Abstract
Morocco is known for its high plant biodiversity, but many plants are poorly valorized. For this reason, this study aims to valorize the methanolic and aqueous extracts of Melitotus albus leaves by studying their antioxidant activity and toxicity. The extracts’ antioxidant activity is assessed using the FRAP, DPPH, CAT, and ABTS methods. The chemical composition was determined using LC–MS analysis and evaluated using in silico studies. The results revealed that the total polyphenol content of the aqueous extract, 259.26 ± 7.79 (mg GAE/g), is higher than that of the methanolic extract, 131.41 ± 12.64 (mg GAE/g). The antioxidant activity by the methods of DPPH, ABTS, and phosphor molybdenum of aqueous extracts (0.087 ± 0.015, 0.014 ± 0.001 and 6.157 ± 1.050 mg eq vit C/g, respectively) is greater than that of methanolic extracts (0.107 ± 0.02, 0.167 ± 0.03, and 0.453 ± 0.014 mg eq vit C/g, respectively). The reducing power of iron (FRAP) shows that the methanolic extract has a greater reducing power than that of the aqueous extract with a low IC50 (0.011 ± 0.003 and 0.199 ± 0.016 mg/mL, respectively). The study of acute and subacute toxicity shows that the administration of the aqueous extract of M. albus at different doses increases the body weight of rats without modifying their general behavior. The M. albus extract had a 99.99% total phenolic content, as determined by LC–MS, consisting of 12 different components. The primary constituents of the extract are chlorogenic acid (43.68%), catechin/epicatechin (24.82%), quercetin-3-O-glucuronic acid (9.91%), naringin (7.64%), and p-hydroxybenzoic/salicylic acid (2.95%). The in-silico study showed that these compounds can passively permeate through the blood and have a beneficial effect on various organs of the body. Based on these results, M. albus can be used as a medicinal plant in phytotherapy, cosmetics, or as a dietary supplement. The bioactive compounds of these plants will require a lot of further effort in terms of isolation and characterization.
1. Introduction
Since ancient times, many herbal remedies have been used in folk medicine. They have been used worldwide for thousands of years as natural medicines with therapeutic and other pharmacological effects. Currently, 80% of people worldwide rely on traditional medicine for their primary healthcare needs, according to data from the World Health Organization (WHO). Preliminary results of a study carried out on behalf of the WHO have shown that the number of people using medicinal plants is significant and increasing, even among young people.1 Only in the past century, the traditional use of whole herbs, plant extracts, or isolated ingredients has left the ground to the era of fully synthetic drugs, weakening the use of herbal remedies.2 Finding novel sources of natural bioactive compounds is currently an interesting approach for the design of novel pharmaceuticals, food supplements, and functional foods.3
The use of medicinal plants or parts of plants (leaves, rhizomes, roots, seeds, and flowers) can take many forms, such as the fresh raw form and preparations in the form of teas, decoctions, powdered plant material, or extracted forms of medicinal agents (juices, water or alcohol extracts, tinctures, essential oils, resins, and balms).4 Many medicinal plants contain large amounts of antioxidants such as polyphenols, which can play an important role in adsorbing and neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides. Many of these phytochemicals possess significant antioxidant capacities that are associated with lower occurrence and lower mortality rates of several human diseases.5 Although some herbal medicines have promising potential and are widely used, many have not been tested and their use is not monitored. It is therefore very difficult to know their potential adverse effects, to identify the safest and most effective therapies and to promote their rational use.6 For this reason, studies looking at the toxicological and pharmacological profiles of medicinal plants, their extracts, and their formulations have increased dramatically over the past few years.7
Melilotus albus belongs to the Fabaceae family. M. albus is an aromatic, herbaceous biennial plant with a strong taproot, trifoliate leaves, and racemes of white flowers. It grows in full sun or partial shade in limestone and loam soils. M. albus is considered an important plant for honey production.8 It is also a wild edible plant rich in vitamin C. The young leaves are eaten in small quantities in mixed salads. The seeds can be used as spices. The whole plant can be dried and used as a flavoring in desserts, sauces, and drinks.9 According to the available literature, the biological properties, content, and bioactive composition of M. albus have been little investigated.10M. albus has been shown to contain high levels of coumarins,11 which explains their use in traditional medicine as an anticoagulant agent and as ointments for external ulcers.12 In recent years, the use of medicinal plants has been supported and encouraged, but measuring antioxidant activity is crucial to obtaining their health advantages. To the best of our knowledge, there are no further studies on the antioxidant activity and toxicological activity of the Moroccan M. albus leaves. We are aware that M. albus’ biological activities have not been thoroughly studied and that their therapeutic potential has not yet been fully investigated. The present study aims to valorize M. albus leaf extracts by investigating the content of phenolic compounds, including antioxidant activity and chemical composition, in silico studies, and their toxicity.
2. Materials and Methods
2.1. Plant Material
The M. albus plant was harvested at its mature age in February 2022 from Taounate, Morocco. The leaves of the plant were sorted and reduced to powder. The powder obtained was stored carefully at room temperature (14.00 ± 2.00 °C) away from light until the day of the preparation of the extracts.
2.2. Extraction Method
The maceration method was used to prepare the methanolic and aqueous extracts to evaluate their antioxidant activity. Ten grams of dried leaves were macerated in 100 mL of methanol or distilled water for 24 h at room temperature (14 °C). The extracts obtained were filtered and evaporated and then stored at 4 °C.
The aqueous extract tested on rats was prepared by the decoction technique, which consists of boiling 10 g of the leaves in 100 mL of distilled water for 30 min. The extract obtained was filtered and concentrated using a steamer.
2.3. Yield
The yield of plant material extraction was defined as the ratio between the mass of the dry extract obtained and the mass of the plant material. The following formula is used to calculate it
where M0 is the plant material, and M1 is the mass in grams of the dry extract.
2.4. Determination of Total Flavonoid Contents
The flavonoid content of the methanolic and aqueous extracts was measured by colorimetric testing using aluminum chloride. 500 μL of aluminum chloride (20%) and 500 μL of quercetin were combined in a 500 μL sample volume and allowed to react for 1 h at room temperature and in complete darkness. We measured the absorbance at 420 nm. Milligrams of quercetin equivalents (mg of QE/g of DW) per gram of dry weight were used to indicate the total flavonoid content of each extract. Using quercetin as the reference, the calibration curve was created.13,14
2.5. Determination of Total Phenolic Content
The total polyphenol contents of the methanolic and aqueous extracts were calculated using the Folin-Ciocalteu method.15 A known dilution of the extract in 0.5 and 2 mL of sodium carbonate at 7% was combined with 2.5 mL of 10% Folin-Ciocalteu after two hours of a dark, room–temperature reaction. Jasco v-530 was used to measure the absorbance at 760 nm. The polyphenol content is expressed in milligrams of gallic acid equivalents per gram of dry weight of the extract (mg of GAE/g DW). Gallic acid at different concentrations (from 0.13 to 0.013 mg/mL) was utilized as a standard to create a calibration curve.16
2.6. Antioxidant Activity
2.6.1. DPPH Free Radical Scavenging Test
Using the Wu17 method, the antioxidant activity of the methanolic and aqueous extracts to scavenge the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was evaluated. At different concentrations, 0.1 mL of the sample or standard was mixed with 1.5 mL of the methanol extract that contained 0.1 mmol of DPPH. The mixture’s absorbance at 517 nm was measured using a spectrophotometer after 30 min of incubation at room temperature in the dark. The positive control was BHT. The formula below was used to compute the percentage inhibition
where A0 is the absorbance of the negative control and As is the absorbance of the sample.
2.6.2. Power Reduction Capability (FRAP)
The methanolic and aqueous extracts’ power to reduce iron utilizing antioxidants was evaluated using the method outlined by Oyaizu.18 500 μL of phosphate buffer (0.2 M, pH 6.6) and 500 μL of potassium ferricyanide [K3Fe (CN) 6] 1% are added to 200 μL of the extract. After incubating the solution obtained at 50 °C for 20 min, 500 μL of trichloroacetic acid 10% was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 mL) was mixed with 500 μL of distilled water and 100 μL of FeCl3 (0.1%). Absorbance was measured at 700 nm. Quercetin was used as the standard. The result was expressed in IC50 (mg/mL). Plotting the absorbance against the matching extract concentration allowed us to determine the extract concentration (IC50) that corresponds to 0.5 absorbances.19
2.6.3. ABTS Radical Scavenging Activity
According to Re et al.,20 ABTS + radical scavenging activity was conducted. The ABTS + cationic radical was obtained by the reaction between 100 μL of 70 mM potassium persulfate K2S2O8 and 10 mL of 2 mM ABTS (diammonium salt). Incubation of the resulting mixture was carried out in the dark for 24 h at room temperature before use. 2850 μL of ABTS + solution is mixed with 150 μL of extract or Trolox (positive control). At 734 nm, absorbance values were measured in comparison to a blank after 30 min of incubation in the dark. The following formula was used to express the extract’s antiradical power as a percentage inhibition of the ABTS + radical
where As is the absorbance of the sample and Ac is the absorbance of the control. The IC50 values were calculated as the concentrations providing 50% of the initial ABTS + radical inhibition.
