Abstract
Background
CRISPR/Cas9 system to treat human-related diseases has achieved significant results and, even if its potential application in cancer research is improving, the application of this approach in clinical practice is still a nascent technology.
Main body
CRISPR/Cas9 technology is not yet used as a single therapy to treat tumors but it can be combined with traditional treatment strategies to provide personalized gene therapy for patients. The combination with chemotherapy, radiation and immunotherapy has been proven to be a powerful means of screening, identifying, validating and correcting tumor targets. Recently, CRISPR/Cas9 technology and CAR T-cell therapies have been integrated to open novel opportunities for the production of more efficient CAR T-cells for all patients. GMP-compatible equipment and reagents are already available for several clinical-grade systems at present, creating the basis and framework for the accelerated development of novel treatment methods.
Conclusion
Here we will provide a comprehensive collection of the actual GMP-grade CRISPR/Cas9-mediated approaches used to support cancer therapy highlighting how this technology is opening new opportunities for treating tumors.
Keywords: GMP procedures, CRISPR/Cas-9, Cancer therapy
Background
The incidence and mortality of cancer still remains the principal health issue worldwide. Despite countless progress, much still needs to be done to improve the outcomes of those patients without a valid therapeutic alternative. The Advanced Therapy Medicinal Products (ATMP) oriented to a precision and individualized treatment for the patients have opened a new era for cancer treatment. In this scenario, the genome editing offers a powerful tool for the development of new strategies for treating cancer.
Good Manufacturing Practice (GMP) guidelines
ATMPs offer a new powerful opportunity for treating, and in some instances, curing diseases (such as cancer) for which there are often no other available treatments. While this has offered an important new therapeutic tool, it has also raised the need to produce drugs following regulations, modalities, and quality standards that ensure safety for patients. In fact, ATMPs are characterized by a very different modalities, use different cell types and, mostly, for the different manufacturing protocols. In particular, ATMP production is a complex manufacturing process and the procedures are still evolving to meet these unique needs. In this regards GMP [1] are the mandatory guidelines governing ATMPs manufacturing. Noteworthy, GMP compliance is mandatory for all products intended for the market and those used for clinical trials.
These guidelines describe the minimum quality standard that a medicines manufacturer must follow to ensure that products are consistently produced and controlled. These are designed to minimize the risks involved in any pharmaceutical production which cannot be avoided or eliminated even testing the final product [2]. Furthermore it is very important to note that the guidelines do not intend to place any restrains on the development of new concepts of new technologies, rather intend to ensure the quality, safety, efficacy and traceability of the product. In fact, any alternative approaches may be implemented by the manufacturers, the important thing is to demonstrate that the alternative approach can meet the same quality standard. Based on the previous considerations, it is important to make the appropriate assessments of the technologies that are being developed and employed for the ATMPs production, before moving from research scale to clinical or commercial manufacturing. For this reason, it is essential to have a very good process development phase. The main goal of process development is to reach a very robust manufacturing process with high efficiency, cost containment, maintenance of quality and safety standards, and overall risk reduction as additional key objectives. To this end, several preclinical studies have already been developed for ready clinical translation [3–5].
CRISPR/Cas9 technology mechanism of action
Discovered for the first time in 1987 as a defense mechanism in prokaryotes [6]Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) has greatly improved the field of precise genome editing. The CRISPR system relies on RNA ‘guides’ that drives the site-specific binding of CRISPR-associated (Cas) proteins for mediating DNA or RNA cleavage [7]. The CRISPR system includes three principal types (I, II and III) and 12 subtypes [8]. The type II relies on a single Cas protein, Cas9, to target a specific sequence of DNA. For this reason, the CRISPR/Cas9 has become the most widely adopted genome editing tool [9]. The requirements for recognizing and cut a specific DNA sequence, once paired with a guide RNA (gRNA), are as follows: 1) a site-specific complementarity between a 20-nucleotides (nt) targeting sequence, called the protospacer, that is a part of the CRISPR RNA (crRNA), which together with the transacting crRNA (tracrRNA) generated a single guide RNA (sgRNA), which recruits the Cas9 nuclease to specific DNA sequences, 2) an NGG protospacer adjacent motif (PAM) sequence located at the 3´ of the targeting crRNA/protospacer sequence. It has been observed that, without the PAM sequence, the Cas9 nuclease cannot cleave the target sequence, also if fully complementary to the sgRNA [10].
Once these two criteria are met, the DNA sequence could be targeted and cut by the Cas9/sgRNA system. The design of a specific sgRNA guide sequence allows the detection of double-strand breaks (DSBs) sites where [11] Cas9 binds and cleaves the target DNA sequences, complementary to the crRNA. DSBs, located at approximately − 3 nucleotides before the PAM sequence, are introduced in the target sequence and then the endogenous DNA DSB repair mechanisms rebuild the breaks. The DNA repair machinery is initiated via two most common pathways: non-homologous end joining (NHEJ), which is the predominant repair pathway in most mammalian cells; the less-frequent homology-directed repair (HDR). The NHEJ frequently results in genomic insertions or deletions (indels) which can introduce frameshift mutations that can result in truncated and/or non-functional proteins. Whereas the HDR uses the donor DNA template to precisely repair DSBs for gene modification [12, 13]. In the genome editing procedure, it is possible to design a DNA template, with high homology to the specific target gene locus, containing the aimed genetic change. The procedure of the genome editing could be very challenging because the efficiency of HDR-mediated gene insertion is significantly lower than NHEJ-mediated INDEL formation [14]. Hence, the editing outcomes are the result of the interaction between these two different repair pathways. Furthermore, the CRISPR/Cas9 system can accurately modify the DNA sequences by generating multiple DSBs at specific sites in the genome and, using multiple guide RNAs, it can achieve a multiple genome editing of the target sequence [9]. Because CRISPR/Cas9 system is more effective and easier to perform compared to the other gene editing technologies, such as zinc-finger nucleases (ZFNs) and transcription activator like effector nucleases (TALENs) [15, 16], it can be advantageously applied in the clinical trials that incorporate gene editing for cancer treatment.
