Abstract
Purpose:
To identify clinical parameters that are prognostic for improved overall survival (OS) after yttrium-90 radioembolization (RE) in patients with liver metastases from colorectal cancer (CRC).
Materials and Methods:
A total of 131 patients who underwent RE for liver metastases from CRC, treated at 2 academic centers, were reviewed. Twenty-one baseline pretreatment clinical factors were analyzed in relation to OS by the Kaplan-Meier method along with log-rank tests and univariate and multivariate Cox regression analyses.
Results:
The median OS from first RE procedure was 10.7 months (95% confidence interval [CI], 9.4–12.7 months). Several pretreatment factors, including lower carcinoembryonic antigen (CEA; ≤20 ng/mL), lower aspartate transaminase (AST; ≤40 IU/L), neutrophil-lymphocyte ratio (NLR) <5, and absence of extrahepatic disease at baseline were associated with significantly improved OS after RE, compared with high CEA (>20 ng/mL), high AST (>40 IU/L), NLR ≥5, and extrahepatic metastases (P values of <.001, <.001, .0001, and .04, respectively). On multivariate analysis, higher CEA, higher AST, NLR ≥5, extrahepatic disease, and larger volume of liver metastases remained independently associated with risk of death (hazard ratios of 1.63, 2.06, 2.22, 1.48, and 1.02, respectively).
Conclusions:
The prognosis of patients with metastases from CRC is impacted by a complex set of clinical parameters. This analysis of pretreatment factors identified lower AST, lower CEA, lower NLR, and lower tumor burden (intra- or extrahepatic) to be independently associated with higher survival after hepatic RE. Optimal selection of patients with CRC liver metastases may improve survival rates after administration of yttrium-90.
Colorectal cancer (CRC) is the third most common cancer diagnosis in the United States (1). More than one-half of patients with CRC present with or develop liver metastases during the course of the disease (2). Although surgical resection is a potentially curative treatment for hepatic metastases, this approach is feasible in only 20% of patients owing to medical comorbidities, underlying hepatic dysfunction, or extent of tumor burden (3,4). Radio-frequency ablation (RFA) has been investigated as a potentially curative and less invasive alternative to surgical resection, but tumor size and location can limit its feasibility (3,5). A number of other locoregional therapies are available, including stereotactic body radiation therapy, cryotherapy, hepatic intra-arterial pump chemotherapy, transarterial chemoembolization, and transarterial radioembolization (RE). RE with yttrium-90 (90Y) resin microspheres is approved by the Food and Drug Administration for treatment of liver metastases from CRC (6–11). The treatment has been shown in multiple institutional studies to be safe and has been used and compared with transarterial chemoembolization in patients with a greater hepatic disease burden (12–14).
It is difficult to predict which patients have the best outcomes after RE. Previous studies identified lung shunt fraction and markers of hepatic function as having prognostic implications in patients both with primary hepatic malignancies and with hepatic metastases treated by RE (15,16). Similarly, elevated bilirubin, high alkaline phosphatase, high tumor volume, increased number of previous therapies, low albumin, and presence of extrahepatic disease have been correlated with poor prognosis after RE in patients with metastatic CRC (12,13). The purpose of the present report was to elucidate and further identify prognostic factors in a patient population with metastatic CRC treated with RE at 2 different academic centers.
MATERIALS AND METHODS
Patients
Institutional Review Board approval was obtained before data collection, and owing to the retrospective nature requirement of informed consent was waived. One hundred thirty-one patients undergoing RE at 2 separate institutions from 2007 to 2014 were identified and included in the analysis. Patients were not operative candidates (because of patient- or tumor-related factors) and had failed multiple previous chemotherapy regimens. All patients were treated with 90Y resin microspheres. The study population included 84 men (64.1%) and 47 women (35.9%) with an overall mean age of 59 years. All patient baseline characteristics are summarized in Table 1. Most patients (71.8%) had hepatic metastases at the time of their initial CRC diagnosis, 68% of the patients had received greater than 2 lines of chemotherapy, and 79% had undergone resection of their primary colorectal tumor. The colorectal primary tumor was right-sided in 19% of patients, left sided-in 68% of patients, and unknown in 16% of patients. Right-sided tumors were located in the cecum, ascending colon, hepatic flexure, or transverse colon, and left-sided tumors were defined as originating in the splenic flexure, descending colon, sigmoid colon, or rectum. Previous hepatic local or regional therapies were administered in 26% and 33% of patients, respectively, and included resection, transarterial chemoembolization, RFA, cryoablation, stereotactic body radiotherapy, and hepatic artery infusion pump therapy, as described in Table 1. Some patients received multiple types of prior hepatic locoregional therapies.
