Abstract
Purpose/objectives:
Lung tumor motion may be impacted by heartbeat in addition to respiration. This study seeks to quantitatively analyze heart-motion-induced tumor motion and to evaluate its impact on lung cancer radiotherapy.
Methods/materials:
Fluoroscopy images were acquired for 30 lung cancer patients. Tumor, diaphragm, and heart were delineated on selected fluoroscopy frames, and their motion was tracked and converted into temporal signals based on deformable registration propagation. The clinical relevance of heart impact was evaluated using the dose volumetric histogram of the redefined target volumes.
Results:
Correlation was found between tumor and cardiac motion for 23 patients. The heart-induced motion amplitude ranged from 0.2 to 2.6 mm. The ratio between heart-induced tumor motion and the tumor motion was inversely proportional to the amplitude of overall tumor motion. When the heart motion impact was integrated, there was an average 9% increase in internal target volumes for 17 patients. Dose coverage decrease was observed on redefined planning target volume in simulated SBRT plans.
Conclusions:
The tumor motion of thoracic cancer patients is influenced by both heart and respiratory motion. The cardiac impact is relatively more significant for tumor with less motion, which may lead to clinically significant uncertainty in radiotherapy for some patients.
Keywords: Frequency analysis, Cardiac motion, Respiratory motion, Lung tumor, Fluoroscopy, Deformable image registration
Radiation therapy for thoracic cancer patients suffers from the constant motion of the tumor. Much research has been conducted to address respiration-induced tumor motion in radiotherapy with various approaches [1–7], such as voluntary breath control and the utilization of internal target volume (ITV) in treatment planning. In clinical implementation, most motion management approaches acquire tumor motion information through the respiration-correlated four-dimensional computed tomography (4DCT) [8]. These approaches, although attempting to tackle tumor motion, do not accurately account for the impact of cardiac motion.
Cardiac motion, along with the respiratory motion, cyclically alters the spatial locations of anatomic structures in the thoracic volume. Cardiac motion is involuntary, and with higher frequency compared to respiration. Due to the frequency and phase difference, the heart impact appears as inter-phase motion uncertainty in respiration-correlated 4DCT but cannot be fully captured as restricted by the relatively low sampling rate [9] and relatively short scanning time of CT. Research has been conducted on the impact of cardiac motion on lung tumors [10–12] using fluoroscopy imaging. In previous studies, to highlight the target motion in fluoroscopy images, a metal fiducial was implanted close to the tumor, and its motion was recorded as the tumor motion for analysis. The procedure is invasive and may not be applicable to many patients, plus the correlation between the surrogate and actual tumor motion was not fully investigated. Fortunately, the latest development of deformable image registration (DIR) and its clinical application has enabled the direct tracking of tumor motion without invasive implantation.
The objectives of this study were to (1) implement an object constrained DIR tool to extract the heart-induced tumor motion; (2) quantify the heart-induced tumor motion via frequency domain analysis; (3) conduct statistical study on the quantified cardiac impact, and (4) evaluate the potential clinical influence of cardiac motion on radiotherapy.
Methods and materials
Patients
30 patients who received external beam radiotherapy (EBRT) for thoracic cancer from October 2011 through May 2013 were enrolled onto a prospective Institutional Review Board approved study. Radiation was delivered to patients in 1.8 Gy or 2 Gy per day to a median dose of 62 Gy (min: 48.6 Gy, max: 66 Gy). To avoid using preliminary results to guide the clinical practice, we did not include SBRT patients in our study. However, for selected patients who met the criteria of SBRT, we generated experimental SBRT plans following the clinical protocol at the institute. These experimental plans were used for the evaluation of the potential cardiac impact in clinical cases.
Image acquisition
Patients underwent respiration correlated retrospective 4DCT scanning for treatment planning purposes using a GE Lightspeed™ 16 CT scanner (Waukesha, WI) equipped with the Advantage 4DCT system. The 4DCT images were generated retrospectively by acquiring images in the cine mode at the interval determined by , where was the length of patient’s respiratory cycle. The generated images were sorted into 10 different image sets corresponding to different respiratory phases, denoted as from 0% to 90%.
For all patients, 15-s fluoroscopy was acquired weekly in the anterior–posterior (AP) direction at 8 frames per second as part of the pre-treatment patient setup procedure. All fluoroscopy was acquired by the kilo-voltage On Board Imaging (OBI) system of Varian© (Palo Alto, CA) using the same imaging protocol. The overall radiation dose from fluoroscopy was estimated and deemed clinically negligible.
