Abstract
The reported incidence of persistent hypoperfusion despite complete recanalization as surrogate for impaired microvascular reperfusion (IMR) has varied widely among clinical studies, possibly due to differences in i) definition of complete recanalization, with only recent Thrombolysis in Cerebral Infarction (TICI) grading schemes allowing distinction between complete (TICI3) and partial recanalization with distal occlusions (TICI2c); ii) operational definition of IMR; and iii) consideration of potential alternative causes for hypoperfusion, notably carotid stenosis, re-occlusion and post-thrombectomy hemorrhage. We performed a systematic review to identify clinical studies that carried out brain perfusion imaging within 72 hrs post-thrombectomy for anterior circulation stroke and reported hypoperfusion rates separately for TICI3 and TICI2c grades. Authors were contacted if this data was missing. We identified eight eligible articles, altogether reporting 636 patients. The incidence of IMR after complete recanalization (i.e., TICI3) tended to decrease with the number of considered alternative causes of hypoperfusion: range 12.5–42.9%, 0–31.6% and 0–9.1% in articles that considered none, two or all three causes, respectively. No study reported the impact of IMR on functional outcome separately for TICI-3 patients. Based on this systematic review, IMR in true complete recanalization appears relatively rare, and reported incidence highly depends on definition used and consideration of confounding factors.
Keywords: Acute ischemic stroke, no-reflow, perfusion imaging, reperfusion, thrombectomy
Introduction
Based on extensive pre-clinical literature dating back almost 60 years, the no-reflow phenomenon has been defined as an absence of microvascular carbon black filling/perfusion despite resumption of either brain circulation after transient global ischemia or arterial recanalization after focal cerebral ischemia, causing additional neuronal death and tissue necrosis.1 –4 Given the major mechanistic implications with respect to ischemic stroke, no-reflow has been the matter of extensive studies in animal models that suggest that the microvascular obstruction that underlies no-reflow may reflect two main mechanisms, both involving the neurovascular unit and potentially amenable to intervention: i) intravascular clogging from a variety of mainly inflammation-related processes; and ii) peri-capillary pericyte constriction.2,5 –11 The no-reflow phenomenon may contribute to an important degree to impaired microvascular circulation, and some authors have even equated the phenomenon of “no-reflow” with microcirculatory failure and interchanged the terminology, 12 even if incomplete microvascular reperfusion may include other causes, such as circulatory failure or vasogenic oedema.
Despite clear clinical relevance given its potential impact on functional outcome, impaired microvascular reperfusion despite complete recanalization (denoted IMR in what follows) has until recently attracted little attention in the clinical community, probably because of the limited opportunities of early recanalization in acute ischemic stroke (AIS), even after intravenous thrombolysis (IVT), and the unlikely opportunity to assess vessel status early after IVT. Thus, assessment of recanalization after IVT, when indicated, would employ magnetic resonance angiography (MRA) or CT angiography (CTA) usually obtained 24 h to several days later, therefore potentially including delayed recanalization, when tissue outcome is already settled. Furthermore, MRA/CTA do not allow to detect residual distal arterial occlusions, which can cause tissue hypoperfusion mimicking IMR.
A new era opened in 2015 with the advent of mechanical thrombectomy (MT), which aims to recanalize selected patients with large-vessel occlusion (LVO). 13 This major progress directly impacts the investigation of IMR because: i) end-procedure digital subtraction angiography (DSA) allows the degree and precise timing of recanalization to be determined, when no-reflow may still impact the (salvaged) brain tissue; ii) MT allows high rates of complete recanalization (currently around 75% 14 ) making no-reflow potentially widely prevalent; and iii) although recanalization markedly benefits functional outcome, still around 50% of patients do not resume an independent life, so-called ‘futile recanalization’ – defined by the occurrence of poor functional outcome (modified Rankin scale score at 3 months >2) despite successful angiographic recanalization (defined as a modified Thrombolysis in Cerebral Infarction [TICI] score 2 b–3) 15 -, to which IMR may contribute. 16 Partial or complete prevention of no-reflow in animal models makes it of even greater clinical relevance.5,8,17,18 Accordingly, interventions against IMR could be administered before, during or just after MT. 19
In-keeping with this new scenario, a series of clinical articles aiming to investigate “no-reflow” after MT-induced recanalization have recently appeared.20 –33 They all used persistent cerebral hypoperfusion as surrogate, evaluated with computed tomography- or magnetic resonance imaging-based perfusion imaging. Two key comments should be made at this point: Firstly, hypoperfusion has not to this day been formally validated as surrogate for no-reflow in pre-clinical studies. Second, no-reflow in ischemic brain has been shown to occur in vessels <100 µm diameter in animals models, below the resolution of perfusion imaging, with patency above those diameters in the territory. 34 Hence, it is unclear if the hypoperfusion reported after recanalization in human patients is related only to “no-reflow”, which is why in the present review article we elected to use the more generic term IMR.
Importantly, there has been significant variability in reported incidence, severity and impact on functional outcome of IMR among these studies, which may have caused confusion in the clinical community. Potential reasons for these discrepancies may include methodological differences, notably regarding the operational definition and method of assessment used for IMR, as well as differences in populations studied. The most obvious source of confusion has however been the definition used for ‘complete recanalization’, which has substantially evolved over time. Thus, the TICI recanalization grading system has only recently been implemented, and TICI systems allowing distinction between near-complete and complete recanalization even more so. The latter include the mTICI with 2c classification scheme (to be referred to as new-mTICI in what follows) and the eTICI grading scheme (Table 1).35 –39 In turn, only those recently published reports that included the TICI2c grading have relevance to IMR, whose definition implies complete recanalization - i.e., new-m/eTICI3. Additional major confounders for the assessment of IMR include potential alternative causes of hypoperfusion such as hemodynamic proximal carotid stenosis, re-occlusion and post-thrombectomy hemorrhage, and potentially also the timing of and method used to assess hypoperfusion.
Table 1.