Table 1.
Baseline Characteristics
| Characteristic | Parameter | Value |
|---|---|---|
| Sex | Female | 47 (35.9%) |
| Male | 84 (64.1%) | |
| Age, y | 59 ± 1 | |
| ECOG performance status | 0 | 74 (64.9%) |
| 1 | 33 (29.0%) | |
| 2 | 6 (5.3%) | |
| 3 | 1 (0.9%) | |
| Site | Medical center 1 | 43 (32.8%) |
| Medical center 2 | 88 (67.2%) | |
| CEA, ng/mL | 37.3 (0–7690.5) | |
| ALT, IU/L | 33.5 (10–291) | |
| AST, IU/L | 39 (13–207) | |
| ALP, IU/L | 138 (43–1,554) | |
| Albumin, g/dL | 3.9 (2.6–4.7) | |
| Bilirubin, mg/dL | 0.5 (0.2–2.9) | |
| NLR class | 1 | 81 (64.3%) |
| 2 | 45 (35.7%) | |
| Extrahepatic metastases | Yes | 59 (46.1%) |
| No | 69 (53.9%) | |
| Lesion size, cm | <5 (1.5–20.8) | |
| Tumor volume, % | 12.6 (0.8–76.0) | |
| Metastasis to liver | Synchronous | 94 (71.8%) |
| Metachronous | 37 (28.2%) | |
| Lesion category | Dominant | 35 (27.1%) |
| Diffuse | 94 (72.9%) | |
| Previous chemotherapy lines | 0 | 2 (1.6%) |
| 1 | 39 (30.5%) | |
| 2 | 50 (39.1%) | |
| 3 | 22 (17.2%) | |
| ≥4 | 15 (11.7%) | |
| Hepatic local therapy | Yes | 34 (26.0%) |
| No | 97 (74.0%) | |
| Hepatic regional therapy | Yes | 43 (32.8%) |
| No | 88 (67.2%) | |
| Type of hepatic locoregional therapy* | Resection | 18 (13.7%) |
| Transarterial chemoembolization | 10 (7.6%) | |
| Cryoablation | 2 (1.5%) | |
| HAIP | 2 (1.5%) | |
| RFA | 16 (12.2%) | |
| SBRT | 4 (3.0%) | |
| Primary colorectal cancer removal | Yes | 104 (79.4%) |
| No | 27 (20.6%) | |
| Side of origin of primary colorectal tumor | Left | 89 (67.9%) |
| Right | 25 (19.1%) | |
| Unknown | 21 (16.0%) | |
| Lung shunting, % | 4 (0–18) | |
| Treatment approach | Sequential lobar | 67 (51.1%) |
| Single administration | 64 (48.9%) | |
| Radiation activity, GBq | 1.51 ± 0.04 |
Note–Values are presented as n(%), mean ± SD, or median (range). ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate transaminase; CEA = carcinoembryonic antigen; ECOG = Eastern Cooperative Oncology Group; HAIP = hepatic artery infusion pump; NLR = neutrophil-lymphocyte ratio; RFA = radiofrequency ablation; SBRT = stereotactic body radiotherapy.
Some patients underwent more than one type of hepatic locoregional therapy.
Procedures before Therapy
Patients were eligible for treatment after consensus evaluation by specialists in medical oncology, surgical oncology, interventional radiology, and radiation oncology. Clinical examination and baseline laboratory evaluation, including complete blood count (CBC), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), albumin, total bilirubin, and the tumor marker carcinoembryonic antigen (CEA), were obtained for all patients. Neutrophil-lymphocyte ratio (NLR) was calculated as the ratio of neutrophils to lymphocytes. NLR was dichotomized by a threshold of 5, (if <5, NLR class 1; if ≥ 5, NLR class 2). All patients underwent baseline imaging, including triphasic contrast-enhanced hepatic computerized tomography (CT), triphasic magnetic resonance imaging (MRI), and/or positron-emission tomography–CT as clinically appropriate. The maximum lesion size was defined as the greatest dimension of the largest lesion, and the tumor volume percentage was defined as the percentage of whole liver volume involved with tumor.
