Abstract
Purpose:
For inoperable stage I (T1-T2N0) small cell lung cancer (SCLC), national guidelines recommend chemotherapy with or without conventionally fractionated radiation therapy. The present multi-institutional cohort study investigated the role of stereotactic ablative radiation therapy (SABR) for this population.
Methods and Materials:
The clinical and treatment characteristics, toxicities, outcomes, and patterns of failure were assessed in patients with histologically confirmed stage T1-T2N0M0 SCLC. Kaplan-Meier analysis was used to evaluate the survival outcomes. Univariate and multivariate analyses identified predictors of outcomes.
Results:
From 24 institutions, 76 lesions were treated in 74 patients (median follow-up 18 months). The median age and tumor size was 72 years and 2.5 cm, respectively. Chemotherapy and prophylactic cranial irradiation were delivered in 56% and 23% of cases, respectively. The median SABR dose and fractionation was 50 Gy and 5 fractions. The 1- and 3-year local control rate was 97.4% and 96.1%, respectively. The median disease-free survival (DFS) duration was 49.7 months. The DFS rate was 58.3% and 53.2% at 1 and 3 years, respectively. The median, 1-year, and 3-year disease-specific survival was 52.3 months, 84.5%, and 64.4%, respectively. The median, 1-year, and 3-year overall survival (OS) was 17.8 months, 69.9%, and 34.0% respectively. Patients receiving chemotherapy experienced an increased median DFS (61.3 vs 9.0 months; P=.02) and OS (31.4 vs 14.3 months; P=.02). The receipt of chemotherapy independently predicted better outcomes for DFS/OS on multivariate analysis (P=.01). Toxicities were uncommon; 5.2% experienced grade ≥2 pneumonitis. Post-treatment failure was most commonly distant (45.8% of recurrence), followed by nodal (25.0%) and “elsewhere lung” (20.8%). The median time to each was 5 to 7 months.
Conclusions:
From the findings of the largest report of SABR for stage T1-T2N0 SCLC to date, SABR (≥50 Gy) with chemotherapy should be considered a standard option.
Summary
For inoperable stage I (T1-T2N0) small cell lung cancer, the national guidelines have recommended chemotherapy with or without conventionally fractionated radiation therapy. The findings from present multi-institutional cohort study demonstrated that stereotactic ablative radiation therapy, together with chemotherapy, provides appropriate local control and survival and should thus be considered for these patients.
Introduction
Stereotactic body radiation therapy (SBRT), also termed stereotactic ablative radiation therapy (SABR), results in high local control (LC) with minimal treatment morbidity and is currently the standard of care for the management of inoperable early-stage non—small cell lung cancer (NSCLC) (1–3). Recent prospective data from surgery-eligible patients have suggested potential equipoise, or even improved outcomes, with SABR compared with surgery (4–6).
However, for resectable stage I (non—nodal) small cell lung cancer (SCLC), the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines currently recommend lobectomy and mediastinal lymph node dissection, with adjuvant chemoradiation if node-positive and chemotherapy if node-negative (7). However, for nonoperative patients (most commonly medically inoperable or those refusing surgery), chemotherapy with or without conventionally fractionated RT is the current NCCN recommendation (7). Although SABR—associated with high LC and patient convenience and low toxicity and costs—could effectively treat these T1-T2N0 SCLC lesions, a relative lack of published experience using this approach is available, with only 3 case series reported, none with >8 patients (8–10).
Because of the superior local control of SABR compared with conventionally fractionated RT for NSCLC (11, 12) and the improved outcomes of RT and chemotherapy for limited-stage SCLC (13), it is important to determine whether current guidelines should be modified to include SABR in the T1-T2N0 SCLC patient population (14). This is an especially pertinent issue as of late, with the recent approval of low-dose computed tomography screening for lung cancer in eligible patients (15, 16). Hence, although quite debatable, a substantial increase could occur in the diagnosis of early-stage NSCLC and SCLC, just as was observed in the National Lung Screening Trial (17, 18).
To determine the role of SABR in the management of stage I SCLC, we compiled the cumulative experience from 24 institutions to examine the survival outcomes, toxicities, and patterns of failure after SABR in this population.
