Abstract

Polo-like kinase 3 (Plk3) is involved in tumor development with a tumor suppressive function. However, the effect of Plk3 on the chemoresistance remains unclear. It has been documented that activation of the PI3K/AKT signaling pathway by PTEN loss significantly enhances chemoresistance in nonsmall-cell lung cancer (NSCLC). This study aims to evaluate the PTEN regulation by Plk3 and identify targets and underlying mechanisms that could be used to relieve chemoresistance. Our results showed that silencing Plk3 reduced PTEN expression and activated PI3K/AKT signaling by dephosphorylating and destabilizing PTEN in NSCLC cells. Reducing Plk3 expression promoted drug resistance to cisplatin (DDP), while overexpressing Plk3 promoted DDP sensitivity. However, these effects were attenuated when MK2206, a PI3K/AKT inhibitor, was applied. In conclusion, upregulation of Plk3 sensitized NSCLC cells toward DDP, which provides a potential target to restore DDP chemoresponse. We provided novel evidence that the PTEN/PI3K/AKT signaling pathway could be regulated by Plk3 through phosphorylation of PTEN and highlighted the critical role of Plk3 in the DDP resistance of NSCLC.
1. Introduction
Nonsmall-cell lung cancer (NSCLC) is one of the histopathological types of lung cancer, accounting for approximately 80–85% of lung cancer cases.1 For patients with early-stage NSCLC who have no contraindications, surgical resection is an indicated choice. For patients with advanced unresectable tumors, radiation therapy or a combination of radiation therapy and chemotherapy may achieve long-term survival.2 However, only a small number of cases demonstrate satisfied outcomes since NSCLC patients are less sensitive to chemotherapy based on cisplatin (DDP) treatment.3 Alleviating DDP resistance in NSCLC patients will enable a better outcome of chemotherapy.
Polo-like kinase 3 (Plk3) is a serine/threonine protein kinase associated with cell cycle regulation. It appears that Plk3 also participates in the regulation of stress responses in mammals.4 Later studies have revealed that Plk3 is involved in tumor development with a tumor suppressive function.5 In many human malignancies, such as colorectal cancer,6 colon cancer,7 hepatocellular carcinoma,8 gastric cancer,9 and squamous cell lung carcinoma,10 Plk3 is found to be downregulated and implicated in diverse progressions during the tumorigenesis. However, a document focusing on the effects of Plk3 on chemoresistance is limited.
PTEN is a dual phosphatase that has been found to possess both protein and lipid phosphatase activities. In recent years, more and more studies have demonstrated that PTEN serves as a tumor suppressor and metabolic regulator.11 An impaired and/or loss of function of PTEN is involved in tumor development across many cancer types.12 PTEN participates in regulating various progressions of tumor development, such as angiogenesis, metastasis, tumor cell proliferation, and apoptosis.11 Notably, PTEN also regulates chemoresistance in diverse cancers. PTEN loss significantly enhances chemoresistance to DDP in bladder cancer.13 Overexpressed PTEN alleviates the resistance of ovarian cancer cells to DDP by regulating the expression of KRT10.14 An in vivo study proved that recombinant adenovirus-mediated overexpression of PTEN and KRT10 improves the inhibitory effect of cisplatin on ovarian cancer cell xenograft tumor growth.15 Recently, Plk3 has been found to phosphorylate and stabilize PTEN phosphatase.16 Hence, we aimed to investigate whether Plk3 could regulate the chemoresistance of NSCLC by modulating PTEN and the underlying mechanisms. The results showed that Plk3 expression was downregulated in NSCLC and regulated DDP resistance of NSCLC cell lines through the PI3K/AKT signaling pathway. Furthermore, PTEN plays a crucial role in mediating the functions of Plk3.
2. Materials and Methods
2.1. Bioinformatics Analysis
Differential gene expression levels of Plk3, GSK3B, and CSNK2A2 between normal, lung adenocarcinoma (LUAD), and squamous cell carcinoma (LUSC) subtypes were obtained from the Gene Expression Profiling Interactive Analysis 2 (GEPIA2) database (http://gepia2.cancer-pku.cn/#index).
2.2. Cell Culture and Construction of Drug-Resistant Cell Lines
The human bronchial epithelial cell line (16HBE) and NSCLC cell lines A549 and H1299 were purchased from Procell Life Science and Technology Co., Ltd. (Procell, Wuhan, China). 16HBE and H1299 cells were cultured in Roswell Park Memorial Institute 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS; Gibco, Invitrogen, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a moist incubator with 5% CO2 at 37 °C. A549 cells were cultured in Ham’s F-12K medium supplemented with 10% heat-inactivated FBS (Gibco) and 1% penicillin/streptomycin (Sigma-Aldrich) in a moist incubator with 5% CO2 at 37 °C. The DDP-resistant A549 cells (A549/DDP) and DDP-resistant H1299 cells (H1299/DDP) were established as follows: A549 and H1299 cells were subjected to continuous low-dose DDP (0.5–8 μg/mL, Sigma-Aldrich) for 2 months with normal activity. To maintain the DDP-resistant phenotype, A549/DPP and H1299/DPP cells were cultured in the presence of 1 μg/mL DDP. A549/DPP and H1299/DPP cells were cultured for 1 week in a DDP-free medium to eliminate the influence of residual DDP in the culture medium prior to the experiments.
