Abstract
Agriculture waste has increased annually due to the global food demand and intensive animal production. Preventing environmental degradation requires fast and effective agricultural waste treatment. Aerobic digestion or composting uses agricultural wastes to create a stabilized and sterilized organic fertilizer and reduces chemical fertilizer input. Indeed, conventional composting technology requires a large surface area, a long fermentation period, significant malodorous emissions, inferior product quality, and little demand for poor end results. Conventional composting loses a lot of organic nitrogen and carbon. Thus, this comprehensive research examined sustainable and adaptable methods for improving agricultural waste composting efficiency. This review summarizes composting processes and examines how compost additives affect organic solid waste composting and product quality. Our findings indicate that additives have an impact on the composting process by influencing variables including temperature, pH, and moisture. Compost additive amendment could dramatically reduce gas emissions and mineral ion mobility. Composting additives can (1) improve the physicochemical composition of the compost mixture, (2) accelerate organic material disintegration and increase microbial activity, (3) reduce greenhouse gas (GHG) and ammonia (NH3) emissions to reduce nitrogen (N) losses, and (4) retain compost nutrients to increase soil nutrient content, maturity, and phytotoxicity. This essay concluded with a brief summary of compost maturity, which is essential before using it as an organic fertilizer. This work will add to agricultural waste composting technology literature. To increase the sustainability of agricultural waste resource utilization, composting strategies must be locally optimized and involve the created amendments in a circular economy.
1. Introduction
Agricultural waste is any waste generated during agricultural operations, primarily crop residue and livestock waste. Because of the rising population, urbanization, and changes in consumer behavior, agricultural waste creation rises every year globally.1,2 Most of the agricultural waste generated as a byproduct of agricultural production is released into the environment without being treated, being burned on a large scale, or being disposed of at random. This results in resource depletion, soil and water pollution, fires, as well as more serious ecological and environmental issues.3 Agricultural waste combustion produces a lot of smoke and hazardous pollutants, primarily CO, CO2, and NOX and other hazardous and poisonous gases. Therefore, the critical concerns pertaining to the sustainable growth of human society is how to manage such growing quantities of solid waste. Organic garbage makes up the majority (46%) of the total solid waste.1 Therefore, how to deal with the environmental pollution caused by agricultural waste has become a major problem that developing countries urgently need to solve. The organic portion of wastes, on the contrary, is a valuable organic resource that can be recycled and turned into value-added bioproducts by the application of different energy recovery processes.4−6 Therefore, an adequate management of organic solid waste is essential.2
Conversely, the widespread agricultural practices under inorganic fertilizers has led to an acceleration in environmental contamination. The aforementioned areas demonstrate specific evidence. (1) The soil exhibits compacting, limited cultivability, and reduced capacity to function as a soil buffer. The nutritional content in the soil is uneven as a result of the excessive and unnecessary use of nitrogen (N) fertilizer, which is abundant in nitrogen, while regions dedicated to farming are experiencing a continual deficiency in potassium (K) and phosphorus (P).7 (3) Both the quantity and quality of agricultural products suffer as a result of the reduction in fertilizer benefits. Food and vegetables, for instance, lack quality which evolved into a key characteristic of modern agricultural goods. (4) It contaminated the soil health, harming the populations of helpful microbes. Numerous chemicals pollute the environment in which people live and pose a major hazard to their health. The food chain is the entry point for these chemical compounds into the ecological cycle.8,9
The problems of improper treatment of agricultural wastes and low comprehensive utilization levels have become increasingly prominent, which has become a shortcoming of rural environmental governance. The harmless treatment and productive use of agricultural waste will minimize the release of hazardous chemicals, reducing soil, air, water, and other environmental pollution. Therefore, the transformation of agricultural waste products, including as straw, livestock waste, and poultry manure, into an organic fertilizer that is both highly effective and environmentally friendly holds significant importance in stimulating the sustainable growth of the agricultural economy and enhancing the ecological environment.10 Combining the domestic situation and foreign studies, many applied and sustainable agricultural waste treatment technologies can be utilized and promoted such as the carbonization and activation utilization, feed utilization, biotransformation, fertilizer utilization, anaerobic composting, and aerobic/microbial composting.11 The processes of composting and vermicomposting are two methods for converting agricultural waste into natural fertilizer through biological breakdown.6,12,13 The resulting amendments can be utilized to increase carbon retention in soil.14−17 Application of compost results in enhanced soil structure, decreased erosiveness, and increased water-retentiveness.18
1.1. Aerobic Digestion Process
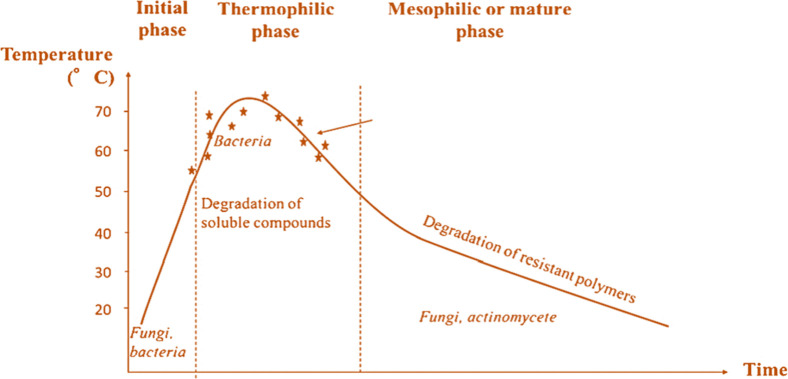
Aerobic digestion (composting) is a biochemical fermentation process that uses microorganisms to convert biodegradable organic matter into stable humus under controlled conditions. Because it does not harm the environment, especially the soil, compost/organic fertilizer is produced.19 Compost or organic fertilizer is the result of the transformation of organic matter from an unstable condition into a stable humus substance, which does not harm the environment, especially the soil environment. The composted material experiences large changes in volume and weight throughout the composting process. Weight and volume typically decrease by 30% to 50%19 as a result of the decomposition and conversion of volatile substances like carbon. Based on this original composting technique, the modern composting process was established and is broken down into aerobic composting and anaerobic composting. The final product is stable and pathogen- and phytotoxic-free.20 A composting cycle includes initial activation, the thermophilic phase, and the maturation phase (Figure 1). Microbial populations mineralize sugars during the early activation, which lasts 1–3 days and creates CO2, NH3, organic acids, and heat.21
Figure 1.
Composting temperature variations and the resulting microbial populations and organic molecules. Phases during which pathogen microorganisms are eliminated are marked with stars.22
This stage raises composting pile temperature. In the thermophilic phase, temperature peaks. Composting is best at 40–65 °C, where pathogens die at 55 °C. Table 1 shows the temperature and heat duration needed to kill common pathogens during composting. Here, thermophilic bacteria degrade lignin, cellulose, and lipids.21Table 2 shows microorganism development at different temperatures.23 Microbial activity decreases due to biodegradable chemical reduction, lowering temperature during mesophilic maturation. Microbial populations vary with composting pile temperature.22,24 Bacteria predominate above 60 °C, whereas fungi are absent.25
Table 1. Sanitized Conditions for Various Widespread Pathogens in the Digestion Process.
| Pathogens | Sanitary temperature | Sanitized time |
|---|---|---|
| Salmonella typhi | 55–60 °C | 30 min |
| Salmonella | 56 °C OR 60 °C | 60 min OR 14–24 min |
| Shigella | 55 °C | 60 min |
| Escherichia coli | 55 °C OR 60 °C | 60 min OR 15–20 min |
| Amoeba | 68 °C | 60 min |
| Hookless striped worm | 71 °C | 5 min |
| Ancylostoma americanus | 45 °C | 50 min |
| Brucella abortus | 61 °C | 3 min |
| Micrococcus pyogenes | 50 °C | 10 min |
| Streptococcus fermentans | 54 °C | 10 min |
| Mycobacterium bovis | 55 °C | 45 min |
Table 2. Interaction between Fermentation Temperature and Microbial Agent.
| Temperature/°C | Mesophilic | Thermophile | Hyperthermophile |
|---|---|---|---|
| 25–38 | Excited state | N/A | N/A |
| 38–45 | Inhibited state | Start to grow | N/A |
| 45–55 | Destruction state | Excited state | N/A |
| 55–60 | Bacterial flora decline | Inhibition state (slight) | N/A |
| 60–70 | - | Inhibition state (obvious) | Start to grow |
| >70 | - | Destruction state | Growth period |
As a result, temperature and the relative presence of microbes are solid indicators of the compost’s development, and Figure 2 clearly shows this process. The phases of heating up, elevated temperatures, cooling, and decomposition constitute a full composting process. Different bacteria, actinomycetes, fungus, and protozoa are found in each stage. Until a stable humic material is created, the microbes utilize the agricultural waste and stage products as a source of food and energy at each stage.
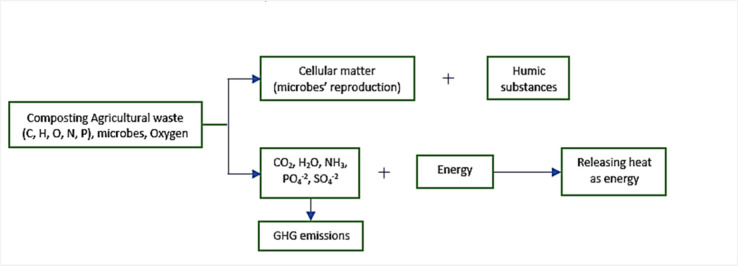
Figure 2.
Schematic diagram of the aerobic composting reaction process.
1.1.1. Principles of the Aerobic Composting Process
Composting and aerobic digestion are substitutes for each other, and both processes use microbes to break down agricultural waste.16 The type of biological waste can influence the choice of organic substrate during the fermentation process. The bacillus that is resistant to high temperatures, a lack of carbon dioxide, and oxygen is dried to create the microbial fertilizer inoculum, which can then be kept for a long period. When Xi and He26 and Li and He27 introduced various additions to the fermentation of biological waste, the findings indicated the inoculum’s effects clearly, and within a short period of time, they had completely decomposed high-quality organic fertilizer.28−30 The completed organic fertilizer can be used on regular farms to enhance soil quality and make it more conducive to growing “green food” and raising the quality of that food.
Aerobic bacteria, actinomycetes, fungi, etc. oxidize small fermentation substrate molecules to fuel biological growth, aeration, temperature, moisture, pH, C/N, particle size, etc. They also aid in the growth and reproduction of microorganisms, decompose a portion of macromolecular organic materials, and generate additional bacteria to advance the fermentation process. To create humus soil, which may be utilized to improve soil, the organic matter is fermented. The aerobic digestion of agricultural waste can be represented by the chemical reaction equation shown below:
| 1 |
| 2 |
| 3 |
| 4 |
Most of the time, aerobic composting of agricultural waste takes place in an environment that is natural. Temperature, moisture, pH, C/N ratio, and particle size affect rapid aerobic composting fermentation.21,31 Microorganisms and agricultural waste type are internal factors, while ventilation, oxygen supply, temperature, moisture content, pH value, C/N, particle size, etc. (Table 3) affect aerobic composting.31−33
Table 3. Various Compost Factors and Their Significance.
| Factor | Significances | Literature | |
|---|---|---|---|
| Properties of compost substrates | C/N ratio | Low C/N ratio substrates lose nitrogen by NH3 volatilization, while high C/N ratio substrates compost more slowly. | (33) |
| Biochemical composition | It demonstrates their ability for biodegradation, or stable feedstocks are difficult to disintegrate during composting. | (32,34) | |
| Particle size | Small particle substrates—likely to encourage the formation of clumps. | (21) | |
| Large particle substrates— difficult to disintegrate. | |||
| Moisture | Acts as a conduit for the transfer of nutrients within the compost mixture and has an impact on gas exchange within the compost heap. | (31) | |
| pH | Influences NH3 volatilization in addition to microbial activity. | (35) | |
| Environmental factors | Temperature | Indicates the level of microbial activity and the stage of composting. High temperature is beneficial for compost sanitary. | (33,36) |
| Aeration | Compost aeration has a substantial impact on the quality of the compost as well as the GHG emission. | (37,38) | |
1.2. Limitations of Conventional Agricultural Waste Composting
Agricultural waste contains a lot of organic matter and high levels of nitrogen, phosphorus, and potassium, which are required for crop growth. Common processes for turning agricultural waste into organic fertilizer include aerobic composting. Composting is a more effective way to treat agricultural waste than other methods because it improves soil structure and acceleration of the geochemical process pertaining to the availability of essential nutrients for crops, along with the enhancement of soil fertility levels.39
On the other hand, traditional composting methods exhibit several drawbacks. These include a substantial requirement for surface area, an extended fermentation period, significant emission of malodorous substances during the fermentation process, suboptimal product quality, and limited demand for substandard products. The negative reputation places restrictions on how widely the procedure can be used and promoted. Moreover, conventional aerobic composting is occasionally ineffective for decomposition due to the physiochemical characteristics of organic waste. These properties reduce composting temperature, which reduces decomposition and sanitation. NH3, H2S, and other pollutants can also occur from conventional aerobic composting.40 Furthermore, the loss of organic nitrogen and carbon during the composting process is significant. However, by raising the treatment temperature above 70 °C, pathogens in animal manure can be destroyed in just 10 to 30 min.41
The presence of elevated levels of nitrogen in livestock manure gives rise to a significant issue in conventional agricultural waste composting, namely, the release of various nitrogen compounds into the atmosphere; i.e., NH3, NxO, NOx, CH4, VOCs, and other molecules that are chemically related to these are all examples of these compounds. The primary component of the gaseous emissions is NH3, and conventional composting significantly wasted nitrogen resources by emitting ammonia at a rate of between 70 and 88%.42 These odor emissions are hazardous to the environment because NH3 is offensive, irritating, and smelly. The compost’s value as a fertilizer is also reduced by the NH3 loss. Compost quality has been significantly impacted by the loss of carbon and nitrogen, and the acidification induced by the released ammonia gas has decreased biodiversity. A number of environmental issues have been brought on by the greenhouse gases created, which have increased global warming.
1.3. Research Purpose and Significance
Considering the preceding discourse, it is vital to do research on expeditious and innocuous approaches for generating compost of high quality. An enhanced and environmentally viable aerobic composting process holds significant potential for effectively managing the substantial volume of valuable organic waste. Based on the domestic and foreign research on agricultural solid waste composting, it is worth exploring the composting of additives and agricultural straw waste into resources, which is also important for the optimization of agricultural straw waste composting technology and the enhancement of organic fertilizer standards. To expedite and sustain thermophile temperature levels, composting practices employ a range of physicochemical and microbiological techniques. These techniques include the incorporation of bulking agents, the regulation of ventilation, and the application of compost additives/conditioners during the initial phase of composting.43 The reduction in the carbon-to-nitrogen (C/N) ratio inside the compost matrix is a significant factor that contributes to the emission of greenhouse gases. Therefore, carbon-rich additives (such as wood chips, mushroom residues, rice bran, biochar, minerals, etc.) in the composting system have become an important way to regulate nitrogen loss and control greenhouse gas emissions.44,45
In order to explore the impact of additives on the composting process of agricultural waste, as well as the resultant compost quality and microorganism composition, this study offers a novel approach and theoretical framework for enhancing the optimization of agricultural solid waste composting. This study examines the composting process of normal agricultural straw waste, with the addition of biochar/mineral as a conditioner. The aim is to investigate the impact of these additives on the microbiological, chemical, and physical parameters of the composting system. The present study aimed to investigate the variations observed in composting techniques, the quality of compost produced, and the reactions exhibited by crucial microbial populations upon the introduction of compost additives during the composting process.
This research study’s value and significance can also be seen in its useful results, which have provided valuable pieces of reference information for developing effective strategies to produce quality organic fertilizer from agricultural wastes within a short duration. Thus, this study’s results could serve as vital reference information for effective aerobic composting systems, operational guidelines, optimization of process parameters, process scaling up to a large industrial scale, resource recovery/recycling, and efficient waste management. Moreover, the results of this study have, without doubt, contributed immensely to the academic body of knowledge and to bridging the existing research gaps in the field of research studies.