2.6.4. Total Antioxidant Capacity
The production of a green phosphate Mo(V) complex after the reduction of Mo(VI) to Mo(V) in an acidic pH was used to measure the total antioxidant capacity.21 The reagent solution (0.6 mol/L sulfuric acid, 4 mmol/L ammonium molybdate, and 28 mmol/L sodium phosphate) was diluted to 1 mL and then 25 L of the extract was added. 90 min of incubation at 95 °C is followed by cooling to room temperature. Jasco v-530 was used to detect the absorbance at 695 nm. The amount of vitamin C equivalent in grams of dry weight (mg of vitamin C E/g of DW) is used to measure total antioxidant capability.
2.7. Toxicity Study
Acute and subacute toxicity was performed on rats, male and female. The animals obtained from the Faculty of Sciences Dhar El Mahraz were between 4 and 6 weeks old and weighed between 70 and 100 g of body weight. They were placed in cages under animal facility conditions.
2.7.1. Acute Toxicity
After administration of the substance, the rats were observed for 14 days, several times a day, according to the guidelines of the OECD and CCAC.22,23 The rats were divided into four batches of five individuals and acclimatized for 3 days before starting the experiment. They were kept on an empty stomach for 18 h before the administration of the various extracts. The first batch (controls) received distilled water, while the second, third, and fourth groups received a single oral administration of M. albus’s aqueous extract at doses of 100, 200, and 600 mg/kg. Changes in general behavior, mortality, and body weight of rats from each batch were monitored for 14 days.
2.7.2. Subacute Toxicity
The study was conducted according to OECD guideline 407 (OECD, 2008b).24 Three groups of five mice were used, and groups 2, 3, and 4 received M. albus aqueous extract at doses of 100, 200, and 600 mg/kg, while group 1 (control groups) received the distilled form of water by mouth. The rats were treated daily for 28 days, during which time changes in general behavior, mortality, and body weight were measured daily. On the 29th day, all the rats were anesthetized and sacrificed to remove their organs (liver, kidneys, and spleen); thus, the blood was collected in dry tubes to carry out biochemical analyses, including ASAT, ALAT, ALP, CREA, and UREA.
2.8. LC–MS/MS Analysis of the M. albus Extract
The chemical profile of the M. albus extract was established using ultrahigh-performance liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry (LCMS8060, Shimadzu Italy, Milan). Specifically, the source settings were configured as follows: a nebulizing gas flow rate of 2.9 L/min, a heating gas flow rate of 10 L/min, an interface temperature of 300 °C, a Linear Ion Trap detector temperature of 250 °C, a thermal block temperature of 400 °C, and a drying gas flow rate of 10 L/min. We developed an internal database that includes polyphenol derivatives through qualitative analysis. The separation of compounds and standards was carried out on a C18 column with dimensions of 3 × 100 mm and a particle size of 2.6 μm (Phenomenex, Torrance, CA, USA). The mobile phase consisted of acetonitrile (A) and water with 0.01% formic acid (B). The M. albus extract was added to a mixture of acetonitrile and water in a 1:1 ratio. The solution (20 μL) was then diluted with acetonitrile (980 μL) and injected into the instrument for analysis. A molecule was considered positive if its area under the curve was greater than that of the blank sample. In cases of very similar structures, the distinction was made using retention time, with the instrument configured to record the molecular mass in the third quadrupole.25,26
2.9. Prediction Molecular Docking Analysis
An in-silico study was carried out on 12 chemical compounds extracted from M. albus, a plant of the Fabaceae family. In the first stage, the molecules under investigation were examined by their physicochemical and pharmacokinetic properties of adsorption, distribution, excretion, metabolism, excretion, and toxicity (ADMET) based on the five rules of Lipinski and the predictive model of Egan BOILD-Egg. Thereafter, the major compound’s inhibition mechanism was evaluated using molecular docking technology toward antioxidant, antifungal, and antibacterial proteins. The physicochemical and pharmacokinetic features of ADMET were predicted using SWISADME and PKCSM online servers.27,28 Chlorogenic acid, as the major compound, as extracted with the highest value of area (AUC of 43.68%), was docked to the NADPH oxidase from Lactobacillus, the secreted aspartic protease from Candida albicans, and the crystal structure of the FimH lectin domain from Escherichia coliK12, encoded in protein data bank (PDB) by 2CDU, 1ZAP, and 4XO8, respectively. Three-dimensional crystal structures of oxidoreductase, aspartic protease, and cell adhesion proteins were uploaded from the RCSB PDB, with resolutions of 1.8, 2.50, and 1.7 Å, respectively. The targeted proteins were prepared by removing water molecules and all cocrystallized ligands, suspended to each protein, and adding Gasteiger charges. Then, chlorogenic acid was docked to each targeted protein using AutoDock 4.2 software.29 The grid boxes were centralized on each macromolecule (protein), putting the maximum sizes 126, 126, and 126 in their three-dimensional structures with a spacing of 0.375 Å. In the last step, 3- and 2-dimensional interactions of the produced (ligand–protein) complexes were visualized with the help of Discovery Studio 2021 software30 to examine their potential inhibition toward antioxidant, antifungal, and antibacterial proteins.
2.10. Statistical Analysis
The results were expressed as means of three experiments ± standard deviation (±SD). For the choice of the type of statistical analysis (parametric or nonparametric), the verification of the homogeneity of variances and normality was checked. The significance of the difference between the means has been verified by the student’s t-test and the analysis of variances (One and two-way ANOVA). Even, Tukey’s multiple range tests at p < 0.05 were conducted using GraphPad Prism 8.0.2.
3. Results and Discussion
3.1. Yield of M. albus Extracts
The yield of extraction depends on the harvest season, the plant component used, the extraction method, and therefore the choice of the solvents used and their physical and chemical properties, particularly their polarity.31 The preparation of the extracts from M. albus was carried out with two solvents: distilled water and methanol. For each extract, the yield is determined relative to 10 g of dry plant material and expressed as a percentage. The results obtained are reported in Table 1.
Table 1. Yield of M. albus Extracts (%)a.
| aqueous extract (%) | methanolic extract(%) | |
|---|---|---|
| macerations | 7.5 ± 1.10a | 2.31 ± 0.89b |
| decoction | 20.00 ± 0.89 | N.D |
Means (±SD, n = 3) followed by different letters in the same line indicate a significant difference according to Tukey’s multiple interval tests at p < 0.05. N.D: Not determined.
3.2. Total Polyphenol and Flavonoid Contents
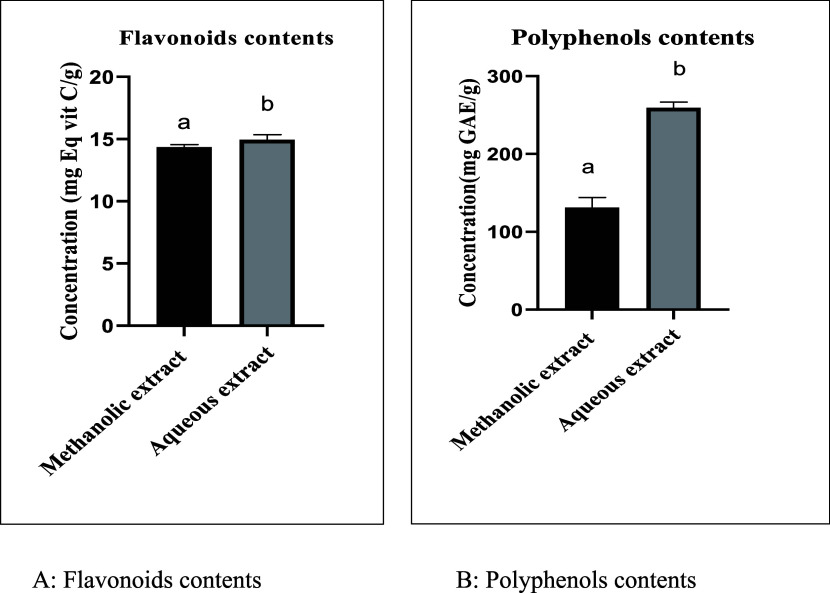
An essential factor in determining the antioxidant capacity of samples is the concentration of phenolic compounds and their antioxidant activity. Figure 1 lists the measurements’ findings. The Folin-Ciocalteu and aluminum chloride colorimetry methods, respectively, were employed to determine the total phenolic content and total flavonoid content.
