Abstract
BACKGROUND
The Endometriosis Health Profiles (EHPs), the EHP-30 and EHP-5, are patient-reported outcome measures that were developed to measure the health-related quality of life (HRQoL) of women living with endometriosis. Prior to their development, a systematic review was undertaken which identified that the HRQoL of women living with endometriosis was poorly understood, with only three medical and one surgical study identified.
OBJECTIVE AND RATIONALE
The 20-year anniversary of the EHP-30 provided a timely opportunity to assess how the tools have been used and explore what the findings tell us about the impact of endometriosis and its associated treatments upon women’s QoL. Applying robust systematic review methodology, following PRISMA guidelines, we sought to answer: How many studies have used the EHP and for what purpose?; What are the demographic characteristics and international context of the studies?; What is the methodological nature and quality of the studies?; Which interventions have been assessed and what are the reported EHP outcomes?; and Can the EHP outcomes of these interventions be analysed using a meta-analysis and, if so, what do the results show?
SEARCH METHODS
The electronic databases MEDLINE, CINAHL, PsycINFO, PubMed, and Google Scholar were searched from the year the EHP was first published, in 2001 to 26 February 2020 using the search terms ‘EHP30’, ‘EHP5’, ‘EHP-30’, ‘EHP-5’, ‘endometriosis health profile 30’, and ‘endometriosis health profile 5’. We updated the searches on 9 April 2021. All included studies were quality assessed using the Mixed Methods Appraisal Tool (MMAT).
OUTCOMES
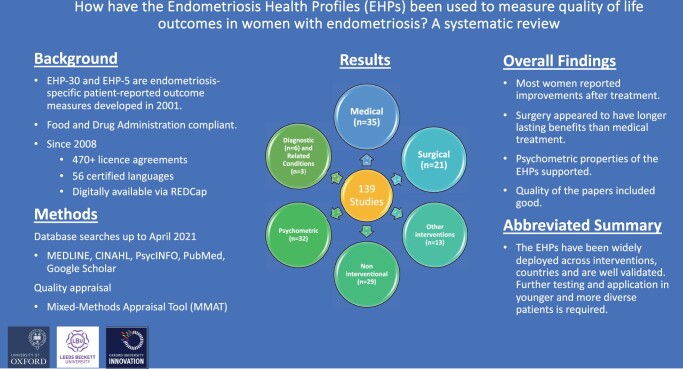
The review included 139 papers. In clinical intervention studies, the EHPs were deployed most frequently to measure the outcomes of medical (n = 35) and surgical (n = 21) treatment. The EHPs were also used in 13 other intervention studies, 29 non-interventional studies, 32 psychometric/cross cultural validation studies; six diagnostic studies, and in three other studies to measure outcomes in related conditions. They were mainly deployed in studies undertaken in Europe and North America. Overall, regardless of the nature of the intervention, most women reported improvements in HRQoL after treatment. Surgical interventions generally resulted in significant improvements for the longest amount of time. There was also evidence that when participants stopped taking medication their EHP scores worsened, perhaps reinforcing the temporary impact of medical treatment. Younger patients reported more negative impact upon their HRQoL. Further evidence using classical test theory to support the EHPs’ robust psychometric properties, including acceptability, dimensionality, reliability, validity (including cross-cultural), and responsiveness, was demonstrated, particularly for the EHP-30. Strikingly, using anchor-based methods, EHP-30 responsiveness studies demonstrate the largest mean changes in the ‘control and powerlessness’ domain post-intervention, followed by ‘pain’. MMAT outcomes indicated the quality of the papers was good, with the exception of five studies. A meta-analysis was not undertaken owing to the heterogeneity of the interventions and papers included in this review.
WIDER IMPLICATIONS
Women with endometriosis face a lifetime of surgical and/or medical interventions to keep the condition under control. Less invasive treatments that can lead to improved longer term physical and psycho-social outcomes are needed. The EHPs are reliable, valid, acceptable, and responsive tools, but more assessment of EHP outcomes using modern psychometric methods and in the context of women from ethnically diverse backgrounds and in routine clinical care would be beneficial. Given the brevity of the EHP-5, it may be the most appropriate version to use in routine clinical practice, whereas the longer EHP-30, which provides more granularity, is more appropriate for research.
Keywords: Endometriosis Health Profile 30, Endometriosis Health Profile 5, endometriosis, health-related quality of life, patient-reported outcome measures, systematic review, endometriosis treatment
Graphical abstract
A summary of Endometriosis Health Profile use.
Introduction
Endometriosis, a common gynaecological disease with an estimated prevalence of 10%, is defined as the presence of endometrial-like tissue in extra-uterine locations (Zondervan et al., 2020). It affects women of reproductive age and typically regresses after the menopause. Symptoms include chronic pelvic pain (CPP), painful periods (dysmenorrhoea), pain on defaecation (dyschezia), pain on intercourse (dyspareunia), sub-fertility, and other symptoms such as fatigue (Bulletti et al., 2010). There is no cure and the effectiveness of the limited treatment options varies. Those available focus on symptom control, including pain relief, using analgesics, hormonal therapy, surgery, and, where relevant, fertility treatment. Consequently, endometriosis imposes heavy demands on women and healthcare professionals with resulting high costs.
Recognizing the value of measuring the quality of life of affected women in routine clinical practice and research, the Endometriosis Health Profiles (EHPs) were developed, including the long form version (EHP-30) (Jones et al., 2001) and the shorter EHP-5 (Jones et al., 2004a). Before the EHPs were developed, the impact of endometriosis and associated interventions on women’s health-related quality of life (HRQoL) was little understood. For example, a 2002 systematic review identified only four studies (three medical; one surgical) that had assessed treatment outcomes using a patient-reported outcome measure (PROM), all of which were carried out in developed countries (Jones et al., 2002).
The domain and scoring algorithms of the EHPs
Originally developed in English at the University of Oxford, the EHP-30 consists of a core instrument with five scale scores covering: Pain; Control and powerlessness; Social support; Emotional well-being, and Self-image. In addition, six (optional) supplementary modules can be deployed alongside the core instrument covering areas of health status that may not affect every endometriosis sufferer. These cover: Work; Relationship with child/children; Sexual intercourse/functioning; Feelings about medical profession; Feelings about treatment, and Feelings about infertility. Practitioners can choose these modules (in any combination) to assess specific areas of HRQoL that are relevant to their research/clinical practice and the patient.
The core section outcomes can be presented at an individual item level, domain level, or as an overall summary score (i.e. a total score, which includes all 30 items). On the modular section, only the outcomes at an item level or domain level are appropriate. The EHP-30 core and modular sections combined take, on an average, less than 15 min to complete (Nogueira-Silva et al., 2015).
The EHP-5 was developed to provide a briefer version of the tool by taking one item from each of the five core scales (five items) and one from each of six modular scales (six items). The items were chosen based on the highest to total correlation within the scale (Jones et al., 2004a).
The EHPs are compliant with the US Food and Drug Administration’s (FDA) recommended guidance for developing PROMs (FDA, 2009). They were developed using data derived from systematic reviews of the literature (Jones et al., 2002) and in-depth interviews with affected women to ensure content validity (Jones et al., 2004b). These measures provided the first psychometrically established instrument, designed specifically to evaluate the impact of endometriosis and its associated treatments, or other related interventions, from the woman’s perspective (Jones et al., 2001, 2004c, 2006). This valuable information empowers women to express the impact of the disease on their well-being; it can also help clinical management and decision-making by enabling healthcare professionals to monitor progress.
Portfolio of licence agreements and translations
Since 2008, the EHPs have accumulated over 474 licence agreements through Oxford University Innovation’s Clinical Outcomes team, of which 67% have been in publicly funded treatment or academic studies. The remainder is commercial, awarded to privately funded healthcare providers, pharmaceutical companies, or digital platform providers serving commercial users.
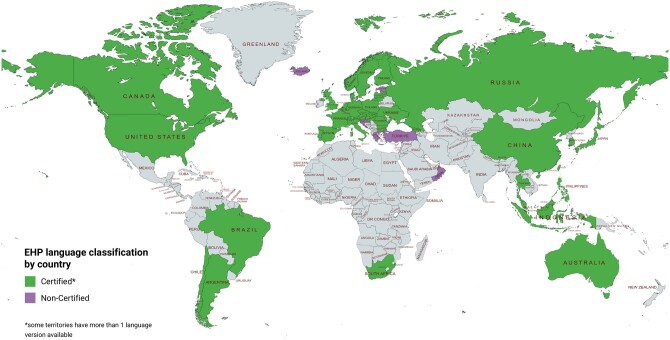
The EHPs have 56 certified language versions for use across the globe (i.e. translated and linguistically validated in strict accordance with sector good practices). Some territories have more than one language version covering different populations, e.g. Switzerland: German, French, and Italian, bringing the total number of language versions to 456. There are also eight non-certified language versions (that may not have strictly followed sector good practices), which have been produced by academic or publicly funded groups in close collaboration with the Clinical Outcomes team (Fig. 1).
Figure 1.
Endometriosis Health Profile language translation map. The Endometriosis Health Profiles (EHPs) have 56 certified language versions for use across the globe. Certified means the EHPs have been translated and linguistically validated in strict accordance with sector good practices.
Modes of EHP administration
The EHPs are available in paper and e-versions. They have been faithfully reproduced into a few eCOA (electronic Clinical Outcome Assessment) libraries and migrated digitally multiple times, most recently into a REDCap friendly survey which is available, subject to licence, to download and install. For each eCOA, the digital reproduction has been assessed by the Clinical Outcomes Team at Oxford to ensure comparability of results acquired from the eCOA modality to the more conventional paper completions. This enables the EHPs to be distributed to patients digitally and provides the functionality to collect and analyse data more easily, especially relevant in routine care. A user manual is freely available from Oxford University Innovation, which guides users on the application and scoring of the EHP measures.
Professional and regulatory endorsement
The American Society for Reproductive Medicine (ASRM) and European Society of Human Reproduction and Embryology (ESHRE) recommend the EHP-30 for use as the secondary outcome measure in clinical trials to assess endometriosis-associated pain (an 11-point numerical rating scale is recommended as the primary outcome) (Vincent et al., 2010). The EHP-30 is also recommended in other national endometriosis guidelines (Grundström et al., 2020a).
Rationale
The 20-year anniversary of the EHP-30 provides an opportunity to assess how the measures have been used globally and explore how endometriosis and its treatments impact upon self-reported quality of life. While other reviews are available, none focus exclusively on the EHP (Bourdel et al., 2019). The new Women’s Health Strategy in England acknowledges that still not enough is known about endometriosis and how it impacts affected women (Department of Health & Social Care, 2021). Therefore, this systematic review aims to identify and synthesise the literature relating to the use of the EHP instruments over the last 20 years, and specifically answer the following questions:
How many studies have used the EHPs and for what purpose?
What are the demographic characteristics and global context of the studies?
What is the methodological nature and quality of the studies?
What interventions have been assessed and what are the patient-reported EHP outcomes?
Can the EHP outcomes of these interventions be assessed by meta-analysis and, if so, what do the results show?
Methods
Protocol registration
The protocol for this systematic review was published on Prospero: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021226485.
Search strategy
Publications in all languages in electronic databases (MEDLINE, CINAHL, PsycINFO, PubMed, and Google Scholar) were searched between 2001 (when the EHP was first published) and 26 February 2020, using the search terms ‘EHP30’, ‘EHP5’, ‘EHP-30’, ‘EHP-5’, ‘endometriosis health profile 30’, and ‘endometriosis health profile 5’. We re-ran the searches on 9 April 2021 to capture any papers published since the initial searches were conducted.
Inclusion and exclusion criteria
The review focuses on a wide range of interventions: surgical (e.g. hysterectomy and laparoscopy), medical (e.g. GnRH analogues, long-acting reversible contraceptives, and the combined oral contraceptive), educational (e.g. self-management interventions), and holistic (e.g. acupuncture, yoga).
We also included studies that have used the EHPs to undertake further psychometric testing, and cross-cultural translation and validation studies. All study types (quantitative, qualitative, and mixed methods) that included empirical EHP data were eligible for inclusion, including but not limited to randomised controlled trials (RCTs), cohort studies, case studies, cross-sectional studies, and qualitative studies. Studies that used the EHPs to measure outcomes in related conditions, such as CPP, were also included but analysed separately.
Literature reviews (e.g. systematic reviews) of the EHPs were recorded separately but were excluded. Studies that mentioned the EHPs but did not report any outcome data were excluded, as were manuscripts written in a foreign language, case reports, opinion pieces, editorials, comments, news, and letters.
Screening and quality assessment
RAYYAN—an online application that facilitates systematic review teams to undertake, organise, and manage screening of literature (Ouzzani et al., 2016)—was used. Titles and abstracts were screened and assessed against the inclusion and exclusion criteria by F.T. Full texts were downloaded and inspected to assess if: the article met the inclusion criteria; or the inclusion/exclusion criteria could not be determined based on the title and abstract alone. If F.T. could not determine the inclusion/exclusion criteria of full text articles, then the team made the final decision after discussion.
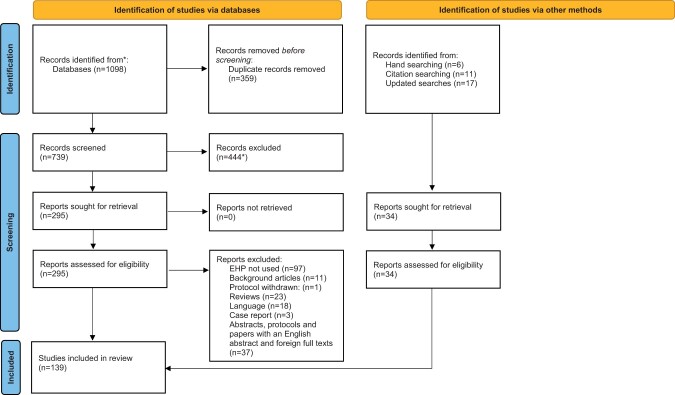
Included articles were used to identify additional articles. A standardised data extraction form was created using a Microsoft Excel spreadsheet. The first reviewer read the full text of each included study and extracted the data using a standardised data extraction form; a second reviewer checked the details. A PRISMA flow-diagram to display the screening process was also produced (Fig. 2).
Figure 2.
PRISMA flow chart of the selection of studies in a systematic review to determine the use of the Endometriosis Health Profiles to measure quality of life outcomes in women with endometriosis. *Two studies were screened out at the title stage but were later found to be eligible and were put back into the final sample. EHP, Endometriosis Health Profile.
Areas of ambiguity that arose during the screening and data extraction process (e.g. uncertainty around eligibility) or that required further clarification (e.g. nature of the clinical intervention) were resolved in regular weekly meetings involving F.T., K.B., G.L.J., and S.H.K. when clinical endometriosis expertise was needed. Several authors (n = 7) of the included papers were contacted to seek clarity on unreported data or where additional details were needed.
All included studies were assessed for quality using the Mixed Methods Appraisal Tool (MMAT) by K.B. and were discussed during the team meetings if there was any uncertainty (Hong et al., 2018). This tool allows most common types of study design and methodology to be appraised, incorporating different sections for each qualitative, quantitative, and mixed methods studies (Hong et al., 2018). The MMAT asks two initial screening questions: whether there are clear research questions, and whether the data collected enable those questions to be answered? Full appraisal of the papers may not be appropriate if these criteria are not satisfied. Abstracts and protocols were not quality assessed. In addition, papers reporting findings of post hoc analyses were also not appraised since it is difficult to do so within MMAT, which largely asks questions about the original study design and sample. The MMAT requires the review team to agree on a cut-off value for acceptable complete outcome data and apply this uniformly across the included studies. We determined studies to have complete outcome data where there was a withdrawal/drop-out rate of up to 20% for studies of 12 months or less, or of up to 30% for studies of over 12 months (Dettori, 2011; Viswanathan and Berkman, 2012).