1.4. Development Approaches in Aerobic Composting Technology: Additive-Mediated Composting
In accordance with the discussion above, it is crucial to research quick and risk-free ways to create high-quality compost. The direction of optimizing the country’s agricultural solid waste composting technology is mainly to shorten the composting time, reduce the generation and emission of waste gas from composting, and reduce the composting quality deterioration caused by the loss of composting nutrients.46 Thus, improving the composting process for these issues is crucial for agricultural waste treatment and disposal. Temperature, pH, carbon–nitrogen ratio, seed germination index, ammonium nitrogen, and nitrate nitrogen all affect composting quality and smoothness.47 Due to high refractory cellulose, agricultural waste composting is slowed by a lack of organic materials. Additionally, most nutrients like carbon, nitrogen, and others are lost during composting, lowering compost quality. Due to high composting temperatures and insufficient carbon sources in the substrate,48 nitrogen is volatilized and lost as NH3, NO, N2O, and N2.49 Moreover, NH3, NO, and N2O are polluting gases; NH3 is the main source of odor; and N2O is a greenhouse gas.50
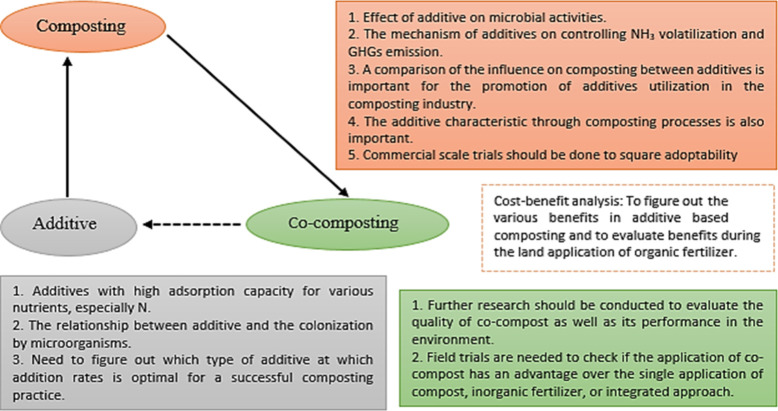
Therefore, adding readily available carbon sources/organic matter to the composting system of agricultural straw waste has become a feasible optimization method. Exploring the effect of adding readily available carbon sources (OM) and inorganic matter on the composting process, the effect of nitrogen retention and the effect of functional microbial communities optimize the agricultural waste composting. Compost conditioner refers to additives that are added in small amounts (relative to the mass of raw biomass materials in compost) to significantly optimize and adjust the composting process or composting products (Figure 3). Conditioners include some cheap crude chemical additives (ferrous sulfate, sodium humate, superphosphate),51 biochar,52 and carbon-rich biomass (corn stalks, sawdust, waste mushroom culture substrate).53
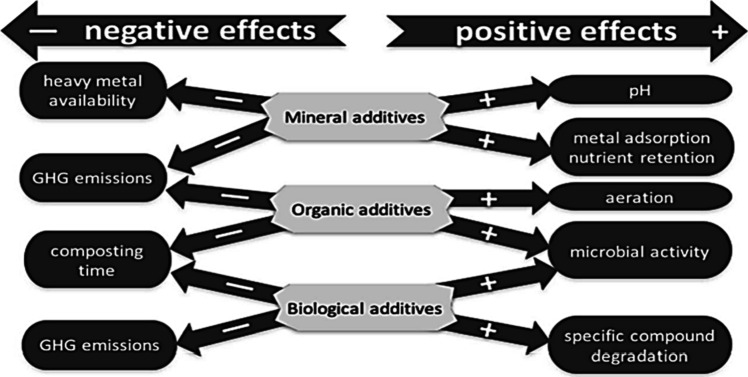
Figure 3.
Prospective consequences, both good and bad, of biological, organic, and inorganic additives. When selecting an additive, a choice between these consequences must be established.
These additives can be categorized into three distinct groups: mineral, organic, and biological.21,54
(1) Organic additives: The C/N ratio of many livestock and poultry manures is relatively low, and it is added with various carbon-rich substances to the pile to adjust the C/N to 25:1–35:1.55 Wei and Yuan56 used corn stalks to adjust the initial C/N of cow dung compost to 24.84, which is conducive to the rapid decomposition of compost. Li and Yuan57 used straw and urea to adjust the carbon–nitrogen ratio of the pile and found that straw can improve the ventilation of the pile, promote the decomposition of the pile, and reduce the loss of nitrogen. Mei and Li58 found that adding straw to manure compost can increase the C/N ratio of the compost and reduce the nitrogen loss rate. Zhuang and Shan59 found that adding sawdust to cow dung compost can increase the C/N ratio, porosity, and degradable carbon content of the compost and promote microbial composting. A highly stable organic composting component, bio charcoal, a pyrolysis output with a high aromatic content, is also gaining popularity.60,61 Composts’ ability to retain carbon can be enhanced through biochar, which would help to slow global warming.62,63 Biochar has the functions of improving the quality of composting, adsorption of pollutants, and reducing greenhouse gas emissions. It has been a research hotspot in the field of compost additives in recent years.64
(2) Inorganic/mineral additives: Microbial reproduction and metabolism depend on pH. The optimal pH for methanogens is 6.5–7.5. It will struggle to thrive below pH 6, reducing methane emissions.65 Feces acidification increases hydrogen ions, inhibits NH4+-N to NH3, and prevents nitrogen loss.66 Kupper and Häni67 showed that compared to the control group spring, summer, and autumn NH3 emissions fell 66%, 44%, and 71%. Aboltins and Melece68 conducted a meta-analysis of 89 literatures on greenhouse gas and NH3 emission reduction in the process of livestock and poultry manure treatment and concluded that manure acidification can simultaneously reduce CH4, N2O, and NH3 emissions. Wang and Xue69 conducted a systematic assessment of the greenhouse gas and NH3 emissions of the entire cow or pig manure treatment chain and found that the acidification of biogas slurry can reduce CH4 emissions by 87%, which can reduce the greenhouse gas and NH3 emissions of the entire treatment chain. In addition to improving compost porosity, temperature, oxygen content, methanogen inhibition, and CH4 emissions, high-porosity, high-specific-surface-area mineral additives can adsorb nitrate nitrogen and N2O in the pile, reducing N2O emissions.70 These additions benefit from their wide availability and low cost as industrial waste.71 Adding more than 10% of the dry weight (more than 4% of the wet weight) of calcium super phosphorus to pig manure composting reduces NH3, CO2, CH4, and N2O emissions and improves carbon and nitrogen storage, according to Xie and Tran.72 Li and Wang73 and Mei and Li58 reported that when calcium superphosphate was added to chicken dung NH3 emissions dropped 31.1% compared to the control. Few studies have examined carbon, nitrogen, and humic material changes during organic–inorganic co-composting, despite the necessity of using fertilizers to prevent nitrogen loss. The production method, biological effectiveness of fertilizers, and nutrient content of compost may all benefit from clarification of these changes. How the addition of a mineral additive to organic–inorganic aerobic co-composting affected the amounts of carbon, nitrogen, and humic compounds was investigated.74 The data showed that adding inorganic fertilizers did not affect compost fermentation. This ingredient increased compost bin temperature, pH, and oxygen, speeding organic–inorganic co-composting fermentation.75
(3) Biological agents: The inoculation of biological agents can promote compost maturity, shorten the fermentation time, and help the nitrogen preservation and harmlessness of compost.76 The microorganisms in the biological agent can convert a large amount of NH4+-N in the manure of livestock and poultry through nitrification into nitrate and then generate N2 through denitrification, and NH4+-N can also be fixed into microbiological protein nitrogen, thereby reducing NH4+-N content, reducing the synthesis of NH3.77 Zhou and Liang78 added a genetically enriched stable microbial community CC-E in feces from cattle, and the amount of NH3 emissions within 20 days after addition to the heap was reduced by 63% compared with the control. Mao and Zhang79 found that bamboo charcoal was compounded with two kinds of bacterial powder and then added to the manure pile, and the peak emissions of CH4, N2O, and NH3 were significantly lower than those of the control. Wang and Xu80 believed that adding biological carbon composite bacteria to pig dung could significantly reduce NH3 and N2O emissions: they decreased by 70.54% compared with the control group, and N2O emissions decreased by 29.01%, which enhanced the carbon and nitrogen storage effect of the pile.81,82
1.5. Research Trends of Additive-Mediated Aerobic Composting
Through introducing an inoculum or organic or mineral components that alter aeration, temperature, moisture, pH, nutrients available, etc. during the composting process, additives can directly or indirectly modify the indigenous microbial community (Table 4).
Table 4. Overview of the Different Additives Used in Agricultural Waste Aerobic Composting and Their Major Effects.
| Feedstock | Additives | Influences |
|---|---|---|
| Sewage sludge + rapeseed marc | Bamboo biochar (600 °C) 0%, 1%, 3%, 5%, 7%, 9% (FW) | Adding 9% biochar into the composting feedstock decreased TN loss by 64.1% and produced more stable Cu2+ and Zn2+ compost.132 Improved porosity and compost maturity.121 |
| Poultry waste | Pine chips biochar (400 °C) @ 0%, 5%, 20% (DW) | The increase in biochar addition rates resulted in higher pH and peak pile temperatures. A fall in NH3 emissions and 52% less total N loss.42 |
| Poultry waste | Wood biochar (300–450 °C) @ 50% (FW) | Composting had a major effect by the addition of biochar. Higher biological waste degradation, compost maturityk and less odor emissions and N loss.60 |
| Poultry manure + apple pomace, rice straw, and oak bran | Wood biochar (400–600 °C) 2% (v/v) | Increased the decomposition of organic matter despite a decline in microbial biomass. A wide variety of fungus in compost added with charcoal.133 |
| Cattle manure + apple pomace, rice straw, and rice bran | Wood biochar (400–600 °C) 20% (w/w) | The increased aeration resulted in decreased methanogens (McrA) while methanotrophs (pmoA) grew.37 |
| Poultry waste + sawdust | Nutshell, hard wood shaving, chicken litter @ 5%, 10% (fresh weight basis) | This produced increased respiration rates which showed higher OM degradation and increased microbial activity. The compost showed lower NH3 emissions with enhanced maturity in compost.111 |
| Poultry waste + tomato stalk | 1% commercial biochar | Adding biochar increased pile’s temperature and extended thermophilic phase and exerted a less significant impact on bacterial diversity.120 |
| Sewage sludge + wood chip | Woody material 4% (wet weight basis) | Increasing pile temperature and disintegration of organic matter, while showing lower NH3 emissions in the first week of composting.134 |
| Sewage sludge + rice straw | Wood (500–600 °C) 6% 12%, 18% (wet weight) | Due to increased porosity of sewage sludge, OURs improved and accelerated humification and degradation.46 |
| Poultry manure + barley straw | Holm oak biochar (650 °C) 3% (wet weight) | Holm oak biochar improved aeration, reducing composting time by 4 weeks (20%) and increasing stabilization and detoxifying while promoting organic matter decomposition. There is no visible impact on CO2, CH4, or N2O emission and reducing N loss by 15%.123,135 |
| Poultry manure + wheat straw | Woodchips 5%; 10% (wet weight) | The addition of woodchip biochar has shortened composting time due to higher pile peak temperature.83 |
| Poultry manure + rice straw | Rice straw (400–500 °C) 2% (dry weight) | Modified microbial genetic makeup and increased C catabolic capability.136 |
| Cow manure/poultry manure + apple pomace, rice straw, and rice bran | Hard wood (550 °C) 10% (v/v) | Stability and recalcitrant nature of the compost were improved with microbial communities. Improving FA fractions.137 |
| Municipal solid waste + green waste | Holm oak (650 °C) 10% (dry weight) | It accelerated the decomposition of organic matter and reduced the emission of GHGs. Decreased N losses and greater concentration of P that is readily available.114 |
| Poultry litter + sugar cane straw | (Green waste + poultry) biochar (550 °C) 10% (dry weight) | Reduced total GHG emissions, improved N retention, and decreased NH3 emissions because the adsorption capacity of biochar may fix the nutrients. A 60% less loss of NH3 and 51% reduction in TN losses.138,139 |
| Sewage sludge + wheat straw | Wheat straw 2%, 4%, 6%, 8%, 12%, 18% (dry weight) | The amendment encouraged humification and the decomposition of organic substances with low N losses. The heavy metal and emission of GHG (NH3, CH4, and N2O) were decreased by 58.03–65.17%, 92.85–95.34%, and 95.14–97.28%, respectively, but CO2 emissions rose.140,141 |
| River sediment + rice straw, bran, and vegetable | Rice straw (500 °C) 2% | Rice straw affected the diversity of the bacterial community and suppressed the availability of heavy metal.142,143 |
| Layer manure + saw dust | Corn stalk; Bamboo; Woody; Layer manure; Coir (450–500 °C) 10% (wet weight) | The nitrification and pile temperature were increased, and the emissions of NH3 and CH4 were reduced.144,145 |
| Fishpond sediment + green waste, rock phosphate | Coir (450 °C) 0%, 20%, 30% (v/v) | A 24 day reduced compost production time, improved nitrification, enzyme activity, microbial population, nutrient content, and better grade compost.35 |
| Chicken manure + tomato stalk | Wheat straw 1% (w/w, wet weight) | Quick thermophilic phase attainment, higher temperature, and longer duration. Raising germination index.120 |
| Green waste + spent mushroom | Coconut husk fiber 20–30% (w/w, dry weight) | Improving particle size distribution and the free air space. Increasing the nutritional and CEC contents.115 |
| Cow dung + hydrilla + sawdust | Wood 5% (w/w, wet weight) | This showed a prolonged thermophilic phase with 39% reduction in air-filled porosity and a 45% increase in TN.146 |
| Sewage sludge + wheat straw | Wheat straw 8–12% (w/w, dry weight) | Speeds up the humification process with a decrease in the odorous index and volatile fatty acids147 |
| Sewage sludge + paddy straw | Wood 6–18% (w/w, wet weight) | A rise in the rate of O2 absorption. Adding 13–26% more FA-like chemicals and 15–30% more HA-like compounds, respectively.148 |
| Poultry manure + rice husk + apple pomace | Oak 2% (v/v) | The increased rate of enzymatic activity accelerated the humification process by 10% by increasing HS carbon.133 |
| Manure | Charcoal 9% and 28% | With pH drop and greater GI, it accelerated the thermophilic phase change. Cu and Zn mobility decreased by 35%, 65%, and 39%, while the TKN loss decreased.105 Reducing NH3 and CH4 losses. A 27–32% drop in CO2-equivalent GHG emissions.38 A 6.9%–7.4% increase in C–CO2 emissions.83 |
| Manure | Biochar 50% (fresh weight) | Biochar significantly improved humification. Maintaining the nitrogen and organic materials in compost.60 |
| Manure | Chestnuts, leaf litter (25%) | Except for Zn, the co-composts’ heavy metal content was within the allowable limit.149 |
| Manure | Phosphogypsum 10–30% dw | In manure composted with mineral additives, TC, TN, and mineral N in the finished compost product were unaffected. The EC and TS content increased while pH decreased. The composting showed a significant reduction in CH4 emissions. By modifying the nitrification process, the N2O emission was decreased. There is no negative impact on the germination index or the breakdown of organic materials.150,151 |