Hepatic arteriography was performed to assess the hepatic vascular anatomy and, if needed, to coil embolize the gastroduodenal artery, right gastric artery or any extrahepatic collateral arteries to prevent extrahepatic distribution of microspheres (17–19). Patients were evaluated for pulmonary and extrahepatic shunting with the use of single-photon-emission computerized tomography (SPECT) imaging after technetium-99m macro-aggregated albumin (MAA) infusion through the proper hepatic artery, common hepatic artery, left and right hepatic arteries, or accessory vessels as determined by the planned treatment site and patient anatomy (19–22). Patients with >20% shunt according to MAA-SPECT were excluded from RE (17,19). Chemotherapy was typically withheld for 2 weeks before and after RE.
The Eastern Cooperative Oncology Group (ECOG) Performance Status, age, sex, tumor volume percentage, percentage lung shunting, presence of extrahepatic disease, presence of dominant or diffuse lesions, presence of synchronous lesions, removal of primary colorectal cancer, previous hepatic regional therapy, and previous hepatic tumor removal were recorded for all patients. Synchronous metastases were defined as liver metastases occurring at diagnosis or within 2 months after the initial diagnosis. Metachronous metastases were those diagnosed greater than 2 months after the initial CRC diagnosis.
Radiation Treatment Planning and Administration
The 90Y dosage for each patient was calculated according to liver and tumor volumes contoured on triphasic CT or MRI scans with the use of Eclipse (Varian, Palo Alto, California) or Pinnacle (Philips, Amsterdam, Netherlands) treatment planning systems (17). The prescribed dose was administered into the proper hepatic artery for bilobar treatment and left or right hepatic artery for unilobar or sequential bilobar treatment (18).
Statistics
Patient characteristics were summarized with the use of descriptive statistics. According to Shapiro-Wilk normality test, age and radiation activity were in normal distribution, and their means and standard deviations are presented. Other continuous variables were nonnormally distributed, and their medians and ranges are presented. Overall survival (OS) was evaluated by Kaplan-Meier survival analysis and described accordingly with the median and 95% confidence interval (CI). Univariate Cox regression analyses were used to assess the ability of various factors to predict survival times, including age, sex, CEA, ALT, AST, ALP, albumin, bilirubin, ECOG performance status, metastases other than liver, lesion size, tumor volume percentage, metastasis to liver, lesion category, hepatic tumor removal, side of colorectal primary, hepatic locoregional therapy, primary colorectal tumor removal, lung shunting, treatment approach, and radiation activity. Multivariate Cox regression analysis was done in a backward selection model with elimination level P = .05. To better interpret their influence on survival in univariate and multivariate Cox regression analyses, certain continuous variables (AST, ALT, ALP, and CEA) were dichotomized into low levels and high levels. The cutoffs were selected with consideration of the medians and the clinical laboratory reference ranges (AST, 40 IU/L; ALT, 40 IU/L; ALP, 147 IU/L; and CEA, 20 ng/mL). For all significant variables on multivariate Cox regression analysis, median survival times were calculated, and OSs were compared by means of log-rank test. All tests were 2-sided, and statistical significance was assumed at P < .05. All statistics were performed with the use of SAS University Edition for Windows (SAS Institute, Cary, North Carolina).
RESULTS
Survival and Prognostic Factors
There were 117 cancer-related deaths during follow-up, with a median survival time of 10.7 months (95% CI, 9.4–12.7 months, with 10.7% censored; 1-year OS, 44.3%; 3-year OS, 8.7%) from first RE procedure. Among all 21 prognostic factors investigated in univariate Cox regression analysis, higher CEA, ALP, AST, and NLR class, presence of extrahepatic metastases, and larger percentage tumor volume were correlated with worse OS (Table 2). On multivariate backward Cox regression analysis, higher CEA, AST, NLR, presence of extrahepatic metastases, and increased tumor volume percentage continued to be strong independent predictors of decreased OS (Table 3).
Table 2.