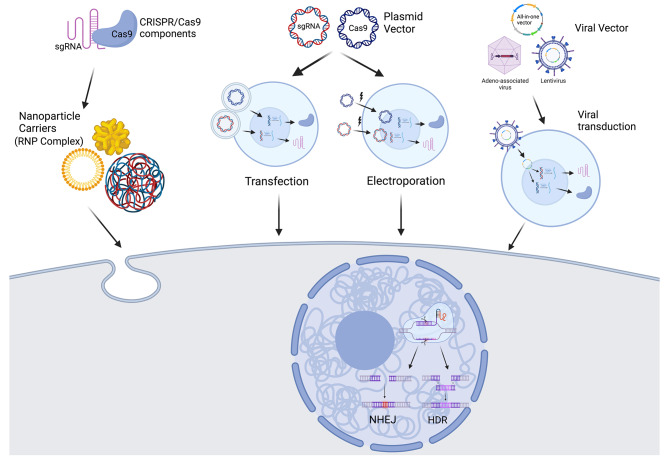
The CRISPR/Cas9 system is mostly employed in ex vivo strategies to perform gene editing in cells that are then reinfused into the patient. The most commons delivery technologies for gene editing are broadly classified as viral, such as lentivirus, retrovirus, adenovirus and adeno-associated virus, or non-viral vectors, such as electroporation, nanoparticles and cell squeezing (Fig. 1).
Fig. 1.
CRISPR/Cas9 mechanism of action. Cas9 and sgRNA vehiculation to edit the nuclear target sequence by nanoparticles when assembled to form RNP complex (left); delivery of the elements as single plasmids of expression through lipo-assisted transfection reagent or by electroporation (center); Viral transduction of Cas9 and sgRNA carrying vector (right). DNA repair machinery (NHEJ, HDR) is activated when the nucleus is reached by the CRISPR/Cas9 system
CRISPR/Cas9 clinical applications
The first clinical application of CRISPR/Cas9 system was performed by Lu et al. in 2016, when they carried out in human phase I clinical trial of CRISPR/Cas9 PD-1-edited T cells in patients with advanced non-small-cell lung cancer [17, 18]. Rising from this study, many other clinical trials that use CRISPR/Cas9 in cancer treatment or using gene edited CAR T-cells or Tumor Infiltrating Lymphocytes (TIL) cells have been established (Table 1). Considering this new and powerful opportunity for cancer treatment, it is very important to develop safe and efficient delivery CRISPR/Cas9 system vectors to target the tissues and cells. To be used in clinical trials it is mandatory that these strategies for CRISPR delivery are manufactured following GMP procedures.
Table 1.
Clinical Trials using CRISPR/Cas9 technology in Cancer Immunotherapy
| NCT Number | Study Design | Target Gene | Phases | Cell Type | Tumor Type |
|---|---|---|---|---|---|
| NCT04438083 | A Safety and Efficacy Study Evaluating CTX130 | TRAC; β2M; CD70 | Phase 1 | CAR T-Cells | Renal Cell Carcinoma |
| NCT04417764 | TACE Combined With PD-1 Knockout Engineered T Cell | PD-1 | Phase 1 | Engineered T-Cells | Hepatocellular Carcinoma |
| NCT04244656 | A Safety and Efficacy Study Evaluating CTX120 | TCR; MHC I | Phase 1 | CAR T-Cells | Multiple Myeloma |
| NCT02793856 | PD-1 Knockout Engineered T Cells | PD-1 | Phase 1 | Engineered T-Cells | Metastatic Non-small Cell Lung Cancer |
| NCT03081715 | PD-1 Knockout Engineered T Cells | PD-1 | Completed | Engineered T-Cells | Esophageal Cancer |
| NCT03545815 | CRISPR-Cas9 Mediated PD-1 and TCR Gene-knocked Out Mesothelin-directed CAR-T Cells | PD-1; TCR | Phase 1 | CAR T-Cells | Multiple solid tumor |
| NCT03398967 | Safety Study of Universal Dual Specificity CD19 and CD20 or CD22 CAR-T Cell Immunotherapy | TRAC; CD52 | Phase 1/ Phase 2 | CAR T-Cells | B-cell Acute Lymphoblastic Leukemia |
| NCT02867332 | PD-1 Knockout Engineered T Cells | PD-1 | Phase 1 | Engineered T-Cells | Renal Cell Carcinoma |
| NCT05812326 | PD-1 Knockout Anti-MUC1 CAR-T Cells | PD-1 | Phase 1/ Phase 2 | Engineered T-Cells | Breast Cancer |
| NCT02867345 | PD-1 Knockout Engineered T Cells | PD-1 | Unknown | Engineered T-Cells | Prostate Cancer |
| NCT05662904 | Genetic Ablation of CD33 in HSC | CD33 | Phase 1 | Hematopoietic Stem Cells | Acute Myeloid Leukemia |
| NCT03044743 | PD-1 Knockout EBV-CTLs for Advanced Stage Epstein-Barr Virus (EBV) Associated Malignancies | PD-1 | Phase 1/ Phase 2 | Engineered T-Cells | Gastric Carcinoma; Nasopharyngeal Carcinoma; T cell Lymphoma; Adult Hodgking |
| NCT03057912 | TALEN and CRISPR/Cas9 in the Treatment of HPV-related Cervical Intraepithelial Neoplasia | E6;E7 | Phase 1 | Engineered T-Cells | Cervical Intraepithelial Neoplasia |
| NCT05066165 | NTLA-5001 in Subjects With Acute Myeloid Leukemia | Phase 1/ Phase 2 | CAR T-Cells | Acute Myeloid Leukemia | |
| NCT03747965 | PD-1 Gene-knocked Out in Mesothelin-directed CAR-T Cells | PD-1 | Phase 1 | CAR T-Cells | Mesothelin Positive Multiple Solid Tumors |
| NCT05643742 | A Safety and Efficacy Study Evaluating CTX112 | TRAC; β2M; CD70 | Phase 1/ Phase 2 | CAR T-Cells | B Cell-Malignancies |
| NCT04502446 | A Safety and Efficacy Study Evaluating CTX130 | TRAC; β2M; CD70 | Phase 1 | CAR T-Cells | B Cell-Malignancies |
| NCT03166878 | UCART019 in Patients With Relapsed or Refractory CD19 Tumors | TRAC;CD52 | Phase 1/ Phase 2 | CAR T-Cells | Leukemia and Lymphoma |
| NCT05566223 | CISH Inactivated TILs in the Treatment of NSCLC | CISH | Phase 1/ Phase 2 | Engineered T-Cells | Non small cell lung cancer |
| NCT05795595 | A Safety and Efficacy Study Evaluating CTX131 | TRAC; β2M; CD70 | Phase 1/ Phase 2 | CAR T-Cells | Renal cell carcinoma; Cervical Carcinoma; Pancreatic Adenocarcinoma; Malignant Pleural Mesothelioma |
| NCT04035434 | A Safety and Efficacy Study Evaluating CTX110 | TRAC; β2M; CD70; | Phase 1/ Phase 2 | CAR T-Cells | B Cell-Malignancies |
| NCT04426669 | CISH depletion using CRISPR/Cas9 in Tumor Infiltrating Lymphocytes | CISH | Phase 1/ Phase 2 | Engineered T-Cells | Gastrointestinal Cancer |
| NCT05037669 | Allogeneic CRISPR-edited T Cells Engineered to Express Anti-CD19 Chimeric Antigen Receptor | TCR, HLA-I; HLA-II | Phase 1 | CAR T-Cells | Acute Myeloid Leukemia; Chronic Lymphocytic Leukemia; Non Hodgkin Lymphoma |
| NCT02863913 | PD-1 Knockout Engineered T Cells for Muscle-invasive Bladder Cancer | PD-1 | Phase 1 | Engineered T-Cells | Bladder Cancer |