Figure 1.
Flavonoid and polyphenol content of extracts from Melitotus albus: (A) flavonoid contents; (B) polyphenol contents. Means (±SD, n = 3), Different letters (a and b) indicate a significant difference according to Tukey’s multiple interval tests at p < 0.001.
The flavonoid content of M. albus extracts is expressed in milligrams equivalent of vitamin C per gram of extract (mg Eq vit C/g). According to the results (Figure 1), the flavonoid contents of the aqueous extracts are comparable with the values of the methanolic extracts, respectively, of the order of 14.96 ± 0.21 (mg Eq vit C/g) and 14.35 ± 0.419 (mg Eq vit C/g). Flavonoids present several properties, but the one related to their ability to scavenge free radicals and act as antioxidants is indubitably the most relevant. Within flavonoid classes, the antioxidant capacity varies depending on the type of functional group and its arrangement around the nuclear structure.32 In comparison with other studies, Stefanović et al.,10 have studied the plant M. albus by maceration with acetone, ethyl acetate, and ethanol; they found that total flavonoid content ranged from 36.96 to 132.76 mg RUE/g in M. albus extracts.
The polyphenol content of the extracts studied was expressed in milligrams equivalent of gallic acid per gram of extract (mg GAE/g). The results (Figure 1) show that the total polyphenol content of the aqueous extracts 259.26 ± 7.79 (mg GAE/g) is higher than that of the methanolic extracts 131.41 ± 12.64 (mg GAE/g). Phenolic compounds have been recently widely studied due to their biological effects, which could be beneficial for human health.33 These benefits are mainly related to both their direct and indirect antioxidant actions. Polyphenols can not only donate electrons to oxidant species, scavenge free radicals, and chelate metal ions34 but can also indirectly attenuate the production of reactive oxygen species by either improving antioxidant enzymes’ activity or inhibiting enzymes that induce pro-oxidant effects.35 In comparison with other results, Stefanović et al.10 found that the content of the results varied from 14.80 mg GAE/g (ethanol extract) to 28.80 mg GAE/g (acetone extract). So according to the results obtained, we can say that extraction with water and methanol is better than other solvents.
3.3. Antioxidant Activity of M. albus Leaf Extracts
Many authors link various biological characteristics of plants to secondary metabolic products of plants, particularly phenolic compounds. Because of this, the disclosure of such substances has evolved into an essential initial step toward ideal utilization. The ABTS+, DPPH, ferric reducing power, and total antioxidant capacity assays have all been used to investigate the antioxidant properties of M. albus leaf extracts.
There are currently very few published data on the phenolic component content of the plant species M. albus. Table 2 shows the antioxidant activity of methanolic and aqueous extracts by the method of DPPH, FRAP, and expressed ABTS. According to these results, it is noted that the inhibiting power of the DPPH radical of the aqueous extract is greater than that of the methanolic extract, and of BHT with IC50 values of the order of 0.087 ± 0.01, 0.107 ± 0.02, and 0.12 ± 0.00 mg/mL, respectively. The DPPH method is the most frequently used for in vitro antioxidant activity evaluation. It is demonstrated that phenolic compounds generally exhibit significant scavenging effects against the DPPH free radical.36,37 Also, they are considered as anti-inflammatory, and antibacterial, agents due to their antioxidant and free radical scavenging properties.38−40
Table 2. IC50 Values Were Found for the Methanolic and Aqueous Extract of the Plant Studieda.
| DPPH (mg/mL) | FRAP (mg/mL) | ABTS (mg/mL) | |
|---|---|---|---|
| methanolic extract | 0.107 ± 0.02a | 0.010 ± 0.003a | 0.167 ± 0.03a |
| aqueous extract | 0.087 ± 0.01b | 0.199 ± 0.016b | 0.014 ± 0.001b |
| BHT | 0.118 ± 0.00a | ||
| quercetin | 0.033 ± 0.0004a | ||
| trolox | 0.01 ± 0.002b |
Means (±SD, n = 3) followed by different letters in the same column indicate a significant difference according to Tukey’s multiple interval tests at p < 0.05.
The antioxidant effect on the FRAP method of the extracts studied shows that the methanolic extract 0.010 ± 0.003 (mg/mL) has a remarkable reducing power 0.010 ± 0.00 (mg/mL) than that of the aqueous extract 0.199 ± 0.016 mg/mL and that of quercetin used as a reference antioxidant.
The aqueous extract reveals a higher ABTS-reducing activity than the methanolic extract with IC50 values of around 0.014 ± 0.001 and 0.167 ± 0.03, (mg/mL), respectively. However, this activity remains lower than that of the reference antioxidant Trolox (0.01 ± 0.002) (mg/mL).
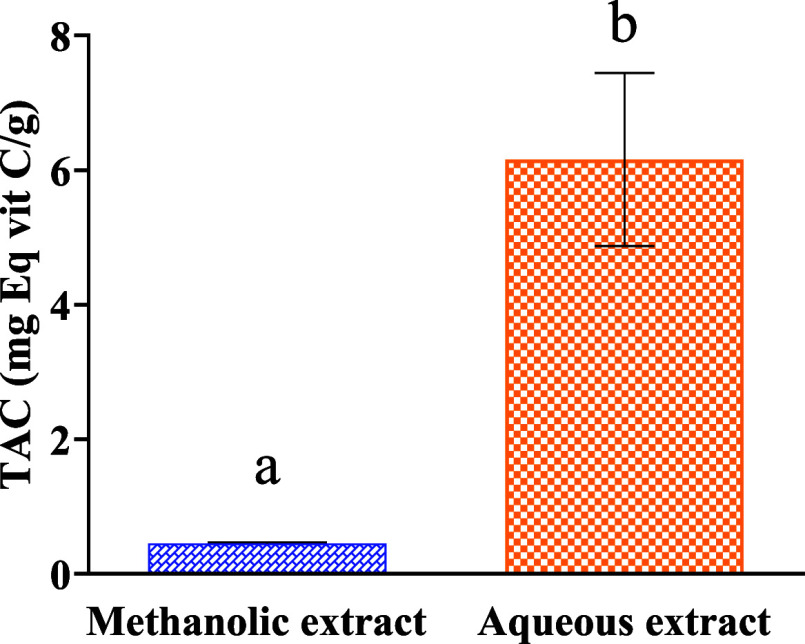
3.4. Total Antioxidant Capacity
Figure 2 shows the total antioxidant activity of the different extracts expressed in milligrams equivalent of vitamin C per gram extract. According to the results obtained, it is noted that the aqueous extract has a total antioxidant activity of the order of (6.157 ± 1.050 mg eq vitC/g) which is higher than that of the methanolic extract (0.453 ± 0.014 mg vitC/g).
Figure 2.
Total antioxidant capacity of M. albus extracts (mg EqVit C/g). Different letters (a and b) indicate a significant difference according to Student’s t-test at p < 0.05.
Due to great structural diversity, the antioxidant profiles differ greatly from one plant to another. The activity of natural extracts depends on the plant compounds as well as the type and polarity of the extraction solvent and the isolation procedure.41
In this study, a positive relationship between the contents of phenolic compounds and the antioxidant activity of the tested extracts was found. The results obtained are in good agreement with the literature data where the authors confirmed the correlation between antioxidant activity and the content of phenolic compounds.42−44
3.5. Acute Toxicity
The effect of this extract on the general behavior, body weight, and organ weight of rats is used to determine the acute toxicity of the aqueous extract of M. albus leaves.
3.5.1. Effects of M. albus Leaf Extract on General Behavior in Rats
Data on the acute toxicity of M. albus extracts are rare in the literature, and this is the first study to address this area of research.
Table 3 illustrates the results of single oral administration of M. albus leaf extract at different doses (100, 200, and 600 mg/kg) in rats. Observations for 14 days show no signs of toxicity (vomiting, tremors, sleep, aggressiveness, diarrhea, and mobility). In addition, this administration did not cause the death of the rats in all of the batches treated.
Table 3. Effects of Extract M. albus on General Behavior in the Rata.
| doses |
||||
|---|---|---|---|---|
| signs | 100 mg/kg | 200 mg/kg | 600 mg/kg | |
| number of animals | 5 | 5 | 5 | 5 |
| mobility | N | N | N | N |
| aggressiveness | N | N | N | N |
| tremor | N | N | N | N |
| sleep | N | N | N | N |
| vomiting | N | N | N | N |
| diarrhea | N | N | N | N |
| vigilance | N | N | N | N |
| number of dead | 0 | 0 | 0 | 0 |
N: Normal in comparison with the control.
