Abstract
Purpose:
Bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of TGFβ receptor II (a TGFβ “trap”) fused to a human IgG1 mAb blocking programmed death-ligand 1 (PD-L1), was evaluated as treatment in patients with locally advanced or persistent, recurrent, or metastatic (P/R/M) cervical cancer.
Patients and Methods:
In this multicenter, open-label, phase Ib trial (NCT04551950), patients with P/R/M cervical cancer received bintrafusp alfa 2,400 mg once every 3 weeks plus cisplatin or carboplatin plus paclitaxel with (Cohort 1A; n = 8) or without (Cohort 1B; n = 9) bevacizumab; patients with locally advanced cervical cancer received bintrafusp alfa 2,400 mg every 3 weeks plus cisplatin plus radiation, followed by bintrafusp alfa monotherapy maintenance (Cohort 2; n = 8). The primary endpoint was safety; secondary endpoints included efficacy (including objective response rate) and pharmacokinetics.
Results:
At the data cutoff of April 27, 2022, patients in Cohorts 1A, 1B, and 2 had received bintrafusp alfa for a median duration of 37.9, 31.1, and 16.7 weeks, respectively. Two dose-limiting toxicities (grade 4 amylase elevation and grade 3 menorrhagia) unrelated to bintrafusp alfa were observed in Cohort 1B and none in other cohorts. Most treatment-emergent adverse events of special interest were grades 1–2 in severity, most commonly anemia (62.5%–77.8%) and bleeding events (62.5%–77.8%). Objective response rate was 75.0% [95% confidence interval (CI), 34.9–96.8], 44.4% (95% CI, 13.7–78.8), and 62.5% (95% CI, 24.5–91.5) in Cohorts 1A, 1B, and 2, respectively.
Conclusions:
Bintrafusp alfa had manageable safety and demonstrated clinical activity, further supporting the investigation of TGFβ/PD-L1 inhibition in human papillomavirus–associated cancers, including cervical cancer.
Translational Relevance.
Bintrafusp alfa, a first-in-class bifunctional fusion protein composed of the extracellular domain of TGFβ receptor II (a TGFβ “trap”) fused to a human IgG1 mAb blocking programmed death-ligand 1 (PD-L1), has demonstrated clinical activity and manageable safety in patients with human papillomavirus (HPV)-associated malignancies, including cervical cancer. This phase Ib study evaluated the safety of bintrafusp alfa in combination with chemotherapy ± bevacizumab in 17 patients with persistent, recurrent, or metastatic cervical cancer or in combination with chemoradiotherapy in 8 patients with locally advanced cervical cancer. Bintrafusp alfa had a manageable safety profile with no new safety signals and demonstrated clinical activity. The clinical activity seen in these settings further supports the efficacy of bintrafusp alfa demonstrated in previous studies with HPV-associated cancers and may reflect the underlying role of TGFβ in the pathophysiology of cervical cancer.
Introduction
Persistent human papillomavirus (HPV) infection is implicated in 99% of cervical cancers and is linked to activation of TGFβ and programmed death-ligand 1 (PD-L1) signaling pathways (1–3). The standard-of-care treatment for locally advanced cervical cancer, defined as IB3-IVA by the International Federation of Gynecology and Obstetrics (FIGO 2018), is concurrent chemoradiation with weekly cisplatin (4), with newer treatment approaches failing to improve current long-term outcomes (5, 6). Both the OUTBACK trial (for adjuvant chemotherapy after chemoradiation; NCT01414608) and the CALLA trial (for durvalumab, a mAb targeting PD-L1, in combination with concurrent chemoradiotherapy; NCT03830866) did not demonstrate improved long-term clinical outcomes for these therapies (5–8). However, the KEYNOTE-A18 trial (NCT04221945) for the anti-PD-1 therapy pembrolizumab with concurrent chemoradiotherapy demonstrated an improvement in progression-free survival (PFS) versus concurrent chemoradiotherapy in an interim analysis (9).
For recurrent, persistent, and metastatic cervical cancer, the standard-of-care treatment is platinum-based chemotherapy with or without bevacizumab, with a median overall survival (OS) of 16.8 months and 13.3 months, respectively, in a clinical trial setting (1). Pembrolizumab in combination with platinum-based chemotherapy, with or without bevacizumab, is also approved for patients whose tumors express PD-L1 [combined positive score (CPS) ≥1; ref. 10]. Approval was based on the results of the phase III KEYNOTE-826 trial (NCT03635567), which demonstrated significantly longer PFS and OS with pembrolizumab than with placebo (11, 12).
With anti-PD-1 and PD-L1 therapies demonstrating mixed results, limited improvements in OS and PFS, and treatment eligibility being restricted to PD-L1 expression in first-line therapy, there remains significant unmet clinical needs for patients with locally advanced and metastatic cervical cancer. Recent studies have investigated the potential of dual-inhibition approaches and bispecific immunotherapies (13–16). TGFβ has been shown to promote epithelial–mesenchymal transition and angiogenesis as well as immune escape of tumor cells (2, 17–20), suggesting that simultaneous inhibition of two nonredundant immunosuppressive pathways (TGFβ and PD-L1) might improve outcomes in cancers with increased TGFβ activation, such as those associated with HPV (13–16). Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the extracellular domain of the human TGFβ receptor II (TGFβRII or TGFβ “trap”) fused via a flexible linker to the C-terminus of each heavy chain of an IgG1 antibody blocking PD-L1 (13, 14). By blocking both the immunosuppressive TGFβ and the cell-intrinsic PD-L1 interaction, bintrafusp alfa is hypothesized to enhance the anticancer effect observed with agents targeting either pathway alone or in combination. In a pooled analysis of patients from phase I (INTR@PID 001; NCT02517398) and phase II (NCI 012; NCT03427411) studies, bintrafusp alfa demonstrated clinical activity and manageable safety in patients with HPV-associated malignancies, with a confirmed objective response rate (ORR) of 30.5%, including 30% for 33 patients with heavily pretreated recurrent/metastatic cervical cancer (21, 22). Most bintrafusp alfa–associated adverse events (AE) were mild to moderate in severity (16, 22).
This phase Ib open-label study evaluated the safety and tolerability of bintrafusp alfa in combination with other anticancer therapies in patients with locally advanced or persistent, recurrent, or metastatic cervical cancer.