In a very interesting study, Palmer DC et al. [3] developed a clinical scale and GMP-compliant manufacturing process for highly efficient and precise CRISPR/Cas9 CISH knockout (KO) in human T cells and TIL. In several clinical trials the genome editing of the biological component of the study is associated to chemotherapy. Based on the study of Palmer and colleagues, a phase I/II trial has been started for patients with metastatic gastrointestinal epithelial cancer (NCT04426669), in which Cyclophosphamide, Fludarabine and Aldesleukin are administered combined with TIL in which the gene encoding CISH has been inactivated using the CRISPR/Cas9 System. To be administered to the patients the TIL production and the gene editing procedure need to be performed in a GMP grade environment with a quality system that guarantees the final release of genetically modified cells. Another interesting application for CRISPR/Ca 9 system is in the cancer immunotherapy with CAR T-cells. It has been demonstrated that PD-1 deficient CAR T-cells have an improved antitumor activity in vitro [19] while a previous study and clinical trials (NCT02808442 and NCT02746952), performed using TALEN as gene editing system, have showed how disrupting genes encoding T cell receptor (TCR) α and β chains in the infused CAR T-cell product can prevent graft-versus-host disease (GVHD) appears [20]. Based on these results several other clinical trials have been established. In the phase I study for patients with mesothelin positive multiple solid tumors (NCT03545815), a CRISPR/Cas9 mediated gene knock-out of PD-1 and endogenous TCR for CAR T-cells is performed. Following a similar strategy, a phase I trial to assess the safety and feasibility of administering pre-manufactured allogeneic T cells from healthy donors expressing CD19.CAR T-cells lacking expression of HLA class I, HLA class II molecules and endogenous TCR through CRISPR/Cas9 mediated genome-editing of beta-2 microglobulin (β2M), CIITA and T cell receptor alpha chain, respectively (Table 1, NCT05037669).
All of these studies showed the fundamental GMP grade manufacturing role for producing a CRISPR/Cas9 gene edited cell product for clinical trials. In fact several studies are now performed for developing production process for improving clinical scale manufacturing of genetically modified cells for clinical trials [4, 21]. In this review we will provide a comprehensive collection of the actual GMP-grade CRISPR/Cas9-mediated approaches used to support cancer therapy, highlighting how this technology is opening new opportunities for treating tumors.
Main text
CRISPR/Cas9 gene-editing of immune check-points
Immunotherapy is a novel approach to fight the growth and invasion of tumor cells by inducing the stimulation of the immune system [22]. It involves cytokine therapy, oncolytic virus therapy, dendritic cell (DC) therapy, cancer vaccine, adoptive cellular immunotherapy (ACT), immune checkpoint blockade, and antibody-drug conjugate (ADC). Additionally, CAR T-cells therapy has demonstrated high efficacy for hematological and recently for solid tumors [23, 24].
Tumor immunity promotes tumor progression by modifying tumor biological features [25], selecting tumor cells adapted to the microenvironment [26] or creating a favorable tumor microenvironment [27]. Among the factors that play an important role in tumor immunity, immune checkpoints molecules such as PD-1 and CTLA4 deserve a special mention. Under physiological conditions, PD-1, expressed on T-cells, binds its physiological ligand, PD-L1, expressed on tumor cells. This interaction may impair the activity of T-cells and prevent further damage induced by cytotoxic effector molecules and autoimmunity.
In recent years immune checkpoint blockade became one of the most important therapeutic options for cancer. Several anti- PD-1/PD-L1 antibodies (Nivolumab, Pembrolizumab, and Atezolizumab) have shown significant advantages in certain malignancies such as melanoma, non-small-cell lung cancer (NSCLC) and urothelial carcinoma, and have been approved by the Food and Drug Administration [28–30]. However, specific side effects remain [31, 32], and the overall survival rate is not significantly improved [33].
CRISPR/Cas9 is an RNA-guided endonuclease, which is widely used as a simple and fast method to modify the DNA of mammalian cells [34]. In primary T-cells, researchers have conducted several studies to test the effectiveness of CRISPR/Cas9 in vitro. Schumann and colleagues introduced prassembled sgRNA and Cas9 endonuclease into human CD4+ primary T-cells using electroporation. This delivery resulted in inducing site-specific mutations in CXCR4 and PD-1 genes [35]. Su S. and colleagues conveyed CRISPR/Cas9 system through electroporation in the peripheral CD8+ T-cells of cancer patients or healthy individuals. Disruption of PD-1 In T-cells increased immune responses against cancer antigens [36, 37].
The knock-out of PD-1 in T-cell lymphocytes reduces the number of regulatory T-cells (Treg) or impairs Treg activity and recruits more effector cells. In addition, it can modulate the production of cytokines and activate caspases, inhibiting tumor proliferation in vivo and in vitro and improving survival [37–39].
In light of the recent pre-clinical results, CRISPR/Cas9 technology seems to be a good candidate to provide a powerful and effective protocol for editing genes that express checkpoint inhibitors, in particular PD-1, in a wide range of immune cells to block immune checkpoints [33].
Thus, in recent years, numerous clinical trials have been started with the aim to evaluate the potential of gene editing and to translate this knowledge into clinical settings [40].
All therapeutic drugs, including CRISPR/Cas9, during clinical trials must follow GMP procedures in order to minimize steps involved in any pharmaceutical production that cannot be eliminated through final product testing. Since immunotherapy obtained by the CRISPR/Cas9 system has not yet been deeply programmed in clinical practice, many clinical trials are in progress. In fact, in vitro, the combined effect of CRISPR/Cas9 with immunotherapies has been demonstrated such as the improvement of antibody performance [41, 42], the modulation of TME and immune cell activity [43–46] and reprogramming MHC specificity (correcting MHC mismatches) [47].
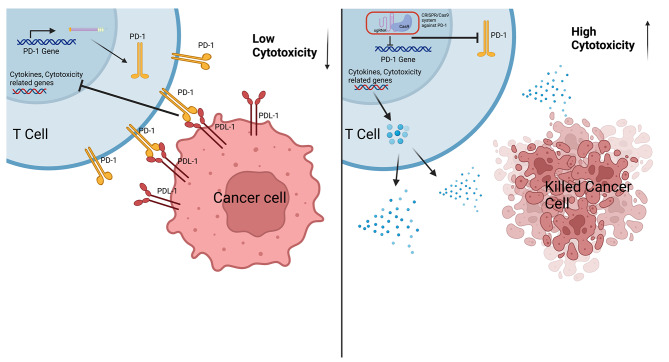
But the main focus regards the editing of T-cells. Among the targets of CRISPR/Cas9, PD-1 is the most targeted checkpoint in T-cell. In particular, many clinical trials have focused their attention on the autologous origin T-cell, in which CRISPR/Cas9 system is used to deplete PD-1 (Fig. 2).
