Abstract
Background:
The incidence of, risk factors for, and outcomes after the development of ascites are poorly described for contemporary patients with cirrhosis
Methods:
We examined data for a 20% random sample of United States Medicare enrollees with cirrhosis and Part D prescription coverage from 2008-2019, excluding patients with heart failure and diuretic use prior to cirrhosis. Among 63,364 persons with cirrhosis, we evaluated the incidence of ascites using an Aalen-Johansen estimator. We evaluated risk factors for ascites, mortality, and mortality after ascites using multistate modeling. We determined the associations with each outcome for an array of medication exposures including nonselective beta-blockers, antiviral therapy, statins, rifaximin, anticoagulants, and metformin.
Results:
The cumulative incidence of ascites was 5.1%, 9.5%, and 10.7% and 1,3, and 5 years overall. The corresponding data for ascites requiring paracentesis were 1%, 2.1%, and 2.4%. Persons aged <65 years, with alcohol-related cirrhosis, varices, or HE are most likely to develop ascites. The risk of ascites was higher for persons taking any NSBB (including carvedilol) but lower for those taking atorvastatin (but not other statins) and antiviral therapy for Hepatitis C. Incident ascites was associated with increased risk of death, HR 27.6 95%CI(21.7-35.1). Survival following ascites was 1.08 years (interquartile range,IQR,0.26-2.75), 0.38 years (IQR0.1-1.3) for those requiring paracentesis. Lipophilic statins were the only medications associated with lower mortality after ascites requiring paracentesis.
Conclusions:
Ascites is associated with a high risk of death. Very few candidate therapies are associated with reduced the risk of ascites and mortality after ascites development.
Keywords: Liver Disease, NAFLD, Alcohol, Varices
Introduction
Ascites is associated with life-threatening infections, renal dysfunction, malnutrition, and diminished health-related quality of life (HRQOL).1 As such, ascites is a major driver of morbidity and mortality for persons with cirrhosis. Despite its frequency, data are limited regarding the actual incidence, was predictors, and outcomes of ascites among contemporary patients with cirrhosis.
D’amico et al estimate 46% of compensated patients will develop ascites, after which 5-year survival is 20%.2 However, those patients were aged <50 years on average and most of whom had viremic hepatitis C. Since this landmark study, and others,2-6 the epidemiology of cirrhosis has shifted. Driven by emerging risk factors, such as nonalcoholic fatty liver disease (NAFLD),7 patients with cirrhosis are presenting at increasingly older ages with cardiovascular and renal comorbidities,8, 9 all of which may contribute to both ascites development and its competing risks.10, 11 In addition to the need for a better delineation of today’s burden of ascites, there are current knowledge gaps regarding the associations of ascites development with potential pharmacological therapies. There is mounting interest in therapies to potentially forestall disease progression. These include non-selective beta-blockers (NSBB), anticoagulants, rifaximin, and statins.9, 12-14 Data are needed to estimate the potential benefit of future clinical trials aiming to prevent the development of ascites.
Herein, we evaluate the incidence of, associations with, and mortality after ascites in a population-based US cohort of Medicare enrollees with cirrhosis.
Methods
Study Population
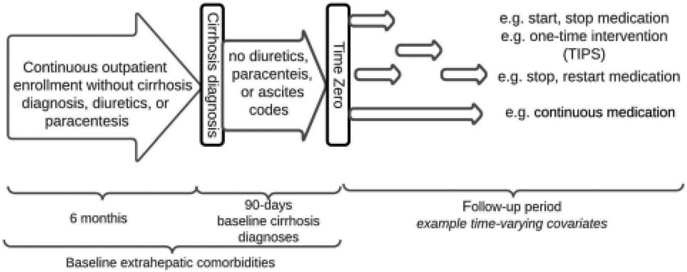
We examined data from a 20% random sample (the second largest available extract of data from this government payer) of US Medicare enrollees with cirrhosis (using a validated algorithm for Medicare data; one of the following: ≥2 outpatient cirrhosis codes or 1 cirrhosis code and ≥1 cirrhosis complication codes15) and continuous Part D (prescription) coverage from 2008 through 2014. A summary of diagnostic codes used is provided in Supplementary Table 1. Medicare beneficiary claims data from inpatient and outpatient encounters are available in deidentified data sets prepared by the Centers for Medicare and Medicaid Services for research purposes. Exclusions summarized in Supplementary Table 2. We required 180 days of continuous outpatient enrollment prior to cirrhosis diagnosis and set 90 days after cirrhosis diagnosis as a landmark and therefore excluded all patients with less than 90-days of outpatient follow up and those with ascites diagnostic codes, paracentesis, loop diuretic or potassium-sparing diuretics at any time prior to or within the landmark period. In addition, we excluded patients who had HE (diagnostic code or lactulose/rifaximin), variceal bleeding (diagnostic code or endoscopic intervention), transjugular intrahepatic portosystemic shunt (TIPS), and congestive heart failure prior to or concurrent with the first cirrhosis code. As this is a study to ascertain risk factors for ascites, all patients who developed ascites between cirrhosis diagnosis and 90-days were excluded. We included patients who were diagnosed with HE during the 90-day period following cirrhosis diagnosis. To allow for adequate covariate acquisition, we included all comorbidities within 365 days prior to the landmark period (effectively 9 months prior to the first diagnosis of cirrhosis). Each beneficiary is assigned an anonymous identifier allowing for longitudinal analyses. Subjects were followed until death, transplant, date of last follow-up, or the end of study (12/31/2017). In order to evaluate the impact of medication usage, we limited our analyses to beneficiaries who had been continuously enrolled in Medicare Part D for 3 months or more before the index/enrollment visit. The study design is summarized in Figure 1. This study was deemed exempt from institutional review board review by the University of Michigan Medical School.
