Abstract
Background:
The current standard of care for patients with newly diagnosed limited-stage small-cell lung cancer (SCLC) is concurrent chemoradiotherapy (CCRT). The prognosis remains poor due to the aggressiveness and high risk of progression or relapse of SCLC even if an initial response is achieved. Therefore, there is an urgent unmet clinical need in this population. The multicenter, phase 3, randomized, placebo-controlled, double-blind KEYLYNK-013 study evaluates the addition of pembrolizumab to CCRT followed by pembrolizumab with or without olaparib in participants with previously untreated limited-stage SCLC. (ClinicalTrials.gov: NCT04624204).
Methods:
Eligible participants aged ≥18 years with newly diagnosed, pathologically confirmed, limited-stage (ie, stage I-III) SCLC will be randomized 1:1:1 to CCRT (ie, etoposide plus carboplatin or cisplatin for 4 cycles and standard thoracic radiotherapy) plus pembrolizumab (Groups A and B) or CCRT plus placebo (Group C). In the absence of disease progression, participants will receive pembrolizumab plus placebo (Group A), pembrolizumab plus olaparib (Group B), or placebo (Group C). Dual primary endpoints are progression-free survival per RECIST version 1.1 by blinded independent central review and overall survival.
Results:
Enrollment began in December 2020 and is ongoing at approximately 150 sites.
Conclusions:
KEYLYNK-013 will provide valuable information on the efficacy and safety of pembrolizumab plus CCRT and pembrolizumab with or without olaparib post CCRT in participants with limited-stage SCLC.
Keywords: Limited-stage SCLC, Pembrolizumab, Olaparib, Concurrent chemoradiotherapy
Introduction
At initial diagnosis, 35% to 40% of small-cell lung cancer (SCLC) cases present as limited-stage disease (stage I-III).1 The standard of care for fit patients with limited-stage SCLC is therefore concurrent chemoradiotherapy (CCRT) with etoposide plus cisplatin or carboplatin and definitive thoracic radiation therapy, but prognosis remains poor due to the high risk of relapse.2,3
Preclinical and clinical studies have provided a rationale for the combination of the monoclonal anti–PD-1 antibody pembrolizumab concurrently with CCRT and in the consolidation setting for SCLC.4,5 Notably, in a single-center, open-label, phase 1/2 study of 40 participants with limited-stage SCLC or other neuroendocrine tumors, treatment with pembrolizumab, etoposide, a platinum agent, and thoracic radiotherapy yielded an objective response rate (ORR) of 79%, median progression-free survival (PFS) of 19.7 months, and median overall survival (OS) of 39.5 months.4 The most common grade 3 to 4 treatment-related adverse event was neutropenia, occurring in 18% of participants. Three cases of grade 3 treatment-related pneumonitis (8% of participants) and 1 case of grade 4 treatment-related respiratory failure (3% of participants) were observed. No grade 5 treatment-related events occurred. These safety findings were consistent with other studies combining immune checkpoint inhibitor therapies concurrently with CCRT.
Proteomic analyses suggest that poly(ADP-ribose) polymerase (PARP) 1 is highly expressed in SCLC.6 Based on evidence from preclinical SCLC models, PARP inhibitors block tumor cell proliferation, significantly increase tumor cell expression of PD-L1 and enhance the antitumor activity of PD-L1 blockade.7,8 Additionally, PARP inhibitors stimulate the innate immune response via the STING pathway, resulting in increased expression of interferon-β and multiple chemokines associated with cytotoxic T-lymphocyte recruitment and activity.8 Finally, PARP inhibition has been shown to potentiate the antitumor effects of cytotoxic chemotherapy or radiation in SCLC cell lines.9 Therefore, combined PARP and immune checkpoint inhibition may be a promising approach to delay disease progression following response to CCRT. Olaparib is a PARP inhibitor currently established as maintenance therapy for advanced, platinum-sensitive ovarian and pancreatic cancer.10 In the KEYLYNK-007 study, olaparib 300 mg twice daily plus pembrolizumab 200 mg every 3 weeks showed antitumor activity and a grade ≥3 treatment-related adverse event rate of 36% in participants with previously treated, advanced solid tumors with homologous recombination repair mutations and/or homologous recombination deficiency.11 The most common treatment-related adverse events observed with this combination were nausea, anemia and fatigue, and pembrolizumab plus olaparib had a safety profile in line with expectations based on the safety profiles of the individual agents.