Data extraction and synthesis
Studies that met the inclusion criteria (n = 139) were examined comprehensively. Our standardised data extraction sheet included the categories shown in Tables 1, 2, and 3. We then undertook a narrative synthesis, pooling similar papers, which were described in textual form (Popay et al., 2006; Aromataris and Pearson, 2014). A meta-analysis was not undertaken owing to the heterogeneity of the interventions and papers included.
Table 1.
Summary of studies that used the Endometriosis Health Profile in the context of a medical intervention.
| Author and year | Country of research | Specific intervention | Study type | Sample size* | Sample mean/median age in years | Sample ethnicity (%) | Stage of endometriosis | EHP measure used | EHP data collection points reported |
|---|---|---|---|---|---|---|---|---|---|
| Medical interventions—GnRH agonists and antagonists | |||||||||
| (Ács et al., 2015) |
Bulgaria, Hungary, Poland,
|
Elagolix, and Leuprorelin Acetate (LA), and placebo | RCT | 174 | 31.7 | White (100) | NR | EHP-5 core section | Baseline, 4, 8, 12, 16, 20, 24 weeks and follow-up. |
| (Agarwal et al. 2020) | Not stated but post hoc analysis | Elagolix vs placebo | RCT (Post hoc analysis of EM-I and EM-II trials) | 1368 | 32.2 |
|
NR | EHP-30 core section and sex module | Original data collected at baseline, month 1, 3, 6, and every 3 months at follow-up. |
| (Al-Azemi et al., 2009) | UK | Zoladex followed by HRT (tibolone 2.5 mg) | RCT | 25 (14 = HRT group; 11 = placebo) |
|
NR | NR | EHP-30 core section | Baseline, 6, 18, 30 months |
| (Alshehre et al., 2020) | UK | GnRHa (Triptorelin SR) with add-back therapy (ABT) using Tibolone | Single-arm open-label trial | 27 | 33.35 | NR | NR | EHP-30 core and modular sections. | Baseline, 6, 12, 18, 24, 30 months. |
| (Carr et al., 2013) | USA | Elagolix vs placebo | RCT |
|
For both groups the median = 33years (21–47) |
|
I-IV | EHP-5 core section | Baseline, 8 and 24 weeks. |
| (Carr et al., 2014) | USA | Elagolix vs subcutaneous depot medroxyprogesterone acetate (DMPA-SC) | RCT | 252 (Elagolix 150 mg = 84; Elagolix 75 mg = 84; DMPA-SC = 84) | Elagolix 150 mg = 32.4; Elagolix 75 mg = 31.4; DMPA-SC = 31.6) |
|
I-IV | EHP-5 core section |
|
| (Diamond et al., 2014) | USA | Elagolix vs placebo | RCT |
|
|
|
I-IV | EHP-5 core section | Baseline, 4, 8, 12, 16, 20, 24 and 30 weeks |
| (Donnez et al., 2020) | USA, Europe | Linzagolix vs placebo | RCT | 323 (Placebo = 53; 50 mg = 49;75 mgFD = 56; 75 mgTD = 58; 100 mg = 51;200 mg = 56) |
|
|
NR | EHP-30 core section | Baseline, 12, 24 weeks |
| (Leyland et al., 2019) | Not stated but post hoc analysis | Elagolix vs placebo | RCT (Post hoc analysis of EM-I and EM-II trials) | 1384 (no dyspareunia = 57; any dyspareunia = 1297) |
|
|
NR | EHP-30 sex module | Baseline, 1 month, 3 months, 6 months |
| (Osuga et al., 2021) | Japan | Relugolix vs placebo or Leuprorelin | RCT | 487 (Relugolix 10 mg = 103; 20 mg = 100; 40 mg = 103. Leuprorelin 3.75 mg = 82. Placebo = 99) |
|
NR | NR | EHP-30 core section | Baseline, week 12 12 |
| (Pokrzywinski et al., 2020c) |
|
Elagolix vs placebo | RCT (Post hoc analysis of EM-I and EM-II trials) | 1686 (EM-I = 871; EM-II = 815) |
|
White (EM-I = 87.1, EM-II = 89.2) | NR | EHP-30 core section and sex module | Baseline, 1 month, 3 months, 6 months, 9, 12, 15, 18 months |
| (Surrey et al., 2018) | USA, Puerto Rico, Canada | Elagolix vs placebo | RCT | 569 (EM-III Elagolix 150 mg = 149; 200 mg = 138. EM-IV Elagolix 150 mg = 142; 200 mg = 140) |
|
|
NR | EHP-30 core section and sex module | Baseline, 12 months |
| (Taylor et al., 2017) |
|
Elagolix vs placebo | RCT |
|
|
|
NR | EHP-30 core section and sex module | Baseline, 1 month, 3 months, 6 months |
| (Taylor et al., 2020) | Not stated but post hoc analysis | Elagolix vs placebo | RCT (Post hoc analysis of EM-I and EM-II trials) | 1686 (Placebo = 734; Elagolix 150 mg = 475; 200 mg = 477) | Placebo = 32.4; Elagolix 150 mg = 32.3; 200 mg = 32.3) |
|
NR | EHP-30 core section and sex module | Baseline, 1 month, 3 months, 6 months |
| Medical interventions—GnRH with contraceptive | |||||||||
| (Crosignani et al., 2006) | Europe, Asia, Latin America (countries not specified) and New Zealand | Injectable contraception (DMPA-SC) vs Leuprolide | RCT |
|
|
|
NR | EHP-30 core section and sex module | Baseline, 6, and 18 months |
| (Granese et al., 2015) | Italy | Dienogest and Estradiol Valerate (E2V) vs Leuprorelin Acetate (LA) | RCT | 78 (E2V = 39; LA = 39) |
|
NR | I-IV | Unclear-EHP-5 used but it’s not clear what sections | Baseline, 9 months |
| (Schlaff et al., 2006) | USA, Canada | Injectable contraceptive (DMPA-SC) vs Leuprorelin Acetate | RCT | 274 (DMPA-SC = 136; LA = 138) | DMPA-SC = 29.2; LA = 32.1. |
|
NR | EHP-30 core section and sex module | Baseline, 6, 18 months |
| Medical interventions—hormonal contraceptive | |||||||||
| (Barra et al., 2020) | Italy | Dienogest (DNG) | Cohort study | 83 | 32.8 | NR | NR. | EHP-30 core section | Baseline, 6, 12, 24, 36 months |
| (Carvalho et al., 2018) | Brazil | Etonogestrel (ENG)-releasing contraceptive implant vs Levonorgestrel-releasing intrauterine system (LNG-IUS) | RCT | 103 (ENG = 51; LNG-IUS = 52) |
|
|
I-IV | EHP-30 core and modular sections | Baseline, 6 months |
| (Ebert et al., 2017) | Germany, Austria, France, Finland, Czech Republic, Spain | Dienogest (DNG) | Open-label single-arm study | 111 | 15.4 |
|
NR | EHP-30 core section | Baseline, 12, 24, 52 weeks |
| (Egekvist et al., 2019) | Denmark | Oral contraceptives, oral gestagens, and/or the levonorgestrel-releasing intrauterine device (LNG-IUS) | Cohort study | 80 | 38.6 | NR | NR | EHP-30 core section | Baseline, 6, 12 months |
| (Ferrero et al., 2020) | Italy | Etonogestrel (ENG)-releasing implant | Cohort study | 43 | 32.8 | White (83.7), Other (16.3) | NR | EHP-30 core section | Baseline, 6 months, 12 months, 24 months |
| (Flores et al., 2015) | Mexico | Levonorgestrel-releasing intrauterine system (LNG-IUS) | Open non-comparative study | 29 | 31.7 | NR | II-IV | Modified version of the EHP-30 | Baseline, 6 months |
| (Middleton et al., 2017) | UK | Injectable contraceptive (DMPA) vs Levonorgestrel-releasing intrauterine system (LNG-IUS) | RCT pilot | 77 | 31 |
|
I-IV | EHP-30 pain module only | Unclear |
| (Morotti et al., 2014) | Italy | Dienogest (DNG) | Pilot open-label single-arm trial | 25 | 33.4 | NR | NR | EHP-30 core section | Baseline, 6 months |
| (Scala et al., 2018) | Italy | Norethindrone Acetate (NETA) vs Oral contraception (OC) | Open label comparative study |
|
|
|
NR | EHP-30 core section | Baseline, 12 months |
| (Taniguchi et al., 2020) | Japan | Tokishakuyakusan add-on therapy with low-dose oral contraceptive pills | Open-label single-arm trial | 9 | 31.4 | NR | NR | EHP-30 core section | Baseline, following 3 menstrual cycles |
| (Techatraisak et al., 2019) |
|
Dienogest (DNG) | Cohort study | 865 | 34.4 | Asian (100) | I-IV | EHP-30 core and modular sections | Baseline, 6 months |
| (Yela, 2020) | Brazil | Progestin | Cross-sectional study | 58 | 37.2 |
|
NR | EHP-30 core and modular sections | N/A |
| (Yong, 2020) | Canada | Combined hormonal contraceptive (CHC) | Cross-sectional study |
|
|
NR | I-IV | EHP-30 pain module | N/A |
| Other medical interventions | |||||||||
| (Ekin et al., 2021) | Turkey | New Cross linked Hyaluronan Gel (NCH gel) vs sterile saline solution | Pilot RCT |
|
|
NR | NR | EHP-5 core and modular sections | Baseline, 3, 6 months |
| (Khodaverdi et al., 2021) | Iran | Superior Hypogastric Plexus (SHP) block | Open-label pilot trial | 16 | 33 | NR | IV | EHP-5 core and modular sections | Baseline, 1, 4, 12, 24 weeks |
| (Mathiasen et al., 2019) | Denmark | Assisted reproductive technologies (ART) | Cohort study | 154 (-Endometriosis undergoing ART Yes/No = 52/50 -Without endometriosis undergoing ART = 52) |
|
NR | NR | EHP-30 core section | Baseline, 28–40 days |
| (Van der Houwen, 2014) | Netherlands | Assisted reproductive technologies | Cohort study | 75 (IUI = 25; IVF = 25; IVF-Long = 25) | IUI = 32; IVF = 34; IVF-Long = 34 |
|
III-IV | EHP-30 core and modular sections | Baseline, 29 days |
| (Wickström et al., 2013) | Sweden | Lidocaine vs placebo | RCT | 42 (Lidocaine = 24; placebo = 18) | (Lidocaine = 33.08; placebo = 33.4) | NR | NR | EHP-30 core section and sex module | Baseline, 6, 12 months |
For consistency, we have cited the sample size upon which age and ethnicity were calculated.
The women were allocated to more than one group.
NR, not reported; RCT, randomised controlled trial; EHP, Endometriosis Health Profile.
Table 2.
Summary of studies that used the Endometriosis Health Profile in the context of a surgical intervention.
| Author and year | Country | Specific intervention | Study type | Sample size* | Sample mean/median age in years | Sample ethnicity | Stage of endometriosis | EHP measure used | EHP data collection points |
|---|---|---|---|---|---|---|---|---|---|
| Hysterectomy | |||||||||
| (De la Hera-Lazaro et al., 2016) | Spain | Hysterectomy, one/two-sided adnexectomy bowel resection, rectum-vaginal nodule resection, bladder resection, vaginal resection | Non-randomised interventional study | 46 | 38.6 | NR | IV | EHP-5 core and modular sections | Baseline, 6 months |
| (Kent et al., 2016) | UK | Laparoscopic surgery | Cohort study | 137 | 36.7 | NR | IV | EHP-30 core and modular sections | Baseline, 2 months, 6 months, 12 months |
| (Sandström et al., 2020) | Sweden | Hysterectomy | Cross-sectional study | 137 | 41 | NR | Not staged-IV | EHP-30 core section | 37–107 months |
| (Tan et al., 2013) | UK | Abdominal hysterectomy and bilateral salpingo-oophorectomy | Cohort study | 16 | 38.1 | NR | I-IV | EHP-30 core and modular sections | Baseline, 3 months |
| Laparoscopic Surgery | |||||||||
| (Barton-Smith, 2010) | UK | Harmonic scalpel excision vs carbon dioxide laser vaporisation | RCT | 133 (Excised=66; Vaporised=67) |
|
NR | I-III | EHP-30 core and sex module | Baseline, 3, 6, 12 months |
| (Delbos et al., 2018) | France | Simple shaving, shaving exclusively/in part by plasma vaporisation (plasma), or resection. | Cross sectional study |
|
|
NR | NR | EHP-5 core and modular sections | Collected twice during one interview |
| (Ekine et al., 2020) | Hungary | Laparoscopic excision | Cohort study | 87 | 34.2 | NR | I-IV | Unclear -EHP-36 and modules are unfamiliar. | Baseline, 6, 12, 24 months. |
| (Gallicchio et al., 2015) | USA | White light imaging+narrow band imaging (WL/NBI) vs white light imaging only (WL/WL) | RCT | 148 (WL/NBI=110; WL/WL=38). | WL/NBI=33.2; WL/WL=30.6). |
|
I-IV | EHP-30 core section | Baseline, 3, 6 months. |
| (Ghai et al., 2020) | UK | Conservative and radical surgery | RCT (Post hoc analysis of two trials) | 198 (102 superficial; 96 recto-vaginal endometriosis). |
|
NR | NR | EHP-30 core and modular sections | Baseline and 12-month post-surgery. |
| (Meuleman et al., 2009) | Belgium | Laparoscopic excision | Cohort study | 56 | 32 | NR | II-IV | EHP-30 core section | Baseline, median follow-up was 29 months (range 6–76 months) |
| (Meuleman et al., 2011) | Belgium | Bowel resection and reanastomosis at the end of a CO2 laser laparoscopic radical excision of endometriosis | Cohort study | 45 | 30 | NR | III-IV | EHP-30 core section | Baseline, median follow up of 27 months (range: 16–40) months |
| (Meuleman et al., 2014) | Belgium | Laparoscopic excision of moderate-severe endometriosis in women with and without bowel resection and reanastomosis. | Cohort study | 203 (Study group=76; Control=127). | (Study group=32.9 and Control=32.1) | NR | III-IV | EHP-30 core and modular sections | Baseline, 6, 12, 18, 24 months |
| (Minas and Dada, 2014) | UK | Laparoscopic ablation with and without excision | Cross-sectional study | 49 | NR | NR | I-IV | EHP-5 core and modular sections | Collected twice post-surgery to ask about pre-and-post-QoL outcomes |
| (Misra et al., 2020) | UK | Laparoscopic treatment with electrodiathermy vs helium thermal coagulator | RCT | 192 (Diathermy=96; Helium=96) | Diathermy=28.9 Helium=29.03 | NR | I-III | EHP-30 core section | 6, 12, and 36 weeks |
| (Protopapas et al., 2014) | Greece | Laparoscopic excision | Cohort Study | 36 | 29.2 | NR | I-IV | EHP-30—unclear which sections | Baseline, 6, 12, 18, 24 months |
| (Rindos et al., 2020) | USA | Laparoscopic excision | Cohort Study | 46 | 32.5 |
|
I-IV | EHP-30 core section | Baseline, 1 month, and 2.6- to 6.8-year post-surgery |
| (Soto et al., 2017) | USA | Laparoscopy (Lap) vs robotic surgery (RS) | RCT | 73 (RS=35; Lap=38) |
|
|
I-IV | EHP-30 core and modular sections | Baseline, 6 weeks, 6 months |
| (Tiringer et al., 2020) | Austria | Laparoscopic excision | Cohort study | 115 | 32 | NR | NR | EHP-30 core section | Baseline, and 6–10 weeks post-operatively. |
| (Turco et al., 2020) | Unclear—Italy | Segmental colorectal resection | Cohort study | 50 | 38 | NR | III-IV | EHP-30 core and modular sections | Baseline, median follow-up 42.5 months (range 12–157 months) |
| (Yong et al., 2018) | Canada | Minimally invasive surgery including excision (and other interdisciplinary treatments) | Cohort study | 497 (1-year follow-up=278; Lost to follow-up=219) |
|
NR | I-IV | EHP-30 sex module only | Baseline, 12 months |
| Medical and surgical interventions | |||||||||
| (Vercellini et al., 2013) | Italy | Second-line conservative surgery (Surgery) at laparoscopy or low-dose progestin treatment (Progestin) | Cohort study | 154 (Progestin=103 Surgery=51) |
|
NR | III-IV | EHP-30 core and modular sections | Baseline, 3, 6, and 12 months |
For consistency, we have cited the sample size upon which age and ethnicity were calculated.