| Manure | Compost inoculum 33% dw | Accelerating the succession of the microbial community which reduces the time needed for composting. After the thermogenic phase, harmful bacteria in compost are eliminated.152 |
| Manure | Biochar, sawdust 5%, 10% (wet weight) | This resulted in higher respiration rate, increased bacterial activity, and reduced nitrate leaching and NH3 emission.111 |
| Manure | Ash 0–20% | Ash amendment reduced the amount of nitrogen loss, quick OM mineralization, and rise in humic acid. The improved aeration of pile produced less odorous fumes.153 |
| Manure | 10%, Straw | The highest temperature and organic matter degradation.154 |
| Manure | Zeolite 0.4, 1.0, 2.5, and 6.25% | Considerable fall in the concentration of ammonia nitrogen. The substrates’ temperature remained in the thermophilic range. Zeolite showed 60% less ammonia volatilization and pH less than 5. A decrease in soluble P because of the development of low solubility and slow release of the N source.155,156 |
| Manure | Bentonite 0%, 2.5%, 5%, 7.5%, and 10% dw | No significant variations in pH and temperature but promoted OM decomposition. The TKN content increased while lowering the C/N ratio. Decrease in the amount of extractable heavy metals.97 |
| Manure | Rock-P 0%, 2.5%, 5.0%, and 7.5% (w/w, dw) | The amount of bioavailable Cu fractions decreased. The exchangeable and reducible fractions contained zinc. By complexing the metal ions with inorganic components, you can decrease the availability of metal.157 |
| Manure | Rice straw 25% (w/w, fresh weight) | Increased the quantity of N and P while lowering the amounts of NH4+–N and soluble P fractions. A decline in labile P and increase in pH, a decline in OM, and a reduction in the C/N ratio.158 |
| Manure | Elemental S (2 mol H+ mol–1 S) | There was a 90–95% reduction in the loss of N from aerobic conditions due to ammonia (NH3) volatilization. There was a 60% reduction in NH3 loss.106 |
| Manure | Rock-P 4% fw | Maximum water-soluble P and K release. Improved P and K soil fertility status, higher yield, absorption, and nutrient recoveries. A rise in the amount of accessible phosphorus (41% of the total phosphorus).159,160 |
| Manure | 28%, 3% Rock-P, phosphogypsum | Significant increases in accessible P levels (13 times) were seen in soil. Soil function was altered, and soil biochemical characteristics were improved by the application of P-enriched OMC.161 |
| Manure | Mg hydroxide, phosphoric acid 3.8%, 7.3% and 8.9% of dw | Initial N content loss to total nitrogen loss fell from 35% to 12%, 5%, and 1%. The final compost increased total nitrogen by 10–12 g/kg and NH4+–N by 8–10 g/kg. Mature was best. Best Mg and P salt dosages are 20% of starting nitrogen.162,163 |
| Manure | 5%, 20% fw biochar | Temperatures and CO2 reached at significantly greater levels. Poultry litter that has been modified with biochar breaks down more quickly. Ammonia emissions decreased by as much as 64%. Losses of total N were decreased by up to 52%.42 |
| FW | 50% fw sawdust | Faster acidification and composting timeframes and a lower pH upon completion. As a result of increased airflow through the particles, the pace of composting quickened and temperature increased.96 |
| FW and GW | Ash (8%, 16%) and (25%, 50%) | The addition of ash had no negative effects and met with all legal requirements. Measurements of soil respiration showed that composts with additives performed better.164 Ash led to a 75% increase in the volume of water that could be stored. As basal respiration, organic, soluble, and microbial biomass carbon levels increased, the activity of the enzymes b-glucosidase, l-asparaginase, alkali phosphatase, and arylsulphatase all reduced in the composted products.98 |
| FW | Microbial inoculum (500 mL solution (1:20)) | Development of stable, mature compost. Within a week of the microbial inoculum, the thermophilic phase was produced. The germination index (>80%) and self-heating test.110 |
| FW | Biochar (300–450 °C) 10%, 15% (w/w) | Enhanced the physiochemical makeup of the finished compost and the composting process. Attained the thermophilic temperature quickly, which affected OM degradation by 14.4–15.3%, NH4 concentration by 37.8–45.6%, and NO3 concentration by 50–62%.61 |
| FW | Coal fly ash (25%, 33%, 50%), lime (2%, 4%) | Additives inactivated the pathogens, maintaining a pH of 12 for around 4 days. Effective in minimizing poststabilization regrowth and devitalizing the pathogens.100 |
| FW | Cornstalks, sawdust, spent mushroom 15% (5% each) | Compost achieved the highest maturity (germination index rose from 53% to 111% and the C/N ratio dropped from 23 to 16). Minimal effect on NH3 emissions but reduced leachate formation, CH4, and N2O emissions. Reduced overall greenhouse gas emissions (to 33 kg CO2-eq t–1 dry matter).45 |
| FW | 100 g of Na acetate | Acetate raised the pH level to a value between 5.2 and 5.5. A favorable impact on organic material degradation and reducing propionic and butyric acid generation.165 |
| GW | Biochar, clay 10%, 25%, and 50% | Biochar reduces (44%) carbon mineralization during co-composting and produces lower emissions of CO2.166 |
| GW | 10%, Rock-P, sediment | There was an increase in nitrogen oxide emissions but a decrease in methane, ammonia, and hydrogen sulfide emissions of about 35.5–65.5%. During the composting of manure, the overall emissions of greenhouse gases (GHG) were reduced by about 34.7%. Produced more humic acid, as evidenced by the E4/E6 ratio. Delayed biological organic matter decomposition and created mature compost with increased electrical conductivity.167 |
| GW | Jaggery, fly ash (5% each) | Additives showed a big impact on cellulose activity and microbial development. The C/N ratio was reduced by more than 8%.85 |
| GW | Biochar (20%, 30%), spent mushroom (35%, 55%) | Biochar boosts compost nutrients. Improved compost’s dehydrogenase activity, temperature, particle size distribution, open air space, cation exchange capacity, nitrogen transformation, organic matter degradation, humification, element concentrations, and seed germination toxicity.115 |
| MSW | Bagasse, paper, peanut shell, sawdust (10–40%) | Bagasse biochar has optimized the moisture up to 60% and produces more FAS.102 |
| MSW | Rice straw 10%, 20%, and 30% | Decrease in emissions of sulfur compounds that are noxious. Decrease in emissions of VFAs, alcohols, aldehydes, ketones, aromatics, and ammonia.168 |
| MSW | 5% and 10% of Zeolite | The finished compost’s ammonia content decreased. The rates of ammonia uptake were 74.94 and 87.98%.169 |
| MSW | 40% (w/w) of reed straw, 12% zeolite, plastic tubes, woodchips (50% each), and inoculum (2.5 and 5 mL kg–1 dry MSW) | Range of cumulative emissions of N2O, CH4, and CO2 was 92.8, 5.8, and 260.6 mg kg–1 DM to 274.2, respectively. The range of cumulative NH3 emission was 3.0 to 8.1 g kg–1 DM.170 The emission factors given have lower values.171 Increased the rate of maturity and humification.172 |
| SWS | 12% and 1% Biochar, lime 10%, 15%, 30%, and 1% zeolite and lime | Accelerated disintegration rate, decreased the emission of N2O, CH4, and ammonia, and greater levels of the substances fulvic acid (3.79%) and humic acid (17.23%). HM bioavailability was successfully decreased (34.81% Cu, 56.7% Zn, 87.96% Pb, and 86.5% Ni). High mature compost with increased nutrient concentrations in compost by increasing the adsorption of ammonium ions and decreasing ammonia loss and N2O emission.36,173 |
| SWS | Bamboo charcoal @ 0%, 1%, 3%, 5%, 7%, 9% (w/w) | Considerably lessen nitrogen loss with the total nitrogen loss decreased by 64.1%. Reduced up to 44.4% and 19.3%, respectively, in the mobility of Cu and Zn in the sludge.132 |
| SWS | Woodchips, rice husk @ 1:1 to 1:4 (biosolids:woodchips) | Compost pore space, oxygen, operating temperature, and heavy metal concentrations were according to CCME (1996) standards.174 |
| SWS | Yeast inoculum (8.13 × 107 and 5.37 × 106 CFU/g-ds) | During the heating stage, raw materials and acetic acid were degraded.91 |
| SWS | 5%, 10%, and 15% Coal fly ash and 1.5% and 3% lime | The 28-day composting period could be shortened (composting time by 35%) while increasing the breakdown efficiency by high pH. The amount of heavy metals was within the permitted range.100 |
| SWS | 25%, 33%, and 50% Coal fly ash and 2% and 4% lime | Bacterial colony was totally rendered inert. Slowed the regrowth after stabilization.175 |
| SWS | Lime 2.0% (w/w) | Percentage of compounds like humic acid rose from 20.5% to 40.9% and 20.6% to 32.6%, respectively, through expediting the maturation process and improving the transition of organic matter. The copper’s transformation was only marginally impacted, and zinc’s transformation from exchangeable and reducible fractions to oxidizable and residual fractions was improved.176 |
| SWS | 25% Zeolite | Zeolites completely eliminated Ni, Cr, and Pb, as well as a sizable portion (more than 60%) of Cu, Zn, and Hg. Low metal concentrations (<1 mg/kg) were found in zeolite leachates.177 |
| SWS | 20% Red mud 30% fly ash | Germination index and electric conductivity both rose, whereas the pH and total organic carbon decreased. Sludge’s toxicity was eliminated, and its stabilization was expedited. By raising the residual fractions, the amount of heavy metals overall was decreased.178 |
| Sewage sludge | 10% Coal fly ash and 20% phosphate rock | The earthworm development, reproduction, and metal concentrations (apart from Zn and Cd) were all significantly greater. The mixtures’ concentrations of total metal and total organic carbon (TOC) decreased.179 |
| Rice straw | Red mud, 25 g (w/w) | Significant changes were seen in the pH, water extractable organic carbon (WEOC), and total organic carbon (TOC). The heavy metals that have had the greatest efficiency loss. Following the addition of the additives, the microbial biomass in the treated soil rose.180 |
| Chicken manure + sawdust | Straw biochar (0.42 cm3/g pore size) 25% (w/w, wet weight) | The cornstalk biochar, free load bacteria, mixed load bacteria, and separate load bacteria groups reduced NH3 by 12.43%, 5.53%, 14.57%, and 22.61%, respectively. Total nitrogen loss, electrical conductivity, water-soluble carbon, and ammonium nitrogen decreased. Increased seed germination, microbial diversity, and lactic acid bacteria during composting.181 |
1.5.1. Temperature Profile
The composting process is significantly influenced by temperature, which not only reflects the level of composting and the growth rate of microorganisms but also reduces pathogen hazards in living organism-derived materials.83,84 When microbial activity is stimulated by additives, the thermophilic phase commences early and lasts longer than would be the case with conventional composting.15,85,86 Commercial components like zeolite, kaolinite, chalk, ashes, and sulfates or biochar accelerated agricultural and food waste composting by 2–3 weeks.87 After 50–60 days, biochar-modified compost stabilized.61 Biochar, zeolite, jaggery, and polyethylene glycol raise composting temperatures quickly in animal dung, food waste, and green waste.85,88,89 Similar temperature trends have been found in composting with biological or organic additives.90,91 These additions may accelerate temperature rise due to increased microbial biomass and activity. Research has indicated that throughout the composting process of the same material the temperature rises rapidly after adding biochar to the compost, generally can enter the high temperature period 6–7 days in advance, prolong the high temperature retention time, promote the rapid degradation of organic matter in the compost, and significantly accelerate the composting process.42,64,88 This may be because the rich pore structure of biochar provides a favorable environment for microbial activities, and enhanced microbial activities release heat, which increases the temperature of the pile.
1.5.2. Moisture Content of the Matrix
The oxygen intake, microbial activity, and decomposition rate of composting depend on moisture. Therefore, an adequate moisture content minimizes the time it takes for composting to mature.21,92 A range of around 50–60% of water is thought to be the optimum amount of water for organic matter biodegradation.55,93 High humidity, however, could encourage anaerobic conditions and odor development during composting. Because they can partially absorb leachate, fibrous materials94 are utilized as bulking agents to reduce organic waste moisture.94,95 Air flow through sawdust particles increased water absorption and degradation, according to Chang and Chen.96 Water losses may be reduced by the inclusion of materials having strong water retention capabilities, like clays. Due to bentonite’s ability to swell, Li and Wang97 demonstrated that the buffered initial moisture decrease and improved water retention capacity when composting green wastes with ash98 or adding phosphate rock35 to green waste composting improved water retention capacity and buffered initial moisture loss. Eggshells may reduce biological activity but not water retention. However, research shows that biochar composting increases the moisture content of the same material. A biochar-containing system has more moisture due to its high water holding capacity (WHC).99
1.5.3. pH Value
The pH level of the pile has a significant impact on the environment in which microorganisms can survive and regulate the movement of heavy metals inside the pile. Microbial activity is influenced by pH variations during composting, resulting in decreasing initially and an increase in the latter stages.31 Adding pH-raising chemicals to acid feedstocks such as food waste improves composting.100 A study found that adding an inoculum community raised composted food waste pH from 4.3 to 6.3.90 Higher biological activity breaks down acids and organics. Bulking materials including bagasse, paper, peanut shells, sawdust, and Ca-bentonite raise composting pH like fly ash, lime, or red mud.101−103 Alkaline additions may slow metabolism. Initial sludge cocomposition with 25% fly ash had less thermophilic bacteria, according to Wong and Fang.104 Because bamboo charcoal or zeolite absorbs ammonia from organic nitrogen mineralization, these additives may minimize pH rise during the thermophilic phase. Lower pH may limit nitrogen loss because ammonia volatilizes at high pH.105 Finally, adding elemental sulfur to poultry manure composting significantly decreased pH,106 primarily as a result of H2SO4 being produced during elemental sulfur’s oxidation, which raised the proportion of H+ ions.