Comparison of existing TICI (thrombolysis in cerebral infarction) classification schemes.
| TICI grade | Original TICI 35 | Modified TICI 36 | Modified TICI with 2c (“New m-TICI”)37,38 | eTICI 39 |
|---|---|---|---|---|
| 0 | No perfusion | No reperfusion | No reperfusion | 0% reperfusion |
| 1 | Minimal perfusion | Minimal reperfusion | Minimal reperfusion | Reduction in thrombus but without any resultant filling of distal branches |
| 2a | Partial filling<2/3 territory | Partial filling<1/2 territory | Partial filling <1/2 territory | Reperfusion of 1–49% of the territory |
| 2b | Partial filling≥2/3 territory | Partial filling≥1/2 territory | Partial filling ≥1/2 territory | - 2b50: reperfusion of 50–66% of the territory- 2b67: reperfusion of 67–89% of the territory |
| 2c | – | – | Near complete perfusion except for slow flow or distal emboli in a few distal cortical vessels | Extensive reperfusion of 90–99% of the territory |
| 3 | Complete perfusion | Complete reperfusion | Complete reperfusion | Complete (100%) reperfusion |
The aims of the present systematic review were to i) identify published articles on IMR/hypoperfusion after MT-induced recanalization of LVO-AIS of the anterior circulation that used the new-mTICI or eTICI grading systems; ii) summarize the findings regarding IMR incidence, degree and clinical impact; iii) identify potential reasons behind reported differences, particularly with respect to the three major potential confounders listed above; and, from there, iv) propose towards future research rigorous methodology to investigate, and an operational definition of, IMR after AIS.
Our primary hypothesis is that the incidence of IMR is relatively small in new-m/eTICI3 recanalization, and in turn that IMR is unlikely to be a major contributor to ‘futile (complete) recanalization’. Preliminary results have been presented at the European Stroke Organisation Conference (ESOC) 2023. 40
Methods
Study design
We performed a systematic review of the literature to identify studies that assessed brain perfusion within 72 hrs after thrombectomy and used the TICI grading system. Because, as mentioned above, a reliable assessment of IMR as a pathophysiological process can only be carried out in situations of complete recanalization, we then selected those articles that reported post-MT hypoperfusion using the new-m/eTICI grading systems separately for new-m/eTICI3 recanalizations.
Literature screening
Studies were identified by systematically searching the Pubmed and Embase databases up to April 9th,2023 using the following combination terms: “no-reflow AND thrombectomy”, “no-reflow AND endovascular treatment”, “hypoperfusion AND thrombectomy”, “hypoperfusion AND endovascular treatment”, and “cerebral no-reflow”. We chose to use the term ‘no-reflow’ for the literature search because this term has so far been widely used in the clinical literature to indicate persistent hypoperfusion despite recanalization. Abstracts were screened and only articles reporting clinical perfusion studies performed after thrombectomy or EVT were retained for further analysis. References of the selected articles, as well as their citations, were screened for additional relevant articles. Only English languages articles were assessed. We did not include overlapping articles (i.e., that used the same database), review articles, study protocols, and published abstracts. Two independent researchers (JCB, ATS) screened titles/abstracts and hand-searched the chosen publications.
The inclusion criteria were the following: 1) clinical study in adult patients (≥18 years old); 2) study assessing recanalization after MT for anterior circulation LVO according to recent (new-m/e) TICI classification systems;37 –39 3) brain perfusion study performed within 72 hrs after MT using MR or CT; and 4) study reported the number of patients with hypoperfusion. However, only those articles that reported hypoperfusion separately for new-m/eTICI scores 2c and 3 were finally retained. In case this data was missing, corresponding authors were personally contacted and invited to provide it.
Studies were excluded a priori if: 1) the TICI grading was not used; 2) “complete recanalization” included m/eTICI2c, but without possibility from the published data to distinguish m/eTICI2c from new-m/eTICI3 patients, and response not provided by the corresponding authors; 3) m/eTICI2c grading not used; and 4) perfusion assessed with methods not allowing brain mapping, such as DSA or transcranial Doppler.
For each identified article, we then determined whether the study excluded patients/data with carotid stenosis, re-occlusion or post-thrombectomy confluent hemorrhage (or quantitated hypoperfusion in peri-hematoma areas, which may not represent IMR 41 )
Standard protocol approvals, registrations, and patient consents
The current work is a systematic review of already published studies, and therefore, no patient consent or ethics approval was required.
Data availability [database]
Datasets used for this systematic review will be made available upon reasonable request.
Results
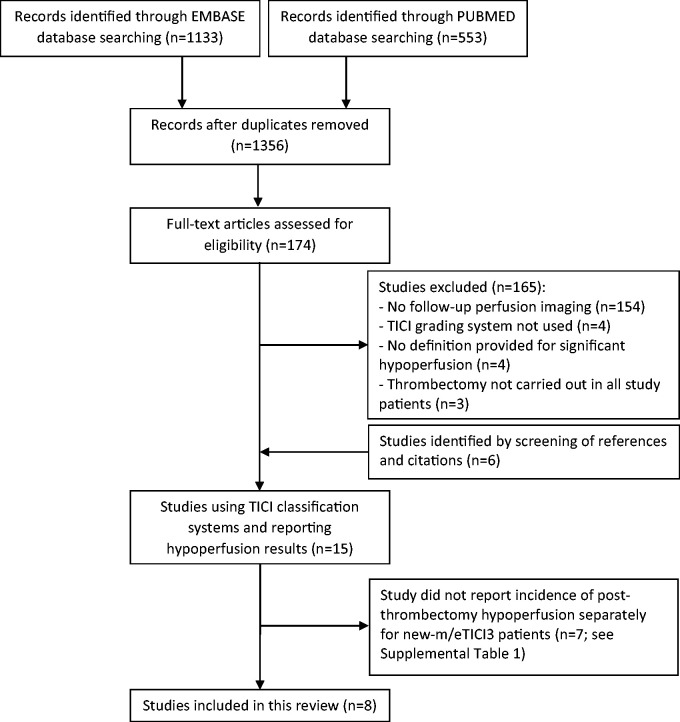
Our search identified 1356 articles, and 15 articles that reported findings from brain perfusion imaging performed within 72 hrs after thrombectomy and used a TICI grading system to characterize post-procedure recanalization were retained for further analysis.20 –33,42–44 Figure 1 shows the PRISMA diagram. Note that out of these 15 articles, 6 were identified by screening references and citations.23,26,28,31,43,44
Figure 1.