Terminology
The definition of GTV (gross tumor volume), CTV (clinical target volume), ITV, and PTV (planning target volume) complied with ICRU62 [7] and the clinical practice at the facility where the proposed study was conducted.
The tumor motion definition used in this study accounts for the intra-fractional shape, size, and location change of the tumor. We assumed the tumor self-deformation was ignorable compared to the respiration and cardiac impact. The motion between any two give time points was defined as the displacement of the center of mass (COM) of the tumor between the corresponding images.
Image processing
4DCT images were transferred to the Eclipse treatment planning system (TPS) (Varian Medical Systems, Palo Alto, CA). All target volumes were manually delineated for treatment planning.
The motion information in the fluoroscopy images was analyzed with a software package driven by the in-house deformable registration propagation algorithms [13,14]. The software interface and the structures we used to retrieve the motion signals were illustrated in Supplementary Fig. 1. First DIR was performed between the digitally reconstructed radiograph (DRR) of the corresponding 4DCT phase and a fluoroscopy frame to project the tumor into the 2D domain of fluoroscopy images, forming the initial tumor template . To propagate, one frame was registered non-rigidly to its direct neighbors, i.e., to and , constrained by . The registration generated a displacement map between the source () and target (). By deforming using , we derived in the target frames, and the propagation continued until displacement between all neighboring frames had been calculated.
Cardiac motion analysis
For patients included in this study, the average respiratory frequency was 0.2 s−1, while the average heartbeat frequency was 1.4 s−1. The frequency difference enabled the separation of cardiac motion signals from the respiratory motion through frequency domain analysis.
In the first step of the decoupling process we retrieved the cardiac motion signal by depriving the respiration impact from heart motion. The respiratory motion frequency was determined directly using right diaphragm motion signal . The heart motion (in form of heartwall motion) in fluoroscopy images was usually a combination of the heartbeat and the respiration motion. To derive the cardiac motion frequency, we performed Fourier transformation of the motion signal of the heart, and filtered out the low frequency component in heart motion using a tentative cutoff frequency. The cardiac motion without respiratory impact was reconstructed using the filtered frequency signal.
The choice of the cutoff frequency affected the decoupling process. To effectively decouple the motion, we performed correlation analysis between the respiratory motion, and heart motion with and without the respiratory impact. We evolved the value of the cutoff frequency based on the analysis result until the reconstructed heart motion was no longer correlated with the respiratory motion. The heartbeat frequency was determined from the filtered heart motion signal .