2.3. Cell Transfection
The recombinant plasmids pcDNA3.1/Plk3 (Plk3-OE), pcDNA3.1/PTEN (PTEN-OE), small interfering RNA targeting Plk3 (si-Plk3), si-PTEN, and negative control (si-NC) were obtained from Guangzhou RiboBio Co., Ltd. (RiboBio, Guangzhou, China). For cell transfection, indicated cells (1 × 105 per well) were incubated for 24 h, and then the plasmids (3 μg per well) or siRNAs (400 pmol per well) were transfected into the cells by Lipofectamine 2000 (Invitrogen). At the indicated times after transfection, the cells were harvested for subsequent testing.
2.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNAs were purified using TRIzol reagent (Invitrogen), and then 1 μg of total RNA was used for cDNA synthesis with 20 μL of PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan). The obtained cDNA was subjected to PCR using an SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA, USA). For each sample, mRNA levels were normalized against the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control. Primers used for Plk3 detection: forward 5′-GCG CGA GAA GAT CCT AAA TG-3′, reverse 5′-GAT CTG CCG CAG GTA GTA GC-3′; for ABCB1 detection: forward 5′-TGC TGG AGC GGT TCT ACG-3′, reverse 5′-ATA GGC AAT GTT CTC AGC AAT G-3′; for GAPDH detection: forward 5′-ACC TGA CCT GCC GTC TAG AA-3′, reverse 5′-TCC ACC ACC CTG TTG CTG TA-3′.
2.5. Western Blot
Prepared cellular proteins from indicated cells (50 μg) were resolved with 10% SDS-PAGE, and the Plk3, p-gp, Akt, p-Akt, PTEN, and p-PTEN expression levels were quantified by Western blot analysis following the previously described procedure.17 The primary antibodies against Plk3, p-gp, Akt, p-Akt, PTEN, p-PTEN, and GAPDH and the appropriate HRP-conjugated secondary antibodies were purchased from Affinity Biosciences (Changzhou, China) and Abcam (Cambridge, MA, USA). Western blot signals were visualized by enhanced chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The method of Western blot quantification analysis using ImageJ involves selecting and measuring a white reference standard in the image, calculating correction factors, and adjusting the color calibration.
2.6. CCK-8 Assay
A commercial CCK-8 reagent (Beyotime, Shanghai, China) was used to analyze NSCLC cell viability in response to DDP incubation. Transfected NSCLC cells plated in 96-well plates were incubated with DDP (0, 4, 8, 16, or 32 μg/mL). Afterward, 20 μL of CCK8 reagent was added to the NSCLC cells and incubated for 4 h. Absorbance was determined using a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm. The 50% inhibiting concentration (IC50) value was respectively calculated.
2.7. Flow Cytometry
The apoptotic rates of different cell lines were determined by using an annexin V-FITC/PI-staining assay kit (Beyotime, Nantong, China). Cells (5 × 105 per well) were collected and centrifuged at 1000g for 5 min. Afterward, the cells were resuspended in 195 μL of annexin V-FITC binding buffer and 5 μL of annexin V-FITC. Next, cells were incubated with 10 μL of propidine iodide (PI; 50 mg/mL) in the dark for 20 min. The cell apoptosis was immediately analyzed by flow cytometry (Beckman Coulter, Brea, CA, USA).
2.8. Statistical Analysis
Statistical analysis was performed using Prism, version 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as means ± the standard deviation. Comparisons between the two groups were performed by the Student’s t-test. Comparisons among multiple groups were performed by ANOVA with Tukey’s posthoc method. p values of less than 0.05 were considered to be significant.
3. Results
3.1. Plk3 Expression Is Downregulated in NSCLC Clinical Samples and Cells
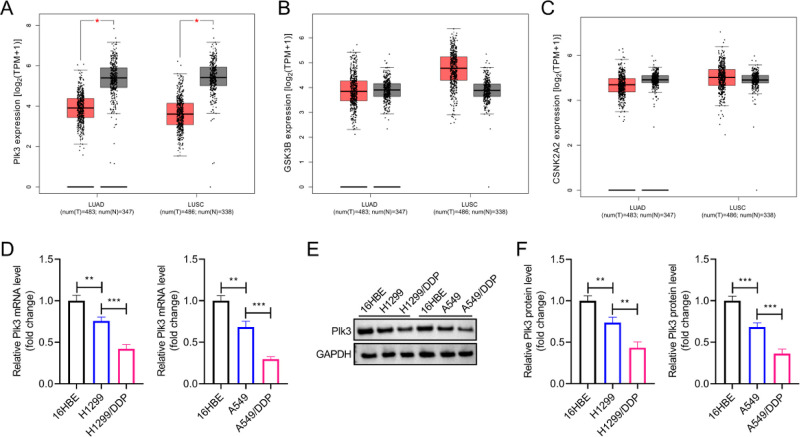
NSCLC encompasses multiple subtypes, including LUAD, LUSC, and large-cell cancers. Among them, LUAD and LUSC are the two major NSCLC subgroups. Utilizing GEPIA2, we assessed the Plk3 expression levels among normal samples and LUAD and LUSC subtypes. We found significantly lower expression levels of Plk3 in both LUAD and LUSC samples (Figure 1A). Despite previous reports suggesting that multiple kinases regulate the biological activity of PTEN, no significant differences were observed in their expression levels between normal and NSCLC tissues, including GSK3B and CSNK2A2 (Figure 1B,C).11 Moreover, Plk3 expression levels, including mRNA and protein levels, were markedly decreased in A549 and H1299 cells compared to human bronchial epithelial 16HBE cells. Lower expression levels of Plk3 were observed in A549/DDP and H1299/DDP cells (Figure 1D–F).