Fig. 1. Study design.
All patients had continuous outpatient enrollment for 6 months prior to their index cirrhosis diagnosis, none of whom received diuretics or a paracentesis. After their diagnosis, patients were required to have a 90-day landmark period without diuretics, paracenteses, ascites codes, transplant, or mortality. Medication exposures and transjugular intrahepatic portosystemic shunts (TIPS) were treated as time-varying covariates
Ascertainment of Incident Ascites
Our primary aim was to describe the incidence of and risk factors for ascites for patients with cirrhosis diagnosed during long-term follow-up. Incident ascites was defined if identified for the first time at least 90 days after first cirrhosis diagnosis based on ICD-9/10 codes,16 combination loop (furosemide, bumetanide, torsemide) and potassium-sparing diuretics (spironolactone, amiloride, eplerenone), or the performance of a paracentesis, whichever came first. We have validated the use of ascites codes and combination diuretics. Combination diuretics have a sensitivity/specificity of 95.2%/86.8%, while the ICD-10 code R18.8 has sensitivity/specificity 90%/68.5%.17 We conducted a sensitivity analysis focusing only on patients requiring paracentesis.
Ascertainment of Risk Factors for Ascites
We sought to examine the association between incident ascites and the use of medication classes that have biological plausibility for the development of ascites (NSBB, anti-viral therapies for hepatitis C), those that are felt based on prior observational data to reduce risk of ascites complications and all-cause mortality (statins, anticoagulants, metformin, rifaximin).13, 14, 18, 19 We included other medications to serve as negative controls including cardioselective beta-blockers, other hypolipidemics, and insulin.(Supplementary Table 1). Baseline medication exposures were defined as those which were used within 180-days prior to or 90-days after cirrhosis diagnosis. Thereafter, medication exposures were treated as time-varying covariates accounting for the timing and amount of medication dispensed over the course of follow-up.
For complete description of the cohort and risk-adjustment we also included age, sex, race, comorbidities,20 etiology of liver disease, complications of cirrhosis within the first 90-days of cirrhosis, and baseline evaluation by a gastroenterologist/hepatologist (prior to or within 90-days of cirrhosis diagnosis). Patients could have multiple causes of cirrhosis (e.g. viral and alcohol-related liver disease). As performed by other investigators,21, 22 we also classified a group of patients with likely NAFLD or non-alcohol, non-viral-related cirrhosis (no diagnostic codes for viral hepatitis, auto-immune or biliary disease, alcohol-related use disorder or alcohol-related organ injury). As 1% had hepatitis B, we combined these patients in ‘viral hepatitis’.
Analyses
All data were derived from a landmark analysis, setting cohort entry as 90-days after the first diagnosis of cirrhosis in order to mitigate the risk of delays in coding and immortal time bias.23 Cumulative incidence curves were drawn to demonstrate the risk of ascites overall and stratified by age (≥65 or younger), etiology of cirrhosis, varices and/or HE diagnosed within 90-days following cirrhosis diagnosis, and the use of non-selective beta-blockers. Competing risks included death and liver transplantation. The probability of ascites at 1 year, 3-years, and 5-years was estimated using an Aalen-Johansen estimator, a method to compute state occupation probabilities for multistate disease models.24 All analyses were performed using R and SAS (SAS Institute Inc., Cary, NC, USA).
Multistate model
We employed a competing-risk illness-death multistate model. All individuals start in the ascites-free initial state (cirrhosis), may move to state 2 (ascites) and afterwards enter state 3 (death). The endpoint state, also called the absorbing state is death and the intermediate state is ascites. We then addressed the time-dependent contribution of ascites and other exposures to the risk of death using time-dependent cox model. In addition to the medications, TIPS was also treated as a time-varying covariate. For further details regarding the statistics of our models, please refer to the Supplement.
Results
Demographics and Clinical Characteristics
We included 63,364 Medicare-enrollees with cirrhosis who were followed for a median 3.9 (IQR 2.4-5.9) years per-person. The key features of this cohort are their age (median 72 years), 64% urban dwelling, 77% Caucasian, 40% from southern US, and 42% receiving disability.(Table 1). The plurality had alcohol-related cirrhosis. Less than half were under the care of a gastroenterologist/hepatologist. Most patients had comorbid hypertension, roughly one-third had dyslipidemia or pulmonary disease, one-sixth had peripheral vascular disease, one-seventh had cerebrovascular disease.