Fig. 2.
CRISPR/Cas9-mediated editing of T-cell. Interaction between PD-1 receptor transcribed by T-cells and PD-L1 ligand expressed on cancer cells. Activation of PD1/PD-L1 checkpoint inhibits cytokine production and cytotoxic activity of T-cell (left). CRISPR/Cas9-mediated editing of the PD-1 sequence inhibits PD-1 expression allowing extracellular cytokines release and improving T-cell killing activity (right)
For example, in order to evaluate the safety of CRISPR/Cas9 technology, Lu et al. used the CRISPR/Cas9 method to obtain PD-1 depletion in T-cells from patients with non-small-cell lung cancer (NSCLC). The editing of autologous T-cell was followed by ex vivo reinfusion, hypothesizing that may ameliorate T-cell response. PD-1-edited T-cells were modified by co-transfection performing electroporation of Cas9 and sgRNA plasmids. Monitoring of T-cells modifications by next generation sequencing resulted in mutation frequency of off-target events of about 0.05% at 18 candidate sites. The authors conclude that clinical application of CRISPR/Cas9 gene-edited T-cells is generally safe and feasible and that this approach is clinically feasible (NCT02793856) [18].
The knockout of PD-1 performed using the CRISPR/Cas9 system is also used in Epstein-Barr virus cytotoxic lymphocytes (EBV-CTL) cells to treat patients affected by EBV positive advanced stage malignancies. Also in this case, the editing is obtained through CRISPR/Cas9-mediated PD-1 knockout in T-cells of autologous origin. The authors evaluate adverse events after each cycle by Common Terminology Criteria for adverse events as primary endpoint. Tumor and immunological markers are also evaluated as secondary endpoints to monitor the efficacy of the anti-tumor effect (NCT03044743) [48].
Among combination therapies involving PD-1 disruption in T-cells, an important study analyzes the safety and efficacy of a therapeutic vaccine in combination with the depletion of PD-1 carried out by the CRISPR/Cas9 system in the treatment of advanced prostate cancer. The therapeutic vaccine consists in a customized product involving the use of a recombinant fusion protein (PAP-GM-CSF) to stimulate the production of the antigen that would increase the immune system activity to kill tumor cells [49]. The strategy used by the authors was once again the engineering of patient’s T-cells through CRISPR/Cas9 technology to disrupt PD-1 gene. The therapeutic vaccine and PD-1 knockout T cells will be infused back to the patient in 3 times with a 2-week interval and the safety and efficacy effect will be evaluated at the end of the study (NCT03525652).
Another trial investigates the safety and effect of transcatheter arterial chemoembolization (TACE), a minimally invasive therapy that combines local delivery of chemotherapy with a procedure called embolization, in combination with engineered T-cells modified by CRISPR/Cas9 on PD-1 gene in patients with advanced hepatocellular carcinoma. TACE would block the blood supply of the tumor to achieve ischemic, hypoxic and necrotic effects (NCT04417764).
Among immune-checkpoint, the role of Cytokine-inducible SH2 domain-containing protein (CISH)has recently been deeply understood. CISH belongs to the suppressor of cytokine signaling (SOCS) family of negative feedback regulators that have been demonstrated a pivotal role in lymphoid cell function and development. Thus, it is a novel intra-cellular immune checkpoint and an important negative regulator of T-cell able to impair their activity [50].
Tumor Infiltrating Lymphocytes (TIL) have demonstrated efficacy in some malignancies, principally melanoma. Efficacy in most common solid tumors was shown through the selection of cancer neoantigen-specific TIL. Combined therapy with checkpoint inhibitor molecules has also been employed with the aim to increase the efficacy of the therapies with the autologous TILs. Since genetic engineering of T-cells performed by CRISPR/Cas9 that may ameliorate anti-tumor activity is now possible, researchers have improved and optimized a CRISPR/Cas9 based methodology to achieve precise and efficient editing in primary human T-cells without affecting cell function or viability, obtaining the inhibition of undruggable intracellular checkpoint. Thus, researchers are trying to edit the gene encoding this new intracellular checkpoint target, CISH, in TIL obtained from patients with metastatic cancers. Trials that regard the targeting of CISH in TIL through CRISPR/Cas9 involve Metastatic Gastrointestinal Cancers (NCT04426669) and NSCLC (NCT05566223). In these trials the safety and efficacy of genetically modified T-cell selected for anti-tumor activity for solid tumors are evaluated in the setting of novel target that involved checkpoint inhibitor [51].
In the last years scientists have evaluated the possibility to use TALEN and CRISPR/Cas9 to treat human cervical intraepithelial neoplasia induced by Human Papillomavirus (HPV) without invasion. In fact, the infection of HPV is the main causative factor of cervical intraepithelial neoplasia (CIN) and cervical cancer. HPV vaccines strategy allows to target the two most important oncoproteins expressed by HPV16 and HPV18, E6 and E7, which are also constitutively expressed by cancer cells [52, 53]. Numerous strategies have been applied to develop therapeutic vaccines using vectors, peptides/proteins, DNA and genome editing tools. Vector, peptide and protein vaccines are used in particular to treat HPV16 infection, whereas DNA vaccines and the vaccines that use genome editing tools are mostly polyvalent vaccines used for the treatment of both HPV16 and HPV18 and target E6 and E7 genes. The important roles of E6 and E7 playing in HPV-driven carcinogenesis make them attractive targets for therapeutic interventions. Furthermore some experimental studies demonstrated that using TALEN and CRISPR/Cas9 as genome editing tool may induce depletion of E6 and E7 genes, significantly decreasing the expression of E6/E7, inducing cell death and inhibiting cell lines growth [54, 55].
The efficacy and safety of E6/E7 disruption induced by TALEN and CRISPR/Cas9 technology in treating HPV Persistency and HPV-related Cervical Intraepithelial Neoplasia is under evaluation of a specific clinical study (NCT03057912).
NTLA-5001 is an investigational CRISPR/Cas9-engineered T-cell receptor (TCR)-T cell therapy in development for the treatment of all genetic subtypes of acute myeloid leukemia (AML) using a WT1-targeting TCR. This study is conducted to evaluate the safety, tolerability, cellular kinetics (CK), activity, and pharmacodynamics (PD) of NTLA-5001 in participants with AML (NCT05066165).