The addition of pembrolizumab to CCRT in the treatment of participants with newly diagnosed, limited-stage SCLC unselected for homologous recombination repair mutations and/or homologous recombination deficiency, followed by pembrolizumab with or without olaparib in the absence of disease progression, is being investigated in the randomized, placebo-controlled, double-blind, phase 3 KEYLYNK-013 trial.
Participants and Methods
Study Design and Objectives
In the KEYLYNK-013 study (ClinicalTrials.gov identifier NCT04624204), participants with pathologically confirmed, limited-stage SCLC by the American Joint Committee on Cancer version 8 criteria12 will be randomized 1:1:1 to receive pembrolizumab in combination with CCRT followed by pembrolizumab and olaparib placebo (Group A), pembrolizumab in combination with CCRT followed by pembrolizumab and olaparib (Group B), or pembrolizumab placebo plus CCRT followed by pembrolizumab placebo and olaparib placebo (Group C) (Figure 1). Participants, investigators, and the sponsor will be blinded to treatment assignment in Groups A, B, and C. The dual primary endpoints of the study are PFS per RECIST version 1.1 as assessed by blinded independent central review (BICR) and OS. Secondary endpoints include safety and tolerability, ORR and duration of response (DOR) per RECIST version 1.1 by BICR, the effect of PD-L1 expression levels on PFS, OS, ORR, and DOR, and patient-reported outcomes. Additional biomarkers, event-free survival and time to subsequent therapy are exploratory endpoints.
Figure 1.
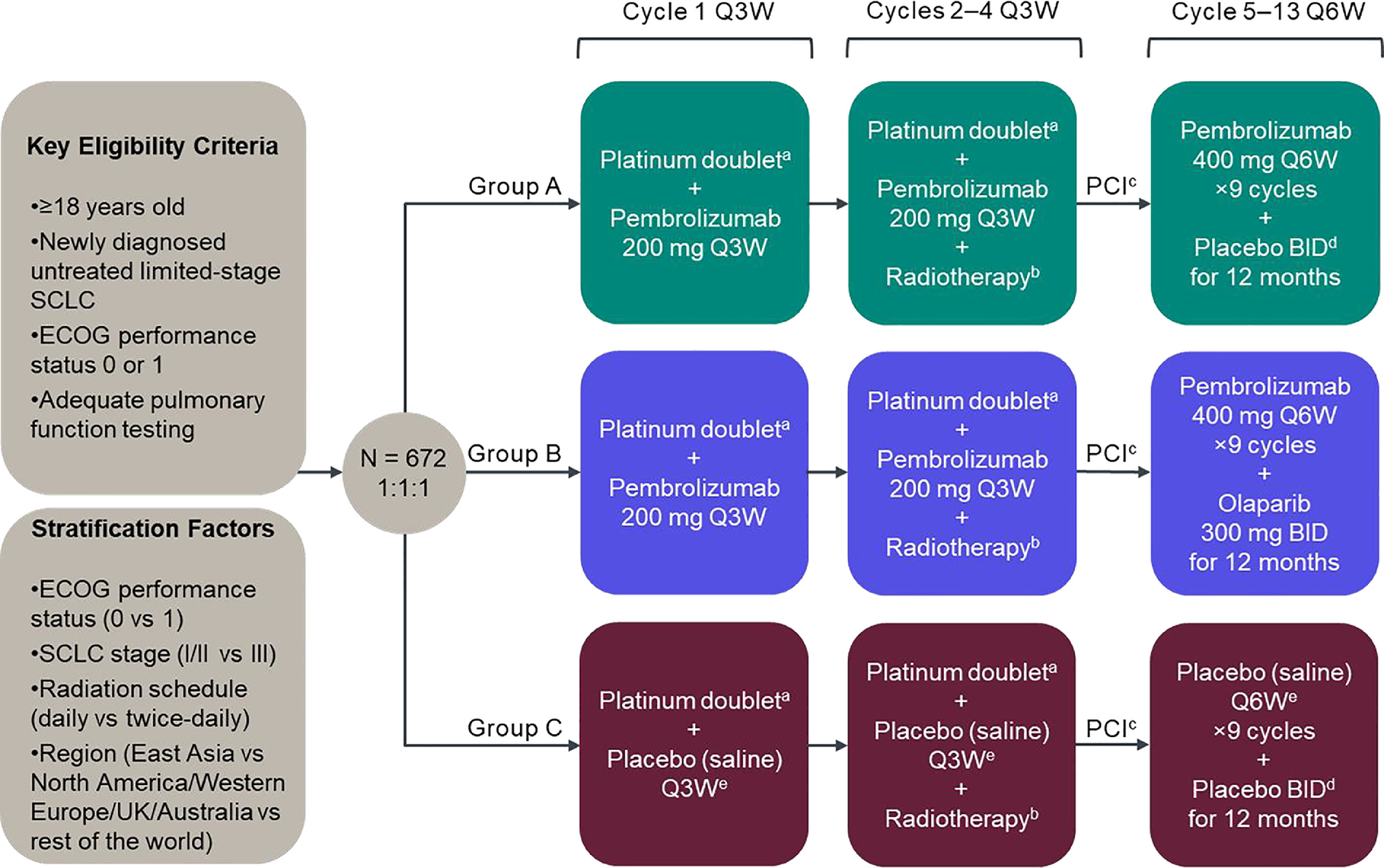