Patients and Methods
Study design
This study (NCT04551950) was a multicenter, open-label, phase Ib trial evaluating the safety of first-line bintrafusp alfa according to the following treatment groups: patients in Cohort 1A were treated with bintrafusp alfa 2,400 mg once every 3 weeks plus cisplatin or carboplatin plus paclitaxel plus bevacizumab; patients in Cohort 1B were treated with bintrafusp alfa 2,400 mg every 3 weeks plus cisplatin or carboplatin plus paclitaxel; and patients in Cohort 2 were treated with bintrafusp alfa 2,400 mg every 3 weeks plus cisplatin plus radiation, followed by maintenance with bintrafusp alfa monotherapy (2,400 mg every 3 weeks). This dose was determined to be the recommended phase II dose of bintrafusp alfa in combination with chemotherapies that are administered every 3 weeks based upon population pharmacokinetics (popPK) modeling of data from two phase I studies of bintrafusp alfa (23).
Patients continued treatment until confirmed disease progression per RECIST 1.1, death, unacceptable toxicity, or study withdrawal. In Cohort 1, patients continued chemotherapy until complete response (CR; as clinically indicated) and/or unacceptable toxicity due to chemotherapy, after which, bintrafusp alfa (plus bevacizumab in Cohort 1A) may have been continued until 2 years or unacceptable toxicity; in Cohort 2, patients continued maintenance treatment (bintrafusp alfa) for a maximum of 2 years (or beyond at the discretion of the investigator).
The study included a 28-day screening period and a dose-limiting toxicity (DLT) observation period of 4 weeks. Safety follow-up visits occurred at 28 days and 12 weeks after the last dose of study intervention; long-term follow-up was performed every 12 weeks after the safety follow-up.
This study was conducted at 13 sites across three countries (United States: five sites; Japan: five sites; Spain: three sites).
Patient eligibility criteria
Key inclusion criteria for Cohort 1 (A and B) included adult patients with documented persistent, recurrent, or metastatic squamous cell carcinoma, adenosquamous carcinoma, or adenocarcinoma of the cervix; no prior treatment with systemic chemotherapy (including neoadjuvant and adjuvant regimens); and tumors not amenable to curative treatment (such as with surgery and/or radiation). Prior radiation with or without radiosensitizing chemotherapy was allowed in Cohort 1 (A and B). Documented evidence of cervical adenocarcinoma, squamous cell carcinoma, or adenosquamous carcinoma FIGO 2018 stages IB2 to IVA and no prior chemotherapy or radiotherapy for cervical cancer were inclusion criteria for Cohort 2. Key exclusion criteria included patients with active central nervous system metastases causing clinical symptoms or metastases that required therapeutic intervention.
The protocol was reviewed and approved by the Institutional Review Board/International Ethics Committee before the study was initiated, and it was conducted in accordance with the Declaration of Helsinki, Council for International Organisations of Medical Sciences, International Ethical Guidelines, applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice (GCP), the Japanese Ministerial Ordinance on GCP, and other applicable laws and regulations. All patients provided written informed consent before enrolling in the study.
Outcomes
Primary study endpoints included occurrence of a DLT within the DLT period (within 4 weeks after first administration of bintrafusp alfa) and AEs. DLTs were assessed by the investigator and/or sponsor according to NCI Common Terminology Criteria for Adverse Events version 5.0 and judged to be related to study intervention and confirmed by the safety monitoring committee. The full definition of DLTs is presented in the Supplementary Materials and Methods and includes grade 4 nonhematologic toxicity or hematologic toxicity lasting ≥7 days despite medical intervention, grade ≥3 bleeding events occurring within 5 days of bintrafusp alfa treatment (regardless of causality), and grade 5 toxicity. Secondary study endpoints included pharmacokinetics, and tertiary/exploratory endpoints included OS, PFS, ORR, and duration of objective response (DOR); disease control rate (DCR) was also analyzed.
Statistical analyses
The data cut-off date was April 27, 2022. For ORR and DCR, 95% confidence intervals (CI) were calculated using the Clopper–Pearson method. Median OS, PFS, and DOR (and 95% CIs) were calculated according to the Brookmeyer and Crowley method and Kaplan–Meier analyses. Continuous variables were summarized using number, mean, median, SD, minimum, and maximum, and categorical variables were summarized using frequency counts and percentages.
Data availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany (https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When the healthcare business of Merck KGaA, Darmstadt, Germany has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.
Results
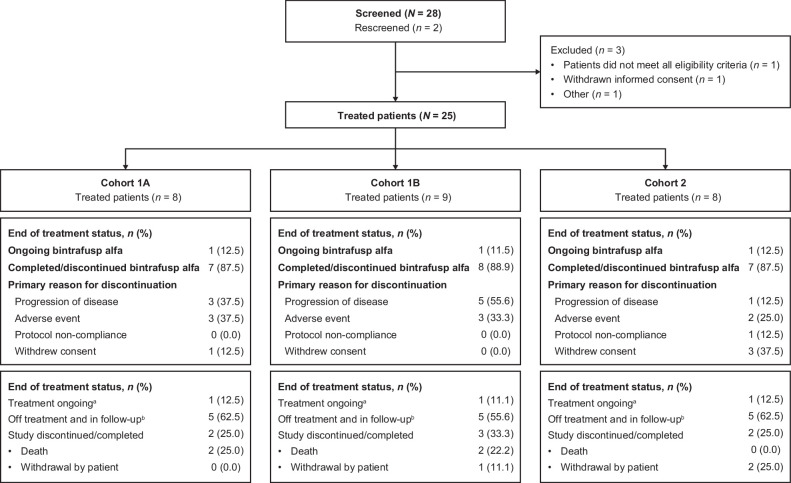
As of April 27, 2022, 8 patients in Cohort 1A, 9 in Cohort 1B, and 8 in Cohort 2 received bintrafusp alfa for a median duration of 37.9, 31.1, and 16.7 weeks, respectively (Supplementary Table S1). All patients completed the DLT period, and 12.5%, 11.1%, and 12.5% of patients in Cohorts 1A, 1B, and 2 (n = 1 for each cohort), respectively, remained on bintrafusp alfa treatment at data cutoff (Fig. 1).
Figure 1.
Patient disposition. aAt least one study intervention ongoing. bAll treatments either completed, discontinued, or patients received no treatment.
Demographic and baseline disease characteristics were mostly well balanced across the three cohorts (Table 1). Squamous cell carcinoma was most common in Cohort 1A (50%) and Cohort 1B (55.6%), and all patients treated in Cohort 2 had squamous cell carcinoma. Median time since initial cancer diagnosis was 3.3 and 19.6 months in Cohort 1A and Cohort 1B, respectively; median time since initial diagnosis in Cohort 2 was 1.1 months (Supplementary Table S1). The study representativeness with respect to patients with cervical cancer is presented in Supplementary Table S2.
