Abstract
A new bush frog species is described from Yunnan, China, based on phylogenetic analyses, species delimitation analyses, and morphological comparisons. Raorchesteshekouensissp. nov. is distinguished from all other congeners by a combination of 11 morphological characters. The new species brings the current number of Raorchestes species in China to ten, nine of which are distributed in Yunnan. Molecular analyses supported an unnamed lineage previously recorded as “Raorchestesgryllus” in northern Vietnam. Further studies including additional samples are necessary to clarify the species diversity and boundaries of Raorchestes in China and Indochina.
Key words: Indochina, “Raorchestesgryllus”, Raorchesteshekouensis sp. nov., species diversity, taxonomy
Introduction
The genus Raorchestes Biju, Shouche, Dubois, Dutta & Bossuyt, 2010, which currently contains 76 species (Frost 2023), is one of the most speciose genera within the family Rhacophoridae. Members of Raorchestes are characterized by a small body size (15–45 mm), lack of vomerine teeth, transparent/translucent vocal sac when calling, and direct development (Biju et al. 2010; Vijayakumar et al. 2014). Raorchestes is widely distributed in South and Southeast Asia, from India to Nepal, Myanmar, Thailand, and Laos to southwestern China, Vietnam, Cambodia, and West Malaysia (Frost 2023).
Most Raorchestes species were initially assigned to the genus Philautus Gistel, 1848 (Bossuyt and Dubois 2001); however, Yu et al. (2009) and Li et al. (2009) revealed that frogs traditionally classified in Philautus consisted of two groups rather than being a monophylum, and Li et al. (2009) proposed the name Pseudophilautus Laurent, 1943 for the group primarily distributed on the Indian subcontinent, which itself consists of two reciprocally monophyletic groups, i.e., a radiation with notably large diversity in the Western Ghats of India and a radiation with large diversity in Sri Lanka. Biju et al. (2010) later erected the genus Raorchestes for the clade with substantial diversity in the Western Ghats to distinguish it from Pseudophilautus sensu stricto, a clade of 80 species largely restricted to Sri Lanka (Biju et al. 2010; Meegaskumbura et al. 2019). Based on phylogenetic analysis, Li et al. (2013) suggested that Raorchestes and Pseudophilautus formed a sister group of Kurixalus Ye, Fei & Dubois, 1999; however, more recent studies based on wider genus-level sampling suggested Raorchestes is sister to Pseudophilautus (Vijayakumar et al. 2014; Chan et al. 2018; Garg et al. 2021) or the clade composed of Raorchestes and Pseudophilautus is sister to Mercurana Abraham, Pyron, Ansil, Zachariah & Zachariah, 2013 (Meegaskumbura et al. 2019).
As one of the most diverse groups in the Rhacophoridae family, Raorchestes frogs form a distinct radiation with more than 80% of the known species distributed in South Asia, especially in India. As such, most research attention has been paid to the taxonomy and evolution of Indian Raorchestes. For examples, Vijayakumar et al. (2014) reported on Raorchestes relationships within the Western Ghats, naming nine species and recognizing 15 clades within the Western Ghats complex; Vijayakumar et al. (2016) revealed that geological processes, Quaternary glaciations, and ecological gradients drove diversification of Raorchestes frogs in the Western Ghats; and Garg et al. (2021) named five species in the Western Ghats and delimited Raorchestes into 16 species groups.
The diversity of Raorchestes in southwestern China, Indochina, the Himalayas, and northeastern India is markedly lower than that in the Western Ghats. To date, only 16 species are known from these areas, including R.andersoni (Ahl, 1927), R.annandalii (Boulenger, 1906), R.cangyuanensis Wu, Suwannapoom, Xu, Murphy & Che, 2019, R.dulongensis Wu, Liu, Gao, Wang, Li, Zhou, Yuan & Che, 2021, R.gryllus (Smith, 1924), R.hillisi Jiang, Ren, Guo, Wang & Li, 2020, R.huanglianshan Jiang, Wang, Ren & Li, 2020, R.longchuanensis (Yang & Li, 1978), R.malipoensis Huang, Liu, Du, Bernstein, Liu, Yang, Yu & Wu, 2023, R.manipurensis (Mathew & Sen, 2009), R.menglaensis (Kou, 1990), R.parvulus (Boulenger, 1893), R.rezakhani Al-Razi, Maria & Muzaffar, 2020, R.sahai (Sarkar & Ray, 2006), R.shillongensis (Pillai & Chanda, 1973), and R.yadongensis Zhang, Shu, Liu, Dong & Guo, 2022 (Frost 2023). Of these 16 species, nine are known in China (i.e., R.andersoni, R.cangyuanensis, R.dulongensis, R.hillisi, R.huanglianshan, R.longchuanensis, R.malipoensis, R.menglaensis, and R.yadongensis), all from the border areas of Yunnan, except for R.yadongensis, which is only known from southern Tibet (Zhang et al. 2022). Moreover, the distribution of the R.andersoni is also recorded in southern Medog, Tibet (e.g., Chen et al. 2020; Fei et al. 2012), R.andersoni was originally described “on level marshy flats on the banks of the Nampoung [= Nanben River] in the centre of the Kakhyen Hills”, Yingjiang County, Yunnan, China by Anderson (1878), and it was once recognized as Thelodermaandersoni by Li et al. (2009). However, Hou et al. (2017) suggested that it possibly belonged to Raorchestes on the basis of morphological similarities to Philautuslongchuanensis, subsequently Chen et al. (2020) transferred T.andersoni (Ahl, 1927) to the genus Raorchestes based on the molecular evidence. Raorchestesparvulus was originally described from Karin Bia-po in Myanmar by Boulenger (1893) and previously recorded from China by Yu et al. (2019) based on specimens from Menglun, Yunnan. However, Jiang et al. (2020) considered that the record of R.parvulus from Yunnan was misidentified and revised it to R.menglaensis.
Six Raorchestes species are known from Southeast Asia, i.e., R.parvulus (Boulenger, 1893), R.gryllus, R.menglaensis, R.huanglianshan, R.longchuanensis, and R.malipoensis (Frost 2023). However, the taxonomic status of R.gryllus is problematic. This species was originally described from Langbian Plateau in Lam Dong Province, southern Vietnam, and has been widely reported in Vietnam (Lam Dong, Dak Lak, Gia Lai, and Kon Tum, Lao Cai, Cao Bang, Vinh Phu, and Bac Thai) and Laos (Sepian, Boloven Highlands, Champasak Province) (Bourret 1937, 1939, 1942; Orlov et al. 2002, 2012; Teynié et al. 2004; Nguyen et al. 2009). Biju et al. (2010) confirmed the affiliation of R.gryllus with the genus Raorchestes based on molecular data from Li et al. (2009). However, those specimens used in Li et al. (2009) were collected from Pac Ban, Tuyen Quang, northern Vietnam, and Orlov et al. (2012) considered records of R.gryllus in this region to be highly improbable. Furthermore, the species contains a series of tubercles along the outer side of the forearm and foot, and a dermal projection on the snout (Smith 1924), very similar to members of Kurixalus, and differing in egg capsule appearance from other Raorchestes species, with thick and semi-transparent eggs in R.gryllus compared to transparent eggs in other Raorchestes species (Orlov et al. 2012).
Recently, Poyarkov et al. (2021) suggested the transfer of R.gryllus to Kurixalus based on unpublished molecular data of specimens from the type locality and unpublished morphological data from type material, implying that the so-called “R.gryllus” specimens from northern Vietnam used in previous phylogenetic analyses (e.g., Li et al. 2009, 2013; Nguyen et al. 2014; Wu et al. 2019) are not actually true K.gryllus, but represent an unnamed species. Moreover, Huang et al. (2023) considered that the specimen of R. UI ROM30288 from Pac Ban, Tuyen Quang, northern Vietnam was misidentified and revised it to R.malipoensis. This suggests that other records of the species from Vietnam and Laos need further examination.
Yunnan Province harbors the highest amphibian species diversity in China (AmphibiaChina, 2022), with many new species described in recent years (e.g., Gan et al. 2020; Liu et al. 2021; Wang et al. 2022). During recent field surveys in Hekou, Yunnan, China, we collected eight specimens of Raorchestes. Morphological comparison and phylogenetic analysis indicated that these specimens could be distinguished from all other members of the genus Raorchestes, except for the R. UI ROM 38828 from northern Vietnam in molecular analysis, indicating that the eight specimens from Hekou and the ROM38828 specimen from northern Vietnam represent a new species.
