Abstract
Angiotensin receptor-neprilysin inhibitors (ARNIs) greatly benefit functional capacity and longevity in heart failure with reduced ejection fraction (HFrEF). Angiotensin receptor-neprilysin inhibitors remain underutilized and unstudied, however, in left ventricular assist device (LVAD) recipients, in spite of their underlying HFrEF. In this case series, we studied the feasibility and short-term efficacy of ARNI utilization in 21 LVAD patients. Angiotensin receptor-neprilysin inhibitor initiation was successful in most, resulting in significant consolidation of blood pressure (BP) medical management and marked improvements in both functional capacity and diuretic requirements. Angiotensin receptor-neprilysin inhibitors are safe, feasible, and within a short timeframe benefit BP and heart failure control in LVAD recipients.
Keywords: angiotensin receptor-neprilysin inhibitor, left ventricular assist device, heart failure, hypertension
Angiotensin receptor-neprilysin inhibitors (ARNIs) greatly improve long-term survival1 and functional capacity2 for patients with heart failure (HF) with reduced ejection fraction (HFrEF). Angiotensin receptor-neprilysin inhibitors also lower blood pressure (BP) and often reduce diuretic requirements. These effects would fulfill an unmet need for patients with left ventricular assist devices (LVAD), who still suffer poor long-term survival, and, in many cases, limited functional capacity and residual HF symptoms.3 It stands to reason that ARNIs should benefit LVAD recipients, given recent literature revealing the benefits of other guideline-directed medical therapy (GDMT) in patients with LVADs.4 That said, ARNIs remain underutilized and unstudied in the LVAD population. In this study, we sought to assess the feasibility and efficacy of ARNI treatment in LVAD patients.
Methods
This was a case series of all consecutive LVAD subjects at our center who initiated sacubitril-valsartan from 2018 to 2020. Sacubitril-valsartan was initiated in the setting of persistent hypertension or HF symptoms in spite of medication and LVAD speed optimization. Patients were included in the ARNI “failure” group if sacubitril-valsartan was discontinued within 3 months due to side effects; otherwise, patients were included in the “success” group. Baseline clinical, laboratory, and echocardiographic characteristics were obtained for all. Three-month characteristics were obtained for successfully initiated patients. The research was approved by the Hopkins Institutional Review Board. Data were summarized using mean ± SD. Continuous variables were compared using t-tests, categorical variables using χ2 tests, and paired data using paired t-tests. Statistical analysis and graphics were performed using Stata-15 (StataCorp) and Prism (GraphPad), respectively.
Results
Twenty-one patients on stable LVAD support were initiated on sacubitril-valsartan. Patients were 54 ± 10 years old and 19 ± 23 months (median 12, IQR: 6–17, range: 2.5–100) post-LVAD implantation. Seventeen were male (81%); 62% were African-American and 33% Caucasian. Two (9%) had a HeartMate II (Abbott) LVAD, 14 (67%) HeartWare (Medtronic) LVAD, and five (24%) HeartMate 3 (Abbott) LVAD; 12 were bridge-to-transplant (BTT) (57%) while nine (43%) were destination therapy (DT).