Flowchart illustrating the literature search implemented in the present systematic review.
Table 2 presents the relevant data for the eight articles (overall number of patients: 636) that fulfilled all our inclusion/exclusion criteria and reported post-MT hypoperfusion incidence separately for new-m/eTICI3 and m/eTICI2c recanalization (or whose authors provided this data following personal email contact), and were therefore included in the final analysis.20,22 –26,30,32,33 Of these 8 articles, 5 reported retrospective analysis of prospectively collected data,20,22,23,26,30,33 and 3 included patients from randomized controlled trials.24,25,32 No study reported data regarding pre-thrombectomy thrombolysis or use of antithrombotics either prior to the stroke or during/following the endovascular procedure according to presence/absence of post-MT hypoperfusion. Note that all studies save one 25 had as overall (explicit or implicit) assumption that the better the reperfusion after thrombectomy, the better the clinical outcome. Laredo et al 25 tested the hypothesis that intra-arterial thrombolysis administered after complete recanalization would prevent or dissolve microvascular thrombi/emboli.
Table 2.
Relevant data regarding the eight published articles retained for the present systematic review, stratified by the number of excluded potential alternative causes for post-thrombectomy hypoperfusion (i.e., ICA stenosis, intracranial reocclusion, areas with hemorrhage). Note that no study excluded only one of these alternative causes.
| First author, year | N patients/onset-to-recanalization time (median[IQR] min) | Type of study | TICI classification used | Post-MT perfusion imaging timing/method | Definition of hypoperfusion | Exclusion of ICA stenosis | Exclusion of intracranial reocclusion | Exclusion of areas with hemorrhage | N (%) of m/eTICI2c patients with hypoperfusion | N (%) of new-m/eTICI3 patients with hypoperfusion | Impact on clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies excluding 3/3 potential alternative causes for hypoperfusion | |||||||||||
| ter Schiphorst, 2021/202320,33*$ | 33/196 [154–230]& | Retrospective | mTICI including 2c | 24hr/MRI ASL/GE Healthcare | Visual assessment in the initially occluded artery territory | Yes | Yes | Yes§ | 3/22 (13.6%) | 1/11 (9.1%) | No¤ |
| Luijten, 2023 32 $ | 27/107[61–367]@ | Patients from randomized controlled trial | mTICI including 2c | 24hr/MRI-ASL | ≥40% decrease in post-MT CBF in affected hemisphere compared to mirror region | Yes | Yes | YesΔ | 0/9 (0%) | 0/18 (0%) | Not assessable |
| Studies excluding 2/3 potential alternative causes for hypoperfusion | |||||||||||
| Ng, 2022 24 | 130/≤270& | Patients from randomized controlled trial | eTICI | 24hr/CTP or PWI/RAPID | Visual assessment of CBV and CBF maps within infarct, confirmed by >15% decrease vs mirror region | Yes | Yes | Only for quantitative analysis¥ | 15/73 (20.5%) | 18/57 (31.6%) | Yes across eTICI2c and eTICI3 |
| Mujanovic, 2022 26 | 338/169[88–376]# | Retrospective | eTICI | ≈24hr/CTP or PWI/Olea Sphere | Presence of Tmax ≥4 sec deficit within the initially occluded artery territory | Not specified | Yes | Yes∞ | 25/144 (17%) | 0/194 (0%) | Yes in eTICI2c patients; not assessable in eTICI3 |
| Studies excluding 0/3 potential alternative causes for hypoperfusion (or not mentioning them) | |||||||||||
| Potreck, 2021 22 | 29/248 [125–343] | Retrospective | mTICI including 2c | 24hr/PWI/Olea Sphere | Visual assessment within the infarct on CBF maps | No | No | No | 3/13 (23.1%)£ | 2/16 (12.5%)£ | No |
| Tan, 2021 23 | 31/282 [216–390] | Retrospective | mTICI including 2c | 24-36hr/CTP or PWI/RAPID | <90% reduction of pre-MT Tmax > 6s lesion volume in the affected hemisphere | No | No | Not specified | 3/15 (20%) | 3/16 (18.8%) | Not reported |
| Laredo 2022 25 $ | 12/≤1440 | Placebo group patients from randomized controlled trial | eTICI | 48hr/PWI/ MIStar | Presence of imaging deficit Tmax > 6sec outside or within the infarct | Not specified | Not specified | Not specified | 3/5 (60%) | 3/7 (42.9%) | Not reported |
| Hong, 2023 30 $ | 36/≤1440 | Retrospective | mTICI including 2c | 24-48hr/CTP/ MIStar | DT > 3 sec reperfusion index < 0.9 | No | Not specified | Not specified | 2/6 (33.3%) | 10/30 (33.3%) | Not reported |
*: The data for this study were extracted from two successive publications, the first using a ROI template and stringent quantitative perfusion criteria, the second exploring the whole affected vascular territory based on visual assessment; $: Data provided by the author following personal request; @: stroke onset-to-imaging time; &: stroke onset-to-inclusion time; #: stroke onset-to-door time; §: patients with parenchymal hematoma were excluded, and areas with HI-2 according to ECASS classification 45 were excluded from analysis. Δ: Exclusion of patients with parenchymal hematomas; ¥: Hemorrhagic areas not excluded in qualitative analysis of hypoperfusion and excluded in the quantitative analysis, but hypoperfusion was measured in peripheral hematoma area; ∞: Exclusion of “bleedings and hemorrhagic tissue transformation”; £: Among the 5 m/eTICI2c-3 patients with hypoperfusion, 4 (80%) had hemorrhagic transformation: 2 each with mTICI3 and mTICI2c recanalization; ¤: Incomplete microvascular reperfusion was not statistically associated with functional independence at 3 months after exclusion of patients with missing follow-up or strokes in multiple territories.