Table 1.
Baseline patient demographics and disease characteristics (N = 25).
| Cohort 1A | Cohort 1B | Cohort 2 | |
|---|---|---|---|
| n = 8 | n = 9 | n = 8 | |
| Age, median (range), years | 43 (34–63) | 47 (39–63) | 49 (26–70) |
| Geographic region, n (%) | |||
| Asia | 3 (37.5) | 3 (33.3) | 3 (37.5) |
| North America | 3 (37.5) | 1 (11.1) | 3 (37.5) |
| Europe | 2 (25.0) | 5 (55.6) | 2 (25.0) |
| ECOG performance status, n (%) | |||
| 0 | 8 (100.0) | 6 (66.7) | 5 (62.5) |
| 1 | 0 | 3 (33.3) | 3 (37.5) |
| Histology, n (%) | |||
| Squamous cell carcinoma | 4 (50.0) | 5 (55.6) | 8 (100.0) |
| Adenocarcinoma | 3 (37.5) | 4 (44.4) | 0 |
| Othera | 1 (12.5) | 0 | 0 |
| Concurrent chemoradiation, n (%) | 1 (12.5) | 2 (22.2) | 0 |
| Months since initial cancer diagnosis, median (Q1–Q3) | 3.3 (1.0–27.8) | 19.6 (11.9–43.2) | 1.1 (0.9–1.8) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
aThis patient had poorly differentiated adenocarcinoma and histology was described as “other”.
Safety
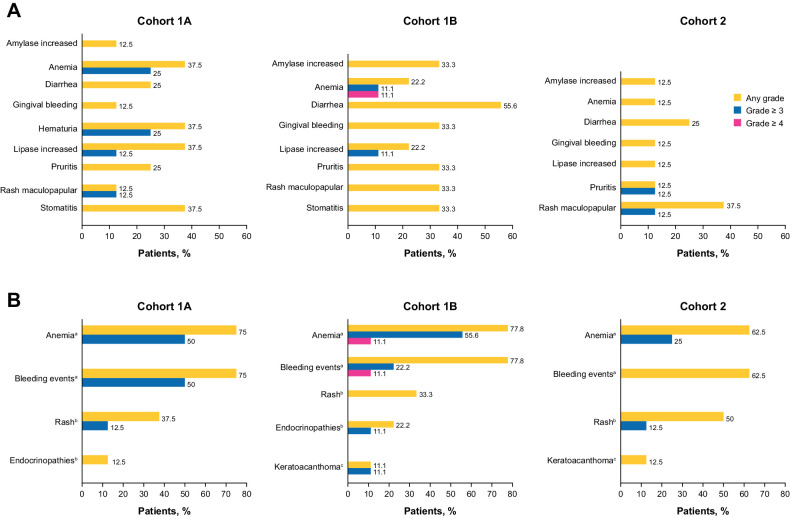
No DLT was observed in Cohort 1A or Cohort 2; two DLTs (grade 4 amylase elevation related to carboplatin and paclitaxel and grade 3 menorrhagia unrelated to study intervention) unrelated to bintrafusp alfa treatment were observed in Cohort 1B, neither of which led to treatment discontinuation. No new safety signals were observed in any cohorts (Fig. 2).
Figure 2.
Bintrafusp alfa-related AEs occurring in ≥20% of patients (A) and AESIs (B). aBintrafusp alfa–related AE. birAE related or unrelated to bintrafusp alfa and/or other study drugs. cTGFβ inhibition–mediated skin AE related or unrelated to bintrafusp alfa and/or other study drugs.
Bintrafusp alfa–related AEs of any grade occurred in 87.5%, 100.0%, and 87.5% of patients in Cohorts 1A, 1B, and 2, respectively. The most common bintrafusp alfa–related AEs in Cohorts 1A, 1B, and 2, respectively, were anemia (37.5%, 22.2%, and 12.5%), diarrhea (25.0%, 55.6%, and 25.0%), stomatitis (37.5%, 33.3%, and 0%), amylase increased (12.5%, 33.3%, and 12.5%), lipase increased (37.5%, 22.2%, and 12.5%), hematuria (37.5%, 0%, and 0%), pruritus (25.0%, 33.3%, and 12.5%), and rash maculopapular (12.5%, 33.3%, and 37.5%; Fig. 2A). No treatment-related deaths occurred.
Treatment-emergent AEs led to permanent discontinuation of bintrafusp alfa in 37.5%, 33.3%, and 12.5% of patients in Cohorts 1A, 1B, and 2, respectively. The most common AEs that led to permanent discontinuation of bintrafusp alfa in Cohort 1A were small intestinal hemorrhage (12.5%), hematuria (12.5%), and pelvic pain (12.5%). The most common AEs that led to permanent discontinuation of bintrafusp alfa in Cohort 1B were vaginal hemorrhage (22.2%), anemia (11.1%), and squamous cell carcinoma (11.1%). The most common AE that led to permanent discontinuation of bintrafusp alfa in Cohort 2 was acute kidney injury (12.5%).
Treatment-emergent serious AEs (SAE) were reported in 87.5%, 44.4%, and 75.0% of patients in Cohorts 1A, 1B, and 2, respectively (Supplementary Table S3), of which 50%, 44.4%, and 37.5% of SAEs in Cohorts 1A, 1B, and 2, respectively, were considered by the investigator as related to bintrafusp alfa. The most common SAEs were hematuria (25.0%, Cohort 1A), urinary tract infection (25.0%, Cohort 1A; 11.1%, Cohort 1B; 12.5%, Cohort 2), and vaginal hemorrhage (25.0%, Cohort 1B).
The most common treatment-emergent AEs of special interest (AESI) were anemia (75.0%, 77.8%, and 62.5% in Cohorts 1A, 1B, and 2, respectively) and bleeding events (75.0%, 77.8%, and 62.5% in Cohorts 1A, 1B, and 2, respectively), and most AESIs were grade 1–2 in severity (Fig. 2B). Bleeding events were managed and resolved with standard treatment (Supplementary Table S3). Immune-related AEs (irAE) occurred in 50.0%, 44.4%, and 50.0% in Cohorts 1A, 1B, and 2, respectively. The most common irAEs were rash maculopapular, which occurred in 1 patient in each cohort, and hypothyroidism, which occurred in 1 patient in Cohorts 1A and 1B. Two patients in Cohort 2 reported rash. In Cohort 1A, 1 grade 3 irAE was observed (rash maculopapular), grade 3 secondary adrenocortical insufficiency in 1 patient was the only grade 3 irAE in Cohort 1B, and 1 patient in Cohort 2 reported grade 3 irAEs (pruritus and rash maculopapular). TGFβ inhibition–mediated skin AEs occurred in 1 patient in Cohorts 1B and 2 (11.1% and 12.5%, respectively) and was keratoacanthoma in both.