KEYLYNK-013 study design. AUC, area under the curve; BID, twice daily; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; PCI, prophylactic cranial irradiation; Q3W, every 3 weeks; Q6W, every 6 weeks; UK, United Kingdom. aPlatinum doublet chemotherapy comprises etoposide 100 mg/m2 on days 1, 2 and 3 of each cycle and investigator’s choice of carboplatin AUC 5 mg/mL/min or cisplatin 75 mg/m2 on day 1 of each cycle. Chemotherapy treatments will be administered intravenously Q3W for 4 cycles. bRadiotherapy is administered twice daily (45 Gy in 30 twice-daily fractions of 1.5 Gy over 3 weeks, 5 days/week) or daily (66 Gy in 33 daily fractions of 2 Gy over 6.5 weeks, 5 days/week), starting concurrently with chemotherapy on day 1 of cycle 2. cPCI occurs after early response assessment (imaging 12 weeks after randomization) and is strongly recommended for participants who achieve complete or partial response following CCRT and is at the investigator’s discretion for participants with stable disease. PCI should be administered no later than 6 weeks after the last dose of cycle 4. The standard dose for PCI to the whole brain is 25 Gy in 10 daily fractions (2.5 Gy/fraction). dOlaparib matching placebo. ePembrolizumab matching placebo.
Participants
Detailed participant inclusion and exclusion criteria are shown in Table 1. Briefly, participants must be ≥18 years of age, have histologically or cytologically confirmed, newly diagnosed stage I-III SCLC, and received no prior therapy (chemotherapy, radiotherapy, surgery) for SCLC. Eligible participants must have an Eastern Cooperative Oncology Group performance status of 0 or 1, adequate organ function, and should not be expected to require tumor resection during the study.
Table 1.
