 0.09 (0.92) | 0.03 (0.94) | 0.03 (0.90) |
| Patient-reported outcome measures | |||
| Dysmenorrhoea NRS score∥ | |||
| Mean (SD) | 7.2 (1.63) | 7.1 (1.66) | 7.0 (1.65) |
| <7—n (%) | 112 (40.7%) | 117 (42.2%) | 111 (44.9%) |
| ≥7—n (%) | 163 (59.3%) | 160 (57.8%) | 136 (55.1%) |
| Non-menstrual pelvic pain NRS score∥ | |||
| Mean (SD) | 5.7 (1.91) | 5.7 (1.93) | 5.5 (1.98) |
| <4—no. (%) | 57 (20.7%) | 61 (22.0%) | 69 (27.9%) |
| ≥4—no. (%) | 218 (79.3%) | 216 (78.0%) | 178 (72.1%) |
| Dyspareunia NRS score∥ | |||
| <7—no. (%) | 112 (40.7%) | 117 (42.2%) | 111 (44.9%) |
| ≥7—no. (%) | 163 (59.3%) | 160 (57.8%) | 136 (55.1%) |
| EHP-30 pain domain¶ | |||
| n (%) | 274 | 271 | 247 |
| Mean (SD) | 56.2 (14.9) | 57.3 (17.1) | 56.2 (16.4) |
| Analgesic use—no. (%)** | |||
| Non–opioids | 245 (89.1) | 249 (89.9) | 220 (89.1) |
| Opioids | 95 (34.5) | 109 (39.4) | 96 (38.9) |
All analyses were performed on the modified intention-to-treat population defined as all enrolled patients who took at least one study drug. BMD, bone mineral density; CT, combination therapy; EHP-30, Endometriosis Health Profile 30-item Questionnaire; NRS, Numerical Rating Scale.
Race was reported by the patient. ‘Other’ includes Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, other, and multiple.
Z-scores are based on analysis of corrected BMD data as assessed by a central radiology laboratory.
Scores for dysmenorrhoea, dyspareunia, and non-menstrual pelvic pain range from 0 (no pain) to 10 (worst pain imaginable) and were recorded in an electronic daily diary.
EHP-30 assesses the impact of pain to limit normal daily activity including ability to participate in social events, jobs, standing, sitting, walking, exercising, appetite, and sleep on a scale of 0–100, with higher scores denoting greater functional impact of pain.
Baseline refers to the time before the 35-day run-in period where patients were assigned specific analgesics on the National Cancer Institute’s Common Terminology for Adverse Events (version 5.0).
Efficacy
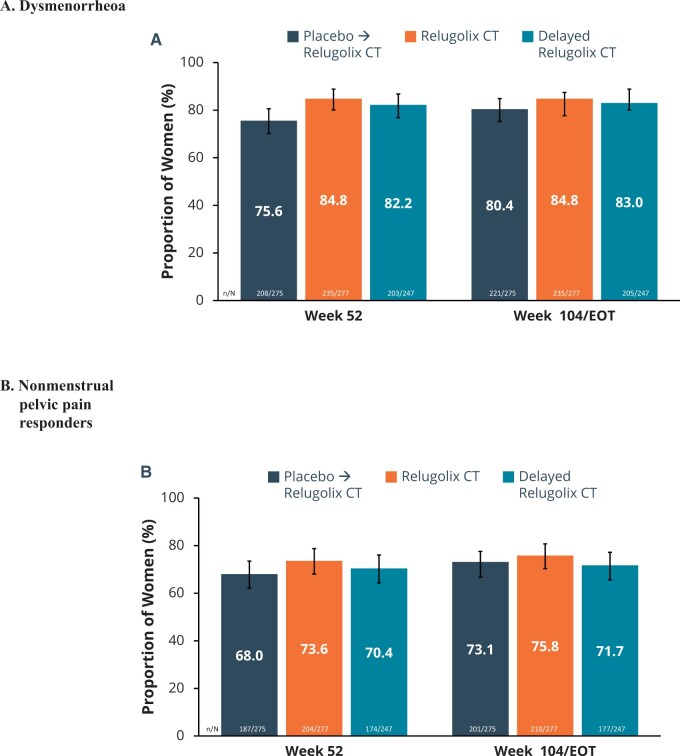
Among patients in the relugolix CT group, at Week 52, 84.8% (95% CI: 80.1%, 88.8%) of patients met the dysmenorrhea responder definition and 73.6% (95% CI: 68.0%, 78.7%) of patients met the NMPP responder definition. These rates of response were sustained through Week 104/EOT: with 84.8% (95% CI: 80.1%, 88.8%) for dysmenorrhea and 75.8% (95% CI: 70.3%, 80.7%) for NMPP (Fig. 2). As expected, patients who were in the placebo → relugolix CT group showed improvement in dysmenorrhea and NMPP scores soon after commencing relugolix CT, with Week 104/EOT responder rates of 80.4% and 73.1%, that were generally consistent with those observed in the relugolix CT group. Findings for patients who were in the delayed relugolix CT group showed similar responder rates for dysmenorrhea and NMPP as those patients who had been treated with relugolix CT. Similar to the pivotal studies (Giudice et al., 2022), the robustness of the results of the primary endpoints was supported by consistent findings across subgroups defined by age, race, BMI, and geography. The responder rates in the subgroups were comparable to those in the overall population (Supplementary Tables S1 and S2).