Efficacy
Exploratory endpoints included evaluation of efficacy (ORR, DOR, OS, and PFS); DCR was also analyzed. ORR was 75.0% (95% CI, 34.9–96.8), 44.4% (95% CI, 13.7–78.8), and 62.5% (95% CI, 24.5–91.5) in Cohort 1A, Cohort 1B, and Cohort 2, respectively (Table 2; Supplementary Figs. S1A, S1B, and S2); DCR was 87.5% (95% CI, 47.3–99.7), 66.7% (95% CI, 29.9–92.5), and 62.5% (95% CI, 24.5–91.5), respectively. Median DOR was 7.4 months [95% CI, 4.2– not evaluable (NE)] in Cohort 1A and not reached in Cohort 1B and Cohort 2. DOR rate at 6 months was 66.7% (95% CI, 19.5–90.4) in Cohort 1A and 100% (95% CI, 100.0–100.0) in Cohorts 1B and 2. At data cutoff, 2/6 (33%), 1/4 (25%), and 3/5 (60%) responders had ongoing responses in Cohorts 1A, 1B, and 2, respectively.
Table 2.
Tumor response.
| Cohort 1A | Cohort 1B | Cohort 2 | |
|---|---|---|---|
| n = 8 | n = 9 | n = 8 | |
| Best overall response, n (%) | |||
| Complete response | 1 (12.5) | 2 (22.2) | 3 (37.5) |
| Partial response | 5 (62.5) | 2 (22.2) | 2 (25.0) |
| Stable disease | 1 (12.5) | 2 (22.2) | 0 (0.0) |
| Progressive disease | 0 (0.0) | 2 (22.2) | 1 (12.5) |
| Not evaluable | 1 (12.5) | 1 (11.1) | 2 (25.0) |
| Confirmed ORR, % (95% CI) | 75.0 (34.9–96.8) | 44.4 (13.7–78.8) | 62.5 (24.5–91.5) |
| Confirmed DCR, % (95% CI) | 87.5 (47.3–99.7) | 66.7 (29.9–92.5) | 62.5 (24.5–91.5) |
| Median DOR, % (95% CI) | 7.4 (4.2–NR) | NR (10.4–NR) | NR (NR–NR) |
Abbreviations: CI, confidence interval; DCR, disease control rate; DOR, duration of response; NR, not reached; ORR, objective response rate.
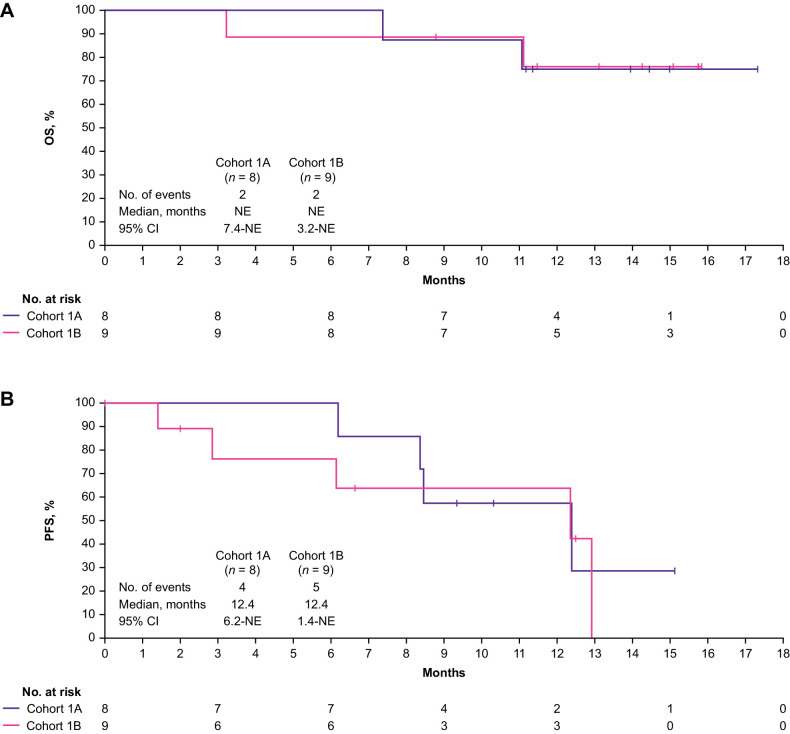
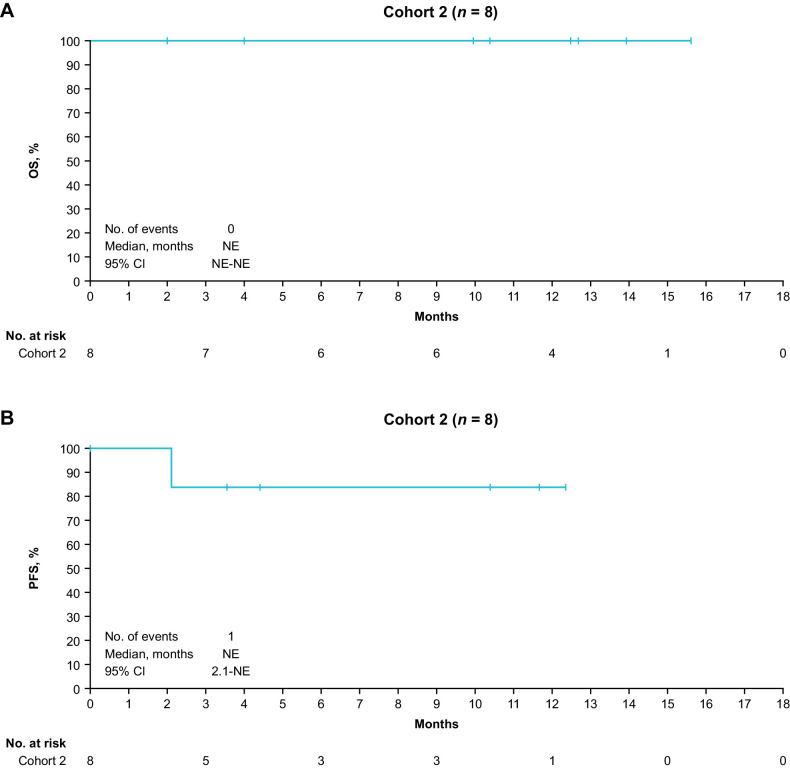
At data cutoff, median OS was not reached in Cohorts 1A or 1B (Fig. 3A); OS rate at 12 months was 75.0% (95% CI, 31.5–93.1) and 76.2% (95% CI, 33.2–93.5) in Cohorts 1A and 1B, respectively. Median PFS was 12.4 months (95% CI, 6.2–NE) and 12.4 months (95% CI, 1.4–NE) in Cohorts 1A and 1B, respectively (Fig. 3B), and the PFS rate at 12 months was 57.1% (95% CI, 17.2–83.7) and 63.5% (95% CI, 23.8–86.6) in each cohort. Median OS and PFS were not reached in Cohort 2 (Fig. 4A and B). In Cohort 2, the OS and PFS rates at 12 months were 100% (95% CI, 100.0–100.0) and 83.3% (95% CI, 27.3–97.5), respectively.