1.5.4. Pile Aeration
According to Gao and Li,107 forced aeration through pipes108 and mechanically moving the composted material38,109,110 are the best ways to provide the appropriate aeration needed for composting. Biochar, residual crops straw, woodchip or sawdust, and crushed branches boost the composting pile’s natural aeration and porosity, reducing the cost of pile turnover or forced aeration.88 The presence of bulking agents, which have a low moisture content and a large number of pores, results in the formation of inter- and intraparticle voids.102 The inclusion of biochar, which has a porous structure, has the potential to significantly improve the aeration of compost.89 There exists a clear correlation between the surface area that is subjected to microbial attack and the biological oxidation rate.33
1.5.5. Organic Matter
According to research, biochar promotes more to the decomposition of soluble organic carbon during composting than it does in systems with no biochar addition.111 The incorporation of biochar to organic matter that has been decomposed enhances the porosity of the material because of the enormous porosity and variety of pores in biochar. Through the stimulation of microbial and enzymatic activity, biochar also effectively expedited the decomposition of organic matter.112,113 The absorption of molecules like NH3, NH4+, H2S, and SO42– by biochar is the cause of the acceleration of the rate of decomposition of organic matter.114 Functional groups on biochar’s surface increased the chemical absorption of nutrients and organic carbon, minimizing effluent loss from compost.115 Biochar also increased compost aeration, aiding heap operations. Wang and Tu’s116 and Awasthi and Wang’s36 experiments on sewage sludge–wheat straw compost (biochar from wheat straw, 600 °C) corroborated this finding. Biochar-enriched compost yields higher humus acids.36,116
1.5.6. Microbial Biodiversity
In composting, various types of bacteria play different roles in the degradation of lignin. Due to the synergistic effect of various types of bacteria, lignin completes the entire chemical process of degradation in composting. Therefore, in the process of composting, it is necessary to understand the amount, diversity, and group structure of the microbial ecosystem in the compost. Due to their nutrient and readily available carbon levels, additives affect compost microbial populations via affecting pile temperature, moisture, and aeration.117 In the enzymatic decomposition of cellulose during the composting of green wastes, the additive increased the quantity of microorganisms.85 Fishpond sediments, wasted mushroom substrate, and charcoal were also added with similar outcomes.115,118−120 Biochar regulates moisture and aeration, which affects composting temperature, and its porous nature may stimulate microbial activity.89 Biochar application rates above 20% can slow organic material biodegradation.121
1.5.7. Key Nutrients
The inorganic nitrogen in compost transforms during the process from NH4+ to NO3. The bioavailability of nitrogen declines as the process proceeds onward.122 According to a study by López-Cano and Roig,123 composting two-phase olive mill waste with sheep dung at 650 °C with 4% (dry weight) oak wood biochar helped nitrification. Biochar slows ammonification and creates a nitrifying bacteria-friendly environment. Biochar increased compost nitrogen. With biochar, NH4+ or volatile NH3– adsorption on charcoal lowers compost N losses.105,123 In a study by López-Cano and Roig,123 BC compost had twice as much NO3– as compost without biochar. In addition to nitrogen, compost contains P, K, Ca, Mg, Na, and S. P and K are found in larger concentrations than the other macroelements in composted material, depending on the type. Usually unaffected by composting materials, the effectiveness of compost as a soil fertilizer is diminished when such components are lost during the composting process.122 Because biochar contains the previously mentioned macroelements, adding it to compost organic matter improves the compost’s fertilizing characteristics.113
1.5.8. Organic Pollutants
Black carbon (BC) and other carbonaceous sorbents, for example, strongly bind to organic contaminants.124 Thus, considerable BC sorption reduces organism bioaccumulation, reducing the risk of contaminated matrices. PCBs, PCDD/F, pesticides, linear alkylbenzenesulfonates (LAS), nonylphenol (NP), and polycyclic aromatic hydrocarbons (PAH) may be found in composts.125 They may be present in substantial quantities in composts made from sewage sludge. According to microbial activity, certain pollutants are converted into metabolites or mineralized; some contaminants remain in compost; whereas others are leached.125 Because biochar absorbs organic pollutants from water, soil, and sewage sludge, its bioavailable concentration decreases,126 and microorganisms that might be able to degrade them are stimulated. There have not been any studies done yet on how biochar affects the amount of contaminants in compost. However, studies on sewage sludge show that adding biochar for 30 days reduces the bioavailable portion of PAHs by 17.4% to 58.0%. Studies on biochar aging show that it reduces its affinity for organic pollutants.127
1.5.9. Humic Substances
The metabolic activities that decompose and convert plant and microbial leftovers generate humus (HS), a complex heterogeneous combination of polydisperse compounds. It is Earth’s main organic carbon reservoir. HS can stabilize soil structure, control the carbon and nitrogen cycle, stimulate plant and microorganism growth, limit heavy metal mobility and toxicity, and preserve plant growth and terrestrial life. Humic compounds are classified by solubility as fulvic acid (FA), humic acid (HA), and humin. Additives improve composting performance, microbial activity, speed, and humification. Microbial agents, regulators, metal oxides, humus precursors, and bulking agents are common compost additives.128 Järup129 discovered that biochar increased compost quality, specifically FA generation. The lignocellulosic part of the carton can boost water retention and HS formation when employed as a filler.130 Sener and Sehirli131 found that iron oxide promoted FA to HA conversion, which increased with iron oxide concentration. Protein precursors (amino acids) can boost HS and HA synthesis in lignocellulosic biomass composts.131Table 4 shows the comprehensive literature on different compost additives used and their effects on various compost characteristics, nutrient retention, global warming reduction potential, better compost quality, and finally the biological diversity. The compost additives made the compost more stable and mature to use as soil conditioner, while suppressing the availability of heavy metals into the soil. The soil applied with compost can be more efficient in water holding capacity or high water productivity for crops. Table 4 showed the effect agricultural waste composting amended with various additives (type of additives) prepared under different preparation conditions.
1.6. Environmental Assessment of Additive-Mediated Composting
Ammonia (NH3), CO2, N2O, CO, and other gaseous products of the organic matter’s degradation are released during composting. Most of those are greenhouse gases with damage to the environment. Due to the loss of nutrients in the pile as well as their impact on climate change, the release of those gases should be reduced. Some research results show that the emission of CO2 causes a loss of 11.4–22.5% of the total carbon in chicken manure, and the emission of CH4 causes a loss of 0.004–0.2% of the total carbon; while the emission of N2O causes a loss of 0.05–0.1% of total nitrogen, and the emission of NH3 causes a loss of 0.8–26.5% of total nitrogen.182
1.6.1. Odor Emissions
Composting releases NH3 and sulfur compounds, which smell bad. Due to NH3 emissions, which damage the environment and stink, compost loses agronomic value. Shao and Zhang168 found that adding rice straw (1:5) to the composting pile of municipal wastes improved oxygen transmission and reduced the overall production of foul-smelling gases that contained sulfur. Similar outcomes have been attained with the addition of ash and biochar42,111,132 addition. For this, natural zeolite can be added, with the amount affecting odor reduction. Additives may increase ammonia losses by stimulating microbial activity.61,183 The ammonia emissions, however, were unaffected by organic inputs including sawdust, cornstalks, and wasted mushroom substrates.45,184 Chemical additives might reduce sulfur-containing chemical odors without harming composting by lowering dimethyl sulphide and dimethyl disulfide emissions.184,185 Chemical additives such magnesium hydroxide–phosphoric acid absorbent mixtures, calcium superphosphate,167 and other phosphate and magnesium salts can reduce ammonia losses during composting.
1.6.2. GHG Emissions
In Table 5, it is depicted how much greenhouse gas (GHG) was produced during the composting of various at-the-start feedstocks with various additives. Despite extensive research, there are little findings on GHG emissions from co-composting systems. Additionally, in national GHG records, only CH4 and N2O emissions from composting are considered; CO2 emissions of a biogenic origin are not taken into account.102 The compost pile’s feedstock and aeration rate may affect N2O and CH4 emission. The amount of added bulking agent and composting pile rotation frequency must be regulated to reduce GHG emissions, particularly gaseous N losses.15,171,186 Under aerobic conditions, incomplete nitrification or denitrification can cause N2O losses, or under anaerobic conditions, where a lack of oxygen causes nitrate accumulation, N losses can happen. Different additives affect N2O emissions differently depending on the feedstock and type. Mineral additives like phosphogypsum lowered N2O emissions from composted manure by increasing SO42– concentration or modifying the nitrification process.151 Sawdust significantly reduced N2O emissions during kitchen trash composting,95 whereas woodchips and polyethylene tubes as bulking agents had no effect on municipal waste composting.171 When oxygen levels in a composting pile are too low or there are anaerobic zones present, CH4 emissions typically result.138 CH4 emissions seem to depend more on addition qualities of feedstock properties than N2O emissions. Thus, methanotrophic bacteria to improve CH4 oxidation and lower CH4 emissions and organic fillers to bulk up anaerobic zones to prevent expansion45,171 or capture released gases45,171 can lower CH4 emissions.173 The efficacy with which additives reduce CH4 emissions may depend on their physical characteristics, such as particle size. For instance, after adding some organic bulking agents, like sawdust and wasted mushrooms, compact zones with even higher CH4 emissions may occur,171 and due to their higher surface area, tiny biochar particles may capture more gas. CO2 emissions may depend on the addition, especially how easily organic molecules degrade (Figure 4).
Table 5. GHG Emissions from Different Feedstock Compost Amended with Additives.
| Gaseous
emissions (reported on 100 days) |
||||||
|---|---|---|---|---|---|---|
| Feedstock | Additive | Duration (days) | CO2 (g kg–1) | N2O (g kg–1) | CH4 (g kg–1) | Literature |
| Cattle manure | Crop straw | 99 | 166.67 | 9.01 | 0.078 | (191) |
| Woodchips | 99 | 147.07 | 9.02 | 0.085 | ||
| Sewage sludge | Cotton stalks | 105 | 239.77 | - | - | (21) |
| Poultry manure, olive wastewater | Cotton stalks | 105–140 | 196–200 | - | - | |
| Sewage sludge, olive wastewater | Corn stalks | 112–119 | 260–270 | - | - | |
| City refuse | Sorghum residues | 133 | 179.31 | - | - | |
| Duck manure | Reed straw + Zeolite | 31 | 405.84 | 0.14 | <0.01 | |
| Cattle and poultry manure | Barley waste | 31 | 300 | 0.08 | 1.19 | (38) |
| Animal manure + food waste (75:25) | Plastic tubes (3:1) | 28 | 225.71 | 0.02 | 0.08 | (38) |
| Plastic tubes (6:1) | 28 | 233.21 | 0.02 | 0.13 | ||
| Woodchips (3:1) | 28 | 171.43 | 0.03 | 0.12 | ||
| Woodchips (6:1) | 28 | 178.57 | 0.05 | 0.14 | ||
| Biochar (3:1) | 28 | 147.14 | 0.01 | 0.02 | ||
| Biochar (6:1) | 28 | 162.14 | 0.01 | 0.04 | ||
| Barley straw (3:1) | 28 | 179.64 | 0.01 | 0.08 | ||
| Lupine residues (3:1) | 28 | 118.57 | 0.01 | 0.68 | ||
| Kitchen waste | Sawdust | 28 | - | 0.78 | 0.15 | (95) |
| Cornstalks | 28 | - | 0.4 | - | ||
| Spent mushroom substrate | 28 | - | 1.04 | 0.05 | ||
| Municipal wastes | Woodchips | 24 | - | <0.01 | 0.01 | (171) |
| Polyethylene tubes | 24 | - | <0.01 | <0.01 | ||
| Hen manure + Sawdust | Straw (450–500 °C), 10% fw | 15 | - | - | - | (181) |
| Poultry manure + Wheat straw | Bamboo (450–500 °C), 2–10% dw | 42 | 5.5–72.6 | 12.4–81.6 | 12.5–72.9 | (192) |
| Chicken manure + Wheat straw | Chicken manure (550–600 °C), 2–10% dw | 42 | - | 4.7–15.1 | 20.5–61.5 | (193) |
| Layer manure + Sawdust | Cornstalk, Bamboo woody, Layer manure Coir (450–500 °C), 5% dw | 15 | - | - | 15.5–26.1 | (144) |
| Sewage sludge + Wheat straw | Wheat straw (450–500 °C), 10% fw | 56 | - | 95.1–97.3 | 92.8–95.3 | (140) |
| Poultry litter + Sugar cane straw | Green waste, Poultry litter (500 °C), 2–18% dw | 60 | - | 68.2–74.9 | 77.8–83.3 | (139) |
| Green waste + Municipal solid waste | Holm oak (650 °C), 10% dw | 90 | 52.9 | 14.2 | 95.1 | (114) |
| Cattle manure + Rice-chaff | Wheat straw (450 °C), 3% dw | 65 | - | 54.1 | - | (194) |
| Hen manure + Barley strawEffect of Additives on Heavy Metals | Hardwood + Softwood (4:1) (500–700 °C), 27% dw | 31 | 21.5–22.9 | 16.1–35.3 | 77.9–83.6 | (38) |
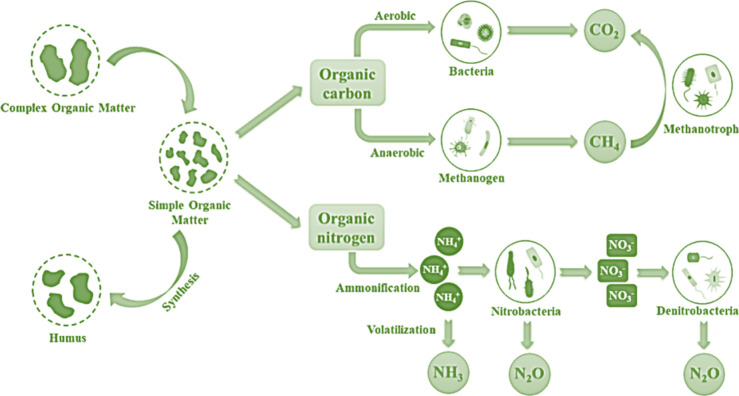
Figure 4.
Schematic showing the microbiological processes involved in the production of greenhouse gases during composting.
Paper, straw, peat, and other easily decomposable organic materials enhance these emissions,106 while lignin-rich organic materials decrease them.38,106 Comparing biochar to conventional compost throughout the composting process shows inconsistent impacts on CO2 emissions, either increasing88,187 or decreasing.38 Vermicomposting also showed such inconsistent results.83,166,188 However, reducing composting CO2 emissions without impacting biodegradation is difficult.166,189
When biochar was added to cow dung compost, Jindo and Sonoki137 discovered that the formation of CH4 was slowed while the oxidation was increased, reducing the overall CH4 emission. In their study of the impact of adding biochar during the composting of manure, Wang and Lu190 discovered that the addition of biochar greatly decreased the overall N2O emissions, particularly during the late composting phase. Adding biochar to compost can considerably lower N2O emissions while decomposing chicken manure. By incorporating 20% more biochar than the control, N2O emissions can be cut by 59.8%. This is mostly because biochar is alkaline, and the high pH value during composting greatly alters the richness of denitrifying bacteria, causing a decrease in N2O-producing bacterial communities and an increase in N2O-consuming bacterial communities. Municipal sludge compost can cut greenhouse gas emissions by 10.39% by adding biochar. Composting has many drawbacks, and one of them is that a significant amount of NH3 is volatilized during the high temperature stage, which results in significant nitrogen loss. According to Steiner and Das42 adding 20% biochar to the composting of nitrogen-rich chicken dung not only sped up the process but also cut the emission of NH3 by 64%. Czekała and Malińska88 reduced NH3 emissions by 30% and 44%, respectively, by adding 5% and 10% biochar to compost.
Chowdhury and de Neergaard38 found that adding 27% dry weight of biochar to composted chicken manure made from 80% hardwood and 20% softwood reduced CH4 emissions by 27 to 32%. The compost contained 12% dry biochar. The compost with biochar had 80% lower CH4 emission than the compost without biochar due to its better oxygen conditions. Similar research was done on sewage sludge and wheat straw compost. Awasthi and Wang173 examined how biochar and zeolites affect composting gas emissions. Wheat straw was pyrolyzed at 500–600 °C to form biochar, and 12% (dry weight) was added to compost.
Biochar made up 3% of the compost mixture. When biochar was added to compost, the overall N2O emission was reduced by 25.9%. The authors provided the following explanation for why there was less emission from compost including biochar than compost without biochar: reduced NO2, which is a precursor to nitrous oxide conversion, biochar, which elevates the composted mixture’s pH, and increased bacteria that reduce N2O and decreased enzymes that promote its formation.179 Other research claims that adding biochar to decomposed material increases gas emissions. Compost made from chicken manure with biochar (27%) emits 6–8% more CO2 than compost made from chicken dung without biochar, according to Chowdhury and de Neergaard.38 The increased aeration of the composted mass brought on by the addition of biochar is likely what resulted in the higher CO2 emission seen in the research mentioned above. High porosity and specific surface area are typical characteristics of biochar, which unquestionably contribute to enhanced aeration of decomposed matter. Additionally, it speeds up the compost material’s decomposition, causing CO2 emissions.
There are reports that show that biochar does not affect gas emissions during composting; however, it may decrease or increase greenhouse gas emissions. The results of two studies, one by Sánchez-García smf Alburquerque135 on the emission of the same gases during composting of poultry manure with barley straw and one by López-Cano and Roig123 found no difference in CO2, CH4, and N2O emission (holm oak wood) biochar.123,135 As opposed to the preceding investigations, the composted materials’ various qualities, coupled with biochar properties and composting conditions, may have contributed to the observed variances. The immobilization of NH3 is mainly caused by two processes: (1) the activity of nitrifying bacteria—biochar fosters the growth of nitrifying bacteria by providing an environment that is conducive to their growth. (2) Biochar absorbed NH3 and NH4+ during composting—biochar has an affinity for NH3, reducing its availability and losses.
1.7. Passivation
When livestock and poultry are fed mineral-rich feed over an extended period of time, the resulting manure has high concentrations of heavy metals like Cu, Zn, and Cd and easily pollutes the environment when applied to farmland. When livestock and poultry are fed mineral-rich feed over an extended period of time, the resulting manure has high concentrations of heavy metals like Cu, Zn, and Cd and easily pollutes the environment when applied to farmland. Duan and Yang195 added biochar, microbiological agents, and chemical adsorbents to the pig manure compost for aerobic composting in order to lower the amount of heavy metals in the compost. They also studied the effects of various passivators on Cu and Zn in the composting system. Biochar was found to be the best passivator after extensive comparison and analysis of the passivation from the perspectives of passivation impact, input cost, biomass, etc. Li and Wang196 observed that when rice husk composting included oak charcoal the total content of Cu and Zn increased, but the proportion of accessible Cu and Zn steadily declined. The passivation rate of Cu reached 65.94% when Zhou and Meng197 added peanut shell charcoal to the compost made from a mixture of manure and straw. With a passivation rate of 57.2%, the addition of maize straw charcoal resulted in the best passivation rate for Pb. The result of passing Cd through sawdust charcoal is 94.67% passivation. Proper biochar addition can strengthen the bioavailability of heavy metals by promoting the composting process and fungal residue while also converting Zn and Cu to the direction of low activity.192 Li and Song194 arrived to an alternative conclusion. In the composting of manure and sludge, they thought biochar had an activating impact on Pb but no discernible passivation effect on Zn, Cu, or Cd. There is an ongoing debate over the effect of biochar on the bioavailability of heavy metals in animal and poultry manure, and more research is required.