Univariate Cox Regression Analysis
| Parameter | HR | 95% CI | P Value |
|---|---|---|---|
| ALP, high vs low | 2.17 | 1.48–3.18 | <.0001 |
| AST, high vs low | 1.952 | 1.35–2.83 | .0004 |
| NLR, class 2 vs 1 | 2.18 | 1.45–3.28 | .0002 |
| Extrahepatic metastases, yes vs no | 1.46 | 1.01–2.10 | .046 |
| CEA, high vs low | 1.87 | 1.29–2.71 | .001 |
| Tumor volume percentage | 1.02 | 1.003–1.03 | .01 |
ALP = alkaline phosphatase; AST = aspartate transaminase; CEA = carcinoembryonic antigen; CI = confidence interval; HR = hazard ratio; NLR = neutrophil-lymphocyte ratio.
Table 3.
Multivariate Cox Regression Analysis
| Parameter | HR | 95% CI | P Value |
|---|---|---|---|
| CEA, high vs low | 1.63 | 1.10–2.42 | .02 |
| AST, high vs low | 2.06 | 1.39–3.06 | .0003 |
| NLR, class 2 vs 1 | 2.22 | 1.46–3.38 | .0002 |
| Extrahepatic metastases, yes vs no | 1.48 | 1.01–2.16 | .04 |
| Tumor volume percentage | 1.02 | 1.003–1.03 | .02 |
AST = aspartate transaminase; CEA = carcinoembryonic antigen; CI = confidence interval; HR = hazard ratio; NLR = neutrophil-lymphocyte ratio.
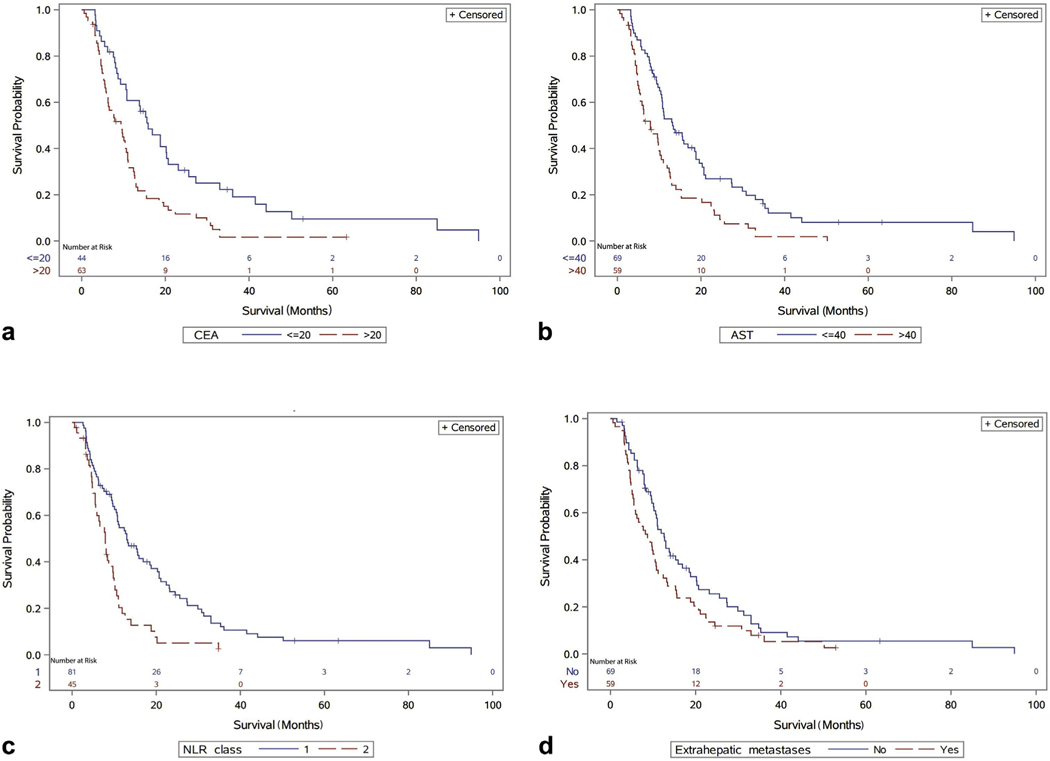
Patients with low CEA (≤20 ng/mL; n = 44) at the time of RE had an improved survival (P < .001), with a median survival time of 15.9 months (95% CI, 10.6–20.72 months), compared with those with high CEA (>20 ng/mL; n = 63), who had a median survival of 9.6 months (95% CI, 5.9–11.0 months; Fig 1a). Low AST (≤40 IU/L; n = 69) was correlated with improved survival (P < .001), with a median survival time of 13.2 months (95% CI, 10.6–18.8 months), and high AST (>40 IU/L; n = 59) was consistent with poorer survival outcomes, with a median survival of 7.9 months (95% CI, 5.5–10.2 months; Fig 1b). Patients with NLR class 1 had a longer OS (P = .0001), with a median survival time of 13.0 months (95% CI, 10.5–18.5 months), compared with those with NLR class 2, who had a median survival of 7.9 months (95% CI, 5.5–9.7 months; Fig 1c). Patients without extrahepatic disease had a longer OS (P = .04), with a median survival time of 12.6 months (95% CI, 10.1–15.9 months), compared with those with extrahepatic metastases, who had a median survival of 8.7 months (95% CI, 5.5–10.7 months; Fig 1d).