Figure 3.
OS (A) and PFS (B) for Cohort 1A and Cohort 1B. NE, not evaluable; OS, overall survival; PFS, progression-free survival.
Figure 4.
OS (A) and PFS (B) for Cohort 2. NE, not evaluable; OS, overall survival; PFS, progression-free survival.
Pharmacokinetic profile
The pharmacokinetic profile was assessed in 7 patients in Cohort 1A, 8 in Cohort 1B, and 2 in Cohort 2 at Cycle 1 up to the 504-hour (21 days) sampling timepoint and at day 43. Following a single dose, the geometric mean AUC0–504 reached 159,000 μg•hour/mL [32.2% coefficient of variation (CV)], and the geometric mean concentration at end of infusion (Ceoi) was 849 μg/mL (17.0% CV). Overall, observed exposure ranges were consistent with those predicted using the phase I popPK model, developed with monotherapy data (23). The pharmacokinetic parameters following a single dose are shown in Supplementary Table S4, and individual exposures at day 1 are shown in Supplementary Fig. S3. The target concentration at the end of dosing interval (geometric mean Ctrough of >100 μg/mL) was achieved by day 43.
Discussion
Bintrafusp alfa demonstrated a manageable safety profile with chemotherapy with or without bevacizumab in patients with persistent, recurrent, or metastatic cervical cancer and with concurrent chemoradiotherapy in patients with locally advanced cervical cancer. There were no new safety signals and no treatment-related deaths. The ORR with bintrafusp alfa was 75.0%, 44.4%, and 62.5% for Cohorts 1A, 1B, and 2, respectively. Median OS was not reached in the three cohorts and median PFS was 12.4 months, 12.4 months, and not reached in Cohorts 1A, 1B, and 2, respectively.
Bintrafusp alfa in combination with platinum-based chemotherapy has shown clinical activity in patients with locally advanced or persistent, recurrent, or metastatic cervical cancer. This study demonstrated efficacy outcomes with bintrafusp alfa that are in line with historical data in these settings, with observed ORRs of 44.4%–75.0%, median PFS of 12.4 months–not reached, and median OS not reached across all cohorts. We would note that the follow-up duration was too short to provide a meaningful comparison for PFS and OS, but this does help to establish the clinical activity of bintrafusp alfa in locally advanced or persistent, recurrent, or metastatic cervical cancer.
Regarding the frontline setting, the phase III KEYNOTE-826 trial (NCT03635567) showed that the addition of pembrolizumab to chemotherapy, with or without bevacizumab, improved clinical outcome in patients with persistent, recurrent, or metastatic cervical cancer compared with those who received chemotherapy with or without bevacizumab (ORR 66% vs. 51% for intention-to-treat population; median PFS 10.4 vs. 8.1 months; and median OS 29.6 vs. 17.4 months) for the CPS ≥ 10 group (11, 12). Moreover, clinical benefit was observed in the pembrolizumab + chemotherapy arm compared with the placebo + chemotherapy arm, regardless of bevacizumab use (11, 12).
In the CheckMate 358 study (NCT02488759), nivolumab, an anti-PD-1 antibody, was studied in combination with ipilimumab, an anti–CTL-associated antigen-4 (CTLA-4) antibody, for patients with recurrent or metastatic cervical cancer. An interim analysis of patients without prior systemic therapy found the median PFS to be 13.8 months and 8.5 months with nivolumab every 2 weeks plus ipilimumab every 6 weeks and nivolumab plus ipilimumab every 3 weeks for four doses followed by nivolumab every 2 weeks, respectively. Median OS was not reached for either cohort after a median follow-up of 10.7 months and 13.9 months, respectively (24). Several ongoing clinical trials are studying immune checkpoint inhibitors in the frontline setting for recurrent or metastatic cervical cancers, such as the FERMATA phase III trial (anti-PD-1 BCD-100 in combination with platinum-based chemotherapy with and without bevacizumab, NCT03912415) and the BEATcc phase III trial (platinum chemotherapy plus paclitaxel with bevacizumab and anti-PD-L1 atezolizumab, NCT03556839); results from these trials will help determine the potential benefits of these newer immune checkpoint inhibitors in this setting (25).
In patients with locally advanced cervical cancer, the phase III OUTBACK trial (NCT01414608) found that the addition of adjuvant chemotherapy to chemoradiation did not significantly improve OS or PFS over chemoradiation alone (5-year OS rate 72% vs. 71%, respectively, HR: 0.90; 95% CI, 0.70–1.17; P = 0.81; 5-year PFS rate 63% vs. 62%, respectively, HR: 0.86; 95% CI, 0.69–1.08; P = 0.58), highlighting the unmet need in patients with locally advanced cervical cancer (6).