Figure 2.
Dysmenorrhoea and non-menstrual pelvic pain responders. The dual primary endpoints of both trials were the proportion of (A) dysmenorrhoea and (B) non-menstrual pelvic pain responders at Week 104. Error bars represent 95% CI. A responder was a woman who achieved a predefined, clinically meaningful reduction from baseline in Numerical Rating Scale scores (0 = no pain, 10 = worst pain imaginable) for the specific pain type with no increase in analgesic use. The clinically meaningful threshold for dysmenorrhea was 2.8 points and for non-menstrual pelvic pain was 2.1 points. EOT, end of treatment.
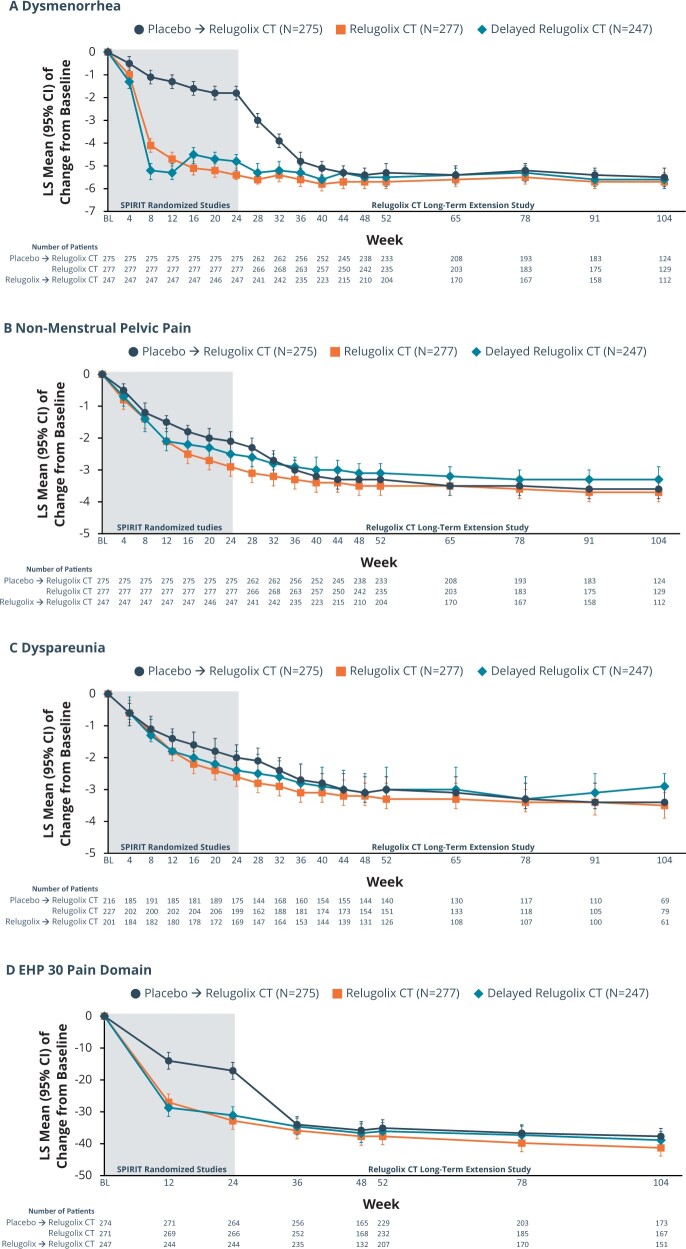
In the relugolix CT group, the pivotal study baseline least squares (LS) mean dysmenorrhea NRS score of 7.4 decreased to 1.2 at Week 52, representing an 83.9% reduction from baseline and a reduction in pain scores from severe to mild. At Week 104, the LS mean reduction in the dysmenorrhea score was maintained (1.2 [0.17]), representing an 84.0% decrease from baseline (Fig. 3A). In the pivotal studies, the post-baseline LS mean dysmenorrhea NRS score in the placebo group was higher than in the relugolix groups through Week 24 (Giudice et al., 2022). Following transition to relugolix CT in the LTE study, there was a sharp decline in the score between Weeks 24 and 36. With continued treatment, the LS mean dysmenorrhea score in this group became comparable to the scores in the relugolix group, and the mean dysmenorrhea score at Week 104 was 1.5, representing a 78.8% reduction from baseline. Findings for patients who were originally randomized to delayed relugolix CT group showed similar responder rates for dysmenorrhea.
Figure 3.
Change from baseline in average dysmenorrhea NRS score, non-menstrual pelvic pain NRS score, dyspareunia Numerical Rating Scale score, and EHP-30 pain domain. Change from baseline in average (A) dysmenorrhea NRS score, (B) non-menstrual pelvic pain NRS score, (C) dyspareunia numerical rating scale score, and (D) EHP-30 pain domain. During the first 24 weeks of treatment, patients receive one of three randomized treatments: relugolix CT, relugolix for 12 weeks followed by relugolix CT for 12 weeks, or placebo. Starting at Week 24, the beginning of the SPIRT long-term extension study, all patients received relugolix CT. The score range for the Numerical Rating Scale (NRS) for dysmenorrhea, non-menstrual pelvic pain (NMPP), and dyspareunia was 0 (no pain) to 10 (pain as bad as you can imagine). The score range for the EHP-30 pain domain ranged from 0 to 100, with higher scores denoting a greater functional impact of pain. CT, combination therapy; EHP-30, Endometriosis Health Profile-30.