Table 1.
Baseline characteristics of study cohort
| Variables | No ascites (n = 50,099) | Ascites (n = 13,265) | P value | |
|---|---|---|---|---|
| Demographics | Age | 72.7 (16.3) | 71.5 (14.9) | <0.001 |
| Black race | 12.7% (6356) | 10.7% (1422) | <0.001 | |
| White race | 75.9% (38,049) | 77.9% (10,340) | <0.001 | |
| Male sex | 54.6% (27,363) | 58.0% (7695) | <0.001 | |
| Urban | 64.0% (32,062) | 64.1% (8506) | 0.79 | |
| Midwest | 18.0% (9011) | 19.3% (2560) | 0.001 | |
| Northeast | 18.8% (9431) | 18.6% (2465) | 0.53 | |
| South | 40.3% (20,184) | 39.2% (5206) | 0.04 | |
| West | 21.9% (10,980) | 22.0% (2923) | 0.78 | |
| Disability | 41.5% (20,773) | 43.9% (5816) | <0.001 | |
| Medicaid | 28.5% (14,269) | 31.3% (4154) | <0.001 | |
| Cirrhosis features | Alcohol-related | 32.0% (16,041) | 49.4% (6558) | <0.001 |
| Likely NAFLD | 39.5% (19,810) | 25.9% (3439) | <0.001 | |
| Viral cirrhosis | 28.4% (14,248) | 24.6% (3268) | <0.001 | |
| Varices (within 90-day of diagnosis) | 4.0% (1988) | 12.2% (1612) | <0.001 | |
| HE (within 90-day of diagnosis) | 5.1% (2531) | 12.1% (1601) | <0.001 | |
| Gastroenterology consultation | 40.5% (20,279) | 46.0% (6098) | <0.001 | |
| Comorbidities | Myocardial infarction | 6.0% (3011) | 6.1% (807) | 0.76 |
| PVD | 17.2% (8640) | 16.0% (2126) | 0.001 | |
| CVD | 15.7% (7880) | 13.6% (1807) | <0.001 | |
| Hypertension | 71.6% (35,865) | 69.0% (9157) | <0.001 | |
| Hyperlipidemia | 38.6% (19,320) | 33.4% (4431) | <0.001 | |
| COPD | 30.8%(15,417) | 29.1% (3861) | <0.001 | |
| CKD | 7.2% (3626) | 7.2% (955) | 0.88 | |
| Diabetes | 10.0% (5002) | 13.0% (1726) | <0.001 | |
| Medications | Propranolol/Nadolol | 2.2% (1086) | 6.6% (877) | <0.001 |
| Carvedilol | 1.8% (919) | 2.6% (340) | <0.001 | |
| Selective beta-blocker | 17.1% (8542) | 16.4% (2171) | 0.06 | |
| Simvastatin | 5.5% (2757) | 6.2% (823) | 0.002 | |
| Atorvastatin | 3.2% (1627) | 2.8% (377) | 0.02 | |
| Other statins | 4.0% (2006) | 4.0% (527) | 0.87 | |
| Fibrates/niacin | 5.1% (2573) | 4.1% (544) | <0.001 | |
| Hepatitis C therapy | 3.7% (1869) | 2.6% (349) | <0.001 | |
| Anticoagulants | 0.6% (298) | 0.5% (65) | 0.17 | |
| Metformin | 6.5% (3251) | 9.9% (1318) | <0.001 | |
| Insulin | 7.2% (3617) | 9.9% (1310) | <0.001 |
Many patients had both hepatitis C and alcoholic cirrhosis. Patients with cirrhosis but neither viral hepatitis nor any alcohol use disorder or injury or other liver disease were classified as likely NAFLD. HE = Hepatic encephalopathy, CVD = cerebrovascular disease, PVD = peripheral vascular disease, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease. Two-tailed p-values were obtained using Chi-squared testing for categorical variables and Student’s T-testing for continuous variables
Incident Ascites
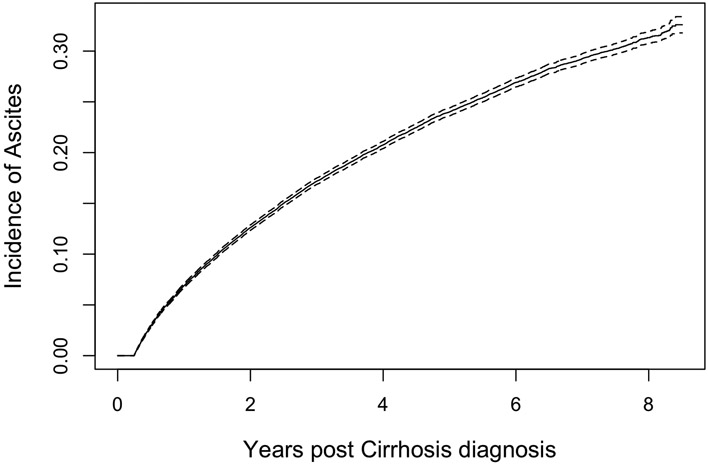
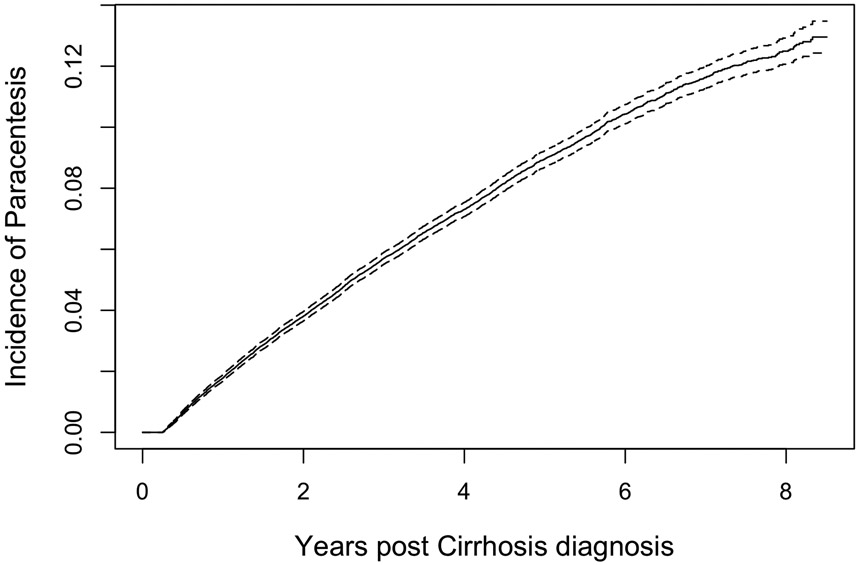