Methods and Materials
The present study was an institutional review board—approved multi-institutional analysis for biopsy-proven, primary stage I SCLC. All 24 institutions contributed every such patient from their prospectively collected SABR databases or through retrospective medical record review of SABR patients, using a standardized data collection format. All data were reviewed by 3 investigators. Patients with a history of SCLC were excluded, with the exception of a single patient with limited-stage SCLC who had been without evidence of disease for 12 years before developing a new solitary lung nodule pathologically confirmed to be SCLC. Two cases of presumed synchronous primaries in different lobes that were pathologically confirmed were included, without evidence of nodal or distant metastasis. Although heterogeneity in treatment at each institution was present, SABR was delivered in 3 to 5 fractions, and all protocols accounted for respiratory motion and used principles of image-guided RT. The individual institutions determined the specifics of post-treatment imaging, and the responses were evaluated using the Response Evaluation Criteria in Solid Tumors. The collected information included details of the demographic data, oncologic history, initial and/or ancillary workup, tumor characteristics, age at diagnosis and at SABR completion, treatment details, time to failure (and corresponding locations), and time to death. Toxicities were assessed by the treating physician at the initial occurrence using the Common Terminology Criteria for Adverse Events, version 4.0.
Data analysis was performed using MP14 statistical software (StataCorp, College Station, TX). Fisher’s exact test was used to assess measures of association in frequency tables. The Wilcoxon rank-sum test was used to evaluate the equality of the population distributions. The survival function was performed using Kaplan-Meier estimates. The log-rank test was used to examine equality across groups. P<.05 was considered statistically significant. Statistical tests were based on a 2-sided significance level.
The survival time was calculated from SABR completion to the first occurrence of the considered event (eg, recurrence). Overall survival (OS) was defined as the interval from SABR completion to death from any cause. Disease-specific survival (DSS) was defined as the interval from SABR completion to death from SCLC. Hence, patients who died of non-SCLC causes were considered dead for the OS curves but censored for the DSS curves. Disease-free survival (DFS) was defined as the interval from SABR completion to the first recurrence of disease.
The patterns of failure were categorized as local (in-field only), nodal (regional, including supraclavicular), and distant. However, in this comorbid population, new lung lesions that develop during follow-up after SABR are often not biopsied and are considered either second primaries or recurrences (ie, following patterns of NSCLC). These isolated or so-called oligo lung lesions are often treated definitively; thus, the term “elsewhere lung failure” has been increasingly used, recognizing that innumerable pulmonary nodules still represent a type of distant failure.
The Cox proportional hazards model was used for multivariate analysis to assess the effect of several factors of significance on the endpoints. All factors with P ≤.25 on univariate analysis were included in the multivariable analysis, with each factor eliminated in a stepwise manner until the most significant variables were identified. The Wald test was used to assess the role of covariates in the model.
Results
Patients and treatment
A total of 76 lesions were treated in 74 patients at 24 institutions from 2005 to 2015. The clinical characteristics of this patient cohort are summarized in Table 1. The median age was 72 years, the median smoking history was 50 pack-years, and 25.7% of the patients had a history of previous noncutaneous malignancies. Of the 76 lesions, 67 (88.2%) were categorized as medically inoperable, nearly all because of cardiopulmonary comorbidities. The median tumor size was 2.2 cm (range 0.7–7.2). The lesions were predominantly T1 (56 of 76; 73.7%) or T2a (15 of 76; 19.7%). At baseline, 52 of 74 patients (70.3%) had an Eastern Cooperative Oncology Group performance status of 0 to 1.
Table 1.
Clinical characteristics of the study population
| Parameter | n (%)* |
|---|---|
| Age at diagnosis (y) | |
| Median | 72 |
| Range | 44–105 |
| Ethnicity | |
| White | 66 (89.2) |
| African-American | 6 (8.1) |
| Other | 2 (2.7) |
| Gender | |
| Male | 37 (50.0) |
| Female | 37 (50.0) |
| Smoking history (pack-years) | |
| Median | 50 |
| Range | 10–162 |
| Persistent smoking at last follow-up | |
| Yes | 10 (13.5) |
| No | 64 (86.5) |
| History of malignancy † | |
| None | 55 (74.3) |