RCT, randomised controlled trial; NR, not reported; EHP, Endometriosis Health Profile.
Table 3.
Summary of Endometriosis Health Profile psychometric studies and cross-cultural adaptations.
| Author and year | Country | Aim of study | Sample size* | Sample mean/median age in years | Sample ethnicity | Stage of endometriosis | EHP measure used |
|---|---|---|---|---|---|---|---|
| (Aubry et al., 2017) | France | To compare the French version of the EHP-5 with the EQ-5D. | 216 | 33.2 | NR | I-IV | EHP-5 core and modular sections |
| (Chauvet et al., 2017) | France | To evaluate the French version of the EHP-30. | 913 | 33.4 | NR | NR | EHP-30 core and modular sections |
| (Chauvet et al., 2018) | France | To assess the content validity of the French version of the EHP-30 and SF-36. | 913 (Comments Yes=339; Comments No=574) |
|
NR | NR | EHP-30 core and modular sections |
| (Deal et al., 2010b) | USA | To use the EHP-30 core questionnaire during the construct validity testing of the Endometriosis Treatment Satisfaction Questionnaire (ETSQ). | Quantitative study=158 | Quantitative study=32.2 |
|
NR | EHP-30 core section |
| (Deal et al., 2010a) | USA | To use the EHP-30 core questionnaire during the construct validity testing of a daily electronic Endometriosis Pain and Bleeding Diary (EPBD). | Quantitative study=128 | Quantitative study=33.9 | NR | NR | EHP-30 core section |
| (Fauconnier et al., 2017) | France | To assess the psychometric properties of the French version of the EHP-5. |
|
With endometriosis=34.6 Controls=34.7 | NR | NR | EHP-5 core and modular section |
| (Goshtasebi et al., 2011) | Iran | To develop and validate the Iranian version of the EHP-5. | 199 | 31.4 | NR | NR | EHP-5 core section |
| (Grundström et al., 2020a) | Sweden | To cross-culturally assess the Swedish version of EHP-30 core questionnaire. | 18 | 33.3 | NR | NR | EHP-30 core section |
| (Grundström et al., 2020b) | Sweden | To evaluate the psychometric properties of the Swedish version of the EHP-30. | 128 | 38 | NR | NR | EHP-30 core section |
| (Jenkinson et al., 2008) | USA | To evaluate the EHP-30 in modular and summary form in a USA sample. | 225 | 30.5 |
|
NR | EHP-30 core section |
| (Jia et al., 2013) | China | To develop a simplified Chinese version of the EHP-30 and evaluate its psychometric properties. | 336 | 33.5 |
|
NR | EHP-30 core and modular section |
| (Jones et al., 2001) | UK | To develop the UK version of the EHP-30. |
|
Group C=32.5 | NR | NR | EHP-30 core and modular sections |
| (Jones et al., 2004a) | UK | To develop the UK version of the EHP-5. |
|
|
NR | NR | EHP 5 core and modular sections |
| (Jones et al., 2004c) | UK | To evaluate the sensitivity to change of the UK version of the EHP-30. | 40 | 34.3 | NR | NR | EHP-30 core and modular sections |
| (Jones et al., 2006) | UK | To test the data quality of the UK version of the EHP-30. | 610 | 34.7 | NR | NR | EHP-30 core and modular sections |
| (Khong et al., 2010) | Australia | To evaluate the EHP-30 in an Australian population. | 195 | 34.6 | NR | NR | EHP-30 core and modular sections |
| (Maiorana et al., 2012) | Italy | To evaluate the Italian version of the EHP-30. | 98 | 34.4 | NR | NR | EHP-30 core section, unclear if the modular section used |
| (Marí-Alexandre et al., 2022) | Spain | To evaluate the Spanish version of the EHP-30 core questionnaire. | 223 (Histologically confirmed=124; Clinical evidence=99) |
|
|
NR | EHP-30 core section |
| (Moradi et al., 2019) | Australia | To use the EHP-5 (with an altered recall of 12 months) during the concurrent validity testing of the Endometriosis Impact Questionnaire (EIQ). | 423 | Mean 32.64 | NR | NR | EHP-5 core and modular sections |
| (Nogueira-Silva et al., 2015) | Portugal | To evaluate the Portuguese version of the EHP-30. | 152 | 34.7 |
|
I-IV | EHP-30 core and modular sections. |
| (Nojomi et al., 2011) | Iran | To evaluate the Persian version of the EHP-30. | 100 | 39.5 | NR | NR | EHP-30 core and modular sections |
| (Oppenheimer et al., 2019) | France | To use the EHP-5 during the construct validity and responsiveness testing of the French version of the Sexual Activity Questionnaire (SAQ). | 267 (Completed SAQ at T1 Yes/No=136/131) |
|
NR | I-IV | EHP-5, but sections NR |
| (Pokrzywinski et al., 2020b) | USA |
|
1686 (EM-I=871; EM-II=815) |
|
|
NR | EHP-30 core section and sex module |
| (Pokrzywinski et al., 2020a) | USA | To evaluate the responsiveness and responder thresholds of the EHP-30 core and sexual functioning domain in the context of elagolix for moderate-to-severe endometriosis-associated pain. | As per Pokrzywinski et al. (2020b) | As per Pokrzywinski et al. (2020b) | As per Pokrzywinski et al. (2020b) | As per Pokrzywinski et al. (2020b) | As per Pokrzywinski et al. (2020b) |
| (Roomaney and Kagee, 2018) | South Africa | To use the EHP-30 to assess the reliability and validity of the Stellenbosch Endometriosis Quality of life measure (SEQOL). |
|
|
NR | NR | EHP-30 core and work, sex, and infertility |
| (Selcuk et al., 2015) | Turkey | To test the Turkish version of the EHP-5. | 58 | 33.8 | NR | NR | EHP-5 core and modular sections |
| (van de Burgt et al., 2011) | Netherlands | To test the Dutch version of the EHP-30. | 371 | NR | NR | NR | EHP-30 core and modular sections |
| (van de Burgt et al., 2013) | Netherlands | To assess the responsiveness of the Dutch version of the EHP-30. | 228 | 34.5 (18–56) | NR | NR | EHP-30 core and modular sections |
| (Verket et al., 2018) | Norway | To test the Norwegian (Bokmål) version of the EHP-30. | 157 | 35.2 | NR | NR | EHP-30 core and modular sections |
| (Wickström et al., 2017) | Sweden | To evaluate the responsiveness of the Swedish version of the EHP-30 core section+sexual functioning domain. | 42 | 33.2 | NR | NR | EHP-30 core section and sexual functioning module |
| (Wickström and Edelstam, 2017) | Sweden | To use the EHP-30 to evaluate whether the MIDs generated by an endometriosis VAS scale significantly affected QoL. | 37 (>50% on VAS=13; <50% on VAS=23) |
|
NR | NR | EHP-30 core section and sexual functioning module |
| (Wyrwich et al., 2018) | USA | To use the EHP-5 to validate the Endometriosis Daily Pain Impact Diary (EDPID). | 126 | 33.0 |
|
NR | EHP-5 core and modular sections |
For consistency, we have cited the sample size upon which age and ethnicity were calculated.
EHP, Endometriosis Health Profile; VAS: Visual analogue scale.
Results
Study selection
A PRISMA flow diagram (Page et al., 2021) is shown in Fig. 2. The initial database searches produced 1098 papers in total. After removing 359 duplicates, 444/739 (60.1%) papers were excluded at the title and abstract stage leaving 295 papers that were assessed for eligibility against the inclusion/exclusion criteria. A further 153 papers were excluded (Fig. 2), plus 37 abstracts, protocols, and texts with English abstracts but foreign full texts. A further 11 papers, identified from the excluded reviews, were included in the final synthesis. The updated searches (9 April 2021) identified 17 additional eligible papers and six identified by hand searching. One initially included paper was later retracted and therefore excluded (Mira et al., 2015). Thus, in total, 139 papers were included in this review.
Descriptive summary of the studies
The EHPs were deployed most frequently to measure the outcomes of medical (n = 35) (Table 1) and surgical treatment (n = 21) (Table 2). The EHPs were also used in 13 studies to measure the outcomes of other interventions (Supplementary Table S1), in 29 non-interventional studies (Supplementary Table S2), and 32 psychometric/cross cultural validation studies (Tables 3, 4, and 5, and Supplementary Table S3). An additional six studies were diagnostic, and three deployed the EHPs in other related conditions, e.g. CPP and adenomyosis; their findings are not reported in the text but can be found in Supplementary Table S4.
Table 4.
Comparison of the Endometriosis Health Profile-30 responsiveness/MCID results using a subjective ‘general health status’ anchor-based method question following an intervention.
|
Jones et al. (2004c) Surgery (conservative)* |
van de Burgt et al. (2013
) Mixed (medical and surgical)** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean score change (n) |
Effect size (n) |
Mean score change (n) |
Effect size (n) |
|||||||
| Somewhat better | About the same | Overall group | Better group | No change group | Somewhat better | About the same | Overall group | Better group | No change group | |
| EHP-30 Core Domains | ||||||||||
| Pain | −24.8 | −5.6 | −0.9 (39) | −1.8 (19) | −0.2 (17) | −11.5 (29) | −3.3 (86) | −0.4 (227) | −1.1 (80) | −0.1 (87) |
| Control and powerlessness | −35.2 | −7.6 | −1.1 (39) | −2.3 (19) | −0.3 (17) | −12.5 (29) | −3.9 (87) | −0.4 (228) | −1.1 (80) | −0.1 (87) |
| Emotional well-being | −7.6 | −1.5 | −0.5 (38) | −1.1 (18) | −0.1 (17) | −5.5 (29) | −1.6 (87) | −0.3 (228) | −0.9 (80) | −0.1 (87) |
| Social support | 1.7 | −2.9 | −0.2 (38) | −0.6 (19) | −0.1 (17) | −10.0 (28) | −0.8 (86) | −0.3 (226) | −0.8 (79) | 0.0 (86) |
| Self-image | −9.9 | 1.85 | −0.3 (39) | −0.9 (19) | 0.1 (18) | −3.2 (29) | −5.0 (86) | −0.3 (227) | −0.6 (80) | −0.2 (86) |
| EHP-30 Modular Domains | ||||||||||
| Work | — | — | −0.6 (35) | −1.6 (15) | 0.0 (17) | −5.7 (23) | −2.3 (68) | −0.2 (180) | −0.7 (57) | −0.1 (68) |
| Children | — | — | −0.7 (10) | −1.4 (6) | 0.0 (4) | −17.5 (5) | −5.9 (32) | −0.5 (65) | −1.1 (17) | −0.2 (32) |
| Sexual functioning | — | — | −0.4 (32) | −1.1 (13) | 0.1 (16) | −4.4 (23) | −8.4 (64) | −0.3 (190) | −0.6 (62) | −0.3 (64) |
| Medical profession | — | — | −0.4 (26) | −0.7 (10) | −0.3 (7) | 2.5 (17) | −2.6 (49) | −0.2 (145) | −0.3 (43) | −0.1 (49) |
| Treatment | — | — | −0.2 (18) | −0.4 (3) | −0.1 (6) | −7.8 (15) | 0.2 (51) | −0.2 (147) | −0.4 (39) | 0.0 (51) |
| Infertility | — | — | 0.1 (19) | −0.5 (8) | 0.5 (8) | 2.2 (13) | 1.3 (36) | −0.1 (117) | −0.6 (34) | 0.1 (36) |
| SF-36 Domains | ||||||||||
| Pain | 11.1 | 3.1 | 0.5 (40) | 1.0 (19) | 0.1 (18) | / | / | / | / | / |
| Energy/vitality | 10.5 | 2.7 | 0.5 (39) | 1.3 (19) | 0.2 (17) | / | / | / | / | / |
| General health | 2.4 | −2.2 | 0.1 (39) | 0.4 (19) | 0.0 (17) | / | / | / | / | / |
| Mental health | 8.7 | 1.0 | 0.4 (39) | 0.9 (19) | 0.2 (17) | / | / | / | / | / |
| Physical | 8.6 | 0.6 | 0.3 (38) | 0.7 (19) | 0.1 (17) | / | / | / | / | / |
| Role (emotional) | 3.0 | 11.1 | −0.3 (40) | 0.5 (19) | 0.3 (18) | / | / | / | / | / |
| Role (physical) | 22.7 | −2.8 | 0.3 (40) | 0.9 (19) | 0.0 (18) | / | / | / | / | / |
| Social functioning | 22.2 | 5.6 | 0.4 (40) | 1.1 (19) | 0.2 (18) | / | / | / | / | / |
Jones et al. (2004c) and van de Burgt et al. (2013) reported the improvement in score and for the purposes of showing consistency across other studies, we’ve reversed the sign to show the absolute change in score because in the EHP, low is better. Other indicators of responsiveness have also been used (e.g. the standardised response mean in Jones et al., 2004c).
EHP: 0 = best health status, 100 = worst health status.
SF-36: 0 = worst health status, 100 = best health status.
Four months after conservative surgery based upon the global health perception question in the SF-36.
Six months after any treatment (could have been medical or surgical) using the same anchor question as reported in Jones et al. (2004c).
Not calculated due to the small number of responses.
Not reported.
MCID, minimally clinically important difference; EHP, Endometriosis Health Profile.
Table 5.
Comparison of the Endometriosis Health Profile-30 responsiveness/MCID results using a subjective ‘pain’ anchor-based question following an intervention.
|
Pokrzywinski et al. (2020a) Elagolix and placebo* |
Wickström and Edalstam (2017) Pertubation + lignocaine and placebo** |
Wickström et al. (2017) Pertubation + lignocaine and placebo*** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Improved |
No change/worsened |
Improved | Not improved | Better or pain free |
Worse or same |
Somewhat better^**** | |||||
| Mean score change (n) |
Mean score change (n) |
Median score change (n) | Mean score change (n) | Mean score change (n) | Effect size | Mean score change (n) | Effect size | Mean score change (n) | |||
| EM-I | EM-II | EM-I | EM-II | ||||||||
| EHP-30 Core | |||||||||||
| Pain | −31.1 (564) | −30.8 (544) | −5.5 (151) | 7.3 (152) | −21.6 (12) | 0 (19) | −22.0 (19) | −1.22 | −1.7 (13) | −0.09 | −19.9 (17) |
| Control and powerlessness | −40.8 (566) | −36.0 (551) | −8.8 (153) | −8.6 (154) | −33.3 (12) | 0 (20) | −28.3 (19) | −1.24 | −2.1 (14) | −0.10 | −25.7 (17) |
| Emotional well-being | −21.1 (566) | −18.9 (546) | −6.3 (154) | −4.9 (153) | −8.3 (11) | 0 (19) | −18.1 (17) | −1.04 | 7.1 (13) | 0.35 | −13.9 (15) |
| Social support | −22.8 (574) | −20.6 (551) | −0.7 (156) | −1.9 (154) | −9.4 (12) | −6.3 (19) | −16.8 (19) | −0.84 | −3.8 (13) | −0.17 | −12.9 (17) |
| Self-image | −20.3 (569) | −17.8 (550) | −1.0 (155) | −2.6 (153) | −16.7 (12) | 0 (19) | −8.8 (19) | −0.51 | 1.9 (13) | 0.11 | −6.9 (17) |
| EHP Modular | |||||||||||
| Work | / | / | / | / | / | / | / | / | / | / | / |
| Children | / | / | / | / | / | / | / | / | / | / | / |
| Sexual functioning | −22.1 (400) | −21.9 (393) | −2.5 (119) | −4.9 (104) | −20 (9) | 5 (17) | −7.3 (16) | −0.30 | 5 (10) | 0.15 | −4.5 (14) |
| Medical profession | / | / | / | / | / | / | / | / | / | / | / |
| Treatment | / | / | / | / | / | / | / | / | / | / | / |
| Infertility | / | / | / | / | / | / | / | / | / | / | / |
| Work | / | / | / | / | / | / | / | / | / | / | / |
| Children | / | / | / | / | / | / | / | / | / | / | / |
| Sexual functioning | / | / | / | / | / | / | / | / | / | / | / |
| Pain VAS (mm) | / | / | / | / | Somewhat better −38.6 (18) | Same −7.4 (11) | / | / | / | / | / |
EHP: 0 = best health status, 100 = worst health status.