Biochar’s usefulness in reducing heavy metal bioavailability and mobility during composting and using composts created with biochar to soil is not supported by all studies. Holm oak wood-derived biochar had no significant effect on the total and water-soluble content of Cu, Zn, Ni, Pb, and Cr, according to López-Cano and Roig.123 Most investigations have shown that composting reduces heavy metal bioavailability. However, biochar efficiency and composting raw materials reduce metal bioavailability. The decrease in bioavailability in the research may be due to functional groups reducing oxygen. Mostly due to microbial oxidation, biochar surface cation exchange capacity improves.198 Increasing CEC and biochar sorption ability may also affect soluble organic matter sorption with various functional groups.39,199 The biochar in the compost then holds onto the DOC, which is another factor adsorbing heavy metals.
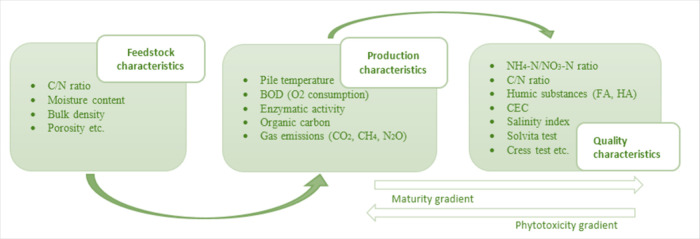
1.8. Research Trends of Compost Maturity Evaluation
To be safe in soil, compost must be stable or mature and have no phytotoxic chemicals or plant or animal illnesses. The following compost groups are based on fertilization preparation: when stable, microbiological processes stop, mature, as phytotoxins are reduced, and both, the compost is “finished”. Sometimes “stable” and “mature” are interchangeable. Microbial mineralization and humification stabilize compost organic matter, which is called compost maturity. Composting product performance depends on age, as is widely known. Standardizing compost maturity procedures is difficult due to the complexity and diversity of composting materials and settings. However, compost maturity is assessed by physical, chemical, biological, and spectroscopic methods. Composting parameters were divided by Azim and Soudi,200 and their relationships are displayed in Figure 5.
Figure 5.
Classification of composting parameters.200
Several factors change during composting, allowing for compost assessment.201 Many criteria have been set for compost maturity; however, most only apply to municipal garbage composts. Maturity factors include physical, chemical, biological, and microbiological activity (Table 6).
Table 6. Compost Quality Is Currently Characterized by Current Parameters Studied in the Literature.
| Physical | Odor, color, temperature, particle size, and inert materials | |
|---|---|---|
| Chemical | C, N analyses | C/N ratio in solid and water extract |
| Cation exchange capacity | CEC, CEC/total organic-C ratio, etc. | |
| Water-soluble extract | pH, EC, organic-C, ions, etc. | |
| Mineral nitrogen | NH4-N content, NH4-N/NO3-N ratio | |
| Pollutants | Heavy metals and organics | |
| Organic matter quality, humification | Lignin, complex carbohydrates, lipids, sugars, etc. make up the organic composition | |
| Elemental and functional group investigations, molecular weight distribution, the E4/E6 ratio, pyrolysis GC-MS, NMR, FTIR, fluorescence, and other methods define humification indices and humic-like substances | ||
| Biological | Microbial activity indicators | O2 uptake/consumption, CO2 production, self-heating test, and biodegradable components |
| Enzyme activity (phosphatases, dehydrogenases, proteases, etc.) | ||
| ATP content | ||
| Nitrogen mineralization–immobilization potential, nitrification, etc. | ||
| Microbial biomass | ||
| Phytotoxicity | Germination and plant growth tests | |
| Others: Viable weed seed, pathogen, and ecotoxicity tests | ||
Nitrification indicates compost ripeness. When NH4–-N drops and NO3–-N appears, the composting material is ready.202 Zucconi203 set a maximum of 0.04% for mature municipal rubbish compost since excessive NH4–-N indicated unsterilized material. Bernai and Paredes204 defined NH4-N/NO3-N ratios below 0.16 as compost maturity indices for all origins (Table 7).
Table 7. Maturity Indices Established for Composts of Different Sources.
| Parameter | Limiting value | Reference |
|---|---|---|
| Water-soluble (C/N) | 5–6 | (137) |
| Germination index | >50% | (111) |
| NH4-N | <0.4 g/kg | (205) |
| C/N | <20 preferably <10 | (137) |
| CO2 release rate | ≤120 mg CO2/kg/h | (114) |
| Water-soluble organic-C | ≤10 g/kg; <17 g/kg | (114) |
| Water-soluble (C/N) | ≤16 | (205) |
| Water-soluble organic-C/total organic-N | ≤0.70; <0.55 | (137) |
| CEX | ≤60 g/kg | (206) |
| CFA | ≤12.5 g/kg | (206) |
| CEX/water-soluble organic-C | ≥6.0 | (206) |
| C/N | <12 | (205) |
| NH4–N/NO3–N | <0.16 | (114) |
| NH4–N | <0.4 g/kg | (137) |
| Mineralizable C in 70 days | <30% | (114) |
| NO3–N/CO2–C ratio (per day) | >8 | (207) |
| Water-soluble organic-C | ≤4.0 g/kg | (205) |
The design and implementation of quality requirements help form a compost material market that supports waste composting.208,209 However, government and nongovernment organizations have set compost quality standards,210−214 which include inert pollution, organic contaminants and heavy metals, sanitization (pathogens and phyto-pathogens), maturity and stability, weed seeds, water, OM, and nutritional content. International harmonization of such standards is needed. Researchers have long recognized the importance of maturity and stability in compost quality. However, the most reliable indices appear to be the only way to determine composted material maturity/stability.215 The CCQC maturity assessment216 considered composts with a C/N ratio >25 immature. At C/N of 25, a group stability and maturity test was chosen (Table 8). According to the TMECC (2002) and CCQC maturity index, the material is highly mature, mature, or immature.
Table 8. Assessment of Maturity Using the CCQC Maturity Index C/N Ratio of 25.
| Stability
thresholds | ||||
|---|---|---|---|---|
| Method | Units | Very stable | Stable | Unstable |
| Specific oxygen uptake rate | mg O2/g OM/d | <3 | 3–10 | >10 |
| CO2 evolution rate | mg CO2-C/g OM/d | <2 | 2–4 | >4 |
| Dewar self-heating test | Dewar index | V | V | <V |
| Headspace CO2 (solvita) | Color code | 7–8 | 5–6 | 1–4 |
| Biologically available C | mg CO2-C/g C/d | <2 | 2–4 | >4 |
| Maturity
thresholds | ||||
|---|---|---|---|---|
| Method | Units | Very mature | Mature | Immature |
| NH4-N | mg/kg dw | <75 | 75–500 | >500 |
| NH4-N/NO3-N | - | <0.5 | 0.5–3.0 | >3 |
| Seedling germination | % of control | >90 | 80–90 | <80 |
| Seedling vigor | % of control | >95 | 85–95 | <85 |
| In-vitro germination index | % of control | >90 | 80–90 | <80 |
| Earthworm bioassay | % weight gain | <20 | 20–40 | >40 |
| NH3 (solvita) | Color code | 5 | 4 | 3–1 |
| VFA | mmol/g dw | <200 | 200–1000 | >1000 |
1.9. Effect of Additives on Compost Maturity and Quality
Jindo and Sonoki137 studied the effects of biochar (10% fresh weight) made from broad-leaved tree (Quercus serrata) wood at 550 °C on organic matter during cattle or chicken dung composting. The C/N ratio and HA/FA compost maturity indicators were identified. Both treatments with and without biochar had lower C/N ratios during composting due to substrate mineralization or nitrogen increase after carbon decomposition.137,217 Compared to the substance being composted without the addition of biochar, the C/N ratio decreased noticeably less with the addition of biochar. A further study by Zhang and Chen113 suggests that biochar-derived carbon and decreased compound mineralization in composts may cause this effect. Since bacteria cannot consume biochar carbon, the C/N ratio must not change considerably with carbon stability.42,218 Additionally, Jindo and Sonoki137 noted that the inclusion of biochar increased the value of HA/FA, which dictated the extent of polymerization of humification process products. This resulted from humus components adhering to the biochar’s surface and speeding up the production of aromatic polymers.137 Adding biochar to compost increases its GI value, which implies it removes phytotoxins faster.105 Biochar may aid compost maturation. Biochar, an NH3– and water-soluble NH4+ absorber, reduces nitrogen loss during manure composting. The humus content of the compost can be increased, and the composting process can be greatly accelerated by the addition of biochar. According to Steiner and Das,42 adding 20% biochar to piles of animal and poultry dung can cut the overall nitrogen loss by 52%.
1.10. Feasibility of Composting Technology
1.10.1. Plant Growth Stimulator
Few studies, including none that were conducted in the field, evaluated the effect of co-composts on plant growth.164,219 The germination index serves as a biological measure for assessing the toxicity and maturity of compost. It is commonly utilized in the process of analyzing the effect that co-composts have on plants.220 A compost is considered phytotoxin-free if the germination index is greater than 50%. Since additives affect nutrient availability, their presence may affect this germination index. However, some studies found that bentonite and alkaline materials inhibit plant growth.97,221 Composting pig dung with bamboo charcoal and bamboo vinegar, as demonstrated by Chen and Huang,222 resulted in an increase of this index of up to 95%. Furthermore, the application of co-compost can alter how much nutrition plants absorb. Zayed and Abdel-Motaal223 demonstrated that the biological additive amended composting improved the plant uptake of phosphorus while reducing bacterial development in the rhizosphere. Similar to this, mineral additions can improve plants’ access to nutrients. Phosphate rock-enriched compost gave seedlings more phosphorus than a standard growing medium,224 while adding waste mica boosted biomass output, absorption, and P and K recoveries.159 Furthermore, metal transfers to plants were restricted by the use of particular amendments that could absorb heavy metals. Finally, the few co-compost field studies conducted revealed potential for improved soil fertility and potential soil revegetation in damaged areas. Kuba and Tschöll164 found that co-compost with 16% wood ash increased plant cover on ski slopes better than mineral and organic fertilizers. Similar findings were made by Chowdhury and Bolan219 who found that using co-composts (biowastes with an alkaline amendment) to replant an urban dump soil increased soil fertility.
1.10.2. Economical and Practical Aspects
The composting method is great for making soil amendments, but its economic sustainability depends on start-up costs, production volume, feedstock quality, and local end-product prices.225 Lim and Lee225 estimate that a composting facility costs 4.37 million USD annually (initial investment, operation, and maintenance) and benefits 1.10 million USD. Composting takes a long time;31 therefore, utilizing chemicals to speed up the process can save money. It is not economical to screen and reuse additives. To generate a high-quality product, additives must be inexpensive and effective. Jaggery and polyethylene glycol, for example, which are pricey,85 but also bentonite or allophane,226 which are affordable and plentiful, might help the composting process. To lower the cost of composting, it is also necessary to optimize the additive to organic waste ratio. For instance, a 1:1 proportion (sawdust/sludge) proved effective and cost-effective compared to a 3:1 proportion. Low sawdust ratios, however, resulted in a lower-quality final product, as evidenced, for instance, by a low germination index.227
Furthermore, compared to high sawdust proportions, a low sawdust proportion requires a longer composting procedure. Finally, there are a number of trade-offs to consider while choosing additives. The additives must be personalized to the region, the location of the composting facility, and the season while considering the additive cost, accessibility throughout both space and time (e.g., agricultural leftovers collected periodically), and abundance. In addition, vermicomposting also makes money since it produces better end products than traditional composting does.228 We believe that adding worms will improve composting by increasing food availability, stabilizing carbon, speeding up composting, and reducing GHG emissions.229 Compared to standard composting, with or without additions, this method may have a higher ROI and lower annual cost. However, a thorough economic study is required to take into consideration the financial benefits and drawbacks of the various composting methods. Vermicomposting, for instance, might make pile turning less expensive, but the amount of space needed to handle an equivalent volume of organic waste may be substantially larger than in conventional composting units, raising expenses. Additionally, vermicomposting may not sanitize the wastes, necessitating additional composting.
1.11. Future Perspectives
As previously mentioned, adding compost additives aided in the breakdown of biomass, improved the quality of the compost, and mitigated several problems that can arise with conventional composting. However, there are still several unknowns. As a result, we highlighted a few points of view that will serve as a roadmap for more research (Figure 6).
Figure 6.
Future applications of biochar as an addition in the composting of organic solid waste are schematically illustrated.
2. Conclusion
Agriculture waste is now a substantial global source of environmental degradation, and the sustained development of agriculture depends on the proper and prompt management of this enormous amount of agricultural waste. However, chemical fertilizer overuse degrades agricultural yield and pollutes. Therefore, the use of organic fertilizer is required in agriculture to produce goods of improved quality without the use of chemicals. Recycling agricultural waste using traditional composting is experiencing protracted composting issues. Because agricultural waste has a high nitrogen concentration, it produces hazardous substances during typical composting, including ammonia (NH3), nitrous oxide (N2O), nitric oxide (NO), methane (CH4), and volatile organic compounds (VOCs).230 This review studied the composting of agricultural waste amended with various mineral, organic, and biological additives to enhance the process variables and product quality. Increased soil fertility produces food and fiber, and soil organic matter stores C and mitigates climate change during composting procedures. These compost additives were amended to (1) attain the thermophilic period quickly and shorten the composting period, (2) improve nutrient content retention while decreasing metal mobility, and (3) restrict emissions of odor and GHG. There have been reports of some co-composts having detrimental impacts on plant growth, though. To truly comprehend the additive impacts, more research is needed because their application during composting results in end products with diverse features that affect soil characteristics in various ways. Thus, to improve soil health and reduce environmental concerns, more research is needed to link co-compost characteristics, soil parameters, and plant growth, according to species, and to make the system more cost-effective, and additive quality and quantity must be considered. Consider creating a composting system that is cost-effective. In the process of composting, additives have an impact on the following factors: (1) early compost maturity, (2) increased compost pH, (3) more nutrient retention (Ca, Mg, N, etc.), (4) higher nitrification ratio, (5) stability of humic-like substances, (6) heavy metal passivation (reduces bioavailability), and (7) GHG emission reduction. Before using compost as an organic fertilizer, it is important to determine its maturity. However, it is difficult to standardize the techniques for determining compost maturity due to the variety of conditions, the complexity of the composting material, and process.
Acknowledgments
The authors extend their appreciation to National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (NRF 2021R1F1A1055482). The authors also extend their appreciation to the Deanship of Scientific Research at King Khalid University for support this work through large group Research Project under grant number RGP2/277/44.
Author Contributions
+ Rana Shahzad Noor and Adnan Noor Shah contributed equally to this work. Rana Shahzad Noor, Adnan Noor Shah, and Muhammad Bilal Tahir: Conceptualization of the study. Muhammad Umair, Muhammad Nawaz, Amjed Ali, and Sezai Ercisli: Design & Development of the review. Rana Shahzad Noor, Nader R. Abdelsalam, Hayssam M. Ali, and Seung Hwan Yang: Formal analysis, Visualization, Writing of original draft. Sami Ullah and Mohammed Ali Assiri: Reviewed, Supervised, and Edited.
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (NRF-2021R1F1A1055482).
The authors declare no competing financial interest.