The cumulative incidence curves for ascites are shown in Figure 2. Incident ascites was diagnosed in 21% (n=13,265) after median of 1.7 years (IQR0.8-3.1). Among these, 4,732 required paracentesis (most of whom would also receive ascites codes and diuretics), 7,237 were identified by code (1,545 of whom would require diuretics), and 1,206 by combination diuretics without a code or paracentesis. The cumulative incidence of ascites was 5.1%, 9.5%, and 10.7% and 1,3, and 5 years overall. The corresponding incidence for ascites requiring paracentesis was 1%, 2.1%, and 2.4%.
Figure 2A: The Cumulative Incidence of Ascites.
The incidence of ascites defined by diagnostic codes, combination diuretics and/or paracentesis is presented, accounting for the competing risk of death
Clinical Risk factors for ascites
In Table 2, we present the cumulative incidence of ascites overall and according to baseline clinical factors. Persons who are younger than 65 years, have alcohol-related cirrhosis, varices, and HE are most likely to develop ascites. The group at highest risk for ascites are those who are diagnosed with other complications of cirrhosis within 90-days after the time of their index cirrhosis diagnosis. In Table 3, we present the results of the multistate model. Here varices and HE are both strongly associated with incident ascites, both with hazard ratios (HR) of 2.1 95%CI(2.0-2.2).
Table 2.
Probability of incident ascites at one and three years
| Category | Any Ascites* Probability ± SE |
Ascites requiring Para centesis Probability ± SE |
||
|---|---|---|---|---|
| One year | Three year | One year | Three year | |
| Overall | 5.13 ± 0.09 | 9.49 ± 0.12 | 1.01 ± 0.04 | 2.09 ± 0.06 |
| Age < 65 | 5.57 ± 0.14 | 10.75 ± 0.20 | 1.09 ± 0.06 | 2.39 ± 0.10 |
| Age ≥ 65 | 4.81 ± 0.11 | 8.57 ± 0.15 | 0.95 ± 0.05 | 1.86 ± 0.07 |
| Varices | 12.1 ± 0.55 | 23.4 ± 0.75 | 2.21 ± 0.25 | 6.36 ± 0.45 |
| No Varices | 4.71 ± 0.09 | 8.66 ± 0.12 | 0.94 ± 0.04 | 1.83 ± 0.06 |
| HE | 12.0 ± 0.51 | 17.4 ± 0.62 | 2.17 ± 0.23 | 3.86 ± 0.32 |
| No HE | 4.65 ± 0.09 | 8.93 ± 0.12 | 0.93 ± 0.04 | 1.96 ± 0.06 |
| Varices and HE | 20.7 ± 2.32 | 31.0 ± 2.90 | 5.24 ± 1.28 | 6.47 ± 1.71 |
| Alcohol | 7.45 ± 0.18 | 13.31 ± 0.24 | 1.53 ± 0.08 | 3.24 ± 0.13 |
| Viral | 4.43 ± 0.16 | 8.41 ± 0.22 | 0.87 ± 0.07 | 1.64 ± 0.11 |
| NAFLD** | 3.4 ± 0.12 | 6.53 ± 0.17 | 0.61 ± 0.05 | 1.27 ± 0.08 |
All estimated probabilities generated using the Aalen–Johansen estimator. The categories were defined based on clinical factors that occurred during the first 90-days after the cirrhosis diagnosis. (*)Any ascites includes diagnostic codes, combination diuretics, and paracentesis. (**) Patients with cirrhosis but neither viral hepatitis nor any alcohol use disorder or injury or other liver disease were classified as likely NAFLD = Nonalcoholic Fatty Liver Disease. HE = Hepatic encephalopathy. Viral etiology combines both hepatitis C and hepatitis B
Table 3.