For NMPP NRS scores over time, the pivotal study baseline LS mean scores were 5.9 for the relugolix CT and 5.9 in the placebo group. In the relugolix CT group, the LS mean NRS score decreased to 2.2 at Week 52, representing a 63.5% decrease from baseline and a reduction in pain from moderate to mild (Fig. 3B). At Week 104, the LS mean reduction in the NMPP score was maintained (1.8 [0.17]), representing a 68.9% decrease from baseline. In the pivotal study, the LS mean NMPP NRS score in the placebo group remained higher than in the relugolix groups through Week 24 (Giudice et al., 2022). Following transition to relugolix CT in the LTE study, starting at Week 28, the LS mean NMPP score in placebo → relugolix CT group decreased noticeably and subsequently became similar to the scores in the relugolix CT group. The mean NMPP score at Week 104 was 2.0, representing a 67.8% decline from baseline. Findings for patients who were originally in the delayed relugolix CT group showed similar responder rates for NMPP as the patients who had been treated with relugolix CT for 104 weeks.
After 104 weeks of treatment, in the relugolix CT group, 95.3% of women had minimal to no dysmenorrhea (NRS ≤ 1) and 70.5% had minimal to no NMPP (NRS ≤1) (Supplementary Fig. S2).
Furthermore, in the relugolix CT group, similar observations as for NMPP were observed for overall pelvic pain and dyspareunia by NRS score by visit, with steady declines in pain through Week 104, with reductions from baseline of 69.4% and 58.6%, respectively (Supplementary Fig. S3 and S3C). For patients treated with placebo who transitioned to relugolix CT, response to treatment was consistent with that observed in the relugolix groups during the first 24 weeks in the pivotal phase 3 studies (Giudice et al., 2022).
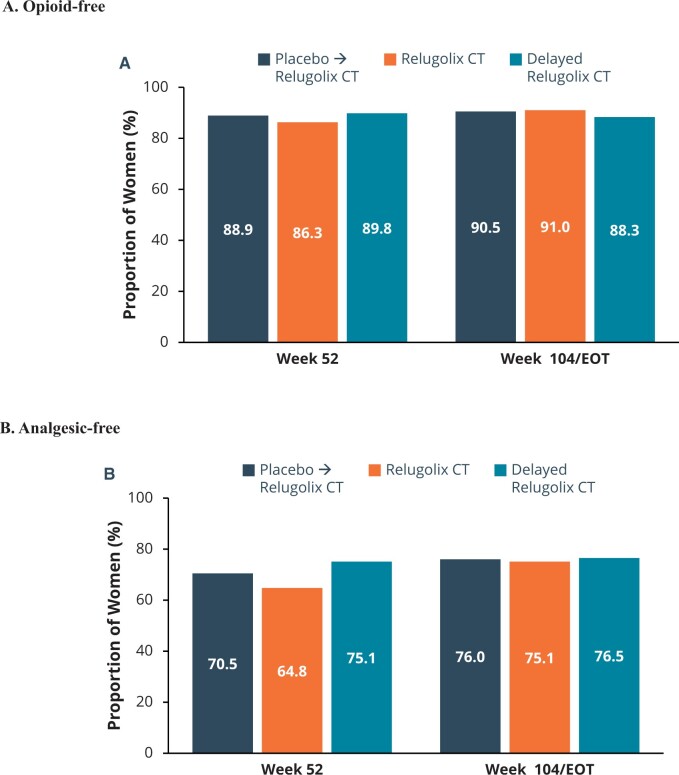
These improvements in pain were associated with better function as reflected by reduction in the EHP-30 pain domain score (Supplementary Fig. 3D) and less use of opioids and analgesics overall through 104 weeks of treatment. The proportion of women classified as responders based on reduction in EHP-30 pain domain was 83.6%, 81.2%, and 79.5% at Week 52 and 88.6%, 85.4%, and 86.1% at Week 104 in the relugolix CT, delayed relugolix CT, and placebo→relugolix CT groups, respectively. There was nearly universal use of analgesics at baseline in the LTE study population (92.8% for relugolix CT, 92.3% for delayed relugolix CT, and 92.7% for placebo→relugolix CT). With relugolix CT through 104 weeks, there was a substantial number of women who became analgesic-free (7.2% analgesic-free at baseline versus 75.1% analgesic-free at Week 104/EOT) and opioid-free (60.6% opioid-free at baseline versus 91.0% opioid-free at Week 104/EOT) (Fig. 4). Similar findings were observed in the subjects who transitioned from relugolix monotherapy (i.e. delayed relugolix CT) and from placebo to relugolix CT.
Figure 4.