VAS: Visual analogue scale (pain only); 0 mm=no pain, 100 mm=worst pain imagined.
Three months after treatment with elagolix. Patient Global Impression of Change (seven response categories) was used to estimate changes in pain intensity. Improved, Very much improved, much improved, minimally improved; No change or worse, No change, minimally worse, much worse, very much worse.
Four/six months following treatment with perturbation with lignocaine using an anchor measure derived from a VAS.
Six months after treatment with perturbation with lignocaine. Modified version of the general quality of life question on the SF-36 (five response categories) to estimate changes in pain intensity. Better or pain free, Somewhat better, much better; Worse or same, Somewhat worse, much worse, about the same.
Effect sizes not reported.
These MIDs were also reported in Wickström et al. (2013).
Not relevant.
MCID, minimally clinically important difference; EHP, Endometriosis Health Profile.
Most studies used the EHP-30, either the core alone (n = 39) or core and all the modular components (n = 34). Seventeen studies used the EHP-30 in addition to specific modular components (e.g. alongside the sexual functioning or work modules). Thirteen studies opted to use specific modules only, without the core measure. Five studies used a modified version of the EHP-30, with a mix/rewording of items/domains taken from the core and modular sections. Seven studies used the EHP-5 (core component only), or core and modular components (n = 13). One study used a modified version of the EHP-5 core and modular sections. The remaining studies did not clearly report either the EHP measures or domains used (n = 10). Some used the EHPs without the ‘not applicable’ box for the modular components (Fauconnier et al., 2017).
A cohort study was the most common design for evaluating the impact of a surgical intervention (n = 12), while an RCT was the most used design for medical interventions (n = 20), including two pilot studies and four post hoc analyses. The locations where the research was undertaken are shown in Tables 1, 2, and 3 and Supplementary Tables S1, S2, and S4.
In terms of demographic characteristics, 90/139 studies did not report participants’ race/ethnicity. Of those that did, 10 simply reported the percentage of ‘White’ or ‘Caucasian’ participants: plus ‘others’ (n = 6), or no information on the remainder (n = 4). Three studies reported including a wholly ‘White’ (n = 1), ‘Asian’ (n = 1), or European’ (n = 1) sample. Thirty-two studies included a mix of ‘White’, ‘Black’, and/or minority ethnic women. One study reported the percentage of ‘Black’ versus ‘non-Black’ participants, another Han versus non-Han participants and two more White versus non-White. Finally, the study by Cosma and Benedetto (2020) compared and mapped guidelines on the diagnosis and treatment of endometriosis to develop an algorithm of the endometriosis care pathway; thus, no participants were recruited.
Quality appraisal
Of the 139 studies, 15 were not fully appraised because their design was not MMAT compatible (review, n = 1; review of guidelines to develop a diagnostic/classification system, n = 1; analysis of data previously collected, n = 13). An additional paper was similarly not fully appraised as the research aims were unclear and at odds with the data collection and analysis carried out. Of the 123 fully appraised studies, 90 (73.2%) were of high quality (4–5 criteria satisfied); 29 moderate quality (2–3 criteria satisfied), and four low quality (0–1 criteria satisfied).
What do the EHPs tell us about the impact of medical treatments for endometriosis?
Table 1 shows the impact on HRQoL of medical interventions: GnRH agonists and antagonists (n = 14), GnRH agonists with contraceptives (n = 3), hormonal contraceptives only (n = 13), and other types of medical intervention, for example perturbation with lidocaine (n = 5).
GnRH agonists and antagonists
Three RCTs (Al-Azemi et al., 2009; Donnez et al., 2020; Osuga et al., 2021) and one single-arm open-label trial (Alshehre et al., 2020) were conducted to treat endometriosis-associated pain and/or HRQoL. All four reported improved pain and/or HRQoL following treatment; two (Al-Azemi et al., 2009; Alshehre et al., 2020) found deterioration after treatment cessation.
Most of the studies assessed the efficacy of elagolix in treating endometriosis-associated moderate-to-severe pain. Four Phase II trials using the EHP-5 (Carr et al., 2013, 2014; Diamond et al., 2014; Ács et al., 2015) demonstrated improved HRQoL in all patients that was greatest for those in the elagolix arms. Two subsequent Phase II studies using the EHP-30, plus the modular sexual functioning domain (EM-I and EM-II) (as reported in Taylor et al. (2017)), demonstrated significantly improved scores after treatment, although some improvements were dose dependent and differed during follow-up. We refer the reader to the comprehensive review of the EM-I and EM-II trials, including the EHP-30 outcomes (Archer et al., 2020) for further in-depth results and information about the comparators in each study.
There have been two extension studies (EM-III and EM-IV), both using the EHP-30 plus the sexual functioning domain (Surrey et al., 2018), and four post hoc studies (Leyland et al., 2019; Agarwal et al., 2020; Pokrzywinski et al., 2020c; Taylor et al., 2020). In the extension studies, there was overall improvement from baseline in HRQoL following 12 months of treatment in both dose groups. Three post hoc studies pooled data from EM-I and EM-II to explore whether women with moderate-severe endometriosis-associated pain, randomised to elagolix 150 mg od or 200 mg bd, showed greater clinical and HRQoL improvement compared to placebo (Agarwal et al., 2020; Pokrzywinski et al., 2020c; Taylor et al., 2020). EHP scores improved significantly in women whose dyspareunia (Agarwal et al., 2020) and dysmenorrhoea and non-menstrual pelvic pain (Pokrzywinski et al., 2020c) also improved. Taylor et al. (2020) similarly found that treatment was associated in a dose-dependent manner with greater improvements in HRQoL compared with placebo. Leyland et al. (2019) pooled EM-I and EM-II data to evaluate the effects of elagolix on endometriosis-associated dyspareunia. Using the EHP-30 sexual functioning domain, they concluded that up to 6 months of treatment improved dyspareunia in a dose-dependent manner.
GnRH agonists with contraceptives
Crosignani et al. (2006) and Schlaff et al. (2006) found that depot medroxyprogesterone acetate (DMPA-SC 104) reduced endometriosis-associated pain as effectively as leuprolide, but with more bleeding. Granese et al. (2015) reported that dienogest plus estradiol valerate and leuprorelin acetate seemed equally efficacious in preventing endometriosis-associated CPP recurrence in the first 9 months after treatment.
Hormonal contraceptives only
Thirteen studies (Table 1) used the EHP-30 to appraise a range of contraceptives including intrauterine devices (levonorgestrel-releasing intrauterine system: LNG-IUS), subdermal implants (etonogestrel (ENG)-releasing contraceptive implant), injectable contraceptives (DMPA), and oral contraceptives (e.g. dienogest, norethindrone acetate: NETA).
Results from two RCTs (Middleton et al., 2017; Carvalho et al., 2018), three open-label single-arm trials (Morotti et al., 2014; Ebert et al., 2017; Taniguchi et al., 2020), one open non-comparative study (Flores et al., 2015), and one open-label comparative study (Scala et al., 2018) demonstrated improvements in all or some of the EHP-30 domains following treatment. However, one study did not calculate domain scores but indicated an increase in the proportion of women selecting ‘never’ in response to the questions (Ebert et al., 2017). A single-arm pilot trial (Taniguchi et al., 2020) assessed Tokishakuyakusan—a Japanese Kampo medicine—as an add-on therapy to hormonal contraceptives: participants reported some improvement in symptoms and some aspects of HRQoL, which did not reach statistical significance.
Four cohort studies demonstrated improvements in HRQoL with hormonal contraceptives. Barra et al. (2020) found significant improvements in EHP-30 scores (with the exception of the self-image and control and powerlessness domains) after 6 months of treatment. Scores further improved at 12 months and stabilised at 24 and 36 months. Ferrero et al. (2020) reported improvements in all EHP-30 core domains following 6 months of treatment, with further improvements limited to the pain domain at 12 months and the emotional well-being domain at 24 months. Egekvist et al. (2019) found a non-significant improvement in HRQoL across a 12-month follow-up period. In an interim analysis, Techatraisak et al. (2019) observed improvements in EHP-30 core and modular domains 6 months after treatment, especially for pain outcomes.
There were two cross-sectional studies. Despite some inconsistencies in the manuscript and incorrect domain labelling, Yela et al. (2020) reported worse HRQoL in the sexual functioning, emotional well-being, infertility, and social support domains in women treated for deeply infiltrating endometriosis (DIE). Yong et al. (2020) found that women who used combined oral contraception continually (as opposed to cyclically) and discontinued treatment owing to side-effects had poorer HRQoL.
Other medical interventions
Five studies tested the effectiveness of other medical interventions using the EHP (Table 1). Using the EHP-30, Wickström et al. (2013) found, in an RCT of perturbation with lidocaine (local anaesthetic) versus placebo, that only social support had significantly improved for the treatment group at 6 months, which disappeared by 12 months.
Using the EHP-5, significant improvements in HRQoL were observed following superior hypogastric plexus block for endometriosis-associated CPP (Khodaverdi et al., 2021) and New Cross linked Hyaluronan Gel (NCH gel) after laparoscopic surgery for DIE (Ekin et al., 2021). However, in Ekin et al. (2021), it is unclear how the EHP-5 was analysed and whether the results refer to the core or modular components or both.
Two studies used the EHP-30 to assess the effects of ART. Owing to limited sample sizes, van der Houwen et al. (2014) only provided a descriptive analysis of the domain scores pre- and post-ART. Mathiasen (2019) showed that controlled ovarian stimulation during ART did not worsen HRQoL.
What do the EHPs tell us about the impact of surgical treatments for endometriosis?
A range of radical and conservative surgical outcomes have been measured using the EHPs (Table 2). Following hysterectomy, three studies used the EHP-30 (Tan et al., 2013; Kent et al., 2016; Sandström et al., 2020) and one the EHP-5 (De la Hera-Lazaro et al., 2016). Overall, they demonstrated an improvement in HRQoL, although infertility concerns may persist (Tan et al., 2013; Kent et al., 2016) (Table 2). Interestingly, Kent et al. (2016) observed that hysterectomy plus bilateral salpingo-oophorectomy provided significantly better HRQoL at 12 months compared to conservative surgery on all EHP-30 domains scores (P < 0.05) with the exception of the modular domains, relationship with children, sex, and feelings about the medical profession (P > 0.05).
Laparoscopic surgery
Sixteen papers used the EHPs to measure HRQoL following laparoscopic surgery, i.e. excision, ablation, vaporisation, resection, or shaving (Table 2). Two retrospective cohort studies, both by the same authors, reported improved HRQoL after laparoscopic surgery for DIE with colorectal extension (Meuleman et al., 2009, 2011). However, it is unclear which questionnaire was used as the scales relating to general health, physical health, and quality of professional life are not EHP domains. In a later prospective study, Meuleman et al. (2014) comparing women with and without bowel resection and reanastomosis, all EHP-30 domains 6 months after surgery (except the relationship with children domain, which was not analysed because of small numbers) showed significant improvement, although after 12 months no further improvement was observed.
Among the other 13 studies (Table 2), Protopapas et al. (2014) stated their patients completed the EHP-30 but did not report the pre- or post-surgery scores. In the Ekine et al. (2020) study, it is unclear which measure was used as they referred to the EHP-36 and the description of its content did not clearly match the current EHP domain structure (i.e. it measures demographics, physical, mental, and socioeconomic well-being). All other laparoscopic surgery resulted in HRQoL improvements from baseline in EHP-30 domains (Barton-Smith, 2010; Gallicchio et al., 2015; Soto et al., 2017; Yong et al., 2018; Ghai et al., 2020; Misra et al., 2020; Rindos et al., 2020; Tiringer et al., 2020; Turco et al., 2020) and the EHP-5 (Minas and Dada, 2014; Delbos et al., 2018).
Surgical versus medical interventions
Only one relevant study was found: second-line laparoscopic excision versus low-dose progestin for severe endometriosis-associated deep dyspareunia (Vercellini et al., 2013) (Table 2). At baseline and 3-month follow-up, women treated medically had significantly worse EHP-30 scores than those treated surgically. The surgical group experienced a rapid improvement in HRQoL; however, this declined over time whereas the medical treatment group continued slowly to improve. Consequently, at 12 months all EHP scores had improved for both groups.
What do the EHPs tell us about the impact of other interventions for endometriosis?
Eleven studies used the EHP-30 and two studies the EHP-5 to measure HRQoL following a variety of interventions (Supplementary Table S1): ultrasound therapy (Philip et al., 2020), transcutaneous electrical nerve stimulation (Mira et al., 2020); laser therapy (Thabet and Alshehri, 2018); repetitive transcranial magnetic stimulation (Pinot-Monange et al., 2019); mindfulness (Kold et al., 2012; Hansen et al., 2017); yoga (Gonçalves et al., 2017); acupuncture (Wayne, 2008; Ahn et al., 2009; de Sousa et al., 2016); Chinese herbal medicine (Flower et al., 2011), and educational interventions (Sayed and Aboud, 2018; Simonsen et al., 2020).
What do the EHPs tell us about the impact of endometriosis upon women’s quality of life?
Twenty-nine non-interventional studies used the EHP-30 (n = 26) or EHP-5 (n = 3) to explore the impact of endometriosis upon sexual functioning (n = 5) (Shum et al., 2018; Bao et al., 2019; van Poll et al., 2020; Wahl et al., 2020; Martins et al., 2022); psychological well-being (n = 6) (Friedl et al., 2015; Rush and Misajon, 2018; van Aken et al., 2018; González-Echevarría et al., 2019; Roomaney et al., 2020; Škegro et al., 2021); work/productivity (n = 2) (Fourquet et al., 2011; Hansen et al., 2013); pain (n = 5) (Hansen et al., 2014; Stratton et al., 2015; van Aken et al., 2017; de Freitas Fonseca et al., 2018; McPeak et al., 2018); general HRQoL (n = 7) (Matasariu et al., 2017; Soliman et al., 2017, 2020; Verket et al., 2018; Florentino et al., 2019; Ali Nor et al., 2020; Mundo-López et al., 2020); sleep (n = 2) (Leone Roberti Maggiore et al., 2017; Arion et al., 2020), and quality of care (n = 2) (Apers et al., 2018; Ng et al., 2020) (Supplementary Table S2).
Despite the heterogeneity of these studies, some interesting findings emerged. For example, there was some evidence that younger women report poorer HRQoL than older women; that perceptions of medical care are related to psychological well-being and some treatment outcomes, and that symptoms impact functioning at work. The studies also identified factors contributing to poorer HRQoL, such as fatigue or poorer quality of sleep, higher levels of pain, endometriosis severity, greater number of symptoms or symptom severity, and poorer psychological health.
What do we know about the psychometric properties of the EHPs?
Thirty-two studies performed psychometric/cross cultural validation of the EHPs (Table 3). Supplementary Table S3 shows the results of the psychometric tests, and the findings from the responsiveness and/or minimally important difference (MID) analyses are reported separately in Tables 4 and 5.
