Abstract
The URS2 region of the Saccharomyces cerevisiae HO upstream region contains 10 binding sites for the Swi4p/Swi6p transcription factor and confers Swi4p dependence for transcription. Using a hybrid promoter, UASGAL (upstream activation sequence of GAL1)-URS2R, in which the GAL1-10 regulatory region is fused to the proximal 360 bp of URS2, we isolated mutants in which Swi4p is no longer required for transcription. Mutations of SIN4, ROX3, SRB8, SRB9, SRB10, SRB11, and two novel genes, NUT1 and NUT2, relieve the requirement of Swi4p for expression of this reporter. We found that NUT1 (open reading frame [ORF] YGL151w) is a nonessential gene, that NUT2 (ORF YPR168w) is essential, and that both Nut1p and Nut2p encode nuclear proteins. Deletion of NUT1 causes a constitutive, Swi4p-independent phenotype only in combination with the nut2-1 allele or an allele of CCR4. In contrast, inactivation of a temperature-sensitive allele of NUT2, nut2-ts70, alone causes constitutivity. nut1Δ nut2-1 cells and sin4Δ cells exhibit Swi4p-independent expression of an ho-lacZ reporter but not of an intact ho gene. Likewise, a pPHO5-lacZ construct is constitutively expressed in nut1 nut2 mutants relative to their wild-type counterparts. These results suggest that Nut1p, Nut2p, Sin4p, and Ccr4p define a group of proteins that negatively regulate transcription in a subtle manner which is revealed by artificial reporter genes.
Cells express only a subset of genes at a given time. The remaining genes are quiescent. How eukaryotic genes are maintained in a repressed state is a fundamental issue that remains poorly understood (reviewed in reference 21). Specific DNA binding proteins are known to bind promoters of some genes and inhibit gene expression. In addition, for many genes, the arrangement of nucleosomes across the promoter is thought to block transcription. Other genes are repressed due to their location in heterochromatic regions of the chromosome.
The yeast Saccharomyces cerevisiae is a valuable experimental organism for identifying components that maintain the quiescent state of genes. Numerous screens have been done in S. cerevisiae for mutations that restore or enhance transcription of inactive genes. For example, a screen for mutants that restore transcription of a HIS4 locus that has been inactivated by a transposon insertion into its promoter has identified a group of genes known as the SPT (suppressor of Ty) genes (62). The SPT gene products include basal transcription components such as Spt15p, the TATA binding protein (17), chromatin components such as histones (11), and other proteins of undetermined function such as Spt6p, which has been shown to interact with histones (8). Nonet and Young have identified mutations which restore viability to strains whose RNA polymerase II enzyme is compromised by truncation of the carboxyl-terminal domain of the Rpb1p subunit (41). Many of the suppressors of Rpb1p truncations (SRB) encode proteins that copurify with the RNA polymerase II holoenzyme (20, 29, 59). For example, SRB10 and SRB11 encode a cyclin-dependent kinase and cyclin which copurify with the holoenzyme (26, 29).
Studies of the regulation of specific genes have also been invaluable in the search for proteins that negatively regulate transcription. For example, the SUC2 gene is not expressed when cells are grown in glucose-containing medium. Mutations that allow expression of the SUC2 gene in the presence of glucose have been found in the genes SSN6, SRB8, SRB9, SRB10, SRB11, SIN4, ROX3, and RGR1 (54). Sin4p, Rgr1p, and Rox3p are components of a mediator activity that allows yeast RNA polymerase II to be stimulated by activators in vitro (18, 28). Biochemical studies suggest that Srb10p, Srb11p, Sin4p, Rgr1p, and Rox3p are intimately involved with RNA polymerase II function. Independently, genetic studies implicate these proteins in the negative regulation of gene expression (10, 13, 19, 46, 49, 54, 56, 61).
We have been characterizing the negative regulation of transcription of the HO gene in S. cerevisiae. HO is transcribed only in haploid mother cells at Start, the G1-to-S phase transition (22, 35). Thus, HO is potentially repressed in haploids in daughter cells and in cells that are outside of Start. Indeed, a daughter-specific repressor of HO, Ash1p, has been identified (7, 53). It is not known, however, if there exist cell cycle-specific repressors of transcription (38). The 0.7-kb region, URS2 (Fig. 1, line 1), of the HO upstream region is required for Start-specific expression of HO and for dependence on the transcription factors Swi4p and Swi6p (36–38). Swi4p binds specifically to 10 sites in URS2 (3), called Swi4p cell cycle boxes (SCBs) (9, 37). Swi4p contains a specific DNA binding domain, four ankyrin repeats, and a domain for interaction with Swi6p (4, 5, 43, 51). Together Swi4p and Swi6p activate the transcription of Start-specific genes such as CLN1, CLN2, and PCL1 as well as artificial reporters containing multimerized SCBs (3, 9, 39, 42). Both Swi4p and Swi6p are absolutely required for HO transcription.
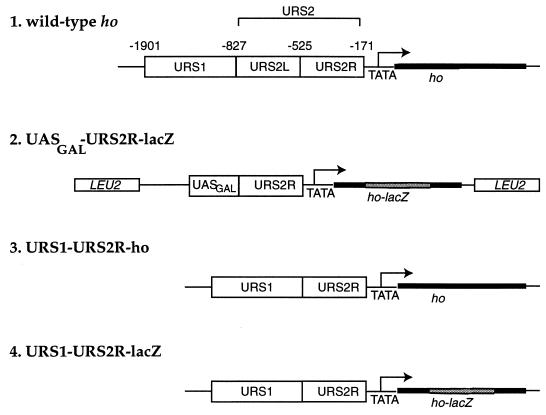
FIG. 1.
Structures of the intact HO gene (line 1) and reporter constructs UASGAL-URS2R-lacZ reporter (line 2), URS1-URS2R-ho allele (line 3), and URS1-URS2R-lacZ (line 4) are depicted. Numbering indicates the nucleotide positions of the endpoints of each region relative to the HO ATG codon. Nucleotide positions for lines 2 to 4 are as for line 1.
The URS2 region is responsible for dependence of HO transcription on Swi4p and Swi6p, as deletion of URS2 allows transcription of HO in the absence of these proteins. Conversely, insertion of the URS2 region between the GAL1 upstream activation sequence (UASGAL) and a TATA box confers Swi4p dependence on UASGAL-driven expression (38). Thus, in the absence of Swi4p, URS2 prevents expression of the HO gene despite the presence of potent activation sequences upstream, either UASGAL or URS1, the far upstream region of the HO promoter (Fig. 1, line 1).
To characterize regulation by URS2, we screened for mutants, named nut (negative regulation of URS2) mutants, which are defective in the Swi4p dependence of UASGAL-URS2R-lacZ, a synthetic reporter gene containing part of URS2. We describe two novel genes, NUT1 and NUT2, that are required for the Swi4p dependence of UASGAL-URS2R-lacZ.
MATERIALS AND METHODS
Strains.
Standard techniques for strain construction and mutant analysis were used (45). Key yeast strains are listed in Table 1. Except where indicated, all strains are isogenic to RT228, a strain derived from W303 and K1107 as follows. The SWI4 locus of strain K1107 (gift of Kim Nasmyth, Institute of Molecular Pathology, Vienna, Austria) was deleted by using pJO98 (42) digested with EcoRI and SalI to create RT211. This strain, RT211, was crossed to W303 to produce the segregant RT228. To create strains isogenic to RT228, the mating-type locus of RT228 was converted to MATα by two-step gene replacement (47) using pSC9 (gift of S. Chu, University of California, San Francisco). An isogenic SWI4 strain was created by two-step gene replacement using pRKT427.
TABLE 1.