Percentage of women who were opioid-free and analgesic-free at Week 52/Week 104. Secondary efficacy endpoints included opioid and analgesic use. Graphs in Fig. 4 depict observed data for the Week 52 assessment, while the final assessment includes data for patients at either the Week 104 visit or the end of treatment (EOT) visit. At Week 104/EOT, 91% of patients in the relugolix CT group were opioid-free (A) and 75% of patients were analgesic-free (B), with generally consistent results in women in the placebo→relugolix CT and delayed relugolix CT groups. CT, combination therapy.
Safety
Treatment with relugolix CT for up to 104 weeks was generally well tolerated. No new safety trends emerged with long-term treatment up to 104 weeks. In the relugolix CT group, 73.6% and 93.1% of women had at least one AE during participation in the 80 weeks of LTE study and cumulatively over the 104-week treatment period, respectively (Table 2). Few patients were reported to have grade 3 or higher events (5.4% during the LTE study, 10.8% cumulatively), or serious AEs (2.5% during the LTE study, 4.0% cumulatively). There was no disproportionate increase in the incidence of either serious or non-serious AEs in the relugolix CT group up to 104 weeks of treatment relative to what was observed through Week 24. There were no fatal AEs.
Table 2.
Adverse events in the three study groups in the 80 weeks of LTE study and cumulatively over the 104-week treatment period.
| Placebo → Relugolix CT | Relugolix CT | Delayed Relugolix CT | ||||
|---|---|---|---|---|---|---|
| (N = 275) |
(N = 277) |
(N = 247) |
||||
| Cumulative | LTE | Cumulative | LTE | Cumulative | LTE | |
| Adverse events * (AE)—n (%) | ||||||
| Any AE | 249 (90.5%) | 215 (78.2%) | 258 (93.1%) | 204 (73.6%) | 224 (90.7%) | 177 (71.7%) |
| AE Leading to study treatment discontinuation | 23 (8.4%) | 22 (8.0%) | 19 (6.9%) | 15 (5.4%) | 23 (9.3%) | 17 (6.9%) |
| AE related to study drug | 177 (64.4%) | 135 (49.1%) | 172 (62.1%) | 94 (33.9%) | 175 (70.9%) | 93 (37.7%) |
| Grade 3 or higher AE | 42 (15.3%) | 30 (10.9%) | 30 (10.8%) | 15 (5.4%) | 34 (13.8%) | 23 (9.3%) |
| Serious AE | 20 (7.3%) | 18 (6.5%) | 11 (4.0%) | 7 (2.5%) | 20 (8.1%) | 19 (7.7%) |
| Fatal AE | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse events reported in >10% in any group—n (%) | ||||||
| Headache | 121 (44.0%) | 38 (13.8%) | 146 (52.7%) | 38 (13.7%) | 119 (48.2%) | 18 (7.3%) |
| Nasopharyngitis | 46 (16.7%) | 22 (8.0%) | 63 (22.7%) | 24 (8.7%) | 30 (12.1%) | 14 (5.7%) |
| Hot flush | 40 (14.5%) | 22 (8.0%) | 41 (14.8%) | 9 (3.2%) | 106 (42.9%) | 8 (3.2%) |
| Urinary tract infection | 24 (8.7%) | 18 (6.5%) | 31 (11.2%) | 12 (4.9%) | 25 (10.1%) | 14 (5.1%) |
| Vulvovaginal mycotic infection | 16 (5.8%) | 15 (5.5%) | 31 (11.2%) | 28 (10.1%) | 12 (4.9%) | 12 (4.9%) |
| Toothache | 14 (5.1%) | 5 (1.8%) | 30 (10.8%) | 10 (3.6.%) | 12 (4.9%) | 5 (2.0%) |
| Back pain | 24 (8.7%) | 15 (5.5%) | 28 (10.1%) | 11 (4.0%) | 18 (7.3%) | 4 (1.6%) |
| Nausea | 22 (8.0%) | 10 (3.6%) | 28 (10.1%) | 8 (2.9%) | 13 (5.3%) | 3 (1.2%) |
Adverse events were coded using the Medical Dictionary for Regulatory Activities and severity of adverse events was evaluated by the investigator based on the National Cancer Institute’s Common Terminology for Adverse Events (version 5.0). AE, adverse events; CT, combination therapy.
In the relugolix CT group, over the cumulative 104-week treatment period in the phase 3 pivotal studies and the LTE study, the most frequently reported AEs included headache (52.7%), nasopharyngitis (22.7%), and hot flush (14.8%). Within the LTE population, none of the headaches were reported as severe, and one resulted in discontinuation. The incidence of hot flush during the LTE study of up to 80 weeks was 3.2%. For most of these preferred terms, the AEs were reported within the first 24 weeks of treatment and generally there was no evidence of a time-dependent increase in events.