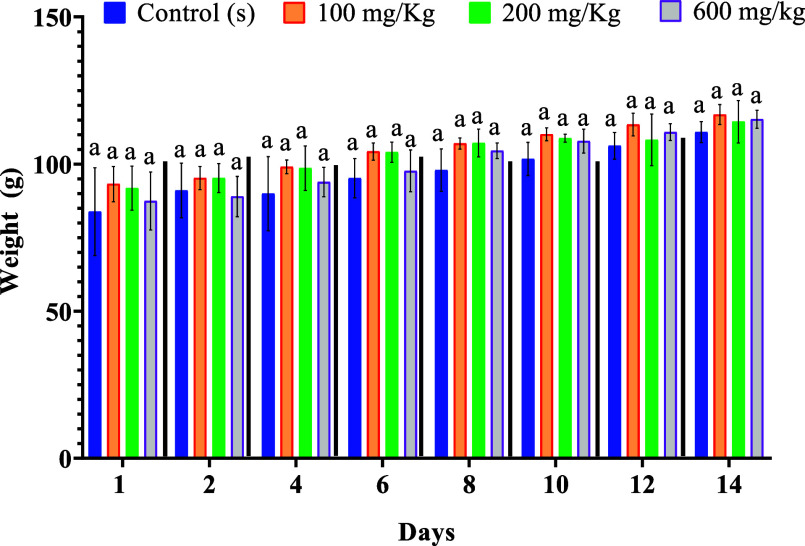
3.5.2. Effect of the Extract M. albus on the Evolution of the Body Weight of the Rat
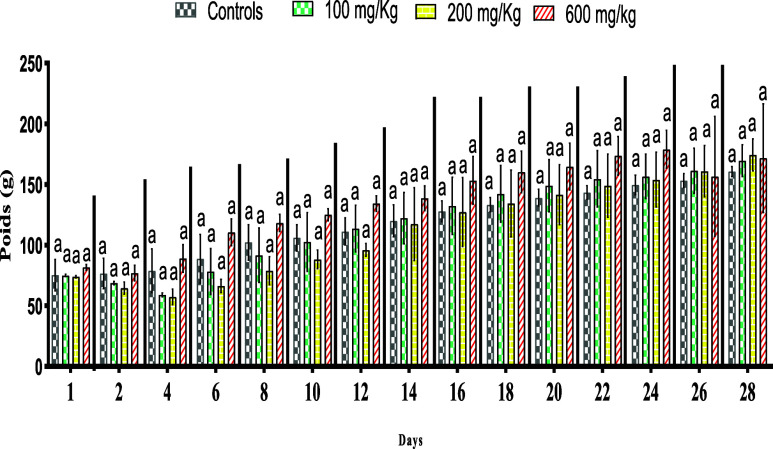
According to Figure 3, the examination of the weight evolution of the rats shows that the groups treated with the different doses (100, 200, 600 mg/kg) gradually gained weight during the 14 days (Figure 3).
Figure 3.
Effects of extract M. albus on changes in body weight rats. The letter (a) indicates no significant difference at p < 0.05.
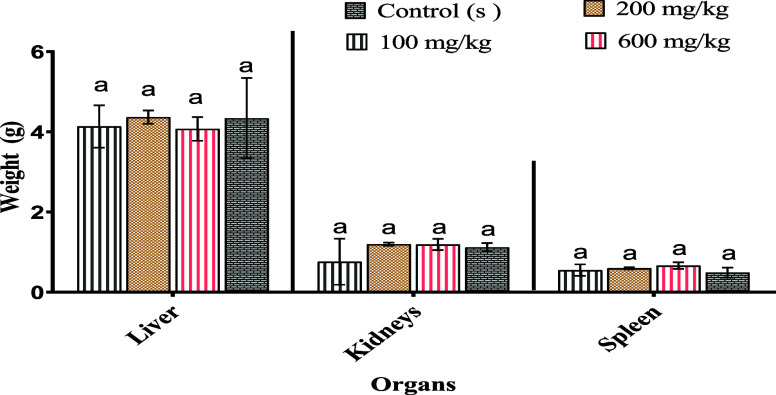
3.5.3. Effects of the Aqueous Extract of M. albus on the Organs of Rats
The results obtained are presented in Figure 4, and it appears from these results that the administration of the aqueous extract at different doses does not influence the relative weights of the noble organs (liver, spleen, and kidneys) in comparison with the control.
Figure 4.
Variation in relative organ weights of the control and treated animals. The letter (a) indicates no significant difference at p < 0.05.
3.6. Subacute Toxicity
By examination of the impact of this extract on the general behavior, body weight, and organ weight of rats, the subacute toxicity of the aqueous extract of the leaves of M. albus is studied.
3.6.1. Effects of Extract of M. albus on the General Behavior of Rats
Table 4 illustrates the daily oral administration for 28 days of the M. albus extract at different doses (100, 200, and 600 mg/kg) in rats. Observations over 28 days show no signs of toxicity (vomiting, sleep, aggression, tremors, diarrhea, and mobility), and no death was noted for the treated rats and the control rats.
Table 4. Effects of Extract M. albus on the General Behavior in Ratsa.
| doses |
||||
|---|---|---|---|---|
| signs | aqueous extract | 100 mg/kg | 200 mg/kg | 600 mg/kg |
| number of animals | 3 | 3 | 3 | 3 |
| mobility | N | N | N | N |
| aggressiveness | N | N | N | N |
| tremor | N | N | N | N |
| sleep | N | N | N | N |
| vomiting | N | N | N | N |
| diarrhea | N | N | N | N |
| vigilance | N | N | N | N |
| number of dead | 0 | 0 | 0 | 0 |
N: Normal in comparison with the control.
3.6.2. Effect of the Extract M. albus on the Evolution of the Body Weight of the Rat
Effects of the Subacute Toxicity of the aqueous extract of M. albus leaves on body weight: All treated and controlled animals gained weight. This gain was more pronounced in animals treated at a dose of 600 mg/kg than in the controls. M. albus extract induced a slight weight increase in rats (Figure 5).
Figure 5.
Effect of the extract of M. albus on the evolution of body weight in albino rats. Means (±SD, n = 5). The use of superscript letters (a) indicate no significant differences at p < 0.05.
3.6.3. Effect of Extract on Organ Weight
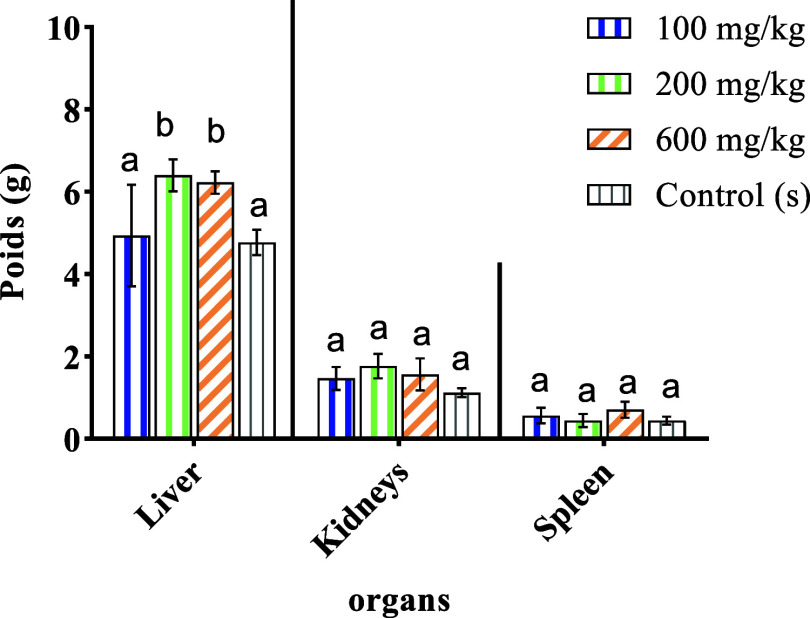
The results obtained are listed in Figure 6. It appears from these results that the administration of the aqueous extract to rats at doses of 100, 200, and 600 mg/kg does not influence the weights of organs (liver, kidneys, and spleen). On the other hand, there is a slight increase in the kidneys of the treated rats in comparison with the control rats.
Figure 6.
Variation in relative organ weights (g) of the control and treated animals. Different letters (a and b) indicate a significant difference at p < 0.05.