Key Inclusion and Exclusion Criteria for the KEYLYNK-013 Study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
| |
| • Age ≥18 years | • Extensive-stage SCLC, defined as stage IV or T3–4 that cannot be encompassed in a tolerable radiation plan |
| • Histologically/cytologically confirmed, newly diagnosed, limited-stage (stage I-III by AJCC eighth edition) SCLC that can be safely treated with radiotherapy | • Known MDS/AML, or features suggestive of MDS/AML |
| • At least 1 measurable lesion per RECIST version 1.1 as assessed by the local investigator | • Documented >10% weight loss from baseline in the preceding 3 months |
| • No evidence of metastatic disease | • Prior olaparib or other PARP inhibitor therapy or prior anti-PD-1, PD-L1, PD-L2 or other stimulatory or co-inhibitory T-cell receptor therapy |
| • No prior chemotherapy, radiotherapy or surgery for limited-stage SCLC | • Major surgery <4 weeks prior to initiation of study treatment |
| • Not expected to require tumor resection during the course of the study | • Received colony-stimulating factors ≤28 days prior to initiation of study treatment |
| • Provision of newly obtained (strongly preferred) or archival tumor tissue sample for PD-L1 assessment | • Known additional malignancy that is progressing or required active treatment ≤5 years prior to initiation of study treatment |
| • ECOG PS of 0 or 1 | • Uncontrolled, potentially reversible cardiac conditions |
| • Adequate pulmonary function testing, defined as a FEV1 >50% of predicted normal volume and a DLCO >40% of predicted normal value | • History of/current pneumonitis or interstitial lung disease |
| • Adequate organ function | • Active infection requiring systemic therapy |
| • Life expectancy at least 6 months | • Diagnosis of immunodeficiency or is receiving chronic systemic steroid therapy ≤7 days prior to initiation of study treatment |
| • Written informed consent | • Active autoimmune disease requiring systemic treatment in the past 2 years |
| • Known history of HIV or Hepatitis B or active Hepatitis C infection | |
Abbreviations: AJCC = American Joint Committee on Cancer; AML = acute myeloid leukemia; DLCO = carbon monoxide lung diffusing capacity; ECOG PS = Eastern Cooperative Oncology Group performance status; FEV = forced expiratory volume; HIV = human immunodeficiency virus; MDS = myelodysplastic syndrome; PARP = poly (ADP-ribose) polymerase; SCLC = small-cell lung cancer.
Treatments
Participants in Groups A, B and C will receive etoposide and investigator’s choice of carboplatin or cisplatin for 4 cycles concurrently with standard thoracic radiotherapy (Figure 1). Participants in Groups A and B will receive pembrolizumab 200 mg intravenously every 3 weeks concurrently with CCRT, followed by pembrolizumab 400 mg every 6 weeks plus olaparib matched placebo or olaparib 300 mg administered orally twice daily, respectively. Participants in Group C will receive pembrolizumab placebo with CCRT, followed by pembrolizumab placebo and olaparib placebo. Pembrolizumab, olaparib and the respective placebos will be administered in the post CCRT setting for up to 9 cycles of pembrolizumab 400 mg every 6 weeks (approximately 12 months), or until specific discontinuation criteria are met (including request to discontinue study treatment, unblinding of treatment assignment, disease progression or recurrence, or pregnancy). Permitted dose modifications of pembrolizumab are limited to interruption or discontinuation. The dosage of olaparib may be reduced to 250 mg twice daily, and then to 200 mg twice daily if needed, with no further dose reductions permitted. Overlapping toxicities between olaparib and pembrolizumab such as pneumonitis and renal dysfunction will be monitored and managed according to the protocol.
Prophylactic cranial irradiation will be strongly recommended for participants who achieve a complete or partial response following CCRT and offered at the investigator’s discretion to participants who have stable disease and no clinical evidence of brain metastases.
Assessments
Tumor response and PFS per RECIST version 1.1 will be evaluated radiographically. The first scan following CCRT should be performed approximately 12 weeks after study treatment initiation. Subsequent radiographic imaging will be performed to assess response every 9 weeks until the end of the second year, every 12 weeks until the end of the third year, every 16 weeks until the end of the fourth year, every 6 months until the end of the fifth year, and annually thereafter until confirmed disease progression, the start of a new anticancer therapy, pregnancy, death, withdrawal of consent or the end of the study.
All adverse event data will be reported from the time of randomization to 30 days after discontinuation of study treatment, except for serious adverse events, which will be collected for up to 90 days following discontinuation of study treatment.
Statistical Analysis
Treatment differences in the dual primary endpoints of PFS per RECIST version 1.1 by BICR and OS will be compared between Groups A and B vs. C using a stratified log-rank test. Hazard ratios will be estimated using a stratified Cox regression model, and event rates over time will be estimated using the Kaplan-Meier method. Efficacy will be evaluated in the intention-to-treat population, comprised of all randomized participants. Safety will be assessed in the as-treated population, comprised of all participants who receive ≥1 dose of study treatment.
External Data Monitoring Committee
An independent, external data and safety monitoring committee will oversee the trial and assess outcomes at protocol-specified interim analyses.