CRISPR/Cas9 gene-editing of CAR T-cells
Over the last 30 years, adoptive T-cell transfer has become the major form of cancer immunotherapy, used, predominantly, in hematological malignancies. With this approach, tumor-specific cytotoxic T-cells are infused into patients, upon lympho-depleting chemotherapy [56–58]. The key potential advantage of this treatment strategy is the ability to reach privileged niches where conventional anticancer therapeutics have struggled to penetrate [59]. CAR T-cells usually identify cell surface antigens present in the natural state on the surface of tumor cells without the necessity of peptide processing or HLA expression for recognition [60]. The two most diffused safety-related problems, due to CAR T-cell administration, have been partially overcome. The first, concerning the targeted destruction of normal cells, is resolved through the identification of tumor-specific cell surface molecules to be targeted. The second concern, regarding the possible induction of a cytokine storm associated with anti-tumor response mediated by large numbers of activated T-cells is strongly overcome utilizing suicide genes such as inducible caspase-9 to halt deleterious responses [61, 62]. The innovative principle of CAR T-cells is to couple the potency of a T-cell with the specificity of an antibody to selectively kill target cells. Modifications applied to subsequent generations of CAR T-cells achieved a very efficient product in which inhibitory domains were eliminated and co-stimulatory domains were introduced.
Engineering a patient’s own T-cells to selectively target and eliminate tumor cells has cured patients with untreatable hematological cancers [62, 63] and the manufacturing of CAR T-cells under GMP is a focal point for this therapeutic modality [64, 65]. The main challenges for CAR T-cell therapy concern solid tumors due to the difficulty to identify truly specific tumor antigens as targets, overcoming tumor antigen escape, improving CAR T-cells trafficking, infiltration and expansion at the tumor site as well as persistence and functions in a hostile tumor microenvironment (TME) [66]. Many clinical trials are on going testing CAR T-cells in brain tumors targeting several antigens such as Disialogangloside GD2, to test the promising data obtained at pre-clinical level [67–71].The same target resulted strongly valid in the NCT05573097 clinical trial against high-risk pediatric neuroblastoma showing a sustained anti-tumor effect [72].
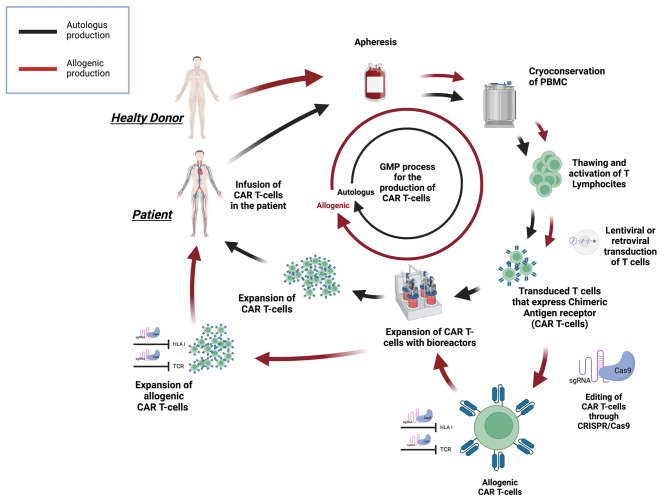
Preparation of clinical-grade CAR T-cells for therapy begins with leukopheresis to obtain large numbers of peripheral blood mononuclear cells followed by cryopreservation of these cells. After being thawed, at the manufacturing facility, the cells are activated by CD3/CD28 stimulation for ex-vivo expansion [73].
Then, genetic modification of T-cell is carried out through transduction with a self-inactivating lentiviral or retroviral vector encoding the transgene of interest. The transgenic T-cells are expanded through different platform (GE bioreactors, G-Rex bioreactors) until sufficient numbers for treatment are obtained, around 300 million cells. Transduction efficiency is measured by flow cytometry and percent of killing activity is evaluated against tumor cell lines expressing the target antigen [74](Fig. 3).
Fig. 3.
CRISPR/Cas9-mediated editing of CAR T-cell. Production of CAR T cell starting from patient for autologous infusion (black line) including apheresis from peripheral blood and cryo-conservation; thawing is followed by stimulation and lentiviral transduction for generation of CAR T-cells. The expansion is achieved by bioreactors and the requested number of CAR T-cells is ready for patient infusion. The process starting from healthy donor (red line) for allogenic CAR T cell production follows the same procedure until CAR transduction. CRISPR/Cas9 editing of HLA and TCR before CAR T-cells expansion generates universal CAR T-cells that can be infused into the patient
The use of virus in CAR T-cells production has showed some disadvantages including an increased risk of tumor development resulting from insertional mutagenesis [75].
Despite the success of CAR T-cells in treating hematological malignancies, challenges such as cytokine release syndrome (CRS) [76], T-cell exhaustion, tumor antigen masking [77, 78] and durability and risk of GVHD remain [79].
For this reason, recently, CRISPR/Cas9 technology has been integrated with CAR-T cell-based treatment to open novel opportunities for the production of more efficient CAR-T cells for all patients [80–82].
Non-viral gene-editing systems can be delivered to primary T cells using electroporation, liposome or nanoparticle transfection methods [83]. The best tool that meets the three crucial criteria which are lack of immunogenicity, compatibility with GMP grade reagents and feasibility on a clinical scale is electroporation [84]. A non-viral protocol to generate gene-specific integrated T-cells was developed in 2021 by Jiqin Zhang et al. An anti-CD19 CAR sequence containing 4-1BB and CD3z was constructed and electroporated into T cells. Through this procedure, cell expansion was not impaired and cell viability was high. In addition, electroporation increased the ratio of CD8+ to CD+4 T cells when compared to lentiviral transduction. Since blocking the PD1-PD-L1 axis has been demonstrate to improve CAR T-cells killing activity, the authors integrated an anti-CD19 sequence into the PD1 gene obtaining a robust clearance of tumor cells. Safety and efficacy assay, followed by GMP-procedures adaptation, was performed to carry out a phase I clinical trial (NCT04213469) in B-NHL patients and in relapsed/refractory B-cell malignancies (NCT04637763, CB010). Data obtained by the trial revealed that the development of non-viral gene-specific targeted CAR T-cells by CRISPR/Cas9 showed high efficiency against the tumor through a simplified manufacturing procedure with reduced preparation time and expenses.