Cross-cultural adaptation and validation
Four papers from our group concluded that the EHP-30 was reliable, valid, and sensitive to change, and both acceptable and understandable to respondents (Jones et al., 2001, 2004a,b,c, 2006). Nineteen other studies have cross-culturally adapted and validated the EHP in French (Aubry et al., 2017; Chauvet et al., 2017, 2018; Fauconnier et al., 2017); Iranian/Persian (Goshtasebi et al., 2011; Nojomi et al., 2011); Swedish (Wickström et al., 2017; Grundström et al., 2020a,b); Chinese (Jia et al., 2013); Australian English (Khong et al., 2010); Spanish (Marí-Alexandre et al., 2022); Italian (Maiorana et al., 2012); Turkish (Selcuk et al., 2015); Dutch (van de Burgt et al., 2011, 2013); Norwegian (Verket et al., 2018); Portuguese (Nogueira-Silva et al., 2015), and US American (Jenkinson et al., 2008). However, only seven of these related to the EHP-5 (Table 3 and Supplementary Table S3). The EHP-5 has also been translated into Croatian, but validation of the tool was underway at the time of publication (Škegro et al., 2021).
The findings overall supported the psychometric validity of the measures in these languages; however, some recommendations for improvement have been made. For example, Grundström et al. (2020a) suggested one question in the Swedish version should be reworded. Maiorana (2012) observed that four items in the Italian version may have been improperly translated making it difficult to differentiate between ‘rarely’ and ‘sometimes’. They also criticised the internal consistency reliability of two domains, social support and self-image, but these achieved alpha values of 0.84 and 0.69 and it appears as though some items may not have been correctly allocated to the pain (n = 7) and control and powerlessness (n = 10) subscales in their analyses, as these should be n = 11 and n = 6, respectively.
Chauvet et al. (2018) suggested the French EHP-30 may not capture all relevant issues for women living in France. Some of the issues raised as missing from the EHP (e.g. fatigue) were included during EHP development (Jones et al., 2004b) but were later removed following psychometric testing. Other issues, such as ‘thanks’ and ‘advice’, were also identified by Chauvet et al. (2018) as missing from the EHP but it is not clear to what extent these would impact upon HRQoL and/or improve the tools.
Dimensionality
To date (with the exception of the study by Maiorana et al., 2012), only classical test theory has been used to explore dimensionality of the EHPs. In some EHP-30 studies, an overall total of the core and/or modular scores was reported; in others, just the domain scores. Most studies included in this review supported the five-factor structure and multi-dimensionality of the EHP-30 core. However, Grundström et al. (2020b) proposed four factors with the domains ‘social support’ and ‘self-image’ loading together. In the Norwegian version, the results of the factor analysis undertaken by Verket et al. (2018) revealed similar findings and, in particular, a lack of validity of the ‘self-image’ domain. The original structure of the EHP-30 was partially confirmed in another study as factor 5 could not be entirely classified as independent, and the subscale ‘pain’, which mainly corresponds to factor 1, was also related to other domains, for example ‘control and powerlessness’ (Marí-Alexandre et al., 2022). The unidimensionality of the EHP-30 core has been demonstrated, supporting the production of a summary score using classical test theory (Jones, 2001; Jenkinson et al., 2008; Nojomi et al., 2011; Nogueira-Silva et al., 2015) but was not when Rasch analysis was undertaken on the Italian EHP-30 core part. A summary score for the EHP-5 is supported by Fauconnier et al. (2017) reporting that the 11 EHP-5 items were also unidimensional, although the ‘not relevant’ boxes were removed during this analysis. Supplementary Table S3 provides further details on rates of data completion and item response distributions (e.g. floor and ceiling effects).
Reliability
All 17 studies that measured the internal consistency reliability of the tools reported Cronbach alpha values >0.7 (except in the study by Maiorana et al., 2012, where self-image was 0.69). Of the six papers that assessed test–retest reliability, three reported intraclass correlation coefficients (ICC) or Spearman’s rho correlations exceeding 0.8 (Jones et al., 2001; Selcuk et al., 2015; Grundström et al., 2020b). The ICC agreement in the Van de Burgt et al. (2011) study ranged from 0.65 to 0.89. The EHP-30 test–retest reliability was high (>0.8) in the other two studies, except for the domains ‘social support’ (0.51), and ‘infertility’ (0.65) (Chauvet et al., 2017), and ‘relationship with children’ (0.67) (Verket et al., 2018).
Validity
Seven studies used the EHP-5 (Wyrwich et al., 2018; Moradi et al., 2019; Oppenheimer et al., 2019) and EHP-30 (Deal et al., 2010a,b; Roomaney and Kagee, 2018; Pokrzywinski et al., 2020b) as measures of external validity to assess the construct validity of newly developed tools. Pokrzywinski et al. (2020b) used the EHP-30 to validate the health-related productivity questionnaire and the remaining studies used existing PROMs including the generic Short Form-36 (SF-36) and the EuroQoL five dimension (EQ-5D) to establish the convergent validity of the EHPs and their translated versions. In a comparison of the EHP-5 and the EQ-5D, the authors found that the EQ-5D had lower construct validity concluding that ‘the EHP-5 appears to be a better candidate than EQ-5D’ as it is ‘simpler and easier to interpret, facilitating evaluation of the baseline quality of life’ (Aubry et al., 2017).
Responsiveness and MIDs
As the EHPs have been used in trials to evaluate how treatment outcomes affect subjective health status, their responsiveness and/or MIDs/minimally clinically important differences (MCIDs) have been assessed. Responsiveness is a measure of longitudinal validity, which assesses the ability of a questionnaire to detect a change (if it truly exists) in health status; MIDs/MCIDs base the magnitude of change in health status on small, but to the patient, noticeable amounts. Synthesising the results was difficult because of methodological differences and the range of reported interventions. Sometimes it was unclear how the MID was calculated; in addition, other tools may have been used, not the EHPs, or EHP data were not provided as a contrast for those feeling worse/staying the same (Wickström et al., 2013). However, where synthesis was possible, the findings from external, anchor-based approaches (e.g. using the patients overall self-report of meaningful change), and distribution-based methods, which are based upon statistical indices of the change in QoL scores (e.g. such as effect sizes) (Revicki et al., 2006), are shown in Tables 4 and 5, respectively.
Two studies calculated MIDs using general changes in health status as the anchor (Jones et al., 2004c; van de Burgt et al., 2013) (Table 4). Jones et al. (2004c) found that the effect sizes were larger in magnitude for the EHP-30 core domains in patients who reported feeling better after conservative surgery (−0.6 to −2.3) compared to the group overall (−0.2 to −1.1) and those who reported no change (+0.1 to –0.3). A similar trend was observed for the modular section. They also observed that, with the exception of the social support domain, a small improvement in well-being following conservative surgery is equivalent to a mean score change of between −7.6 and −35.2 units. van de Burgt et al. (2013) observed smaller changes, i.e. that a small improvement in HRQoL is equivalent to a mean score change of between −3.2 and −12.5 units, depending on the EHP core domain but this was expected as the study was not intervention specific.
Other studies used a specific pain question as the anchor (Table 5). Pokrzywinski et al. (2020a), who assessed the EHP-30’s responsiveness in the context of the EM I and EM II trials, observed similar findings to those of Jones et al. (2004c). They noted moderate to large effect sizes on the EHP-30 core and sexual functioning domains (EM-I range −0.59 to −1.80; EM-II range −0.52 to −1.59), and improved well-being equivalent to mean score changes, depending on the EHP-30 domain, of −20.3 to −40.8 (EM I), and −17.8 to −36.0 (EM II). EHP-30 thresholds of meaningful change for these domains ranged from −20 to −35, with greater changes indicating greater improvement in health status. The authors suggested that the EHP-30 should have domain-specific responder threshold values rather than a ‘one number fits all’ value, and that clinicians should individualise treatment goals by EHP-30 domain and track changes (Pokrzywinski et al., 2020a).
Wickström et al. (2017) evaluated the responsiveness and calculated the MIDs of the Swedish version in their perturbation with lidocaine study. On the core and sexual functioning domains, an improvement in HRQoL was equivalent to a mean score change of between −7.3 and −28.3, with effect sizes ranging from −0.30 to −1.24.
Interestingly, using any anchor-based methods, all five studies demonstrated that improvements in HRQoL were largest in the ‘control and powerlessness’ domain post-intervention, followed by ‘pain’. This pattern was also observed in the effect sizes for the better groups (i.e. for those patients that said they had improved following an intervention), with the exception of van de Burgt et al. (2013) who showed the effect sizes for the ‘pain’ and ‘control and powerlessness’ domains were the same.
Aubry et al. (2017) evaluated the EHP-5’s responsiveness and MCIDs before and 12 months after medical or surgical treatment using the Clinical Global Impression-Improvement scale as the ‘anchor’, which includes seven responses (much better, better, somewhat better, no change, somewhat worse, worse, and much worse), and compared the EHP-5 findings with those for the EQ-5D descriptive system and a visual analogue scale (VAS). The effect sizes demonstrated that for the better group overall, both the EHP-5 and EQ-5D had equivalent responsiveness for the treatment group overall and the surgical treatment group. However, only the EHP-5 was responsive to changes in quality of life after medical treatment (P = 0.014) compared to the EQ-5D descriptive system (P = 0.064) and VAS (P = 0.437). Improvement (somewhat better and better) on the EHP-5 was equivalent to a score change of −4.5. While the analysis included patients both medical and surgical treatments, the EHP-5 was more sensitive than the EQ-VAS and EQ-5D index, that had score changes of 10.2 and 0.26, respectively. Wyrwich et al. (2018) also used the EHP-5 but only as a measure of external validity when determining the responsiveness of another tool.
Discussion
This systematic review, which aimed to identify and synthesise the literature describing the use of EHPs over the last 20 years, found 139 relevant publications. The questionnaire has mostly been deployed in clinical trials (particularly RCTs) demonstrating its value in assessing the outcomes of interventions from the patient’s perspective. Overall, regardless of the nature of the intervention, most women reported improvements in HRQoL after treatment.
Clinical findings
In general, surgical interventions resulted in significant improvements in HRQoL for the longest amount of time and EHP scores worsened when women stopped medication, perhaps reinforcing the temporary impact of medical treatment. Our review also highlights: that less-invasive treatments, which can improve longer term outcomes, are needed; how the condition and its symptoms impact adversely on all areas of women’s lives (including work); and the importance of research involving younger patients who may be experiencing more negative impact upon their HRQoL and psychological well-being, based upon their EHP scores.
Many women currently require multiple surgical and/or medical interventions to control symptoms; hence, more effective therapies have long been needed. Encouragingly, since undertaking the review, the US FDA has approved a combination of relugolix, estradiol, and norethindrone acetate (Myfembree) for moderate-severe endometriosis-associated pain (https://www.pfizer.com/news/press-release/press-release-detail/myovant-sciences-and-pfizer-receive-us-fda-approval) (Giudice et al., 2022). In the company’s FDA submission, the EHP-30 pain domain was used as a secondary outcome to measure the impact on daily activities.
Reporting of ethnicity was a major limitation of most of the studies included in the review because of failure to provide the information, or the use out-of-date terminology and definitions, e.g. ‘White/Non-White’, which led to difficulty understanding the full impact of the EHP scores that can only be improved by better reporting of ancestry/ethic origins (Flanagin et al., 2021). In addition, the EHPs have rarely been used to assess the HRQoL of women residing in Africa and the Middle East for complex reasons: (https://archive.discoversociety.org/2020/06/03/quality-of-life-measurement-in-women-living-with-endometriosis-observations-from-the-use-of-the-endometriosis-health-profile-around-the-world). Hence, efforts to facilitate more assessments of endometriosis-associated HRQoL in women from ethnically diverse backgrounds would be beneficial, as would data from transgender and non-binary patients. Lastly, EHP data from routine clinical practice are lacking. We believe the measures would be of value to clinicians and patients if deployed more widely at aggregate level; however, evidence regarding the meaningfulness of individual scores is uncertain and more research is needed in this area.
Methodological findings
Most of the psychometric studies concluded that the EHPs are reliable, valid, acceptable, and responsive tools and the results of the psychometric analyses appear to support deriving a summary score for the EHP-30 core domain and EHP-5, as previously highlighted by Jones (2001). Given the EHP-5’s brevity, and recent evidence that the core and modular sections are unidimensional and thus efficient to score (Fauconnier et al., 2017), it may be the most appropriate version to use in routine clinical practice, whereas its longer form (EHP-30), which provides more granularity, is more appropriate for research.
While a summary score for the EHP-30 is also possible, its responsiveness has not yet been established and recent evidence suggests the five-domain structure is more robust (Hansen et al., 2022). In addition, providing EHP-30 data at domain, rather than summary, level has been recommended for clinical trials (Vincent et al., 2010). Although we would recommend including the ‘not applicable’ boxes, researchers may sometimes think they are inappropriate and omit them. However, this complicates scoring and interpretation of the EHP data: those patients for whom the EHP domain/item is not relevant may choose not to respond or tick ‘never’, when the issue was actually not relevant.
Most importantly, the responsiveness of the EHP-30 has been demonstrated and emerging evidence suggests the EHP-5 is also responsive. Strikingly, using anchor-based methods, all five EHP-30 studies showed the largest mean changes in the ‘control and powerlessness’ domain post-intervention, followed by ‘pain’. This suggests that women experience the greatest improvements in these domains regardless of the intervention/s. Our findings support the direction of responder thresholds proposed by Pokrzywinski et al. (2020a) of ‘control and powerlessness’ as the largest (-35), followed by ‘pain’ (-30). However, the magnitude of the thresholds differed between studies. The thresholds for comparing medical treatment versus placebo suggested by Pokrzywinski et al. (2020a) seem appropriate; however, these may need to be reduced in the context of other interventions (e.g. surgery), or those samples which include mixed interventions.
The rather surprising finding that ‘control and powerlessness’ is a more salient domain than ‘pain’ perhaps reflects how pain reduction can result in an even greater improvement in patients’ sense of control and power over their endometriosis. Hence, our synthesis concurs with the recommendation of other authors, such as Pokrzywinski et al. (2020a), to strengthen efforts to measure and address the psychological impact of endometriosis. While the EHP-30 pain domain is sometimes chosen as the primary outcome measure in research, we advocate that the ‘control and powerlessness’ domain should be included as a secondary outcome measure in future trials (ideally with other relevant modules). If researchers prefer the brevity of the EHP-5 for clinical trials, we recommend reporting the scores for ‘pain’ and ‘control and powerlessness’ at item level.
The only direct comparisons with generic measures showed that the EHP-30 is overall more responsive than the SF-36 (Jones et al., 2004c) and the EHP-5 more consistently detects treatment changes than the EQ-5D (Aubry et al., 2017). The ASRM and ESHRE currently recommend an 11-point numerical rating scale as the primary outcome in clinical trials assessing endometriosis-associated pain (Vincent et al., 2010). However, some studies continue to use a VAS to measure pain despite recent FDA guidelines (FDA, 2022). Interestingly, all the studies included used (with the exception of Maiorana et al., 2012) classical test theory and not modern psychometric methods, such as Rasch analysis and Item Response Theory. We are re-analysing the EHP-30 using such modern methods to strengthen the psychometric properties of the EHPs, with a focus on the ‘pain’ domain given the FDA’s recommendation regarding VAS.
We identified new instruments specifically designed to measure PROs in endometriosis. Some are disease-specific and the EHP was used as the measure of external validity to aid their development: the Endometriosis Treatment Satisfaction Questionnaire (Deal et al., 2010b); Endometriosis Pain and Bleeding Diary (EPBD) (Deal et al., 2010a); Sexual Activity Questionnaire (SAQ) (Oppenheimer et al., 2019); Endometriosis Daily Pain Impact Diary (Wyrwich et al., 2018); Endometriosis Impact Questionnaire (EIQ) (Moradi et al., 2019), and Stellenbosch Quality of Life questionnaire (Roomaney and Kagee, 2018). The generation of these measures and wide use of the EHP tools possibly reflect best practice guidance over the last 20 years to measure PROs. Other generic PROMS have also been widely used in endometriosis research, e.g. SF-36 (Bourdel et al., 2019; Sima et al., 2021).