Results
Current Status
Enrollment for this study began in December 2020 and is ongoing at approximately 150 sites. The current estimated study completion date is October 2027. Planned sample size is approximately 672 participants.
Discussion
SCLC is an aggressive cancer that is characterized by heterogeneity and sensitivity to chemotherapy regimens, followed by rapid progression or recurrence.1,13 Prognosis for patients with limited-stage SCLC is poor, with a median OS with standard CCRT of 25 to 30 months.2 New treatment options that delay disease progression and prolong survival are urgently needed.
Investigations into the possible role for immune checkpoint inhibitors as consolidation therapy following CCRT for limited-stage SCLC are ongoing. In the STIMULI trial, consolidation therapy with nivolumab plus ipilimumab (4 cycles) followed by nivolumab for up to 12 months was compared vs. observation for participants without progressive disease after CCRT. However, study accrual was closed early and the study failed to meet its primary endpoint of PFS.14 Similar studies, including the phase 3 ADRIATIC study of durvalumab with or without tremelimumab vs. placebo after CCRT, are further investigating this treatment approach. Introduction of immunotherapy earlier in the treatment paradigm, ie, in conjunction with CCRT, may be another strategy in this disease setting. To that effect, the phase 3 NRG-LU005 study is evaluating CCRT plus atezolizumab followed by atezolizumab monotherapy vs. CCRT alone, and the phase 3 KEYLYNK-013 trial is evaluating CCRT plus pembrolizumab followed by pembrolizumab with or without olaparib vs. CCRT alone.
Early data suggest that the addition of pembrolizumab to CCRT and its continuation post CCRT may be an efficacious treatment strategy in limited-stage SCLC.4 Based on the established antitumor activity of pembrolizumab across tumor types, including lung cancer, introducing immune checkpoint inhibition concurrently with CCRT may improve responsiveness to treatment and prolong the duration of benefit for patients with newly diagnosed limited-stage SCLC. In the absence of disease progression, continued pembrolizumab after CCRT may prolong disease control. Early-phase trials have suggested that PARP inhibitors in combination with cytotoxic chemotherapy or an immune checkpoint inhibitor are active in SCLC.15–18 Therefore, the addition of olaparib to continued pembrolizumab post CCRT may contribute further towards antitumor activity and the maintenance of response or stable disease in participants with limited-stage SCLC.
In conclusion, the KEYLYNK-013 study aims to identify whether the addition of pembrolizumab to CCRT and pembrolizumab with or without olaparib following CCRT improves outcomes for participants with newly diagnosed, limited-stage SCLC, a disease setting with few recent advancements in treatment.
Acknowledgments
We thank the participants and their families and caregivers, all primary investigators and their site personnel; Cathy Pietanza for study support (Merck & Co., Inc., Rahway, NJ, USA); and Ina Nikolaeva (Merck & Co., Inc., Rahway, NJ, USA) for medical writing assistance. This study and assistance with manuscript editing were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA. Dr. Rimner was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure
A.R. reports research funding/grants from Merck Sharp & Dohme LLC (MSD), a subsidiary of Merck & Co., Inc, Rahway, NJ, USA, AstraZeneca, Boehringer-Ingelheim, Pfizer, Varian Medical Systems, consulting fees from AstraZeneca, Boehringer-Ingelheim, Cybrexa, MoreHealth, MSD, honoraria from Research-ToPractice, travel support from Philips/Elekta, scientific advisory role for MSD, board membership for ABR, ASTRO, IMIG, ITMIG. W.V.L. reports research funding/grants from MSD, consulting fees from AstraZeneca, MSD. R.C. reports research funding/grants from MSD, Abbvie, AstraZeneca, Bristol Myers Squibb, Clovis, Janssen, Lilly Oncology, Novartis, Pfizer, Roche, Takeda, advisory board roles, consulting fees and honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Janssen, Lilly Oncology, MSD, Novartis, Pfizer, Roche, Sanofi, Takeda, travel support from MSD, Roche, Takeda, stock ownership LOC at the Christie Private Care. S.K.J. reports research funding/grants from MSD, consulting fees and advisory board role from MSD, Syntactx, and IMX Medical. C.M.R. reports research funding/grants from MSD, consulting fees from Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Epizyme, Genentech/Roche, Ipsen, Jazz, Kowa, MSD, Syros, advisory board role for Bridge Medicines, Earli, Harpoon Therapeutics, stock ownership Earli. C.F.