3.6.4. Biochemical Parameters
To evaluate liver function, we examined the hepatic enzymes ALAT, ASAT, and ALP. The analysis of ASAT, ALAT, and ALP revealed no signs of toxicity, as there were no significant differences compared to the negative control group. Furthermore, the different concentrations of the M. albus extract did not have any adverse effects on the kidney function parameters UREA and CREA (Table 5).
Table 5. Impact of M. albus’ Aqueous Extract on Liver and Kidney Parametersa.
| treatment | dose (mg/kg) | ASAT (U/L) | ALAT (U/L) | ALP (U/L) | UREA (mg/dL) | CREA (mg/dL) |
|---|---|---|---|---|---|---|
| control (NaCl 0.9%) | 136.0 ± 2.77a | 48.93 ± 4.10a | 221.3 ± 2.40a | 20.67 ± 2.91a | 0.36 ± 0.04a | |
| aqueous extract of M. albus | 100 | 124.9 ± 3.50a | 55.77 ± 5.67a | 231.7 ± 1.17a | 23.80 ± 3.36a | 0.39 ± 0.04a |
| aqueous extract of M. albus | 200 | 121.4 ± 3.338a | 56.97 ± 4.3a | 216.0 ± 1.89a | 21.37 ± 1.39a | 0.37 ± 0.01a |
| aqueous extract of M. albus | 600 | 124.6 ± 5.84a | 43.07 ± 2.55a | 211.0 ± 2.77a | 25.73 ± 2.75a | 0.36 ± 0.02a |
The use of superscript letters (a) indicates no significant differences at p < 0.05, when comparing all treatments through One-way ANOVA followed by the Tukey test. The presented data represents the mean ± SD (n = 5).
The assumption that herbal preparations/remedies are safe and effective has influenced the indiscriminate consumption of such remedies, most especially among rural communities, where these remedies can be administered for a long period of time without considering the dose or concentration that will bring about toxic side effects.45 In general, changes in relative organ mass reflect the toxicity after exposure to a toxic substance. To a toxic substance, the heart, liver, kidneys, spleen, and lungs are the first organs to be affected by the metabolic reaction provoked by the toxicant.46 Thus, a scientific evaluation of the oral toxicity is necessary and will help determine the dose ranges that are safe for subsequent studies.47 The current study used an animal model to evaluate the acute and subacute toxicity of M. albus. To the best of our knowledge, this is the first study carried out on the toxicity characteristics of the M. albus leaf extract. Completing the achievement of the acute toxicity study, no deaths or significant signs of toxicity (vomiting, tremors, sleep, aggressiveness, diarrhea, and mobility) were observed in animals given a single dose of 100, 200, and 600 mg/kg compared with the control. Rats treated with the aqueous extract of the plant studied showed no effect on the organ weights (spleen, kidneys, and liver) and a normal evolution in body weight, demonstrating the absence of signs of toxicity.
At the end of the subacute toxicity study, animals administered a single dose of 100, 200, or 600 mg/kg did not exhibit any significant signs of toxicity (such as vomiting, tremors, sleeplessness, aggression, diarrhea, or mobility) in comparison to the control group. Rats given the plant’s aqueous extract exhibited a normal evolution of body weight, indicating the lack of any toxic effects. So, the approximate LD50 was found to be greater than the 600 mg/kg dose. However, the liver weight increased significantly. The liver is the central organ of metabolism. In case of liver damage, we observe an increase in transaminase activity (ALT and AST), and when the damage increases, this activity also increases.48 Furthermore, when liver cell membrane is damaged, a variety of enzymes normally located in the cytosol are released into the bloodstream. Measuring the activities of serum marker enzymes like ALT, AST, and ALP, as well as level of serum total bilirubin has provided a powerful tool for the assessment of liver function.49,50 Creatinine and urea are known to be effective indicators of renal function and any rise in the levels of these parameters indicates a marked renal damage.51 The results from this study (Table 5) however suggest that the doses given to rats do not have a negative. Chronic toxicity needs to be assessed in order to verify the product security.
3.7. LC–MS/MS Analysis of the M. albus Extract
The analysis of the phenolic composition of extract of M. albus was studied using UHPLC. The results obtained are presented in Table 6 and Figure S1. LC–MS analysis revealed a total phenolic content of 99.99% (12 compounds) in the M. albus extract. The extract mainly contains chlorogenic acid (43.68%), catechin/epicatechin (24.82%), quercetin-3-O-glucuronic acid (9.91%), naringin (7.64%), and p-hydroxybenzoic/salicylic acid (2.95%).
Table 6. Phenolic Composition by LC–MS/MS of M. albus Extracts.
| compounds | formula | retention time (min) | (M – H)− | AUC | % AUC |
|---|---|---|---|---|---|
| p coumaric acid | C9H8O3 | 0.017 | 1,629,000 | 7,267,528 | 2.41 |
| trans ferulic acid | C10H10O4 | 2.771 | 1,930,000 | 8,621,394 | 2.86 |
| apigenin | C15H10O5 | 1.866 | 2,690,000 | 1,396,681 | 0.46 |
| amentoflavone | C30H18O10 | 2.363 | 5,371,000 | 3,321,777 | 1.10 |
| quercetin-3-O-glucuronic acid | C21H20O13 | 1.981 | 4,770,000 | 29,809,069 | 9.91 |
| quercetin-3-O-hexose deoxyhexose | C27H30O15 | 3.829 | 6,090,000 | 7,676,372 | 2.55 |
| syringic acid | C9H10O5 | 2.000 | 1,970,000 | 1,304,898 | 0.43 |
| p-hydroxybenzoic\salicylic acid | C7H6O3 | 1.597 | 1,370,000 | 8,888,587 | 2.95 |
| caffeic acid | C9H8O4 | 0.027 | 179.0000 | 3,547,055 | 1.17 |
| chlorogenic acid | C16H18O9 | 1.446 | 353.0000 | 131,197,922 | 43.68 |
| cathechin\epicathechin | C15H14O6 | 1.652 | 289.0000 | 74,751,258 | 24.82 |
| narigin | C27H32O14 | 1.973 | 579.0000 | 23,004,065 | 7.64 |
According to the available literature, the phytochemical analysis of M. albus extracts was limited, and this study represents the first comprehensive analysis of its polyphenolic composition using LC–MS. To support our findings, we conducted a comparative analysis of the phytochemical composition of the M. albus extract with that of other Fabaceae family plants. A study conducted by Paun et al. (2020) on Melilotus officinalis plants showed that the most abundant compounds were chlorogenic acid, apigenin, and rosmarinic acid.52 These findings corroborate with our results. Another phytochemical study of Melilotus indicus, belonging to the same plant genus, revealed the presence of compounds nearly identical to those obtained in our study. These compounds include quercetin, gallic acid, caffeic acid, vanillic acid, chlorogenic acid, syringic acid, p-coumaric acid, and m-coumaric acid.53 Furthermore, additional studies have demonstrated the richness of M. albus in polyphenolic compounds.10
3.8. Molecular Docking Analysis
The prediction of physicochemical properties revealed that all examined chemical compounds satisfy the five rules of Lipinski, (molecular weight less than 500 g/mol, molar refractive index included in,40 [130] interval, log P inferior than 5, hydrogen bonds acceptors and hydrogen bonds donors less than 10 and 5, respectively),54 except for four molecules labeled as C4, C5, C6, and C12 which did not meet all declared thresholds, as shown in Table S1.
The prediction of pharmacokinetic properties of ADMET indicated a positive effect of AMES toxicity for five chemical compounds C5, C6, C9, C11, and C12, so the remaining small molecules were predicted as free toxic inhibitors, with a good level of human intestinal absorption (HIA) that exceed 70%, except for the compound labeled C10 (HIA of 17.157%). The level of blood–brain barrier (BBB) permeabilities comprised in [-2,1] Log BB and the level of central nervous system (CNS) permeabilities did not exceed −2 Log PS. In addition, the studied molecules have no inhibitory effect on 2D6, 3A4, 1A2, 2C19, and 2C9 cytochromes, as displayed in Table S2.