In the second step of the decoupling process, we defined the patient specific cutting off frequency for tumor motion decoupling as
| 1 |
The tumor motion observed on the fluoroscopy images were decoupled into two components and corresponding to the heartbeat and the respiratory impact, respectively, and the amplitude of both components of tumor motion was calculated. In Fig. 1 the tumor motion and the separated respiratory and cardiac impact signals were presented.
Fig. 1.
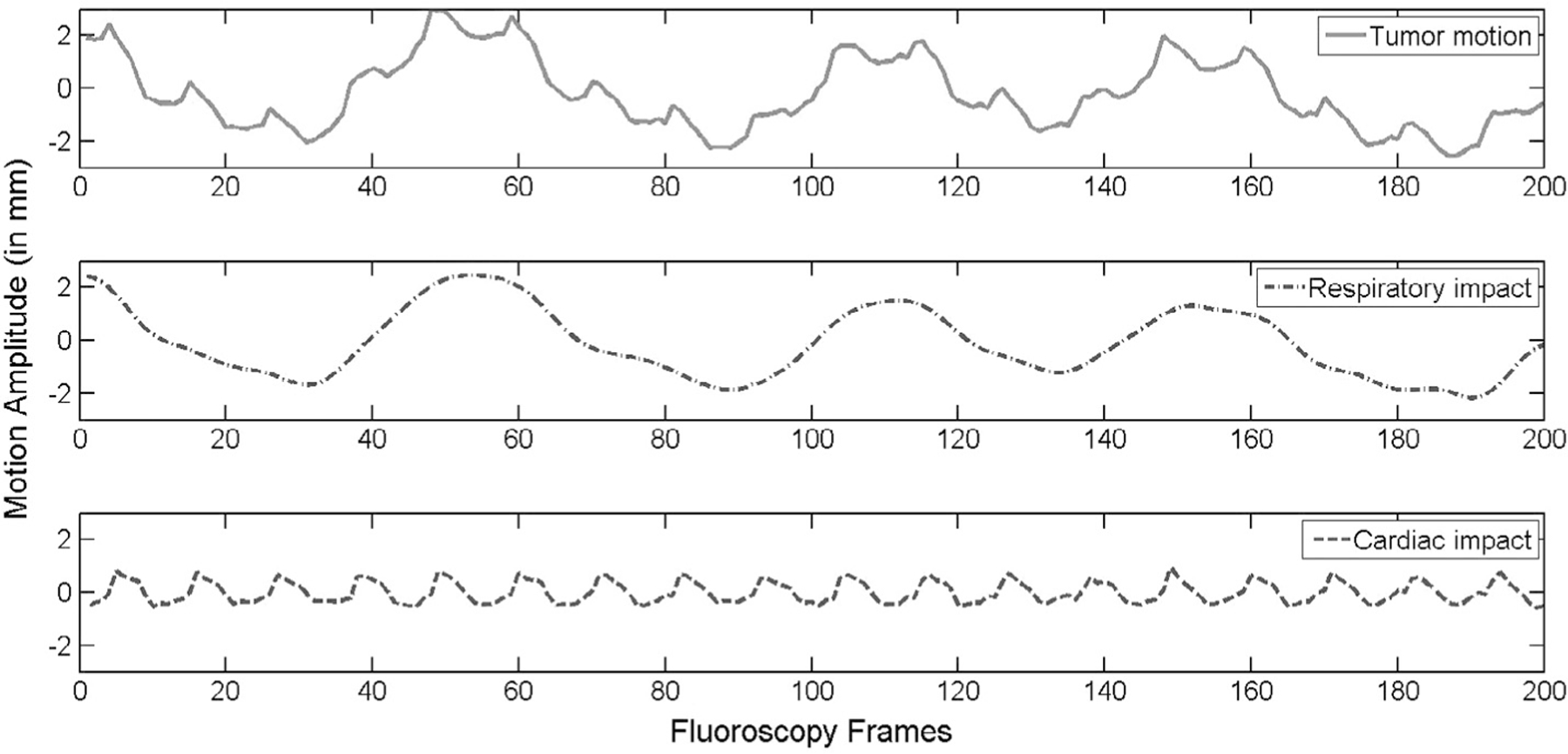
Top: solid line is the tumor motion detected from fluoroscopy. Middle: the dot-dash line denotes the respiratory tumor motion without the cardiac impact. Bottom, the dash line is the cardiac impact signal.
To verify the magnitude of the tumor motion, two experienced radiation oncologists manually contoured the tumor in both 4DCT and selected frames of the fluoroscopy. The tumor motion magnitude was also derived from the manual contours to verify the motion magnitude calculated from DIR.
Statistics
We calculated both the correlation coefficient and the probability of the no-correlation between the tumor motion and the cardiac motion. We define the two motions as correlated if the probability of no-correlation ; highly correlated if the correlation coefficient value was . The average and standard deviations of the correlation-coefficient and cardiac impact amplitude for different lobes and tumor sites have been calculated and studied respectively to determine whether the cardiac impact was location-dependent for lung tumor. In Supplementary Fig. 3 the separated cardiac impact signal was overlaid to the corresponding heart wall motion signal to verify the correlation.
Clinical relevance study
First we conducted a linear regression fit of the cardiac motion impact ratio to the overall tumor motion as a function of the tumor motion amplitude.
Secondly, we regenerated ITVs for each of the patients by expanding the GTVs at the 50% phase with motion margin computed as the sum of the maximum magnitudes of the corresponding cardiac and respiratory induced motion. The volumes of new ITVs were compared with the ones in the original plans. To evaluate the impact of the cardiac motion, the dose coverage of the newly redefined PTVs (ITVs plus setup margin) was computed and compared to the dose coverage of the original PTVs in simulated plans, using identical beam parameters.
Results
For 23 patients whose tumor motion impacted by the heartbeat, the average heart rate is 85.1 beats per minute (max 105, min 73), and the average respiration per minute is 17.1 (max 24, min 13). Table 1 summarized the average amplitude of the tumor motion captured from 4DCT and fluoroscopy through DIR, as well as the cardiac and respiratory impact in fluoroscopy. For comparison, the average amplitude of the tumor motion reconstructed from the manual contours was in the cranial–caudal direction; and in the lateral direction. Paired t-test showed there was no significant difference in amplitude between 4DCT and fluoroscopy derived tumor motion, nor between the motion amplitude derived from DIR and manual contours.