The EHPs have also been adapted to measure outcomes for related conditions, such as CPP (e.g. Cheong et al., 2014) and adenomyosis (Andersson et al., 2019), and non-endometriosis treatments such as ART. van der Houwen et al. (2014) suggest changing the header question to ‘how often did you…’ instead of ‘because of your endometriosis, how often did you…’ to facilitate completing the questionnaire in these circumstances, and we are open to modifying the EHPs on reasonable request. However, the FDA may not recognise results derived from a questionnaire that is not designed for the patient group in question. PROMs for these conditions may, therefore, be needed.
Some commentators consider the EHP-30 takes too long to complete (Wickström and Edelstam, 2017; Roomaney and Kagee, 2018). We find this difficult to understand as, on an average, it takes less than 15 min to complete the EHP-30 long form version (core and modular sections) (Nogueira-Silva et al., 2015) and the overall completion rates in the studies identified are high. Other concerns expressed relate to the lack of a domain on ‘vitality’ or ‘support’, and that the EHP pain scale is only symptom-based (Roomaney and Kagee, 2018). In fact, the items are not purely symptom-based as they also reflect the impact that endometriosis-associated pain may have upon day-to-day functioning: for example, attending social events, undertaking jobs around the home, leisure activities or exercising, sleeping, and other daily-life activities.
Limitations
We have attempted to identify and synthesise the EHP literature since the measure was first published in 2001. Whilst we have tried to be as comprehensive as possible, it is possible that some literature was missed—particularly as one paper that did not mention the EHP in the title or abstract was later identified as relevant to the review (Roomaney and Kagee, 2018). Conversely, some papers that used an EHP were not included because the full text was unavailable in English. Thus, the total number of studies that have used the EHP exceeds 139, which is reflected in the larger number of EHP licences granted. It is also important to mention that the original EHP authors were members of the team that undertook this review: clearly their papers were included. To overcome any potential biases that might have ensued during the quality appraisal process, K.B. who had no prior knowledge or affiliation to the EHP measures undertook this process independently.
In recent years, standards and frameworks for appraising the measurement properties of PROMS have been developed, most notably by the COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) group (e.g. Mokkink et al., 2018). As the purpose of this review was to gather and synthesise the literature to-date on the EHP tools generally (not just their measurement properties), we have described the key psychometric properties using the COSMIN taxonomy as a guide, but we have not applied their risk of bias tool to assess the quality of the studies. This is a similar approach to that undertaken in other recent reviews of quality-of-life measures in endometriosis (Bourdel et al., 2019). We recognise this is an important next step and are currently undertaking such an analysis, using the COSMIN risk of bias framework.
However, we did undertake a thorough review of the responsiveness studies, comparing data based not only on type of approach (anchor and distribution-based) but also on the specific nature of the anchor question (general health status vs pain), which is seldom done. We also quality appraised all the studies using the MMAT. While 97% of the studies exceeded the threshold of a low-quality paper, some caution when interpreting the results is needed. Owing to the considerable heterogeneity across the papers, many of the manuscripts did not fit easily into the MMAT predefined categories (e.g. pilot and feasibility studies). It was also difficult to interpret data from some studies because of the non-standardised interpretation of the scoring. In an attempt to decipher the data, the corresponding authors were contacted and asked to clarify their reporting of the EHP data. One author responded and six authors did not respond. Finally, for studies of 12 months or more, we applied a 30% cut-off as our acceptable level. However, among these studies, there was considerable variation in follow-up times with some studies following up for 36 months or longer. As higher drop-out levels are to be expected in studies with lengthier follow-up periods, the MMAT results on this metric should be interpreted with caution.
Conclusions and implications for future research
The EHP measures have been deployed widely since they were first developed in 2001 and further robust psychometric testing continues to support their reliability, validity, responsiveness, and acceptability. While the EHPs have 56 certified translations with others ongoing, further testing of items in the translated (particularly Italian and Norwegian) versions would be beneficial. Testing should also be extended to samples of younger women and those from ethnic minorities and different populations, especially in Africa. The EHPs’ digital availability via REDCap may help such testing and implementation. Future developments by the EHP authors are focusing on undertaking modern psychometric tests on the tools—specifically to improve measurement of endometriosis-associated pain.
Supplementary Material
Contributor Information
Georgina L Jones, Department of Psychology, School of Humanities and Social Sciences, Leeds Beckett University, Leeds, UK.
Kirsty Budds, Department of Psychology, School of Humanities and Social Sciences, Leeds Beckett University, Leeds, UK.
Francesca Taylor, Department of Psychology, School of Humanities and Social Sciences, Leeds Beckett University, Leeds, UK.
Danielle Musson, Department of Psychology, School of Humanities and Social Sciences, Leeds Beckett University, Leeds, UK.
Justin Raymer, Oxford University Innovation Ltd, Oxford, UK.
David Churchman, Oxford University Innovation Ltd, Oxford, UK.
Stephen H Kennedy, Nuffield Department of Women’s and Reproductive Health, University of Oxford, John Radcliffe Hospital, Oxford, UK.
Crispin Jenkinson, Harris Manchester College, University of Oxford, Oxford, UK.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
All the authors contributed to writing the manuscript. G.L.J.: conceived the idea, and assisted with protocol development, data extraction, and analysis. K.B.: undertook the MMAT, and assisted with protocol development, data extraction, and analysis. F.T.: undertook the literature searches and data extraction, and assisted with protocol development. D.M.: assisted with data extraction and analysis. J.R.: assisted with data extraction and analysis. D.C.: assisted with protocol development. S.H.K.: assisted with protocol development, data extraction, and analysis. C.J.: assisted with protocol development, data extraction, and analysis.
Funding
This systematic review was supported by a grant from the Centre for Psychological Research at Leeds Beckett University.
Conflict of interest
G.L.J., S.H.K., and C.J. are the original developers of the EHP instruments. They receive royalties from commercial licensing, which are facilitated by Oxford University Innovation Limited (a wholly owned subsidiary of the University of Oxford) of which J.R. is an employee. D.C. was also an employee at Oxford University Innovation Limited at the time of working on and producing this review. K.B., D.M., and F.T. have no conflicts of interest to declare.
References
- Ács N, O’Brien C, Jiang P, Burke J, Jimenez R, Garner E, Chwalisz K.. Treatment of endometriosis-associated pain with Elagolix, an oral GnRH antagonist: results from a phase 2, randomized controlled study. J Endometr Pelvic Pain Disord 2015;7:56–62. [Google Scholar]
- Agarwal SK, Soliman AM, Pokrzywinski RM, Snabes MC, Coyne KS.. Clinically meaningful reduction in dyspareunia is associated with significant improvements in health-related quality of life among women with moderate to severe pain associated with endometriosis: a pooled analysis of two phase III trials of Elagolix. J Sex Med 2020;17:2427–2433. [DOI] [PubMed] [Google Scholar]
- Ahn AC, Schnyer R, Conboy L, Laufer MR, Wayne PM.. Electrodermal measures of Jing-Well points and their clinical relevance in endometriosis-related chronic pelvic pain. J Altern Complement Med 2009;15:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Azemi M, Jones G, Sirkeci F, Walters S, Houdmont M, Ledger W.. Immediate and delayed add-back hormonal replacement therapy during ultra long GnRH agonist treatment of chronic cyclical pelvic pain: GnRH agonist and add-back therapy for chronic pelvic pain. BJOG 2009;116:1646–1656. [DOI] [PubMed] [Google Scholar]
- Ali Nor S, Ibrahim S, Elbahlawan G, Abd El-LattifMontaser W.. Assessment the health-related quality of life among endometriosis women in maternal and child health centers in Damietta. Port Said Sci J Nurs 2020;7:204–227. [Google Scholar]
- Alshehre SM, Duffy S, Jones G, Ledger WL, Metwally M.. A prospective, single-centre, single-arm, open label study of the long term use of a gonadotropin releasing hormone agonist (Triptorelin SR, 11.25 mg) in combination with Tibolone add-back therapy in the management of chronic cyclical pelvic pain. Reprod Biol Endocrinol 2020;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JK, Khan Z, Weaver AL, Vaughan LE, Gemzell‐Danielsson K, Stewart EA.. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: a pilot study. Acta Obstet Gynecol Scand 2019;98:1341–1350. [DOI] [PubMed] [Google Scholar]
- Apers S, Dancet EAF, Aarts JWM, Kluivers KB, D’Hooghe TM, Nelen WLDM.. The association between experiences with patient-centred care and health-related quality of life in women with endometriosis. Reprod Biomed Online 2018;36:197–205. [DOI] [PubMed] [Google Scholar]
- Archer DF, Soliman AM, Agarwal SK, Taylor HS.. Elagolix in the treatment of endometriosis: impact beyond pain symptoms. Clin Med Insights Reprod Health 2020;14:263349412096451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion K, Orr NL, Noga H, Allaire C, Williams C, Bedaiwy MA, Yong PJ.. A quantitative analysis of sleep quality in women with endometriosis. J Womens Health (Larchmt) 2020;29:1209–1215. [DOI] [PubMed] [Google Scholar]
- Aromataris E, Pearson A.. The systematic review: an overview. Am J Nurs 2014;114:53–58. [DOI] [PubMed] [Google Scholar]
- Aubry G, Panel P, Thiollier G, Huchon C, Fauconnier A.. Measuring health-related quality of life in women with endometriosis: comparing the clinimetric properties of the Endometriosis Health Profile-5 (EHP-5) and the EuroQol-5D (EQ-5D). Hum Reprod 2017;32:1258–1269. [DOI] [PubMed] [Google Scholar]
- Bao C, Noga H, Allaire C, Williams C, Bedaiwy MA, Sadownik LA, Brotto LA, Smith KB, Yong PJ.. Provoked vestibulodynia in women with pelvic pain. Sex Med 2019;7:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra F, Scala C, Leone Roberti Maggiore U, Ferrero S.. Long-term administration of dienogest for the treatment of pain and intestinal symptoms in patients with rectosigmoid endometriosis. J Clin Med 2020;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Smith P. An investigation into the surgical treatment of endometriosis. Doctoral Thesis. Guildford: University of Surrey, 2010. https://openresearch.surrey.ac.uk/esploro/outputs/99516697002346.
- Bourdel N, Chauvet P, Billone V, Douridas G, Fauconnier A, Gerbaud L, Canis M.. Systematic review of quality of life measures in patients with endometriosis. PLoS One 2019;14:e0208464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletti C, Coccia ME, Battistoni S, Borini A.. Endometriosis and infertility. J Assist Reprod Genet 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B, Dmowski WP, O’Brien C, Jiang P, Burke J, Jimenez R, Garner E, Chwalisz K.. Elagolix, an oral GnRH antagonist, versus subcutaneous depot medroxyprogesterone acetate for the treatment of endometriosis: effects on bone mineral density. Reprod Sci 2014;21:1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr B, Giudice L, Dmowski WP, O’Brien C, Jiang P, Burke J, Jimenez R, Hass S, Fuldeore M, Chwalisz K.. Elagolix, an oral GnRH antagonist for endometriosis-associated pain: a randomized controlled study. J Endometr Pelvic Pain Disord 2013;5:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho N, Margatho D, Cursino K, Benetti-Pinto CL, Bahamondes L.. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: randomized clinical trial. Fertil Steril 2018;110:1129–1136. [DOI] [PubMed] [Google Scholar]
- Chauvet P, Auclair C, Mourgues C, Canis M, Gerbaud L, Bourdel N.. Psychometric properties of the French version of the Endometriosis Health Profile-30, a health-related quality of life instrument. J Gynecol Obstet Hum Reprod 2017;46:235–242. [DOI] [PubMed] [Google Scholar]
- Chauvet P, Guiguet-Auclair C, Comptour A, Denouël A, Gerbaud L, Canis M, Bourdel N.. Feelings and expectations in endometriosis: analysis of open comments from a cohort of endometriosis patients. J Gynecol Obstet Hum Reprod 2018;47:281–287. [DOI] [PubMed] [Google Scholar]
- Cheong Y, Saran M, Hounslow JW, Reading IC.. Are pelvic adhesions associated with pain, physical, emotional and functional characteristics of women presenting with chronic pelvic pain? A cluster analysis. BMC Womens Health 2018;18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YC, Reading I, Bailey S, Sadek K, Ledger W, Li TC.. Should women with chronic pelvic pain have adhesiolysis? BMC Womens Health 2014;14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma S, Benedetto C.. Classification algorithm of patients with endometriosis: proposal for tailored management. Adv Clin Exp Med 2020;29:615–622. [DOI] [PubMed] [Google Scholar]
- Cosma S, Salgarello M, Ceccaroni M, Gorgoni G, Riboni F, La Paglia E, Danese S, Benedetto C.. Accuracy of a new diagnostic tool in deep infiltrating endometriosis: positron emission tomography-computed tomography with 16α-[18F]fluoro-17β-estradiol: endometriosis: radiotracer diagnosis. J Obstet Gynaecol Res 2016;42:1724–1733. [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Luciano A, Ray A, Bergqvist A.. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod 2006;21:248–256. [DOI] [PubMed] [Google Scholar]
- de Freitas Fonseca M, Aragao LC, Sessa FV, Dutra de Resende JA, Crispi CP.. Interrelationships among endometriosis-related pain symptoms and their effects on health-related quality of life: a sectional observational study. Obstet Gynecol Sci 2018;61:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Hera-Lazaro CM, Muñoz-González JL, Perez RO, Vellido-Cotelo R, Díez-Álvarez A, Muñoz-Hernando L, Alvarez-Conejo C, Jiménez-López JS.. Radical surgery for endometriosis: analysis of quality of life and surgical procedure. Clin Med Insights Womens Health 2016;9:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa TR, de Souza BC, Zomkowisk K, da Rosa PC, Sperandio FF.. The effect of acupuncture on pain, dyspareunia, and quality of life in Brazilian women with endometriosis: a randomized clinical trial. Complement Ther Clin Pract 2016;25:114–121. [Google Scholar]
- Deal LS, DiBenedetti DB, Williams VS, Fehnel SE.. The development and validation of the daily electronic Endometriosis Pain and Bleeding Diary. Health Qual Life Outcomes 2010a;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal LS, Williams VSL, DiBenedetti DB, Fehnel SE.. Development and psychometric evaluation of the Endometriosis Treatment Satisfaction Questionnaire. Qual Life Res 2010b;19:899–905. [DOI] [PubMed] [Google Scholar]
- Delbos L, Bouet PE, Catala L, Lefebvre C, Teyssedou C, Descamps P, Legendre G.. Surgery using plasma energy for deep endometriosis: a quality of life assessment. J Gynecol Obstet Hum Reprod 2018;47:359–364. [DOI] [PubMed] [Google Scholar]
- Department of Health & Social Care. Our Vision for the Women’s Health Strategy for England. 2021. https://www.gov.uk/government/publications/womens-health-strategy-for-england/womens-health-strategy-for-england. [Google Scholar]
- Dettori JR. Loss to follow up. Evid Based Spine Care J 2011;2:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MP, Carr B, Dmowski WP, Koltun W, O’Brien C, Jiang P, Burke J, Jimenez R, Garner E, Chwalisz K.. Elagolix treatment for endometriosis-associated pain: results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod Sci 2014;21:363–371. [DOI] [PubMed] [Google Scholar]
- Donnez J, Taylor HS, Taylor RN, Akin MD, Tatarchuk TF, Wilk K, Gotteland JP, Lecomte V, Bestel E.. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone–antagonist: a randomized clinical trial. Fertil Steril 2020;114:44–55. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Dong L, Merz M, Kirsch B, Francuski M, Böttcher B, Roman H, Suvitie P, Hlavackova O, Gude K. et al. Dienogest 2 mg daily in the treatment of adolescents with clinically suspected endometriosis: the VISanne Study to Assess Safety in ADOlescents. J Pediatr Adolesc Gynecol 2017;30:560–567. [DOI] [PubMed] [Google Scholar]
- Egekvist AG, Marinovskij E, Forman A, Kesmodel US, Graumann O, Seyer‐Hansen M.. Conservative treatment of rectosigmoid endometriosis: a prospective study. Acta Obstet Gynecol Scand 2019;98:1139–1147. [DOI] [PubMed] [Google Scholar]
- Ekin M, Kaya C, Erdoğan ŞV, Bahçeci E, Baghaki S, Yaşar L.. The effect of new cross linked hyaluronan gel on quality of life of patients after deep infiltrating endometriosis surgery: a randomized controlled pilot study. J Obstet Gynaecol 2021;41:263–268. [DOI] [PubMed] [Google Scholar]