Materials and methods
Sampling
Field surveys were conducted in March 2019 and April 2023 at Liangzi village, Hekou, Yunnan, China (Fig. 1). Specimens were euthanized, fixed, and preserved in 75% ethanol. Liver tissues were taken and preserved in 99% ethanol. Voucher specimens and tissue samples were deposited at Guangxi Normal University (GXNU), China.
Figure 1.
Map showing type localities of Raorchestes species originally described from China (1–10), type locality of K.gryllus in Vietnam (13), and collection sites of R. UI used in this study (11, 12). Raorchesteshekouensis sp. nov. is known from the type locality (9) and Pac Ban, Tuyen Quang, Vietnam (11).
Morphology and morphometrics
All measurements were made with slide calipers to the nearest 0.1 mm. Morphological characters and measurements followed Du et al. (2020) and included: snout-vent length (SVL); head length (HL); head width (HW); snout length (SL); internarial distance (INS); interorbital distance (IOS); maximum transverse distance of upper eyelid (UEW); eye diameter (ED); tympanum diameter (TD); eye-nostril distance (EN); length of lower arm and hand (LAHL); tibia length (TIL); length of foot and tarsus (TFL); foot length (FL). Morphological measurements of the specimens are given in Table 1. Males and females were identified based on the presence of an external single subgular vocal sac or sac slit opening. Comparative data on the morphology of other Raorchestes species were obtained from previous publications (Boulenger 1893, 1906; Smith 1924; Pillai and Chanda 1973; Yang and Li 1978; Kou 1990; Bossuyt and Dubois 2001; Sarkar and Ray 2006; Fei et al. 2009, 2012; Mathew and Sen 2009; Orlov et al. 2012; Wu et al. 2019, 2021; Al-Razi et al. 2020; Che et al. 2020; Jiang et al. 2020; Zhang et al. 2022; Huang et al. 2023).
Table 1.
Measurements (in mm) of Raorchesteshekouensis sp. nov. specimens from Liangzi, Hekou, Yunnan. Holotype is marked with an asterisk (*).
| Catalog No. | Adults | Sub-adults | ||||||
|---|---|---|---|---|---|---|---|---|
| GXNU YU000159* | GXNU YU000536 | GXNU YU000537 | GXNU YU000538 | GXNU YU000160 | GXNU YU000153 | GXNU YU000154 | GXNU YU000156 | |
| Sex | Male | Male | Male | Male | Female | Male | Female | Female |
| SVL | 17.5 | 17.8 | 16.7 | 16.1 | 21.1 | 14.5 | 12.5 | 12.9 |
| HL | 6.1 | 5.8 | 5.7 | 5.6 | 7.2 | 5.1 | 4.1 | 4.5 |
| HW | 6.9 | 7.1 | 6.1 | 6.1 | 7.6 | 5.3 | 4.9 | 4.5 |
| SL | 2.4 | 1.7 | 1.6 | 1.9 | 2.8 | 1.7 | 1.3 | 1.6 |
| INS | 2.2 | 2.4 | 2.1 | 2.3 | 2.6 | 2.0 | 1.6 | 1.8 |
| IOS | 2.4 | 2.8 | 2.3 | 2.1 | 2.7 | 2.0 | 1.7 | 1.8 |
| UEW | 1.9 | 1.7 | 1.9 | 1.5 | 2.1 | 1.2 | 1.1 | 1.7 |
| ED | 2.5 | 3.1 | 2.7 | 2.4 | 3.0 | 2.3 | 2.0 | 2.0 |
| TD | 1.3 | 1.1 | 1.3 | 1.2 | 1.4 | 0.7 | 0.5 | 0.8 |
| DNE | 1.5 | 1.2 | 1.3 | 1.3 | 1.8 | 1.2 | 1.0 | 1.0 |
| LAHL | 8.5 | 7.4 | 7.9 | 7.1 | 10.1 | 6.9 | 5.8 | 6.0 |
| TIL | 9.2 | 8.8 | 8.4 | 7.8 | 10.6 | 7.7 | 6.1 | 6.1 |
| TFL | 11.4 | 11.0 | 10.4 | 8.9 | 13.6 | 8.8 | 7.8 | 7.5 |
| FL | 6.6 | 6.6 | 5.7 | 5.4 | 8.1 | 5.1 | 4.1 | 4.1 |
DNA sequencing
We extracted genomic DNA from liver tissues stored in 99% ethanol following standard protocols (Vences et al. 2012). We amplified and sequenced the mitochondrial 16S ribosomal RNA (16S) genes using the primer pair L2188 (Matsui et al. 2006) and 16H1 (Hedges, 1994). Polymerase chain reaction (PCR) amplifications were performed in a 50-μL reaction volume, with an initial denaturing step at 95 °C for 4 min, 35 cycles of denaturing at 94 °C for 1 min, annealing at 51 °C for 1 min, and extension at 72 °C for 1 min, with a final extension step of 72 °C for 10 min. Sequencing was conducted using the corresponding PCR primers. All new sequences were deposited in GenBank under accession numbers ON986419–ON986422, OQ029526, OQ859106 and OQ859107 (Table 2).
Table 2.
Information on voucher numbers, localities, and GenBank accession numbers for all specimens used in this study.
| Species | Locality | Voucher No. | 16S | Reference |
|---|---|---|---|---|
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000153 | ON986419 | This study |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000154 | OQ029526 | This study |
| Raorchesteshekouensis sp. nov. | Pac Ban, Tuyen Quang, Vietnam | ROM 38828 | KC465838 | Li et al. (2013) |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000156 | ON986420 | This study |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000159 | ON986421 | This study |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000160 | ON986422 | This study |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000536 | OQ859106 | This study |
| Raorchesteshekouensis sp. nov. | Hekou, Yunnan, China | GXNU YU000537 | OQ859107 | This study |
| Raorchestesandersoni | Medog, Tibet, China | KIZYPX16167 | MW023609 | Chen et al. (2020) |
| Raorchestesandersoni | Medog, Tibet, China | KIZ014104 | MW023610 | Chen et al. (2020) |
| Raorchestesannandalii | Nepal | CDZMTU419 | MT983169 | Khatiwada et al. (2021) |
| Raorchestesagasthyaensis | Western Ghats, India | CESF492 | JX092723 | Vijayakumar et al. (2014) |
| Raorchestesarcheos | Agasthyamalai Massif, Western Ghats, India | CESF1190 | JX092675 | Vijayakumar et al. (2014) |
| Raorchestescangyuanensis | Cangyuan, Yunnan, China | KIZ 015855 | MN475866 | Wu et al. (2019) |
| Raorchestescangyuanensis | Cangyuan, Yunnan, China | KIZ 015856 | MN475867 | Wu et al. (2019) |
| Raorchestescrustai | Elivalmalai Massif, Western Ghats, India | CESF1199 | JX092677 | Vijayakumar et al. (2014) |
| Raorchesteschromasynchysi | Western Ghats, India | CESF1127 | JX092667 | Vijayakumar et al. (2014) |
| Raorchestesdulongensis | Qinlangdang, Yunnan, China | KIZ 035082 | MW537814 | Wu et al. (2019) |
| Raorchestesdulongensis | Qinlangdang, Yunnan, China | KIZ0 35125 | MW537815 | Wu et al. (2019) |
| Raorchestesghatei | Western Ghats, India | CESF1262 | JX092687 | Vijayakumar et al. (2014) |
| Raorchestes UI | Tam Dao, Vinh Phuc, Vietnam | ROM 30298 | MN475869 | Wu et al. (2019) |
| Raorchesteshillisi | Xiding, Yunnan, China | CIB116329 | MT488412 | Jiang et al. (2020) |
| Raorchesteshillisi | Xiding, Yunnan, China | CIB116330 | MT488413 | Jiang et al. (2020) |
| Raorchesteshuanglianshan | Lvchun, Yunnan, China | CIB116353 | MT488415 | Jiang et al. (2020) |
| Raorchesteshuanglianshan | Lvchun, Yunnan, China | CIB116354 | MT488417 | Jiang et al. (2020) |
| Raorchestesleucolatus | Elivalmalai Massif, Western Ghats, India | CESF1147 | JX092669 | Vijayakumar et al. (2014) |
| Raorchesteslongchuanensis | Gongdong, Yunnan, China | KIZ 048468 | MN475870 | Wu et al. (2019) |
| Raorchesteslongchuanensis | Gongdong, Yunnan, China | KIZ048492 | MN475871 | Wu et al. (2019) |
| Raorchestesmalipoensis | Pac Ban, Tuyen Quan, Vietnam | ROM30288 | GQ285674 | Li et al. (2009) |
| Raorchestesmalipoensis | Malipo, Yunnan, China | GXNU 000339 | ON128245 | Huang et al. (2023) |
| Raorchestesmenglaensis | Zhushihe, Yunnan, China | CIB116338 | MT488403 | Jiang et al. (2020) |
| Raorchestesmenglaensis | Zhushihe, Yunnan, China | CIB116340 | MT488404 | Jiang et al. (2020) |
| Raorchestesparvulus | Pulau Langkawi, Malaysia | LSUHC 7596 | MH590202 | Chan et al. (2018) |
| Raorchestesparvulus | Gunung Stong, Malaysia | LSUHC 11118 | MH590201 | Chan et al. (2018) |
| Raorchestesrezakhani | Maulovibazar, Bangladesh | JnUZool-A0319 | MN072374 | Al-Razi et al. (2020) |
| Raorchestesshillongensis | Malki forest, Shilong, Meghalaya, India | R2 | MG980283 | Unpublished |
| Raorchestes sp. 1 | India | CESF420 | JX092712 | Vijayakumar et al. (2014) |
| Raorchestestuberohumerus | Western Ghats, India | 0073PhiTub | EU450004 | Biju and Bossuyt, (2009) |
| Raorchestesuthamani | Western Ghats, India | CESF483 | JX092722 | Vijayakumar et al. (2014) |
| Raorchestesyadongensis | Yadong, Xizang, China | YBU 21222 | OP345440 | Zhang et al. (2022) |
| Raorchestesyadongensis | Yadong, Xizang, China | YBU 21223 | OP345441 | Zhang et al. (2022) |
| Pseudophilautuskani | Western Ghats, India | CESF497 | JX092724 | Vijayakumar et al. (2014) |
| Pseudophilautusamboli | Western Ghats, India | BNHS4399 | EU450025 | Biju and Bossuyt (2009) |
Phylogenetic analysis and species delimitation
To examine the phylogenetic position of the specimens collected from Hekou, Yunnan, China, we reconstructed phylogenetic trees of the genus Raorchestes based on sequences of the 16S rRNA (16S) genes. Furthermore, 35 homologous sequences of other Raorchestes species were obtained from GenBank (Table 2). Pseudophilautuskani (Biju & Bossuyt, 2009) and Pseudophilautusamboli (Biju & Bossuyt, 2009) were selected as outgroups based on Wu et al. (2021). All sequences were aligned in MEGA v. 7.0 (Kumar et al. 2016) using the ClustalW tool and both ends of the sequence were trimmed to minimize missing characters.