Although autologous T-cells have shown promising results in many cases, there are many patients that cannot be treated in this way or for lymphocyte repertoire depletion due to myeloablative therapies, or for intrinsic defect of autologous T-cells. These limitations can be overcome by developing universal genetically engineered CAR T-cells derived from allogenic donor T-cells where TCR and HLA-I are silenced. CRISPR/Cas-9 can be used to knock-out β2M of donor CAR T-cells, a component that forms heterodimers with HLA-I and is requested for HLA-I surface expression, and to silence TCRα subunit constant (TRAC) or TCRβ gene (TCRB) to eliminate the recognition of alloantigen of the recipient. Although at a preclinical level, this study demonstrates that CRISPR/Cas9-mediated multiplex gene editing is applicable and a relay promising strategy [85]. Indeed, a recent phase I clinical trial (CARBON) shows how CTX-110, an anti CD19 CAR T-cell in which MHC I complex has been eliminated by CRISPR/Cas9 editing of TCRA and β2M administrated in patient with relapsed/refractory Diffuse Large B-cell Lymphoma (DLBCL) resulted highly efficient (NCT04035434).
Antigen-escape-mediated relapse is another limitation CAR T therapy and the use of multiantigen targeting could allow the optimization of the response. Yongxian Hu et al. proposed combined approach using universal CD19/CD22 dual targeting CAR T-cells in which TRAC and CD52 gene region is disrupted by using CRISPR/Cas9 technology. The phase I clinical trial (NCT04227015) in adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia showed a safety profile and prominent anti-leukemia activity, especially for patients that were ineligible for autologous CAR T-cells administration [86]. Recently gene editing supported also allogenic “off-the-shelf” CAR T-cells targeting B-Cell Maturation Antigen (BCMA) in multiple myeloma (CTX-120) using CRISPR/Cas9 system to eliminate TCR and MHC class I, coupled with specific insertion of the CAR at the TRAC locus [87]. Results from animal models showed complete tumor regression and phase I study is ongoing in patients with refractory or relapsed multiple myeloma (NCT04244656). A valid study was performed in clear renal cell carcinoma through the development of allogenic CRISPR/Cas9-engineered CAR T-cells. It was designed to insert an anti-CD70 CAR cassette into the TRAC locus to disrupt TRAC, b2M and CD70 CTX-130). The results from phase I trial (NCT04438083) showed safety and encouraging antitumor activity [88].
Conclusions
The latest advancements in GMP procedures are allowing an efficient improviement of personalized medicine. In particular immunotherapy is strongly taking advantage of clinical manufacturing platforms to cure patients who are refractory to previous therapy or who relapse upon a first period of remission. CRISPR/Cas9 technology has become the most widely used gene-editing tool in cancer immunotherapy favoring differentiation and persistence of genetically modified T-cells. The discovery of cancer-selected markers remains one of the principle obstacles while the use of allogenic CRISPR/Cas9-modified CAR T-cells is overcoming the difficulties to treat relapsing cancer cells showing an antigen different from that expressed at the onset of the disease. CAR T-cells generated against multiple tumor targets are preventing relapsing events. The manufacturing processes still comprised procedures performed manually even if supported by semi-automated manner, which result in product variability and very high cost. The development of a more controlled and cost-effective manufacturing process remains the pivotal aim to ensure CAR T-cells therapy for all patients.
Acknowledgements
Figures were created with Biorender (https://www.biorender.com/).
Abbreviations
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- Cas9
CRISPR Associated Protein 9
- CAR
Chimeric Antigen Receptor
- GMP
Good Manufacturing Practice
- ATMP
Advanced Therapy Medicinal Products
- gRNA
Guide RNA
- NT
Nucleotide
- crRNA
CRISPR RNA
- tracrRNA
Transacting crRNA
- sgRNA
Single Guide RNA
- PAM
Protospacer Adjacent Motif
- DSBs
Double-Strand Breaks
- NHEJ
Non-Homologous End Joining
- HDR
Homology-Directed Repair
- INDEL
Insertions or Deletions
- ZFNs
Zinc-Finger Nucleases
- TALENs
Transcription Activator Like Effector Nucleases
- PD-1
Program Cell Death 1
- TIL
Tumor Infiltrating Lymphocytes
- CISH
Cytokine-Inducible SH2 Domain-Containing Protein
- KO
Knock Out
- TCR
T-Cell Receptor
- GVHD
Graft Versus Host Disease
- HLA
Human Leukocyte Antigen
- β2M
Beta-2 Microglobulin
- CIITA
Class II Trans Activator
- DC
Dendritic Cell
- ACT
Adoptive Cellular Immunotherapy
- ADC
Antibody-Drug Conjugate
- CTLA4
Cytotoxic T-Lymphocyte Antigen 4
- PD-L1
Program Cell Death Ligand-1
- NSCLC
Non Small Cell Lung Cancer
- CXCR4
Chemokine Receptor Type 4
- TReg
T-Regulatory-Cell
- TME
Tumor Microenvironment
- MHC
Major Histocompatibility Complex
- EBV-CTL
Epstein-Barr Virus Cytotoxic Lymphocytes
- PAP-GM-CSF
Prostatic Acid Phosphatase-Granulocyte Macrophage-Colony Stimulating Factor
- TACE
Transcatheter Arterial Chemoembolization
- SOCS
Suppressor of Cytokine Signaling
- HPV
Human Papillomavirus
- CIN
Cervical Intraepithelial Neoplasia
- AML
Acute Myeloid Leukemia
- WT-1
Wilms Tumor-Suppressor Gene-1
- CK
Cellular kinetics
- PD
Pharmacodynamics
- CRS
Cytokine Release Syndrome
- B-NHL
B-Lymphoma Non Hodgking
- TRAC
TCRα Subunit Constant
- TCRB
T Cell Receptorβ
- DLBCL
Diffuse Large B-cell Lymphoma
- BCMA
b-Cell Maturation Antigen
Authors contributions
MC, SI and VF conceived and wrote the manuscript; CQ intellectually contributed to the manuscript composition; FL provided intellectual input and critically revised the manuscript.
Funding
This work was supported by the Italian Ministry of Health with “Current Research” funds.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Locatelli F and Folgiero V are co-last authors.
References
- 1.European commision. EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines. 2003. https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en.
- 2.European medicine agency. Good manufacturing practice. https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/compliance-research-and-development/good-manufacturing-practice.