In another recent trial in patients with locally advanced cervical cancer, the phase III CALLA trial (NCT03830866) found that durvalumab plus chemoradiotherapy did not significantly improve PFS over placebo plus chemoradiotherapy (24-month PFS rate 65.9% vs. 62.1%, respectively, HR: 0.84; 95% CI, 0.65–1.08; P = 0.174; ORR 82.6% vs. 80.5%, respectively; ref. 5). However, the phase III KEYNOTE-A18/ENGOT-cx11/GOG-3047 trial (chemoradiotherapy with or without pembrolizumab; NCT04221945) met its primary endpoint of significantly superior PFS for the chemotherapy + pembrolizumab arm versus chemotherapy alone at an interim analysis (9). Results are pending from other ongoing trials in patients with locally advanced cervical cancer, such as the phase II ATEZOLACC trial (for atezolizumab; NCT03612791) and the phase II ATOMICC trial [anti-PD-1 dostarlimab (TSR-042) as maintenance therapy for patients at high risk after chemoradiation; NCT03833479; ref. 25]. The results from these studies will help define the role of immunotherapy in the management of patients with locally advanced cervical cancer. Simultaneous blockade of complementary immunosuppressive pathways such as the TGFβ pathway [which leads to resistance to anti-PD-(L)1 immunotherapy) along with the PD-L1 pathway may also have clinical potential in these patients. The manageable safety of bintrafusp alfa demonstrated in this study warrants further exploration of these dual-targeted therapies.
Further studies are also necessary to better understand the performance of immune checkpoint inhibitors in the subgroup of patients with CPS <1% in the recurrent/metastatic setting. Bintrafusp alfa may represent an attractive therapeutic approach to overcome the immunosuppressive tumor microenvironment with scarce T-cell infiltration that characterizes this particular subgroup of patients with CPS <1%. Further investigations are also needed to investigate the efficacy and safety of bintrafusp alfa in the post-immunotherapy setting.
Overall, observed exposure ranges in this study were consistent with those predicted using the phase I popPK model, which was developed based on monotherapy data (23, 26). In addition, pharmacokinetic profiles were similar with and without bevacizumab, confirming low victim drug–drug interaction potential for bintrafusp alfa with small-molecule drugs and protein therapeutics, which support identical dosing regimen for all cohorts used in this study (27). The target Ctrough associated with target occupancy for all four targets of bintrafusp alfa (TGFβ1, TGFβ2, TGFβ3, and PD-L1; ref. 23) has been reached in this study, similar to other studies with bintrafusp alfa. The previous report also suggested a low perpetrator drug–drug interaction potential of bintrafusp alfa toward substrates of CYP3A4 (27), which includes chemotherapies (such as paclitaxel used in this study) and commonly used concomitant medications.
The incidence of bleeding AEs in this study was relatively high (75.0%, 77.8%, and 62.5% for Cohorts 1A, 1B, and 2, respectively), consistent with the previously reported association between the cervical cancer type and probability of bleeding (28). The higher incidence of bleeding events observed with bintrafusp alfa in the current study has been seen in other clinical studies of bintrafusp alfa as well, in which a higher frequency of low-grade bleeding events has been observed than with immune checkpoint inhibitors or targeted agents (29–31). Moreover, exposure-safety relationship for bleeding AEs was established in previous studies and this analysis suggested that the cervical cancer tumor type was associated with a higher probability of AEs in addition to exposure (31). Mechanistically, the association of TGFβ inhibition with bleeding events may be related to the inhibition of the TGFβ2 isoform, a hematopoietic regulator (31). As bintrafusp alfa has a higher affinity for the TGFβ1 and TGFβ3 isoforms (23), dose reduction may be a feasible management approach to reduce the probability of bleeding events while retaining pharmacologic activity (31).
Also, because bintrafusp alfa was combined with bevacizumab (and bleeding is a known AE with this antiangiogenic agent), there was a possibility of additive toxicity of the two drugs. However, the bleeding events in the bintrafusp alfa + chemotherapy arm were similar to those in the bintrafusp + chemotherapy + bevacizumab arm in this study. Other clinical studies that combine blockade of VEGF/VEGFR, TGFβ, and PD-L1 have also not demonstrated an additive toxicity with anti-VEGF/VEGFR and anti-TGFβ agents (32). However, this should be interpreted with caution due to the small number of patients, lack of randomized clinical trials, and limited published data on the combination of these two agents. The clinical activity seen with bintrafusp alfa, in combination with bevacizumab without additive toxicity, suggests that perhaps the colocalized inhibition of TGFβ and PD-L1 is not potentiating the antiangiogenic activity of bevacizumab, but is instead resulting in effects within the tumor microenvironment (33).
Limitations of this study include its open-label design, which may have influenced investigators’ judgment in evaluating safety events and treatment discontinuation, the small number of patients, and the absence of a comparator arm.
Conclusion
In conclusion, this phase I study of bintrafusp alfa identified no new safety signals with first-line bintrafusp alfa plus chemotherapy with or without bevacizumab in patients with persistent, recurrent, or metastatic cervical cancer or bintrafusp alfa plus chemoradiotherapy in locally advanced cervical cancer. Despite this being a phase Ib clinical trial with a small sample size, bintrafusp alfa was efficacious in this patient population. The clinical activity observed here may reflect the underlying role of TGFβ in the pathophysiology of cervical cancer, particularly the relationship between HPV, TGFβ, and cervical cancer.
Supplementary Material
Supplementary Methods
Table S1. Median duration of therapy. IQR, interquartile range; NA, not applicable.
Table S2. Study representativeness.
Table S3. Serious treatment-related adverse events. Bleeding events: a One patient had two consecutive hematuria events, and another patient also had two events, the first episode related to bevacizumab, the second episode due to bintrafusp alfa. All hematuria events resolved within 4-6 weeks. b One patient treated with blood transfusion and vaginal tamponade, resolved within 2 days; other patient required blood transfusion and event resolved within 3 weeks. c Patient presented with bloody diarrhea and stomach discomfort, assessed as being caused by late radiation therapy and a rectal ulcer due to bevacizumab use; resolved with fasting and fluid replacement within 2 weeks. d Mucosal bleeding assessed as being caused by bintrafusp alfa and bevacizumab use; resolved with blood transfusion. e One event resolved with tumor compression by gauze and blood transfusion within a few days. f Bleeding in the duodenum resolved with standard gastrointestinal medication and fluid replacement within one week. g Rectal bleeding along with urinary tract infection; patient treated in emergency room with antibiotics and discontinuation of heparin for prior thrombosis, symptoms resolved within 2 weeks. AE, adverse event; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Table S4. Pharmacokinetic exposure summary. AUC, area under the curve; Ceoi, concentration at end of infusion; Ctrough, concentration at the end of the dosing interval; CV, coefficient of variation; NA, not applicable; popPK, population pharmacokinetic; SD, standard deviation.
Figure S1. Change from baseline in sum of diameters of all target lesions per RECIST v1.1 for (A) Cohort 1A and (B) Cohort 1B. CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure S2. Change from baseline in sum of diameters of all target lesions per RECIST v1.1 for Cohort 2. CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure S3. Patient-level concentration profile of bintrafusp alfa at Day 1 postdose.