Table 1.
Summary of image-derived tumor motion. Lateral and longitudinal tumor motion in 4DCT and fluoroscopy (first and second row); Lung and heart impact (third and fourth row); and the correlation between heart motion and the tumor motion (high frequency component). The tumor motion amplitude in 4DCT ranged from 0.2 to 3.8 mm in lateral, and 0.2 to 16.7 mm in longitudinal direction; the tumor motion amplitude in fluoroscopy ranged from 0.5 to 4.3 mm in lateral, and 0.3 to 14.5 mm in longitudinal direction; the respiration-induced tumor motion amplitude ranged from 0.2 to 3.9 mm in lateral, and 0.1 to 14.5 mm in longitudinal direction; and the heart-induced tumor motion amplitude ranged from 0.2 to 1.6 mm in lateral, and 0.2 to 2.6 mm in longitudinal direction.
| Empty Cell | Empty Cell | Lat. motion | Lng. motion |
|---|---|---|---|
| 4DCT | Mean (mm) | 1.43 | 4.52 |
| standard deviation (mm) | 0.72 | 4.41 | |
| Fluoroscopy | Mean (mm) | 1.84 | 4.38 |
| standard deviation (mm) | 1.16 | 4.39 | |
| Lung impact | Mean (mm) | 1.42 | 3.60 |
| standard deviation (mm) | 1.09 | 3.55 | |
| Heart impact | Mean (mm) | 0.52 | 0.82 |
| standard deviation (mm) | 0.31 | 0.65 | |
| Correlation between heart and tumor motion | Mean | 0.48 | 0.48 |
| standard deviation | 0.22 | 0.18 |
We found that the cardiac motion and the tumor motion were correlated in the lateral direction for 18 out of 30 patients, with 5 highly correlated; the motion was correlated in the cranial-caudal direction in 20 out of 30 patients, with 6 highly correlated. No significant dependence was found between the cardiac impact (in correlation coefficient) and the tumor location in terms of lobe (left or right, upper lobes or not), or with the stage of the malignancy. However, there was a significant difference between the amplitude of the cardiac impact to tumors in the left and right lobes. The cardiac impact to the left lung tumor was larger in amplitude. The correlation co-efficient and the probability for non-correlation were summarized in Table 2.
Table 2.
Comparison of cardiac impact between left and right lung tumors. Statistical analysis on correlation between tumor motion and cardiac impact, and the comparison of the cardiac impact to left and right lung tumor and are the correlation coefficients between cardiac impact and the tumor motion. The mean and standard deviation of cardiac impact, correlation coefficient for lateral motion, and correlation coefficient for sup–inf motion were calculated for left and right lungs respectively. The results indicated that the cardiac impact magnitude is different for left and right lungs, but the correlation of cardiac impact to tumor motion is indifferent for left and right lung tumors. Patients who had no correlation between cardiac and tumor motion (7 out of 30) were not included in the table.
| Patient | Lobe | Cardiac impact (in mm) | No. of correlation in lateral | No. of correlation in sup–inf | ||
|---|---|---|---|---|---|---|
| All right (n = 13) | 0.65 ± 0.45 | 0.45 ± 0.25 | 11 | 0.38 ± 0.13 | 12 | |
| All left (n = 10) | 1.20 ± 0.68 | 0.34 ± 0.23 | 7 | 0.51 ± 0.28 | 8 | |
| Left vs. right | p < 0.05 | p > 0.05 | p > 0.05 |
No strong correlation was found between the heart impact and the tumor distance from the heart (central or peripheral), either. This can be partly explained by the fact that some central tumors, although geometrically closer to the heart, were attached to surrounding stable tissues.
The heart impact was quantified and evaluated as a function of the overall tumor motion amplitude. It was observed that the heart impact becomes more critical for patients with smaller tumor motion magnitude, as shown in Fig. 2.
Fig. 2.