Figure 1.
Plk3 expression is downregulated in NSCLC clinical samples and cell lines. (A–C) Differential gene expression levels of Plk3, GSK3B, and CSNK2A2 between normal, LUAD, and LUSC subtypes were analyzed using GEPIA2 based on the TCGA and GTEx databases. (D) RT-qPCR assay was performed to measure the Plk3 mRNA levels in different cell lines. (E,F) Western blot analysis was conducted for the detection of Plk3 protein levels in different cell lines. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Overexpression of Plk3 Increases the Sensibility of DDP-Resistant NSCLC Cells to DDP
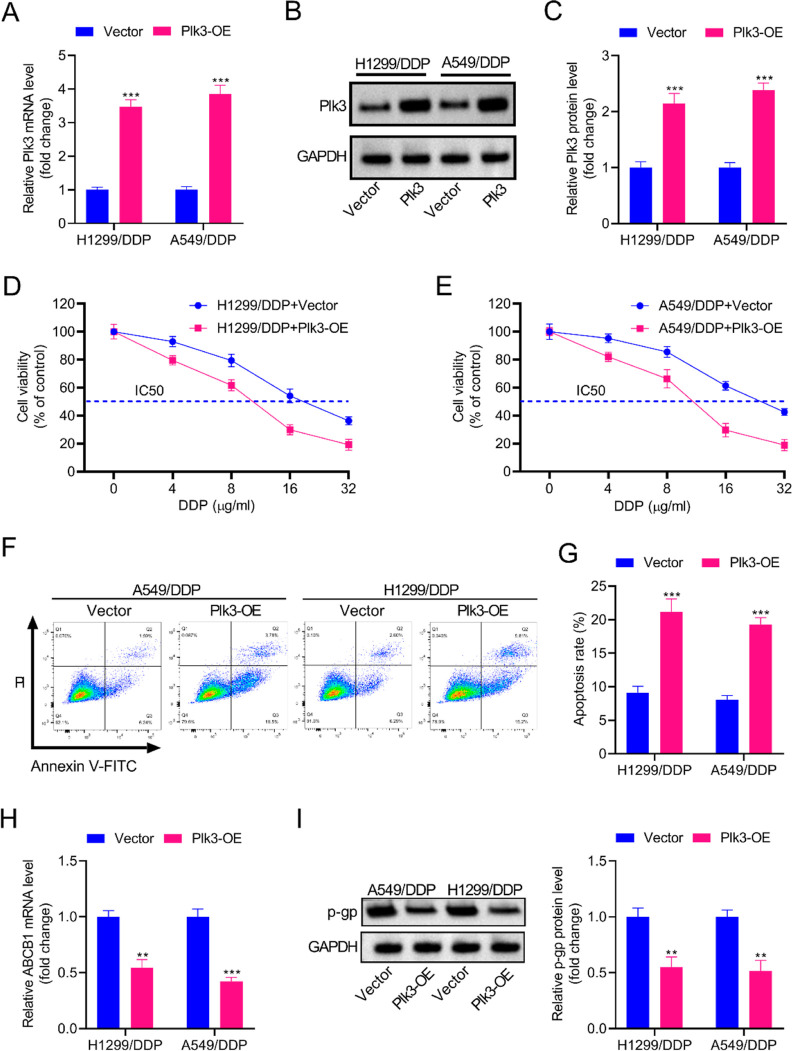
In order to explore the role of Plk3 in regulating DDP resistance, Plk3-overexpressing A549/DDP cells and H1299/DDP cells were respectively constructed. As shown in Figure 2A–C, Plk3 expression was dramatically upregulated in A549/DDP cells and H1299/DDP cells after transfection with the Plk3-OE plasmid. The CCK-8 assay showed that the IC50 values of DDP in A549/DDP-Plk3-OE and H1299/DDP-Plk3-OE cells were significantly reduced when compared with parental cells, implying that Plk3 suppressed the DDP resistance of A549/DDP cells and H1299/DDP cells (Figure 2D,E). The results of flow cytometry demonstrated that Plk3 overexpression promoted cell apoptosis in A549/DDP cells and H1299/DDP cells (Figure 2F,G). Figure 2H,I revealed that the mRNA levels of ABCB1 and the protein levels of p-gp (encoded by the ABCB1 gene) were downregulated in A549/DDP-Plk3-OE and H1299/DDP-Plk3-OE compared to parental cells, which might explain the effects of Plk3 on DDP sensitivity.
Figure 2.
Overexpression of Plk3 enhances the DDP sensitivity of DDP-resistant NSCLC cells. (A–C) Plk3-overexpressing A549/DDP cells and H1299/DDP cells were, respectively, constructed. Results of RT-qPCR and Western blot showed that Plk3 expression was dramatically upregulated in A549/DDP cells and H1299/DDP cells after transfection with the Plk3-OE plasmid. (D,E) Plk3 suppressed the DDP resistance of A549/DDP cells and H1299/DDP cells, as shown by reduced IC50 values of DDP. (F,G) Flow cytometry demonstrated that Plk3 overexpression promoted A549/DDP and H1299/DDP cell apoptosis. (H,I) The mRNA levels of ABCB1 and the protein levels of p-gp (encoded by the ABCB1 gene) were downregulated by Plk3, as shown by RT-qPCR and Western blot. **p < 0.01, ***p < 0.001.