The association between incident ascites and clinical and demographic factors in a multistate model
| Incident ascites | Death before ascites | Death after ascites | ||||
|---|---|---|---|---|---|---|
| sHR 95%CI | p | sHR 95%CI | p | sHR 95%CI | p | |
| Age | 1.001 (1.000, 1.003) | 0.052 | 1.030 (1.029, 1.032) | <0.001 | 1.017 (1.015, 1.019) | <0.001 |
| Race (Black) | 0.818 (0.773, 0.867) | <0.001 | 1.024 (0.961, 1.090) | 0.467 | 1.156 (1.066, 1.255) | <0.001 |
| Race (Other) | 0.973 (0.920, 1.030) | 0.352 | 0.808 (0.751, 0.870) | <0.001 | 1.010 (0.930, 1.097) | 0.812 |
| Male | 1.054 (1.017, 1.093) | 0.004 | 1.376 (1.318, 1.437) | <0.001 | 1.324 (1.257, 1.395) | <0.001 |
| Varices | 2.100 (1.974, 2.234) | <0.001 | 0.993 (0.886, 1.113) | 0.898 | 0.865 (0.790, 0.948) | 0.002 |
| HE | 2.111 (2.000, 2.227) | <0.001 | 1.709 (1.592, 1.835) | <0.001 | 1.160 (1.080, 1.246) | <0.001 |
| SBB | 1.002 (0.954, 1.051) | 0.947 | 0.982 (0.930, 1.037) | 0.515 | 0.947 (0.885, 1.013) | 0.115 |
| Other NSBB | 1.548 (1.438, 1.666) | <0.001 | 0.852 (0.737, 0.985) | 0.031 | 0.953 (0.860, 1.056) | 0.361 |
| carvedilol | 1.299 (1.163, 1.450) | <0.001 | 1.121 (0.979, 1.285) | 0.099 | 0.890 (0.764, 1.035) | 0.130 |
| simvastatin | 0.969 (0.899, 1.044) | 0.405 | 0.954 (0.872, 1.044) | 0.305 | 0.911 (0.820, 1.012) | 0.082 |
| atorvastatin | 0.861 (0.776, 0.957) | 0.005 | 0.799 (0.704, 0.906) | <0.001 | 0.864 (0.740, 1.009) | 0.064 |
| other statins | 0.942 (0.860, 1.031) | 0.192 | 0.810 (0.722, 0.910) | <0.001 | 0.931 (0.818, 1.061) | 0.284 |
| Antiviral therapy | 0.772 (0.693, 0.861) | <0.001 | 0.843 (0.723, 0.983) | 0.029 | 0.899 (0.761, 1.062) | 0.210 |
| anticoagulants | 1.102 (0.863, 1.408) | 0.435 | 0.822 (0.619, 1.093) | 0.177 | 0.649 (0.441, 0.956) | 0.029 |
| metformin | 1.373 (1.290, 1.461) | <0.001 | 0.803 (0.728, 0.885) | <0.001 | 0.918 (0.840. 1.004) | 0.060 |
| other cholesterol | 0.747 (0.684, 0.816) | <0.001 | 0.786 (0.710, 0.872) | <0.001 | 0.866 (0.762, 0.985) | 0.029 |
| insulin | 1.279 (1.201, 1.362) | <0.001 | 1.311 (1.214, 1.416) | <0.001 | 1.071 (0.983, 1.168) | 0.118 |
| Rifaximin | 5.715 (5.244, 6.207) | <0.001 | 5.366 (4.690, 6.139) | <0.001 | 3.335 (3.138, 3.544) | <0.001 |
| GI consult | 1.137 (1.097, 1.178) | <0.001 | 0.856 (0.820, 0.894) | <0.001 | 0.866 (0.823, 0.911) | <0.001 |
| Etiology (NAFLD) | 0.504 (0.482, 0.526) | <0.001 | 0.915 (0.872, 0.960) | <0.001 | 1.016 (0.954, 1.082) | 0.619 |
| etiology (Viral) | 0.628 (0.601, 0.657) | <0.001 | 0.793 (0.749, 0.840) | <0.001 | 1.053 (0.989, 1.121) | 0.106 |
| TIPS | 1.700 (1.269, 2.277) | <0.001 | 5.376 (3.787, 7.632) | <0.001 | 1.477 (1.293, 1.686) | <0.001 |
| ascites | 36.418 (29.284, 45.291) | <0.001 | ||||
Hazard ratios are derived from multistate models with time-varying covariates (medications and transjugular intrahepatic portosystemic shunt, TIPS) for the outcomes after ascites. The etiologies, NAFLD (nonalcoholic fatty liver disease) and viral, are presented as relative to alcohol-related liver disease as a reference. All values are adjusted simultaneously for the other variables in the table and also by geographic region, urban/rural, Medicaid coinsurance, social security disability, and comorbidities (vascular disease, heart disease, pulmonary disease, chronic kidney disease, diabetes, hypertension, hyperlipidemia)
Medication use is associated with ascites incidence
The risk of ascites was highest for persons taking any NSBB (including carvedilol). The risk of ascites was lower for users of atorvastatin (but not other statins, including simvastatin), fibrates/niacin, and antiviral therapy for HCV. Rifaximin and diabetic therapies such as metformin and insulin were associated with higher risk of ascites, while anticoagulants were not associated with the risk of ascites.(Table 3) Supplementary Table 3 provides the estimates without time-varying covariates to demonstrate the stability of baseline associations. Supplementary Table 4 presents a sensitivity analysis where ascites is defined by paracentesis requirement and in this case all statins were not associated with incident ascites.