| Lung, early-stage NSCLC | 10 (13.5) |
| Lung, locally advanced NSCLC | 4 (5.4) |
| Lung, SCLC‡ | 3 (4.1) |
| Genitourinary | 3 (4.1) |
| Head and neck | 1 (1.4) |
| Lymphoma | 2 (2.7) |
| Breast | 2 (2.7) |
| Melanoma | 1 (1.4) |
| Malignancy within 1 y of diagnosis | |
| Yes | 8 (10.8) |
| No | 66 (89.2) |
| Previous thoracic irradiation | |
| Yes | 4 (5.4) |
| No | 70 (94.6) |
| Indication for SABR | |
| Medically inoperable | 67 (88.2) |
| Other active cancer | 4 (5.3) |
| Refused surgery | 3 (3.9) |
| Other/unknown | 2 (2.6) |
| Lobe of lung | |
| Right upper | 21 (27.6) |
| Left upper | 19 (25.0) |
| Right lower | 15 (19.7) |
| Left lower | 12 (15.8) |
| Right middle | 6 (7.9) |
| Unknown | 3 (3.9) |
| Location | |
| Peripheral | 63 (82.3) |
| Central | 13 (17.1) |
| Lesion size (cm) | |
| Median | 2.2 |
| Range | 0.7–7.2 |
| Lesion group | |
| ≤1 cm | 6 (7.8) |
| 1.1–2.0 cm | 29 (38.2) |
| 2.1–3.0 cm | 21 (27.6) |
| 3.1–4.0 cm | 14 (18.4) |
| 4.1–5.0 cm | 1 (1.3) |
| >5.1 cm | 5 (6.6) |
| AJCC clinical T stage | |
| T1a | 35 (46.1) |
| T1b | 21 (27.6) |
| T2a | 15 (19.7) |
| T2b | 4 (5.3) |
| T3 | 1 (1.3) |
| Baseline staging PET performed | |
| Yes | 67 (90.5) |
| No | 7 (9.5) |
| SUVmax on pre-SABR PET | |
| Median | 7.6 |
| Range | 1.3–27.7 |
| Mediastinal nodal sampling | |
| Performed | 19 (25.0) |
| Not performed | 57 (75.0) |
| ECOG performance status at diagnosis | |
| 0 | 16 (21.1) |
| 1 | 36 (47.4) |
| 2 | 14 (18.4) |
| 3 | 9 (11.8) |
| Unknown | 1 (1.3) |
Abbreviations: AJCC = American Joint Committee on Cancer; ECOG = Eastern Cooperative Oncology Group; NSCLC = non–small cell lung cancer; PET = positron emission tomography; SABR = stereotactic body radiation therapy; SCLC = small cell lung cancer; SUVmax = maximum standard uptake value.
Parameters applicable to either the number of patients (n=74) or number of lesions (n=76).
Data do not sum to 100% owing to patients with synchronous or metachronous neoplasms.
Two patients had synchronous lesions in different lobes without any evidence of nodal or distant disease; hence, the lesions were treated as separate primaries. One patient had history of limited-stage small cell carcinoma in another lung lobe and had been without evidence of disease for 12 years.
The treatment parameters for this population are listed in Table 2. The most frequent SABR dose and fractionation was 50 Gy in 5 fractions (36.8%), followed by 50 Gy in 4 fractions (23.9%), and 54 Gy in 3 fractions (10.5%). The median total SABR dose was 50 Gy (range 30–60). Chemotherapy was administered to 45 patients (59.2%), not given to 27 (35.5%), and likely to have been given (although unconfirmed) in 4 patients (5.3%). The most common chemotherapy protocol was as follows: a platinum agent and etoposide (95.9%), administered for 4 cycles (61.2%), after completion of SABR (67.3%). Prophylactic cranial irradiation was performed in 17 patients (23.0%), all of whom were treated at a dose of 25 Gy in 10 fractions.
Table 2.
Treatment characteristics of the study population
| Parameter | n (%) |
|---|---|
| SABR dose and fractionation | |
| 50 Gy in 5 fractions | 28 (36.8) |
| 50 Gy in 4 fractions | 18 (23.9) |
| 54 Gy in 3 fractions | 8 (10.5) |
| 60 Gy in 5 fractions | 6 (7.9) |
| 60 Gy in 3 fractions | 5 (6.6) |
| 48 Gy in 4 fractions | 5 (6.6) |
| Other | 6 (7.9) |
| Total SABR dose (Gy) | |
| Median | 50 |
| Range | 30–60 |
| SABR dose group | |
| ≥60 Gy | 12 (15.8) |
| 50–59 Gy | 55 (72.4) |
| 40–49 Gy | 8 (10.5) |
| <40 Gy | 1 (1.3) |
| Biologically effective dose* (Gy) | |
| Median | 112.5 |
| Range | 72–180 |
| Biologically effective dose group | |
| <100 Gy | 3 (3.9) |
| 100–129 Gy | 53 (69.7) |
| 130–149 Gy | 7 (9.2) |
| ≥150 Gy | 13 (17.1) |
| Receipt of PCI † | |
| Yes | 17 (23.0) |
| No | 53 (71.6) |
| Unknown | 4 (5.4) |
| Receipt of chemotherapy | |
| Yes | 45 (59.2) |
| No | 27 (35.5) |
| Unknown, but most likely‡ | 4 (5.3) |
| Type of chemotherapy | |
| Cisplatin/etoposide | 28 (57.1) |
| Carboplatin/etoposide | 19 (38.8) |
| Other/unknown | 2 (4.1) |
| Cycles of chemotherapy | |
| 1 | 3 (6.1) |
| 2 | 4 (8.2) |
| 3 | 4 (8.2) |
| 4 | 30 (61.2) |
| 6 | 4 (8.2) |
| 8 | 1 (2.0) |
| Unknown | 3 (6.1) |
| Timing of chemotherapy | |
| After SABR | 33 (67.3) |
| Before SABR | 12 (24.5) |
| Before SABR or concurrently | 2 (4.1) |
| Before SABR, concurrently, or after SABR | 1 (2.0) |
| Unknown | 1 (2.0) |
| Primary tumor response § | |
| Complete response | 19 (25.0) |
| Partial response | 29 (38.2) |
| Stable | 13 (17.1) |
| Progression | 3 (3.9) |
| Unknown | 12 (15.8) |
| Size of residual lesion (cm) | |
| Median | 1.3 |
| Range | 0.4–3.5 |
| SUVmax of residual lesion | |
| Median | 1.9 |
| Range | 0–6.1 |
Abbreviations: SABR = stereotactic body radiation therapy; PCI = prophylactic cranial irradiation; SUVmax = maximum standardized uptake value.