References
- Hoornweg D.; Bhada-Tata P.; Kennedy C. Environment: Waste production must peak this century. Nature 2013, 502 (7473), 615–617. 10.1038/502615a. [DOI] [PubMed] [Google Scholar]
- Lohri C. R.; et al. Treatment technologies for urban solid biowaste to create value products: a review with focus on low-and middle-income settings. Reviews in Environmental Science and Bio/Technology 2017, 16, 81–130. 10.1007/s11157-017-9422-5. [DOI] [Google Scholar]
- Yu Q.; et al. A review of crop straw pretreatment methods for biogas production by anaerobic digestion in China. Renewable and Sustainable Energy Reviews 2019, 107, 51–58. 10.1016/j.rser.2019.02.020. [DOI] [Google Scholar]
- Abdullah A.; et al. Potential for sustainable utilisation of agricultural residues for bioenergy production in Pakistan: An overview. Journal of Cleaner Production 2021, 287, 125047. 10.1016/j.jclepro.2020.125047. [DOI] [Google Scholar]
- Marshall R. E.; Farahbakhsh K. Systems approaches to integrated solid waste management in developing countries. Waste management 2013, 33 (4), 988–1003. 10.1016/j.wasman.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Schott A. B. S.; Wenzel H.; la Cour Jansen J. Identification of decisive factors for greenhouse gas emissions in comparative life cycle assessments of food waste management–an analytical review. Journal of Cleaner Production 2016, 119, 13–24. 10.1016/j.jclepro.2016.01.079. [DOI] [Google Scholar]
- Savci S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. 10.1016/j.apcbee.2012.03.047. [DOI] [Google Scholar]
- Sharma N.; Singhvi R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. International Journal of Agriculture. Environment and Biotechnology 2017, 10, 675–680. 10.5958/2230-732X.2017.00083.3. [DOI] [Google Scholar]
- Bhatt M.; Labanya R.; Joshi H.C. Influence of Long-term Chemical fertilizers and Organic Manures on Soil Fertility - A Review. Universal Journal of Agricultural Research 2019, 7, 177. 10.13189/ujar.2019.070502. [DOI] [Google Scholar]
- Noor R. S.; et al. Effect of compost and chemical fertilizer application on soil physical properties and productivity of sesame (Sesamum Indicum L.). Biomass Conversion and Biorefinery 2023, 13, 1–11. 10.1007/s13399-020-01066-5. [DOI] [Google Scholar]
- Obi F. O.; Ugwuishiwu B. O.; Nwakaire J. N. Agricultural waste concept, generation, utilization and management. Nigerian Journal of Technology 2016, 35 (4), 957–964. 10.4314/njt.v35i4.34. [DOI] [Google Scholar]
- Lashermes G.; et al. Indicator of potential residual carbon in soils after exogenous organic matter application. European Journal of Soil Science 2009, 60 (2), 297–310. 10.1111/j.1365-2389.2008.01110.x. [DOI] [Google Scholar]
- Peltre C.; et al. RothC simulation of carbon accumulation in soil after repeated application of widely different organic amendments. Soil Biology and Biochemistry 2012, 52, 49–60. 10.1016/j.soilbio.2012.03.023. [DOI] [Google Scholar]
- Ceglie F. G.; et al. The challenge of peat substitution in organic seedling production: optimization of growing media formulation through mixture design and response surface analysis. PLoS One 2015, 10 (6), e0128600 10.1371/journal.pone.0128600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A. B.; et al. Agri-food sludge management using different co-composting strategies: study of the added value of the composts obtained. Journal of Cleaner Production 2016, 121, 186–197. 10.1016/j.jclepro.2016.02.012. [DOI] [Google Scholar]
- Wang M.; et al. Comparison of additives amendment for mitigation of greenhouse gases and ammonia emission during sewage sludge co-composting based on correlation analysis. Bioresource technology 2017, 243, 520–527. 10.1016/j.biortech.2017.06.158. [DOI] [PubMed] [Google Scholar]
- Malińska K.; et al. Biochar amendment for integrated composting and vermicomposting of sewage sludge–the effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour. Technol. 2017, 225, 206–214. 10.1016/j.biortech.2016.11.049. [DOI] [PubMed] [Google Scholar]
- Diacono M.; Montemurro F. Long-term effects of organic amendments on soil fertility. Sustainable agriculture 2011, 2, 761–786. 10.1007/978-94-007-0394-0_34. [DOI] [Google Scholar]
- Fukushima M.; et al. Effects of the maturity of wood waste compost on the structural features of humic acids. Bioresource technology 2009, 100 (2), 791–797. 10.1016/j.biortech.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Wichuk K. M. W. M.; McCartney D. A review of the effectiveness of current time–temperature regulations on pathogen inactivation during composting. Journal of Environmental Engineering and Science 2007, 6 (5), 573–586. 10.1139/S07-011. [DOI] [Google Scholar]
- Bernal M.; Alburquerque J.; Moral R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresource technology 2009, 100 (22), 5444–5453. 10.1016/j.biortech.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Insam H. M.Chapter 3: Microbiology of the composting process. In Compost Science and Technology; Diaz L. F., et al. Eds.; Elsevier, 2007; pp 25–48. [Google Scholar]
- Rastogi M.; Nandal M.; Khosla B. Microbes as vital additives for solid waste composting. Heliyon 2020, 6 (2), e03343 10.1016/j.heliyon.2020.e03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta C. M.; et al. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Management 2014, 34 (3), 607–622. 10.1016/j.wasman.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Klamer M.; Bååth E. Microbial community dynamics during composting of straw material studied using phospholipid fatty acid analysis. FEMS Microbiology Ecology 1998, 27 (1), 9–20. 10.1111/j.1574-6941.1998.tb00521.x. [DOI] [Google Scholar]
- Xi B.; et al. Effect of multi-stage inoculation on the bacterial and fungal community structure during organic municipal solid wastes composting. Bioresour. Technol. 2015, 196, 399–405. 10.1016/j.biortech.2015.07.069. [DOI] [PubMed] [Google Scholar]
- Li M. X.; et al. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 2021, 264, 128549. 10.1016/j.chemosphere.2020.128549. [DOI] [PubMed] [Google Scholar]
- Wan L.; et al. Effect of inoculating microorganisms in chicken manure composting with maize straw. Bioresour. Technol. 2020, 301, 122730. 10.1016/j.biortech.2019.122730. [DOI] [PubMed] [Google Scholar]
- Sánchez O. J.; Ospina D. A.; Montoya S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag 2017, 69, 136–153. 10.1016/j.wasman.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Schäpper D.; et al. Application of microbioreactors in fermentation process development: a review. Anal Bioanal Chem. 2009, 395 (3), 679–95. 10.1007/s00216-009-2955-x. [DOI] [PubMed] [Google Scholar]
- Onwosi C. O.; et al. Composting technology in waste stabilization: On the methods, challenges and future prospects. Journal of Environmental Management 2017, 190, 140–157. 10.1016/j.jenvman.2016.12.051. [DOI] [PubMed] [Google Scholar]
- Viaene J.; et al. Co-ensiling, co-composting and anaerobic co-digestion of vegetable crop residues: Product stability and effect on soil carbon and nitrogen dynamics. Scientia Horticulturae 2017, 220, 214–225. 10.1016/j.scienta.2017.03.015. [DOI] [Google Scholar]
- De Bertoldi M., et al. Compost Science and Technology; Elsevier, 2007. [Google Scholar]
- Vandecasteele B.; et al. Feedstock mixture composition as key factor for C/P ratio and phosphorus availability in composts: role of biodegradation potential, biochar amendment and calcium content. Waste and biomass valorization 2017, 8, 2553–2567. 10.1007/s12649-016-9762-3. [DOI] [Google Scholar]
- Zhang L.; Sun X. Addition of fish pond sediment and rock phosphate enhances the composting of green waste. Bioresour. Technol. 2017, 233, 116–126. 10.1016/j.biortech.2017.02.073. [DOI] [PubMed] [Google Scholar]
- Awasthi M. K.; et al. Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. Journal of Cleaner Production 2016, 135, 829–835. 10.1016/j.jclepro.2016.07.008. [DOI] [Google Scholar]
- Shen Y.; et al. Influence of aeration on CH4, N2O and NH3 emissions during aerobic composting of a chicken manure and high C/N waste mixture. Waste Management 2011, 31 (1), 33–38. 10.1016/j.wasman.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A.; de Neergaard A.; Jensen L. S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. 10.1016/j.chemosphere.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Zeng G.; et al. Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour. Technol. 2010, 101 (1), 222–7. 10.1016/j.biortech.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Jurado M.; et al. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. 10.1016/j.biortech.2015.03.059. [DOI] [PubMed] [Google Scholar]
- Li T.; Dong H.; Tao X. Research progress on disinfection technology of wastewater from animal production. Journal of Agricultural Science and Technology (Beijing) 2013, 15 (2), 137–143. 10.3969/j.issn.1008-0864.2013.02.22. [DOI] [Google Scholar]
- Steiner C.; et al. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual 2010, 39 (4), 1236–42. 10.2134/jeq2009.0337. [DOI] [PubMed] [Google Scholar]
- Zhang C.; et al. Influence of inoculating white-rot fungi on organic matter transformations and mobility of heavy metals in sewage sludge based composting. Journal of hazardous materials 2018, 344, 163–168. 10.1016/j.jhazmat.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Santos A.; et al. Gaseous emissions and process development during composting of pig slurry: the influence of the proportion of cotton gin waste. Journal of Cleaner Production 2016, 112, 81–90. 10.1016/j.jclepro.2015.08.084. [DOI] [Google Scholar]
- Yang F.; et al. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013, 93 (7), 1393–1399. 10.1016/j.chemosphere.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang J.; et al. Phanerochaete chrysosporium inoculation shapes the indigenous fungal communities during agricultural waste composting. Biodegradation 2014, 25, 669–680. 10.1007/s10532-014-9690-5. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Liang Z.; Zhang Y. Evolution of physicochemical properties and bacterial community in aerobic composting of swine manure based on a patent compost tray. Bioresour. Technol. 2022, 343, 126136. 10.1016/j.biortech.2021.126136. [DOI] [PubMed] [Google Scholar]
- Dietrich M.; Fongen M.; Foereid B. Anaerobic digestion affecting nitrous oxide and methane emissions from the composting process. Bioresource Technology Reports 2021, 15, 100752. 10.1016/j.biteb.2021.100752. [DOI] [Google Scholar]
- Zhong X.-Z.; et al. Insight into the microbiology of nitrogen cycle in the dairy manure composting process revealed by combining high-throughput sequencing and quantitative PCR. Bioresource technology 2020, 301, 122760. 10.1016/j.biortech.2020.122760. [DOI] [PubMed] [Google Scholar]
- Tucker C.; Ferrenberg S.; Reed S. C. Modest residual effects of short-term warming, altered hydration, and biocrust successional state on dryland soil heterotrophic carbon and nitrogen cycling. Frontiers in Ecology and Evolution 2020, 8, 467157. 10.3389/fevo.2020.467157. [DOI] [Google Scholar]
- Wang M.; et al. Comparison of composting factors, heavy metal immobilization, and microbial activity after biochar or lime application in straw-manure composting. Bioresour. Technol. 2022, 363, 127872. 10.1016/j.biortech.2022.127872. [DOI] [PubMed] [Google Scholar]
- Zhou L.; et al. Effect of biochar addition on copper and zinc passivation pathways mediated by humification and microbial community evolution during pig manure composting. Bioresour. Technol. 2023, 370, 128575. 10.1016/j.biortech.2023.128575. [DOI] [PubMed] [Google Scholar]
- Zhu P.; et al. Reducing odor emissions from feces aerobic composting: additives. RSC Adv. 2021, 11 (26), 15977–15988. 10.1039/D1RA00355K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.; et al. Evaluation of the synergistic effects of biochar and biogas residue on CO2 and CH4 emission, functional genes, and enzyme activity during straw composting. Bioresour. Technol. 2022, 360, 127608. 10.1016/j.biortech.2022.127608. [DOI] [PubMed] [Google Scholar]
- Doublet J.; et al. Influence of bulking agents on organic matter evolution during sewage sludge composting; consequences on compost organic matter stability and N availability. Bioresour. Technol. 2011, 102 (2), 1298–1307. 10.1016/j.biortech.2010.08.065. [DOI] [PubMed] [Google Scholar]
- Wei Y.; et al. Anaerobic co-digestion of cattle manure and liquid fraction of digestate (LFD) pretreated corn stover: Pretreatment process optimization and evolution of microbial community structure. Bioresource technology 2020, 296, 122282. 10.1016/j.biortech.2019.122282. [DOI] [PubMed] [Google Scholar]
- Li D.; et al. Effects of carbon/nitrogen ratio and aeration rate on the sheep manure composting process and associated gaseous emissions. Journal of Environmental Management 2022, 323, 116093. 10.1016/j.jenvman.2022.116093. [DOI] [PubMed] [Google Scholar]
- Mei J.; et al. Effects of potassium persulfate on nitrogen loss and microbial community during cow manure and corn straw composting. Bioresour. Technol. 2022, 363, 127919. 10.1016/j.biortech.2022.127919. [DOI] [PubMed] [Google Scholar]
- Zhuang M.; et al. Different characteristics of greenhouse gases and ammonia emissions from conventional stored dairy cattle and swine manure in China. Sci. Total Environ. 2020, 722, 137693. 10.1016/j.scitotenv.2020.137693. [DOI] [PubMed] [Google Scholar]
- Dias B. O.; et al. Use of biochar as bulking agent for the composting of poultry manure: Effect on organic matter degradation and humification. Bioresour. Technol. 2010, 101 (4), 1239–1246. 10.1016/j.biortech.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Waqas M.; et al. Optimization of food waste compost with the use of biochar. Journal of environmental management 2018, 216, 70–81. 10.1016/j.jenvman.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Lehmann J. A handful of carbon. Nature 2007, 447 (7141), 143–144. 10.1038/447143a. [DOI] [PubMed] [Google Scholar]
- Lehmann J.; Gaunt J.; Rondon M. Bio-char Sequestration in Terrestrial Ecosystems – A Review. Mitigation and Adaptation Strategies for Global Change 2006, 11 (2), 403–427. 10.1007/s11027-005-9006-5. [DOI] [Google Scholar]
- Yin Y.; et al. Research progress and prospects for using biochar to mitigate greenhouse gas emissions during composting: a review. Science of The Total Environment 2021, 798, 149294. 10.1016/j.scitotenv.2021.149294. [DOI] [PubMed] [Google Scholar]
- Gao Y.; et al. Insights into biodegradation behaviors of methanolic wastewater in up-flow anaerobic sludge bed (UASB) reactor coupled with in-situ bioelectrocatalysis. Bioresour. Technol. 2023, 376, 128835. 10.1016/j.biortech.2023.128835. [DOI] [PubMed] [Google Scholar]
- Gao X.; et al. Emission of volatile sulphur compounds during swine manure composting: Source identification, odour mitigation and assessment. Waste Management 2022, 153, 129–137. 10.1016/j.wasman.2022.08.029. [DOI] [PubMed] [Google Scholar]
- Kupper T.; et al. Ammonia and greenhouse gas emissions from slurry storage-A review. Agriculture, ecosystems & environment 2020, 300, 106963. 10.1016/j.agee.2020.106963. [DOI] [Google Scholar]
- Aboltins A.; Melece L.; Priekulis J. Model for ammonia emissions’ assessment and comparison of various dairy cattle farming systems and technologies. Agronomy Research 2019, 17, 322–332. 10.3969/j.issn.1008-0864.2013.02.22. [DOI] [Google Scholar]
- Wang Y.; et al. Mitigating ammonia emissions from typical broiler and layer manure management–A system analysis. Waste Management 2019, 93, 23–33. 10.1016/j.wasman.2019.05.019. [DOI] [PubMed] [Google Scholar]
- Wang S.; Ang H. M.; Tadé M. O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72 (11), 1621–1635. 10.1016/j.chemosphere.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Gomes H. I.; Mayes W. M.; Rogerson M.; Stewart D. I.; Burke I. T. Alkaline residues and the environment: a review of impacts, management practices and opportunities. Journal of Cleaner Production 2016, 112, 3571–3582. 10.1016/j.jclepro.2015.09.111. [DOI] [Google Scholar]
- Xie S.; Tran H.-T.; Pu M.; Zhang T. Transformation characteristics of organic matter and phosphorus in composting processes of agricultural organic waste: research trends. Materials Science for Energy Technologies 2023, 6, 331. 10.1016/j.mset.2023.02.006. [DOI] [Google Scholar]