- 3.Palmer DC, Webber BR, Patel Y, Johnson MJ, Kariya CM, Lahr WS, et al. Internal checkpoint regulates T cell neoantigen reactivity and susceptibility to PD1 blockade. Med. 2022 doi: 10.1016/j.medj.2022.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shy BR, Vykunta VS, Ha A, Talbot A, Roth TL, Nguyen DN, et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat Biotechnol. 2023 doi: 10.1038/s41587-022-01418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basar R, Daher M, Uprety N, Gokdemir E, Alsuliman A, Ensley E, et al. Large-scale GMP-compliant CRISPR-Cas9-mediated deletion of the glucocorticoid receptor in multivirus-specific T cells. Blood Adv. 2020 doi: 10.1182/bloodadvances.2020001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987 doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012 doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 8.Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020 doi: 10.1038/s41579-019-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided Hum Genome Eng via Cas9 Sci. 2013 doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath P, Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010 doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- 12.Jiang F, Doudna JA. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 2017 doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 13.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014 doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyer W, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010 doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaj T, Gersbach CA. Barbas CF3. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013 doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010 doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 17.Cyranoski D. CRISPR gene-editing tested in a person for the first time. Nature. 2016 doi: 10.1038/nature.2016.20988. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020 doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 19.Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017 doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin R, Graham C, Yallop D, Jozwik A, Mirci-Danicar OC, Lucchini G, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020 doi: 10.1016/S0140-6736(20)32334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balke-Want H, Keerthi V, Gkitsas N, Mancini AG, Kurgan GL, Fowler C, et al. Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing. Mol Cancer. 2023 doi: 10.1186/s12943-023-01799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015 doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turtle CJ, Hanafi L, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016 doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Hu Y, Xue J, Li J, Yi J, Bu J, et al. Advances in immunotherapy for triple-negative breast cancer. Mol Cancer. 2023 doi: 10.1186/s12943-023-01850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balkwill F, CharlKellie A, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005 doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005 doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019 doi: 10.1093/annonc/mdz288. [DOI] [PubMed] [Google Scholar]
- 29.Chism DD. Urothelial Carcinoma of the bladder and the rise of Immunotherapy. J Natl Compr Canc Netw. 2017 doi: 10.6004/jnccn.2017.7036. [DOI] [PubMed] [Google Scholar]
- 30.Stege H, Haist M, Nikfarjam U, Schultheis M, Heinz J, Pemler S, et al. The Status of Adjuvant and Neoadjuvant Melanoma Therapy, New Developments and Upcoming challenges. Target Oncol. 2021 doi: 10.1007/s11523-021-00840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with Anti-programmed Death-1/Programmed death Ligand 1 therapy. J Clin Oncol. 2017 doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018 doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Chen C, Guo Y, Hu S, Sun Z. Effect of CRISPR/Cas9-Edited PD-1/PD-L1 on Tumor Immunity and Immunotherapy. Front Immunol. 2022 doi: 10.3389/fimmu.2022.848327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013 doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su S, Hu B, Shao J, Shen B, Du J, Du Y, et al. CRISPR-Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients. Sci Rep. 2016 doi: 10.1038/srep20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Peng Y, Hublitz P, Zhang H, Dong T. Genetic abrogation of immune checkpoints in antigen-specific cytotoxic T-lymphocyte as a potential alternative to blockade immunotherapy. Sci Rep. 2018 doi: 10.1038/s41598-018-23803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su S, Zou Z, Chen F, Ding N, Du J, Shao J, et al. CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology. 2016 doi: 10.1080/2162402X.2016.1249558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Shi L, Zhang W, Han J, Zhang S, Fu Z, et al. CRISPR knock out of programmed cell death protein 1 enhances anti-tumor activity of cytotoxic T lymphocytes. Oncotarget. 2017 doi: 10.18632/oncotarget.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian X, Gu T, Patel S, Bode AM, Lee M, Dong Z. CRISPR/Cas9 - an evolving biological tool kit for cancer biology and oncology. NPJ Precis Oncol. 2019 doi: 10.1038/s41698-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsui CK, Barfield RM, Fischer CR, Morgens DW, Li A, Smith BAH, et al. CRISPR-Cas9 screens identify regulators of antibody-drug conjugate toxicity. Nat Chem Biol. 2019 doi: 10.1038/s41589-019-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ministro JH, Oliveira SS, Oliveira JG, Cardoso M, Aires-da-Silva F, Corte-Real S, et al. Synthetic antibody discovery against native antigens by CRISPR/Cas9-library generation and endoplasmic reticulum screening. Appl Microbiol Biotechnol. 2020 doi: 10.1007/s00253-020-10423-3. [DOI] [PubMed] [Google Scholar]
- 43.Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, et al. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. J Clin Invest. 2019 doi: 10.1172/JCI121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Li Z, Shen M, Wang Y, Wang L, Li J, et al. Programmable Unlocking Nano-Matryoshka-CRISPR precisely reverses immunosuppression to Unleash Cascade amplified adaptive Immune response. Adv Sci (Weinh) 2021 doi: 10.1002/advs.202100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019 doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018 doi: 10.1136/gutjnl-2017-314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelton W, Waindok AC, Pesch T, Pogson M, Ford K, Parola C, et al. Reprogramming MHC specificity by CRISPR-Cas9-assisted cassette exchange. Sci Rep. 2017 doi: 10.1038/srep45775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Yan J, Su S, Shao J, Zhao Y, Xu Q, Yang Y, Zou Z, Huang X. J. Wei. A phase I/II trial of CRISPR-Cas9-mediated PD-1 knockout Epstein-Barr Virus cytotoxic lymphocytes (EBV-CTLs) for advanced stage EBV associated malignancies. 2018;29:64 – 5.