Assuming an α/β ratio of 10.
PCI, when delivered, was administered at a dose of 25 Gy in 10 fractions in all cases.
In these patients, chemotherapy was most probably administered but could not be corroborated definitively owing to loss of follow-up.
In accordance with Response Evaluation Criteria in Solid Tumors.
Clinical outcomes
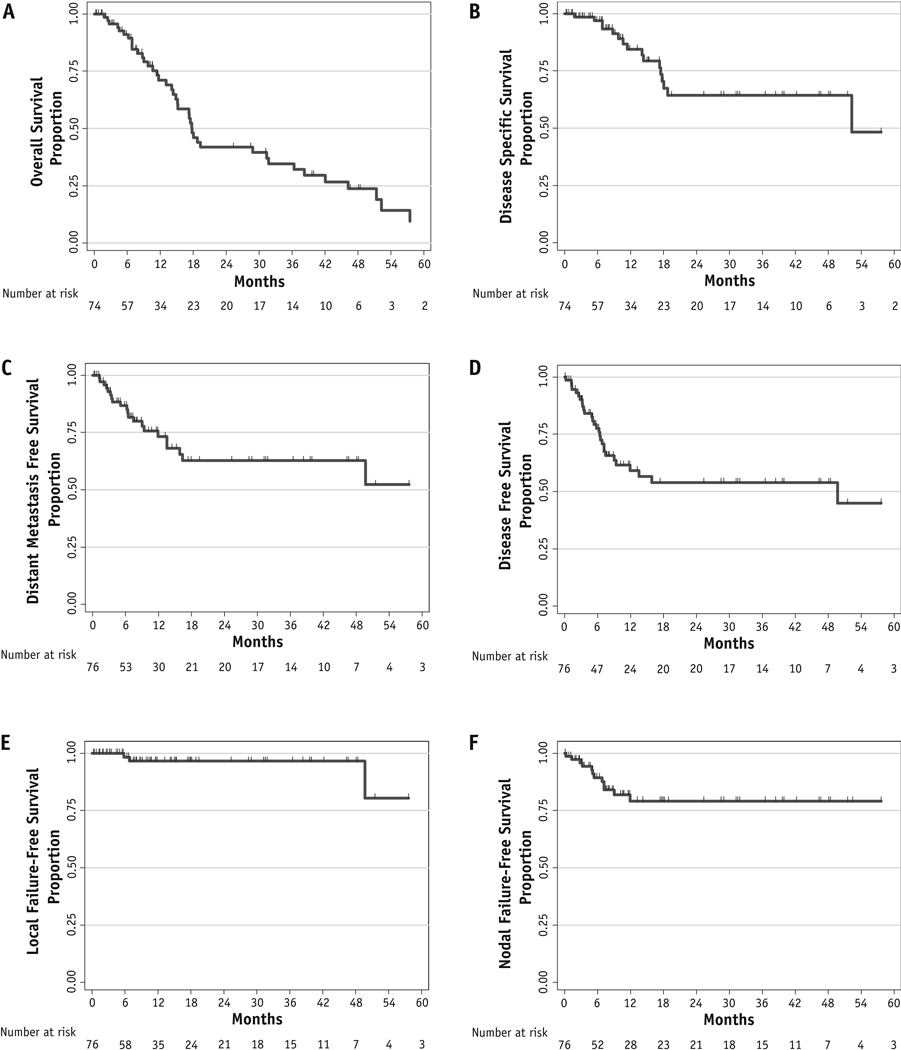
The median follow-up period was 18 months (range 0.1–62.3) after SABR completion. A complete response occurred in 19 lesions (25.0%), a partial response in 29 (38.2%), stable disease in 13 (17.1%), and progression in 3 (3.9%). Figure 1 presents the Kaplan-Meier survival analysis. The 1- and 3-year LC rate was 97.4% and 96.1%, respectively; the 1- and 3-year local failure-free survival rates were both 96.6%. The distant metastasis-free survival rate was 73.2% and 62.7% at 1 and 3 years, respectively. The median DFS period was 49.7 months, which corresponded to a DFS rate of 59.1% and 54.0% at 1 and 3 years, respectively. The median DSS period was 52.3 months, for a rate of 84.4% and 64.4% at 1 and 3 years, respectively. The 1- and 3-year OS rate was 71.1% and 34.6%, respectively, and the median OS period was 17.8 months.