Acknowledgments
The authors thank the patients and their families, investigators, coinvestigators, and study teams at each of the participating centers and at the healthcare business of Merck KGaA, Darmstadt, Germany. Medical writing support was provided by Rebecca Yao, PhD, and Joyce Lee, PhD, of MediTech Media Asia Pacific, which was funded by the healthcare business of Merck KGaA (CrossRef Funder ID: 10.13039/100009945) and was previously part of an alliance between the healthcare business of Merck KGaA and GlaxoSmithKline, in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
The trial was sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945) and was previously part of an alliance between the healthcare business of Merck KGaA and GlaxoSmithKline. The healthcare business of Merck KGaA provided the trial drugs. The investigators worked with the healthcare business of Merck KGaA on the trial design, collection and analysis of data, and interpretation of results.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 929
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A. Oaknin reports non-financial support from ESMO and GCIG; other support from ASCO, GOG, and SEOM; personal fees from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, the healthcare business of Merck KGaA, F. Hoffmann-La Roche, Genmab, GlaxoSmithKline, ImmunoGen, Itheos, Merck & Co., Mersana Therapeutics, Novocure, OneXerna Therapeutics, Inc., PharmaMar, Regeneron, Sattucklabs, Seagen, and Sutro Biopharma; personal fees and other support from AstraZeneca, PharmaMar and Roche; and grants from AbbVie Deutschland, Advaxis Inc., Aeterna Zentaris, Amgen, Aprea Therapeutics AB, Bristol Myers Squibb, Clovis Oncology Inc, Eisai Ltd, F. Hoffmann–La Roche Ltd, Immunogen Inc, Merck & Co., Millennium Pharmaceuticals Inc, PharmaMar SA, Regeneron Pharmaceuticals, and Tesaro Inc. outside the submitted work. S.A. Ghamande reports grants from Merck KGaA during the conduct of the study; personal fees from GSK, Seagen, and Esai outside the submitted work; and institutional support for multiple clinical trials by the following pharma: GSK, the healthcare business of Merck KGaA, Iovance, Jounce, Eisai, Takeda, Clovis, AstraZeneca, Sutro, and Seagen. M. Gil-Martin reports personal fees from GSK, MSD, and Clovis outside the submitted work. C. Garcia-Duran reports personal fees from GSK and MSD-AstraZeneca outside the submitted work. M. Sato reports personal fees from Merck Biopharma Co., Ltd. during the conduct of the study. S.P. Chaudhary reports other support from EMD Serono during the conduct of the study. Y. Vugmeyster reports personal fees from EMD Serono during the conduct of the study; in addition, Y. Vugmeyster has a patent for work with EMD Serono pending. K. Hasegawa reports personal fees from Merck & Co. outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
A. Oaknin: Conceptualization, data curation, formal analysis, methodology, writing–original draft, writing–review and editing. S.A. Ghamande: Data curation, formal analysis, writing–original draft, writing–review and editing. Y. Kasamatsu: Data curation, formal analysis, writing–original draft, writing–review and editing. M. Gil-Martin: Data curation, formal analysis, writing–original draft, writing–review and editing. J.F. Grau-Bejar: Data curation, formal analysis, writing–original draft, writing–review and editing. C. Garcia-Duran: Data curation, formal analysis, writing–original draft, writing–review and editing. M. Sato: Formal analysis, supervision, writing–original draft, project administration, writing–review and editing. A. Siddiqui: Conceptualization, formal analysis, supervision, methodology, writing–original draft, project administration, writing–review and editing. S.P. Chaudhary: Conceptualization, supervision, methodology, writing–original draft, project administration, writing–review and editing. Y. Vugmeyster: Conceptualization, formal analysis, supervision, methodology, writing–original draft, project administration, writing–review and editing. K. Hasegawa: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing.
References
- 1. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annal Oncol 2017;28:iv72–83. [DOI] [PubMed] [Google Scholar]
- 2. Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, et al. Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol 2014;5:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang W, Lu Y-P, Yang Y-Z, Kang J-R, Jin Y-D, Wang H-W. Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J Obstet Gynaecol 2017;43:1602–12. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Rassy E, Coutte A, Achkar S, Espenel S, Genestie C, et al. Current standards in the management of early and locally advanced cervical cancer: update on the benefit of neoadjuvant/adjuvant strategies. Cancers (Basal) 2022;14:2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mileshkin LR, Moore KN, Barnes E, Gebski V, Narayan K, Bradshaw N, et al. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: the randomized phase III OUTBACK trial (ANZGOG 0902, RTOG 1174, NRG 0274). J Clin Oncol 39:18s, 2021. (suppl; abstr LBA3). [Google Scholar]
- 6. Monk B, Toita T, Wu X, Limón JC, Zhou Q, Tarnawski R, et al. Durvalumab, in combination with and following chemoradiotherapy, in locally advanced cervical cancer: results from the phase 3 international, randomized, double-blind, placebo-controlled CALLA trial. International Gynecologic Cancer Society (IGCS)2022Sept 29-Oct 01; New York, United States [Google Scholar]
- 7. NIH. Study of durvalumab with chemoradiotherapy for women with locally advanced cervical cancer (CALLA). NCT03830866. 2023. Available from: https://clinicaltrials.gov/ct2/show/NCT03830866.
- 8. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 9. Merck. Merck announces phase 3 KEYNOTE-A18 trial met primary endpoint of progression-free survival (PFS) in patients with newly diagnosed high-risk locally advanced cervical cancer. 2023. Available from: https://www.merck.com/news/merck-announces-phase-3-keynote-a18-trial-met-primary-endpoint-of-progression-free-survival-pfs-in-patients-with-newly-diagnosed-high-risk-locally-advanced-cervical-cancer/.
- 10. US Food and Drug Administration. Keytruda full prescribing information. 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125514s128lbl.pdf.