ASL: arterial spin labeling; CTP: computed tomography perfusion; ICA: internal carotid artery; MRI: magnetic resonance imaging; MT: mechanical thrombectomy; PWI: perfusion weighted imaging; TICI: thrombolysis in cerebral infarction (mTICI: modified TICI; eTICI: expanded TICI); CBF: cerebral blood flow; CBV: cerebral blood volume; DT: delay time.
As shown in Table 2, two studies only out of the 8 eligible studies20,32 excluded the three major alternative causes of post-MT hypoperfusion, namely ipsilateral carotid stenosis, intracranial re-occlusion, and confluent hemorrhagic transformation within or abutting the hypoperfused area. In these two studies, the incidence of hypoperfusion was 9.1% and 0% in mTICI3 patients, and 13.6% and 0% in mTICI 2c, respectively. Two studies excluded 2 of the 3 confounders: one study that excluded both hemorrhagic areas and intracranial reocclusions 26 reported hypoperfusion in 0% and 17% of eTICI3 and eTICI 2c patients, respectively, but did not indicate whether carotid stenosis was also cause of exclusion. The other study 24 reported hypoperfusion in 31.6% and 20.5% of eTICI3 and eTICI 2c patients, respectively, and excluded carotid stenosis and intracranial re-occlusions. In this study, in order to quantitatively confirm visually-identified hypoperfusions, a ROI was used that excluded hemorrhagic areas – a procedure that would not avoid the issue of peri-hematoma hypoperfusion. In the remaining four studies, potential confounders either were not excluded, or are not mentioned as exclusion criteria, with an incidence of hypoperfusion ranging from 12.5% to 42.9% in m/eTICI3 patients, and from 20% to 60% in m/eTICI2c patients.22,23,25,30 However, in one of these studies, the authors noted that among the five mTICI2c-3 patients with hypoperfusion, 4 (85%) had hemorrhagic transformation 22 (both mTICI3 patients with hypoperfusion had hemorrhage).
In sum, the incidence of post-MT hypoperfusion in m/eTICI3 patients according to the number of considered potential confounders was the following: 0-9.1% in studies excluding all three factors; 0-31.6% in studies excluding two factors; and 12.5-42.9% in studies excluding none (no study considered only one confounder).
Out of the 8 eligible studies, three only reported on the impact of post-MT hypoperfusion on functional outcome.20,24,26,33 One study (published in two parts) found no significant association,20,33 while the other two studies found a negative association.24,26 In one, 24 presence of hypoperfusion was independently associated with 90-day functional dependence and mortality across eTICI2c and eTICI3 populations, while in the other 26 it was associated with worse outcomes in eTICI2c patients (no instance of hypoperfusion in eTICI3 patients). However, none of the studies that reported hypoperfusion in new-m/eTICI3 specifically assessed its functional impact in this recanalization grade. Regarding the remaining 5 studies, 4 did not report the functional impact of hypoperfusion,22,23,25,30 while in the fifth the prevalence of hypoperfusion was zero in both mTICI2c and mTICI3 patients. 32
The remaining 7 studies that fulfilled the inclusion criteria but were not eligible for the final analysis21,27 –29,31,43,44 did not use the new-mTICI or eTICI classification systems or did not report the findings separately for m/eTICI2c and new-m/eTICI3 (or their authors did not respond to our emails). For the sake of completeness, Supplemental Table 1 summarizes their methods and main findings.
Discussion
In order to evaluate the incidence of IMR in a population with ‘true’ complete recanalization following LVO-AIS, only those studies that assessed post-MT hypoperfusion in TICI3 patients separately from TICI2c and other less complete recanalization grades were deemed eligible. This is because post-MT hypoperfusion assessed with older TICI schemes could reflect distal small-artery or arteriolar occlusions just as well as genuine IMR. Accordingly, we systematically searched for articles that assessed perfusion within the follow-up infarct or the whole initially affected hemisphere within 72 hrs of thrombectomy-induced complete recanalization according to recent TICI schemes.
Out of 15 initially identified articles that used any TICI scheme, 8 fulfilled the above stringent criteria.20,22 –26,30,32,33 The remainder either used old TICI schemes, did not report the incidence of hypoperfusion separately for new-m/eTICI3 and TICI 2c, or did not respond to our repeated email invitation to provide such data.21,27 –29,31,43,44
One key finding of our study is that the incidence of post-MT hypoperfusion in m/eTICI3 patients varied considerably across studies but tended to be smaller as the number of alternative causes (i.e., carotid stenosis, reocclusion and post-thrombectomy hemorrhage) were considered by design: 0–9.1% in studies excluding all factors, 0–31.6% in studies excluding two out of the three factors, and 12.5–42.9% in studies excluding none of them. Regarding m/eTICI2c, the incidence of hypoperfusion appears overall higher than in TICI3 but also shows the same trend as for TICI3 according to the number of alternative causes considered in the protocol: 0–13.6% in studies excluding all three of the latter, 17–20.5% in those excluding two, and 23–60% in those excluding none (one study only reported a higher prevalence of post-MT hypoperfusion in TICI2c as compared to m/eTICI3 24 ).
Over and above the above major finding, the present work highlights methodological issues that may confound reported incidences of IMR, and in turn account for previous apparent discrepancies. This is supported not just by the impact of the number of considered key confounding factors on hypoperfusion incidence in new-m/eTICI 3, but also by the very large variability (0% to 61%) in post-MT hypoperfusion incidence after ‘complete recanalization’ among the initially identified 15 studies (Table 2 and Supplemental Table).