Phylogenetic relationships were inferred based on maximum likelihood (ML) and Bayesian inference (BI) analyses. BI analysis was conducted in MrBayes v. 3.2.6 (Ronquist et al. 2012). The best-fitting model (GTR + I + G) was chosen using the Akaike Information Criterion (AIC) in JModelTest v. 2.1.10 (Darriba et al. 2012). Four Monte Carlo Markov chains were started from a random tree. The chains were run for three million generations and sampled every 100 generations, with the first 25% of sampled trees discarded as burn-in. The remaining trees were used to create a consensus tree and to estimate Bayesian posterior probabilities (BPP). ML analysis was performed using RAxML v. 8.2.10 (Stamatakis 2014) under the GTR + I + G model. Tree searches were performed 100 times with 1000 bootstrap (BS) replicates to assess node support. Nodes with BPP ≥ 0.95 and BS ≥ 70 were considered well supported. Additionally, uncorrected pairwise genetic distances (p-distances) between species in 16S rRNA sequences were calculated using MEGA v. 7.0. (Kumar et al. 2016).
We used two approaches, i.e., the Bayesian Poisson Tree Processes (bPTP; Zhang et al. 2013) and Assemble Species by Automatic Partitioning (ASAP; Puillandre et al. 2021), to delimit species boundaries. The bPTP method was run on the bPTP server (http://species.h-its.org/) using the tree generated by Bayesian phylogenetic analysis and default parameters. For the ASAP method, the simple distance (p-distance) model was used and the partitioning with the lowest ASAP score was selected as the best, as per Puillandre et al. (2021).
Results
Phylogenetic analysis and genetic divergence
The obtained sequence alignment was 552 bp long and included 211 variable sites and 152 parsimony informative sites. Phylogenetic analysis (Fig. 2) revealed that the specimens from Hekou, Yunnan, China, R.malipoensis and R. UI from northern Vietnam formed a monophyletic group, which itself contained three distinct branches, one consisting of the specimens from Hekou and a specimen of R. UI from Pac Ban, Tuyen Quang, Vietnam (ROM 38828) with strong support (BPP = 100, BS = 100) and short internal branch lengths, one consisting only of R. UI from Tam Dao, Vinh Phuc, Vietnam (ROM 30298), and one consisting of the recently named bush frog species R.malipoensis, which included a specimen previously mistaken of “R.gryllus” from Pac Ban, Tuyen Quang, Vietnam (ROM 30288). The clade containing specimens from Hekou was recovered as the sister to R.malipoensis with strong support. The bPTP analysis delimited the three lineages into three candidate species (Fig. 3). The ASAP analysis identified 10 partitions (Fig. 3) and the best partition (score = 2.5) also grouped the three lineages into three candidate species. The 16Sp-distances between the clade consisting of Hekou specimens and the other Raorchestes lineages included in this study ranged from 2.5% (R.malipoensis) to 12.9% (R.archeos), greater than the divergence between R.hillisi and R.yadongensis (2.0%; Table 3).
Figure 2.
Bayesian phylogram of Raorchestesparvulus group estimated from 16S rRNA showing placement of Raorchesteshekouensis sp. nov. Nodal support values are shown above branches as Bayesian posterior probability (BPP) / ML bootstrap support (BS), and the symbol “-” indicates value below 50.
Figure 3.
ASAP species delimitation within Raorchestes based on 16S sequences. ASAP analysis generated 10 partitions and ranked them using the lowest ASAP score as the best option, and the best partition is highlighted in red. Black and gray vertical bars indicate results of bPTP species delimitation.
Table 3.
Uncorrected p-distance (%) in 16S rRNA sequences of Raorchestes species used in this study.
| ID | Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R.hekouensis sp. nov. | |||||||||||||||||||||||||
| 2 | R.malipoensis | 2.5 | ||||||||||||||||||||||||
| 3 | R. UI ROM30298 | 3.7 | 2.9 | |||||||||||||||||||||||
| 4 | R.longchuanensis | 4.1 | 3.5 | 3.2 | ||||||||||||||||||||||
| 5 | R.rezakhani | 5.3 | 5.0 | 4.6 | 4.8 | |||||||||||||||||||||
| 6 | R.andersoni | 5.4 | 4.9 | 4.9 | 4.4 | 5.2 | ||||||||||||||||||||
| 7 | R.tuberohumerus | 6.0 | 6.2 | 5.4 | 5.3 | 6.6 | 6.5 | |||||||||||||||||||
| 8 | R.menglaensis | 6.0 | 5.5 | 4.7 | 4.4 | 6.7 | 5.9 | 5.5 | ||||||||||||||||||
| 9 | R.annandalii | 6.1 | 5.7 | 5.3 | 4.6 | 5.1 | 4.4 | 7.0 | 6.4 | |||||||||||||||||
| 10 | R.hillisi | 6.1 | 5.2 | 3.5 | 4.5 | 5.2 | 5.3 | 5.6 | 5.5 | 5.5 | ||||||||||||||||
| 11 | R.parvulus | 6.1 | 6.8 | 6.9 | 5.2 | 8.2 | 6.7 | 7.3 | 2.5 | 6.5 | 7.3 | |||||||||||||||
| 12 | R.dulongensis | 6.2 | 5.7 | 3.7 | 3.9 | 5.6 | 4.8 | 6.8 | 5.8 | 5.9 | 3.7 | 7.5 | ||||||||||||||
| 13 | R.leucolatus | 6.6 | 6.1 | 5.9 | 5.1 | 7.3 | 6.2 | 3.3 | 5.3 | 6.4 | 6.5 | 5.9 | 7.3 | |||||||||||||
| 14 | R.yadongensis | 6.7 | 5.1 | 3.9 | 4.4 | 6.0 | 4.9 | 5.5 | 5.5 | 5.3 | 2.0 | 6.7 | 3.7 | 5.7 | ||||||||||||
| 15 | R.ghatei | 7.1 | 6.7 | 5.2 | 5.4 | 5.7 | 5.0 | 5.7 | 6.5 | 5.9 | 5.7 | 7.7 | 5.8 | 4.9 | 5.2 | |||||||||||
| 16 | R.cangyuanensis | 7.6 | 6.6 | 5.8 | 5.8 | 6.5 | 4.4 | 7.3 | 5.8 | 6.0 | 6.2 | 7.6 | 6.4 | 6.9 | 5.4 | 6.5 | ||||||||||
| 17 | R.huanglianshan | 7.6 | 6.8 | 6.0 | 5.5 | 6.3 | 6.3 | 7.1 | 4.7 | 6.7 | 5.8 | 5.5 | 6.1 | 7.0 | 5.6 | 6.9 | 6.4 | |||||||||
| 18 | R. sp 1 | 8.5 | 8.0 | 7.2 | 6.3 | 7.0 | 8.1 | 8.1 | 9.0 | 7.6 | 8.1 | 10.6 | 7.8 | 8.0 | 8.0 | 7.7 | 9.7 | 9.9 | ||||||||
| 19 | R.uthamani | 8.7 | 7.9 | 8.6 | 8.2 | 9.0 | 8.3 | 8.9 | 8.6 | 9.2 | 8.5 | 8.6 | 8.8 | 6.8 | 8.3 | 9.1 | 9.9 | 9.0 | 11.5 | |||||||
| 20 | R.shillongensis | 8.9 | 8.0 | 7.3 | 6.8 | 5.8 | 7.3 | 8.5 | 8.0 | 7.0 | 7.9 | 9.2 | 7.7 | 7.8 | 7.5 | 8.4 | 9.8 | 8.2 | 7.4 | 10.6 | ||||||