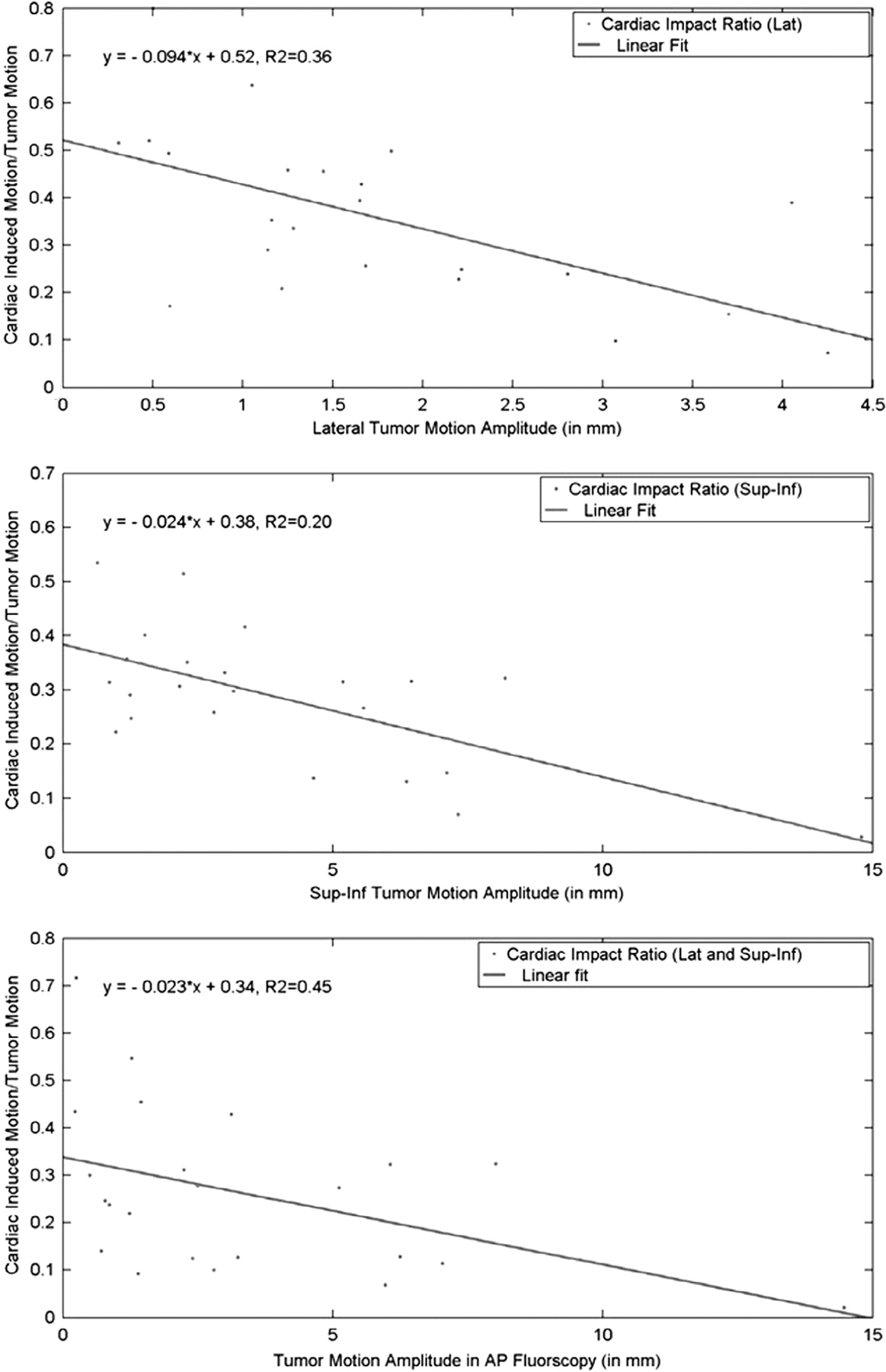
The ratio between the cardiac-motion-induced and the respiration-induce tumor motion as a function of the magnitude of the overall tumor motion. Linear fitting equations are shown for the lateral (top), longitudinal (middle), and the total (bottom) motion respectively.
Compared to the original 4DCT-based ITVs, the redefined ITVs, which were generated with the potential maximal tumor movement, exhibited an average 9% increase in volume (ranging from −19.4% to 55.7%), and increased by more than 5% (ranging from 5.1% to 55.7%) for 15 patients. The prescription dose coverage decreased from 95% of PTV to 84.5% (Fig. 3) for one patient, indicating that the cardiac motion might potentially introduce non-trivial dosimetric effects during thoracic tumor radiotherapy.