Figure 1.
OS in months after radioembolization: (a) stratified by CEA (P < .001); (b) stratified by aspartate transaminase (AST; P < .001); (c) stratified by NLR class (1, <5; 2, ≥5; P = .0001); (d) stratified by extrahepatic metastases (P = .04).
There was no significant difference in OS between patients who underwent resection of the primary colorectal tumor (median, 11.0 months; 95% CI, 9.7–14.0 months) and those who did not undergo resection of the primary colorectal tumor before RE (median, 8.7 months; 95% CI, 5.9–11.1 months; P =.17; Fig 2a). In addition, there was no significant difference in OS between patients who received ≤2 lines of chemotherapy before RE and patients who received >2 lines of chemotherapy before RE (P = .54; Fig 2b). Patients with a right-sided primary tumor location had a median OS of 8.0 months, whereas patients with a left-sided colorectal primary had a median OS of 12.4 months, although this difference did not reach statistical significance (P = .09; Fig 2c). There was also no difference in survival between patients who had received previous hepatic locoregional therapy (median, 11.9 months; 95% CI, 7.6–15.5 months) versus those with no previous hepatic therapy (median; 10.2 months, 95% CI, 8.4–12.7 months; P = .83).
Figure 2.
OS in months after RE: (a) stratified by primary CRC resection (P = .17); (b) stratified by previous chemotherapy lines (P = .54); (c) stratified by tumor sidedness (P = .09).
DISCUSSION
The prognosis of patients with metastatic CRC is affected by a diverse set of clinical factors (tumor/disease factors, underlying hepatic function, and inflammatory state), and the present study analyzed a set of parameters to identify 5 independent prognostic factors after RE: 3 indices of disease burden, 1 marker of hepatic function, and 1 inflammatory marker. This study has demonstrated that lower pre-RE CEA, lower AST, lower NLR, absence of extrahepatic disease, and lower hepatic disease burden were associated with improved OS after RE in patients with metastatic CRC. In addition to these factors being indicative of overall prognosis, they may also help in selecting candidates for RE.
Elevated CEA is expected to correlate with increased tumor burden and worse survival. A cutoff of 130 ng/mL has been used in a predictive scoring system for OS (23), but this cutoff was higher than in most other related studies. Typically, levels of CEA >20 ng/mL are suggestive of disease progression and metastasis (24,25), and this value stratified the current study population in terms of OS (P < .001). High levels of AST imply impaired liver function and hepatocyte damage, and the present study showed a correlation between AST elevation and poorer OS after RE. This correlation suggests that an isolated laboratory test indicative of underlying liver dysfunction can also indicate prognosis. Fengler et al similarly found that transaminase elevation >2.5 times the upper limit of normal is associated with worse OS after RE (26). Lower albumin and higher bilirubin are other metrics of impaired liver function that correlate with worse survival in other studies (27,28). Notably in the present study, modestly elevated AST and CEA were predictive of survival, in contrast to other studies that used much higher cutoff points for analysis. This is possibly a result of consistent laboratory testing done on the day of the RE procedure.