| 21 | R.charius | 9.2 | 9.3 | 9.1 | 8.4 | 8.6 | 8.9 | 7.8 | 8.1 | 10.1 | 9.2 | 8.9 | 9.7 | 8.0 | 8.9 | 8.5 | 9.8 | 7.7 | 10.9 | 7.9 | 10.4 | |||||
| 22 | R.agasthyaensis | 9.7 | 9.3 | 9.3 | 9.4 | 9.6 | 9.3 | 9.1 | 9.0 | 11.0 | 8.5 | 9.4 | 9.4 | 7.8 | 9.0 | 10.4 | 10.9 | 9.9 | 12.5 | 6.8 | 11.9 | 9.2 | ||||
| 23 | R.chromasynchysi | 9.8 | 9.1 | 9.1 | 7.4 | 8.8 | 7.8 | 9.4 | 8.7 | 9.7 | 8.5 | 9.1 | 8.0 | 7.8 | 7.4 | 7.2 | 8.8 | 8.6 | 10.3 | 5.7 | 9.5 | 7.1 | 7.3 | |||
| 24 | R.crustai | 12.6 | 11.4 | 10.3 | 11.6 | 10.6 | 10.7 | 10.4 | 10.7 | 12.8 | 9.6 | 11.4 | 10.3 | 9.4 | 9.1 | 10.2 | 11.8 | 10.4 | 14.7 | 5.3 | 13.0 | 7.9 | 6.2 | 9.5 | ||
| 25 | R.archeos | 12.9 | 11.2 | 12.5 | 12.2 | 12.1 | 12.4 | 12.6 | 11.3 | 12.2 | 11.1 | 11.8 | 12.6 | 11.8 | 10.1 | 13.5 | 12.8 | 11.7 | 14.7 | 9.1 | 15.8 | 10.1 | 8.2 | 10.1 | 9.2 |
Taxonomic account
. Raorchestes hekouensis sp. nov.
3E4731DD-A46D-5E36-A4A1-1751669D45AA
https://zoobank.org/4175879C-5620-49B0-B016-8489657C6069
Table 1 , Figs 4 , 5 , 6 , 7 , 8
Figure 4.
Photographs of holotype of Raorchesteshekouensis sp. nov. (GXNU YU000159) in life. Lateral view (A), dorsal view (B), ventral view (C), fingers (D), toes (E), crotch (F).
Figure 5.
Photographs of Raorchesteshekouensis sp. nov. holotype (GXNU YU000159) in preservative, dorsal view (A), ventral view of hand (B), ventral view of foot (C), ventral view (D).
Figure 6.
Photographs of Raorchesteshekouensis sp. nov. paratype (GXNU YU000160) in preservative, dorsal view (A), dorsal view of hand (B), ventral view of foot (C), ventral view (D).
Figure 7.
Photographs of Raorchesteshekouensis sp. nov. paratype (GXNU YU000156) in preservative, dorsal view (A), and ventral view (B).
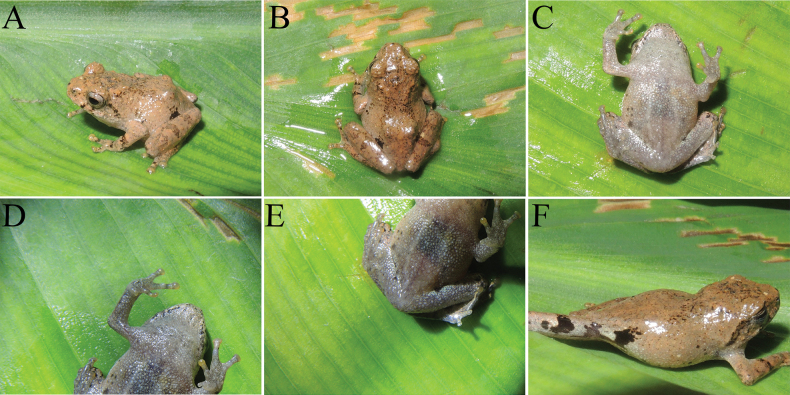
Figure 8.
Photographs of Raorchesteshekouensis sp. nov. paratype (GXNU YU000537) in life, dorsal view (A), and lateral view (B).
Chresonymy.
Raorchestesgryllus (Li et al. 2013).
Type material.
Holotype. GXNU YU000159, adult male, collected on 25 March 2019 by Shuo Liu from Liangzi, Hekou, Yunnan, China (22°49'N, 103°44′E, 1200 m a.s.l.; Fig. 1).
Paratypes. Adult female (GXNU YU000160), three sub-adults (GXNU YU000153, GXNU YU000154, and GXNU YU000156) with the same collection information as the holotype, and three adult males (GXNU YU000536, GXNU YU000537 and GXNU YU000538) collected at the same locality as the holotype on 4 April 2023 by Lingyun Du and Shuo Liu.
Etymology.
The specific epithet hekouensis is named after the type locality, Hekou County, Yunnan, China. We suggest “Hekou bush frog” as its English common name, and “Hé Kǒu Guàn Shù Wā (河口灌树蛙)” as its Chinese common name.
Diagnosis.
Raorchesteshekouensis sp. nov. is distinguished from all other relevant congeners by a combination of the following characters: (1) small body size (male SLV 16.1–17.5 mm, n = 4; female 21.1 mm, n = 1); (2) tympanum distinct; (3) tips of all fingers and toes expanded into discs with circummarginal grooves; (4) rudimentary webbing on toes; (5) all fingers and toes with lateral dermal fringes; (6) inner metacarpal tubercle present and outer metacarpal tubercle indistinct; (7) heels meeting when limbs held at right angles to body; (8) discs of fingers and toes yellow; (9) male with external single subgular vocal sac; (10) distinct X-shaped dark brown marking on back; (11) inner metatarsal tubercle oval, outer metatarsal tubercle absent.
Description of holotype.
GXNU YU000159, adult male, body size small (SVL 17.5 mm); head wider than long (HW = 6.9 mm, HL = 6.1 mm); snout rounded in profile, projecting beyond lower jaw, snout length almost equal to diameter of eye (SL = 2.4 mm; ED = 2.5 mm); canthus rostralis rounded, loreal region slightly concave; internarial distance slightly less than interorbital distance, and wider than maximum width of upper eyelid (INS = 2.2 mm; IOS = 2.4 mm; UEW = 1.9 mm); tympanum distinct (TD = 1.3 mm); tongue pyriform, with deep notch at posterior tip; vomerine teeth absent; temporal fold distinct; dorsolateral fold absent. Length of forelimb and hand slightly shorter than half of snout-vent length (LAHL = 8.5 mm, SVL = 17.5); relative fingers lengths: I < II < IV < III; tips of all four fingers expanded into discs with circummarginal grooves; lateral dermal fringes on all fingers; subarticular tubercles distinct, rounded; supernumerary tubercles absent; no webbing between fingers; inner metacarpal tubercle present, outer metacarpal tubercle indistinct; nuptial pads present on first and second fingers in male. Hindlimbs relatively slender, thigh length (TIL = 9.2) shorter than tibia length (TL = 11.4), but greater than foot length (FL = 6.6); tibiotarsal articulation reaching anterior of eye when hindlimb stretched alongside body; heels meeting when limbs held at right angles to body; relative toe lengths: I < II < III < V < IV; tips of toes with well-developed discs with circummarginal grooves; all toes with lateral dermal fringes; subarticular tubercles distinct, rounded; supernumerary tubercles absent; rudimentary webbing between toes; inner metatarsal tubercle rounded, outer metatarsal tubercle absent. Dorsal surfaces rough, dorsum, dorsal surface of limbs, snout, between eyes, and upper eyelid shagreened with numerous tubercles; flank of body, dorsal part of forelimbs, thighs, and tibia relatively smooth, scattered with sparse granules; throat, chest, and ventral surfaces of forelimbs smooth; abdomen, underside of thigh, and around vent with granules; dorsolateral folds absent; dorsal, dorsal surface of limbs and around vent with several beige patches.