Yeast strains useda
| Strain | Genotype |
|---|---|
| W303ab | MATa ade2 his3 leu2 ura3-52 trp1 can1 ho |
| K1107 | HMLa MATa HMRa his3 leu2 ura3-52 trp1 can1 ho-lacZ46 ade− |
| RT228 | HMLa MATa HMRa swi4ΔHIS3 his3 leu2 ura3-52 trp1 can1 ho ade− |
| RT233c | MATa ho::uasΔURA3::ho-lacZ |
| RT241c | MATα SWI4 LEU2::pRKT188 |
| RT243 | MATa swi4ΔHIS3 NUT LEU2::pRKT188 |
| RT259 | MATa swi4ΔHIS3 nut9-1 LEU2::pRKT188 |
| RT267 | MATa swi4ΔHIS3 nut7-2 LEU2::pRKT188 |
| RT269 | MATa swi4ΔHIS3 nut3-1 LEU2::pRKT188 |
| RT271 | MATa swi4ΔHIS3 nut1-1 nut2-1 LEU2::pRKT188 |
| RT300c | MATa swi4ΔHIS3 sin4ΔLEU2 LEU2::pRKT188 |
| RT335c | MATa sin4ΔLEU2 ho::uasΔURA3::ho-lacZ |
| RT362c | MATα swi4ΔTRP1 nut3-1 LEU2::pRKT188 |
| RT364c | MATα swi4ΔHIS3 nut1-1 nut2-1 LEU2::pRKT188 |
| RT379c | MATa swi4ΔHIS3 nut8-1 LEU2::pRKT188 |
| RT381c | MATa swi4ΔHIS3 nut6-2 LEU2::pRKT188 |
| RT387c | MATα swi4ΔTRP1 nut7-2 LEU2::pRKT188 |
| RT420c | MATα swi4ΔHIS3 nut1-1 NUT2 LEU2::pRKT188 |
| RT421c | MATα swi4ΔHIS3 NUT1 nut2-1 LEU2::pRKT188 |
| RT564 | MATa SWI4 NUT1 NUT2 LEU2::pRKT188 |
| RT575b | MATa/α |
| RT576b | MATa/α nut2ΔhisG::URA::hisG/NUT2 |
| RT609 | MATα swi4ΔHIS3 nut1ΔTRP1 nut21-609 LEU2::pRKT188 |
| RT688 | MATa SWI4 LEU2::pRKT229 |
| RT689 | MATa swi4ΔHIS3 LEU2::pRKT229 |
| RT692 | MATa SWI4 LEU2::pRKT208 |
| RT693 | MATa swi4ΔHIS3 LEU2::pRKT208 |
| RT718 | MATa swi4ΔHIS3 nut1ΔTRP1 nut2-1::URA3 LEU2::pRKT229 |
| RT720 | MATα SWI4 nut1ΔTRP1 nut2-1::URA3 LEU2::pRKT229 |
| RT748 | MATa swi4ΔHIS3 NUT1 nut2-ts70 LEU2::pRKT229 |
| RT781b | MATa NUT2 pRKT517 |
| RT783b | MATa nut2ΔhisG pRKT517 |
| RT784 | MATa/α nut1ΔTRP1/NUT1 nut2-ts70/NUT2 LEU2::pRKT229/LEU2::pRKT229 pRKT353 |
| RT784-1a | MATα NUT1 nut2-ts70 LEU2::pRKT229 pRKT353 |
| RT784-1d | MATα nut1ΔTRP1 NUT2 LEU2::pRKT229 pRKT353 |
| RT784-2c | MATα nut1ΔTRP1 nut2-ts70 LEU2::pRKT229 pRKT353 |
| RT784-6b | MATα NUT1 NUT2 LEU2::pRKT229 pRKT353 |
| RT817 | MATa swi4ΔHIS3 srb10ΔURA3 LEU2::pRKT229 |
| RT821 | MATa swi4ΔHIS3 srb11ΔURA3 LEU2::pRKT229 |
| RT849c | MATa srb10ΔURA3 ho::uasΔURA3::ho-lacZ |
| RT850c | MATa nut1ΔTRP1 nut2-1::URA3 ho::uasΔURA3::ho-lacZ |
| RT854 | MATa swi4ΔHIS3 srb8ΔURA3 LEU2::pRKT229 |
| RT890 | MATa urs2LΔho |
| RT930 | MATa swi4ΔHIS3 srb9ΔURA3 LEU2::pRKT229 |
| RT952 | MATa swi4ΔHIS3 NUT urs2LΔho |
| RT953 | MATa swi4ΔHIS3 nut1ΔTRP1 nut2-1::URA3 urs2LΔho |
| RT954c | MATa swi4ΔHIS3 nut1ΔTRP1 nut2-1::URA3 urs2LΔho-lacZ |
| RT973c | MATa SWI4 NUT urs2LΔho-lacZ |
| RT976c | MATa swi4ΔHIS3 urs2LΔho-lacZ |
| RT1118 | MATa swi4ΔHIS3 srb10Δ urs2LΔho |
| RT1119c | MATa swi4ΔHIS3 sin4ΔLEU2 urs2LΔho-lacZ |
| RT1120c | MATa swi4ΔHIS3 sin4ΔLEU2 urs2LΔho |
All strains are isogenic to RT228 except as noted otherwise. urs2LΔ is referred to in the text as URS1-URS2R.
Isogenic to W303.
Derived from K1107 and W303.
(i) Deletion of SIN4 and SRB genes.
SIN4 was deleted in a haploid strain by using plasmid M1381 (gift of David Stillman, University of Utah). The SRB genes were deleted in strain RT689 by using the following plasmids: to disrupt SRB8, pSL315 (20); to disrupt SRB9, pWS44-11 (gift of Marian Carlson, Columbia University) (54); to disrupt SRB10, pMW14 (gift of Madhu Wahi, University of California, San Francisco) (61); to disrupt SRB11, RY7036 (gift of Richard Young, Massachusetts Institute of Technology).
(ii) Deletion of NUT1.
NUT1 was deleted by using plasmid pRKT365 after digestion with NotI and SfiI, which release the disruption cassette. Deletion of the genomic NUT1 locus was verified by PCR.
(iii) Deletion of NUT2.
One NUT2 allele of RT575, an a/α W303 diploid, was deleted by transformation with pRKT432 after digestion with SalI and NotI. Strains with deleted alleles were identified by PCR.
(iv) URS1-URS2R-HO.
Plasmid pRKT619 was targeted to the ho locus by digestion with NruI and transformation into yeast strains RT690 and RT848 followed by selection for uracil prototrophy. Loop-outs were selected on plates containing 5-fluoro-orotic acid (5-FOA) (47) and verified by PCR. Alleles generated by this procedure, which deletes URS2L, are referred to as urs2LΔ in Table 1 and as URS1-URS2R in Results.
Mutant isolation.
Strain RT243 was mutagenized with UV irradiation to 95% inviability and plated on YEP dextrose so that all mutants were independently derived. Colonies were replica plated onto YEP galactose plates covered with Whatman no. 50 filters. After overnight growth, filters were removed and subjected to a filter β-galactosidase assay (55). Blue colonies were recovered from the original YEP dextrose plate. The 14 original mutant isolates are strains RT259 through RT271. Mutations were assigned to complementation groups by mating matΔ derivatives of mutant strains to MATα derivatives obtained by a standard backcross. The Nut phenotype of matΔ/MATα diploids was assayed by β-galactosidase filter assays on YEP galactose plates.
β-Galactosidase assays.
Plate and liquid β-galactosidase assays were performed as described elsewhere (55). For assays of liquid cultures, cells were grown to mid-log phase (optical density at 600 nm of 0.1 to 1.2). Assays were performed in triplicate. Standard deviations of all reported values were less than 10% of experimental values.
Complementation and cloning of NUT genes. (i) SRB gene complementation.
Plasmid pRKT439 was isolated from the Rose genomic library (44) as a plasmid that complements the mutant phenotype of RT387, a nut7-2 mutant. To determine if strains RT259 (nut9-1), RT381 (nut6-2), and RT379 (nut8-1) are mutant in other SRB genes, these strains were transformed with plasmids containing SRB8 (pMW18; gift of M. Wahi), SRB9 (pWS8; gift of M. Carlson) (54), or SRB11 (pSK5; gift of S. Kuchin and M. Carlson) (26). Complementation of the Nut− phenotype was scored by β-galactosidase filter assays on YEP galactose plates.
(ii) ROX3 complementation.
To determine if nut3-1 is defective in ROX3, RGR1, or SIN4, RT269 was transformed with URA3 plasmids containing ROX3 (pIT218; gift of M. Carlson) (54), RGR1 (pM2597), and SIN4 (pM1305; gift of D. Stillman) (24). Allelism with ROX3 was determined by integration of HindIII-cut pIT225 (gift of M. Carlson) (54) into strain RT689. This strain was crossed to nut3 mutant strain RT362.
(iii) NUT1 complementation.
Plasmid pRKT353 was isolated from the Rose genomic library in YCp50 (44). pRKT354, a derivative of this plasmid obtained by removing the SalI-SphI fragment of pRKT353, complements the Nut− phenotype of RT271. YGL151w is the only complete reading frame on this plasmid. pRKT355, which contains a SalI-XbaI deletion that disrupts YGL151w, lacks the complementing activity. To determine if the insert on pRKT353 is allelic to NUT1, the 2.3-kb HindIII fragment from pRKT353 was subcloned into the HindIII site of pRS306 (52) to generate pRKT356. pRKT356 was targeted to the genomic locus of the insert after digestion with BstEII and transformation to uracil prototrophy in the NUT1 NUT2 strain RT243. When the progeny from crosses of this strain to nut1 nut2 RT364 were analyzed, only 1 of 49 URA3 spores was phenotypically Nut−, indicating that URA3 is tightly linked to the NUT1 locus.
(iv) NUT2 complementation.
Plasmid pRKT404 was isolated from the Rose genomic library. The SalI-HindIII fragment of the genomic insert from pRKT404 was subcloned into pRS306 to generate pRKT417. Integration of AflII-digested pRKT417 marks the locus of the insert with URA3. Analysis of 16 tetrads from a cross between nut2-1 and a strain bearing an integration of AflII-digested plasmid pRKT417 demonstrated that the insert on pRKT404 was allelic to nut2-1 since none of 16 Nut− segregants carried the integrated pRKT417 allele. To determine which open reading frame complemented the nut2-1 defect, derivatives of pRKT404 were made by deleting the EcoRI fragment (pRKT447), the SphI-AflII fragment (pRKT448), and the XbaI-AflII fragment (pRKT450) and by filling in the AflII site, which inactivates the reading frame YPR169w (pRKT449). This analysis demonstrated that YPR168w is the open reading frame that complemented the nut2-1 defect.
(v) NUT21/CCR4 complementation.
Plasmid pRKT562 was isolated from the Rose genomic library by complementation of the mutant phenotype of RT609. The SalI-NotI fragment was excised from pRKT562 to generate plasmid pRKT564, which still complemented the mutant phenotype. The SpeI-NotI fragment from pRKT562 was subcloned into the SpeI and NotI sites of pRS306 to generate plasmid pRKT566. When integrated at the URA3 or CCR4 locus, pRKT566 complemented the mutant phenotype. CCR4 is the only gene contained on the SpeI-NotI fragment. Finally, this construct was used to mark the CCR4 locus by integration of the SfiI-digested plasmid. Crosses established that CCR4 is tightly linked to nut21-1, since in 10 tetrads, no recombination was observed between nut21-1 and the marked allele of CCR4.
Sequence analysis.
Database searches with the Nut1p and Nut2p protein sequences were performed by XREF (6), with additional support from the Wisconsin package of the Genetics Computer Group. Nucleotide positions in the HO promoter are numbered relative to the HO ATG codon such that the A immediately preceding the ATG is −1.
Plasmids.
Standard methods for DNA manipulations were as described previously (50). Plasmids generated for this study are listed in Table 2.
TABLE 2.