Fig. 1.
Kaplan-Meier curves for the cohort illustrating overall survival (A), disease-specific survival (B), distant metastasis-free survival (C), disease-free survival (D), local failure-free survival (E), and nodal failure-free survival (F).
To identify the factors associated with several endpoints, univariate analysis was performed (Tables E1-E5; available online at www.redjournal.org). After adjustment for potential confounders, multivariate analysis determined several factors associated with various endpoints (Table 3). Receipt of chemotherapy was an independent predictor of both improved DFS (hazard ratio [HR] 0.37, 95% confidence interval [CI] 0.17–0.82; P=.01) and OS (HR 0.41, 95% CI 0.21–0.80; P=.01). The median OS for patients who received and did not receive chemotherapy was 31 and 14 months, respectively. Tumor size >2 cm was also associated with worse OS (HR 2.80, 95% CI 1.32–5.94, P=.01). Finally, when examining the total RT dose as a continuous variable, an association with greater local control was observed (HR 0.71, 95% CI 0.54–0.94; P=.02).
Table 3.
Multivariate analysis using Cox proportional hazards model
| Parameter | HR | 95% CI | P value |
|---|---|---|---|
| OS | |||
| Receipt of chemotherapy (yes vs no) | 0.41 | 0.21–0.80 | .010 |
| Tumor size (>2 cm vs ≤2 cm) | 2.80 | 1.32–5.94 | .008 |
| Presence of nodal failure (yes vs no) | 3.88 | 1.73–8.75 | .001 |
| DFS | |||
| Receipt of chemotherapy (yes vs no) | 0.37 | 0.17–0.82 | .014 |
| Response of primary tumor (partial vs complete) | 3.61 | 1.20–10.87 | .023 |
| LC | |||
| Total radiation dose (continuous variable) | 0.71 | 0.54–0.94 | .018 |
Abbreviations: CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; LC = local control; OS = overall survival.
Statistically significant variables associated with OS, DFS, and LC are listed.
At the latest available follow-up examination, 43 patients (58.1%) had died. The cause of death for 10 patients (23.3%) was unknown; 16 patients (37.2%) had died of SCLC, 8 patients (18.6%) of cardiopulmonary causes, 3 (7.0%) of other malignancies, and 6 (14.0%) of other causes.
Patterns of failure
The patterns of failure were categorized into 4 groups: local (in-field), nodal, elsewhere lung (including out-of-field), and distant (Table 4). Of the 74 patients, 32 (43.2%) experienced a total of 46 recurrences. At the last follow-up visit, local recurrence had developed in 4 patients (8.7% of all failures). In all cases, these local recurrences were synchronously accompanied by other recurrences. In contrast, distant metastases were the most common failure, accounting for nearly one half (45.7%) of post-SABR failures. The most frequent metastases were to the liver (46.4%), bone (25.0%), and brain (14.3%). None of the 4 patients with treatment failure in the brain had received prophylactic cranial irradiation. Nodal and elsewhere lung failures represented 26.1% and 19.6% of all recurrences, respectively, and were often associated with concomitant recurrences in other locations. The median time to distant, nodal, and elsewhere lung failures in all patients was 5 to 10 months for each failure type (Table 4).
Table 4.