Outcomes
Overall, 16,125 patients died during follow-up after 2.1 years (IQR1.0-3.7), 1,483 patients underwent transplantation after 3.8 years (IQR2.0-5.8), and 45,756 were censored at last follow-up after 4.6 years (IQR3.0-6.5). After developing ascites, the median survival was 1.08 years (IQR0.26-2.75), less for those requiring paracentesis, 0.38 years (IQR0.1-1.3).
After developing ascites, 355 (2.7%) patients underwent TIPS, after 2.2 years (IQR1.24-3.64). SBP was diagnosed in 1,173 (8.8%) patients within 0.4 years (IQR0.02-1.31). Factors associated with mortality as a competing risk for ascites included baseline male sex, HE, and metabolic or cardiovascular comorbidities. Conversely, factors associated with lower mortality as a competing risk included other NSBB, statins, and gastroenterology consultation.(Table 3)
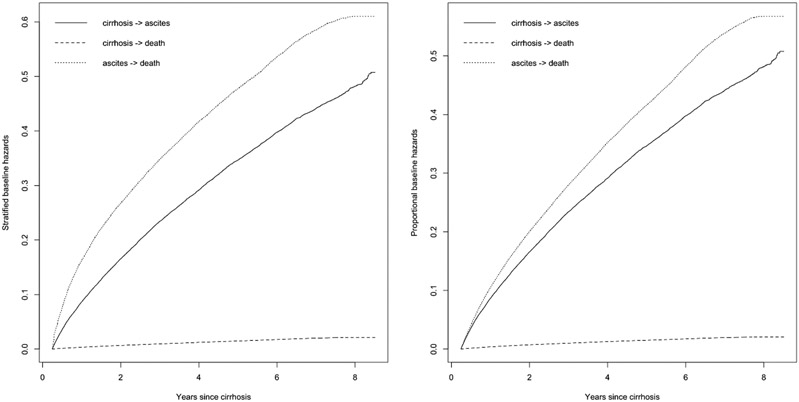
The hazard of death is raised substantially by the development of ascites.(Figure 3) In Table 3, it is shown that ascites as a time-dependent variable is associated with an increased risk of death, HR 27.6 95%CI(21.7-35.1). As in Table 3, the candidate pharmacotherapies are not associated with lower mortality after ascites with the exception of anti-coagulants, HR 0.63 95%CI(0.43-0.94). In contrast, rifaximin was associated with higher mortality, as was TIPS.
Figure 3: Multistate modeling of risks of ascites, mortality, and mortality after ascites.
In this multistate model presented using baseline factors only (A) and time-varying covariates in a proportional hazards model (B), death prior to ascites is rare while it is very common following the incidence of ascites.
We conducted a sensitivity analysis where ascites was defined by paracentesis requirement alone.(Supplementary Table 4) In this context, most relationships were similar with a few exceptions. First, anticoagulants were no longer associated with any outcome but both lipophilic statins (atorvastatin, simvastatin) were associated with lower mortality after ascites. Second, gastroenterology/hepatology consultation was not associated with mortality after ascites.
Discussion
In this longitudinal cohort reflective of contemporary elderly American patients with cirrhosis, we address important knowledge gaps regarding the incidence of, risk factors for, and outcomes after ascites. We show that ascites is common, however less so than older reports and particularly for those with NAFLD. We highlight that even among contemporary patients, outcomes after ascites are dismal. Finally, we show that there are few promising chemopreventative agents.
The incidence of ascites
At 5-years the cumulative incidence of ascites was 10.7% overall and for ascites requiring paracentesis it was 2.4%. The incidence is highest among those diagnosed with varices and/or HE within 90-days of their index cirrhosis diagnosis as well as those with alcohol-related liver disease. It is lowest among persons with likely NAFLD-cirrhosis. Our probabilities of incident ascites are consistent with those described by D’Amico.3 However ours are substantially lower when focusing on patients requiring paracentesis. We make two conclusions. First, our incidence data highlights either the potential for increases in early-stage or over-diagnosis of ascites likely as a function of widespread abdominal imaging or that the severity of ascites among older persons may be fundamentally different than that which was observed in historical cohorts of middle-aged persons, most of whom had viremic hepatitis C.2-6 Second, while ascites is felt to be the most common complication,2-4 this may not be true for older patients. The incidence of ascites observed in this study is, across subgroups, similar to or less than the incidence of HE from our prior study of Medicare enrollees.8 Further, a recent study from the NASH clinical research network showed that HE, not ascites is the most common first complication.25 Taken together, these data highlight how for older persons with cirrhosis and those with NASH, the specific burden of decompensations may be different than younger patients with alcohol or viral related liver disease.