Plasmids used
| Name | Description |
|---|---|
| pRKT186 | pBR322.ho::uasΔURA3::ho-lacZ |
| pRKT208 | pRS305.UASGAL-lacZ |
| pRKT229, pRKT188 | pRS305.UASGAL-URS2R-lacZ |
| pRKT353 | YCp50.NUT1 |
| pRKT354 | YCp50.NUT1ΔSalI-SphI |
| pRKT355 | YCp50.NUT1ΔSalI-XbaI |
| pRKT356 | pRS306.2.3kb HindIII adjacent to NUT1 |
| pRKT363 | pGEM11.NUT1 |
| pRKT365 | pGEM11.nut1ΔTRP1 |
| pRKT404 | YCp50.NUT2 |
| pRKT417 | pRS306.NUT2 |
| pRKT427 | pRS306.SWI4 |
| pRKT432 | pBluescript.nut2ΔhisG.URA3.hisG |
| pRKT439 | YCp50.NUT7/SRB10 |
| pRKT447 | YCp50.NUT2(EcoRIΔ) |
| pRKT448 | YCp50.NUT2(XbaI-SphIΔ) |
| pRKT449 | YCp50.NUT2(AflIIΔ) |
| pRKT450 | YCp50.NUT2(XbaI-AflII) |
| pRKT515 | pRS306.nut2-1 |
| pRKT517 | pRS316.NUT2 |
| pRKT519 | YCp50.nut2-1 |
| pRKT535 | pRS306.URA3.NUT2-HA |
| pRKT542 | pRS306.URA3.HA-NUT1 |
| pRKT559 | pRS306.nut2-ts70 |
| pRKT562 | YCp50.NUT21 |
| pRKT564 | YCp50.NUT21(ΔSalI-NotI) |
| pRKT566 | pRS306.NUT21(SpeI-NotI) |
| pRKT619 | pRS306.urs2LΔho |
(i) lacZ reporters.
Two UASGAL-URS2R-lacZ reporters, pRKT229 and pRKT188, were used. To create the UASGAL-URS2R-lacZ reporter pRKT229, the XhoI-HindIII fragment of pRKT225, which contains the 354-bp SspI-HindIII fragment of URS2 filled in and ligated into the SalI site of pBluescript, was cloned into the XhoI and HindIII sites of pRKT208. To create pRKT188, the 406-bp SspI fragment of URS2 was ligated into the HindIII site of a plasmid closely related to pRKT208. This construct generates a small duplication of the region between the HindIII site at −171 and the SspI site at −122. pRKT208 was generated in two steps. First, the HindIII-BglII fragment of pJO11, a derivative of the ho-lacZ fusion from Russell et al. (48), was ligated into pRS305 (52) cut with HindIII and BamHI. This plasmid was digested with ApaI, filled in with T4 polymerase, and ligated with the Sau3A-AvaI fragment containing UASGAL which had been filled in with T4 polymerase. To create pRKT533, URS1 with an ApaI site and an engineered XhoI site (at position −871 of HO) was digested with these enzymes and ligated into the ApaI and XhoI sites upstream of URS2 in the reporter construct. To create pRKT619, the ApaI-to-HindIII fragment from pRKT533 was cloned into the ApaI and HindIII sites of pRS306.
(ii) UAS-less reporter.
A fragment containing the URA3 gene was ligated into a HindIII- and SacI-digested pBR322 plasmid containing the HO upstream region and gene to generate plasmid pRKT186. pRKT186 has the HO upstream region replaced with URA3. Strain RT233 was generated by transformation of strain K1107 with SalI- and PstI-digested pRKT186. The resulting allele at the HO locus, ho::uasΔURA3::ho-lacZ, lacks a UAS and is similar in construction to a UAS-less reporter plasmid used by others (24).
(iii) NUT1 deletion plasmid.
The 5.2-kb XhoI fragment containing YGL151w was subcloned into the SalI site of pGEM11(f+) to generate pRKT363. Plasmid CY253, containing TRP1, was digested with BglII and HincII and ligated to pRKT363 cut with BglII and EcoRV. This plasmid was then digested with BglII and NcoI, filled in with Klenow DNA polymerase, and religated to remove the entire 5′ coding region of YGL151w. This construct, pRKT365, deleted all of the NUT1 open reading frame except the region coding for the carboxyl-terminal 53 amino acids.
(iv) NUT2 deletion plasmid.
The 9-kb SalI-HindIII fragment was subcloned into pBluescript KS+. The EcoRI-BamHI fragment was then removed by digestion and recircularization of the vector. Divergent primers containing BamHI sites that anneal at the ATG and stop codon of YPR168w were used in the PCR to amplify the flanking regions of YPR168w. The PCR product was digested with BamHI and recircularized. The resulting plasmid was digested with BamHI and ligated to the BamHI-BglII fragment containing the hisG-URA3-hisG cassette from pNKY51 (1) to generate pRKT432.
(v) NUT1 tags.
The SacI-XhoI fragment of pRKT363 containing the entire NUT1 locus was cloned into the SacI and XhoI sites of pRS306 (52). Uracil-substituted single-stranded DNA was prepared from this plasmid by using the phage VCSM13 (Stratagene) and Escherichia coli CJ236. By using site-directed mutagenesis (27), a BamHI site was inserted at the amino-terminal end of the NUT1 open reading frame. The resulting plasmid was digested with BamHI, and annealed oligonucleotides encoding the hemagglutinin (HA) tag were ligated in frame. This plasmid, pRKT542, complemented a nut1Δ mutation.
(vi) NUT2 tags.
The XbaI-ClaI fragment containing the NUT2 locus was subcloned into the XbaI and ClaI sites of pRS306. A BamHI site at the carboxyl terminus was inserted by the same method as for NUT1. The HA tag was ligated into this site in frame to create plasmid pRKT535. For immunofluorescence, this construct was digested with BssHII and integrated into the genomic NUT2 locus. This plasmid, which complemented the inviability of a nut2Δ, is otherwise wild type in sequence.
Gap repair of nut2-1.
The nut2-1 allele was gap repaired from the strain RT271. Plasmid pRKT447 was digested with XbaI and BssHII, gel purified, and transformed into RT271. The gap-repaired plasmid pRKT519 was recovered by electroporation into XL1-Blue cells (Stratagene). The sequence of the allele was determined by dideoxynucleotide sequencing using primers complementary to NUT2. The XbaI-ClaI fragment containing the nut2-1 allele was subcloned into pRS306 to generate pRKT515. This plasmid was used to replace the genomic NUT2 locus with the nut2-1 allele, using two-step gene replacement (47).
Immunofluorescence.
Indirect immunofluorescence was performed as described by Sil and Herskowitz (53). The HA-11 antibody (Babco) was used as the primary antibody at a 1:1,000 dilution.
Northern analysis.
Northern analysis was performed as described by Sil and Herskowitz (53).
Temperature-sensitive alleles of NUT2.
Temperature-sensitive alleles of NUT2 were generated by PCR mutagenesis as described by Muhlrad et al. (34). One allele, nut2-ts70, was cloned into pRS306 to generate plasmid pRKT559. This plasmid was used to introduce nut2-ts70 into the genomic NUT2 locus by two-step gene replacement (47).
RESULTS
The UASGAL-URS2R-lacZ reporter is Swi4p dependent.
To characterize the ability of URS2 to confer Swi4p dependence for transcription, we generated a reporter gene based on the UASGAL-URS2 allele of HO created by Nasmyth (38). To assay Swi4p dependence, the UASGAL from the GAL1-10 intervening region was fused to the ho TATA box which drives the expression of an ho-lacZ reporter gene. The 360 bp URS2R (right) from −528 to −171 was inserted between the UASGAL and the TATA element to generate the UASGAL-URS2R-lacZ reporter (Fig. 1, line 2). An identical reporter lacking URS2R, UASGAL-lacZ, was used as a control.
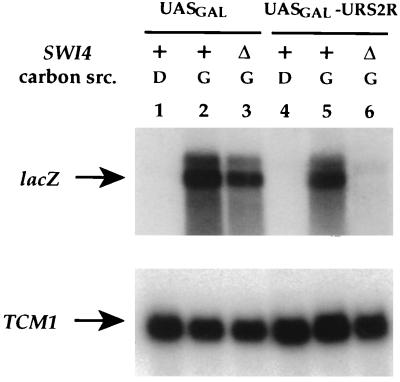
Both UASGAL-lacZ and UASGAL-URS2R-lacZ were dependent on growth in galactose medium for expression (Fig. 2, lanes 1 versus 2 and 4 versus 5). However, during growth in galactose medium, UASGAL-URS2R-lacZ was not expressed in the absence of Swi4p (Fig. 2, lane 6) whereas UASGAL-lacZ was (Fig. 2, lane 3). Thus, the URS2R segment confers Swi4p-dependent expression on the UASGAL-URS2R-lacZ reporter. URS2R is close to the minimum region (−474 to −171) that confers Swi4p dependence for UASGAL-containing reporters (37, 58a).
FIG. 2.
The UASGAL-URS2R-lacZ reporter is dependent on Swi4p and galactose. Expression of the UASGAL-lacZ reporter (pRKT229) was assayed in a wild-type (RT692; lanes 1 and 2) and an isogenic swi4Δ (RT693; lane 3) strain. Expression of the UASGAL-URS2R-lacZ reporter was assayed in a wild-type (RT688; lanes 4 and 5) and an isogenic swi4Δ (RT689; lane 6) strain. RNA was isolated from these strains grown in YEP galactose (G; lanes 2, 3, 5, and 6) or YEP dextrose (D; lanes 1 and 4) liquid medium at 30°C. RNA was analyzed by Northern blotting and hybridized with probes for lacZ and TCM1 as a control for RNA content.
Isolation of mutants that exhibit Swi4p-independent expression.
To identify gene products that might function at URS2R to confer Swi4p dependence, we screened for mutations that relieve the Swi4p dependence of UASGAL-URS2R-lacZ in galactose medium. swi4Δ deletion strains grown on galactose plates formed white colonies because the lacZ reporter was not expressed, whereas mutant strains formed blue colonies due to lacZ expression. From 80,000 mutagenized swi4Δ cells, 14 mutants were isolated. Mutants were categorized as either weak suppressors or strong suppressors of the SWI4 deletion (Table 3). For example, strain RT267, which produced 10 U of β-galactosidase activity, is representative of the weak class of suppressors. In contrast, strain RT271, which produced 80 U of β-galactosidase activity, is representative of the strong class of suppressors. The wild-type parental strain expressed less than 1 U of activity. We also noted that all mutants isolated exhibited similar secondary phenotypes. In particular, they invaded the agar of YEP dextrose plates to much greater extent than the parental strain and were more flocculent than the parental strain when grown in liquid culture.