Proximal carotid stenosis, post-MT re-occlusion and post-thrombectomy parenchymal hemorrhage can all cause hypoperfusion potentially mistakable for IMR. As Table 2 shows, all three confounders were considered in only 2 of the 8 eligible studies. Tandem occlusions affect about 15–20% of patients undergoing MT, 46 only part of which are treated at the end of the procedure, while their treatment per se may cause distal embolization. Severe ‘hemodynamic’ (70-90%) proximal stenosis is also prone to cause pre-stroke chronic hypoperfusion. 47 Early re-occlusion, which is evaluated as part of routine brain imaging ∼24hrs after thrombectomy, occurs in ∼5–6% of successful procedures48,49 and may therefore contaminate the findings. On a related note, repeated procedural passes 29 (which may cause thrombus fragmentation) and ‘infarcts in new territories’ 50 (defined as imaging-proven infarct in a vascular territory outside that of the original target occlusion before MT) may also affect the prevalence of post-MT hypoperfusions via potentially undetectable distal occlusions. Post-thrombectomy parenchymal hemorrhage is a major cause of hypoperfusion within as well as around the hematoma. 41 In one study, 24 hypoperfused areas were detected visually, and their significance was verified if CBF or CBV was reduced >15% relative to mirror areas in a ROI that excluded any local hemorrhage, which however may not have ruled out peri-hematoma hypoperfusion. 41 In another study, 22 all instances of hypoperfusion in mTICI3 patients were found post-hoc to be associated with local hemorrhage. Only studies in animal models may establish if IMR, and more precisely no-reflow, may co-exist with significant hemorrhagic transformation.
Additional between-study differences that may partly account for the observed discrepancies entail the operational definition used for post-MT hypoperfusion and how it was assessed, as well as the population studied. First, the variable timing for assessment (24-48hr; Table 2) may have affected the findings, as suggested by a study that found more frequent hypoperfusions within 6 hr vs 24 h after thrombectomy. 31 Delayed infarct reperfusion/hyperperfusion reflecting secondary capillary neovascularization has long been described. 51 Furthermore, stroke onset-to-recanalization delay, which varied widely among studies (Table 2), may also affect the incidence of post-MT hypoperfusion. 24 Second, the imaging modality used to assess reperfusion differed among the 8 studies: two each used ASL20,32 or PWI,22,25 one used CTP, 30 and the remaining three used CTP or PWI indifferently.23,24,26 Even though ASL has been validated against conventional modalities in AIS patients 52 and CTP and PWI are known to be well correlated, 53 these modalities might differ in sensitivity to IMR. On a theoretical basis, the two main approaches for the measurement of brain perfusion, namely using a freely diffusible tracer such as water (as with 15 O-PET or MR-based ASL) or measuring the vascular transit of an intravascular tracer (such as with dynamic-susceptibility-contrast PWI or perfusion CT) should be sensitive to impaired microvascular - including capillary - perfusion, although the former would be more sensitive to and specific for it. Third, studies vastly differed regarding the definition of significant post-MT hypoperfusion with respect not only to the perfusion software employed (Table 2) but also the hypoperfusion parameter and cut-off used. Thus, it was variably defined as i) a reduction <90% of the baseline Tmax > 6 sec lesion volume, 23 ii) the presence of a defect on Tmax > 6s, Tmax > 4s or ASL maps inside or outside the follow-up infarct,25,26,33 iii) the presence of a defect on CBF maps within the follow-up infarct, 22 iv) a > 40% CBF reduction relative to mirror region,20,32,33 or v) a > 15% reduction in either CBF or CBV relative to mirror region within visually-identified CBF or CBV defects. 24 Some of these definitions deserve comments. For instance, a 15% CBF cut-off may appear permissive as it classically represents the upper limit of CBF asymmetries in normal subjects or unaffected regions. 24 The same applies to Tmax > 4s, since penumbral ischemia is widely considered as Tmax ≥6s,54,55 as well as to <90% of the pre-MT Tmax >6s volume, given the statistical noise intrinsic to contrast-based perfusion mapping. Accordingly, using a >40% instead of >15% hypoperfusion threshold in one study 24 as expected reduced the prevalence of detected post-MT hypoperfusion from 31.6% to 6%. Importantly in this respect, no-reflow may adopt a ‘mottled’ instead of uniform pathological appearance in animal models. 20 Unfortunately, unbiased clinical studies comparing in vivo perfusion maps to post-mortem no-reflow to establish validated cut-offs are unlikely to ever be available. Furthermore, given the reported differences in perfusion maps and values between proprietary software, 56 harmonization across studies would be optimal, or by default the software used should be mentioned in the publication. A fourth potential confound is the extent of hypoperfusion to be considered significant IMR. Some studies used visual assessment with or without providing hypoperfusion extent,20,22,32,33 while others considered as IMR any hypoperfusion detected by automatic software regardless of extent (and did not provide the latter),23,30 and still others used an index of remaining hypoperfusion relative to pre-MT hypoperfusion, consistently using the 90% cut-off (again not providing involved volumes) 26 (Table 2). In one study, 24 median hypoperfusion volume was 14.3 ml (IQR 8.6–31.1 ml); yet it appears quite small in some published figures. In another study, median hypoperfusion volume was 0.6 mL (IQR: 0.0–3.2) within, and 2.5 mL (1.8–9.1) outside the DWI lesion, 25 pointing to some very small hypoperfused volumes of uncertain clinical relevance. Future studies should consider using a volume cut-off, or at least systematically report involved volumes. More importantly, all the above hypoperfusion characteristics will have to be validated in large-animal MCA occlusion studies combining in vivo perfusion imaging in co-registration with post-mortem assessment of IMR, and more specifically no-reflow. 57 To this day, there is a “gap” between on one hand, the no-reflow phenomenon in the ischemic territory as determined by neuropathology, and, on the other hand, impaired microvascular reperfusion as described in reports of clinical approaches. It is indeed unclear whether the events captured by the articles included in this review are arteriolar or venular, or (more likely) a composite of the microcirculation at the resolution of the imaging (which would have no-reflow embedded, but not detected). Furthermore, the resolution of current clinical imaging techniques is of several millimeters, not on par with the microscopic definition of no-reflow. Hence, the ability of the individual imaging modalities to identify perfusion reduction as it might relate to true no-reflow will only be evaluable through extensive animal studies.