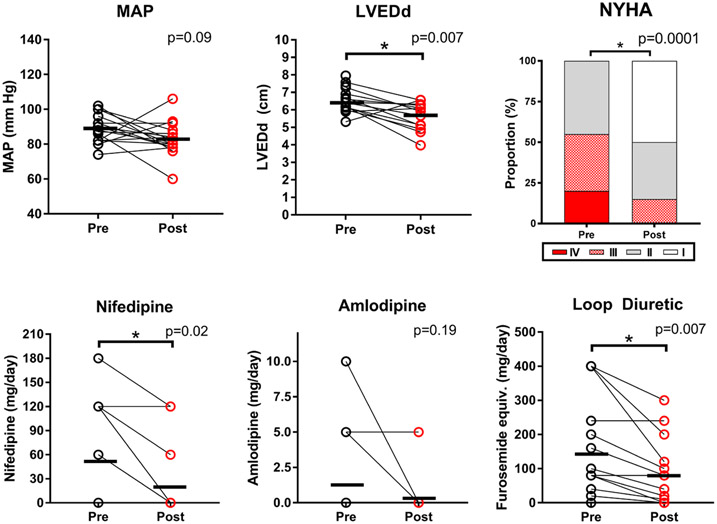
Sixteen patients (76%) successfully initiated sacubitril-valsartan and were assessed at baseline and 3 months later. Sacubitril-valsartan was kept at 24/26 mg BID in 37%, up-titrated to 49/51 mg BID in 44%, and reached 97/103 mg BID in 19%. Mean arterial pressure (MAP) trended lower at 3 months (89 ± 8 to 83 ± 10 mm Hg, p = 0.09) (Figure 1 and Table 1). Angiotensin receptor-neprilysin inhibitor allowed for concomitant reduction in calcium channel blocker (CCB) dosage in 82% of subjects (Figure 1 and Table 1) and complete discontinuation in 60% (Table 1). New York Heart Association (NYHA) functional capacity also improved significantly (Figure 1 and Table 1). Daily loop diuretic requirements also decreased significantly: furosemide equivalents fell from 143 ± 145 to 79 ± 94 mg/day, p = 0.007 (Figure 1 and Table 1). Of note, antihypertensive and diuretic doses were stable for the month before ARNI initiation (Table 1).
Figure 1.
Blood pressure and heart failure changes 3 months following angiotensin receptor-neprilysin inhibitor (ARNI) initiation in LVAD patients. Key variables pre- and postsuccessful initiation of ARNI (n = 16). Mean arterial pressure (MAP) decreased slightly, but not statistically significantly. Calcium channel blocker use decreased. New York Heart Association (NYHA) Functional capacity significantly improved. Loop diuretic requirements significantly decreased as well. There were significant decreases in left ventricular end-diastolic dimensions (LVEDd). LVAD, left ventricular assist device.
Table 1.
Patient Characteristics of ARNI Failure Versus Success Cases and, Among Success Cases, Pre- and Post-ARNI
| ARNI Failure | ARNI Success | p | |||||
|---|---|---|---|---|---|---|---|
| Overall (n = 21) |
Pre-ARNI (n = 5) |
Pre-ARNI (n = 16) |
Post-ARNI (n = 16) |
Failure vs. Success |
Success: Pre vs. Post |
Med Dose 1-Month Prior vs. Pre |
|
| Mean (SD), n (%) |
Mean (SD), n (%) |
Mean (SD), n (%) |
Mean (SD), n (%) |
||||
| Demographics | |||||||
| Age (years) | n/a | 56.0 (5) | 53.3 (11) | n/a | 0.59 | n/a | n/a |
| Body mass index (kg/m2) | 34.1 (8) | 30.6 (6) | 35.2 (8) | n/a | 0.24 | n/a | n/a |
| Time from LVAD (months) | 19.6 (23) | 18.1 (14) | 20.1 (26) | n/a | 0.88 | n/a | n/a |
| Female gender | 4 (19%) | 1 (20%) | 3 (19%) | n/a | 0.95 | n/a | n/a |
| Medication use | |||||||
| Carvedilol (mg/day) | 15 (18) | 26 (24) | 11 (15) | 8 (11) | 0.10 | 0.33 | 0.10 |
| 1-month prior (mg/day) | 12 (14) | 21 (20) | 9 (11) | ||||
| Metoprolol succinate (mg/day) | 10 (34) | 0 (0) | 13 (39) | 14 (39) | 0.49 | 0.33 | 0.33 |
| 1-month prior (mg/day) | 8 (24) | 0 (0) | 9 (27) | ||||