3.3. Plk3 Silencing Decreases the Sensitivity of DDP-Sensitive NSCLC Cells to DDP via the PI3K/AKT Signaling
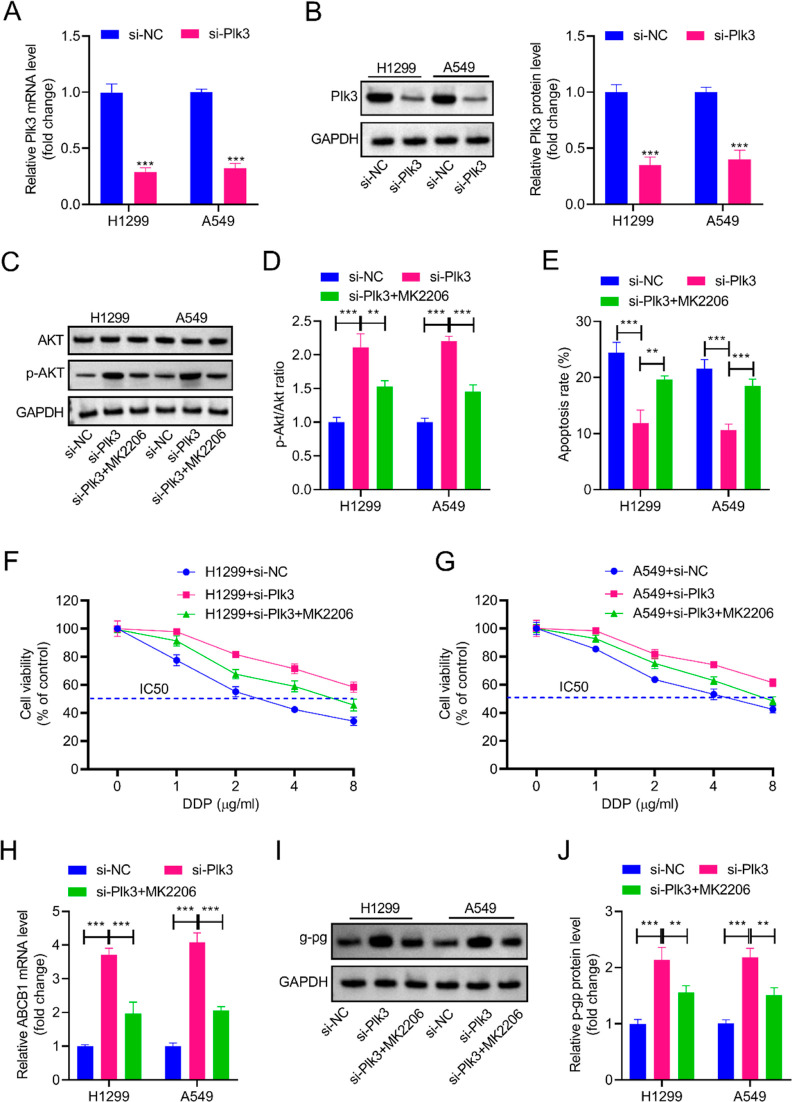
Plk3-silencing cells were successfully established in A549 and H1299 cells through transfection with si-Plk3, as confirmed by RT-qPCR and Western blotting (Figure 3A,B). Plk3-silencing A549 and H1299 cells showed higher levels of p-Akt/Akt ratio, which could be partially reversed by MK2206 (10 μM), an inhibitor of PI3K/AKT signaling (Figure 3C,D). Knockdown of Plk3 caused an obvious decrease in cell apoptosis as well as an increase in the IC50 values of DDP in A549 and H1299 cells. Interfering with MK2206 blocked the effects of si-Plk3 on cell apoptosis and IC50 values of DDP (Figure 3E–G). A549 and H1299 cells transfected with si-Plk3 showed increased mRNA levels of ABCB1 and protein levels of p-gp. MK2206 treatment attenuated the inductive effects of si-Plk3 on ABCB1/p-gp expression (Figure 3H–J). These results suggested that PI3K/AKT signaling was involved in the regulatory effect of Plk3 on DDP sensitivity.
Figure 3.
Knockdown of Plk3 decreases the DDP sensitivity of NSCLC cells via regulating PI3K/AKT signaling. (A,B) Plk3-silencing A549/DDP and H1299/DDP cell lines were respectively constructed through transfection with si-Plk3. RT-qPCR and Western blot showed that Plk3 expression was dramatically downregulated after transfection with si-Plk3. (C,D) A549 and H1299 cells transfected with si-NC or si-Plk3 were incubated with MK-2206 (10 μM) for 24 h in the presence of 2 μg/mL DDP. Plk3-silencing A549 and H1299 cells showed higher levels of the p-Akt/Akt ratio, which could be partially reversed by MK2206. (E) Flow cytometry showed that the si-Plk3-caused decrease in cell apoptosis was mitigated by MK2206. (F,G) Knockdown of Plk3 caused an obvious increase in the IC50 values of DDP in A549 and H1299 cells. While interfering with MK2206 blocked the effects of si-Plk3 on the IC50 values of DDP. (H–J) A549 and H1299 cells transfected with si-Plk3 showed increased mRNA levels of ABCB1 and protein levels of p-gp, which could be attenuated by MK2206. **p < 0.01, ***p < 0.001.