TABLE 3.
nut and sin4Δ mutations alleviate Swi4p-dependent reporter gene expression
| Genotype | β-Galactosidase activitya |
|---|---|
| SWI4 | 100 |
| swi4Δ | <1 |
| swi4Δ nut7-2 | 10 |
| swi4Δ nut1-1 nut2-1 | 80 |
| swi4Δ sin4Δ | 270 |
β-Galactosidase activity produced by the UASGAL-URS2R-lacZ fusion reporter was assayed in strains grown in galactose medium at 30°C and normalized such that the activity present in SWI4+ derivatives of RT243 (19.7 Miller units) is 100. Expression of the reporter was analyzed in the swi4Δ parental background (RT243), in a weak (RT267) and a strong (RT271) mutant, and in a strain deleted for SIN4 (RT300). Except for RT300, which is closely related, these strains are isogenic.
To determine if the mutant phenotypes were due to recessive mutations, we mated MAT deletion derivatives of each mutant to a wild-type MATα swi4Δ strain. All 14 mutants appeared recessive, as they did not express the UASGAL-URS2R-lacZ reporter gene. Mutations in the lacZ reporter are expected to be dominant. Therefore, these mutants were likely to carry mutations in genes required for transcriptional regulation of the reporter.
Each mutant was backcrossed to a MATα swi4Δ UASGAL-URS2R-lacZ strain to determine if the defect segregated as a single-gene trait. In 9 of the 10 mutants, the defect in reporter gene regulation segregated as a single-gene trait in more than seven tetrads. However, in one strong mutant, strain RT271, the defect segregated as a two-gene trait (see below).
Because the mutations were recessive, we were able to perform complementation tests by mating matΔ derivatives of each mutant with MATα derivatives obtained from backcrossing. Mutations that failed to complement were provisionally assigned to the same complementation group. From this analysis (data not shown), we deduced that at least nine separate complementation groups were defined (summarized in Table 4).
TABLE 4.
Summary of genes identified
| Complementation groupa | No. of alleles | Gene | Open reading frame |
|---|---|---|---|
| NUT1 | 1 | NUT1 | YGL151w |
| NUT2 | 1 | NUT2 | YPR168w |
| NUT3 | 1 | ROX3 | YBL093c |
| NUT6 | 5 | SRB8b | YCR081w |
| NUT7 | 2 | SRB10b | YPL042c |
| NUT8 | 1 | SRB9b | YDR443c |
| NUT9 | 1 | SRB11b | YNL025c |
| NUT21 | 1 | CCR4 | YAL021c |
Mutants defective in the complementation groups NUT4 and NUT5 are both complemented by a high-copy-number plasmid containing RGR1 (58a). Further analysis will be required to determine if either or both of these complementation groups are allelic to RGR1 as mutations in nut4 and nut5 could exhibit intra-allelic complementation.
Allelism tests were not performed.
Weak Nut− mutants carry mutations in the genes SRB8, SRB9, SRB10, and SRB11.
To determine the nature of the defect in strains of the weak phenotype class, we cloned NUT7 by complementation (see Materials and Methods). Sequencing of the insert of the complementing plasmid indicated that it contained SRB10. Mutations in this gene were previously identified as a suppressor of truncations in the carboxyl-terminal domain of RNA polymerase II, as a modifier of the glucose-repressed state of SUC2 (54), and as a modifier of α2-mediated repression of a-specific genes (61). Plasmid pMW11, which contains only SRB10 and none of the adjacent genes from the original complementing plasmid, also complemented the defect in nut7-2. Further, as for the original nut7 alleles, deletion of SRB10 had a weak defect in reporter gene regulation (Table 5).
TABLE 5.
The SRB10 class of genes is required for Swi4p-dependent reporter gene expression
| Genotype | β-Galactosidase activitya |
|---|---|
| SWI4 SRB | 100 |
| swi4Δ SRB | 0.4 |
| swi4Δ srb8Δ | 5.1 |
| swi4Δ srb9Δ | 6.6 |
| swi4Δ srb10Δ | 5.3 |
| swi4Δ srb11Δ | 4.9 |
β-Galactosidase activity from the UASGAL-URS2R-lacZ reporter was measured in strains RT688, RT689, RT854, RT930, RT817, and RT821 during growth in YEP galactose medium at 30°C. Results were normalized so that the activity in the SWI4 SRB strain (55 Miller units) was 100. All strains are isogenic.
These data strongly suggest that nut7-2 is an allele of SRB10 and raised the possibility that the remaining weak constitutive mutants, nut6, nut8, and nut9, are defective in the genes SRB8, SRB9, or SRB11, since mutation of these SRB genes causes phenotypes similar to those caused by mutations in SRB10 (54). To test this hypothesis, we transformed nut6, nut8, and nut9 mutants with centromere plasmids containing SRB8, SRB9, and SRB11. SRB8 complemented the Nut− phenotype of nut6-2; SRB9 complemented nut8-1; SRB11 complemented nut9-1. Furthermore, deletion of SRB8, SRB9, or SRB11 caused a partial relief of the Swi4p dependence of the UASGAL-URS2R-lacZ reporter (Table 5). These results show that the genes SRB8, SRB9, SRB10, and SRB11 are required for appropriate regulation of the reporter.
Mutation of ROX3 or SIN4 causes a Nut− phenotype.
To determine the identities of the remaining genes, we compared the phenotype of the nut mutants with that of a sin4Δ deletion in our strain background. Loss of SIN4 function has been previously shown to bypass the Swi4p requirement for transcription of ho-lacZ reporters (24, 32). Likewise, deletion of SIN4 allowed transcription of the UASGAL-URS2R-lacZ reporter in swi4Δ strains (Table 3). The nut3 mutant complemented a sin4Δ strain, suggesting that nut3 is not an allele of SIN4. Since mutations in RGR1 and ROX3 have phenotypes like mutations in SIN4 (13, 23, 54), we tested whether centromere plasmids bearing these genes could complement nut3-1. A plasmid that contained the ROX3 open reading frame alone complemented the mutant phenotype, whereas plasmids containing RGR1 or SIN4 did not. Further, we found that nut3 was allelic to the ROX3 gene since nut3 segregated away from a ROX3 locus that was marked with the URA3 gene in eight tetrads. Therefore, mutation of either ROX3 or SIN4 causes inappropriate expression of the UASGAL-URS2R-lacZ reporter.
Mutant RT271 is defective in two genes, NUT1 and NUT2.
The mutant phenotype in RT271, which exhibits a strong defect in the Swi4p dependence of UASGAL-URS2R-lacZ, segregated as if it were due to mutations in two unlinked loci that we designated nut1 and nut2. In 32 tetrads, 7 parental ditype tetrads, 4 nonparental ditype tetrads, and 21 tetratype tetrads were observed. This ratio is consistent with the expected 1:1:4 ratio for segregation of two unlinked genes, when at least one gene is far from its centromere. Further, when one mutant locus, NUT1, was homozygous whereas the other locus, NUT2, was heterozygous, single-gene segregation (2:2 segregation) of the phenotype was observed in 14 tetrads.
These data indicated that two unlinked loci must be mutated to allow expression of the reporter construct in the absence of Swi4p. By quantitating β-galactosidase activity, we determined that expression was at background levels when only one of the two loci was mutated in nut1 NUT2 or NUT1 nut2 strains (Table 6). In contrast, expression was robust, to the levels of strains with Swi4p activity, in a strain in which both loci were mutated. Therefore, the genes NUT1 and NUT2 both contribute to the negative regulation of UASGAL-URS2R-lacZ reporter in strains deleted for SWI4.
TABLE 6.
Mutations in both NUT1 and NUT2 are required for Swi4p-independent reporter expression
| Genotype | β-Galactosidase activitya |
|---|---|
| SWI4 NUT1 NUT2 | 100 |
| swi4Δ NUT1 NUT2 | 1.4 |
| swi4Δ nut1-1 NUT2 | 2.6 |
| swi4Δ NUT1 nut2-1 | 0.8 |
| swi4Δ nut1-1 nut2-1 | 108 |
β-Galactosidase activity from the UASGAL-URS2R-lacZ reporter was measured in strains RT241, RT243, RT420, RT421, and RT271 during growth in YEP galactose medium at 30°C. Results are normalized so that the activity in the SWI4 NUT1 NUT2 strain (20.6 Miller units) is 100.
NUT1 encodes a novel nonessential protein.
To clone NUT1 and NUT2, we transformed the double mutant strain with a genomic library, reasoning that a centromere plasmid containing either gene should restore the Swi4p dependence of UASGAL-URS2R-lacZ. We obtained two plasmids that differed in their restriction maps. The insert from one plasmid was tightly linked to nut1, whereas the insert of the other plasmid was tightly linked to nut2 (see Materials and Methods).
Subcloning of the NUT1 insert delimited the complementing activity to a single open reading frame, YGL151w, which encodes a large polypeptide of 1,132 amino acids and has no significant homologs. Deletion of NUT1 caused the same phenotype as the original nut1-1 allele. Specifically, in the absence of SWI4, deletion of NUT1 alone did not cause reporter gene expression in strains wild-type for NUT2 but did permit high-level expression of the reporter in strains that carried the nut2-1 mutation (data not shown). In conclusion, NUT1 encodes a large novel protein which is not essential for cell viability.
NUT2 encodes a novel essential protein.
By subcloning the insert from the other complementing plasmid, we determined that the NUT2 gene is YPR168w. This open reading frame encodes a protein of 157 amino acids which has sequence homologs of unknown function including the human expressed sequence tag yx99c06.r1 and the Caenorhabditis elegans open reading frame T09A5.6 (Fig. 3).
FIG. 3.
Alignment of Nut2p with human and C. elegans homologs. The human expressed sequence tag yx99c06.r1 (GenBank accession no. N40234) and the C. elegans open reading frame T09A5.6 (SwissProt P45966) are aligned with Nut2p. Black highlighting indicates positions conserved among these proteins. The amino acid positions of the Nut2p sequence are indicated above the alignment. • denotes the position mutated to lysine in the nut2-1 allele.