A further important consideration regarding assessment of IMR is whether the hypoperfusion affects the infarcted tissue, non-infarcted tissue, or both. Based on pre-clinical literature,1,4,18 no-reflow classically results in tissue pan-necrosis/infarction. Accordingly, no-reflow should in principle be searched only within follow-up infarcted areas. 20 However, across eligible studies, hypoperfusion was variably assessed within the infarcted area, over the affected vascular territory or across the whole affected hemisphere (Table 2). Whether post-MT hypoperfusion remote from/surrounding the infarct may represent no-reflow is unclear, although Del Zoppo et al. reported microvascular obstruction affecting the penumbra in a geographically heterogeneous fashion. 9 It is therefore plausible that peri-infarct hypoperfusion may reflect patchy/moderate no-reflow within the rescued penumbra, which may in turn evolve as selective neuronal loss, a phenomenon described in both animal models and man.58,59 Additional potential causes of hypoperfusion within or around the infarct, include vasogenic edema 60 and failure of collateral reperfusion, which has been shown to cause hypoperfusion in clinical studies carried out prior to recanalization. 61 Furthermore, mild-to-moderate hypoperfusion remote from the infarct may also reflect infarct-induced disconnection – i.e., diaschisis.20,62 Diaschisis-induced hypoperfusion is however not expected to involve blood flow slowing, i.e., MTT/Tmax delay, a marker of vascular obstruction. Finally, persistent hypoperfusion vs reperfusion within the infarct refers to the concept of incomplete/heterogeneous infarction,24,63,64 according to which occurrence of no-reflow within the ischemic lesion would result in more complete infarction with fewer preserved tissue islands, in turn influencing functional outcome. Further studies, particularly in large-animal models, are needed to address these complex issues.
A final potential confounder in studies of post-MT hypoperfusion is population studied. Studies eligible for the present review included patients either prospectively recruited or retrospectively collected from prospective databases, which is more prone to selection bias. Thus, perfusion imaging might be less systematically performed in patients who deteriorate due to IMR. To ensure lack of major bias, it is recommended in retrospective studies to compare the clinical-radiological data between the included and excluded patients, 20 acknowledging this remains imperfect. Although prospective studies avoid this type of bias, the use of stringent inclusion criteria may also bias the population studied and affect generalizability.
From a clinical standpoint, the most critical issue is whether IMR impacts functional outcome, potentially culminating in ‘futile recanalization’, but also as any reduction in mRS across the board. Of the eight eligible studies, three only reported hypoperfusion impact on outcome, and none separately for new-m/eTICI3 patient subsets. Furthermore, in the two studies that considered all three major confounders, the incidence of post-MT hypoperfusion in new-m/eTICI3 was very small or absent. No reliable conclusion on functional impact can therefore be made at this point. A prominent study reported an independent negative association between hypoperfusion across eTICI2c/3 subsets and functional outcome. 24 Although this association was adjusted for the presence of hemorrhagic transformation, peri-hematoma hypoperfusion was not formally excluded (as already pointed out), while a specific analysis of the eTICI3 subset was not presented. Although it remains entirely possible, and actually plausible, that post-MT hypoperfusion regardless of its cause negatively impacts outcome and in turn that performing perfusion imaging after MT may have clinical utility, several mechanisms apart from or in addition to no-reflow may underlie this effect.
Despite the hurdles encountered so far, studying cerebral IMR in human stroke remains a critically important goal given its pharmacological alleviation in pre-clinical models. 5 The recently published CHOICE trial, 65 included in this review, 25 reported a clinical benefit from intra-arterial alteplase administered at the end of successful MT, which was mainly attributed to no-reflow prevention. However, because CHOICE defined successful recanalization as ≥eTICI2b50, outcome could have improved via alleviation of distal occlusions. More generally, use of thrombolytics or antithrombotics prior to the stroke or to thrombectomy, or their use during or following the endovascular procedure, is an important clinical variable to record and analyze in future clinical studies of post-MT hypoperfusion.
The main practical objective of the present study was to identify potential causes underlying the wide differences in incidence of post-MT hypoperfusion reported so far in the clinical literature, and from there derive a rigorous methodology to investigate IMR. Based on the above, we would recommend that future studies i) focus on TICI3 separately from TICI2c, assessed by an independent interventionalist; ii) carefully exclude all of the three major alternative causes of hypoperfusion and record the number of passes and use of thrombolysis or antithrombotics; and iii) assess hypoperfusion as early as possible and no later than 72 hrs after MT, and preferably serially so. Regarding perfusion methodology and threshold, we would recommend i) using derivatives of the mean transit time over CBF itself, which is sensitive to non-ischemic processes such as diaschisis; ii) use validated thresholds for significant ischemia, e.g. Tmax ≥ 6s; 54 iii) consider a meaningful cut-off volume, e.g. ≥5 mls, and iv) assess hypoperfusion separately within the follow-up infarct and the remainder of the affected vascular territory. Table 3 summarizes these recommendations.
Table 3:
Recommendations for the assessment of incomplete microvascular reperfusion despite complete recanalization.
| Studied population | |
| Recanalization definition | Include only patients with new-mTICI or eTICI 3 recanalization (assessed by an independent interventionalist) |
| Confounding factors | |
| Hemorrhage | Exclude patients with parenchymal hematoma; exclude from analysis areas with confluent hemorrhage and peri-hemorrhage areas |
| Stenosis | Exclude patients with hemodynamic-degree carotid stenosis not treated during the endovascular procedure |
| Re-occlusion | Exclude patients with re-occlusion on follow-up imaging |
| Methodology for the assessment of incomplete microvascular reperfusion | |
| Timing | Perform perfusion imaging as early as possible and no later than 72 hrs after thrombectomy |
| Perfusion parameter | Preferably use derivatives of the mean transit time over CBF |
| Hypoperfusion cutoff | Use validated thresholds for significant ischemia, e.g., Tmax ≥6s |
| Hypoperfusion volume cutoff | Consider a meaningful cut-off volume, e.g., ≥5 mls, and report the volume |
| Brain zones to be assessed | Separately the follow-up infarct and the remainder of the originally affected vascular territory |
The following two methodological points regarding our study are worth emphasizing. First, we suspected from the outset that many potentially eligible articles would not be detected by standard key-words literature search either because of the variety of approaches used to assess post-MT perfusion as well as terminology used, or because post-MT hypoperfusion was not the main objective of the study. Accordingly, our systematic search identified 9 only of the 15 studies reported in Results,20 –22,24,25,27,29,30,32 while the remaining 6 studies were extracted from the references or citations of published articles.23,26,28,31,43,44 Thus, to our best knowledge all articles relevant to the subject of this review and published as of April 9th, 2023 have been identified. Second, although extensively considered, a meta-analysis would not have been appropriate given the substantial between-study heterogeneity in operational definition of post-MT hypoperfusion used and variability in number of major confounding factors taken into account.