3.4. Plk3 Phosphorylates and Stabilizes PTEN
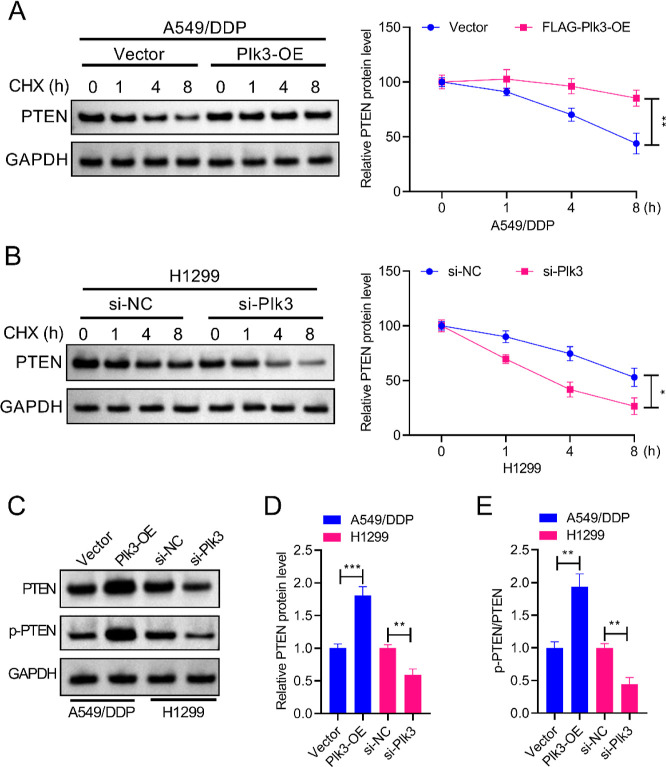
In A549/DDP cells and A549/DDP-Plk3-OE cells, treatment with cycloheximide (CHX) (0.1 mg/mL) for the specified durations caused decreased PTEN protein levels. In addition, A549/DDP-Plk3-OE cells exhibited higher expression levels of PTEN than did control A549/DDP cells (Figure 4A). Simultaneously, incubation with CHX (0.1 mg/mL) also led to markedly decreased PTEN protein levels in both H1299 and H1299-si-Plk3 cells. Knockdown of Plk3 caused expression levels of PTEN to be lower than those of control H1299 cells, implying that Plk3 prevented the degradation of the PTEN protein (Figure 4B). In addition, Plk3 overexpression induced expression levels of PTEN and p-PTEN in A549/DDP cells, and Plk3 knockdown suppressed PTEN and p-PTEN expression in H1299 cells (Figure 4C–E). Collectively, these findings implied that Plk3 promoted the phosphorylation of PTEN and contributed to the stabilization of PTEN by preventing the degradation of the PTEN protein.
Figure 4.
Plk3 phosphorylates and stabilizes PTEN in both DDP-sensitive NSCLC cells and DDP-resistant NSCLC cells. (A,B) For CHX treatment, the cells were respectively incubated with CHX (0.1 mg/mL) for 0, 1, 4, or 8 h. A western blot was then performed to measure PTEN protein degradation. (C–E) Western blot also showed that Plk3 overexpression induced expression levels of PTEN and p-PTEN in A549/DDP cells, and Plk3 knockdown suppressed PTEN and p-PTEN expression in H1299 cells. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. Plk3 Inhibits PI3K/AKT Signaling by Regulating PTEN
To further confirm whether the regulatory effect of Plk3 on PI3K/AKT signaling was mediated by PTEN, A549/DDP cells were transfected with si-PTEN to knock down PTEN. The decreased p-Akt/Akt ratio caused by Plk3 overexpression was elevated after transfection with si-PTEN (Figure 5A,B). Meanwhile, H1299 cells were transfected with PTEN-OE to overexpress PTEN. Knockdown of Plk3 significantly increased the p-Akt-to-Akt ratio, which could be reversed by PTEN overexpression (Figure 5C,D). The results indicated that Plk3 inhibited the activation of PI3K/AKT signaling by regulating PTEN. Collectively, Plk3 increases the chemosensitivity of NSCLC cells to cisplatin through PTEN-mediated inhibition of the PI3K/AKT signaling pathway.
Figure 5.