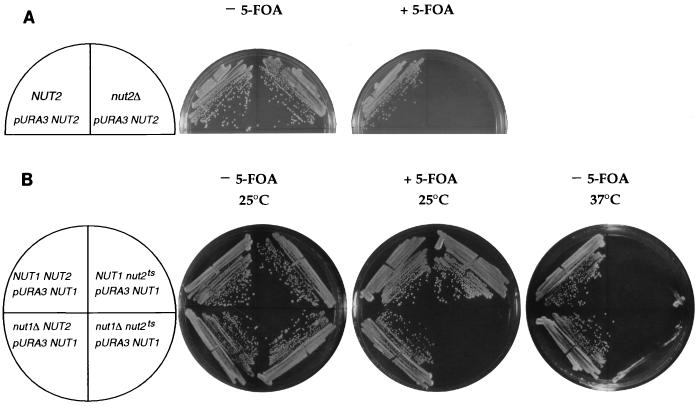
To determine the phenotype of complete loss of function of NUT2, we generated a marked deletion of NUT2 in a diploid yeast strain. Upon sporulation, only two spores of each tetrad formed colonies of 10 tetrads analyzed. These spores were invariably NUT2 since they did not bear the marker from the deletion. Therefore, the spores that failed to form colonies lacked the NUT2 gene, although these spores did germinate, as verified by microscopic inspection. A further indication that NUT2 is essential is the inability of nut2Δ strains to lose a URA3 centromere plasmid containing the NUT2 gene. These nut2Δ strains were unable to grow in the presence of 5-FOA, which selects against the URA3 NUT2 plasmid, although their isogenic NUT2 siblings readily lost this plasmid (Fig. 4A). Thus, the NUT2 gene is essential for viability.
FIG. 4.
NUT2 is an essential gene that exhibits synthetic lethality with NUT1. (A) NUT2 is an essential gene. Isogenic strains that are NUT2 (RT781) or nut2Δ (RT783) were maintained with a centromere plasmid containing URA3 and NUT2. Strains were streaked on synthetic complete medium (left) or on synthetic complete medium containing 5-FOA (right) and incubated for 3 days. (B) A temperature-sensitive allele of NUT2, nut2-ts70, is synthetically lethal with a deletion of NUT1. Isogenic strains derived from RT784 that are NUT1 NUT2 (RT784-6b), nut1Δ NUT2 (RT784-1d), NUT1 nut2-ts70 (RT784-1a), and nut1Δ nut2-ts70 (RT784-2c) were constructed bearing pRKT353, a centromere plasmid containing the URA3 and NUT1 genes. Strains were streaked on plates with (center) or without (left and right) 5-FOA and incubated at 25°C (left and center) or 37°C (right) for 3 days.
Given that we were unable to analyze the phenotype of the NUT2 deletion with respect to reporter gene transcription, we confirmed that YPR168w is the NUT2 locus by rescuing the nut2-1 allele from yeast by gap repair (see Materials and Methods). When the NUT2 locus in a swi4Δ nut1Δ strain was replaced with the recovered nut2-1 allele, we found that the UASGAL-URS2R-lacZ reporter was expressed despite the absence of Swi4p. Sequencing of the recovered allele revealed that nut2-1 has a single nucleotide change in YPR168w that converts codon 132 from GAA to AAA, which changes a glutamic acid residue to lysine (Fig. 3). No other mutations were found. This observation confirms that YPR168w is the open reading frame mutated in nut2-1.
Nut1p and Nut2p localize to the cell nucleus.
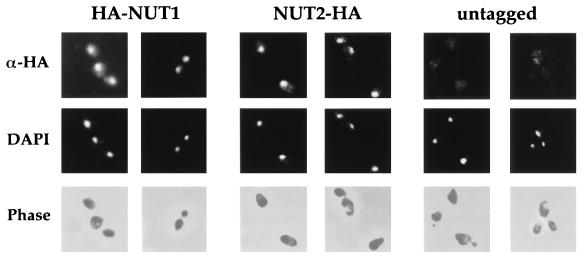
To determine the subcellular localization of Nut1p and Nut2p, we introduced epitope tags into the coding sequence of each gene. An amino-terminal fusion of the Nut1p open reading frame joined to two copies of the HA epitope complemented the Nut− phenotype of nut1Δ nut2-1 strains. Likewise, the Nut2p open reading frame was fused in frame at its carboxyl terminus to two copies of the HA epitope. The Nut2p-HA protein complemented the inviability of a NUT2 deletion. By indirect immunofluorescence using a monoclonal antibody directed against the HA epitope, both the HA-Nut1p fusion protein (Fig. 5, columns 1 and 2) and the Nut2p-HA protein (Fig. 5, columns 3 and 4) were localized to the nuclei of yeast cells. Little if any background signal was observed in untagged strains analyzed in parallel with the same antibodies and under the same conditions (Fig. 5, columns 5 and 6). These data suggest that Nut1p and Nut2p are predominantly localized in cell nuclei, where they might affect gene expression.
FIG. 5.
Nut1p and Nut2p are localized to nuclei. Cells bearing integrated copies of the HA-NUT1 fusion (pRKT542; columns 1 and 2), the NUT2-HA fusion (pRKT535; columns 3 and 4), or no fusion (columns 5 and 6) were harvested in mid-log phase, fixed, and stained with antibodies against the HA epitope (α-HA) as described in Materials and Methods. Localization of the tagged epitope was revealed by rhodamine-conjugated anti-mouse antibodies in the first row. For the same field of cells, DNA was visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining in the second row. Cell outlines were visualized by phase-contrast microscopy in the third row.
Loss of function of NUT2 alone causes Swi4p-independent reporter transcription.
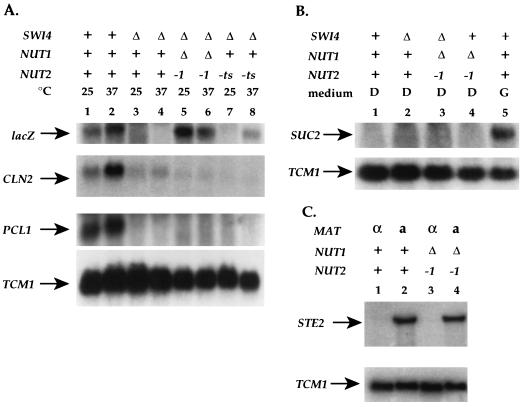
To determine the phenotype of complete loss of NUT2 function, we generated alleles of NUT2 that are temperature sensitive for viability using PCR mutagenesis. One such allele, nut2-ts70, did not support cell growth at the restrictive temperature of 37°C (Fig. 4B, top right sector). nut2-ts70 cells died with abnormal morphology but no specific cell cycle arrest (58a). We generated swi4Δ strains carrying the UASGAL-URS2R-lacZ reporter that were NUT1 NUT2, nut1Δ nut2-1, or NUT1 nut2-ts70. Following growth in YEP galactose medium at 25 or 37°C, we assayed reporter gene expression by Northern analysis (Fig. 6A). Consistent with data from analysis of β-galactosidase activity, the lacZ transcript was apparent in SWI4 strains but absent from swi4Δ strains regardless of temperature (Fig. 6A; compare lanes 1 and 2 with lanes 3 and 4). Deletion of NUT1 in combination with the nut2-1 allele restored transcription of the reporter in the absence of Swi4p (Fig. 6A, lanes 5 and 6). The nut2-ts70 allele did not perturb reporter gene expression at the permissive temperature (Fig. 6A, lane 7) but did cause expression of reporter gene transcription at the restrictive temperature of 37°C (Fig. 6A, lane 8), despite NUT1 activity. We conclude that inactivation of NUT2 alone is sufficient to cause Swi4p-independent expression of the UASGAL-URS2R-lacZ reporter.
FIG. 6.
Inactivation of a temperature-sensitive allele of NUT2 allows Swi4p-independent expression. (A) Expression of the UASGAL-URS2R-lacZ reporter was assayed in wild-type (RT688; lanes 1 and 2), swi4Δ (RT689; lanes 3 and 4), swi4Δ nut1Δ nut2-1 (RT718; lanes 5 and 6), and swi4Δ NUT1 nut2-ts70 (RT748; lanes 7 and 8) strains. RNA was isolated from these strains after growth in YEP galactose liquid medium at 25°C (odd-numbered lanes) or 37°C (even-numbered lanes) for 3 h. RNA was analyzed by Northern blotting and hybridized with probes for lacZ, CLN2, PCL1, and TCM1. The latter serves as an RNA loading control. The TCM1 signal indicates that lanes 2, 3, and 4 contain slightly more RNA than other lanes. All strains are isogenic. (B) Expression of the SUC2 gene was assayed in wild-type (RT688; lanes 1 and 5), swi4Δ (RT689; lane 2), swi4Δ nut1Δ nut2-1 (RT718; lane 3), and SWI4 nut1Δ nut2-1 (RT720; lane 4) strains by Northern analysis. Strains were grown in dextrose (D; lanes 1 to 4) or in galactose (G; lane 5) medium. (C) STE2 expression was assayed in MATα cells (lanes 1 and 3) and MATa cells (lanes 2 and 4) that were either wild type (lanes 1 and 2) or nut1Δ nut2-1 mutants (lanes 3 and 4).
NUT1 is synthetically lethal with a temperature-sensitive allele of NUT2.
After replacement of nut2-ts70 into the genomic NUT2 locus, we sporulated a nut1Δ/+ nut2-ts70/+ diploid to determine the phenotype of nut1Δ nut2-ts70 double mutants. Of 19 tetrads germinated at the permissive temperature, 25°C, no nut1Δ nut2-ts70 double mutants were found although 19 NUT1 nut2-ts70 spores grew up normally. To determine if nut1Δ is indeed synthetically lethal with nut2-ts70, we obtained a double mutant that was rescued by a URA3 NUT1 plasmid. Such double mutants were unable to lose the URA3 NUT1 plasmids, as they failed to grow on 5-FOA whereas their isogenic NUT1 nut2-ts70 siblings did (Fig. 4B). Thus, deletion of NUT1 exacerbates the growth phenotype of the nut2-ts70 allele just as it exacerbates the reporter gene phenotype due to the nut2-1 allele.
Mutation of NUT1 and NUT2 affects expression of the UASGAL-URS2R-lacZ reporter but not the endogenous HO gene.