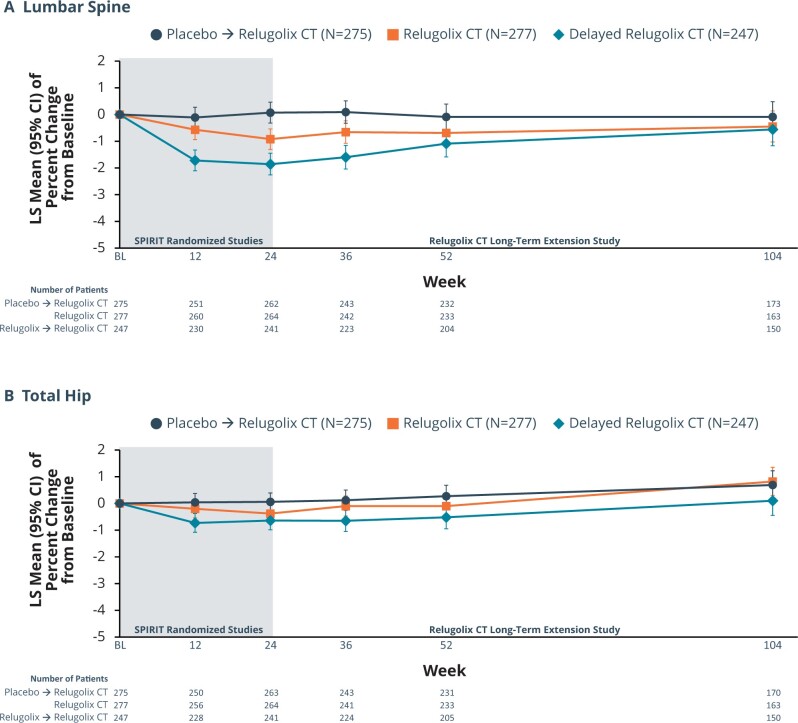
Among patients treated for up to 104 weeks in the relugolix-CT group, the small mean decrease in BMD observed over the initial 24 weeks of treatment did not progress during continued treatment up to 104 weeks. At Week 12, a non-clinically meaningful decline in BMD at the lumbar spine was observed in the relugolix-CT group with mean bone loss <1% through Week 24 (Week 12, −0.57%; Week 24, −0.92%), after which BMD stabilized starting at Week 36, with percentage change from baseline of −0.66%, −0.69%, and −0.45% at Weeks 36, 52, and 104, respectively (Fig. 5A). For total hip BMD, there was evidence of stability with LS mean change from baseline to Week 52 and Week 104 of −0.10% and 0.82%, respectively (Fig. 5B). In the placebo→ relugolix CT group, LS mean percentage changes at Weeks 12, 24, 36, 52, and 104 in BMD at the lumbar spine were −0.11%, 0.07%, 0.09%, −0.09%, and −0.09%, respectively (Fig. 5A). In the delayed relugolix CT group, after the initial decline in lumbar spine BMD observed during the 12 weeks on relugolix monotherapy, results showed a plateau between Weeks 24 and 36 followed by a trend towards recovery through Week 104, likely reflecting the effect of the oestradiol/norethisterone acetate in relugolix CT. LS mean percentage changes from pivotal baseline to Weeks 12, 24, 36, 52, and 104 were −1.72%, −1.86%, −1.60%, −1.09%, and −0.56%.
Figure 5.
Percentage change from baseline in bone mineral density over time for lumbar spine and total hip. During the first 24 weeks of treatment, patients receive one of three randomized treatments: relugolix CT, relugolix for 12 weeks followed by relugolix CT for 12 weeks, or placebo. Starting at Week 24, the beginning of the SPIRT long-term extension study, all patients received relugolix CT. CT, combination therapy. (A) Data for lumbar spine. (B) Data for hip.
As oestradiol, and progesterone are effectively suppressed, menstrual bleeding declined both in frequency and intensity. In the relugolix CT group, 79.6% of women achieved amenorrhea at Week 52 and 76.9% at Week 104/EOT. In parallel, the number of days of menstrual bleeding declined. Among patients who continued to menstruate, bleeding intensity also declined. Residual bleeding, when present, was primarily limited to patient reports of spotting. AEs of uterine bleeding (e.g. preferred terms of menorrhagia, metrorrhagia, menstruation irregular) were infrequent (6.9% of women), generally had an onset within the first 1–2 months of treatment and were rarely treatment limiting. After discontinuation of treatment, the median time for menstruation resumption was 33 days. Over 90% of patients resumed menses within 2 months of stopping relugolix CT. In patients for whom menstruation did not resume and for whom data were available (6 patients, 2.5%), pregnancy, menopause, surgery, or medical treatment were the explanation.
While all patients were required to follow contraception requirements of the study protocol; there were seven pregnancies reported during the SPIRIT LTE (Supplementary Table S3). Patient compliance data from diary data and study drug accountability in the month prior to estimated conception suggest that two or more doses may have been missed in most of the seven patients. Outcomes from pregnancies with conception dates during the LTE study are as follows: three pregnancies resulted in live births of healthy infants without any complications, one patient had a missed abortion, one a spontaneous abortion, and one an elective abortion, while one patient was lost to follow-up.
There was a total of five fractures associated with trauma during 80 weeks of treatment—one in the relugolix-CT group and four in the delayed relugolix-CT groups.
Discussion
Endometriosis is a chronic disease affecting more than 190 million women worldwide. Thus, an effective therapeutic agent that can provide women with a once-daily treatment option that improves moderate to severe pain associated with the disease while mitigating the known risks of a hypoestrogenic state is an important medical need.