Coloration of holotype in life.
Dorsal surface yellowish brown, with distinct dark brown X-shaped marking on back; blackish line between eyes; tea-brown spots on both sides of lower jaw; dorsal side of limbs with several brown bands; flank near crotch with distinct black region between two creamy white patches, thighs with similar black patch near groin, next to another creamy white patch; ventral surface of throat, chest, ventral side of limbs, and belly opaque creamy white with small black spots and white tubercles; finger and toe discs yellow (Fig. 4).
Coloration of holotype in preservative.
Dorsal color changed to grayish brown; forelimbs and hindlimbs with black-brown bands; patches or spots blackish brown; abdomen and ventral sides of limbs still milky white with several small black spots (Fig. 5).
Male secondary sexual characteristics.
Adult male with nuptial pads on dorsal surface of first and second fingers and external single subgular vocal sac with slit-like opening at posterior of jaw. White lineae masculinae visible on ventral body.
Variation.
Specimen GXNU YU000160 significantly has more black spots on the abdomen and near the cloaca (Fig. 6), specimen GXNU YU000156 differs from the other seven type specimens (GXNU YU000159, GXNU YU000160, GXNU YU000153, GXNU YU000154, GXNU YU000536, GXNU YU000537, and GXNU YU000538) by pale yellow mid-dorsal vertebral stripe from snout to vent, pale yellow stripe along hindlimbs crossing at vent region, mid-ventral stripe from snout to vent and stripe along forelimbs crossing at breast region (Fig. 7), and the specimen GXNU YU000537 has distinctly darker ground color on the dorsal side, especially on the head (Fig. 8).
Distribution.
Currently known from the type locality, Hekou County, Yunnan Province, China, and Bac Pan, Tuyen Quang, Vietnam.
Habitat.
In Yunnan, Raorchesteshekouensis sp. nov. was found in shrubs and herbs on the edge of a small stream near the road at an elevation of ca 1200 m a.s.l. (Fig. 9) on the nights of 25 March 2019 and 4 April 2023. There were many herbaceous plants near the stream, such as Ageratinaadenophora. No male was heard calling and no eggs were observed during our surveys in late March, but there were males calling during our surveys in April. Therefore, the breeding season for this species starts in April.
Figure 9.
Habitat at type locality of Raorchesteshekouensis sp. nov. at Liangzi Village, Hekou, Yunnan, China.
Remarks.
Raorchesteshekouensis sp. nov. is assigned to the genus Raorchestes based on its molecular phylogenetic position and the following morphological characters: relatively small body size (SVL 15.0–45.0 mm); absence of vomerine teeth; large transparent/translucent vocal sac. Due to the close phylogenetic relationship and distribution (Figs 1, 2), we compared the new species with 16 recognized congeners distributed in Southeast Asia, southwestern China, the Himalayas, and northeastern India, as mentioned above. Raorchesteshekouensis sp. nov. is distinguished from all other 16 congeners by a unique combination of characters. A detailed morphological comparison table of currently known Raorchestes species from China is provided (Table 4).
Table 4.
Morphological comparison among currently known species of Raorchestes in China (? = unknown).
| Character | Raorchesteshekouensis sp. nov. | R.cangyuanensis | R.dulongensis | R.menglaensis | R.longchuanensis | R.huanglianshan | R.hillisi | R.andersoni | R.yadongensis | R.malipoensis |
|---|---|---|---|---|---|---|---|---|---|---|
| SVL of adult male (mm) | 16.1–17.5 | 16.1–20.0 | 15.0–19.0 | 15.0–21.6 | 17.8–21.2 | 17.0–19.6 | 15.9–17.7 | 24.0 | 17.8–24.1 | 14.6–19.3 |
| HDL/HDW | HDL < HDW | HDL < HDW | HDL > HDW | HDL ≈ HDW | HDL ≈ HDW | HDL ≤ HDW | HDL > HDW | HDL < HDW | HDL < HDW | HDL < HDW |
| Tympanum | Distinct | Indistinct | Distinct | Indistinct | Distinct | Distinct | Distinct | Distinct | Distinct | Distinct |
| Nuptial pad | Present | Present | Absent | Present | Present | Present | Present | ? | Present | Present |
| Vocal sac | External single subgular vocal sac | External single subgular vocal sac | External single subgular vocal sac | Internal single subgular vocal sac | External single subgular vocal sac | External single subgular vocal sac | External single subgular vocal sac | Internal single subgular vocal sac | External single subgular vocal sac | External single subgular vocal sac |
| Finger web | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Rudimentary | Absent |
| Toe web | Rudimentary | Rudimentary | Rudimentary | Rudimentary or 1/4 | 1/4 webbing | Rudimentary, except between toe I and toe II | Rudimentary, except between toe I and toe II | Rudimentary or 1/3 | Rudimentary | Rudimentary |
| Outer metatarsal tubercle | Absent | Absent | Absent | Present | Absent | Absent | Absent | Absent | Absent | Absent |
| Relative toe lengths | I < II < III < V < IV | I < II < V < III < IV | I < II < V < III < IV | I < II < III ≤ V < IV | I < II < III = V < IV | I < II < III < V < IV | I < II < III < V < IV | I < II < III ≤ V < IV | I < II < III < V < IV | I < II < V < III < IV |
| Reference | This study; Li et al. (2013) | Wu et al. (2019) | Wu et al. (2020) | Kou 1990; Jiang et al. (2020) | Yang and Li 1987; Fei et al. (2009) | Jiang et al. (2020) | Jiang et al. (2020) | Anderson 1978; Fei et al. (2009); Che et al. (2020) | Zhang et al. (2022) | Huang et al. (2023) |
Raorchestesgryllus is still considered a member of Raorchestes in Frost (2023), although Poyarkov et al. (2021) suggested that it should be transferred to the genus Kurixalus. Raorchesteshekouensis sp. nov. can be distinguished from K.gryllus based on the following characters: no webbing between fingers (vs rudimentary webbing between fingers), rudimentary webbing between toes (vs little more than half webbed), heel with no pointed appendage (vs heel with small, pointed appendage), snout rounded (vs snout pointed with dermal tip), and series of tubercles along outer side of forearm and foot absent (vs present). Raorchesteshekouensis sp. nov. differs from R.malipoensis by inner metacarpal tubercle present, outer metacarpal tubercle indistinct (vs inner and outer metacarpal tubercle indistinct), heels meeting when limbs held at right angles to body (vs heels not meeting when limbs held at right angles to body); and relative toe lengths: I < II < III < V < IV (vs I < II < V < III < IV). Raorchesteshekouensis sp. nov. is distinguishable from R.huanglianshan by supernumerary tubercles absent (vs present) and lateral dermal fringes on all fingers and toes present (vs absent). Raorchesteshekouensis sp. nov. differs from R.parvulus by length of lower arm and hand slightly shorter than half of body size (vs longer than half of body size) and supernumerary tubercles absent (vs present on third finger). Raorchesteshekouensis sp. nov. differs from R.menglaensis by external single subgular vocal sac in adult male (vs internal single subgular vocal sac), tympanum distinct in male (vs indistinct), and lateral dermal fringes on all fingers and toes present (vs absent). Raorchesteshekouensis sp. nov. differs from R.cangyuanensis by tympanum distinct in male (vs indistinct) and relative toe lengths: I < II < III < V < IV (vs I < II < V < III < IV). Raorchesteshekouensis sp. nov. differs from R.hillisi by head wider than long (vs head longer than wide) and lateral dermal fringes on all fingers and toes present (vs fingers lacking lateral dermal fringes and toes with weak lateral dermal fringes, except outside of toe I and both sides of toe II). Raorchesteshekouensis sp. nov. differs from R.dulongensis by head wider than long (vs head longer than wide), snout rounded (vs pointed), relative toe lengths: I < II < III < V < IV (vs I < II < V < III < IV), and nuptial pad present (vs absent). Raorchesteshekouensis sp. nov. differs from R.longchuanensis by head wider than long (vs head length almost equal to width) and lateral dermal fringes on all fingers and toes (vs lateral dermal fringes only on fingers I and II and no lateral dermal fringes on toes). Raorchesteshekouensis sp. nov. differs from R.andersoni by tibiotarsal articulation reaching anterior of eye (vs tibiotarsal articulation reaching tip of snout), ventral surface of throat, chest, and belly opaque creamy white, with small black spots (vs chest and belly yellowish, with brown punctuations), and flank near crotch with distinct black region between two creamy white patches (vs irregular large black patch on groin, extending to half of side, with two yellow patches). Raorchesteshekouensis sp. nov. differs from R.yadongensis by lacking webbing between fingers (vs fingers with rudimentary webbing) and tibiotarsal articulation reaching anterior of eye when adpressed (vs tibiotarsal articulation reaching tip of snout when adpressed).