Based on the primary results of UHPLC coupled with high-resolution mass spectrometry, we noted that chlorogenic acid, as the major compound of this extract, which was discovered by the highest value of area (AUC of 43.68%), is predicted as not a toxic agent with a good ADME profile, meeting all five rules of Lipinski.55,56
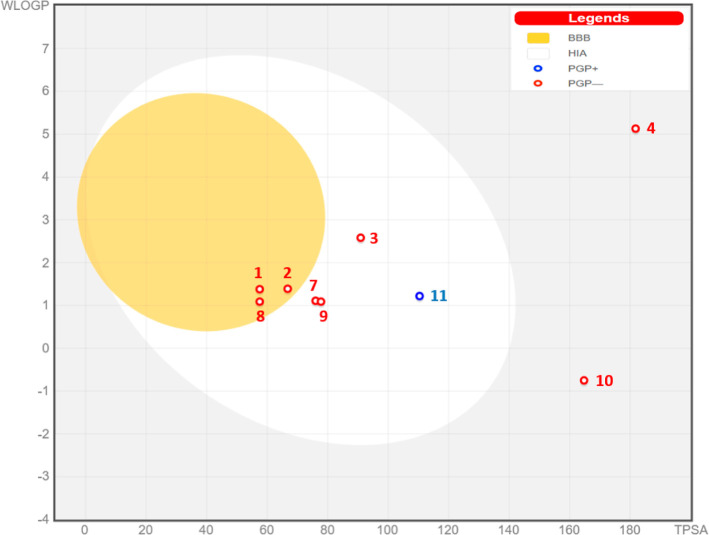
The predictive model of Egan shown in Figure 7 revealed that chemical compounds C1, C2, and C8 are part of the yellow Egan egg; therefore, they were predicted to passively permeate through the BBB. The compounds labeled C3, C7, C9, and C11 are part of white Egan eggs, so they were predicted to be passively absorbed by the gastrointestinal tract. The compound labeled C10 colored in blue was predicted to be rejected from the CNS by the P-glycoprotein. Finally, molecules outside the Egan egg have been declared off-limits to prediction, so they cannot be used as potent inhibitors of the CNS.57
Figure 7.
Predictive model of Egan Boiled-Egg.
The results of molecular docking in 2D and 3D visualizations as pictured in Figure 8 display that the chemical compound namely chlorogenic acid (C10) reacted to NADPH oxidase protein encoded by 2CDU.pdb, forming four conventional hydrogen bonds with Ala300, His10, Ser41, and Gly329 amino acids (A–A) residues, in addition to one Pi-Alkyl bond detected with the Leu299 (A–A) residue. The compound under study was equally docked to the secreted aspartic protease from C. albicans, encoded as 1ZAP.pdb protein, reacting to a variety of chemical bonds, including five hydrogen bonds detected toward Gly85, Asp218, Gly132, Arg192, and Asn131 (A–A) residues, more than one carbon hydrogen bond produced with Ala133 (A–A) residues. Regarding the mechanism of inhibition of this candidat ligand toward the crystal structure of the FimH lectin domain from E. coli K12, coded by 4XO8.pdb, it was noticed that several intermolecular interactions which can explain the activity of the investigated extract, such as an unfavorable Acceptor–Acceptor bond which was produced with Gly14 (A–A) residue, four hydrogen bonds were created with Ser139, Asp140, Asp141, and Gln143, two carbon hydrogen bonds were produced with Glu15 and Phe142 (A–A) residues.
Figure 8.
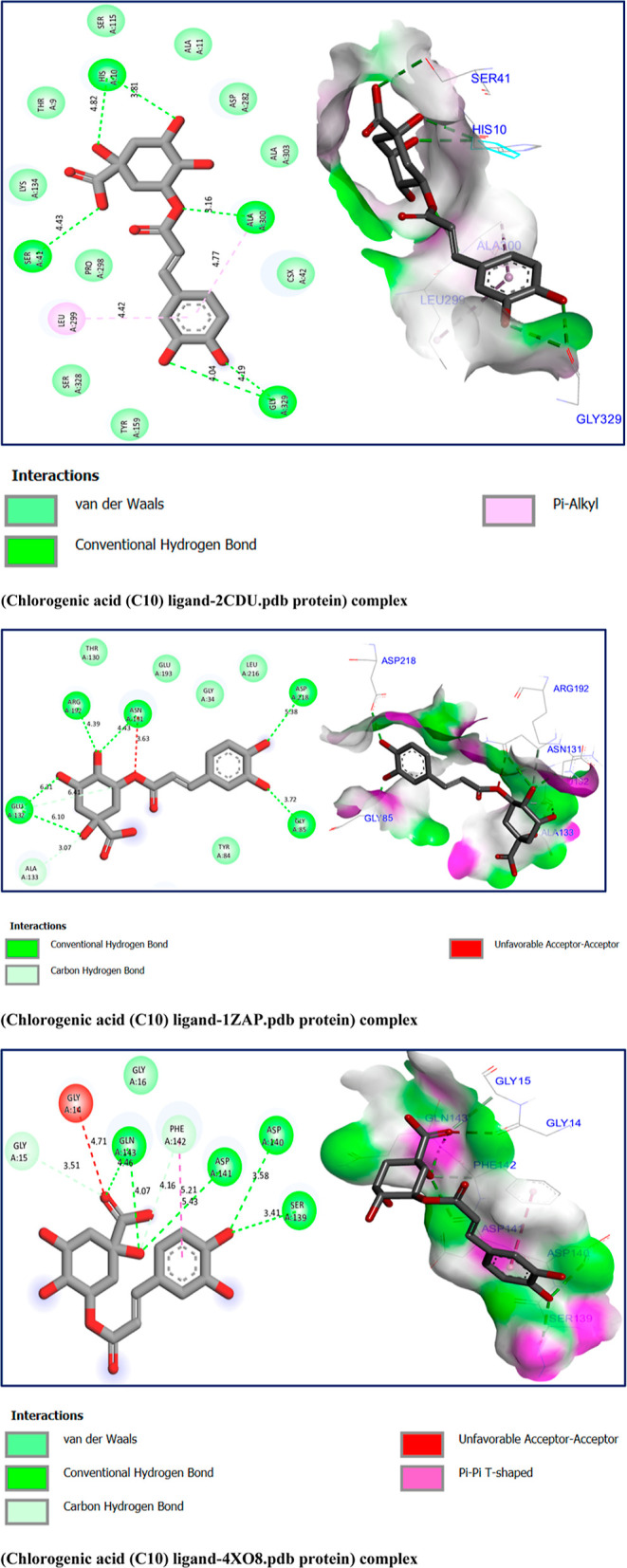
Two- and three-dimensional views of the inhibition mechanism of the major compound’s inhibition mechanism toward 2CDU.pdb, 1ZAP.pdb, and 4XO8.pdb proteins, with binding energies of −6.50, −4.23, and −5.38 kcal/mol, respectively.
In line with the recently published work of M. Jeddi et al., in which they examined the antioxidant, antifungal, and antibacterial effects of two major compounds of essential oil extracted from Lavandula angustifolia mill with the same target proteins, we noticed that our major compound, namely chlorogenic acid (C10), was docked to the active sites of each targeted protein, as declared by M. Jeddi et al.58
4. Conclusions
The objective of this study is to evaluate the flavonoids, polyphenols, and antioxidant activity of the M. albus plant’s methanolic and aqueous extracts. We will further look at the in-silico studies, toxicity activity, and chemical composition. The results show that the extracts of M. albus have remarkable antioxidant activity by all the methods used, which is explained by their richness in phenolic compounds and flavonoids. The aqueous extract is the best solvent for extracting polyphenols and the other antioxidants, DPPH, ABTS, and the total antioxidant capacity of the plant studied. Concerning the acute and subacute toxicity, it appears that the aqueous extract of M. albus does not cause the death of the rats. It would be interesting for a better exploitation of this plant to develop the following lines of research: the estimation of the content of alkaloids, terpenoids, coumarins, starches, etc. The research emphasizes identifying the mechanisms of action of these active compounds as well as other biological activities like anti-inflammatory and anticancer activities.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R110), King Saud University, Riyadh, Saudi Arabia, for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c08314.