- Ekine AA, Fulop I, Rucz A, Koppan A, Siklos P, Koppan M.. The benefits of radical laparoscopic surgery and a modified Endometriosis Health Profile-36 (EHP-36) on quality of life. J Women’s Health Dev 2020;3:379–397. [Google Scholar]
- Fauconnier A, Huchon C, Chaillou L, Aubry G, Renouvel F, Panel P.. Development of a French version of the Endometriosis Health Profile 5 (EHP-5): cross-cultural adaptation and psychometric evaluation. Qual Life Res 2017;26:213–220. [DOI] [PubMed] [Google Scholar]
- Ferrero S, Scala C, Ciccarelli S, Vellone VG, Barra F.. Treatment of rectovaginal endometriosis with the etonogestrel-releasing contraceptive implant. Gynecol Endocrinol 2020;36:540–544. [DOI] [PubMed] [Google Scholar]
- Flanagin A, Frey T, Christiansen SL; AMA Manual of Style Committee. Updated guidance on the reporting of race and ethnicity in medical and science journals. Jama 2021;326:621–627. [DOI] [PubMed] [Google Scholar]
- Florentino AVdA, Pereira AMG, Martins JA, Lopes RGC, Arruda RM.. Quality of life assessment by the Endometriosis Health Profile (EHP-30) questionnaire prior to treatment for ovarian endometriosis in Brazilian women. Rev Bras Ginecol Obstet 2019;41:548–554. [DOI] [PubMed] [Google Scholar]
- Flores RC, Lara EB, Corral LCQ, Chaib RAI, de la O, Pérez LO, Díaz OAG, Flores RC.. Quality of life in women with endometriosis pelvic pain treated with the levonorgestrel-releasing intrauterine system. Open J Obstet Gynecol 2015;05:167–172. [Google Scholar]
- Flower A, Lewith GT, Little P.. A feasibility study exploring the role of Chinese herbal medicine in the treatment of endometriosis. J Altern Complement Med 2011;17:691–699. [DOI] [PubMed] [Google Scholar]
- Fourquet J, Báez L, Figueroa M, Iriarte RI, Flores I.. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 2011;96:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl F, Riedl D, Fessler S, Wildt L, Walter M, Richter R, Schüßler G, Böttcher B.. Impact of endometriosis on quality of life, anxiety, and depression: an Austrian perspective. Arch Gynecol Obstet 2015;292:1393–1399. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Helzlsouer KJ, Audlin KM, Miller C, MacDonald R, Johnston M, Barrueto FF.. Change in pain and quality of life among women enrolled in a trial examining the use of narrow band imaging during laparoscopic surgery for suspected endometriosis. J Minim Invasive Gynecol 2015;22:1208–1214. [DOI] [PubMed] [Google Scholar]
- Ghai V, Jan H, Shakir F, Kent A.. Identifying preoperative factors associated with nonresponders in women undergoing comprehensive surgical treatment for endometriosis. J Minim Invasive Gynecol 2020;27:141–147. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Collins F, De Leo B, Horne AW, Saunders PTK.. Pelvic pain correlates with peritoneal macrophage abundance not endometriosis. Reprod Fertil 2021;2:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, As-Sanie S, Arjona Ferreira JC, Becker CM, Abrao MS, Lessey BA, Brown E, Dynowski K, Wilk K, Li Y. et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022;399:2267–2279. [DOI] [PubMed] [Google Scholar]
- Gonçalves AV, Barros NF, Bahamondes L.. The practice of Hatha yoga for the treatment of pain associated with endometriosis. J Altern Complement Med 2017;23:45–52. [DOI] [PubMed] [Google Scholar]
- González-Echevarría AM, Rosario E, Acevedo S, Flores I.. Impact of coping strategies on quality of life of adolescents and young women with endometriosis. J Psychosom Obstet Gynaecol 2019;40:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshtasebi A, Nematollahzadeh M, Hariri FZ, Montazeri A.. The short form endometriosis health profile (EHP-5): translation and validation study of the Iranian version. J Ovarian Res 2011;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granese R, Perino A, Calagna G, Saitta S, De Franciscis P, Colacurci N, Triolo O, Cucinella G.. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand 2015;94:637–645. [DOI] [PubMed] [Google Scholar]
- Grundström H, Rauden A, Olovsson M.. Cross-cultural adaptation of the Swedish version of Endometriosis Health Profile-30. J Obstet Gynaecol 2020a;40:969–973. [DOI] [PubMed] [Google Scholar]
- Grundström H, Rauden A, Wikman P, Olovsson M.. Psychometric evaluation of the Swedish version of the 30-item endometriosis health profile (EHP-30). BMC Womens Health 2020b;20:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KE, Kesmodel US, Baldursson EB, Kold M, Forman A.. Visceral syndrome in endometriosis patients. Eur J Obstet Gynecol Reprod Biol 2014;179:198–203. [DOI] [PubMed] [Google Scholar]
- Hansen KE, Kesmodel US, Baldursson EB, Schultz R, Forman A.. The influence of endometriosis-related symptoms on work life and work ability: a study of Danish endometriosis patients in employment. Eur J Obstet Gynecol Reprod Biol 2013;169:331–339. [DOI] [PubMed] [Google Scholar]
- Hansen KE, Kesmodel US, Kold M, Forman A.. Long-term effects of mindfulness-based psychological intervention for coping with pain in endometriosis: a six-year follow-up on a pilot study. Nord Psychol 2017;69:100–109. [Google Scholar]
- Hansen KE, Lambek R, Røssaak K, Egekvist AG, Marschall H, Forman A, Kesmodel US.. Health-related quality of life in women with endometriosis: psychometric validation of the Endometriosis Health Profile 30 questionnaire using confirmatory factor analysis. Hum Reprod Open 2022;2022:hoab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon M-P, Griffiths F, Nicolau B, O’Cathain A. et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf 2018;34:285–291. [Google Scholar]
- Jenkinson C, Kennedy S, Jones G.. Evaluation of the American version of the 30-item Endometriosis Health Profile (EHP-30). Qual Life Res 2008;17:1147–1152. [DOI] [PubMed] [Google Scholar]
- Jia SZ, Leng JH, Sun PR, Lang JH.. Translation and psychometric evaluation of the simplified Chinese-version Endometriosis Health Profile-30. Hum Reprod 2013;28:691–697. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Kennedy S.. Development of the short form Endometriosis Health Profile Questionnaire: the EHP-5. Qual Life Res 2004a;13:695–704. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Kennedy S.. The impact of endometriosis upon quality of life: a qualitative analysis. J Psychosom Obstet Gynaecol 2004b;25:123–133. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Kennedy S.. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual Life Res 2004c;13:705–713. [DOI] [PubMed] [Google Scholar]
- Jones G, Jenkinson C, Taylor N, Mills A, Kennedy S.. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the Endometriosis Health Profile Questionnaire. Hum Reprod 2006;21:2686–2693. [DOI] [PubMed] [Google Scholar]
- Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C.. Development of an endometriosis quality-of-life instrument: the Endometriosis Health Profile-30. Obstet Gynecol 2001;98:258–264. [DOI] [PubMed] [Google Scholar]
- Jones GL. The measurement of health-related quality of life in women with endometriosis. D.Phil Thesis. University of Oxford, 2001.
- Jones GL, Kennedy SH, Jenkinson C.. Health-related quality of life measurement in women with common benign gynecologic conditions: a systematic review. Am J Obstet Gynecol 2002;187:501–511. [DOI] [PubMed] [Google Scholar]
- Kent A, Shakir F, Rockall T, Haines P, Pearson C, Rae-Mitchell W, Jan H.. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol 2016;23:526–534. [DOI] [PubMed] [Google Scholar]
- Khan KS, Tryposkiadis K, Tirlapur SA, Middleton LJ, Sutton AJ, Priest L, Ball E, Balogun M, Sahdev A, Roberts T. et al. MRI versus laparoscopy to diagnose the main causes of chronic pelvic pain in women: a test-accuracy study and economic evaluation. Health Technol Assess 2018;22:1–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaverdi S, Alebouyeh MR, Sadegi K, Mehdizadehkashi A, Kaveh M, Entezari SR, Mirzaei H, Khaledi M, Khodaverdi M.. Superior hypogastric plexus block as an effective treatment method for endometriosis-related chronic pelvic pain: an open-label pilot clinical trial. J Obstet Gynaecol 2021;41:966–971. [DOI] [PubMed] [Google Scholar]
- Khong SY, Lam A, Luscombe G.. Is the 30-item Endometriosis Health Profile (EHP-30) suitable as a self-report health status instrument for clinical trials? Fertil Steril 2010;94:1928–1932. [DOI] [PubMed] [Google Scholar]
- Kold M, Hansen T, Vedsted-Hansen H, Forman A.. Mindfulness-based psychological intervention for coping with pain in endometriosis. Nord Psychol 2012;64:2–16. [Google Scholar]
- Leone Roberti Maggiore U, Bizzarri N, Scala C, Tafi E, Siesto G, Alessandri F, Ferrero S.. Symptomatic endometriosis of the posterior cul-de-sac is associated with impaired sleep quality, excessive daytime sleepiness and insomnia: a case–control study. Eur J Obstet Gynecol Reprod Biol 2017;209:39–43. [DOI] [PubMed] [Google Scholar]
- Leyland N, Taylor HS, Archer DF, Peloso PM, Soliman AM, Palac HL, Martinez M, Abrao MS.. Elagolix reduced dyspareunia and improved health-related quality of life in premenopausal women with endometriosis-associated pain. J Endometr Pelvic Pain Disord 2019;11:171–180. [Google Scholar]
- Maiorana A, Scafidi Fonti GM, Audino P, Rosini R, Alio L, Oliveri AM, Milito AM.. The role of EHP-30 as specific instrument to assess the quality of life of Italian women with endometriosis. Minerva Ginecol 2012;64:231–238. [PubMed] [Google Scholar]
- Marí-Alexandre J, García-Oms J, Agababyan C, Belda-Montesinos R, Royo-Bolea S, Varo-Gómez B, Díaz-Sierra C, González-Cantó E, Gilabert-Estellés J.. Toward an improved assessment of quality of life in endometriosis: evaluation of the Spanish version of the Endometriosis Health Profile 30. J Psychosom Obstet Gynaecol 2022;43:251–257. [DOI] [PubMed] [Google Scholar]
- Martins G, Ferreira M, Vilaça M, Ferreira H, Osório F, Nogueira-Silva C, Pereira MG.. Quality of life and sexual satisfaction in women with endometriosis: the moderator role of symptom severity. Psychol Sex 2022;13:952–964. [Google Scholar]
- Matasariu RD, Mihaila A, Iacob M, Dumitrascu I, Onofriescu M, Crumpei Tanasa I, Vulpoi C.. Psycho-social aspects of quality of life in women with endometriosis. Acta Endocrinol (Buchar) 2017;13:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen M, Egekvist AG, Kesmodel US, Knudsen UB, Seyer‐Hansen M.. Similar evolution of pain symptoms and quality of life in women with and without endometriosis undergoing assisted reproductive technology (ART). Acta Obstet Gynecol Scand 2019;98:77–85. [DOI] [PubMed] [Google Scholar]
- McPeak AE, Allaire C, Williams C, Albert A, Lisonkova S, Yong PJ.. Pain catastrophizing and pain health-related quality-of-life in endometriosis. Clin J Pain 2018;34:349–356. [DOI] [PubMed] [Google Scholar]
- Meuleman C, D’Hoore A, Van Cleynenbreugel B, Beks N, D’Hooghe T.. Outcome after multidisciplinary CO2 laser laparoscopic excision of deep infiltrating colorectal endometriosis. Reprod Biomed Online 2009;18:282–289. [DOI] [PubMed] [Google Scholar]
- Meuleman C, Tomassetti C, D’Hoore A, Buyens A, Van Cleynenbreugel B, Fieuws S, Penninckx F, Vergote I, D’Hooghe T.. Clinical outcome after CO2 laser laparoscopic radical excision of endometriosis with colorectal wall invasion combined with laparoscopic segmental bowel resection and reanastomosis. Hum Reprod 2011;26:2336–2343. [DOI] [PubMed] [Google Scholar]
- Meuleman C, Tomassetti C, Wolthuis A, Van Cleynenbreugel B, Laenen A, Penninckx F, Vergote I, D'Hoore A, D'Hooghe T.. Clinical outcome after radical excision of moderate—severe endometriosis with or without bowel resection and reanastomosis: a prospective cohort study. Ann Surg 2014;259:522–531. [DOI] [PubMed] [Google Scholar]
- Middleton LJ, Daniels JP, Weckesser A, Bhattacharya S; PRE-EMPT Trial Collaborative Group. Preventing recurrence of endometriosis by means of long-acting progestogen therapy (PRE-EMPT): report of an internal pilot, multi-arm, randomised controlled trial incorporating flexible entry design and adaption of design based on feasibility of recruitment. Trials 2017;18:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minas V, Dada T.. Laparoscopic treatment of endometriosis and effects on quality of life: a retrospective study using the short form EHP-5 endometriosis specific questionnaire. J Obstet Gynaecol 2014;34:336–340. [DOI] [PubMed] [Google Scholar]
- Mira TAA, Giraldo PC, Yela DA, Benetti-Pinto CL.. Effectiveness of complementary pain treatment for women with deep endometriosis through Transcutaneous Electrical Nerve Stimulation (TENS): Randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2015;194:106. [DOI] [PubMed] [Google Scholar]
- Mira TAA, Yela DA, Podgaec S, Baracat EC, Benetti-Pinto CL.. Hormonal treatment isolated versus hormonal treatment associated with electrotherapy for pelvic pain control in deep endometriosis: randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 2020;255:134–141. [DOI] [PubMed] [Google Scholar]
- Misra G, Sim J, El‐Gizawy Z, Jerreat S, Coia T, Ritchie J, O’Brien S.. Laparoscopic ablation or excision with helium thermal coagulator versus electrodiathermy for the treatment of mild‐to‐moderate endometriosis: randomised controlled trial. BJOG 2020;127:1576–1535. [DOI] [PubMed] [Google Scholar]
- Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, Terwee CB.. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res 2018;27:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D.. The Endometriosis Impact Questionnaire (EIQ): a tool to measure the long-term impact of endometriosis on different aspects of women’s lives. BMC Womens Health 2019;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotti M, Sozzi F, Remorgida V, Venturini PL, Ferrero S.. Dienogest in women with persistent endometriosis-related pelvic pain during norethisterone acetate treatment. Eur J Obstet Gynecol Reprod Biol 2014;183:188–192. [DOI] [PubMed] [Google Scholar]
- Mundo-López A, Ocón-Hernández O, San-Sebastián AP, Galiano-Castillo N, Rodríguez-Pérez O, Arroyo-Luque MS, Arroyo-Morales M, Cantarero-Villanueva I, Fernández-Lao C, Artacho-Cordón F.. Contribution of chronic fatigue to psychosocial status and quality of life in Spanish women diagnosed with endometriosis. Int J Environ Res Public Health 2020;17:3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng N, Wahl K, Orr NL, Noga H, Williams C, Allaire C, Bedaiwy MA, Yong PJ.. Endometriosis and negative perception of the medical profession. J Obstet Gynaecol Can 2020;42:248–255. [DOI] [PubMed] [Google Scholar]
- Nogueira-Silva C, Costa P, Martins C, Barata S, Alho C, Calhaz-Jorge C, Osório F.. Validation of the Portuguese version of EHP-30 (The Endometriosis Health Profile-30). Acta Med Port 2015;28:347–356. [DOI] [PubMed] [Google Scholar]
- Nojomi M, Bijari B, Akhbari R, Kashanian M.. The assessment of reliability and validity of Persian version of the Endometriosis Health Profile (EHP-30). Iran J Med Sci 2011;36:84–89. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer A, Panel P, Rouquette A, Du Cheyron J, Deffieux X, Fauconnier A.. Validation of the Sexual Activity Questionnaire in women with endometriosis. Hum Reprod 2019;34:824–833. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Gemmill JAL, Sinaii N, Stegmann B, Khachikyan I, Chrousos G, Segars J, Stratton P.. Hypothalamic-pituitary-adrenal axis responses in women with endometriosis-related chronic pelvic pain. Reprod Sci 2020;27:1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuga Y, Seki Y, Tanimoto M, Kusumoto T, Kudou K, Terakawa N.. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain in a dose–response manner: a randomized, double-blind, placebo-controlled study. Fertil Steril 2021;115:397–405. [DOI] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip CA, Warembourg S, Dairien M, Lefevre C, Gelet A, Chavrier F, Guillen N, Tonoli H, Maissiat E, Lafon C. et al. Transrectal high‐intensity focused ultrasound (HIFU) for management of rectosigmoid deep infiltrating endometriosis: results of phase‐I clinical trial. Ultrasound Obstet Gynecol 2020;56:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinot-Monange A, Moisset X, Chauvet P, Gremeau AS, Comptour A, Canis M, Pereira B, Bourdel N.. Repetitive transcranial magnetic stimulation therapy (rTMS) for endometriosis patients with refractory pelvic chronic pain: a pilot study. J Clin Med 2019;8:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrzywinski R, Soliman AM, Chen J, Snabes MC, Taylor HS, Coyne KS.. Responsiveness evaluation and recommendation for responder thresholds for endometriosis health profile-30: analysis of two phase III clinical trials. J Womens Health (Larchmt) 2020a;29:253–261. [DOI] [PubMed] [Google Scholar]
- Pokrzywinski RM, Soliman AM, Chen J, Snabes MC, Agarwal SK, Coddington C, Coyne KS.. Psychometric assessment of the health-related productivity questionnaire (HRPQ) among women with endometriosis. Expert Rev Pharmacoecon Outcomes Res 2020b;20:531–539. [DOI] [PubMed] [Google Scholar]
- Pokrzywinski RM, Soliman AM, Chen J, Snabes MC, Coyne KS, Surrey ES, Taylor HS.. Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: analysis of 2 phase III clinical trials. Am J Obstet Gynecol 2020c;222:592.e1. [DOI] [PubMed] [Google Scholar]
- Popay J, Roberts HM, Sowden AJ, Petticrew M, Arai L, Rodgers M, Britten N.. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme. Version 1. 2006. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf.