Raorchesteshekouensis sp. nov. differs from R.rezakhani by nuptial pad present (vs absent), dermal fringes present on fingers (vs absent), rudimentary webbing between toes (vs webbing moderate, formula: I2-2+II1¾-2+III1½-3IV2¾-2-V), and inner metacarpal and inner metatarsal tubercles present (vs absent). Raorchesteshekouensis sp. nov. differs from R.annandalii by snout rounded (vs pointed), supernumerary tubercles in toes absent (vs present), and inner metatarsal tubercle present (vs absent). Raorchesteshekouensis sp. nov. differs from R.shillongensis by inner metatarsal tubercles distinct, outer metatarsal tubercle absent (vs inner metatarsal tubercle indistinct, outer metatarsal tubercle present), and relative toe lengths: I < II < III < V < IV (vs I ≤ II < V ≤ III < IV). Raorchesteshekouensis sp. nov. differs from R.sahai by rudimentary webbing between toes (vs nearly half-webbed in toes) and mid-dorsal line absent (vs dark narrow line originating from interorbital region and extending posteriorly to hindmost part of body). Raorchesteshekouensis sp. nov. differs from R.manipurensis by rudimentary webbing between toes (vs almost 2/3 webbing in toes) and webbing between fingers absent (vs present).
Key to Raorchestes species in China
| 1 | Fingers with rudimentary webbing | R.yadongensis |
| – | Fingers without webbing | 2 |
| 2 | Tympanum indistinct | 3 |
| – | Tympanum distinct | 4 |
| 3 | Fingers and toes with lateral dermal fringes | R.cangyuanensis |
| – | Fingers and toes lacking lateral dermal fringes | R.menglaensis |
| 4 | Internal single subgular vocal sac | R.andersoni |
| – | External single subgular vocal sac | 5 |
| 5 | Nuptial pad absent | R.dulongensis |
| – | Nuptial pad present | 6 |
| 6 | Toes with one-fourth webbing | R.longchuanensis |
| – | Toes not with one-fourth webbing | 7 |
| 7 | Fingers with lateral dermal fringe | 8 |
| – | Fingers lacking lateral dermal fringe | 9 |
| 8 | relative toe lengths: I < II < III < V < IV | Raorchesteshekouensis sp. nov. |
| – | relative toe lengths: I < II < V < III < IV | R.malipoensis |
| 9 | Toes lacking lateral dermal fringe | R.huanglianshan |
| – | Toes with weak lateral dermal fringes, except outside of toe I and both sides of toe II | R.hillisi |
Discussion
The small body size, morphological conservativeness, and remarkably similar characters in the Raorchestes genus have resulted in ambiguities in taxonomy and distribution (Jiang et al. 2020), necessitating the application of molecular identification (Orlov et al. 2012). Morphologically, the types and topotypes of Kurixalusgryllus are very similar to other members of the genus due to the series of tubercles along the outer side of the forearm and feet, small pointed appendage on the heel, and pointed snout with a dermal tip (Smith 1924; Orlov et al. 2012; Poyarkov et al. 2021), with wide variation in living color patterns of K.gryllus from the type locality shown to be very similar to that seen in Kurixalusmotokawai and Kurixalusbanaensis (Nguyen, 2015; see Fig. 10). Therefore, we agree with Poyarkov et al. (2021) that “R.gryllus” from the type locality should be reassigned to Kurixalus. We also consider that the samples of so-called “R.gryllus” from northern Vietnam used in the present study (ROM 38828 and ROM 30298) are not conspecific with K.gryllus from the type locality as they are phylogenetically nested within the genus Raorchestes (Fig. 2).
Figure 10.
Kurixalusgryllus from Dak Lak Province (Chu Yang Sin National Park) and Lam Dong Province (Bidoup-Nui Ba National Park) in southern Vietnam (sourced from Orlov et al. 2012).
Our results showed that the R. UI ROM 38828 from northern Vietnam clustered with Raorchesteshekouensis sp. nov. with a short branch length, indicating that R. UI ROM 38828 belonged to the new species (Fig. 2), and recently Huang et al. (2023) revised the specimen ROM 30288 from northern Vietnam, which had been recorded as R.gryllus, to R.malipoensis so the taxonomic status of the R. UI specimen from northern Vietnam (ROM 30298) needs further confirmation. The genetic divergences between R.malipoensis, R. UI ROM 30298, and Raorchesteshekouensis sp. nov. were greater than the divergence between R.hillisi and R.yadongensis (Table 3), and species delimitations grouped them into three different candidate species (Fig. 3), indicating that the clade comprised of ROM 30298 likely represented an unnamed species, pending further morphological study. Of note, both the ROM 38828 and ROM 30288 specimens were collected from Pac Ban, Tuyen Quang, Vietnam, suggesting the coexistence of R.malipoensis and the new species Raorchesteshekouensis sp. nov. in that region, which means the records of Raorchestes from that region also need verification.
In this study, we used distance-based (ASAP) and tree-based (bPTP) delimitation methods, and the two different species delimitation methods give the same results. The ASAP analysis divides species based on pairwise genetic distance, but it can provide a score for each partitioning result for users to refer to and select partitioning results. The difference is that bPTP delimits species using non-hypermetric phylogenies, and estimates speciation events in terms of a number of substitutions; therefore, it only requires a standard phylogenetic tree as input. The combination of both methods confirms the species delimitation and helps overcome the constraints of each approach (Carstens et al. 2013).
With the description of the new species, there are now ten Raorchestes species known from China, all of which occur in Yunnan except for R.yadongensis, which is only known from southern Tibet, China (Zhang et al. 2022). Recently Garg et al. (2021) assigned the Raorchestes species into 16 species groups and the clade containing species from Southeast and East Asia (e.g., R.parvulus, R.menglaensis, R.cangyuanensis) was placed in the R.parvulus species group. Based on Garg et al. (2021) and our phylogenetic results, the new species also belongs to the R.parvulus group. The continuous discovery of new Raorchestes species from Yunnan in recent years (Wu et al. 2019, 2021; Jiang et al. 2020; Huang et al. 2023; this study) indicates that Raorchestes diversity is seriously underestimated in Yunnan. We expect that more Raorchestes species will be found from southern Yunnan given the unnamed lineage in adjacent northern Vietnam mentioned above, from Tam Dao, Vinh Phuc (ROM 30298). Therefore, further studies employing a wider range of Raorchestes samples across its distribution are necessary to clarify the species boundary in Yunnan.
Due to the placement of “R.gryllus” sensu stricto in Kurixalus, the number of recognized Raorchestes species known from Southeast Asia is decreased to five, including R.parvulus, R.longchuanensis, R.menglaensis, R.malipoensis, and R.huanglianshan based on recent studies (Poyarkov et al. 2021; Jiang et al. 2020; Wu et al. 2022; Huang et al. 2023); our results revealed the existence of an additional but unnamed lineage in northern Vietnam. Previous phylogenetic analyses have also revealed that nominal R.parvulus, which is widely reported across Indochina (Frost 2023), also contains multiple clades that do not form a monophyly (Chan et al. 2018; Wu et al. 2019; Yu et al. 2019; Jiang et al. 2020), indicating that multiple cryptic species may exist within the species. Therefore, Raorchestes species diversity in Southeast Asia may be highly underestimated.
Supplementary Material
Acknowledgements
We thank Qiumei Mo and Mr. Hong Hui for assistance during fieldwork.
Citation
Du L, Xu Y, Liu S, Yu G (2024) A new species of Raorchestes (Anura, Rhacophoridae) from Yunnan Province, China. ZooKeys 1192: 213–235. https://doi.org/10.3897/zookeys.1192.106013
Contributor Information
Shuo Liu, Email: liushuo@mail.kiz.ac.cn.
Guohua Yu, Email: yugh2018@126.com.