Limited evidence for preventative therapy
As expected, we see that antiviral therapy for hepatitis C is associated with a reduced risk of ascites. Although there is enthusiasm for non-etiologic therapies to reduce the burden of portal hypertensive complications among persons with cirrhosis, our data does not support any robust effects for the array of examined therapeutic candidates. While PREDESCI showed that patients with portal hypertension could experience a lower risk of ascites on NSBB,12 this is not observed in our data. Our findings do not refute PREDESCI but underscore how trial findings cannot be generalized to older real-world patients with comorbidities without manometry-confirmed portal hypertension who are on NSBB for cardiovascular (or other) indications. Nor do these results suggest that NSBB are harmful – only that their indications are associated with risk and evidence of improved outcomes will be most likely found in carefully selected subset. Similarly, while we observe a lower risk of ascites associated with atorvastatin, a lipophilic statin previously associated with improved portal hemodynamics,26 we neither observe this effect with simvastatin, nor do we see an effect for atorvastatin when ascites is defined by a paracentesis requirement. Instead, we see reduced risk associated with fibrates and niacin. This could suggest a potential benefit to be explored in a trial or it could implicate confounding by indication. Multiple studies of statins – both simvastatin and atorvastatin – are underway and could provide external validation of these data. Similar observations and conclusions also apply to anticoagulants and metformin. Both therapies had previously been associated with improvements in liver-related outcomes18, 27 but we observed no such associations in the present study which was designed to limit the potential for immortal-time bias using landmark analyses. One of the strengths of this analysis is the simultaneous evaluation of a large array of candidate therapies. Whereas many studies have assessed individual therapies in isolation, in reality patients who receive one therapy (i.e. statin) are likely to receive another (e.g. NSBB, metformin).
Outcomes after ascites
Our data confirms that incident ascites is a watershed moment in the natural history of cirrhosis, markedly increasing the risk of death for afflicted patients. Survival following ascites was 1.08 years (IQR0.26-2.75), 0.38 years (IQR0.1-1.3) for those requiring paracentesis. The specific cause of death is unknown in this dataset. SBP was rare (8.8%). Instead, it is likely that the global impact of ascites on frailty, malnutrition, and renal dysfunction mediates its morbidity and mortality.28, 29 Notably, the use of TIPS was rare (2.7%) and we find a higher risk of death associated with TIPS. While we cannot exclude better HRQOL after TIPS, no improvements in transplant-free survival among older patients are observed in this study.
Few of the pharmacotherapies we evaluated were associated with reduced mortality after ascites. Null associations include NSBB, statins, and metformin. Conversely, medications such as fibrates/niacin and anticoagulants are associated with lower risk. Anticoagulants have been associated with reduced risk of portal veinous thrombosis and bacterial peritonitis in a small, unblinded trial.14 Fibrates have been associated with lower portal pressure in animal models.30 Conversely, in our analyses of patients requiring paracentesis, neither anticoagulants nor other hypolipidemics were associated with improved outcomes. The only medications associated with lower mortality among patients requiring paracentesis were lipophilic statins (simvastatin, atorvastatin).(Supplementary Table 4) The discordant drug associations observed between those that did and did not require paracentesis could clarify the potential benefits of therapy stratified by disease severity. However, given the risk of confounding by indication, clinical trials are needed to confirm our most optimistic associations. Finally, prior data has suggested rifaximin may be associated with improved liver-related outcomes,13, 31 but this was not observed in this analysis. Rifaximin is associated with improved outcomes for those with HE but people with HE, whether on rifaximin or not, experience worse outcomes than those without HE.2, 6, 31 While it is likely these data also reflect this indication bias, the strength of the association between rifaximin and ascites (HR>5) and mortality (HR>3) – while adjusting for HE (among other factors) – is so great as to suggest that trials of rifaxmin for its off-target benefits must be both very large trials and exclude those with HE to detect non-HE related health benefits.
Contextual Factors
These findings must be interpreted in the context of study design. First, these data apply to a cohort of patients who are elderly, have multiple comorbidities, and who were diagnosed in the context of medium-to-long-term follow-up. Second, these administrative data are missing both laboratory and imaging results. The former can be helpful for risk adjustment, though the availability of laboratory data has not previously modified the direction or strength of associations observed in prior validations.31 The latter would have helped ascertain or validate the presence of ascites. Further, though we excluded those with CHF at baseline, combination diuretics can be used for conditions other than cirrhotic ascites. Our sensitivity analyses focusing on patients requiring paracentesis should mitigate this concern. Third, we evaluate the effect of many therapies which could be confounded by indication. The therapeutic associations are therefore intended to be hypothesis generating. Regardless, even if these associations are discounted, by including these therapies in the model, we have better adjusted for the clinical factors which they are intended to address. Finally, as follow-up lasted 3.9 (IQR 2.4-5.9) years, associations with ascites must be interpreted over an intermediate timeframe.