Although the usual patient selection criteria for RE permits limited extrahepatic disease (≤5 sites, each ≤2 cm), even a small volume of extrahepatic disease is likely a harbinger of more widespread disease, despite being considered liver dominant. The present study demonstrates that liver-directed therapies are unlikely to affect outcomes in the setting of extrahepatic disease, and worse OS after RE was noted in these patients (29). Small-volume extrahepatic disease, however, is not a contraindication for RE, because liver involvement can be a major contributor to death (30). In addition to extrahepatic disease burden, the overall hepatic tumor burden is a negative prognostic factor for survival.
NLR is a reflection of inflammatory status and has been proposed as a prognostic marker in a variety of solid tumors after RE (31). Sukato et al and Tohme et al both recently demonstrated that NLR was prognostic in patients with, respectively, hepatocellular carcinoma and metastatic CRC treated with RE (32,33). The present study verified this association as an independent predictor of OS, perhaps related to the ability to invoke an antitumor immune response.
Resection of the primary colorectal tumor did not confer a significant survival benefit, in contrast to a recent report demonstrating that palliative primary tumor resection improved OS after first-line chemotherapy (34). RE has been postulated as having a greater benefit in patients with right-sided tumors, who tend to be more refractory to other therapies, as demonstrated in the combined SIRFLOX, FOXFIRE and FOXFIRE Global studies (35). In the present study, patients with a right-sided colorectal primary tumor had worse OS than those with left-sided tumors (36), although the difference failed to achieve statistical significance. It is possible, however, that use of RE in patients with right-sided primary tumors could impact prognosis more than in patients with left-sided primaries.
Efforts have been made to determine optimal timing and use of RE, and Hickey et al showed that RE after ≤2 lines of chemotherapy independently predicted better survival outcomes (37). The SIRFLOX trial showed improved hepatic progression-free survival (PFS) with up-front RE and first-line chemotherapy; however, the primary end point of improved overall PFS was not met (38). More recently, the combined analysis of the SIRFLOX, FOXFIRE, and FOXFIRE Global studies failed to demonstrate an OS benefit of RE in combination with first-line chemotherapy (35). In the present study, all patients had received previous first-line chemotherapy, and the number of previous chemotherapy lines was not correlated with OS. The largest potential benefit for RE appears to be in chemotherapy-refractory metastatic CRC patients after the disease declares hepatic predominance.
The present study thoroughly analyzed multiple patient-, tumor-, and treatment-related factors for association with an OS benefit and described 5 independent predictors for OS in patients with chemorefractory metastatic CRC treated at 2 academic institutions. Besides inherent issues with retrospective analysis, there was not statistical power or an available validation cohort for generation of a specific scoring system to correlate clinical parameters with OS. Excluding patients treated with RE for other tumor histologies allowed a more homogeneous patient population, but limited the number of patients. In addition, assessment of prognostic histologic biomarkers (Ras mutation, microsatellite instability, BRAF mutation) was not feasible, because the majority of these patients were diagnosed before widespread testing for these markers. The number of patients was less than the large retrospective series by Kennedy et al (39), but included a different set of more specific patient characteristics.
In summary, markers of tumor (CEA), liver function (AST), and inflammation (NLR) as well as degree of tumor burden (both hepatic and extrahepatic disease) are independent predictors for OS in patients with metastatic CRC treated with RE in the chemorefractory setting. Particularly given the negative data for PFS and OS with RE in the first-line setting in the SIRFLOX and combined SIRFLOX, FOXFIRE, and FOXFIRE Global studies, selection of patients for RE is paramount. Further investigation of these factors in larger patient cohorts will help to guide clinicians in a new prognostic model establishment and may aid the optimal selection for Y90 RE among patients with CRC liver metastases. Low disease burden (low CEA, low hepatic tumor burden, no extrahepatic disease), good hepatic function (low AST), and potential for an antitumor immune response (low NLR) are favorable factors that can appropriately select for patients who will be most likely to benefit from RE.
ACKNOWLEDGMENTS
The authors thank the patients who participated in this study and the research administrative nurse Donna Normann, who allowed us to achieve a high-quality database with comprehensive factors.
J.R.O. receives grants and personal fees from Viewray (Oakwood Village, Ohio). P.J.P. receives grants from Philips Healthcare (Andover, Massachusetts) and Varian Medical Systems (Palo Alto, California), has partial ownership of Nuvaira (Minneapolis, Minnesota), is on the speakers’ bureau for Varian Medical Systems, and is a paid consultant for Medtronic/Covidien (Dublin, Ireland) and Johnson & Johnson (New Brunswick, New Jersey).