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32060114, 31872212), Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education (ERESEP2022Z04), and Guangxi Key Laboratory of Rare and Endangered Animal Ecology, Guangxi Normal University (19-A-01-06).
Author contributions
Conceptualization: GY, SL. Formal analysis: LD, YX. Investigation: SL, LD. Software: YX. Writing - original draft: YX, LD.
Author ORCIDs
Lingyun Du https://orcid.org/0000-0002-5761-4017
Shuo Liu https://orcid.org/0000-0001-7825-3006
Guohua Yu https://orcid.org/0000-0002-0220-6550
Data availability
All of the data that support the findings of this study are available in the main text.
References
- Abraham RK, Pyron RA, Ansil BR, Zachariah A, Zachariah A. (2013) Two novel genera and one new species of treefrog (Anura: Rhacophoridae) highlight cryptic diversity in the Western Ghats of India. Zootaxa 3640(2): 177–189. 10.11646/zootaxa.3640.2.3 [DOI] [PubMed] [Google Scholar]
- Ahl E. (1927) Zur Systematik der asiatischen arten der froschgattung Rhacophorus. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1927: 35–47. [Google Scholar]
- Al-Razi H, Maria M, Muzaffar SB. (2020) A new species of cryptic Bush frog (Anura, Rhacophoridae, Raorchestes) from northeastern Bangladesh. ZooKeys 927: 127–151. 10.3897/zookeys.927.48733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AmphibiaChina (2022) The database of Chinese amphibians. Kunming Institute of Zoology (CAS), Kunming, Yunnan, China. http://www.amphibiachina.org/
- Anderson J. (1878) Anatomical and zoological researches: Comprising an account of the zoological results of the two expeditions to Western Yunnan in 1868 and 1875. Vol 1. Bernard Quaritch, London, 703–860 + 969–975. [index] 10.5962/bhl.title.50434 [DOI]
- Biju SD, Bossuyt F. (2009) Systematics and phylogeny of Philautus Gistel, 1848 (Anura, Rhacophoridae) in the Western Ghats of India, with descriptions of 12 new species. Zoological Journal of the Linnean Society 9(155): 374–444. 10.1111/j.1096-3642.2008.00466.x [DOI] [Google Scholar]
- Biju SD, Shouche Y, Dubois A, Dutta SK, Bossuyt F. (2010) A ground-dwelling rhacophorid frog from the highest mountain peak of the Western Ghats of India. Current Science 98(8): 1119–1125. [Google Scholar]
- Bossuyt F, Dubois A. (2001) A review of the frog genus Philautus Gistel, 1848 (Amphibia, Anura, Ranidae, Rhacophorinae). Zeylanica 6(1): 1–112. [Google Scholar]
- Boulenger GA. (1893) Concluding report on the reptiles and batrachians obtained in Burma by Signor L. Fea dealing with the collection made in Pegu and the Karin Hills in 1887–88. Annali del Museo Civico di Storia Naturale di Genova 2(13): 304–347. [Google Scholar]
- Boulenger GA. (1906) Description of two Indian frogs. Journal of the Asiatic Society of Bengal 2(2): 385–386. [Google Scholar]
- Bourret R. (1937) Notes herpétologiques sur L’lndochine Française. XIV. Les Batraciens de la Collection du Laboratoire des Sciences naturelles de l’Université. Descriptions de quinze espèces ou variétés nouvelles. Annexe au Bulletin Général de l’Instruction Publique Hanoi 4: 5–56. [Google Scholar]
- Bourret R. (1939) Notes herpétologiques sur L’lndochine Française XVII. Reptiles et batraciens reçus au Laboratoire des Sciences Naturelles de l’Université au cours de l’année 1938. Descriptions de trois espèces Nouvelles. Annexe au Bulletin Général de l’Instruction Publique Hanoi 6: 13–34. [Google Scholar]
- Bourret R. (1942) Les Batraciens de L’Indochine. Hanoi: Institut Océanographique de l’Indochine, 517 pp.
- Carstens BC, Pelletier TA, Reid N, Satler JD. (2013) How to fail at species delimitation. Molecular Ecology 22(17): 4369–4383. 10.1111/mec.12413 [DOI] [PubMed] [Google Scholar]
- Chan KO, Grismer LL, Brown RM. (2018) Comprehensive multi-locus phylogeny of Old World tree frogs (Anura: Rhacophoridae) reveals taxonomic uncertainties and potential cases of over-and underestimation of species diversity. Molecular Phylogenetics and Evolution 127: 1010–1019. 10.1016/j.ympev.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Che J, Jiang K, Yan F, Zhang YP. (2020) Amphibians and Reptiles in Tibet-Diversity and Evolution. Science Press, Beijing, 803 pp. [In Chinese] [Google Scholar]
- Chen JM, Prendini E, Wu YH, Zhang BL, Suwannapoom C, Chen HM, Jin JQ, Lemmon EM, Lemmon AR, Stuart BL, Raxworthy CJ, Murphy RW, Yuan ZY, Che J. (2020) An integrative phylogenomic approach illuminates the evolutionary history of Old World tree frog (Anura: Rhacophoridae). Molecular Phylogenetics and Evolution 145: 106724. 10.1016/j.ympev.2019.106724 [DOI] [PubMed]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): 772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LN, Liu S, Hou M, Yu GH. (2020) First record of Thelodermapyaukkya Dever, 2017 (Anura: Rhacophoridae) in China, with range extension of Thelodermamoloch (Annandale, 1912) to Yunnan. Zoological Research 41(5): 576–580. 10.24272/j.issn.2095-8137.2020.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei L, Hu SQ, Ye CY, Huang YZ. (2009) Fauna Sinica. Amphibia Vol. 2 Anura. Science Press, Beijing, 957 pp. [In Chinese] [Google Scholar]
- Fei L, Ye CY, Jiang JP. (2012) Colored Atlas of Chinese Amphibians and Their Distributions. Sichuan Publishing House of Science and Technology, Chengdu. [In Chinese]
- Frost DR. (2023) Amphibian Species of the World: and Online Reference. Version 6.1. American Museum of Natural History, New York, USA. https://amphibiansoftheworld.amnh.org/ [Accessed 5 May 2023]
- Gan YL, Yu GH, Wu ZJ. (2020) A new species of the genus Amolops (Anura: Ranidae) from Yunnan, China. Zoological Research 41(2): 188–193. 10.24272/j.issn.2095-8137.2020.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Suyesh R, Das S, Bee MA, Biju SD. (2021) An integrative approach to infer systematic relationships and define species groups in the shrub frog genus Raorchestes, with description of five new species from the Western Ghats, India. PeerJ 9: e10791. 10.7717/peerj.10791 [DOI] [PMC free article] [PubMed]
- Hedges SB. (1994) Molecular evidence for the origin of birds. Proceedings of the National Academy of Sciences of the United States of America 91(7): 2621–2624. 10.1073/pnas.91.7.2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Yu GH, Chen HM, Liao CL, Zhang L, Chen J, Li PP, Orlov NL. (2017) The taxonomic status and distribution range of six Theloderma species (Anura: Rhacophoridae) with a new record in China. Russian Journal of Herpetology 24(2): 99–127. 10.30906/1026-2296-2019-24-2-99-127 [DOI] [Google Scholar]
- Huang JK, Liu XL, Du LY, Bernstein JM, Liu S, Yang Y, Yu GH, Wu ZJ. (2023) A new species of Bush frog (Anura, Rhacophoridae, Raorchestes) from southeastern Yunnan, China. ZooKeys 1151: 47–65. 10.3897/zookeys.1151.95616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Ren JL, Wang J, Guo JF, Wang Z, Liu YH, Jiang DC, Li JT. (2020) Taxonomic revision of Raorchestesmenglaensis (Kou, 1990) (Amphibia: Anura), with descriptions of two new species from Yunnan, China. Asian Herpetological Research 11(4): 263–281. 10.16373/j.cnki.ahr.200018 [DOI] [Google Scholar]
- Khatiwada JR, Wang B, Zhao T, Xie F, Jiang JP. (2021) An integrative taxonomy of amphibians of Nepal: An updated status and distribution. Asian Herpetological Research 12(1): 1–35. 10.16373/j.cnki.ahr.200050 [DOI] [Google Scholar]
- Kou ZT. (1990) A new species of genus Philautus (Amphibia: Rhacophoridae) from Yunnan, China. In: From Water onto Land. China Forestry Press, Beijing, 210–212. [In Chinese]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33(7): 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent RF. (1943) Contribution a l’osteologie et a la systematique des rhacophorides non Africains. Bulletin du Musée Royal d’Histoire Naturelle de Belgique 19: 1–16. [Google Scholar]
- Li JT, Che J, Murphy RW, Zhao H, Zhao EM, Rao DQ, Zhang YP. (2009) New insights to the molecular phylogenetics and generic assessment in the Rhacophoridae (Amphibia: Anura) based on five nuclear and three mitochondrial genes, with comments on the evolution of reproduction. Molecular Phylogenetics and Evolution 53(2): 509–522. 10.1016/j.ympev.2009.06.023 [DOI] [PubMed] [Google Scholar]
- Li JT, Li Y, Klaus S, Rao DQ, Hillis DM, Zhang YP. (2013) Diversification of rhacophorid frogs provides evidence for accelerated faunal exchange between India and Eurasia during the Oligocene. Proceedings of the National Academy of Sciences of the United States of America 110(9): 3441–3446. 10.1073/pnas.1300881110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, He YH, Wang YF, Beukema W, Hou SB, Li YC, Che J, Yuan ZY. (2021) A new frog species of the genus Odorrana (Anura: Ranidae) from Yunnan, China. Zootaxa 4908(2): 263–275. 10.11646/zootaxa.4908.2.7 [DOI] [PubMed] [Google Scholar]
- Mathew R, Sen N. (2009) Studies on little known amphibians of Northeast India. Records of the Zoological Survey of India. Occasional Papers 293: 1–64. [Google Scholar]
- Matsui M, Shimada T, Liu WZ, Maryati M, Khonsue W, Orlov N. (2006) Phylogenetic relationships of oriental torrent frogs in the genus Amolops and its allies (Amphibia, Anura, Ranidae). Molecular Phylogenetics and Evolution 38(3): 659–666. 10.1016/j.ympev.2005.11.019 [DOI] [PubMed] [Google Scholar]
- Meegaskumbura M, Senevirathne G, Manamendra-Arachchi K, Pethiyagoda R, Hanken J, Schneider CJ. (2019) Diversification of shrub frogs (Rhacophoridae, Pseudophilautus) in Sri Lanka–timing and geographic context. Molecular Phylogenetics and Evolution 132(8): 14–24. 10.1016/j.ympev.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Nguyen TT. (2015) Systematic study of the rhacophorid frogs in Vietnam. Dissertation. Kyoto University.