| Amlodipine (mg/day) | 2 (4) | 4 (5) | 1 (3) | 0 (1) | 0.15 | 0.19 | 1.00 |
| 1-month prior (mg/day) | 2 (4) | 5 (6) | 1 (3) | ||||
| Nifedipine (mg/day) | 51 (61) | 60 (60) | 49 (63) | 19 (42) | 0.73 | 0.02 | 0.67 |
| 1-month prior (mg/day) | 45 (64) | 45 (57) | 45 (68) | ||||
| Furosemide equivalents (mg/day) | 116 (136) | 32 (36) | 143 (145) | 79 (94) | 0.11 | 0.007 | 0.12 |
| 1-month prior (mg/day) | 108 (136) | 28 (32) | 133 (146) | ||||
| CCB use, n (%) | 15 (71) | 5 (100) | 10 (63) | 4 (25) | 0.12 | 0.07 | n/a |
| Aldosterone antagonist use, n (%) | 7 (44) | 0 (0) | 7 (44) | 7 (44) | 0.07 | 1.00 | n/a |
| Clinical characteristics | |||||||
| Mean arterial pressure (mm Hg) | 89 (8) | 91 (4) | 89 (8) | 83 (10) | 0.59 | 0.09 | n/a |
| Radial artery pulsatility, n (%) | 0 (0) | 0 (0) | 0 (0) | 7 (44) | n/a | 0.004 | n/a |
| NYHA Functional Class | 0.44 | 0.0001 | n/a | ||||
| I | 1 (5%) | 1 (20%) | 0 (0%) | 8 (50%) | |||
| II | 9 (43%) | 2 (40%) | 7 (44%) | 6 (38%) | |||
| III | 7 (33%) | 1 (20%) | 6 (37%) | 2 (12%) | |||
| IV | 4 (19%) | 1 (20%) | 3 (19%) | 0 (0%) | |||
| LVEDd (cm) | 6.6 (0.8) | 6.8 (1) | 6.5 (0.8) | 5.6 (0.8) | 0.57 | 0.007 | n/a |
| LVAD speed (rpm) | n/a | n/a | n/a | ||||
| HM II | 9,700 (141) | 9,800 (0) | 9,600 (0) | 9,600 (0) | |||
| HM 3 | 5,600 (200) | n/a | 5,600 (200) | 5,580 (228) | |||
| HVAD | 2,744 (147) | 2,755 (124) | 2,740 (161) | 2,718 (170) | |||
| Sodium (mEq/L) | 140 (2) | 140 (3) | 140 (2) | 139 (3) | 0.56 | 0.23 | n/a |
| Potassium (mEq/L) | 4.1 (0.4) | 4.1 (0.2) | 4.2 (0.4) | 4.3 (0.6) | 0.62 | 0.28 | n/a |
| BUN (mg/dl) | 20 (8) | 17 (5) | 21 (9) | 23 (14) | 0.37 | 0.33 | n/a |
| Creatinine (mg/dl) | 1.4 (0.4) | 1.5 (0.4) | 1.4 (0.4) | 1.3 (0.3) | 0.69 | 0.56 | n/a |
Medication doses the month before ARNI are noted in italics.
ARNI, angiotensin receptor-neprilysin inhibitor; BUN, blood urea nitrogen; CCB, calcium-channel blocker; HM 3, HeartMate 3; HM II, HeartMate 2; HVAD, HeartWare VAD; LVAD, left ventricular assist device; LVEDd, Left ventricular end-diastolic dimension; NYHA, New York Heart Association.
Left ventricular dimensions changed with ARNI, resulting in concomitant LVAD adjustments. Left ventricular end-diastolic dimensions (LVEDd) significantly decreased 3 months post-ARNI (6.5 ± 0.8 to 5.6 ± 0.8 cm, p = 0.007) (Figure 1). Additionally, 31% of patients underwent LVAD speed reductions during the initiation period, while only 6% underwent increases. On exam, none had palpable radial pulses pre-treatment but 44% had palpable radial pulses after 3 months (p = 0.004). There were no statistically significant changes in renal function, sodium, or potassium (Table 1).