LC–MS/MS analysis graphs of the M. albus extract; prediction of physicochemical properties of 12 chemical compounds based on the five rules of Lipinski; and prediction of ADMET in-silico pharmacokinetic properties of 12 chemical compounds (PDF)
This research work was supported by Researchers Supporting Project number (RSP2024R110), King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization, International Union for Conservation of Nature and Natural Resources & World Wide Fund for Nature . Guidelines on the Conservation of Medicinal Plants; International Union for Conservation of Nature and Natural Resources: Gland, 1993. (accessed 2023–05–13). https://apps.who.int/iris/handle/10665/41651. [Google Scholar]
- Mollica A.; Stefanucci A.; Macedonio G.; Locatelli M.; Luisi G.; Novellino E.; Zengin G. Chemical Composition and Biological Activity of Capparis Spinosa L. from Lipari Island. South Afr. J. Bot. 2019, 120, 135–140. 10.1016/j.sajb.2018.02.397. [DOI] [Google Scholar]
- Sinan K. I.; Yagi S.; Llorent-Martínez E. J.; Ruiz-Medina A.; Gordo-Moreno A. I.; Stefanucci A.; Mollica A.; Bene K.; Zengin G. Understanding the Chemical Composition and Biological Activities of Different Extracts of Secamone Afzelii Leaves: A Potential Source of Bioactive Compounds for the Food Industry. Molecules 2023, 28 (9), 3678. 10.3390/molecules28093678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škrovánková S.; Mišurcová L.; Machů L.. Antioxidant Activity and Protecting Health Effects of Common Medicinal Plants. In Advances in Food and Nutrition Research; Elsevier, 2012; Vol. 67, pp 75–139. [DOI] [PubMed] [Google Scholar]
- Anderson K. J.; Teuber S. S.; Gobeille A.; Cremin P.; Waterhouse A. L.; Steinberg F. M. Walnut Polyphenolics Inhibit In Vitro Human Plasma and LDL Oxidation. J. Nutr. 2001, 131 (11), 2837–2842. 10.1093/jn/131.11.2837. [DOI] [PubMed] [Google Scholar]
- Ekor M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol 2014, 4, 177. 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicson S. M.; Samuthirapandi M.; Govindaraju A.; Kasi P. D. Evaluation of in Vitro and in Vivo Safety Profile of the Indian Traditional Medicinal Plant Grewia Tiliaefolia. Regul. Toxicol. Pharmacol. 2015, 73 (1), 241–247. 10.1016/j.yrtph.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Josifovic M.; Stjepanovic L.; Jankovic M. M.; Gajic M.; Kojic M.; Diklic H.. Flora of SR Serbia; Academy of Sciences and Arts: Belgrade, Serbia, Serbian, 1972. [Google Scholar]
- Jardin-Secrets . Mélilot blanc (Melilotus albus) : culture, entretien, semis, 2023. (accessed 2023-05–13). https://jardin-secrets.com/melilot-blanc.html.
- Stefanović O. D.; Tešić J. D.; Čomić L. R. Melilotus Albus and Dorycnium Herbaceum Extracts as Source of Phenolic Compounds and Their Antimicrobial, Antibiofilm, and Antioxidant Potentials. J. Food Drug Anal. 2015, 23 (3), 417–424. 10.1016/j.jfda.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker J. R. The Biosynthesis of Coumarin in Melilotus Alba. Biochem. Biophys. Res. Commun. 1963, 14 (1), 17–20. 10.1016/0006-291X(63)90203-1. [DOI] [PubMed] [Google Scholar]
- Sarić M.Medicinal Plants of SR Serbia; SASA: Belgrade, 1989. [Google Scholar]
- Kara M.; Assouguem A.; Abdou R Z.; Bahhou J. Phytochemical Content and Antioxidant Activity of Vinegar Prepared from Four Apple Varieties by Different Methods. Trop. J. Nat. Prod. Res. 2021, 5 (9), 1578–1585. 10.26538/tjnpr/v5i9.9. [DOI] [Google Scholar]
- Hmamou A.; Eloutassi N.; Alshawwa S. Z.; Al kamaly O.; Kara M.; Bendaoud A.; El-Assri E.-M.; Tlemcani S.; El Khomsi M.; Lahkimi A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver Rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27 (3), 854. 10.3390/molecules27030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliauskas G.; Venskutonis P. R.; Van Beek T. A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85 (2), 231–237. 10.1016/j.foodchem.2003.05.007. [DOI] [Google Scholar]
- Kara M.; Assouguem A.; Fadili M. E.; Benmessaoud S.; Alshawwa S. Z.; Kamaly O. A.; Saghrouchni H.; Zerhouni A. R.; Bahhou J. Contribution to the Evaluation of Physicochemical Properties, Total Phenolic Content, Antioxidant Potential, and Antimicrobial Activity of Vinegar Commercialized in Morocco. Molecules 2022, 27 (3), 770. 10.3390/molecules27030770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-C.; Chen H.-M.; Shiau C.-Y. Free Amino Acids and Peptides as Related to Antioxidant Properties in Protein Hydrolysates of Mackerel (Scomber Austriasicus). Food Res. Int. 2003, 36 (9–10), 949–957. 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Oyaizu M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44 (6), 307–315. 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- El Khomsi M.; Kara M.; Hmamou A.; Assouguem A.; Al Kamaly O.; Saleh A.; Ercisli S.; Fidan H.; Hmouni D. In Vitro Studies on the Antimicrobial and Antioxidant Activities of Total Polyphenol Content of Cynara Humilis from Moulay Yacoub Area (Morocco). Plants 2022, 11 (9), 1200. 10.3390/plants11091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Rice-Evans C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26 (9–10), 1231–1237. 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Prieto P.; Pineda M.; Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269 (2), 337–341. 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care Conseil canadien de protection des animaux . Manuel Sur le Soin et L’utilisation des Animaux D’expérimentation, 2nd ed.,; CCPA, 1993; Vol. 1.
- OECD . Guidance Document on the Recognition, Assessment and Use of Clinical Signs as Humane Endpoints for Experimental Animals Used in Safety Evaluation; OECD Environmental Health and Safety Monograph Series on Testing and Assessment No 19. p. 20 2000. http://Oecd_gd19.Pdf.
- OECD . Essai no 407: Toxicité orale à doses répétées—pendant 28 jours sur les rongeurs | READ online 2008. http://oecd-ilibrary.org, https://read.oecd-ilibrary.org/environment/essai-n-407-toxicite-orale-a-doses-repetees-pendant-28-jours-sur-les-rongeurs_9789264070691-fr (accessed 2023-05-13).
- Kandsi F.; Conte R.; Marghich M.; Lafdil F. Z.; Alajmi M. F.; Bouhrim M.; Mechchate H.; Hano C.; Aziz M.; Gseyra N. Phytochemical Analysis, Antispasmodic, Myorelaxant, and Antioxidant Effect of Dysphania Ambrosioides (L.) Mosyakin and Clemants Flower Hydroethanolic Extracts and Its Chloroform and Ethyl Acetate Fractions. Molecules 2021, 26 (23), 7300. 10.3390/molecules26237300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.-H.; Sun H.; Yan G.-L.; Han Y.; Zhao Q.-Q.; Wang X.-J. Chinmedomics: A Powerful Approach Integrating Metabolomics with Serum Pharmacochemistry to Evaluate the Efficacy of Traditional Chinese Medicine. Engineering 2019, 5 (1), 60–68. 10.1016/j.eng.2018.11.008. [DOI] [Google Scholar]
- El Fadili M.; Er-Rajy M.; Ali Eltayb W.; Kara M.; Imtara H.; Zarougui S.; Al-Hoshani N.; Hamadi A.; Elhallaoui M. An In-Silico Investigation Based on Molecular Simulations of Novel and Potential Brain-Penetrant GluN2B NMDA Receptor Antagonists as Anti-Stroke Therapeutic Agents. J. Biomol. Struct. Dyn. 2023, 1–15. 10.1080/07391102.2023.2232024. [DOI] [PubMed] [Google Scholar]
- El fadili M.; Er-rajy M.; Imtara H.; Noman O. M.; Mothana R. A.; Abdullah S.; Zerougui S.; Elhallaoui M. QSAR, ADME-Tox, Molecular Docking and Molecular Dynamics Simulations of Novel Selective Glycine Transporter Type 1 Inhibitors with Memory Enhancing Properties. Heliyon 2023, 9 (2), e13706 10.1016/j.heliyon.2023.e13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgan A. P.; Coffman P. K.; Kocher J.-P. A.; Katzmann D. J.; Sosa C. P. Multilevel Parallelization of AutoDock 4.2. J. Cheminf. 2011, 3 (1), 12. 10.1186/1758-2946-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassault Systèmes . Free Download: BIOVIA Discovery Studio Visualizer; Dassault Systèmes, 2023. (accessed 2023-10–01). https://discover.3ds.com/discovery-studio-visualizer-download. [Google Scholar]