Patterns of failure of the study population
| Incidence and proportions | Location* | Median (range) time to failure (mo) | Concomitant failure |
|---|---|---|---|
| Local failure (n=4) | |||
| 5.3% of all lesions 8.7% of all failures |
In-field (n=4) | 28.2 (5.8–61.3) | Elsewhere lung (n=2) Distant (n=1) Nodal and elsewhere lung (n=1) |
| Nodal failure (n=12) | |||
| 15.8% of all lesions 26.1% of all failures |
Hilum (n=7) Mediastinum (n=7) Supraclavicular (n=2) |
5.2 (0.2–11.9) | Isolated nodal (n=5) Distant (n=3) Distant and elsewhere lung (n=3) Elsewhere lung (n=1) |
| Elsewhere lung failure (n=9) | |||
| 11.8% of all lesions 19.6% of all failures |
Ipsilateral lobe (n=1) Ipsilateral lung (n=2) Contralateral lung (n=3) Unknown (n=3) |
10.2 (0.4–61.3) | Isolated elsewhere lung (n=2) Nodal and distant (n=3) Distant (n=1) Local (n=2) Local and nodal (n=1) |
| Distant failure (n=21) | |||
| 27.6% of all lesions 45.7% of all failures |
Liver (n=13) Bone (n=7) Brain (n=4) Adrenal (n=2) Pleura (n=2) |
6.4 (1.2–49.7) | Isolated distant (n=13) Nodal (n=3) Elsewhere lung (n=1) Nodal and elsewhere lung (n=3) Local (n=1) |
Totals might not sum to those of the first column because many patients developed synchronous failure.
Toxicities
Overall, SABR was associated with limited toxicities. Of the 76 lesions, 9 cases (11.8%) of grade 1, 3 (3.9%) of grade 2, and 1 (1.3%) of grade 3 pneumonitis developed. Additionally, 1 case each of grade 1 dermatitis and grade 2 fatigue occurred. Also, 4 cases (5.3%) of chest wall pain (3 with grade 2 and 1 with an unknown grade) were observed, without any rib fractures. No acute or late esophageal toxicity was observed.
Discussion
Although highly effective for stage T1-T2N0 NSCLC, SABR has rarely been performed or reported for T1-T2N0 SCLC. In the present study, we report the cumulative experience of 74 patients with stage I SCLC treated with SABR from 24 institutions. We have demonstrated that SABR is a safe and effective local modality for this patient population, producing high rates of LC and low rates of treatment-related toxicities. Moreover, chemotherapy is essential for these patients.
These results have important ramifications. Regarding inoperable T1-T2N0 SCLC, NCCN has recommended chemotherapy with or without conventionally fractionated RT, although the latter is not recommended for patients with poor performance status (7). However, for this patient population with expected comorbidities, SABR was very well tolerated and resulted in excellent local control. If one may extrapolate from the NSCLC data, SABR is likely superior to conventionally fractionated RT. Even if local control is equivalent, SABR would still be preferred because of the greater patient convenience, low toxicity, and superior cost-effectiveness profiles compared with conventionally fractionated RT (19, 20). Finally, if such patients develop locoregional recurrence after SABR and chemotherapy, chemoradiation could play a role in salvage therapy, although nodal failure was a strong predictor of worse OS on multivariate analysis. Although the idea of prophylactic mediastinal nodal irradiation is a possibility, no supportive data for T1-T2N0 SCLC are currently available. Finally, the median time to regional or distant recurrence in the present series was 5 to 10 months, less than the corresponding data for NSCLC (range 9–13 months), which could necessitate post-treatment imaging studies earlier than performed for NSCLC (21).
Our results compare favorably to the outcomes from published surgical series of medically operable patients (Table 5). The Medical Research Council phase III trial demonstrated a mean survival of 10 and 6.5 months in the RT and surgical arms, respectively, although the patients in that trial likely had a greater disease volume than the patients in our series (22). In the Lung Cancer Study Group 832 randomized trial, the median survival for those with clinical T1-T2N0 disease was 21 to 24 months (23). The retrospective surgical data from a patient population similar to that in the present study demonstrated 1- and 3-year OS of roughly 80% and 60% in clinical stage IA patients and 60% and 45% in clinical stage IB patients, respectively (24). Another study from Johns Hopkins demonstrated 1- and 3-year OS of 82% and 50%, respectively, for stage I SCLC patients undergoing surgical resection who had received adjuvant chemotherapy (25). Their findings are similar to the data for the corresponding patients in the present study (1- and 3-year OS of 80% and 45%, respectively) and to another relatively recent retrospective series with a 1- and 3-year OS of ~70% and 55%, respectively (26). Taken together, although the vast majority of the patients in the present study were medically inoperable and only a few had undergone pathologic mediastinal staging, the survival was surprisingly comparable to that from surgical reports of operable patients. However, it should not be discounted that less-sensitive imaging studies in older reports might have missed patients with occult distant metastases. Although many of the reported surgical series did not examine DSS, the median 52 months we have reported is quite encouraging and is indicative that many patients died of causes other than SCLC.