Data from the SPIRIT LTE study in patients with endometriosis expanded earlier observations by demonstrating that the efficacy observed during the initial 24 weeks of treatment in the pivotal phase 3 SPIRIT studies was maintained over time with continued treatment. In a population of women with moderate to severe pain associated with endometriosis, treatment with once-daily oral relugolix CT for up to 104 weeks provided sustained and clinically meaningful reductions in dysmenorrhea, NMPP, and dyspareunia, leading to a substantial increase in the percentage of women who were opioid-free (60.6% opioid-free at baseline versus 91% opioid-free at Week 104 in the relugolix CT group) and analgesic-free (7.2% analgesic-free at baseline versus 75% analgesic-free at Week 104 in the relugolix CT group). Most women treated with relugolix CT for up to 104 weeks had minimal to no pain associated with endometriosis both during and between their periods. The significant improvement observed in pain was associated with clinically meaningful improvements in function (as measured by the EHP-30 pain domain). Over half of patients (51.6%) experienced amenorrhea by Week 12 and nearly 80% of patients by Week 52; of the remaining patients, most described only spotting.
The observed cumulative AE profile over 104 weeks was generally consistent with that reported through the first 24 weeks of treatment, with no new safety signals identified. An important safety objective of the SPIRIT studies was to evaluate the extent to which BMD loss expected with GnRH antagonist use could be mitigated with relugolix CT during long-term use. Over 104 weeks of treatment, there was evidence of mean bone loss <1% in the relugolix CT group at the most oestrogen-sensitive anatomic location—the lumbar spine. At Week 12, a small, not clinically meaningful, decline in BMD was observed in the relugolix CT group that likely reflects adaptation to the new steady state of oestradiol concentrations associated with relugolix CT, which are lower than the average concentrations observed over the course of a natural menstrual cycle (Stricker et al., 2006) and are consistent with concentrations observed during the early follicular phase (Barbieri, 1992; Cramer et al., 2002; Stricker et al., 2006). The decline in BMD subsequently plateaued starting at Week 36, and BMD was sustained for the duration of treatment up to 104 weeks with little evidence of progressive bone loss over time.
The oestrogen threshold hypothesis posits that there is a therapeutically effective oestradiol range (30–45 pg/ml) at which signs and symptoms of endometriosis or fibroids improve while minimizing hypoestrogenic adverse effects (Friedman et al., 1990; Barbieri, 1992). Phase 1 studies with relugolix CT supported the hypothesis that helped to justify the rationale for the confirmation phase 3 trials, including mean oestradiol concentrations between 32.6 and 44.5 pg/ml (Duijkers, 2020) and maintenance of bone remodelling balance and fewer vasomotor symptoms with relugolix CT compared with relugolix monotherapy (Lukes et al., 2023). While oestradiol concentrations were not measured in all women in the endometriosis Phase 3 programme, clinical outcomes from the clinical trial studies were internally consistent and similarly supported the oestrogen threshold hypothesis: improvement in endometriosis symptomatology with mitigation of hot flushes and BMD loss.
While relugolix CT was associated with suppression of pituitary gonadal hormone secretion and ovarian sex steroid production, return to ovulation or menses was observed in all study participants (Duijkers, 2020). These data will be the subject of a separate manuscript. While seven pregnancies were observed during the LTE study, evidence suggests that missed doses of relugolix CT may have been associated with a return of ovulatory status and conception, hence the need to be compliant with treatment.
The clinical course of endometriosis can be challenging for the patient. Independent of treatment approach, 50% of patients have recurrence of symptoms over 5 years (Zondervan et al., 2020). Since current medical treatments and surgical interventions for moderate-to-severe endometriosis may offer incomplete pain relief, patients may rely on opioid use to control pain as well as repeated surgeries (Soliman et al., 2018; Lamvu et al., 2019). Regardless of the type of management, long-term treatment to inhibit ovulation or reduce oestrogen production is recommended for this chronic condition (Johnson and Hummelshoj, 2013; Practice Committee of the American Society for Reproductive Medicine, 2014; Zondervan et al., 2020; Becker et al., 2022). Continued pelvic pain, along with severity of disease, has been shown to have significant impact on physical health-related quality of life and are associated with decreased work productivity (Nnoaham et al., 2011).
There have been studies of other GnRH receptor antagonists, for example a 6-month evaluation of high and low-dose elagolix monotherapy (Taylor et al., 2017). While the elagolix higher dose regimen (200 mg twice daily) demonstrated qualitatively higher dysmenorrhea and non-menstrual pain responder rates than the lower dose, it was associated with decline in BMD that limits its treatment duration to 6 months. Low-dose elagolix (150 mg once daily) did not show improvement in dyspareunia or decrease in use of analgesics compared with placebo, and was associated with more bleeding days. Six-month extension studies were conducted for both of the elagolix pivotal trials, reflecting 1 year of treatment. Clinically meaningful responses were demonstrated in 75–78% for dysmenorrhea, 67–69% for NMPP, and 58–60% for dyspareunia at the higher elagolix dose (200 mg twice daily). Clinically meaningful response at the lower dose (150 mg daily) was seen for 52% of women in dysmenorrhea, 67% in NMPP, and 45% in dyspareunia. Hypoestrogenic side effects of hot flashes continued into the extension studies with both doses (Surrey et al., 2018). In these replicate extension studies, for patients treated with low-dose elagolix (150 mg once daily), the mean per cent change in lumbar spine BMD was −0.63% and −1.1% at Weeks 36 and 52, respectively. Patients who received the higher dose of elagolix (200 mg twice daily) had greater declines in BMD, −3.6% and −3.9% at Weeks 36 and 52 (Surrey et al., 2018).