Conclusions
Based on the available literature, the incidence of incomplete microvascular reperfusion in patients with complete (i.e., m/eTICI3) recanalization may have been overestimated in many published articles due to the non-exclusion of potential confounding factors, and actually appears small. Accordingly, its impact on functional outcome, if any, remains unsettled. To address these issues, future work should implement strict operational definitions and rigorous methodology. Although challenging, studying incomplete microvascular reperfusion in living stroke patients is an important goal given the encouraging pre-clinical literature suggesting it is amenable to intervention.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231209069 for Incidence, severity and impact on functional outcome of persistent hypoperfusion despite large-vessel recanalization, a potential marker of impaired microvascular reperfusion: Systematic review of the clinical literature by Adrien ter Schiphorst, Guillaume Turc, Wagih Ben Hassen, Catherine Oppenheim and Jean-Claude Baron in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We would like to thank Dr Ángel Chamorro, Dr Lan Hong, Dr Sven Luijten, Dr Arne Potreck and Dr Cheng Xi for kindly providing additional data following our request.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Jean-Claude Baron https://orcid.org/0000-0002-5264-2588
References
- 1.Ames A, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol 1968; 52: 437–453. [PMC free article] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Schmid-Schonbein GW, Mori E, et al. Polymorphonuclear leukocytes occlude capillaries following Middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991; 22: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 3.Little JR, Kerr FW, Sundt TM., Jr. Microcirculatory obstruction in focal cerebral ischemia: an electron microscopic investigation in monkeys. Stroke 1976; 7: 25–30. [DOI] [PubMed] [Google Scholar]
- 4.Crowell RM, Olsson Y. Impaired microvascular filling after focal cerebral ischemia in monkeys. J Neurosurg 1972; 36: 303–309. [DOI] [PubMed] [Google Scholar]
- 5.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037.. [DOI] [PubMed] [Google Scholar]
- 6.Desilles JP, Syvannarath V, Di Meglio L, et al. Downstream microvascular thrombosis in cortical venules is an early response to proximal cerebral arterial occlusion. J Am Heart Assoc 2018; 7 : 20180301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalkara T. Pericytes: a novel target to improve success of recanalization therapies. Stroke 2019; 50: 2985–2991.. [DOI] [PubMed] [Google Scholar]
- 9.del Zoppo GJ, Sharp FR, Heiss WD, et al. Heterogeneity in the penumbra. J Cereb Blood Flow Metab 2011; 31: 1836–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu B, Zhao Z, Wang N, et al. A systematic observation of vasodynamics from different segments along the cerebral vasculature in the penumbra zone of awake mice following cerebral ischemia and recanalization. J Cereb Blood Flow Metab 2023; 43: 665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J, Lyden PD. Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–152. [DOI] [PubMed] [Google Scholar]
- 12.Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab 2012; 32: 2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 14.Hui W, Wu C, Zhao W, et al. Efficacy and safety of recanalization therapy for acute ischemic stroke with large vessel occlusion: a systematic review. Stroke 2020; 51: 2026–2035. [DOI] [PubMed] [Google Scholar]
- 15.Shahid AH, Abbasi M, Larco JLA, et al. Risk factors of futile recanalization following endovascular treatment in patients with large‐vessel occlusion: systematic review and meta‐analysis. Stroke vasc interv neurol 2022; 2: e000257. [Google Scholar]
- 16.Nie X, Leng X, Miao Z, et al. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke 2023; 54: 873–881. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Zhang WH, Allen EM, et al. GPR39 knockout worsens microcirculatory response to experimental stroke in a sex-dependent manner. Transl Stroke Res 2023; 14: 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowell RM, Olsson Y. Impaired microvascular filling after focal cerebral ischemia in the monkey. Modification by treatment. Neurology 1972; 22: 500–504. [DOI] [PubMed] [Google Scholar]
- 19.Savitz SI, Baron JC, Fisher M. Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke 2019; 50: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 20.Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab 2021; 41: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubiera M, Garcia-Tornel A, Olive-Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke 2020; 51: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 22.Potreck A, Mutke MA, Weyland CS, et al. Combined perfusion and permeability imaging reveals different pathophysiologic tissue responses after successful thrombectomy. Transl Stroke Res 2021; 12: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan Z, Parsons M, Bivard A, et al. Optimal tissue reperfusion estimation by computed tomography perfusion post-thrombectomy in acute ischemic stroke. Stroke 2021; 52: e760–e763. [DOI] [PubMed] [Google Scholar]
- 24.Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology 2022; 98: e790–e801. [DOI] [PubMed] [Google Scholar]
- 25.Laredo C, Rodriguez A, Oleaga L, et al. Adjunct thrombolysis enhances brain reperfusion following successful thrombectomy. Ann Neurol 2022; 92: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mujanovic A, Jungi N, Kurmann CC, et al. Importance of delayed reperfusions in patients with incomplete thrombectomy. Stroke 2022; 53: 3350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai X, Yu F, Tian Q, et al. Clinical significance and influencing factors of microvascular tissue reperfusion after macrovascular recanalization. Transl Stroke Res 2023; 14: 446–454. [DOI] [PubMed] [Google Scholar]
- 28.Rosso C, Belkacem S, Amor-Sahli M, et al. Persistent perfusion abnormalities at day 1 correspond to different clinical trajectories after stroke. J Neurointerv Surg 2023; 15: e26–e32. [DOI] [PubMed] [Google Scholar]