- 49.Thara E, Dorff TB, Pinski JK, Quinn DI. Vaccine therapy with sipuleucel-T (Provenge) for prostate cancer. Maturitas. 2011 doi: 10.1016/j.maturitas.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Naser W, Maymand S, Dlugolenski D, Basheer F, Ward AC. The role of cytokine-inducible SH2 domain-containing protein (CISH) in the regulation of basal and cytokine-mediated myelopoiesis. Int J Mol Sci. 2023 doi: 10.3390/ijms241612757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv J, Qin L, Zhao R, Wu D, Wu Z, Zheng D, et al. Disruption of CISH promotes the antitumor activity of human T cells and decreases PD-1 expression levels. Mol Ther Oncolytics. 2022 doi: 10.1016/j.omto.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018 doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fakhr E, Modic Z, Cid-Arregui A. Recent developments in immunotherapy of cancers caused by human papillomaviruses. Immunology. 2021 doi: 10.1111/imm.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inturi R, Jemth P. CRISPR/Cas9-based inactivation of human papillomavirus oncogenes E6 or E7 induces senescence in cervical cancer cells. Virology. 2021 doi: 10.1016/j.virol.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Hu Z, Ding W, Zhu D, Yu L, Jiang X, Wang X, et al. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J Clin Invest. 2015 doi: 10.1172/JCI78206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang L, Huang Z, Mei H, Hu Y. Immunotherapy in hematologic malignancies: achievements, challenges and future prospects. Signal Transduct Target Ther. 2023 doi: 10.1038/s41392-023-01521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenton GA, Mitchell DA. Cellular Cancer Immunotherapy Development and Manufacturing in the clinic. Clin Cancer Res. 2023 doi: 10.1158/1078-0432.CCR-22-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asmamaw Dejenie T, Tiruneh G, Medhin M, Dessie Terefe G, Tadele Admasu F, Wale Tesega W, Chekol Abebe E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum Vaccin Immunother. 2022 doi: 10.1080/21645515.2022.2114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang HKC, Wang B, Tan HX, Sarwar MA, Baraka B, Shafiq T, et al. CAR T-Cell therapy for Cancer: latest updates and challenges, with a focus on B-Lymphoid malignancies and selected solid tumours. Cells. 2023 doi: 10.3390/cells12121586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Stasi A, Tey S, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011 doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guercio M, Manni S, Boffa I, Caruso S, Di Cecca S, Sinibaldi M, et al. Inclusion of the Inducible Caspase 9 suicide gene in CAR Construct increases Safety of CAR.CD19 T cell therapy in B-Cell malignancies. Front Immunol. 2021 doi: 10.3389/fimmu.2021.755639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker DJ, Arany Z, Baur JA, Epstein JA, June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. 2023 doi: 10.1038/s41586-023-06243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016 doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blache U, Popp G, Dunkel A, Koehl U, Fricke S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat Commun. 2022 doi: 10.1038/s41467-022-32866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan T, Zhu L, Chen J. Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp Hematol Oncol. 2023 doi: 10.1186/s40164-023-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med. 2018 doi: 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Billy E, Pellegrino M, Orlando D, Pericoli G, Ferretti R, Businaro P, et al. Dual IGF1R/IR inhibitors in combination with GD2-CAR T-cells display a potent anti-tumor activity in diffuse midline glioma H3K27M-mutant. Neuro Oncol. 2022 doi: 10.1093/neuonc/noab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Del Baldo G, Del Bufalo F, Pinacchio C, Carai A, Quintarelli C, De Angelis B, et al. The peculiar challenge of bringing CAR-T cells into the brain: perspectives in the clinical application to the treatment of pediatric central nervous system tumors. Front Immunol. 2023 doi: 10.3389/fimmu.2023.1142597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022 doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gargett T, Ebert LM, Truong NTH, Kollis PM, Sedivakova K, Yu W, et al. GD2-targeting CAR-T cells enhanced by transgenic IL-15 expression are an effective and clinically feasible therapy for glioblastoma. J Immunother Cancer. 2022 doi: 10.1136/jitc-2022-005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Bufalo F, De Angelis B, Caruana I, Del Baldo G, De Ioris MA, Serra A, et al. GD2-CART01 for relapsed or Refractory High-Risk Neuroblastoma. N Engl J Med. 2023 doi: 10.1056/NEJMoa2210859. [DOI] [PubMed] [Google Scholar]
- 73.Abou-El-Enein M, Elsallab M, Feldman SA, Fesnak AD, Heslop HE, Marks P, et al. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021 doi: 10.1158/2643-3230.BCD-21-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tyagarajan S, Spencer T, Smith J, Optimizing CAR-T. Cell Manufacturing processes during pivotal clinical trials. Mol Ther Methods Clin Dev. 2019 doi: 10.1016/j.omtm.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner DL, Koehl U, Chmielewski M, Scheid C, Stripecke R, Review Sustainable clinical development of CAR-T cells - switching from viral transduction towards CRISPR-Cas Gene Editing. Front Immunol. 2022 doi: 10.3389/fimmu.2022.865424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei Z, Xu J, Zhao C, Zhang M, Xu N, Kang L, et al. Prediction of severe CRS and determination of biomarkers in B cell-acute lymphoblastic leukemia treated with CAR-T cells. Front Immunol. 2023 doi: 10.3389/fimmu.2023.1273507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quintarelli C, Guercio M, Manni S, Boffa I, Sinibaldi M, Di Cecca S, et al. Strategy to prevent epitope masking in CAR.CD19 + B-cell leukemia blasts. J Immunother Cancer. 2021 doi: 10.1136/jitc-2020-001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Locatelli F, Shah B, Thomas T, Velasco K, Adedokun B, Aldoss I, et al. Incidence of CD19-negative relapse after CD19-targeted immunotherapy in R/R BCP acute lymphoblastic leukemia: a review. Leuk Lymphoma. 2023 doi: 10.1080/10428194.2023.2232496. [DOI] [PubMed] [Google Scholar]
- 79.Ghaffari S, Khalili N, Rezaei N. CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J Exp Clin Cancer Res. 2021 doi: 10.1186/s13046-021-02076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ning L, Xi J, Zi Y, Chen M, Zou Q, Zhou X, et al. Prospects and challenges of CRISPR/Cas9 gene-editing technology in cancer research. Clin Genet. 2023 doi: 10.1111/cge.14424. [DOI] [PubMed] [Google Scholar]
- 81.Dimitri A, Herbst F, Fraietta JA. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol Cancer. 2022 doi: 10.1186/s12943-022-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mueller KP, Piscopo NJ, Forsberg MH, Saraspe LA, Das A, Russell B, et al. Production and characterization of virus-free, CRISPR-CAR T cells capable of inducing solid tumor regression. J Immunother Cancer. 2022 doi: 10.1136/jitc-2021-004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li L, Hu S, Chen X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: challenges and opportunities. Biomaterials. 2018 doi: 10.1016/j.biomaterials.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balke-Want H, Keerthi V, Cadinanos-Garai A, Fowler C, Gkitsas N, Brown AK, et al. Non-viral chimeric antigen receptor (CAR) T cells going viral. Immunooncol Technol. 2023 doi: 10.1016/j.iotech.2023.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017 doi: 10.1038/cr.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-targeted CAR-T cell therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2021 doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 87.Henia Dar D, Henderson Z, Padalia MS, Ashley Porras D, Mu MS, Maeng Kyungah. PhD, Seshidhar Police, PhD, Demetrios Kalaitzidis, PhD, Jonathan Terrett, PhD, Jason Sagert, PhD. Preclinical Development of CTX120, an allogeneic CAR-T cell targeting Bcma. 2018;132.
- 88.Sumanta K, Pal MD, Tran B, Haanen MBBSFRACPJB, PhD3 MD, Hurwitz M, PhD4 MD, Sacher A, Argawal MDN, Tannir MDN, Elizabeth Budde MDL, Harrison MDS, PhD MBBS, Klobuch FRACPS, MD, Patel SS, Karsten V, Srour SA. PhD9, Kaitlyn Cohen, MS8, Ellen B. Gurary, PhD8, Henia Dar, PhD8, Anna Ma, MS8, Anjali Sharma, MD8, MD7. 558 CTX130 allogeneic CRISPR-Cas9–engineered chimeric antigen receptor (CAR) T cells in patients with advanced clear cell renal cell carcinoma: results from the phase 1 COBALT-RCC study. 2022.