- Boudjedjou L.Étude De L’activité Antibactérienne Et Antioxydante Des Extraits De La Partie Aérienne De Pituranthos Scoparius ≪Guezzah≫; Thèses-Algérie, 2019. [Google Scholar]
- Kaleem M.; Ahmad A.. Therapeutic, Probiotic and Unconventional Foods; Academic Press, 2018, pp 137–155. [Google Scholar]
- Shahidi F.; Ambigaipalan P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects - A Review. J. Funct.Foods 2015, 18, 820–897. 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Quiñones M.; Miguel M.; Aleixandre A. Los Polifenoles, Compuestos de Origen Natural Con Efectos Saludables Sobre El Sistema Cardiovascular. Nutr. Hosp. 2012, 27 (1), 76–89. [DOI] [PubMed] [Google Scholar]
- Ballard C. R.; Maróstica M. R.. Bioactive Compounds: Health Benefits and Potential Applications; Elsevier Amsterdam: The Netherlands, 2018. [Google Scholar]
- Silva F. A. M.; Borges F.; Guimarães C.; Lima J. L. F. C.; Matos C.; Reis S. Phenolic Acids and Derivatives: Studies on the Relationship among Structure, Radical Scavenging Activity, and Physicochemical Parameters. J. Agric. Food Chem. 2000, 48 (6), 2122–2126. 10.1021/jf9913110. [DOI] [PubMed] [Google Scholar]
- Amarowicz R.; Naczk M.; Shahidi F. Antioxidant Activity of Various Fractions of Non-Tannin Phenolics of Canola Hulls. J. Agric. Food Chem. 2000, 48 (7), 2755–2759. 10.1021/jf9911601. [DOI] [PubMed] [Google Scholar]
- Atki Y. E.; Aouam I.; Kamari F. E.; Taroq A.; Zejli H.; Taleb M.; Lyoussi B.; Abdellaoui A. Antioxidant Activities, Total Phenol an Flavonoid Contents of Two Teucrium Polium Subspecies Extracts. Moroc. J. Chem. 2020, 8 (2), 8–455. 10.48317/IMIST.PRSM/morjchem-v8i2.17071. [DOI] [Google Scholar]
- El Atki Y.; Aouam I.; Taroq A.; el Kamari F.; Timinouni M.; Lyoussi B.; Abdellaoui A. Antibacterial Effect of Combination of Cinnamon Essential Oil and Thymol, Carvacrol, Eugenol, or Geraniol. J. Rep. Pharm. Sci. 2020, 9, 104. 10.4103/jrptps.JRPTPS_25_19. [DOI] [Google Scholar]
- Della Valle A.; Dimmito M. P.; Zengin G.; Pieretti S.; Mollica A.; Locatelli M.; Cichelli A.; Novellino E.; Ak G.; Yerlikaya S.; Baloglu M. C.; Celik Altunoglu Y.; Stefanucci A. Exploring the Nutraceutical Potential of Dried Pepper Capsicum Annuum L. on Market from Altino in Abruzzo Region. Antioxidants 2020, 9 (5), 400. 10.3390/antiox9050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazic M. Antioxidant Activity of Water Extracts of Some Medicinal Plants from Herzegovina Region. Int. J. Pure Appl. Biosci. 2016, 4 (2), 85–90. 10.18782/2320-7051.2251. [DOI] [Google Scholar]
- J-Stage . Effects of the Interaction of Tannins with Co-existing Substances. VI. : Effects of Tannins and Related Polyphenols on Superoxide Anion Radical, and on 1, 1-Diphenyl-2-picrylhydrazyl Radical, 1989. (accessed 2023-06-16). https://www.jstage.jst.go.jp/article/cpb1958/37/8/37_8_2016/_article/-char/ja/.
- Trigui M.; Hsouna A. B.; Tounsi S.; Jaoua S. Chemical Composition and Evaluation of Antioxidant and Antimicrobial Activities of Tunisian Thymelaea Hirsuta with Special Reference to Its Mode of Action. Ind. Crops Prod. 2013, 41, 150–157. 10.1016/j.indcrop.2012.04.011. [DOI] [Google Scholar]
- Boudjou S.; Oomah B. D.; Zaidi F.; Hosseinian F. Phenolics Content and Antioxidant and Anti-Inflammatory Activities of Legume Fractions. Food Chem. 2013, 138 (2–3), 1543–1550. 10.1016/j.foodchem.2012.11.108. [DOI] [PubMed] [Google Scholar]
- Ben-Arye E.; Samuels N.; Goldstein L. H.; Mutafoglu K.; Omran S.; Schiff E.; Charalambous H.; Dweikat T.; Ghrayeb I.; Bar-Sela G.; Turker I.; Hassan A.; Hassan E.; Saad B.; Nimri O.; Kebudi R.; Silbermann M. Potential Risks Associated with Traditional Herbal Medicine Use in Cancer Care: A Study of Middle Eastern Oncology Health Care Professionals. Cancer 2016, 122 (4), 598–610. 10.1002/cncr.29796. [DOI] [PubMed] [Google Scholar]
- Jothy S. L.; Zakaria Z.; Chen Y.; Lau Y. L.; Latha L. Y.; Sasidharan S. Acute Oral Toxicity of Methanolic Seed Extract of Cassia Fistula in Mice. Molecules 2011, 16 (6), 5268–5282. 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unuofin J. O.; Otunola G. A.; Afolayan A. J. Evaluation of Acute and Subacute Toxicity of Whole-Plant Aqueous Extract of Vernonia Mespilifolia Less. in Wistar Rats. J. Integr. Med. 2018, 16 (5), 335–341. 10.1016/j.joim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Gatsing D.; Aliyu R.; Kuiate J. R.; Garba I. H.; Jaryum K. H.; Tedongmo N.; Tchouanguep F. M.; Adoga G. I. Toxicological Evaluation of the Aqueous Extract of Allium Sativum Bulbs on Laboratory Mice and Rats. Cameroon J. Exp. Biol. 2006, 1 (1), 39–45. 10.4314/cajeb.v1i1.37926. [DOI] [Google Scholar]
- Uličná O.; Greksák M.; Vančová O.; Zlatoš L.; Galbavý Š.; Božek P.; Nakano M. Hepatoprotective Effect of Rooibos Tea (Aspalathus linearis) on CCl 4-Induced Liver Damage in Rats. Physiol. Res. 2003, 52 (4), 461–466. 10.33549/physiolres.930340. [DOI] [PubMed] [Google Scholar]
- Porchezhian E.; Ansari S. H. Hepatoprotective Activity of Abutilon Indicum on Experimental Liver Damage in Rats. Phytomedicine 2005, 12 (1–2), 62–64. 10.1016/j.phymed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Gowda S.; Desai P.; Kulkarni S.; Hull V.; Math A.; Vernekar S. Markers of Renal Function Tests. North Am. J. Med. Sci. 2010, 2, 170–173. [PMC free article] [PubMed] [Google Scholar]
- Paun G.; Neagu E.; Albu C.; Savin S.; Radu G. L. In Vitro Evaluation of Antidiabetic and Anti-Inflammatory Activities of Polyphenolic-Rich Extracts from Anchusa Officinalis and Melilotus Officinalis. ACS Omega 2020, 5 (22), 13014–13022. 10.1021/acsomega.0c00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir A.; Asif M.; et al. Therapeutic Potential of Standardized Extract of Melilotus indicus (L.) All. and Its Phytochemicals against Skin Cancer in Animal Model: In Vitro, In Vivo, and In Silico Studies. ACS Omega 2022, 7 (29), 25772. 10.1021/acsomega.2c03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Delivery Rev. 1997, 23 (1–3), 3–25. 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- El fadili M.; Er-rajy M.; Imtara H.; Kara M.; Zarougui S.; Altwaijry N.; Al kamaly O.; Al Sfouk A.; Elhallaoui M. 3D-QSAR, ADME-Tox In Silico Prediction and Molecular Docking Studies for Modeling the Analgesic Activity against Neuropathic Pain of Novel NR2B-Selective NMDA Receptor Antagonists. Processes 2022, 10 (8), 1462. 10.3390/pr10081462. [DOI] [Google Scholar]
- El fadili M.; Er-rajy M.; Ali Eltayb W.; Kara M.; Assouguem A.; Saleh A.; Al Kamaly O.; Zarougui S.; Elhallaoui M. In-Silico Screening Based on Molecular Simulations of 3,4-Disubstituted Pyrrolidine Sulfonamides as Selective and Competitive GlyT1 Inhibitors. Arab. J. Chem. 2023, 16 (10), 105105. 10.1016/j.arabjc.2023.105105. [DOI] [Google Scholar]
- MDPI . Pharmaceuticals|Free Full-Text|QSAR, ADMET In Silico Pharmacokinetics, Molecular Docking and Molecular Dynamics Studies of Novel Bicyclo (Aryl Methyl) Benzamides as Potent GlyT1 Inhibitors for the Treatment of Schizophrenia, 2022. (accessed 2023-10-01). https://www.mdpi.com/1424-8247/15/6/670. [DOI] [PMC free article] [PubMed]
- Mohammed E.CoLab. https://colab.ws/researchers/40293 (accessed 2023-10-01).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.