Conclusion
This study provides updated estimates of the incidence and prognosis of ascites which should apply widely to older patients with comorbidities who are diagnosed with cirrhosis in the context of routine follow-up. We show that ascites carries a dismal prognosis. We also provide associations with ascites and multiple medications which, whether correlated or causal, can power risk prediction models embedded in the electronic medical record. These associations also provide conservative estimates of benefit (or the lack thereof) for multiple therapies to be considered in trials. Given both poor outcomes and the low probability of success for repurposed medications, innovation will be needed.
Supplementary Material
Figure 2B: The Cumulative Incidence of Ascites Requiring Paracentesis.
The incidence of ascites defined by the need for paracentesis is presented, accounting for the competing risk of death
What is known?
Ascites is a common complication of cirrhosis
Data on the incidence and outcomes of ascites come from older cohorts that may not generalize to today’s patients
Medications like nonselective beta-blockers and statins have been associated with a lower risk of ascites
What is new here?
The cumulative incidence of ascites was 10.7% and 2.4% for ascites requiring paracentesis at 5-years
Incident ascites was associated with increased risk of death, HR 27.6 95%CI(21.7-35.1).
Lipophilic statins were the only medications associated with lower mortality after ascites requiring paracentesis.
Funding:
Elliot Tapper receives funding from the National Institutes of Health through NIDDK K23-DK117055.
Footnotes
Pertinent conflicts of interest: This work was supported in part by an unrestricted research grant from Valeant Pharmaceuticals (makers of Rifaximin) . Valeant had no access to the data and played no role in the analysis. No other authors have any relevant conflicts
References
- 1.Tapper E, Kanwal F, Asrani S, et al. Patient Reported Outcomes in Cirrhosis: A Scoping Review of the Literature. Hepatology (Baltimore, Md.) 2017. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014;39:1180–93. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of hepatology 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G, Morabito A, D'Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68:563–576. [DOI] [PubMed] [Google Scholar]
- 5.Jepsen P, Lash TL, Vilstrup H. The clinical course of alcoholic cirrhosis: development of comorbid diseases. A Danish nationwide cohort study. Liver International 2016;36:1696–1703. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: A Danish population-based cohort study. Hepatology 2010;51:1675–1682. [DOI] [PubMed] [Google Scholar]
- 7.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis–related liver transplantation waitlist additions in the United States. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 8.Tapper EB, Henderson JB, Parikh ND, et al. Incidence of and Risk Factors for Hepatic Encephalopathy in a Population-Based Cohort of Americans With Cirrhosis. Hepatology Communications 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology 2018;68:1498–1507. [DOI] [PubMed] [Google Scholar]
- 10.Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States. Journal of clinical gastroenterology 2015;49:690–696. [DOI] [PubMed] [Google Scholar]
- 11.Sharpton SR, Feng S, Hameed B, et al. Combined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomes. Transplantation 2014;98:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet 2019;393:1597–1608. [DOI] [PubMed] [Google Scholar]
- 13.Salehi S, Tranah TH, Lim S, et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Alimentary pharmacology & therapeutics 2019;50:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253–1260. e4. [DOI] [PubMed] [Google Scholar]
- 15.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology 2012;55:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mapakshi S, Kramer JR, Richardson P, et al. Positive predictive value of international classification of diseases, 10th revision, codes for cirrhosis and its related complications. Clinical Gastroenterology and Hepatology 2018;16:1677–1678. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez JJ, Dziwis J, Patel YA, et al. Identifying Ascites in Patients with Cirrhosis Using Administrative Codes and Diuretic Use: A Multicenter Study. Dig Dis Sci 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan DE, Serper M, John BV, et al. Effects of metformin exposure on survival in a large national cohort of patients with diabetes and cirrhosis. Clinical Gastroenterology and Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 19.Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C–related compensated cirrhosis. Gastroenterology 2016;150:430–440. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 21.Mellinger JL, Shedden K, Winder GS, et al. The High Burden of Alcoholic Cirrhosis in Privately Insured Persons in the United States. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 22.Allen AM, Therneau TM, Larson JJ, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology 2018;67:1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lévesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. Bmj 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 24.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology 2015;62:292–302. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. New England Journal of Medicine 2021;385:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimer N, Grønbæk H, Fred RG, et al. Atorvastatin for prevention of disease progression and hospitalisation in liver cirrhosis: protocol for a randomised, double-blind, placebo-controlled trial. BMJ open 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serper M, Weinberg EM, Cohen JB, et al. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation. Hepatology 2021;73:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JC, Tandon P, Bernal W, et al. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montoliu S, Balleste B, Planas R, et al. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol 2010;8:616–22; quiz e80. [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Vilarrupla A, Laviña B, García-Calderó H, et al. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. Journal of hepatology 2012;56:1033–1039. [DOI] [PubMed] [Google Scholar]
- 31.Tapper EB, Aberasturi D, Zhao Z, et al. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Alimentary Pharmacology & Therapeutics 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.