- Protopapas A, Giannoulis G, Chatzipapas I, Athanasiou S, Grigoriadis T, Haidopoulos D, Loutradis D, Antsaklis A.. Posterior deep infiltrating endometriotic nodules: operative considerations according to lesion size, location, and geometry, during one’s learning curve. ISRN Obstet Gynecol 2014;2014:853902–853908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK.. Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes 2006;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindos NB, Fulcher IR, Donnellan NM.. Pain and quality of life after laparoscopic excision of endometriosis. J Minim Invasive Gynecol 2020;27:1610–1617.e1. [DOI] [PubMed] [Google Scholar]
- Roomaney R., Kagee A.. The construction and validation of the Stellenbosch Endometriosis Quality of life measure (SEQOL). Health Care Women Intl 2018;39:1123–1139. [DOI] [PubMed] [Google Scholar]
- Roomaney R, Kagee A, Heylen S.. Biopsychosocial predictors of symptoms of depression in a sample of South African women diagnosed with endometriosis. Health Care Women Int 2020;41:308–329. [DOI] [PubMed] [Google Scholar]
- Rush G, Misajon R.. Examining subjective wellbeing and health-related quality of life in women with endometriosis. Health Care Women Int 2018;39:303–321. [DOI] [PubMed] [Google Scholar]
- Sandström A, Bixo M, Johansson M, Bäckström T, Turkmen S.. Effect of hysterectomy on pain in women with endometriosis: a population‐based registry study. BJOG 2020;127:1628–1635. [DOI] [PubMed] [Google Scholar]
- Sayed HAE, Aboud SAHH.. Effect of an educational intervention on quality of life and sexual function in women with endometriosis. Int J Secur Netw 2018;3:127. [Google Scholar]
- Scala C, Leone Roberti Maggiore U, Barra F, Venturini PL, Ferrero S.. Norethindrone acetate versus extended-cycle oral contraceptive (Seasonique®) in the treatment of endometriosis symptoms: a prospective open-label comparative study. Eur J Obstet Gynecol Reprod Biol 2018;222:89–94. [DOI] [PubMed] [Google Scholar]
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A.. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril 2006;85:314–325. [DOI] [PubMed] [Google Scholar]
- Selcuk S, Sahin S, Demirci O, Aksoy B, Eroglu M, Ay P, Cam C.. Translation and validation of the Endometriosis Health Profile (EHP-5) in patients with laparoscopically diagnosed endometriosis. Eur J Obstet Gynecol Reprod Biol 2015;185:41–44. [DOI] [PubMed] [Google Scholar]
- Shum LK, Bedaiwy MA, Allaire C, Williams C, Noga H, Albert A, Lisonkova S, Yong PJ.. Deep dyspareunia and sexual quality of life in women with endometriosis. Sex Med 2018;6:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima R-M, Pleş L, Socea B, Sklavounos P, Negoi I, Stănescu A-D, Iordache I-I, Hamoud BH, Radosa MP, Juhasz-Boess I. et al. Evaluation of the SF-36 questionnaire for assessment of the quality of life of endometriosis patients undergoing treatment: a systematic review and meta-analysis. Exp Ther Med 2021;22:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen SM, Strømberg C, Zoffmann V, Hartwell D, Olesen ML.. About me as a person not only the disease—piloting Guided Self‐Determination in an outpatient endometriosis setting. Scand J Caring Sci 2020;34:1017–1027. [DOI] [PubMed] [Google Scholar]
- Škegro B, Bjedov S, Mikuš M, Mustač F, Lešin J, Matijević V, Ćorić M, Elveđi Gašparović V, Medić F, Sokol Karadjole V.. Endometriosis, pain and mental health. Psychiatr Danub 2021;33:;632–636. [PubMed] [Google Scholar]
- Soliman AM, Coyne KS, Zaiser E, Castelli-Haley J, Fuldeore MJ.. The burden of endometriosis symptoms on health-related quality of life in women in the United States: a cross-sectional study. J Psychosom Obstet Gynaecol 2017;38:238–248. [DOI] [PubMed] [Google Scholar]
- Soliman AM, Singh S, Rahal Y, Robert C, Defoy I, Nisbet P, Leyland N.. Cross-sectional survey of the impact of endometriosis symptoms on health-related quality of life in Canadian women. J Obstet Gynaecol Can 2020;42:1330–1338. [DOI] [PubMed] [Google Scholar]
- Soto E, Luu TH, Liu X, Magrina JF, Wasson MN, Einarsson JI, Cohen SL, Falcone T.. Laparoscopy vs. Robotic Surgery for Endometriosis (LAROSE): a multicenter, randomized, controlled trial. Fertil Steril 2017;107:996–1002.e3. [DOI] [PubMed] [Google Scholar]
- Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shah J.. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet Gynecol 2015;125:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey E, Taylor HS, Giudice L, Lessey BA, Abrao MS, Archer DF, Diamond MP, Johnson NP, Watts NB, Gallagher JC. et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol 2018;132:147–160. [DOI] [PubMed] [Google Scholar]
- Tan BK, Maillou K, Mathur RS, Prentice A.. A retrospective review of patient-reported outcomes on the impact on quality of life in patients undergoing total abdominal hysterectomy and bilateral salpingo-oophorectomy for endometriosis. Eur J Obstet Gynecol Reprod Biol 2013;170:533–538. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Tokita Y, Ota I, Yamane E, Komatsu H, Azuma Y, Sato E, Endo Y, Sunada H, Harada T.. Efficacy of Tokishakuyakusan add‐on therapy with low‐dose oral contraceptive pills on endometriosis patients with dysmenorrhea. J Obstet Gynaecol Res 2020;46:2280–2286. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB. et al. Treatment of endometriosis-associated pain with Elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28–40. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Soliman AM, Johns B, Pokrzywinski RM, Snabes M, Coyne KS.. Health-related quality of life improvements in patients with endometriosis treated with Elagolix. Obstet Gynecol 2020;136:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techatraisak K, Hestiantoro A, Ruey S, Banal-Silao MJ, Kim MR, Seong SJ, Thaufik S, Ahlers C, Shin SY, Lee BS.. Effectiveness of dienogest in improving quality of life in Asian women with endometriosis (ENVISIOeN): interim results from a prospective cohort study under real-life clinical practice. BMC Women’s Health 2019;19:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet AAEM, Alshehri MA.. Effect of pulsed high-intensity laser therapy on pain, adhesions, and quality of life in women having endometriosis: a randomized controlled trial. Photomed Laser Surg 2018;36:363–369. [DOI] [PubMed] [Google Scholar]
- Tiringer D, Pedrini AS, Gstöttner M, Husslein H, Küssel L, Perricos A, Wenzl R.. Evaluation of quality of life in endometriosis patients before and after surgical treatment using the EHP30 Questionnaire. Open Access J Clin Surg 2020;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco LC, Scaldaferri F, Chiantera V, Cianci S, Ercoli A, Fagotti A, Fanfani F, Ferrandina G, Nicolotti N, Tamburrano A. et al. Long-term evaluation of quality of life and gastrointestinal well-being after segmental colo-rectal resection for deep infiltrating endometriosis (ENDO-RESECT QoL). Arch Gynecol Obstet 2020;301:217–228. [DOI] [PubMed] [Google Scholar]
- van Aken M, Oosterman J, van Rijn T, Ferdek M, Ruigt G, Kozicz T, Braat D, Peeters A, Nap A.. Hair cortisol and the relationship with chronic pain and quality of life in endometriosis patients. Psychoneuroendocrinology 2018;89:216–222. [DOI] [PubMed] [Google Scholar]
- van Aken MAW, Oosterman JM, van Rijn CM, Ferdek MA, Ruigt GSF, Peeters BWMM, Braat DDM, Nap AW.. Pain cognition versus pain intensity in patients with endometriosis: toward personalized treatment. Fertil Steril 2017;108:679–686. [DOI] [PubMed] [Google Scholar]
- van de Burgt TJM, Hendriks JCM, Kluivers KB.. Quality of life in endometriosis: evaluation of the Dutch-version Endometriosis Health Profile–30 (EHP-30). Fertil Steril 2011;95:1863–1865. [DOI] [PubMed] [Google Scholar]
- van de Burgt TJM, Kluivers KB, Hendriks JCM.. Responsiveness of the Dutch Endometriosis Health Profile-30 (EHP-30) questionnaire. Eur J Obstet Gynecol Reprod Biol 2013;168:92–94. [DOI] [PubMed] [Google Scholar]
- van der Houwen LEE, Schreurs AMF, Schats R, Lambalk CB, Hompes PGA, Mijatovic V.. Patient satisfaction concerning assisted reproductive technology treatments in moderate to severe endometriosis. Gynecol Endocrinol 2014;30:798–803. [DOI] [PubMed] [Google Scholar]
- van Poll M, van Barneveld E, Aerts L, Maas JWM, Lim AC, de Greef BTA, Bongers MY, van Hanegem N.. Endometriosis and sexual quality of life. Sex Med 2020;8:532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellini P, Frattaruolo MP, Somigliana E, Jones GL, Consonni D, Alberico D, Fedele L.. Surgical versus low-dose progestin treatment for endometriosis-associated severe deep dyspareunia II: effect on sexual functioning, psychological status and health-related quality of life. Hum Reprod 2013;28:1221–1230. [DOI] [PubMed] [Google Scholar]
- Verket NJ, Andersen MH, Sandvik L, Tanbo TG, Qvigstad E.. Lack of cross-cultural validity of the Endometriosis Health Profile-30. J Endometr Pelvic Pain Disord 2018;10:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verket NJ, Uhlig T, Sandvik L, Andersen MH, Tanbo TG, Qvigstad E.. Health-related quality of life in women with endometriosis, compared with the general population and women with rheumatoid arthritis. Acta Obstet Gynecol Scand 2018;97:1339–1348. [DOI] [PubMed] [Google Scholar]
- Vincent K, Kennedy S, Stratton P.. Pain scoring in endometriosis: entry criteria and outcome measures for clinical trials. Report from the Art and Science of Endometriosis meeting. Fertil Steril 2010;93:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Berkman ND.. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012;65:163–178. [DOI] [PubMed] [Google Scholar]
- Wahl KJ, Orr NL, Lisonek M, Noga H, Bedaiwy MA, Williams C, Allaire C, Albert AY, Smith KB, Cox S. et al. Deep dyspareunia, superficial dyspareunia, and infertility concerns among women with endometriosis: a cross-sectional study. Sex Med 2020;8:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Kerr CE, Schnyer RN, Legedza ATR, Savetsky-German J, Shields MH, Buring JE, Davis RB, Conboy LA, Highfield E. et al. Japanese-style acupuncture for endometriosis-related pelvic pain in adolescents and young women: results of a randomized sham-controlled trial. J Pediatr Adolesc Gynecol 2008;21:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström K, Bruse C, Sjösten A, Spira J, Edelstam G.. Quality of life in patients with endometriosis and the effect of pertubation with lidocaine—a randomized controlled trial. Acta Obstet Gynecol Scand 2013;92:1375–1382. [DOI] [PubMed] [Google Scholar]
- Wickström K, Edelstam G.. Minimal clinically important difference for pain on the VAS scale and the relation to quality of life in women with endometriosis. Sex Reprod Healthc 2017;13:35–40. [DOI] [PubMed] [Google Scholar]
- Wickström K, Spira J, Edelstam G.. Responsiveness of the Endometriosis Health Profile-30 questionnaire in a Swedish sample: an observational study. Clin Exp Obstet Gynecol 2017;44:413–418. [PubMed] [Google Scholar]
- Wyrwich KW, O’Brien CF, Soliman AM, Chwalisz K.. Development and validation of the endometriosis daily pain impact diary items to assess dysmenorrhea and nonmenstrual pelvic pain. Reprod Sci 2018;25:1567–1576. [DOI] [PubMed] [Google Scholar]
- Yela DA, Quagliato I de P, Benetti-Pinto CL.. Quality of life in women with deep endometriosis: a cross-sectional study. Rev Bras Ginecol Obstet 2020;42:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong PJ, Alsowayan N, Noga H, Williams C, Allaire C, Lisonkova S, Bedaiwy MA.. CHC for pelvic pain in women with endometriosis: ineffectiveness or discontinuation due to side-effects. Hum Reprod Open 2020;2020:hoz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong PJ, Williams C, Bodmer-Roy S, Ezeigwe C, Zhu S, Arion K, Ambacher K, Yosef A, Wong F, Noga H. et al. Prospective cohort of deep dyspareunia in an interdisciplinary setting. J Sex Med 2018;15:1765–1775. [DOI] [PubMed] [Google Scholar]
- Zaharia S. Postoperative scar endometriosis: optimization of diagnosis and treatment. PhD Thesis. Chișinău, Moldova: Nicolae Testemiţanu State University of Medicine and Pharmacy, 2020. https://repository.usmf.md/bitstream/20.500.12710/12199/1/PhD_Summary_S._Zaharia.pdf.
- Zondervan KT, Becker CM, Missmer SA.. Endometriosis. N Engl J Med 2020;382:1244–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.