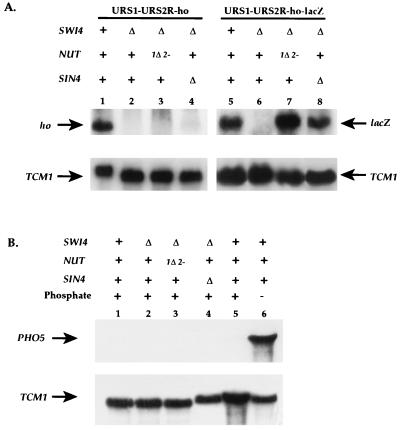
To determine if NUT1 and NUT2 are physiological regulators of HO transcription, we assayed HO transcription in the nut1Δ nut2-1 double mutant. We observed that the nut1Δ nut2-1 double mutant did not affect the Swi4p dependence of the intact ho gene containing URS1-URS2-ho (data not shown). Because the lacZ reporter only contained part of URS2, we constructed an allele of the endogenous ho gene in which the wild-type URS2 region was truncated, leaving only URS2R, the minimal region of URS2 required for Swi4p dependence (Fig. 1, line 3). This allele, URS1-URS2R-ho, required Swi4p (Fig. 7, lane 2) and Swi5p (data not shown) for ho transcription as assayed by Northern hybridization. However, in contrast to the UASGAL-URS2R-lacZ reporter, transcription of ho from the URS1-URS2R-ho gene was not restored in the absence of Swi4p in the nut1Δ nut2-1 mutant (Fig. 7, lane 3).
FIG. 7.
The phenotype of nut1 nut2 mutants is manifest only in the presence of lacZ sequences. (A) HO expression was analyzed for strains containing the URS1-URS2R-ho allele (lanes 1 to 4) or the URS1-URS2R-lacZ allele (lanes 5 to 8). Wild-type strains (RT890 [lane 1] and RT973 [lane 5]) were compared with swi4Δ strains (RT952 [lane 2] and RT976 [lane 6]), swi4Δ nut1Δ nut2-1 strains (RT953 [lane 3] and RT953 [lane 7]), and swi4Δ sin4Δ strains (RT1118 [lane 4] and RT1120 [lane 8]). (B) PHO5 expression was measured in strains grown in the presence (lanes 1 to 5) or absence (lane 6) of inorganic phosphate. Wild-type (lanes 1, 5, and 6) was compared with swi4Δ (RT952; lane 2), swi4Δ nut1Δ nut2-1 (RT953; lane 3), and swi4Δ sin4Δ (RT1118; lane 4) strains.
To analyze the differential effects of NUT1 and NUT2 on URS1-URS2R-ho (Fig. 1, line 3) and UASGAL-URS2R-lacZ (Fig. 1, line 2), we constructed another ho allele that was identical to URS1-URS2R-ho in all respects except that the HO open reading frame was fused to the E. coli lacZ gene within the coding region of the gene (Fig. 1, line 4). We observed that expression of this construct, URS1-URS2R-lacZ, was also dependent on Swi4p (Fig. 7, lane 6) and Swi5p for activity. Unlike URS1-URS2R-ho, URS1-URS2R-lacZ was transcribed in the absence of Swi4p activity when the strain was nut1Δ nut2-1 (Fig. 7, lane 7). Thus, the difference between URS1-URS2R-ho and the reporter UASGAL-URS2R-lacZ can be attributed to the presence of lacZ sequences. This phenotype is similar to the phenotype of SIN4 mutants, which also bypass the requirement of Swi4p for HO expression only when the HO open reading frame is fused to lacZ sequences (Fig. 7, lane 4 versus lane 8) (24, 33, 40). In summary, Nut1p and Nut2p appear essential for the Swi4p dependence of HO alleles compromised by the presence of lacZ sequences.
Mutation of NUT1 and NUT2 affects the expression of another lacZ-containing reporter but not other Swi4p-dependent genes.
To determine if the nut1Δ nut2-1 strain is generally defective in the regulation of gene expression or defective in the regulation of lacZ-containing reporters other than UASGAL-URS2R-lacZ, we assayed the expression of another reporter and other genes in this strain. Transcription of the PCL1 gene is dependent on Swi4p (42), whereas transcription of the CLN2 gene is largely but not completely dependent on Swi4p (14). To determine if mutation of NUT1 or NUT2 enhances the Swi4p-independent transcription of PCL1 or CLN2, we analyzed transcription of these genes in wild-type, swi4Δ, swi4Δ nut1Δ nut2-1, and swi4Δ nut2-ts70 strains (Fig. 6A). We were unable to detect any increase in the Swi4p-independent transcription of these two genes in the nut mutant strains.
We also examined the regulation of genes whose expression is independent of Swi4p but whose repression is dependent on SIN4 or SRB10. The glucose repression of SUC2 transcription is perturbed by mutation of SIN4 or SRB10 (54). We found, however, that SUC2 expression was appropriately repressed in a nut1Δ nut2-1 mutant when cells were grown in glucose (Fig. 6B). The repression of STE2 by α2 is perturbed by mutations in SIN4 and SRB10 (61). The nut1Δ nut2-1 double mutant did not perturb STE2 repression in α cells (Fig. 6C). Finally, the repression of SPO13 in haploid vegetative cells was also unaffected by mutations in NUT1 and NUT2 (data not shown).
Although mutation of NUT1 and NUT2 did not affect the expression of the three genes assayed here, we found that nut1Δ nut2-1 double mutants, like sin4Δ mutants, exhibit a greater than 10-fold elevated expression of a pPHO5-lacZ reporter construct (Table 7, column 2). Under repressing conditions for PHO5 regulation, deletion of SIN4 is known to cause increased expression of this pPHO5-lacZ reporter while not perturbing repression of a PHO5 gene lacking the lacZ moiety (21b). Likewise, the nut1Δ nut2-1 double mutant did not perturb repression of the endogenous PHO5 gene (Fig. 7B). These data demonstrate that mutation of NUT1 and NUT2 affects pPHO5-lacZ similarly to mutation of SIN4. Finally, mutation of SIN4 increases expression of reporters lacking a UAS (Table 7, column 3). Again, nut1Δ nut2-1 double mutants have similar phenotypes, although they exhibit only a 12-fold increase, compared to a 60-fold increase caused by mutation in SIN4.
TABLE 7.
Mutation of SIN4 or of NUT1 and NUT2 elevates expression of pPHO5-lacZ
| Genotype | β-Galactosidase activitya
|
|
|---|---|---|
| pPHO5-lacZb | UAS-less lacZc | |
| NUT+ | 1.0 | 0.1 |
| srb10Δ | 2.8 | 0.1 |
| sin4Δ | 11 | 5.2 |
| nut1Δ nut2-1 | 16 | 1.1 |
Normalized so that the activity in the swi4Δ strain (1.6 Miller units) is 1.0.
Measured in strains RT952, RT1118, RT1120, and RT953 during growth under selection at 30°C in standard synthetic medium which is repressing for pPHO5-lacZ.
Measured in strains RT233, RT849, RT335, and RT850.
Mutation of CCR4 in combination with deletion of NUT1 has a Nut− phenotype.
Because NUT2 is essential for cell viability and is a novel gene, we aimed to identify other genes that function like NUT2. Therefore, we sought other mutants with a Nut2p-like phenotype by screening for constitutive expression of the UASGAL-URS2R-lacZ reporter in cells deleted for SWI4 and NUT1. From this screen, we identified genes that define mutations in at least four different complementation groups (21a). One mutant strain, RT609, was characterized further. This strain contained mutation, designated nut21-1, which exhibits a temperature-sensitive growth defect that cosegregated in seven tetrads with the defect in reporter gene regulation. nut21-1 was not allelic to NUT2 but, like the nut2-1 allele, exhibited a Nut− phenotype in combination with nut1Δ (Table 8). Further, nut21-1 exhibited no phenotype alone or in combination with nut2-1. These results suggest that NUT21 encodes an essential protein that, like Nut2p, functions with Nut1p to regulate expression of the UASGAL-URS2R-lacZ reporter.
TABLE 8.
Mutation of NUT21 synergizes with nut1Δ to cause Swi4p-independent reporter gene expression
| Genotype | β-Galactosidase activitya |
|---|---|
| SWI4 NUT1 NUT21 | 100 |
| swi4Δ NUT1 NUT21 | 0.4 |
| swi4Δ nut1Δ NUT21 | 5.2 |
| swi4Δ nut1Δ nut21-1 | 46 |
| swi4Δ NUT1 nut21-1 | 0.3 |
β-Galactosidase activity from the UASGAL-URS2R-lacZ reporter was measured in strains RT858, RT243, RT569, RT789, and RT709 during growth in YEP galactose medium at room temperature. Results are normalized so the activity in the SWI4 NUT1 NUT2 strain (13.2 Miller units) is 100.
From a centromere genomic library, we isolated a plasmid, pRKT562, that complemented both the defect in reporter gene regulation and the temperature sensitivity of the nut21-1 mutation. Subcloning of the insert on this plasmid established that the gene CCR4 was responsible for the complementing activity (see Materials and Methods). Furthermore, CCR4 was linked to the nut21-1 mutation, as no recombinants between the two loci were obtained in 10 tetrads. These data suggest that mutation of CCR4 in combination with loss of NUT1 function causes Swi4p-independent expression of the UASGAL-URS2R-lacZ reporter. Thus, like Nut2p, Ccr4p might function with Nut1p to negatively regulate UASGAL-URS2R-lacZ.
DISCUSSION
We have identified a novel class of genes which includes NUT1, NUT2, and CCR4 in screening for mutations that relieve the Swi4p dependence of an artificial reporter regulated by the URS2R region of the HO promoter. Nut1p, Nut2p, and Ccr4p formally behave as negative regulators of gene expression. Their function is distinctive in two respects. First, these proteins contribute substantially to the regulation of artificial reporters but not detectably to their endogenous counterparts. Second, these proteins function cooperatively with each other.
Nut1p and Nut2p behave as a negative regulators of transcription.
We found that the 360-bp URS2R segment from the URS2 region is sufficient to confer Swi4p dependence on the UASGAL activation sequences. We propose that in the absence of Swi4p, regulatory mechanisms inhibit activation of transcription by Gal4p in the case of UASGAL-URS2R-lacZ and by Swi5p in the case of HO. In nut1 nut2 double mutants, however, URS2R is unable to inhibit transcription of UASGAL-URS2R-lacZ and URS1-URS2R-lacZ in the absence of Swi4p. Thus, for these reporters, Nut1p and Nut2p act as negative regulators of transcription.