Fig. 3.
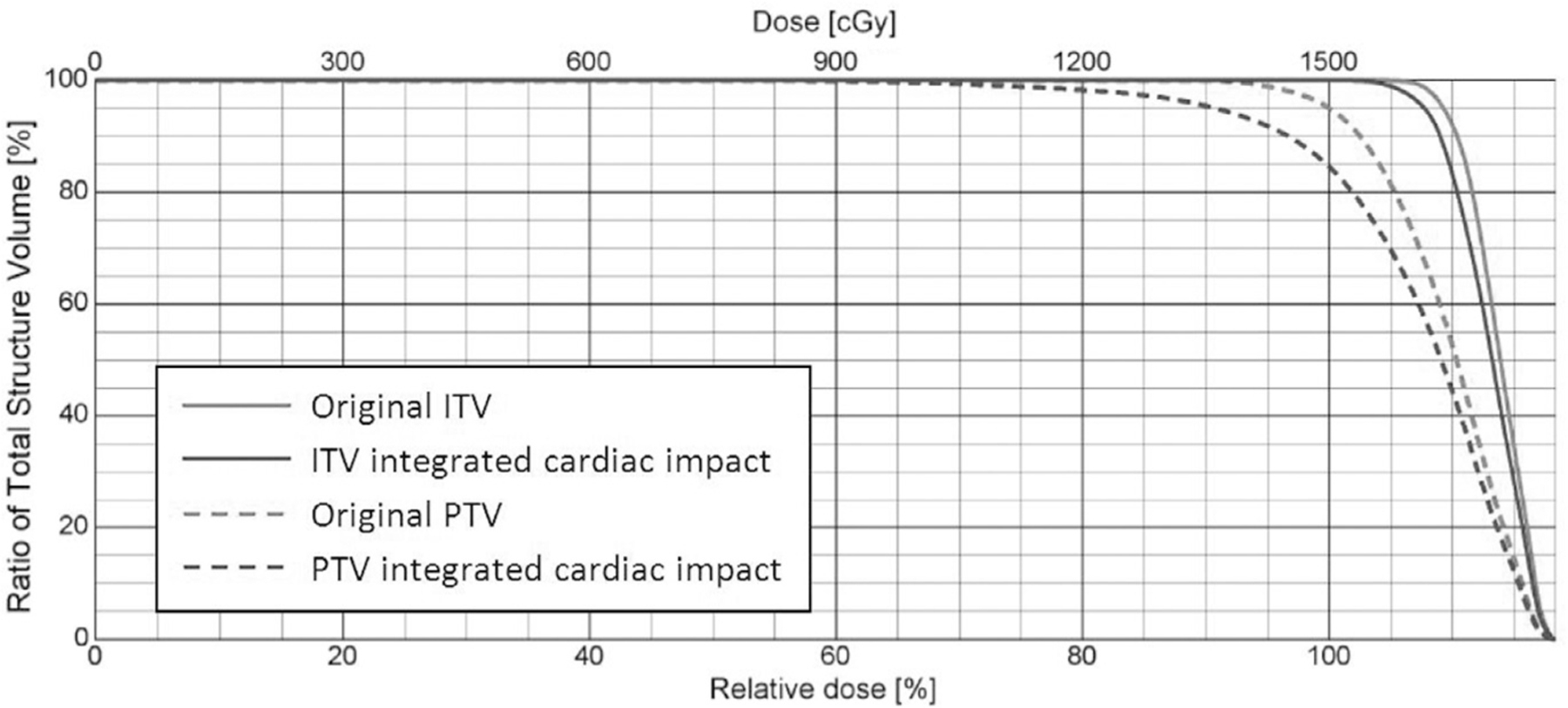
DVH of a simulated SBRT plan with different ITV and PTV definitions. Gray histograms correspond to the PTV and ITV without considering the cardiac induced motion; black histograms correspond to the revised ITV and PTV, taking the cardiac impact into account. Solid lines are histograms of ITVs, and dashed lines are histograms of PTVs.