There were limitations of our study. The study was conducted as an open-label study without a control group over the 80 weeks of the extension period. In addition, there are currently no comparative data to other hormonal medications, and formal comparison of efficacy or safety is not possible with these data. A small number of patients (8.5%) had received treatment with GnRH receptor agonist/antagonist prior to enrolment in SPIRIT (Giudice et al., 2022), which raises a question about their response to treatment with another GnRH receptor antagonist. Treatment response to antecedent therapy was not collected at baseline, so the impact of switching within the drug class cannot be addressed. However, with a low number of study participants with history of GnRH receptor agonist/antagonist use, the impact of this subpopulation on overall study outcomes is unlikely. Finally, a third (37.4%) of the study population terminated study participation early; closer examination of these patients did not show disproportionate attrition within one treatment group. Notably, these patients had completed 24 weeks of the earlier randomized placebo-controlled phase 3 study and agreed to participate in the LTE study, which suggests that they had obtained benefit with and tolerated earlier treatment. Evaluation of efficacy at Week 52 and Week 104 showed similar point estimates and narrow 95% CIs across a variety of endpoints, reflecting the robustness of treatment effect. The most common AEs were similar to those observed in the pivotal phase 3 studies (Giudice et al., 2022), Generally, there was no evidence of an incremental time-dependent increase in AEs (i.e. more than what would be expected given the longer follow-up), including for events that may be related to a hypoestrogenic state or treatment with oestradiol and norethisterone acetate.
In conclusion, the data from this study demonstrate that oral relugolix CT may help fulfil the need for a longer term effective, convenient, and well-tolerated medical treatment for endometriosis. Future areas of study may include examination of the effect of long-term medical control of endometriosis symptoms with relugolix CT on the potential to minimize the need for repeated surgeries.
Supplementary Material
Acknowledgements
The authors would also like to acknowledge editorial support from JD Cox, PhD, and Mayville Medical Communications, in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al., Ann Intern Med 2015; 163:461–4).
Contributor Information
Christian M Becker, Nuffield Department of Women’s & Reproductive Health, Endometriosis CaRe Centre, University of Oxford, Oxford, UK.
Neil P Johnson, Robinson Research Institute, University of Adelaide, Adelaide, Australia.
Sawsan As-Sanie, Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA.
Juan C Arjona Ferreira, Myovant Sciences Inc., Brisbane, CA, USA.
Mauricio S Abrao, Gynecologic Division, A Beneficencia Portuguesa de Sao Paulo, Sao Paulo, Brazil; Obstetrics and Gynecology Department, Faculdade de Medicina FMUSP, Universidade de Sao Paulo, Sao Paulo, SP, Brazil.
Krzysztof Wilk, Obstetrics and Gynecology Department, Boni Fratres Hospital, Katowice, Poland.
So Jung Imm, Myovant Sciences Inc., Brisbane, CA, USA.
Vandana Mathur, Mathur Consulting LLC, Woodside, CA, USA.
Julie S Perry, Myovant Sciences Inc., Brisbane, CA, USA.
Rachel B Wagman, Myovant Sciences Inc., Brisbane, CA, USA.
Linda C Giudice, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco, San Francisco, CA, USA.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
Data will be made available based on reasonable requests.
Authors’ roles
Authors had full access to and reviewed the data analyses and vouch for fidelity of the trial to the protocol. S.J.I. was responsible for data analysis and vouches for the accuracy of the data. The first manuscript draft was written by R.B.W. All authors critically reviewed and provided feedback on all versions.
Funding
This study was funded by Sumitomo Pharma Switzerland GmbH (formerly Myovant Sciences GmbH). Editorial support for the manuscript was funded by Sumitomo Pharma Switzerland GmbH (formerly Myovant Sciences GmbH).
Conflict of interest
C.M.B. reports fees from Myovant, grants from Bayer Healthcare, fees from ObsEva all of which went to the University of Oxford, and Chair of ESHRE Endometriosis Guideline Group; N.P.J. reports personal fees from Myovant Sciences, during the conduct of the study, personal fees from Guerbet, personal fees from Abbott, personal fees from Roche Diagnostics; S.A.-S. reports personal fees from Myovant Sciences, personal fees from Bayer, personal fees from Organon, personal fees from UpToDate; J.C.A.F., S.J.I., J.S.P., and R.B.W. are employees and shareholders of Myovant Sciences; J.C.A.F. and S.J.I. are shareholders of Myovant Sciences (but at time of publicaion are no longer employess of Myovant Sciences); M.S.A. and Dr Dynowski, and K.W. have no conflicts to declare; V.M. is a consultant to Myovant; L.C.G. reports personal fees from Myovant Sciences and Bayer. The authors did not receive compensation for manuscript writing, review, and revision.