ABBREVIATIONS
- AST
aspartate transaminase
- CEA
carcinoembryonic antigen
- CRC
colorectal cancer
- NLR
neutrophil-lymphocyte ratio
- OS
overall survival
- RE
radioembolization
- 90Y
yttrium-90
Footnotes
None of the other authors have identified a conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006; 93:465–474. [DOI] [PubMed] [Google Scholar]
- 3.Mahnken AH, Pereira PL, de Baere T. Interventional oncologic approaches to liver metastases. Radiology 2013; 266:407–430. [DOI] [PubMed] [Google Scholar]
- 4.Blackham AU, Swett K, Levine EA, Shen P. Surgical management of colorectal cancer metastases to the liver: multimodality approach and a single institutional experience. Colorectal Cancer 2013; 2:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddala YK, Stadheim L, Andrews JC, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl 2004; 10:449–455. [DOI] [PubMed] [Google Scholar]
- 6.Coldwell D, Sangro B, Salem R, Wasan H, Kennedy A. Radioembolization in the treatment of unresectable liver tumors. Am J Clin Oncol 2012; 35: 167–177. [DOI] [PubMed] [Google Scholar]
- 7.Kosmider S, Tan TH, Yip D, et al. Radioembolization in combination with systemic chemotherapy as first-line therapy for liver metastases from colorectal cancer. J Vasc Interv Radiol 2011; 22:780–786. [DOI] [PubMed] [Google Scholar]
- 8.van Hazel G, Pavlakis N, Goldstein D, et al. Treatment of 5-FU refractory patients with liver metastasis from colorectal cancer using 90Y resin microspheres plus concomitant systemic irinothecan therapy. J Clin Oncol 2009; 27:4089–4095. [DOI] [PubMed] [Google Scholar]
- 9.Wasan H, Kennedy A, Coldwell D, Sangro B, Salem R. Integrating radioembolization with chemotherapy in the treatment paradigm for unresectable colorectal liver metastases. Am J Clin Oncol 2012; 35:293–301. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015; 6:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Hazel G, Blackwell A, Anderson J, et al. Randomized phase II trial of SIR-Spheres plus 5FU/LV vs. 5FU/LV chemotherapy alone in advanced colorectal cancer. J surg Oncol 2004; 88:78–85. [DOI] [PubMed] [Google Scholar]
- 12.Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival byera and chemotherapy. Eur J Nucl Med Mol Imaging 2014; 41:1861–1869. [DOI] [PubMed] [Google Scholar]
- 13.Maleux G, Deroose C, Laenen A, et al. Yttrium-90 radioembolization for the treatment of chemorefractory colorectal liver metastases: technical results, clinical outcome and factors potentially influencing survival. Acta Oncol 2016; 55:486–495. [DOI] [PubMed] [Google Scholar]
- 14.Nosher JL, Ahmed I, Patel AN, et al. Non-operative therapies for colorectal liver metastases. J Gastrointest Oncol 2015; 6:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henrie AM, Wittstrom K, Delu A, Deming P. Evaluation of liver biomarkers as prognostic factors for outcomes to yttrium-90 radioembolization of primary and secondary liver malignancies. Cancer Biother Radiopharm 2015; 30:305–309. [DOI] [PubMed] [Google Scholar]
- 16.Xing M, Lahti S, Kokabi N, Schuster DM, Camacho JC, Kim HS. 90Y radioembolization lung shunt fraction in primary and metastatic liver cancer as a biomarker for survival. Clin Nucl Med 2016; 41:21–27. [DOI] [PubMed] [Google Scholar]
- 17.Schonewolf CA, Patel B, Gensure RH, et al. Patterns of failure in colorectal patients with liver metastasis after 90Y radioembolization. Am J Clin Oncol 2014; 37:234–240. [DOI] [PubMed] [Google Scholar]
- 18.Liu DM, Salem R, Bui JT, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol 2005; 16:911–935. [DOI] [PubMed] [Google Scholar]
- 19.Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic 90Y radioembolization of chemotherapy refractory colorectal cancer liver metastasis. J Vasc Interv Radiol 2008; 19:1187–1195. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using 90Y microsphere brachytherapy. Int J Radiat Oncol Biol Phys 2007; 6:13–23. [DOI] [PubMed] [Google Scholar]
- 21.Denecke T, Ruhl R, Hildebrandt B, et al. Planning transarterial RE of colorectal liver metastasis with 90Y microspheres: evaluation of sequential diagnostic approach using radiologic and nuclear medicine imaging techniques. Eur Radiol 2008; 18:892–902. [DOI] [PubMed] [Google Scholar]