- Li T.; et al. Ammonia volatilization mitigation in crop farming: A review of fertilizer amendment technologies and mechanisms. Chemosphere 2022, 303, 134944. 10.1016/j.chemosphere.2022.134944. [DOI] [PubMed] [Google Scholar]
- Noor R. S.; et al. Sustainable production and characterization of integrated composting systems of organic biomass and inorganic amendments. Biomass Conversion and Biorefinery 2023, 1–17. 10.1007/s13399-023-03883-w. [DOI] [Google Scholar]
- Noor R. S.; et al. Quantifying the effects of co-composting organic biomass mixtures with inorganic amendments to obtain value-added bio-products. PLoS One 2021, 16 (7), e0253714 10.1371/journal.pone.0253714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ganesh K. S.; Sridhar A.; Vishali S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities-A review. Chemosphere 2022, 287, 132221. 10.1016/j.chemosphere.2021.132221. [DOI] [PubMed] [Google Scholar]
- Ba S.; et al. Meta-analysis of greenhouse gas and ammonia emissions from dairy manure composting. biosystems engineering 2020, 193, 126–137. 10.1016/j.biosystemseng.2020.02.015. [DOI] [Google Scholar]
- Zhou S.; et al. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag 2019, 88, 85–95. 10.1016/j.wasman.2019.03.025. [DOI] [PubMed] [Google Scholar]
- Mao H.; et al. Effects of four additives in pig manure composting on greenhouse gas emission reduction and bacterial community change. Bioresource technology 2019, 292, 121896. 10.1016/j.biortech.2019.121896. [DOI] [PubMed] [Google Scholar]
- Wang Z.; et al. Effects of biochar carried microbial agent on compost quality, greenhouse gas emission and bacterial community during sheep manure composting. Biochar 2023, 5 (1), 3. 10.1007/s42773-022-00202-w. [DOI] [Google Scholar]
- Wakase S.; et al. Investigation of the microbial community in a microbiological additive used in a manure composting process. Bioresour. Technol. 2008, 99 (7), 2687–2693. 10.1016/j.biortech.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Jurado M. M.; et al. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. 10.1016/j.biortech.2015.03.059. [DOI] [PubMed] [Google Scholar]
- Czekala W.; et al. Co-composting of poultry manure mixtures amended with biochar - The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–7. 10.1016/j.biortech.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Gea T.; et al. Optimal bulking agent particle size and usage for heat retention and disinfection in domestic wastewater sludge composting. Waste Management 2007, 27 (9), 1108–1116. 10.1016/j.wasman.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Gabhane J.; et al. Additives aided composting of green waste: effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour. Technol. 2012, 114, 382–8. 10.1016/j.biortech.2012.02.040. [DOI] [PubMed] [Google Scholar]
- Jiang J.; et al. Inoculation with nitrogen turnover bacterial agent appropriately increasing nitrogen and promoting maturity in pig manure composting. Waste Management 2015, 39, 78–85. 10.1016/j.wasman.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Himanen M.; Hänninen K. Effect of commercial mineral-based additives on composting and compost quality. Waste Management 2009, 29 (8), 2265–2273. 10.1016/j.wasman.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Czekała W.; et al. Co-composting of poultry manure mixtures amended with biochar – The effect of biochar on temperature and C-CO2 emission. Bioresour. Technol. 2016, 200, 921–927. 10.1016/j.biortech.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Waqas M.; et al. Optimization of food waste compost with the use of biochar. Journal of Environmental Management 2018, 216, 70–81. 10.1016/j.jenvman.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Manu M. K.; Kumar R.; Garg A. Performance assessment of improved composting system for food waste with varying aeration and use of microbial inoculum. Bioresour. Technol. 2017, 234, 167–177. 10.1016/j.biortech.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Nakasaki K.; Hirai H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag 2017, 65, 29–36. 10.1016/j.wasman.2017.04.019. [DOI] [PubMed] [Google Scholar]
- Iqbal H.; Garcia-Perez M.; Flury M. Effect of biochar on leaching of organic carbon, nitrogen, and phosphorus from compost in bioretention systems. Science of The Total Environment 2015, 521–522, 37–45. 10.1016/j.scitotenv.2015.03.060. [DOI] [PubMed] [Google Scholar]
- Richard T. L.; et al. Moisture Relationships in Composting Processes. Compost Science & Utilization 2002, 10 (4), 286–302. 10.1080/1065657X.2002.10702093. [DOI] [Google Scholar]
- Miner F. D.; Koenig R. T.; Miller B. E. The Influence of Bulking Material Type and Volume On In-house Composting in High-Rise, Caged Layer Facilities. Compost Science & Utilization 2001, 9 (1), 50–59. 10.1080/1065657X.2001.10702016. [DOI] [Google Scholar]
- Yang F.; et al. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 2013, 93 (7), 1393–9. 10.1016/j.chemosphere.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Chang J. I.; Chen Y. J. Effects of bulking agents on food waste composting. Bioresour. Technol. 2010, 101 (15), 5917–5924. 10.1016/j.biortech.2010.02.042. [DOI] [PubMed] [Google Scholar]
- Li R.; et al. Nutrient transformations during composting of pig manure with bentonite. Bioresour. Technol. 2012, 121, 362–368. 10.1016/j.biortech.2012.06.065. [DOI] [PubMed] [Google Scholar]
- Belyaeva O. N.; Haynes R. J. Chemical, microbial and physical properties of manufactured soils produced by co-composting municipal green waste with coal fly ash. Bioresour. Technol. 2009, 100 (21), 5203–5209. 10.1016/j.biortech.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Soares M. A. R.; et al. Assessment of co-composting process with high load of an inorganic industrial waste. Waste Management 2017, 59, 80–89. 10.1016/j.wasman.2016.09.044. [DOI] [PubMed] [Google Scholar]
- Wong J. W.; Fung S. O.; Selvam A. Coal fly ash and lime addition enhances the rate and efficiency of decomposition of food waste during composting. Bioresour. Technol. 2009, 100 (13), 3324–31. 10.1016/j.biortech.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.; et al. Co-composting solid biowastes with alkaline materials to enhance carbon stabilization and revegetation potential. Environmental Science and Pollution Research 2016, 23 (8), 7099–7110. 10.1007/s11356-015-5411-9. [DOI] [PubMed] [Google Scholar]
- Iqbal M. K.; Shafiq T.; Ahmed K. Characterization of bulking agents and its effects on physical properties of compost. Bioresour. Technol. 2010, 101 (6), 1913–9. 10.1016/j.biortech.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Wang Q.; et al. Improving pig manure composting efficiency employing Ca-bentonite. Ecological Engineering 2016, 87, 157–161. 10.1016/j.ecoleng.2015.11.032. [DOI] [Google Scholar]
- Wong J. W. C.; et al. Feasibility of Using Coal Ash Residues as CO-Composting Materials for Sewage Sludge. Environmental Technology 1997, 18 (5), 563–568. 10.1080/09593331808616574. [DOI] [Google Scholar]
- Chen Y. X.; et al. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 2010, 78 (9), 1177–81. 10.1016/j.chemosphere.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Mahimairaja S.; et al. Losses and transformation of nitrogen during composting of poultry manure with different amendments: An incubation experiment. Bioresour. Technol. 1994, 47 (3), 265–273. 10.1016/0960-8524(94)90190-2. [DOI] [Google Scholar]
- Gao M.; et al. The effect of aeration rate on forced-aeration composting of chicken manure and sawdust. Bioresour. Technol. 2010, 101 (6), 1899–1903. 10.1016/j.biortech.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Ogunwande G. A.; Osunade J. A. Passive aeration composting of chicken litter: Effects of aeration pipe orientation and perforation size on losses of compost elements. Journal of Environmental Management 2011, 92 (1), 85–91. 10.1016/j.jenvman.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Chen Z.; et al. Effect of aeration rate on composting of penicillin mycelial dreg. Journal of Environmental Sciences 2015, 37, 172–178. 10.1016/j.jes.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Manu M. K.; Kumar R.; Garg A. Performance assessment of improved composting system for food waste with varying aeration and use of microbial inoculum. Bioresour. Technol. 2017, 234, 167–177. 10.1016/j.biortech.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Khan N.; et al. Maturity indices in co-composting of chicken manure and sawdust with biochar. Bioresour. Technol. 2014, 168, 245–251. 10.1016/j.biortech.2014.02.123. [DOI] [PubMed] [Google Scholar]
- Li R.; et al. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 2015, 36 (5–8), 815–26. 10.1080/09593330.2014.963692. [DOI] [PubMed] [Google Scholar]
- Zhang J.; et al. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–83. 10.1016/j.biortech.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Vandecasteele B.; et al. Biochar amendment before or after composting affects compost quality and N losses, but not P plant uptake. J. Environ. Manage 2016, 168, 200–9. 10.1016/j.jenvman.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Sun X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–84. 10.1016/j.biortech.2014.08.079. [DOI] [PubMed] [Google Scholar]
- Wang C.; et al. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard Mater. 2014, 280, 409–16. 10.1016/j.jhazmat.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Fuchs J. G.Interactions Between Beneficial and Harmful Microorganisms: From the Composting Process to Compost Application. In Microbes at Work: From Wastes to Resources; Insam H., Franke-Whittle I., Goberna M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; pp 213–229. [Google Scholar]
- Zhang D.; et al. Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting. Waste Manag 2017, 68, 56–63. 10.1016/j.wasman.2017.05.042. [DOI] [PubMed] [Google Scholar]
- Meng J.; et al. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour. Technol. 2013, 142, 641–646. 10.1016/j.biortech.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Wei L.; et al. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 2014, 154, 148–154. 10.1016/j.biortech.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Liu N.; et al. Role and multi-scale characterization of bamboo biochar during poultry manure aerobic composting. Bioresour. Technol. 2017, 241, 190–199. 10.1016/j.biortech.2017.03.144. [DOI] [PubMed] [Google Scholar]
- Larney F. J.; et al. Nutrient and trace element changes during manure composting at four southern Alberta feedlots. Canadian Journal of Soil Science 2008, 88 (1), 45–59. 10.4141/CJSS07044. [DOI] [Google Scholar]
- López-Cano I.; et al. Biochar improves N cycling during composting of olive mill wastes and sheep manure. Waste Manag 2016, 49, 553–559. 10.1016/j.wasman.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Semple K. T.; et al. Impact of black carbon on the bioaccessibility of organic contaminants in soil. Journal of hazardous materials 2013, 261, 808–16. 10.1016/j.jhazmat.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Lashermes G.; Barriuso E.; Houot S. Dissipation pathways of organic pollutants during the composting of organic wastes. Chemosphere 2012, 87 (2), 137–43. 10.1016/j.chemosphere.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Stefaniuk M.; Oleszczuk P. Addition of biochar to sewage sludge decreases freely dissolved PAHs content and toxicity of sewage sludge-amended soil. Environ. Pollut. 2016, 218, 242–251. 10.1016/j.envpol.2016.06.063. [DOI] [PubMed] [Google Scholar]
- Hale S. E.; et al. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ. Sci. Technol. 2011, 45 (24), 10445–53. 10.1021/es202970x. [DOI] [PubMed] [Google Scholar]
- Verkleij J. A. C.; et al. Dualities in plant tolerance to pollutants and their uptake and translocation to the upper plant parts. Environmental and Experimental Botany 2009, 67 (1), 10–22. 10.1016/j.envexpbot.2009.05.009. [DOI] [Google Scholar]
- Järup L. Hazards of heavy metal contamination. Br Med. Bull. 2003, 68, 167–82. 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Johri N.; Jacquillet G.; Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 2010, 23 (5), 783–92. 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- Sener G.; Sehirli A. O.; Ayanoglu-Dülger G. Melatonin protects against mercury(II)-induced oxidative tissue damage in rats. Pharmacol Toxicol 2003, 93 (6), 290. 10.1111/j.1600-0773.2003.pto930607.x. [DOI] [PubMed] [Google Scholar]
- Hua L.; et al. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ. Sci. Pollut Res. Int. 2009, 16 (1), 1–9. 10.1007/s11356-008-0041-0. [DOI] [PubMed] [Google Scholar]
- Jindo K.; et al. Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012, 110, 396–404. 10.1016/j.biortech.2012.01.120. [DOI] [PubMed] [Google Scholar]
- Luo X.; et al. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. Journal of Soils and Sediments 2017, 17 (3), 780–789. 10.1007/s11368-016-1361-1. [DOI] [Google Scholar]
- Sánchez-García M.; et al. Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. 10.1016/j.biortech.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Sun D.; et al. Biochar as a novel niche for culturing microbial communities in composting. Waste Manag 2016, 54, 93–100. 10.1016/j.wasman.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Jindo K.; et al. Influence of biochar addition on the humic substances of composting manures. Waste Manag 2016, 49, 545–552. 10.1016/j.wasman.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Agyarko-Mintah E.; et al. Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manag 2017, 61, 138–149. 10.1016/j.wasman.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Agyarko-Mintah E.; et al. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag 2017, 61, 129–137. 10.1016/j.wasman.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Awasthi M. K.; et al. Heterogeneity of biochar amendment to improve the carbon and nitrogen sequestration through reduce the greenhouse gases emissions during sewage sludge composting. Bioresour. Technol. 2017, 224, 428–438. 10.1016/j.biortech.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Liu W.; et al. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235, 43–49. 10.1016/j.biortech.2017.03.052. [DOI] [PubMed] [Google Scholar]
- Chen Y.; et al. Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour. Technol. 2017, 243, 347–355. 10.1016/j.biortech.2017.06.100. [DOI] [PubMed] [Google Scholar]
- Malińska K.; et al. Biochar amendment for integrated composting and vermicomposting of sewage sludge - The effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour. Technol. 2017, 225, 206–214. 10.1016/j.biortech.2016.11.049. [DOI] [PubMed] [Google Scholar]
- Chen W.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag 2017, 61, 506–515. 10.1016/j.wasman.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Khan N.; et al. Physical and chemical properties of biochars co-composted with biowastes and incubated with a chicken litter compost. Chemosphere 2016, 142, 14–23. 10.1016/j.chemosphere.2015.05.065. [DOI] [PubMed] [Google Scholar]
- Jain M. S.; Jambhulkar R.; Kalamdhad A. S. Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour. Technol. 2018, 253, 204–213. 10.1016/j.biortech.2018.01.038. [DOI] [PubMed] [Google Scholar]
- Awasthi M. K.; et al. Beneficial effect of mixture of additives amendment on enzymatic activities, organic matter degradation and humification during biosolids co-composting. Bioresour. Technol. 2018, 247, 138–146. 10.1016/j.biortech.2017.09.061. [DOI] [PubMed] [Google Scholar]
- Zhang J.; et al. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. 10.1016/j.biortech.2014.02.080. [DOI] [PubMed] [Google Scholar]
- Guerra-Rodríguez E.; et al. Evaluation of heavy metal contents in co-composts of poultry manure with barley wastes or chestnut burr/leaf litter. Chemosphere 2006, 65 (10), 1801–1805. 10.1016/j.chemosphere.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Hao X.; et al. The effect of phosphogypsum on greenhouse gas emissions during cattle manure composting. J. Environ. Qual 2005, 34 (3), 774–81. 10.2134/jeq2004.0388. [DOI] [PubMed] [Google Scholar]