Discussion
We investigated the correlation and impact of the cardiac motion to lung tumor motion in patients under radiotherapy. We used DIR between fluoroscopy image frames to retrieve the actual tumor motion without the aid of implanted fiducials. The cardiac motion impact on the tumor motion was quantitatively evaluated for 30 patients, and the result was used to simulate and evaluate potential clinical relevance of the cardiac motion in EBRT. The study showed that by implementing DIR between fluoroscopy frames we could generate accurate and comprehensive tumor motion signals. The cardiac impact could be effectively separated from the tumor motion signal using the frequency filtering technique. The cardiac impact was significant relative to the overall tumor motion and its significance increased as the tumor motion amplitude decreased. Finally, ignoring the cardiac impact might cause inadequate dose coverage for SBRT patients.
The accuracy of DIR has a critical impact on the reliability of the motion analysis. In [17] the error for DIR of lung tumor was analyzed and quantified. It was found that the registration error was proportional (approximately less than 10% in [17]) to the amplitude of the displacement of the target. The finding in [17] supported the use of registration propagation, which consisted of a series of DIR between neighboring fluoroscopy frames with relatively less displacement, for motion signal retraction. Assuming the registration error between any two pairs of images was independent, it was concluded that the accumulative registration error in our study was less than 0.16 mm and 0.09 mm for respiratory-induced and cardiac-induced tumor motion respectively. Therefore, the accumulative error of registration propagation in motion analysis was ignorable compared to the amplitude of the motion signal (average 3.8 mm for respiratory motion, 1.0 mm for cardiac impact). Please refer to the Supplementary document for more mathematical details.
The overall lung tumor motion calculated based on fluoroscopy imaging was quite consistent with previous literature in the cranial-caudal direction, but was statistically larger than the amplitude reported by Seppenwoolde and colleagues [10] in the lateral direction. After decoupling the cardiac impact, the lateral respiratory tumor motion observed on fluoroscopy images was statistically of the same magnitude as derived from 4DCT. This implied that the cardiac motion might not be fully captured in 4DCT as limited by the 4DCT sampling frequency, and fluoroscopy with higher sampling frequency fully captured the cardiac impact in both lateral and sup–inf directions and should be utilized for the quantification of the cardiac impact.
Quantitative analysis showed that the cardiac-induced tumor motion was in the fluoroscopy image domain, and it became more critical as the amplitude of the overall motion of the tumor decreased, as shown in Fig. 2. Based on the linear fittings, the cardiac impact accounts for about 30% of the total tumor motion at the amplitude of 3 mm (in either direction). For most lung tumor patients (usually tumor motion is less than 5 mm under external compression), the cardiac impact accounts for a significant amount of the total tumor motion and cannot be ignored when defining target volumes. By using only 4DCT in treatment planning, there is a possibility that the cardiac impact is not completely included in the ITV.
Our findings were different from what was reported by Seppenwoolde and colleagues [10]. First, we found the cardiac impact exists in 23 out of 30 patients, while in [10] the cardiac impact was detected on 7 out of 20 patients. In [10] the cardiac impact was detected mostly on tumor attached to the aorta but we found that there was no significant difference in correlations between the cardiac impact to central and peripheral tumors. Second, in [10] the cardiac impact was reported to be mostly in the lateral direction, but we found that the cardiac-induced motion was statistically similar in sup–inf (average 0.8 mm) and the lateral direction (average 0.5 mm). Finally in [10] there was no discussion on the relationship between the cardiac impact and the overall tumor motion, but we found there was an inversely proportional relationship between the two. It should be noted that the values are relatively small (0.36 for lateral motion, 0.2 for sup–inf motion, and 0.45 for total cardiac impact) for all linear fittings in Fig. 2. Therefore we can conclude that although there is a relationship between the cardiac impact and the total tumor motion, the actual magnitude of the cardiac impact cannot be accurately predicted using the detected total tumor motion. For each individual patient, the actual cardiac impact has to be retrieved from fluoroscopy images.
By taking into account the cardiac induced motion from fluoroscopy, the ITV volumes had an average increase of 9%. There were two reasons for the increase: (1) the fluoroscopy-captured tumor motion might be larger than the tumor motion in 4DCT for some patients, because of the inclusion of cardiac impact; and (2) due to the independence between cardiac motion and respiration there existed a possibility that at certain time points the cardiac and respiratory impacts were in-phase and the maximal possible tumor motion became the summation of the two individual influences. Due to uncertainties, the volumetric percentage change ranged from −19.4% to 55.7% for original ITVs. However, an average increase of 9% in ITV volume indicated that the cardiac impact information might be only partially captured using the current 4DCT technique.