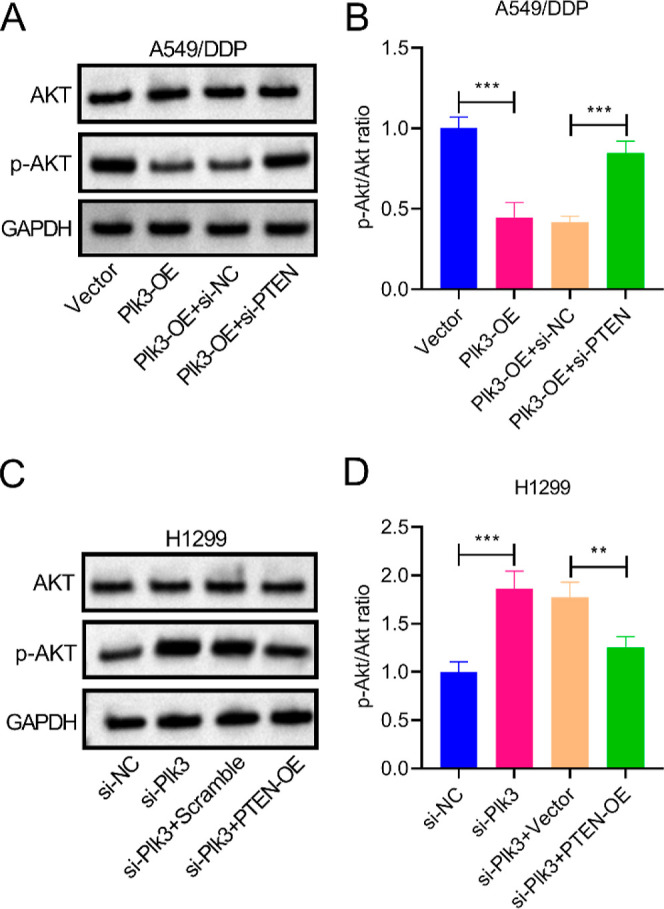
Plk3 inhibits PI3K/AKT signaling by regulating PTEN. (A,B) Decreased p-Akt/Akt ratio caused by Plk3 overexpression was elevated after transfection with si-PTEN. (C,D) Knockdown of Plk3 significantly increased the p-Akt/Akt ratio, which could be reversed by PTEN overexpression. **p < 0.01, ***p < 0.001.
4. Discussion
In addition to well-established regulation of tumor development, a recent study has found that Plk3 may contribute to improving the outcomes of chemoradiotherapy. Rodel et al.18 reported that Plk3 is associated with improved local tumor control and prolonged survival time in anal carcinoma patients who underwent concomitant chemoradiotherapy. Here we found that Plk3 expression was downregulated in NSCLC and regulated the DDP resistance of NSCLC cell lines. It is evident that multidrug resistance mediated by ABCB1/p-gp remains a major cause of chemotherapy failure in cancer.19 We explored the effects of Plk3 on the expression of ABCB1/p-gp, and the results showed that the mRNA levels of ABCB1 and protein levels of p-gp were downregulated in Plk3-overexpressing DDP-resistant NSCLC cells.
Previous in vitro and in vivo studies showed that impaired, mutated, or loss of PTEN is associated with high tumor recurrence, low survival rates, or chemoresistance in lung cancers.20,21 Recent studies have found that Plk3 has the capacity to phosphorylate and stabilize PTEN phosphatase. Xu et al.16 reported that Plk3 null murine embryonic fibroblasts contain a reduced expression level of PTEN. Further investigations prove that purified recombinant Plk3 efficiently phosphorylates PTEN at threonine 366 and serine 370 residues. In our study, we found that Plk3 prevented the degradation of the PTEN protein and promoted the phosphorylation of PTEN in both DDP-sensitive and DDP-resistant NSCLC cells. These findings suggested a potential link between the Plk3/PTEN axis and DDP resistance.
Notably, PTEN is a negative regulator of the PI3K/AKT signaling pathway, which is a major cell growth and survival signaling pathway.22 PTEN inhibits PI3K signaling by dephosphorylating the phosphoinositides. Activation of the PI3K pathway leads to elevated activity of AKT, one of the best-characterized targets of PI3K.23 Activated AKT phosphorylates a plethora of downstream targets to regulate multiple cellular processes. More recently, PTEN/PI3K/AKT has been demonstrated to regulate cancer cell sensitivity to DDP. PTEN reverses chemoresistance to cisplatin by inducing the inactivation of the PI3K/AKT cell survival pathway in human ovarian cancer cells.24 Transmembrane prostate androgen-induced RNA (PMEPA1) interference activates PTEN/PI3K/AKT signaling, thereby enhancing the sensitivity of pancreatic cancer cells to DDP.25 Shi et al.21 demonstrated that associated PTEN downregulation following exosome-derived microRNA-20a (miR-20a) treatment enhances the activation of the PI3K/AKT pathway to promote the progression and DDP resistance of NSCLC. PTEN/PI3K/AKT is involved in the role of miR-25-3p in accelerating DDP tolerance in NSCLC cells.26 MiR-21 also confers cisplatin resistance in gastric cancer, and regulation of the PTEN/PI3K/Akt pathway serves as a potential mechanism.27 This study proved that Plk3 overexpression or Plk3 silencing regulated the activation of PI3K/AKT signaling, which could be reversed by si-PTEN or PTEN overexpression, implying that Plk3 inhibited PI3K/AKT signaling by regulating PTEN.
Hypoxia-inducible factor-1 α (HIF-1α) is a key transcription factor that plays crucial roles in cancer progression through diverse mechanisms such as regulating cancer stem cell maintenance, survival and proliferation of cells, invasion and metastasis, angiogenesis, and treatment resistance.28 Previous work demonstrated that Plk3 may regulate HIF-1α through direct phosphorylation or by regulating PTEN/PI3K/AKT signaling.4 Given the roles of Plk3 in response to hypoxic responses, further studies on the detailed mechanisms of Plk3/PTEN/PI3K/AKT in regulating the HIF-1α pathway are highly warranted.