-F. reports research funding/grants/support from MSD, AstraZeneca, Elekta, honoraria from AstraZeneca, travel support from AstraZeneca, Elekta, MSD, advisory board role for AstraZeneca. B.C.C. reports research funding/grants from Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, GI Innovation, Interpark Bio Convergence Corp, Janssen, Medpacto, MOGAM Institute, MSD, Novartis, Ono, Yuhan, royalties/licenses from Champions Oncology, consulting fees from AstraZeneca, Blueprint Medicines, BMS, Boehringer-Ingelheim, Eli Lilly, Janssen, Medpacto, MSD, Novartis, Ono, Pfizer, Roche, Takeda, Yuhan, scientific advisory role for Bridgebio Therapeutics, Cyrus Therapeutics, Guardant Health, Joseah BIO, KANAPH Therapeutic Inc, board membership for Gencurix Inc, Interpark Bio Convergence Corp, founder role for DAAN Biotherapeutics, and stock ownership Bridgebio Therapeutics, Cyrus Therapeutics, Gencurix Inc, KANAPH Therapeutic Inc, TheraCanVac Inc. T.K. reports research funding/grants from Abbvie, Amgen, AstraZeneca, Blueprint, Chugai, Eli Lilly, Haihe, Merck Serono, MSD, Novartis, Pfizer, Regeneron, Takeda, speaker honoraria from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly, Merck Serono, MSD, Novartis, Ono, Pfizer, Roche, advisory board honoraria from Abbvie, Amgen, AstraZeneca, Beigene, Chugai, Daiichi-Sankyo, Eli Lilly, Glaxo, Merck Serono, MSD, Nippon Kayaku, Novartis, Ono, Pfizer, Taiho, Takeda, and employment (spouse) by Eli Lilly; J.Y. reports research funding/grants from MSD. L.B. reports research funding/grants and consulting fees from MSD. W.C., L.Y. and B.Z. are employees of MSD, and may own stock options in the company.
References
- 1.Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat Res. 2016;170:301–322. [DOI] [PubMed] [Google Scholar]
- 2.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice–daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Small cell lung cancer (Version 3.2021). [DOI] [PubMed]
- 4.Welsh JW, Heymach JV, Guo C, et al. Phase 1/2 trial of pembrolizumab and concurrent chemoradiation therapy for limited-stage SCLC. J Thorac Oncol. 2020;15:1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 6.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardnell RJ, Feng Y, Diao L, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res. 2013;19:6322–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9:646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owonikoko TK, Zhang G, Deng X, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med. 2014;3:1579–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AstraZeneca. Lynparza (olaparib). Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf Accessed May 20. 2022. [Google Scholar]
- 11.Maio MSFR, Yap TA, Ciuleanu T, et al. Olaparib plus pembrolizumab in patients with previously treated advanced solid tumors with homologous recombination repair mutation (HRRm) and/or homologous recombination deficiency (HRD): Initial results of the phase 2 KEYLYNK-007 study. Cancer Res. 2021;81(13_Supplement):CT178. [Google Scholar]
- 12.Brierley JD GM, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. John Wiley & Sons, Ltd; 2017. [Google Scholar]
- 13.Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PetersSP J, Dafni U, Dómine M, et al. LBA84 - Consolidation ipilimumab and nivolumab vs observation in limited stage SCLC after chemo-radiotherapy: results from the ETOP/IFCT 4–12 STIMULI trial. Ann Oncol. 2020;31:S1142–SS215. [DOI] [PubMed] [Google Scholar]
- 15.Farago AF, Yeap BY, Stanzione M, et al. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov. 2019;9:1372–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owonikoko TK, Dahlberg SE, Sica GL, et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small–cell lung cancer: ECOG-ACRIN 2511 study. J Clin Oncol. 2019;37:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietanza MC, Waqar SN, Krug LM, et al. Randomized, double-blind, phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J Clin Oncol. 2018;36:2386–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21:1155–1164. [DOI] [PubMed] [Google Scholar]