For conventional radiation treatments, the volume increase of the redefined PTV may not lead to critical clinical consequences in terms of target coverage. For conventional treatment plans the margin size was usually much larger than the amplitude of the cardiac motion [15,16], making the cardiac impact less significant. In addition, the dosimetry impact caused by the in-phase of the cardiac motion with the respiratory motion may average out over many fractions.
However, for SBRT treatments, cardiac motion might introduce a significant dosimetric alteration under certain circumstances and the effect might become more pronounced. SBRT consists of fewer fractions and has a narrower setup margin to reflect strict immobilization, breathing compression, and image guided patient setup and motion monitoring. Narrow margins make the dosimetry more susceptible to the target motion uncertainty caused by the cardiac motion. As shown in Fig. 3, the cardiac motion might potentially decrease the target volume dose coverage by more than 10% and lead to significant cold spots inside the target volume (the minimum PTV dose was as low as 42% of the prescription dose).
Conclusion
The correlation between the cardiac motion and the tumor motion is confirmed based on the correlation study through motion signal processing in the frequency domain. The cardiac motion impact is independent of the respiration, and is relatively more significant for patients with less tumor motion. For SBRT patients, the cardiac induced target motion may need to be taken into consideration to avoid potential dose coverage compromise. More studies are needed to precisely quantify the dosimetric impacts caused by cardiac motion.
Supplementary Material
Acknowledgement
The study was supported by a Varian Medical Systems research grant.
Footnotes
Conflict of Interest
There is no conflict of interest to be disclosed.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2014.08.007.
References
- [1].Shimizu S, Shirato H, Kagei K, et al. Impact of respiratory movement on the computed tomographic images of small lung tumors in three-dimensional (3D) radiotherapy. Int J Radiat Oncol Biol Phys 2000;46:1127–33. [DOI] [PubMed] [Google Scholar]
- [2].Ozhasoglu C, Murphy MJ. Issues in respiratory motion compensation during external-beam radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:1389–99. [DOI] [PubMed] [Google Scholar]
- [3].Keall PJ, Joshi S, Vedam SS, et al. Four-dimensional radiotherapy planning for DMLC-based respiratory motion tracking. Med Phys 2005;32:942–51. [DOI] [PubMed] [Google Scholar]
- [4].Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874–900. [DOI] [PubMed] [Google Scholar]
- [5].Sharp GC, Jiang SB, Shimizu S, et al. Prediction of respiratory tumour motion for real-time image-guided radiotherapy. Phys Med Biol 2004;49:425–40. [DOI] [PubMed] [Google Scholar]
- [6].Mageras GS, Yorke E, Rosenzweig K, et al. Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system. J Appl Clin Med Phys 2001;2:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prescribing, recording and reporting photon beam therapy. ICRU 62 (Supplement to ICRU report 50); 1999. [Google Scholar]
- [8].Pan T, Lee TY, Rietzel E, et al. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med Phys 2004;31:333–40. [DOI] [PubMed] [Google Scholar]
- [9].Nyquist H Certain topics in telegraph transmission theory. Trans AIEE 1928;47:617–44. [Google Scholar]
- [10].Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002;53:822–34. [DOI] [PubMed] [Google Scholar]
- [11].Berbeco RI, Nishioka S, Shirato H, et al. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol 2005;50:3655–67. [DOI] [PubMed] [Google Scholar]
- [12].Barrett Julia F, Keat Nicholas. Artifacts in CT: recognition and avoidance. Radiographics 2004;24:1679–91. [DOI] [PubMed] [Google Scholar]
- [13].Chen T, Kim S, Goyal S, et al. Object-constrained meshless deformable algorithm for high speed 3D nonrigid registration between CT and CBCT. Med Phys 2010;37:197–210. [DOI] [PubMed] [Google Scholar]
- [14].Chen T, Jabbour S, Qin S, et al. Objected-constrained registration and manifold learning: a new patient setup approach in image guided radiation therapy of thoracic cancer. Med Phys 2013;40:041710–1–041710–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wolthaus JW, Sonke JJ, van Herk M, et al. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys 2008;70:1229–38. [DOI] [PubMed] [Google Scholar]
- [16].van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1121–35. [DOI] [PubMed] [Google Scholar]
- [17].Li S, Glide-Hurst C, Lu M, et al. Voxel-based statistical analysis of uncertainties associated with deformable image registration. Phys Med Biol 2013;58:6481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