- 11. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 2021;385:1856–67. [DOI] [PubMed] [Google Scholar]
- 12. Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. KEYNOTE-826: final overall survival results from a randomized, double-blind, phase 3 study of pembrolizumab + chemotherapy vs placebo + chemotherapy for first-line treatment of persistent, recurrent, or metastatic cervical cancer. J Clin Oncol 41:16s, 2023. (suppl; abstr 5500). [DOI] [PubMed] [Google Scholar]
- 13. Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 2018;10:eaan5488. [DOI] [PubMed] [Google Scholar]
- 14. Birrer MJ, Fujiwara K, Oaknin A, Randall L, Ojalvo LS, Valencia C, et al. The changing landscape of systemic treatment for cervical cancer: rationale for inhibition of the TGF-β and PD-L1 pathways. Front Oncol 2022;12:814169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knudson KM, Hicks KC, Luo X, Chen J-Q, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. OncoImmunology 2018;7:e1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gulley JL, Schlom J, Barcellos-Hoff MH, Wang X-J, Seoane J, Audhuy F, et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol Oncol 2022;16:2117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan Q, Qiu MT, Zhu Z, Zhou JH, Chen L, Zhou Y, et al. Twist induces epithelial-mesenchymal transition in cervical carcinogenesis by regulating the TGF-beta/Smad3 signaling pathway. Oncol Rep 2015;34:1787–94. [DOI] [PubMed] [Google Scholar]
- 18. Hazelbag S, Gorter A, Kenter GG, van den Broek L, Fleuren G. Transforming growth factor-beta1 induces tumor stroma and reduces tumor infiltrate in cervical cancer. Hum Pathol 2002;33:1193–9. [DOI] [PubMed] [Google Scholar]
- 19. Kloth JN, Fleuren GJ, Oosting J, de Menezes RX, Eilers PH, Kenter GG, et al. Substantial changes in gene expression of Wnt, MAPK and TNFalpha pathways induced by TGF-beta1 in cervical cancer cell lines. Carcinogenesis 2005;26:1493–502. [DOI] [PubMed] [Google Scholar]
- 20. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strauss J, Braiteh FS, Calvo E, De Miguel M, Cervantes A, Edenfield WJ, et al. Evaluation of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in cervical cancer: data from phase 1 and phase 2 studies. J Clin Oncol 39:15s, 2021. (suppl; abstr 5509). [Google Scholar]
- 22. Strauss J, Gatti-Mays ME, Cho BC, Hill A, Salas S, McClay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus-associated malignancies. J Immunother Cancer 2020;8:e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vugmeyster Y, Wilkins J, Koenig A, El Bawab S, Dussault I, Ojalvo LS, et al. Selection of the recommended phase 2 dose for bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1. Clin Pharmacol Ther 2020;108:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naumann RW, Oaknin A, Meyer T, Lopez-Picazo JM, Lao C, Bang YJ, et al. Efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol 2019;30:v898–9. [Google Scholar]
- 25. Sherer MV, Kotha NV, Williamson C, Mayadev J. Advances in immunotherapy for cervical cancer: recent developments and future directions. Int J Gynecol Cancer 2022;32:281–7. [DOI] [PubMed] [Google Scholar]
- 26. Vugmeyster YWJ, De Banerjee S, Ojalvo LS, Grenga I, Klopp-Schulze L, Khandewhal A. Extending dosing interval of bintrafusp alfa (M7824) from every 2 weeks to every 3 weeks dosing. 10th American Conference on Pharmacometrics; 2019, 20–23 October 2019; Orlando, FL. [Google Scholar]
- 27. Vugmeyster Y, Locke G, Helwig C, Rolfe PA, Dong JQ, Venkatakrishnan K. Risk assessment of drug-drug interaction potential for bintrafusp alfa with cytochrome P4503A4 substrates: a totality of evidence approach. Clin Transl Sci 2022;15:2838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eleje GU, Eke AC, Igberase GO, Igwegbe AO, Eleje LI. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer. Cochrane Database Syst Rev 2019;3:CD011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kewan T, Covut F, Ahmed R, Haddad A, Daw H. Clinically significant bleeding with immune checkpoint inhibitors: a retrospective cohort study. Eur J Cancer 2020;137:285–7. [DOI] [PubMed] [Google Scholar]
- 30. Leighl NB, Bennouna J, Yi J, Moore N, Hambleton J, Hurwitz H. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer 2011;104:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vugmeyster Y, Grisic AM, Wilkins JJ, Loos AH, Hallwachs R, Osada M, et al. Model-informed approach for risk management of bleeding toxicities for bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1. Cancer Chemother Pharmacol 2022;90:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Wen Q, Ding R. Therapeutic targeting of VEGF and/or TGF-beta to enhance anti-PD-(L)1 therapy: the evidence from clinical trials. Front Oncol 2022;12:905520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020;52:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Table S1. Median duration of therapy. IQR, interquartile range; NA, not applicable.
Table S2. Study representativeness.
Table S3. Serious treatment-related adverse events. Bleeding events: a One patient had two consecutive hematuria events, and another patient also had two events, the first episode related to bevacizumab, the second episode due to bintrafusp alfa. All hematuria events resolved within 4-6 weeks. b One patient treated with blood transfusion and vaginal tamponade, resolved within 2 days; other patient required blood transfusion and event resolved within 3 weeks. c Patient presented with bloody diarrhea and stomach discomfort, assessed as being caused by late radiation therapy and a rectal ulcer due to bevacizumab use; resolved with fasting and fluid replacement within 2 weeks. d Mucosal bleeding assessed as being caused by bintrafusp alfa and bevacizumab use; resolved with blood transfusion. e One event resolved with tumor compression by gauze and blood transfusion within a few days. f Bleeding in the duodenum resolved with standard gastrointestinal medication and fluid replacement within one week. g Rectal bleeding along with urinary tract infection; patient treated in emergency room with antibiotics and discontinuation of heparin for prior thrombosis, symptoms resolved within 2 weeks. AE, adverse event; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2.
Table S4. Pharmacokinetic exposure summary. AUC, area under the curve; Ceoi, concentration at end of infusion; Ctrough, concentration at the end of the dosing interval; CV, coefficient of variation; NA, not applicable; popPK, population pharmacokinetic; SD, standard deviation.
Figure S1. Change from baseline in sum of diameters of all target lesions per RECIST v1.1 for (A) Cohort 1A and (B) Cohort 1B. CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure S2. Change from baseline in sum of diameters of all target lesions per RECIST v1.1 for Cohort 2. CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Figure S3. Patient-level concentration profile of bintrafusp alfa at Day 1 postdose.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of the healthcare business of Merck KGaA, Darmstadt, Germany. All requests should be submitted in writing to the data sharing portal of the healthcare business of Merck KGaA, Darmstadt, Germany (https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html). When the healthcare business of Merck KGaA, Darmstadt, Germany has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, the healthcare business of Merck KGaA, Darmstadt, Germany will endeavor to gain agreement to share data in response to requests.