This interpretation is also consistent with two additional findings. First, mutations in SRB8, SRB9, SRB10, SRB11, SIN4, ROX3, and RGR1 also cause loss of Swi4p-dependent regulation of UASGAL-URS2R-lacZ (Table 4). Given that these genes are required for the repression of a number of endogenous yeast genes including SPO13 (58), IME1 (13), SUC2 (54), and STE2 (61), Nut1p and Nut2p might be similarly required for repression of transcription even though Nut1p and Nut2p are not required for the repression of SPO13, SUC2, and STE2. Second, both Nut1p and Nut2p are localized to the nucleus, where they would be available to regulate transcription directly.
The phenotypes of nut1Δ nut2-1 mutants are context dependent.
We found that the nut1 nut2 double mutation relieved the Swi4p dependence of UASGAL-URS2R-lacZ and URS1-URS2R-lacZ but did not affect the Swi4p dependence of URS1-URS2R-ho or URS1-URS2-ho. These data establish that Nut1p and Nut2p are not absolutely required to regulate transcription of the endogenous HO gene. Possibly, unknown components operate redundantly with Nut1p and Nut2p to regulate HO transcription. The insertion of lacZ sequences might disable these components and thus reveal a requirement for Nut1p and Nut2p. Another possibility is that the presence of lacZ sequences in yeast may simply generate a new situation which requires additional proteins for regulation. We cannot exclude the possibility that mutations in NUT1 and NUT2 would increase the expression of any lacZ-containing reporter. Further experimentation will be necessary to resolve this issue.
In any event, the finding that the nut1 nut2 double mutation primarily affects lacZ-containing reporters suggests that NUT1 and NUT2 may be members of a small group of genes whose phenotypes are limited to artificial reporters. Mutations in SIN4 allow Swi5p-independent expression of a URS1-URS2-lacZ allele but not of URS1-URS2-ho (24, 33, 40). Mutations in SIN4 also cause elevated expression of a phosphate-repressed pPHO5-lacZ construct but not of an identical construct in which the PHO5 promoter directs transcription of the PHO5 gene (21b). We observed that the NUT1 and NUT2 genes are similarly required for the repression of pPHO5-lacZ but do not affect the repression of the endogenous PHO5 gene. Harashima et al. have observed that SIN4 mutations allow constitutive transcription of the PHO5 gene when the PHO5 promoter and open reading frame are integrated at the URA3 locus (19).
Thus, the requirement for Nut1p, Nut2p, and Sin4p is revealed only when the chromosomal environment for gene expression is perturbed by the insertion of bacterial lacZ sequences or by the removal of the gene to another genomic location. One explanation is that these alterations disrupt the normal organization of chromatin across promoter regions. Nucleosomes are important for the regulation of both HO and PHO5 transcription (2, 25, 57). Conceivably, the organization of nucleosomes across the promoters of these genes requires either a suitable chromosomal environment or Nut1p and Nut2p activity. When the chromosomal milieu is disrupted, Sin4p, Nut1p, and Nut2p activity would be essential for positioning the nucleosomes that inhibit transcription at these promoters. Because other models also adequately explain the data, it remains to be determined why the phenotypes of mutations in NUT1, NUT2, and SIN4 are evident only in artificial situations.
Nut1p and Nut2p function cooperatively.
The other unusual property of the nut1 nut2 double mutation is the strong synergy between loss of NUT1 function and the nut2-1 allele (Table 6). An analogous relationship was observed for genes required for glucose repression of SUC2 (60). The finding of two-gene traits often implies functional redundancy between the products of the two genes. Because the original nut1-1 and nut2-1 alleles exhibit no phenotype alone, Nut1p and Nut2p may function in redundant, parallel pathways.
Instead of a simple parallel relationship, we propose that Nut2p is the primary functional moiety whereas Nut1p is a dispensable auxiliary protein that assists Nut2p. Several observations lead to this hypothesis. First, inactivation of a temperature-sensitive Nut2p causes a measurable increase in Swi4p-independent UASGAL-URS2R-lacZ expression despite the presence of wild-type Nut1p. Second, of the two proteins, Nut2p is an essential protein whereas Nut1p is not. Finally, Nut1p appears to assist Nut2p both in the essential function of Nut2p and in regulating the UASGAL-URS2R-lacZ reporter construct. Removal of Nut1p dramatically exacerbates the constitutive reporter phenotype of the nut2-1 allele. Likewise, in the absence Nut1p, the mutant Nut2p encoded by the nut2-ts70 allele is unable to sustain cell viability at any temperature, whereas in the presence of Nut1p, it can support viability at 25°C. Thus, Nut1p contributes substantially to the function of Nut2p.
Nut1p, Nut2p, and Ccr4p may comprise a distinctive class of proteins.
Our finding that Nut1p and Nut2p function cooperatively suggests that other proteins might cooperate with Nut1p. Therefore, we asked if deletion of NUT1 could synergize with mutations in genes other than NUT2 to cause a Nut− phenotype. From analysis of such mutations, we estimate that at least four such genes exist. An allele of CCR4 caused a Nut− phenotype in a nut1Δ background, indicating that Ccr4p may function with Nut1p. Ccr4p was previously identified as a regulator of ADH2 gene expression (15). Its role in gene regulation appears subtle, as the strongest phenotypes were observed on ADH2 genes that were compromised by SPT/CRE mutations or by delta insertions. Ccr4p is a component of a large protein complex that includes Caf1p, Dbf2p, Not1p, Not2p, Not3p, and Not4p (16, 30, 31). The Not proteins appear to be involved in transcriptional repression (12). Our finding that Ccr4p functions with Nut1p to negatively regulate UASGAL-URS2R-lacZ is consistent with these studies. Although we have yet to determine the identity of the remaining genes that function like NUT2, some may encode other proteins in the Ccr4p complex, and likewise Nut1p and Nut2p might physically interact with the Ccr4p complex. Remarkably, Harashima et al. identified two different two-gene traits, one due to mutations in BEL3 and BEL7 and the other due to mutations in BEL5 and BEL6, that affect the repression of PHO5 at a heterologous genomic locus but not at its native locus (19). Not only is the nut1 nut2 mutant similarly defective in two genes, but it shares with these two bel mutants rough colony morphology, flocculent growth in liquid culture, and elevated expression of artificial, albeit different reporter genes. It remains to be determined if bel3, bel5, bel6, and bel7 are alleles of NUT1, NUT2, or CCR4.
In summary, the genetic screens described here have identified Sin4p, Nut1p, Nut2p, and Ccr4p as representatives of a distinctive group of proteins. These proteins all appear to negatively regulate transcription from artificial reporters. While Sin4p and Ccr4p are known to regulate endogenous genes, we do not know if Nut1p and Nut2p do. However, Nut2p may be vital to the regulation of endogenous genes, since Nut2p is essential for viability. The disruptive effect of lacZ sequences may be revealing a requirement for a function dependent on Sin4p, Nut1p, Nut2p, and Ccr4p. Elucidating this function may contribute to our understanding of the processes that negatively regulate eukaryotic transcription.
ACKNOWLEDGMENTS
We are particularly grateful to Meghan Sharp for isolating the Nut− mutants and to Sally Horne for characterization of the NUT2-like genes, during their rotations in the laboratory, and to Sandy Johnson and Megan Grether for improvements on the manuscript. We thank Marian Carlson, Wolfram Hörz, David Stillman, Madhu Wahi, Richard Young, and their colleagues for generously providing plasmids, and Megan Grether, Carol Gross, Wolfram Hörz, Sandy Johnson, Hay-Oak Park, Sylvia Sanders, Shai Shaham, and Anita Sil for numerous discussions during the course of this work.
This work was supported by NIH research grant AI18738 to I.H., a Howard Hughes predoctoral fellowship to R.K.T., and grants from the Markey Foundation and the Herbert W. Boyer Fund.
ADDENDUM IN PROOF
We have recently learned that Nut2p may be a component of the mediator complex (Claes Gustafsson, personal communication; Young-Joon Kim, personal communication). The mediator complex is required for yeast transcriptional activa- tion in vitro and also contains Sin4p, Rox3p, and Rgr1p (see references 18 and 28).