- Nguyen SV, Ho CT, Nguyen TQ. (2009) Herpetofauna of Vietnam. Edition Chimaira, Frankfurt am Main, 768 pp. [Google Scholar]
- Nguyen TT, Matsui M, Duc HM. (2014) A new tree frog of the genus Kurixalus (Anura: Rhacophoridae) from Vietnam. Current Herpetology 33(2): 101–111. 10.5358/hsj.33.101 [DOI] [Google Scholar]
- Orlov NL, Murphy RW, Ananjeva NB, Ryabov SA, Ho CT. (2002) Herpetofauna of Vietnam, a checklist. Part 1. Amphibia. Russian Journal of Herpetology 9: 81–104. [Google Scholar]
- Orlov NL, Poyarkov AN, Vassilieva AB, Ananjeva NB, Nguyen TT, Sang NV, Geissler P. (2012) Taxonomic notes on rhacophorid frogs (Rhacophorinae: Rhacophoridae: Anura) of southern part of Annamite Mountains (Truong Son, Vietnam), with description of three new species. Russian Journal of Herpetology 19(1): 23–64. [Google Scholar]
- Pillai RS, Chanda SK. (1973) Philautusshillongensis, a new frog (Ranidae) from Meghalaya, India. Proceedings of the Indian Academy of Sciences. Section B, Biological Sciences 78(1): 30–36. 10.1007/BF03045421 [DOI] [Google Scholar]
- Poyarkov NA, Nguyen TV, Popov ES, Geissler P, Pawangkhanant P, Thy N, Suwannapoom C, Orlov NL. (2021) Recent progress in taxonomic studies, biogeographic analysis, and revised checklist of amphibians in Indochina. Russian Journal of Herpetology 28(3): 1–110. 10.30906/1026-2296-2021-28-3A-1-110 [DOI] [Google Scholar]
- Puillandre N, Brouillet S, Achaz G. (2021) ASAP: Assemble species by automatic partitioning. Molecular Ecology Resources 21(2): 609–620. 10.1111/1755-0998.13281 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large mod space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Ray S. (2006) Amphibia. Zoological Survey of India, Fauna of Arunachal Pradesh. State Fauna Series 13(1): 285–316. [Google Scholar]
- Smith MA. (1924) New tree-frogs from Indo-China and the Malay Peninsula. 94. Proceedings of the Zoological Society of London, London, 225–234. 10.1111/j.1096-3642.1924.tb01499.x [DOI]
- Stamatakis A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics (Oxford, England) 30(9): 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teynié A, David P, Ohler A, Luanglath K. (2004) Notes on a collection of amphibians and reptiles from southern Laos, with a discussion of the occurrence of Indo-Malayan species. Hamadryad. Madras 29: 33–62. [Google Scholar]
- Vences M, Nagy ZT, Sonet G, Verheyen E. (2012) DNA barcoding Amphibians and reptiles. In: Kress WJ, Erickson DL (Eds) DNA Barcodes: Methods and Protocols, Methods in Molecular Biology, Springer Science + Business Media, LLC, 79–108. 10.1007/978-1-61779-591-6_5 [DOI] [PubMed]
- Vijayakumar SP, Dinesh KP, Prabhu MV, Shanker K. (2014) Lineage delimitation and description of nine new species of bush frogs (Anura: Raorchestes, Rhacophoridae) from the Western Ghats Escarpment. Zootaxa 3893(4): 451–488. 10.11646/zootaxa.3893.4.1 [DOI] [PubMed] [Google Scholar]
- Vijayakumar SP, Menezes RC, Jayarajan A, Shanker K. (2016) Glaciations, gradients, and geography: multiple drivers of diversification of bush frogs in the Western Ghats Escarpment. Proceedings of the Royal Society B, Biological Sciences 283(1836): 20161011. 10.1098/rspb.2016.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li J, Du LY, Hou M, Yu GH. (2022) A cryptic species of the Amolopsricketti species group (Anura, Ranidae) from China-Vietnam border regions. ZooKeys 1112: 139–159. 10.3897/zookeys.1112.82551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Suwannapoom C, Xu K, Chen JM, Jin JQ, Chen HM, Murphy RW, Che J. (2019) A new species of the genus Raorchestes (Anura: Rhacophoridae) from Yunnan Province, China. Zoological Research 40(6): 558–563. 10.24272/j.issn.2095-8137.2019.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Liu XL, Gao W, Wang YF, Li YC, Zhou WW, Yuan ZY, Che J. (2021) Description of a new species of Bush frog (Anura: Rhacophoridae: Raorchestes) from northwestern Yunnan, China. Zootaxa 4941(2): 239–258. 10.11646/zootaxa.4941.2.5 [DOI] [PubMed] [Google Scholar]
- Wu YH, Suwannapoom C, Poyarkov Jr NA, Gao W, Karuno AP, Yuan ZY, Che J. (2022) First record of Kurixalusodontotarsus (Ye et Fei, 1993) and Raorchesteslongchuanensis (Yang et Li, 1978) (Anura: Rhacophoridae) Thailand. Russian Journal of Herpetology 29(1): 1–18. 10.30906/1026-2296-2022-29-1-1-18 [DOI] [Google Scholar]
- Yang DT, Li SM. (1978) In: Yang DT, Su CY, Li SM (Eds) Amphibians and Reptiles of Gaoligongshan, Kunming 8: 37–38. [In Chinese]
- Yu GH, Rao DQ, Zhang MW, Yang JX. (2009) Re-examination of the phylogeny of Rhacophoridae (Anura) based on mitochondrial and nuclear DNA. Molecular Phylogenetics and Evolution 50(3): 571–579. 10.1016/j.ympev.2008.11.023 [DOI] [PubMed] [Google Scholar]
- Yu GH, Liu S, Hou M, Li S, Yang JX. (2019) Extension in distribution of Raorchestesparvulus (Boulenger, 1893) (Anura: Rhacophoridae) to China. Zootaxa 4577(2): 381–391. 10.11646/zootaxa.4577.2.10 [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Kapli P, Pavlidis P, Stamatakis A. (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29(22): 2869–2876. 10.1093/bioinformatics/btt499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HE, Shu GC, Shu FU, Li KE, Liu Q, Wu YY, Dong BJ, Guo P. (2022) A new species of bush frog (Anura, Rhacophoridae, Raorchestes) from southern Xizang, China. Zootaxa 5195(2): 125–142. 10.11646/zootaxa.5195.2.2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data that support the findings of this study are available in the main text.