References
- Barbieri RL. Hormone treatment of endometriosis: the oestrogen threshold hypothesis. Am J Obstet Gynecol 1992;166:740–745. [DOI] [PubMed] [Google Scholar]
- Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, Citrome L, Gurr JA, Mooney LA, Moore BJ. et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015;163:461–464. [DOI] [PubMed] [Google Scholar]
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, King K, Kvaskoff M, Nap A, Petersen K, et al. ; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum Reprod Open 2022;2022:hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Gattrell WT, Gude K, Singh SS.. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril 2017;108:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Barbieri RL, Fraer AR, Harlow BL.. Determinants of early follicular phase gonadotrophin and estradiol concentrations in women of late reproductive age. Hum Reprod 2002;17:221–227. [DOI] [PubMed] [Google Scholar]
- Duijkers I, Migoya EM, Arjona Ferreira JC, Klipping C.. Characterization of pituitary and ovarian hormone concentrations during treatment with relugolix combination therapy. Fertil Steril 2020;114:E81. [Google Scholar]
- Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, et al. ; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400–412. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Lobel SM, Rein MS, Barbieri RL.. Efficacy and safety considerations in women with uterine leiomyomas treated with gonadotropin-releasing hormone agonists: the oestrogen threshold hypothesis. Am J Obstet Gynecol 1990;163:1114–1119. [DOI] [PubMed] [Google Scholar]
- Giudice LC, As-Sanie S, Arjona Ferreira JC, Becker CM, Abrao MS, Lessey BA, Brown E, Dynowski K, Wilk K, Li Y. et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022;399:2267–2279. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod 2013;28:1552–1568. [DOI] [PubMed] [Google Scholar]
- Kuznetsov L, Dworzynski K, Davies M, Overton C; Guideline Committee. Diagnosis and management of endometriosis: summary of NICE guidance. BMJ 2017;358:j3935. [DOI] [PubMed] [Google Scholar]
- Lamvu G, Soliman AM, Manthena SR, Gordon K, Knight J, Taylor HS.. Patterns of prescription opioid use in women with endometriosis: evaluating prolonged use, daily dose, and concomitant use with benzodiazepines. Obstet Gynecol 2019;133:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukes A, Migoya E, Johnson B, Lee TY, Li Y, Arjona Ferreira JC.. A randomized open-label study of relugolix alone or relugolix combination therapy in premenopausal women. Clin Pharmacokinet 2023;62:1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Hitaka T, Imada T, Sasaki S, Yoshimatsu M, Kusaka M, Tanaka A, Nakata D, Furuya S, Endo S. et al. Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem 2011;54:4998–5012. [DOI] [PubMed] [Google Scholar]
- MYFEMBREE (Relugolix, Estradiol, and Norethisterone Acetate) Tablets. Prescribing Information. Brisbane, CA: Myovant Sciences, Inc., 2023. https://www.myfembree.com/static/myfembree-prescribing-information.pdf (3 January 2024, date last accessed).
- Nakata D, Masaki T, Tanaka A, Yoshimatsu M, Akinaga Y, Asada M, Sasada R, Takeyama M, Miwa K, Watanabe T. et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Pharmacol 2014;723:167–174. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT; World Endometriosis Research Foundation Global Study of Women’s Health Consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011;96:366–373.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orilissa (elagolix tablets) Prescribing Information. North Chicago, IL. USA: AbbVie Inc., 2018. https://www.rxabbvie.com/pdf/orilissa_pi.pdf (3 January 2024, date last accessed).
- Orilissa (Elagolix Tablets) Prescribing Information. North Chicago, IL, USA: AbbVie Inc., 2019. https://www.rxabbvie.com/pdf/orilissa_pi.pdf (3 January 2024, date last accessed).
- Osuga Y, Seki Y, Tanimoto M, Kusumoto T, Kudou K, Terakawa N.. Relugolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, in women with endometriosis-associated pain: phase 2 safety and efficacy 24-week results. BMC Womens Health 2021;21:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril 2014;101:927–935. [DOI] [PubMed] [Google Scholar]
- RYEQO® (Relugolix 40 mg, Estradiol 1.0 mg, and Norethisterone acetate 0.5 mg)—Summary of Products Characteristics. Brussels, Belgium: Public Health - European Commission, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/ryeqo#ema-inpage-item-authorisation-details (3 January 2024, date last accessed).
- Singh SS, Gude K, Perdeaux E, Gattrell WT, Becker CM.. Surgical outcomes in patients with endometriosis: a systematic review. J Obstet Gynaecol Can 2020;42:881–888.e11. [DOI] [PubMed] [Google Scholar]
- Soliman AM, Surrey E, Bonafede M, Nelson JK, Castelli-Haley J.. Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Adv Ther 2018;35:408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R.. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med 2006;44:883–887. [DOI] [PubMed] [Google Scholar]
- Surrey E, Taylor HS, Giudice L, Lessey BA, Abrao MS, Archer DF, Diamond MP, Johnson NP, Watts NB, Gallagher JC. et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol 2018;132:147–160. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB. et al. Treatment of endometriosis-associated pain with Elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28–40. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Meana M, Hummelshoj L, Somigliana E, Vigano P, Fedele L.. Priorities for endometriosis research: a proposed focus on deep dyspareunia. Reprod Sci 2011;18:114–118. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P.. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available based on reasonable requests.