- Luo Y.; et al. Effect of phosphogypsum and Dicyandiamide as additives on NH3, N20 and CH4 emissions during composting. J. Environ. Sci. (China) 2013, 25 (7), 1338–45. 10.1016/S1001-0742(12)60126-0. [DOI] [PubMed] [Google Scholar]
- Kato K.; Miura N. Effect of matured compost as a bulking and inoculating agent on the microbial community and maturity of cattle manure compost. Bioresour. Technol. 2008, 99 (9), 3372–80. 10.1016/j.biortech.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Koivula N.; et al. Ash in composting of source-separated catering waste. Bioresour. Technol. 2004, 93 (3), 291–9. 10.1016/j.biortech.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Kulcu R.; Yaldiz O. Composting of goat manure and wheat straw using pine cones as a bulking agent. Bioresour. Technol. 2007, 98 (14), 2700–4. 10.1016/j.biortech.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Lefcourt A. M.; Meisinger J. J. Effect of adding alum or zeolite to dairy slurry on ammonia volatilization and chemical composition. J. Dairy Sci. 2001, 84 (8), 1814–21. 10.3168/jds.S0022-0302(01)74620-6. [DOI] [PubMed] [Google Scholar]
- Venglovsky J.; et al. Evolution of temperature and chemical parameters during composting of the pig slurry solid fraction amended with natural zeolite. Bioresour. Technol. 2005, 96 (2), 181–189. 10.1016/j.biortech.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lu D.; et al. Speciation of Cu and Zn during composting of pig manure amended with rock phosphate. Waste Manag 2014, 34 (8), 1529–36. 10.1016/j.wasman.2014.04.008. [DOI] [PubMed] [Google Scholar]
- LÜ D.-a.; et al. Changes in Phosphorus Fractions and Nitrogen Forms During Composting of Pig Manure with Rice Straw. Journal of Integrative Agriculture 2013, 12 (10), 1855–1864. 10.1016/S2095-3119(13)60400-1. [DOI] [Google Scholar]
- Nishanth D.; Biswas D. R. Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat (Triticum aestivum). Bioresour. Technol. 2008, 99 (9), 3342–3353. 10.1016/j.biortech.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Billah M.; Bano A. Role of plant growth promoting rhizobacteria in modulating the efficiency of poultry litter composting with rock phosphate and its effect on growth and yield of wheat. Waste Manag Res. 2015, 33 (1), 63–72. 10.1177/0734242X14559593. [DOI] [PubMed] [Google Scholar]
- Oliveira S. M.; Ferreira A. S. Change in Soil Microbial and Enzyme Activities in Response to the Addition of Rock-Phosphate-Enriched Compost. Commun. Soil Sci. Plant Anal. 2014, 45 (21), 2794–2806. 10.1080/00103624.2014.954286. [DOI] [Google Scholar]
- Jeong Y.-K.; Hwang S.-J. Optimum doses of Mg and P salts for precipitating ammonia into struvite crystals in aerobic composting. Bioresour. Technol. 2005, 96 (1), 1–6. 10.1016/j.biortech.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Ren L.; et al. Impact of struvite crystallization on nitrogen losses during composting of pig manure and cornstalk. Waste Management 2010, 30 (5), 885–892. 10.1016/j.wasman.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Kuba T.; et al. Wood ash admixture to organic wastes improves compost and its performance. Agriculture, Ecosystems & Environment 2008, 127 (1), 43–49. 10.1016/j.agee.2008.02.012. [DOI] [Google Scholar]
- Yu H.; Huang G. H. Effects of sodium acetate as a pH control amendment on the composting of food waste. Bioresour. Technol. 2009, 100 (6), 2005–11. 10.1016/j.biortech.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Barthod J.; et al. The effects of worms, clay and biochar on CO2 emissions during production and soil application of co-composts. SOIL 2016, 2 (4), 673–683. 10.5194/soil-2-673-2016. [DOI] [Google Scholar]
- Zhang D.; et al. Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting. Waste Management 2017, 68, 56–63. 10.1016/j.wasman.2017.05.042. [DOI] [PubMed] [Google Scholar]
- Shao L. M.; et al. Effects of bulking agent addition on odorous compounds emissions during composting of OFMSW. Waste Manag 2014, 34 (8), 1381–90. 10.1016/j.wasman.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Jannah Abdul Hamid N.; et al. Overview on Use of Zeolites as Bulking Agent to Optimize Organic Waste Composting Process. IOP Conference Series: Earth and Environmental Science 2020, 616 (1), 012050. 10.1088/1755-1315/616/1/012050. [DOI] [Google Scholar]
- Wang J.; et al. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag 2014, 34 (8), 1546–52. 10.1016/j.wasman.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Maulini-Duran C.; Artola A.; Font X.; Sanchez A. Gaseous emissions in municipal wastes composting: Effect of the bulking agent. Bioresour. Technol. 2014, 172, 260–268. 10.1016/j.biortech.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Wei Z.; et al. Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid. Chemosphere 2007, 68 (2), 368–74. 10.1016/j.chemosphere.2006.12.067. [DOI] [PubMed] [Google Scholar]
- Awasthi M. K.; et al. Influence of zeolite and lime as additives on greenhouse gas emissions and maturity evolution during sewage sludge composting. Bioresour. Technol. 2016, 216, 172–81. 10.1016/j.biortech.2016.05.065. [DOI] [PubMed] [Google Scholar]
- Eftoda G.; McCartney D. Determining the Critical Bulking Agent Requirement For Municipal Biosolids Composting. Compost Science & Utilization 2004, 12 (3), 208–218. 10.1080/1065657X.2004.10702185. [DOI] [Google Scholar]
- Wong J. W. C.; Selvam A. Reduction of indicator and pathogenic microorganisms in pig manure through fly ash and lime addition during alkaline stabilization. J. Hazard. Mater. 2009, 169 (1), 882–889. 10.1016/j.jhazmat.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Chen Z.; et al. Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting. Chemosphere 2021, 262, 128342. 10.1016/j.chemosphere.2020.128342. [DOI] [PubMed] [Google Scholar]
- Villasenor J.; Rodriguez L.; Fernandez F. J. Composting domestic sewage sludge with natural zeolites in a rotary drum reactor. Bioresour. Technol. 2011, 102 (2), 1447–54. 10.1016/j.biortech.2010.09.085. [DOI] [PubMed] [Google Scholar]
- He X.; et al. Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour. Technol. 2016, 218, 867–873. 10.1016/j.biortech.2016.07.045. [DOI] [PubMed] [Google Scholar]
- Wang L.; et al. Impact of fly ash and phosphatic rock on metal stabilization and bioavailability during sewage sludge vermicomposting. Bioresour. Technol. 2013, 136, 281–7. 10.1016/j.biortech.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Zhou R.; et al. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. International Biodeterioration & Biodegradation 2017, 118, 73–81. 10.1016/j.ibiod.2017.01.023. [DOI] [Google Scholar]
- Zhang H. Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting. Molecules 2020, 25 (7), 1560. 10.3390/molecules25071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough T. J.; et al. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3 (2), 275–293. 10.3390/agronomy3020275. [DOI] [Google Scholar]
- Jiang T.; et al. Effects of aeration method and aeration rate on greenhouse gas emissions during composting of pig feces in pilot scale. Journal of Environmental Sciences 2015, 31, 124–132. 10.1016/j.jes.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Yuan J.; et al. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. Journal of Environmental Sciences 2015, 37, 83–90. 10.1016/j.jes.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Zang B.; et al. Control of dimethyl sulfide and dimethyl disulfide odors during pig manure composting using nitrogen amendment. Bioresour. Technol. 2017, 224, 419–427. 10.1016/j.biortech.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Szanto G. L.; et al. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 2007, 98 (14), 2659–2670. 10.1016/j.biortech.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wu H.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: a review. Critical Reviews in Biotechnology 2017, 37 (6), 754–764. 10.1080/07388551.2016.1232696. [DOI] [PubMed] [Google Scholar]
- Wu C.; et al. Usage of pumice as bulking agent in sewage sludge composting. Bioresour. Technol. 2015, 190, 516–21. 10.1016/j.biortech.2015.03.104. [DOI] [PubMed] [Google Scholar]
- Bolan N. S.; et al. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–70. 10.1016/j.scitotenv.2012.02.061. [DOI] [PubMed] [Google Scholar]
- Wang C.; et al. Insight into the effects of biochar on manure composting: evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47 (13), 7341–9. 10.1021/es305293h. [DOI] [PubMed] [Google Scholar]
- Xu S.; et al. Greenhouse gas emissions during co-composting of cattle mortalities with manure. Nutrient Cycling in Agroecosystems 2007, 78 (2), 177–187. 10.1007/s10705-006-9083-1. [DOI] [Google Scholar]
- Awasthi M. K.; et al. Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour. Technol. 2020, 303, 122952. 10.1016/j.biortech.2020.122952. [DOI] [PubMed] [Google Scholar]
- Chen H.; et al. Effects of microbial culture and chicken manure biochar on compost maturity and greenhouse gas emissions during chicken manure composting. J. Hazard. Mater. 2020, 389, 121908. 10.1016/j.jhazmat.2019.121908. [DOI] [PubMed] [Google Scholar]
- Li S.; et al. Linking N2O emission from biochar-amended composting process to the abundance of denitrify (nirK and nosZ) bacteria community. AMB Express 2016, 6 (1), 37. 10.1186/s13568-016-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y.; et al. Pollution control in biochar-driven clean composting: emphasize on heavy metal passivation and gaseous emissions mitigation. J. Hazard. Mater. 2021, 420, 126635. 10.1016/j.jhazmat.2021.126635. [DOI] [PubMed] [Google Scholar]
- Li R.; et al. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environmental Technology 2015, 36 (7), 815–826. 10.1080/09593330.2014.963692. [DOI] [PubMed] [Google Scholar]
- Zhou H.; et al. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. 10.1016/j.biortech.2018.02.086. [DOI] [PubMed] [Google Scholar]
- Khan N.; et al. Development of a buried bag technique to study biochars incorporated in a compost or composting medium. Journal of Soils and Sediments 2017, 17 (3), 656–664. 10.1007/s11368-016-1359-8. [DOI] [Google Scholar]
- Zeng G.; et al. Efficiency of biochar and compost (or composting) combined amendments for reducing Cd, Cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. RSC Adv. 2015, 5 (44), 34541–34548. 10.1039/C5RA04834F. [DOI] [Google Scholar]
- Azim K.; et al. Composting parameters and compost quality: a literature review. Organic Agriculture 2018, 8, 141–158. 10.1007/s13165-017-0180-z. [DOI] [Google Scholar]
- Zhou S.; et al. Co-applying biochar and manganese ore can improve the formation and stability of humic acid during co-composting of sewage sludge and corn straw. Bioresour. Technol. 2022, 358, 127297. 10.1016/j.biortech.2022.127297. [DOI] [PubMed] [Google Scholar]
- Finstein M. S.Principles of composting leading to maximization of decomposition rate, odor control, and cost effectiveness. Composting of agricultural and other wastes, 1985.
- Zucconi F. d.Compost specifications for the production and characterization of compost from municipal solid waste. Compost: production, quality and use, 1987; pp 30–50.
- Bernai M.; et al. Maturity and stability parameters of composts prepared with a wide range of organic wastes. Bioresource technology 1998, 63 (1), 91–99. 10.1016/S0960-8524(97)00084-9. [DOI] [Google Scholar]
- Zmora-Nahum S.; et al. Dissolved organic carbon (DOC) as a parameter of compost maturity. Soil Biology and Biochemistry 2005, 37 (11), 2109–2116. 10.1016/j.soilbio.2005.03.013. [DOI] [Google Scholar]
- Raj D.; Antil R. Evaluation of maturity and stability parameters of composts prepared from agro-industrial wastes. Bioresource technology 2011, 102 (3), 2868–2873. 10.1016/j.biortech.2010.10.077. [DOI] [PubMed] [Google Scholar]
- Cooperband L.; et al. Relating compost measures of stability and maturity to plant growth. Compost science & utilization 2003, 11 (2), 113–124. 10.1080/1065657X.2003.10702118. [DOI] [Google Scholar]
- Brinton W. F.Compost quality standards and guidelines: an international view; Woods End Research Laboratory Inc.: ME, 2000; p 10. [Google Scholar]
- Al-Sari M. I.; Sarhan M. A. A.; Al-Khatib I. A. Assessment of compost quality and usage for agricultural use: a case study of Hebron, Palestine. Environmental Monitoring and Assessment 2018, 190 (4), 223. 10.1007/s10661-018-6610-x. [DOI] [PubMed] [Google Scholar]
- BOE. Real Decreto 824/2005, de 8 de julio, sobre productos fertilizantes. Boletín Oficial del Estado 2005, 171, 25592–25669. [Google Scholar]
- BSI. Specification for composted materials; BSI Standards Limited, 2018.
- European Commission. Working document Biological Treatment of Biowaste; European Union Brussels, 2001.
- Ge B.; McCartney D.; Zeb J. Compost environmental protection standards in Canada. Journal of Environmental Engineering and Science 2006, 5 (3), 221–234. 10.1139/s05-036. [DOI] [Google Scholar]
- Thompson W.et al. Test methods for the examination of composting and compost; US Composting Council: Bethesda, MD, 2012. [Google Scholar]
- Zhou S.; et al. The co-addition of biochar and manganese ore promotes nitrous oxide reduction but favors methane emission in sewage sludge composting. Journal of Cleaner Production 2022, 339, 130759. 10.1016/j.jclepro.2022.130759. [DOI] [Google Scholar]
- CCQC. Compost Maturity Index; California Compost Quality Council: Nevada City, 2001. [Google Scholar]
- Zhang L.; Liu Y.; Hao L. Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environmental Research Letters 2016, 11 (1), 014014. 10.1088/1748-9326/11/1/014014. [DOI] [Google Scholar]
- Zhou S.; et al. Biochar-amended compost as a promising soil amendment for enhancing plant productivity: A meta-analysis study. Science of The Total Environment 2023, 879, 163067. 10.1016/j.scitotenv.2023.163067. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.; et al. Co-composting solid biowastes with alkaline materials to enhance carbon stabilization and revegetation potential. Environmental Science and Pollution Research 2016, 23, 7099–7110. 10.1007/s11356-015-5411-9. [DOI] [PubMed] [Google Scholar]
- Zucconi F.; et al. Evaluating toxicity of immature compost. Biocycle 1981, 54–57. [Google Scholar]
- Fang M.; et al. Co-composting of sewage sludge and coal fly ash: nutrient transformations. Bioresource technology 1999, 67 (1), 19–24. 10.1016/S0960-8524(99)00095-4. [DOI] [Google Scholar]
- Chen Y.-X.; et al. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 2010, 78 (9), 1177–1181. 10.1016/j.chemosphere.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Zayed G.; Abdel-Motaal H. Bio-production of compost with low pH and high soluble phosphorus from sugar cane bagasse enriched with rock phosphate. World J. Microbiol. Biotechnol. 2005, 21, 747–752. 10.1007/s11274-004-5407-y. [DOI] [Google Scholar]
- Mihreteab H.; et al. Rock phosphate enriched compost as a growth media component for organic tomato (Solanum lycopersicum L.) seedlings production. Biological Agriculture & Horticulture 2016, 32 (1), 7–20. 10.1080/01448765.2015.1016114. [DOI] [Google Scholar]
- Lim S. L.; Lee L. H.; Wu T. Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. Journal of Cleaner Production 2016, 111, 262–278. 10.1016/j.jclepro.2015.08.083. [DOI] [Google Scholar]
- Bolan N. S.; et al. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. 10.1016/j.scitotenv.2012.02.061. [DOI] [PubMed] [Google Scholar]
- Lei D.; Huang W.; Wang X. Effect of moisture content of substance on fermentation and heat production of cattle manure in aerobic composting. Journal of Ecology and Rural Environment 2011, 27 (5), 54–57. [Google Scholar]
- Doan T. T.; et al. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. 10.1016/j.scitotenv.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Komakech A. J.; et al. Life cycle assessment of biodegradable waste treatment systems for sub-Saharan African cities. Resources, Conservation and Recycling 2015, 99, 100–110. 10.1016/j.resconrec.2015.03.006. [DOI] [Google Scholar]
- Noor R. S.; et al. Enhanced biomethane production by 2-stage anaerobic co-digestion of animal manure with pretreated organic waste. Biomass Conversion and Biorefinery 2023, 13, 2833–2847. 10.1007/s13399-020-01210-1. [DOI] [Google Scholar]