Table 5.
Comparison of survival parameters between our study and surgical series
| Surgical series | Study type | Parameter in surgical series | Parameter in present study |
|---|---|---|---|
| Medical Research Council (22) | Randomized | Mean OS 10.5 mo (RT arm) Mean OS 6 mo (surgery arm) |
Median OS 18 mo |
| Lung Cancer Study Group 832 (23) | Randomized | Median OS 21–24 mo | Median OS 18 mo |
| Osaka, Japan (24) | Retrospective | cIA: 1-y OS 80%; 3-y OS 60% cIB: 1-y OS 60%; 3-y OS 45% |
1-y OS 71% 3-y OS 35% |
| Johns Hopkins (25) | Retrospective | Receiving chemotherapy: 1-y OS 82%; 3-y OS 50% | Receiving chemotherapy: 1-y OS 80%; 3-y OS 45% |
| London, England (26) | Retrospective | 1-y OS ∼70%; 3-y OS ∼55% | 1-y OS 71%; 3-y OS 35% |
Abbreviations: cIA, cIB = clinical stage IA, IB, respectively; OS = overall survival; RT = radiation therapy.
Notably, 1 patient who was 105 years old who developed SCLC experienced a durable response after SABR and died of myelodysplastic syndrome nearly 4 years after SABR. This patient also experienced grade 2 chest wall pain and grade 1 radiation pneumonitis. These responses and tolerance suggest that curative treatment is safe and efficacious in the elderly population, a particularly important aspect regarding SABR for any type of lung cancer.
Because of the uncommon presentation of SCLC with early-stage disease and the current propensity of these patients to subsequently undergo either surgical resection or a conventional chemoradiation course, our study is to date the largest series of this type of patients treated with SABR. However, several limitations must be recognized. In addition to its retrospective nature, our study was limited by the likely heterogeneity in treatment details provided from 24 institutions (contributing, on average, 3 patients per institution), including the workup protocols (eg, pathologic nodal staging), type of treatment (eg, receipt of chemotherapy), and post-treatment imaging studies (within 3 months in most cases). Despite being the largest reported series, the small sample size could be subject to certain biases in survival analysis. Despite this, we found that the clinical outcomes for these patients were quite comparable to the previously cited surgical series. Furthermore, it should be remembered that 10 patients had an unknown cause of death, which might have affected the measured values of DSS. Next, the stratification by receipt of chemotherapy omitted 4 patients who had most likely undergone chemotherapy but without confirmation. Additionally, despite the relatively short follow-up time, the clinical endpoints occurred relatively quickly in these data, including recurrences (median 5–10 months), well within the median follow-up period. Moreover, the inclusion of 2 patients with synchronous lesions could also be debated; however, despite 1 distant recurrence, the other patient was free of disease at the last follow-up visit. Next, although many studies did not define a specific classification for “elsewhere lung failure,” it is an important concept when considering that isolated lesions occurring after initial SABR are rarely biopsied and are often treated definitively. Finally, it is also unclear why distant metastasis-free survival was not significantly improved statistically with the use of chemotherapy despite the improvement in DFS and OS. However, the reason is potentially related to the relatively smaller sample size (n=21) of patients with distant metastases in the present study. The relationship between a partial or complete Response Evaluation Criteria in Solid Tumors response and DFS on multivariate analysis, together with the association of RT dose with LC, is noteworthy and could indicate a suboptimal tumor response with lower doses. However, a biologically effective dose response could not be appreciated such has been reported for stage I NSCLC (11). The role of concurrent chemoradiation (the standard for limited-stage SCLC) and of elective mediastinal nodal irradiation also could not be assessed because the vast majority of patients did not undergo these measures. Finally, the present study did not compare outcomes with the current NCCN recommendation for chemotherapy with or without RT. Thus, it would be inaccurate to state that either method is superior to the other.
A similar unpublished series of 64 patients is currently under study in Japan (27). Although 88% of cases were medically inoperable in the present study, the percentage was 66% in the Japanese cohort, implying a population with fewer comorbidities; chemotherapy was delivered to 36 of 64 patients (56%). Despite the unpublished nature of that work, the preliminary results appear similar to those of the present report regarding the benefits of chemotherapy and the relatively greater incidence of distant metastasis (most commonly to the liver). The 2-year OS and DSS have been reported as 76% and 79%, respectively, indicating less death from competing comorbidities compared with the present study. The 2-year LC rate was reported as 89%, and no grade 3 toxicities developed. These preliminary results are anticipated to corroborate many of the conclusions from our multi-institutional analysis.