Five subjects (24%) failed ARNI initiation within 3 months due to side effects. These included cough (n = 1), dehydration (n = 1), acute kidney injury (n = 1), presyncope (n = 2), hypotension (n = 1), and LVAD suction (n = 3); three subjects incurred multiple side effects. Compared to success patients, patients in the failure group tended to have lower baseline loop diuretic requirements (furosemide equivalent 48 ± 52 vs. 147 ± 148 mg/day, p = 0.09) (Table 1). Otherwise, there were no significant differences between groups with respect to demographics, MAP, medications, and LVEDd (Table 1).
Discussion
In this study, we demonstrate for the first time that ARNI is safe, feasible, and well-tolerated in the majority of LVAD patients. Moreover, ARNIs significantly improve BP control, HF symptoms, and diuretic requirements. These results highlight the potential benefits of ARNI in LVAD recipients and point to the need for further study.
Blood pressure management in LVAD recipients has traditionally focused on MAP control to optimize pump flow and minimize neurologic complications.5 Many centers favor BP control using GDMT, but post-LVAD BP increases often necessitate addition of CCBs, which are generally contraindicated in HFrEF. Angiotensin receptor-neprilysin inhibitors markedly improved BP control in our cohort, thereby allowing both CCB weaning and GDMT maximization. This BP management strategy warrants strong consideration in the LVAD population, given the proven survival benefit of escalating GDMT in LVAD recipients.4 Long-term survival remains a limitation of LVAD support, and improving survival is important not only for patients with DT LVADs, but also for BTT LVAD recipients who now wait longer for heart transplantation ever since the 2018 transplant allocation system took effect.6
Mitigating residual HF and improving quality of life (QOL) remain another unmet need in the LVAD population. Many LVAD recipients still report limitations in functional capacity7 and Kansas City Cardiomyopathy Questionnaire QOL improvements generally plateau at 6 months.8 Angiotensin receptor-neprilysin inhibitors, unlike other GDMT agents, were shown to dramatically improve QOL.2 We too found dramatic improvements in NYHA functional capacity, as well as significant reductions in diuretic requirements. These improvements were especially significant considering patients were on average over a year into LVAD support. These improvements occurred even as MAP remained statistically unchanged, suggesting that they were specific to ARNI and not just BP improvement.
Angiotensin receptor-neprilysin inhibitor initiation proved difficult for some. Reductions in LV dimensions required LVAD speed reductions in almost a third. Among those who failed ARNI initiation, symptoms of presyncope, suction, and dehydration predominated. Angiotensin receptor-neprilysin inhibitor failures tended to have lower baseline diuretic need. Our experience thus cautions against unmonitored ARNI initiation in LVAD recipients with zero or low diuretic requirements. Our sample size, however, limits firmer conclusions.
Conclusions
Limitations of this study include sample size, nonrandomization, short follow-up, and retrospective data collection. That said, our study shows that ARNI initiation is safe and feasible, and effectively improves BP and HF control while optimizing GDMT in the LVAD population. Future efforts will address these limitations with a prospective, randomized evaluation of ARNI in LVAD patients.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
References
- 1.McMurray JJV, Packer M, Desai AS, et al. : Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Chandra A, Lewis EF, Claggett BL, et al. : Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol 3: 498–505, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teuteberg JJ, Cleveland JC Jr, Cowger J, et al. : The Society of Thoracic Surgeons Intermacs 2019 Annual Report: the changing landscape of devices and indications. Ann Thorac Surg 109: 649–660, 2020. [DOI] [PubMed] [Google Scholar]
- 4.McCullough M, Caraballo C, Ravindra NG, et al. : Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol 5: 175–182, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teuteberg JJ, Slaughter MS, Rogers JG, et al. ; ADVANCE Trial Investigators: The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail 3: 818–828, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Rao P, Smith R, Khalpey Z: Potential impact of the proposed revised UNOS thoracic organ allocation system. Semin Thorac Cardiovasc Surg 30: 129–133, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Jakovljevic DG, McDiarmid A, Hallsworth K, et al. : Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol 114: 88–93, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers JG, Aaronson KD, Boyle AJ, et al. : HeartMate II Investigators: Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 55: 1826–1834, 2010. [DOI] [PubMed] [Google Scholar]