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almer A, Rudolph H, Hinnen A, Hörz W. Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements. EMBO J. 1986;5:2689–2696. doi: 10.1002/j.1460-2075.1986.tb04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews B J, Herskowitz I. Identification of a DNA binding factor involved in cell-cycle control of the yeast HO gene. Cell. 1989;57:21–29. doi: 10.1016/0092-8674(89)90168-2. [DOI] [PubMed] [Google Scholar]
- 4.Andrews B J, Herskowitz I. The yeast Swi4 protein contains a motif present in developmental regulators and is part of a complex involved in cell-cycle-dependent transcription. Nature. 1989;342:830–833. doi: 10.1038/342830a0. [DOI] [PubMed] [Google Scholar]
- 5.Andrews B J, Moore L A. Interaction of the yeast Swi4 and Swi6 cell cycle regulatory proteins in vitro. Proc Natl Acad Sci USA. 1992;89:11852–11856. doi: 10.1073/pnas.89.24.11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett D E, Jr, Boguski M S, Spencer F, Reeves R, Goebl M, Hieter P. Comparative genomics, genome cross-referencing and XREFdb. Trends Genet. 1995;11:372–373. doi: 10.1016/s0168-9525(00)89109-x. [DOI] [PubMed] [Google Scholar]
- 7.Bobola N, Jansen R P, Shin T H, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 1996;84:699–709. doi: 10.1016/s0092-8674(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 8.Bortvin A, Winston F. Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science. 1996;272:1473–1476. doi: 10.1126/science.272.5267.1473. [DOI] [PubMed] [Google Scholar]
- 9.Breeden L, Nasmyth K. Cell cycle control of the yeast HO gene: cis- and trans-acting regulators. Cell. 1987;48:389–397. doi: 10.1016/0092-8674(87)90190-5. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, West R W, Jr, Johnson S L, Gans H, Kruger B, Ma J. TSF3, a global regulatory protein that silences transcription of yeast GAL genes, also mediates repression by α2 repressor and is identical to SIN4. Mol Cell Biol. 1993;13:831–840. doi: 10.1128/mcb.13.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark-Adams C D, Norris D, Osley M A, Fassler J S, Winston F. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 1988;2:150–159. doi: 10.1101/gad.2.2.150. [DOI] [PubMed] [Google Scholar]
- 12.Collart M A, Struhl K. NOT1 (CDC39), NOT2 (CDC36), NOT3, and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 1994;8:525–537. doi: 10.1101/gad.8.5.525. [DOI] [PubMed] [Google Scholar]
- 13.Covitz P A, Song W, Mitchell A P. Requirement for RGR1 and SIN4 in RME1-dependent repression in Saccharomyces cerevisiae. Genetics. 1994;138:577–586. doi: 10.1093/genetics/138.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross F R, Hoek M, McKinney J D, Tinkelenberg A H. Role of Swi4 in cell cycle regulation of CLN2 expression. Mol Cell Biol. 1994;14:4779–4787. doi: 10.1128/mcb.14.7.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis C L, Malvar T. The CCR4 gene from Saccharomyces cerevisiae is required for both nonfermentative and spt-mediated gene expression. Genetics. 1990;124:283–291. doi: 10.1093/genetics/124.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draper M P, Salvadore C, Denis C L. Identification of a mouse protein whose homolog in Saccharomyces cerevisiae is a component of the CCR4 transcriptional regulatory complex. Mol Cell Biol. 1995;15:3487–3495. doi: 10.1128/mcb.15.7.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenmann D M, Dollard C, Winston F. SPT15, the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo. Cell. 1989;58:1183–1191. doi: 10.1016/0092-8674(89)90516-3. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson C M, Myers L C, Li Y, Redd M J, Lui M, Erdjument-Bromage H, Tempst P, Kornberg R D. Identification of Rox3 as a component of mediator and RNA polymerase II holoenzyme. J Biol Chem. 1997;272:48–50. doi: 10.1074/jbc.272.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Harashima S, Mizuno T, Mabuchi H, Yoshimitsu S, Ramesh R, Hasebe M, Tanaka A, Oshima Y. Mutations causing high basal level transcription that is independent of transcriptional activators but dependent on chromosomal position in Saccharomyces cerevisiae. Mol Gen Genet. 1995;247:716–725. doi: 10.1007/BF00290403. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner C J, Thompson C M, Zhang J, Chao D M, Liao S M, Koleske A J, Okamura S, Young R A. Association of an activator with an RNA polymerase II holoenzyme. Genes Dev. 1995;9:897–910. doi: 10.1101/gad.9.8.897. [DOI] [PubMed] [Google Scholar]
- 21.Herschbach B M, Johnson A D. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- 21a.Horne, S., and R. K. Tabtiang. Unpublished data.
- 21b.Hörz, W. Personal communication.
- 22.Jensen R, Sprague G F, Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci USA. 1983;80:3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y W, Dohrmann P R, Stillman D J. Genetic and physical interactions between yeast RGR1 and SIN4 in chromatin organization and transcriptional regulation. Genetics. 1995;140:47–54. doi: 10.1093/genetics/140.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y W, Stillman D J. Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:4503–4514. doi: 10.1128/mcb.12.10.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruger W, Peterson C L, Sil A, Coburn C, Arents G, Moudrianakis E N, Herskowitz I. Amino acid substitutions in the structured domains of histones H3 and H4 partially relieve the requirement of the yeast SWI/SNF complex for transcription. Genes Dev. 1995;9:2770–2779. doi: 10.1101/gad.9.22.2770. [DOI] [PubMed] [Google Scholar]
- 26.Kuchin S, Yeghiayan P, Carlson M. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc Natl Acad Sci USA. 1995;92:4006–4010. doi: 10.1073/pnas.92.9.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Bjorklund S, Jiang Y W, Kim Y J, Lane W S, Stillman D J, Kornberg R D. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao S M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Badarinarayana V, Audino D C, Rappsilber J, Mann M, Denis C L. The NOT proteins are part of the CCR4 transcriptional complex and affect gene expression both positively and negatively. EMBO J. 1998;17:1096–1106. doi: 10.1093/emboj/17.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Toyn J H, Chiang Y-C, Draper M P, Johnston L H, Denis C L. DBF2, a cell cycle-regulated protein kinase, is physically and functionally associated with the CCR4 transcriptional regulatory complex. EMBO J. 1997;16:5289–5298. doi: 10.1093/emboj/16.17.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lycan D, Mikesell G, Bunger M, Breeden L. Differential effects of Cdc68 on cell cycle-regulated promoters in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:7455–7465. doi: 10.1128/mcb.14.11.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macatee T, Jiang Y W, Stillman D J, Roth S Y. Global alterations in chromatin accessibility associated with loss of SIN4 function. Nucleic Acids Res. 1997;25:1240–1247. doi: 10.1093/nar/25.6.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muhlrad D, Hunter R, Parker R. A rapid method for localized mutagenesis of yeast genes. Yeast. 1992;8:79–82. doi: 10.1002/yea.320080202. [DOI] [PubMed] [Google Scholar]
- 35.Nasmyth K. Molecular analysis of a cell lineage. Nature. 1983;302:670–676. doi: 10.1038/302670a0. [DOI] [PubMed] [Google Scholar]
- 36.Nasmyth K. At least 1400 base pairs of 5′-flanking DNA is required for the correct expression of the HO gene in yeast. Cell. 1985;42:213–223. doi: 10.1016/s0092-8674(85)80117-3. [DOI] [PubMed] [Google Scholar]
- 37.Nasmyth K. A repetitive DNA sequence that confers cell-cycle START (CDC28)-dependent transcription of the HO gene in yeast. Cell. 1985;42:225–235. doi: 10.1016/s0092-8674(85)80118-5. [DOI] [PubMed] [Google Scholar]
- 38.Nasmyth K. The determination of mother cell-specific mating type switching in yeast by a specific regulator of HO transcription. EMBO J. 1987;6:243–248. doi: 10.1002/j.1460-2075.1987.tb04745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasmyth K, Dirick L. The role of SW14 and SW16 in the activity of G1 cyclins in yeast. Cell. 1991;66:995–1013. doi: 10.1016/0092-8674(91)90444-4. [DOI] [PubMed] [Google Scholar]
- 40.Nasmyth K, Stillman D, Kipling D. Both positive and negative regulators of HO transcription are required for mother-cell-specific mating-type switching in yeast. Cell. 1987;48:579–587. doi: 10.1016/0092-8674(87)90236-4. [DOI] [PubMed] [Google Scholar]
- 41.Nonet M L, Young R A. Intragenic and extragenic suppressors of mutations in the heptapeptide repeat domain of Saccharomyces cerevisiae RNA polymerase II. Genetics. 1989;123:715–724. doi: 10.1093/genetics/123.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogas J, Andrews B J, Herskowitz I. Transcriptional activation of CLN1, CLN2, and a putative new G1 cycline (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell. 1991;66:1015–1026. doi: 10.1016/0092-8674(91)90445-5. [DOI] [PubMed] [Google Scholar]
- 43.Primig M, Sockanathan S, Auer H, Nasmyth K. Anatomy of a transcription factor important for the start of the cell cycle in Saccharomyces cerevisiae. Nature. 1992;358:593–597. doi: 10.1038/358593a0. [DOI] [PubMed] [Google Scholar]
- 44.Rose M D, Broach J R. Cloning genes by complementation in yeast. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. Vol. 194. San Diego, Calif: Academic Press, Inc.; 1991. pp. 195–230. [DOI] [PubMed] [Google Scholar]
- 45.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 46.Rosenblum-Vos L S, Rhodes L, Evangelista C C, Jr, Boayke K A, Zitomer R S. The ROX3 gene encodes an essential nuclear protein involved in CYC7 gene expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5639–5647. doi: 10.1128/mcb.11.11.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. In: Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. Vol. 194. San Diego, Calif: Academic Press, Inc.; 1991. pp. 281–301. [DOI] [PubMed] [Google Scholar]
- 48.Russell D W, Jensen R, Zoller M J, Burke J, Errede B, Smith M, Herskowitz I. Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol Cell Biol. 1986;6:4281–4294. doi: 10.1128/mcb.6.12.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakai A, Shimizu Y, Kondou S, Chibazakura T, Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 51.Sidorova J, Breeden L. Analysis of the SWI4/SWI6 protein complex, which directs G1/S-specific transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:1069–1077. doi: 10.1128/mcb.13.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sil A, Herskowitz I. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 1996;84:711–722. doi: 10.1016/s0092-8674(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 54.Song W, Treich I, Qian N, Kuchin S, Carlson M. SSN genes that affect transcriptional repression in Saccharomyces cerevisiae encode SIN4, ROX3, and SRB proteins associated with RNA polymerase II. Mol Cell Biol. 1996;16:115–120. doi: 10.1128/mcb.16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern M, Jensen R, Herskowitz I. Five SWI genes are required for expression of the HO gene in yeast. J Mol Biol. 1984;178:853–868. doi: 10.1016/0022-2836(84)90315-2. [DOI] [PubMed] [Google Scholar]
- 56.Sternberg P W, Stern M J, Clark I, Herskowitz I. Activation of the yeast HO gene by release from multiple negative controls. Cell. 1987;48:567–577. doi: 10.1016/0092-8674(87)90235-2. [DOI] [PubMed] [Google Scholar]
- 57.Straka C, Hörz W. A functional role for nucleosomes in the repression of a yeast promoter. EMBO J. 1991;10:361–368. doi: 10.1002/j.1460-2075.1991.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strich R, Slater M R, Esposito R E. Identification of negative regulatory genes that govern the expression of early meiotic genes in yeast. Proc Natl Acad Sci USA. 1989;86:10018–10022. doi: 10.1073/pnas.86.24.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Tabtiang, R. K. Unpublished data.
- 59.Thompson C M, Koleske A J, Chao D M, Young R A. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- 60.Vallier L G, Carlson M. Synergistic release from glucose repression by mig1 and ssn mutations in Saccharomyces cerevisiae. Genetics. 1994;137:49–54. doi: 10.1093/genetics/137.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahi M, Johnson A D. Identification of genes required for α2 repression in Saccharomyces cerevisiae. Genetics. 1995;140:79–90. doi: 10.1093/genetics/140.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winston F, Chaleff D T, Valent B, Fink G R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984;107:179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]