Conclusions
From the present multi-institutional experience of SABR for T1-T2N0 SCLC patients, the largest to date, we found SABR to be a safe and effective treatment modality, especially when combined with chemotherapy. This paradigm can offer very high LC and relatively high DFS and DSS. The OS appears numerically similar to that of previously published surgical series for operable patients.
Supplementary Material
Acknowledgments
We thank Drs Kevin Stephans, Neil Woody, and Kyle Wang for their assistance.
Conflict of interest:
M.P.M. has served as a consultant for Abbott, Bristol-Meyers-Squibb, Celldex, Cavion, Elekta, Novartis, Novocure, and Roche, has received research funding from Novocure and Cellectar, and has served in a leadership capacity on the Pharmacyclics board of directors (with stock options). S.H.L. has received research funding from Elekta, STCube Pharmaceuticals, Peregrine, and Roche/Genentech, has served as a consultant for AstraZeneca, and has received honorarium from US Oncology and ProCure.
Footnotes
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer, version 1. Available at: http://www.nccn.org/professionals/physician_gls/PDF/nsclc.pdf. Accessed March 24, 2016.
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simone CB II, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784–1790. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma V. Stereotactic radiotherapy versus surgery for early-stage operable lung cancer: More questions than answers. J Natl Compr Canc Netw 2015;13:1293–1295. [DOI] [PubMed] [Google Scholar]
- 6.Simone CB II, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: Surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Small Cell Lung Cancer, version 1. Available at: http://www.nccn.org/professionals/physician_gls/PDF/sclc.pdf. Accessed March 24, 2016.
- 8.Videtic GM, Stephans KL, Woody NM, et al. Stereotactic body radiation therapy-based treatment model for stage I medically inoperable small cell lung cancer. Pract Radiat Oncol 2013;3:301–306. [DOI] [PubMed] [Google Scholar]
- 9.Shioyama Y, Nakamura K, Sasaki T, et al. Clinical results of stereotactic body radiotherapy for stage I small-cell lung cancer: A single institutional experience. J Radiat Res 2013;54:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ly NB, Allen PK, Lin SH. Stereotactic body radiation therapy for stage I small cell lung cancer: A single institutional case series and review of the literature. J Radiat Oncol 2014;3:285–291. [Google Scholar]
- 11.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94–S100. [DOI] [PubMed] [Google Scholar]
- 12.Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons, and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother Oncol 2009;95:32–40. [DOI] [PubMed] [Google Scholar]
- 13.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890–895. [DOI] [PubMed] [Google Scholar]
- 14.Verma V, Simone CB II, Zhen W. Stereotactic radiotherapy for stage I small cell lung cancer. Oncologist 2016;21:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma V. Lung cancer: Implementing lung-cancer screening—Oncological “grey areas” Nat Rev Clin Oncol 2015;12:256–257. [DOI] [PubMed] [Google Scholar]
- 16.Verma V, Zhen W. Treatment costs of early-stage lung cancers detected by low-dose computed tomography screening. Int J Radiat Oncol Biol Phys 2015;93:207–208. [DOI] [PubMed] [Google Scholar]
- 17.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma V, Beriwal S. Medicare approves coverage for lung cancer screening: The case for symptomatic screening. JAMA Oncol 2015;1: 1027–1028. [DOI] [PubMed] [Google Scholar]
- 19.Lanni TB Jr., Grills IS, Kestin LL, et al. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 2011;34: 494–498. [DOI] [PubMed] [Google Scholar]
- 20.Mitera G, Swaminath A, Rudoler D, et al. Cost-effectiveness analysis comparing conventional versus stereotactic body radiotherapy for surgically ineligible stage I non-small-cell lung cancer. J Oncol Pract 2014;10:e130–e136. [DOI] [PubMed] [Google Scholar]
- 21.Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol 2012;13:802–809. [DOI] [PubMed] [Google Scholar]
- 22.Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus: Ten-year follow-up. Lancet 1973;2:63–65. [DOI] [PubMed] [Google Scholar]
- 23.Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S–330S. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Ann Thorac Surg 2000;70:1615–1619. [DOI] [PubMed] [Google Scholar]
- 25.Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64–72. [DOI] [PubMed] [Google Scholar]
- 26.Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: Time to reevaluate. J Thorac Oncol 2008;3:1267–1271. [DOI] [PubMed] [Google Scholar]
- 27.Shioyama Y, Nagata Y, Komiyama T, et al. Multi-institutional retrospective study of stereotactic body radiation therapy for stage I small cell lung cancer: Japan Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys 2015;93:S101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.