It is worth mentioning that although the research results mentioned above are exciting, there are still some challenging questions that cannot be concluded due to limitations in the experimental conditions. For instance, the A549 and H1299 cell lines used in this study were both derived from the NSCLC tissues of Caucasian males and cultured in vitro. Extensive studies have reported that due to the diversity of genetic backgrounds and differences in hormone secretion levels, there are variations in the response of NSCLC patients from different ethnicities and genders to conventional chemotherapy,29 targeted therapy,30 and immunotherapy.29,31 However, due to limitations in the experimental conditions, this study did not further validate NSCLC cell lines derived from females or other ethnicities. Furthermore, various kinases have been reported to be involved in the regulation of PTEN activity and stability. However, our study did not conduct a comprehensive screening to systematically explore the kinase regulatory network of PTEN. Although it has been reported that Plk3 stabilizes PTEN by phosphorylating the threonine 366 and serine 370 residues, our results do not elucidate whether these residues are crucial for regulating drug resistance.16 Considering the current limitations in research conditions and experimental priorities, we intend to investigate the kinase network governing PTEN stability in our forthcoming work, elucidating the critical residues involved in PTEN-mediated drug resistance and shedding light on the molecular mechanisms of PTEN in chemotherapy resistance.
5. Conclusions
In summary, our results showed that Plk3 expression was downregulated in NSCLC and regulated the DDP resistance of NSCLC cell lines. Downregulation of Plk3 contributed to the dephosphorylation and destabilization of PTEN, thus resulting in the activation of PI3K/AKT signaling in NSCLC cells. We provide novel evidence that Plk3-mediated regulation of the PTEN/PI3K/AKT signaling pathway played a very significant player in the DDP resistance of NSCLC and thus served as a therapeutic target for chemotherapy in NSCLC.
Data Availability Statement
Publicly available data sets analyzed by GEPIA2 could be obtained from the Cancer Genome Atlas (TCGA; https://portal.gdc.cancer.gov/) and Genotype-Tissue Expression (GTEx; https://www.gtexportal.org/home/datasets) databases. Other experimental data in this research are available from the corresponding author upon reasonable request.
Author Contributions
M.X. and X.D. contributed equally to this research. M.X., X.D., and Q.L. designed the study. M.X., X.D., and N.X. performed the experiments. Z.Z. and M.Y. analyzed the data. The first draft of the manuscript was written by M.X. and X.D., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
References
- Yang T.; Xiong Y.; Zeng Y.; Wang Y.; Zeng J.; Liu J.; Xu S.; Li L. S. Current status of immunotherapy for non-small cell lung cancer. Front. Pharmacol 2022, 13, 989461. 10.3389/fphar.2022.989461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Zimmermann S.; Parikh K.; Mansfield A. S.; Adjei A. A. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin. Proc. 2019, 94 (8), 1599–1622. 10.1016/j.mayocp.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Xiao L.; Lan X.; Shi X.; Zhao K.; Wang D.; Wang X.; Li F.; Huang H.; Liu J. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Dis. 2017, 8 (5), e2803 10.1038/cddis.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.; Dai W.; Li C. Polo-like kinase 3, hypoxic responses, and tumorigenesis. Cell Cycle 2017, 16 (21), 2032–2036. 10.1080/15384101.2017.1373224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.; Wang Q.; Jiang Y.; Zhang Y.; Vega-Saenzdemiera E.; Osman I.; Dai W. Roles of Polo-like kinase 3 in suppressing tumor angiogenesis. Exp. Hematol. Oncol. 2012, 1 (1), 5. 10.1186/2162-3619-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou B.; Sun H.; Zhao J.; Xu Z.; Liu Y.; Feng H.; Peng Z. Polo-like kinase 3 inhibits glucose metabolism in colorectal cancer by targeting HSP90/STAT3/HK2 signaling. J. Exp. Clin. Cancer Res. 2019, 38 (1), 426. 10.1186/s13046-019-1418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W.; Liu T.; Wang Q.; Rao C. V.; Reddy B. S. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int. J. Oncol. 2002, 20 (1), 121–126. 10.3892/ijo.20.1.121. [DOI] [PubMed] [Google Scholar]
- Pellegrino R.; Calvisi D. F.; Ladu S.; Ehemann V.; Staniscia T.; Evert M.; Dombrowski F.; Schirmacher P.; Longerich T. Oncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinoma. Hepatology 2010, 51 (3), 857–868. 10.1002/hep.23467. [DOI] [PubMed] [Google Scholar]