- 29.Luby M, Merino JG, Davis R, et al. Association of multiple passes during mechanical thrombectomy with incomplete reperfusion and lesion growth. Cerebrovasc Dis 2022; 51: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong L, Ling Y, Zhang Y, et al. Reperfusion measurements, treatment time, and outcomes in patients receiving endovascular treatment within 24 hours of last known well. CNS Neurosci Ther 2023. ; 29: 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luby M, Hsia AW, Lomahan CA, et al. Post-ischemic hyperemia following endovascular therapy for acute stroke is associated with lesion growth. J Cereb Blood Flow Metab 2023; 43: 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luijten SPR, Bos D, van Doormaal PJ, et al. Cerebral blood flow quantification with multi-delay arterial spin labeling in ischemic stroke and the association with early neurological outcome. Neuroimage Clin 2023; 37: 103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ter Schiphorst A, Oppenheim C, Baron JC. Reader response: prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology 2023; 100: 217–218. [DOI] [PubMed] [Google Scholar]
- 34.Mori E, del Zoppo GJ, Chambers JD, et al. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke 1992; 23: 712–718. [DOI] [PubMed] [Google Scholar]
- 35.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109-137–e137. [DOI] [PubMed] [Google Scholar]
- 36.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almekhlafi MA, Mishra S, Desai JA, et al. Not all “successful” angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol 2014; 20: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cimflova P, Singh N, Kappelhof M, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg 2023; 6: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019; 11: 433–438. [DOI] [PubMed] [Google Scholar]
- 40.Ter Schiphorst A, Ben Hassen W, Turc G, et al. ESOC 2023 abstract book. European Stroke Journal 2023; 8: 3–669.36793746 [Google Scholar]
- 41.Tamm AS, McCourt R, Gould B, et al. Cerebral perfusion pressure is maintained in acute intracerebral hemorrhage: a CT perfusion study. AJNR Am J Neuroradiol 2016; 37: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brugnara G, Herweh C, Neuberger U, et al. Dynamics of cerebral perfusion and oxygenation parameters following endovascular treatment of acute ischemic stroke. J NeuroIntervent Surg 2021; 14: neurintsurg-2020-017163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks MP, Lansberg MG, Mlynash M, et al. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke 2014; 9: 860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albers GW, Goyal M, Jahan R, et al. Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke 2015; 46: 2786–2794. [DOI] [PubMed] [Google Scholar]
- 45.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 46.Feil K, Herzberg M, Dorn F, et al. Tandem lesions in anterior circulation stroke: analysis of the German Stroke Registry-Endovascular treatment. Stroke 2021; 52: 1265–1275. [DOI] [PubMed] [Google Scholar]
- 47.Klijn CJ, Kappelle LJ, Tulleken CA, et al. Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke 1997; 28: 2084–2093. [DOI] [PubMed] [Google Scholar]
- 48.Oliveira R, Correia MA, Marto JP, et al. Reocclusion after successful endovascular treatment in acute ischemic stroke: systematic review and meta-analysis. J Neurointerv Surg 2023; 15: 964–970. [DOI] [PubMed] [Google Scholar]
- 49.Dhoisne M, Puy L, Bretzner M, et al. Early reocclusion after successful mechanical thrombectomy for large artery occlusion-related stroke. Int J Stroke 2023; 18: 712–719. [DOI] [PubMed] [Google Scholar]
- 50.Singh N, Cimflova P, Ospel JM, et al. Infarcts in a new territory: insights from the ESCAPE-NA1 trial. Stroke 2023. ; 54: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 51.Baron JC, Bousser MG, Comar D, et al. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations, and clinical applications in cerebral ischemic disorders. Eur Neurol 1981; 20: 273–284. [DOI] [PubMed] [Google Scholar]
- 52.Bivard A, Krishnamurthy V, Stanwell P, et al. Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke 2014; 45: 127–133. [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Bivard A, Levi CR, et al. Comparison of computed tomographic and magnetic resonance perfusion measurements in acute ischemic stroke: back-to-back quantitative analysis. Stroke 2014; 45: 1727–1732. [DOI] [PubMed] [Google Scholar]
- 54.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke 2008; 39: 870–877. [DOI] [PubMed] [Google Scholar]
- 55.Rao V, Christensen S, Yennu A, et al. Ischemic core and hypoperfusion volumes correlate with infarct size 24 hours after randomization in DEFUSE 3. Stroke 2019; 50: 626–631. [DOI] [PubMed] [Google Scholar]
- 56.Suomalainen OP, Martinez-Majander N, Sibolt G, et al. Comparative analysis of core and perfusion lesion volumes between commercially available computed tomography perfusion software. Eur Stroke J 2023; 8: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taha A, Bobi J, Dammers R, et al. Comparison of large animal models for acute ischemic stroke: which model to use? Stroke 2022; 53: 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baron JC, Yamauchi H, Fujioka M, et al. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 2014; 34: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guadagno JV, Jones PS, Aigbirhio FI, et al. Selective neuronal loss in rescued penumbra relates to initial hypoperfusion. Brain 2008; 131: 2666–2678. [DOI] [PubMed] [Google Scholar]
- 60.Ng FC, Churilov L, Yassi N, et al. Microvascular dysfunction in blood-brain barrier disruption and hypoperfusion within the infarct posttreatment are associated with cerebral edema. Stroke 2022; 53: 1597–1605. [DOI] [PubMed] [Google Scholar]
- 61.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feeney DM, Baron JC. Diaschisis. Stroke 1986; 17: 817–830. [DOI] [PubMed] [Google Scholar]
- 63.Ng F, Venkatraman V, Parsons M, et al. Gradient of tissue injury after stroke: rethinking the infarct versus noninfarcted dichotomy. Cerebrovasc Dis 2020; 49: 32–38. [DOI] [PubMed] [Google Scholar]
- 64.Lassen NA. Incomplete cerebral infarction–focal incomplete ischemic tissue necrosis not leading to emollision. Stroke 1982; 13: 522–523. [DOI] [PubMed] [Google Scholar]
- 65.Renu A, Millan M, San RL, et al. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE Randomized Clinical Trial. JAMA 2022; 327: 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231209069 for Incidence, severity and impact on functional outcome of persistent hypoperfusion despite large-vessel recanalization, a potential marker of impaired microvascular reperfusion: Systematic review of the clinical literature by Adrien ter Schiphorst, Guillaume Turc, Wagih Ben Hassen, Catherine Oppenheim and Jean-Claude Baron in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Datasets used for this systematic review will be made available upon reasonable request.