- 22.Lowandowski RJ, Sato KT, Atassi B, et al. Radioembolization with 90Y microsphere: angiographic and technical considerations. Cardiovasc Intervent Radiol 2007; 30:571–592. [DOI] [PubMed] [Google Scholar]
- 23.Damm R, Seidensticker R, Ulrich G, Breier L, Steffen IG, Seidensticker M. Y90 radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer 2016; 16:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpha-fetoprotein Bell H. and carcinoembryonic antigen in patients with primary liver carcinoma, metastatic liver disease, and alcoholic liver disease. Scand J Gastroenterol 1982; 17:897–903. [DOI] [PubMed] [Google Scholar]
- 25.Lokich JJ, Ellenberg S, Gerson B. Criteria for monitoring carcinoembryonic antigen: variability of sequential assays at elevated levels. J Clin Oncol 1984; 2:181–186. [DOI] [PubMed] [Google Scholar]
- 26.Fendler WP, Ilhan H, Paprottka PM, et al. Nomogram including pretherapeutic parameters for prediction of survival after SIRY of hepatic metastases from colorectal cancer. Eur Radiol 2015; 25: 2693–2700. [DOI] [PubMed] [Google Scholar]
- 27.Dunfee BL, Mulcahy MF, Salem R, et al. Yttrium-90 radioembolization for liver malignancies: prognostic factors associated with survival. J Vasc Interv Radiol 2010; 21:90–95. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy AS, Ball D, Cohen SJ, et al. Pre-90Y hepatic radiotherapy hemoglobin and liver functions to predict overall survival in unresectable, chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2014; 32:abstr 292. [Google Scholar]
- 29.Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastasis using 90Y microspheres. Cancer 2009; 115: 1849–1858. [DOI] [PubMed] [Google Scholar]
- 30.Saxena A, Chua TC, Bester L, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg 2010; 251:910–916. [DOI] [PubMed] [Google Scholar]
- 31.d’Emic N, Engelman A, Molitoris J, Sharma NK, Moeslein FM, Chuong MD. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patient treated with selective internal radiation therapy. J Gastrointest Oncol 2016; 7:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukato DC, Tohme S, Chalhoub D, Han K, Zajko A, Amesur N. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol 2015; 26:816–824.e1. [DOI] [PubMed] [Google Scholar]
- 33.Tohme S, Sukato D, Chalhoub D, McDonald KA, Zajko A, Amesur N. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Ann Surg Oncol 2015; 22:1701–1707. [DOI] [PubMed] [Google Scholar]
- 34.He WZ, Rong YM, Jiang C, Liao FX, Yin CX, Guo GF. Palliative primary tumor resection provides survival benefits for the patients with metastatic colorectal cancer and low circulating levels of dehydrogenase and carcinoembryonic antigen. Chin J Cancer 2016; 35:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasan HS, Gibbs P, Sharma NK, et al. ; FOXFIRE investigators; SIRFLOX Investigators; FOXFIRE-Global Investigators. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX and FOXFIRE-Global): a combined analysis of three multicenter, randomized phase 3 trials. Lancet Oncol 2017; 18: 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoyama T, Kashiwabara K, Oba K, et al. Clinical impact of tumor location on the colon cancer survival and recurrence: analyses of pooled data from three large phage III randomized clinical trials. Cancer Med 2017; 6: 2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey R, Lewandowski RJ, Prudhomme T, et al. Y90 radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med 2016; 47:665–671. [DOI] [PubMed] [Google Scholar]
- 38.van Hazel GA, Neinemann V, Sharma NK, et al. SIRFLOX: Randomized phage III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patient with metastatic colorectal cancer. J Clin Oncol 2016; 34:1723–1731. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy A, Cohn M, Coldwell DM, et al. Updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases. J Gastrointest Oncol 2017; 8: 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]