- Xie M.; Sun M.; Zhu Y. N.; Xia R.; Liu Y. W.; Ding J.; Ma H. W.; He X. Z.; Zhang Z. H.; Liu Z. J.; Liu X. H.; De W. Long noncoding RNA HOXA-AS2 promotes gastric cancer proliferation by epigenetically silencing P21/PLK3/DDIT3 expression. Oncotarget 2015, 6 (32), 33587–33601. 10.18632/oncotarget.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S.; Lu X.; Zhang Z.; Meng R.; Li M.; Xia S. Identification and assessment of PLK1/2/3/4 in lung adenocarcinoma and lung squamous cell carcinoma: Evidence from methylation profile. J. Cell. Mol. Med. 2021, 25 (14), 6652–6663. 10.1111/jcmm.16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y.; Chen J.; He L.; Stiles B. L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamaspishvili T.; Berman D. M.; Ross A. E.; Scher H. I.; De Marzo A. M.; Squire J. A.; Lotan T. L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15 (4), 222–234. 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.; Wang B.; Huang J.; Huang M.; Lin T. YTHDC1 positively regulates PTEN expression and plays a critical role in cisplatin resistance of bladder cancer. Cell Prolif. 2023, 56 (7), e13404 10.1111/cpr.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Wang K.; Liu W.; Hao Q. PTEN overexpression improves cisplatin-resistance of human ovarian cancer cells through upregulating KRT10 expression. Biochem. Biophys. Res. Commun. 2014, 444 (2), 141–146. 10.1016/j.bbrc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Wu H.; Wang K.; Liu W.; Hao Q. Recombinant adenovirus-mediated overexpression of PTEN and KRT10 improves cisplatin resistance of ovarian cancer in vitro and in vivo. Genet. Mol. Res. 2015, 14 (2), 6591–6597. 10.4238/2015.June.18.1. [DOI] [PubMed] [Google Scholar]
- Xu D.; Yao Y.; Jiang X.; Lu L.; Dai W. Regulation of PTEN stability and activity by Plk3. J. Biol. Chem. 2010, 285 (51), 39935–39942. 10.1074/jbc.M110.166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Li Y.; Hu C.; Chen Y.; Chen Z.; Chen Z. S.; Zhang J. Y.; Fang S. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol. Cancer 2022, 21 (1), 103. 10.1186/s12943-022-01524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodel F.; Martin D.; Helmke C.; Balermpas P.; Fokas E.; Wieland U.; Rave-Frank M.; Kitz J.; Matthess Y.; Raab M.; Strebhardt K.; Rodel C. Polo-like kinase 3 and phosphoT273 caspase-8 are associated with improved local tumor control and survival in patients with anal carcinoma treated with concomitant chemoradiotherapy. Oncotarget 2016, 7 (33), 53339–53349. 10.18632/oncotarget.10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellhoner H.; Weiss A.; Schulz A.; Adermann K.; Braitbard O.; Bar-Sinai A.; Hochman J. Reversing ABCB1-mediated multi-drug resistance from within cells using translocating immune conjugates. J. Drug Target 2012, 20 (5), 445–452. 10.3109/1061186X.2012.685473. [DOI] [PubMed] [Google Scholar]
- Sirhan Z.; Alojair R.; Thyagarajan A.; Sahu R. P. Therapeutic Implications of PTEN in Non-Small Cell Lung Cancer. Pharmaceutics 2023, 15 (8), 2090. 10.3390/pharmaceutics15082090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.; Zhu W.; Huang Y.; Zhuo L.; Wang S.; Chen S.; Zhang B.; Ke B. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin. Transl. Med. 2022, 12 (7), e989 10.1002/ctm2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A.; Blanco-Aparicio C.; Renner O.; Link W.; Leal J. F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8 (3), 187–198. 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- Perez-Ramirez C.; Canadas-Garre M.; Molina M. A.; Faus-Dader M. J.; Calleja-Hernandez M. A. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics 2015, 16 (16), 1843–1862. 10.2217/pgs.15.122. [DOI] [PubMed] [Google Scholar]
- Wu H.; Cao Y.; Weng D.; Xing H.; Song X.; Zhou J.; Xu G.; Lu Y.; Wang S.; Ma D. Effect of tumor suppressor gene PTEN on the resistance to cisplatin in human ovarian cancer cell lines and related mechanisms. Cancer Lett. 2008, 271 (2), 260–271. 10.1016/j.canlet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Cheng T.; Xie P.; Wang L.; Chen H.; Cheng Z.; Zhou J. PMEPA1 interference activates PTEN/PI3K/AKT, thereby inhibiting the proliferation, invasion and migration of pancreatic cancer cells and enhancing the sensitivity to gemcitabine and cisplatin. Drug Dev. Res. 2022, 83 (1), 64–74. 10.1002/ddr.21844. [DOI] [PubMed] [Google Scholar]
- Sun B.; Hu N.; Cong D.; Chen K.; Li J. MicroRNA-25-3p promotes cisplatin resistance in Non-small-cell lung carcinoma (NSCLC) through adjusting PTEN/PI3K/AKT route. Bioengineered 2021, 12 (1), 3219–3228. 10.1080/21655979.2021.1939577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. M.; Huang C.; Li X. F.; Yu M. Z.; He Y.; Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 2013, 306, 162–168. 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Rashid M.; Zadeh L. R.; Baradaran B.; Molavi O.; Ghesmati Z.; Sabzichi M.; Ramezani F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. 10.1016/j.gene.2021.145796. [DOI] [PubMed] [Google Scholar]
- Inoue C.; Miki Y.; Suzuki T. New Perspectives on Sex Steroid Hormones Signaling in Cancer-Associated Fibroblasts of Non-Small Cell Lung Cancer. Cancers 2023, 15 (14), 3620. 10.3390/cancers15143620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suay G.; Garcia-Cañaveras J. C.; Aparisi F.; Lahoz A.; Juan-Vidal O. Sex Differences in the Efficacy of Immune Checkpoint Inhibitors in Neoadjuvant Therapy of Non-Small Cell Lung Cancer: A Meta-Analysis. Cancers 2023, 15 (18), 4433. 10.3390/cancers15184433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reungwetwattana T.; Liang Y.; Zhu V.; Ou S. H. I. The race to target MET exon 14 skipping alterations in non-small cell lung cancer: The Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer 2017, 103, 27–37. 10.1016/j.lungcan.2016.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available data sets analyzed by GEPIA2 could be obtained from the Cancer Genome Atlas (TCGA; https://portal.gdc.cancer.gov/) and Genotype-Tissue Expression (GTEx; https://www.gtexportal.org/home/datasets) databases. Other experimental data in this research are available from the corresponding author upon reasonable request.