Abstract
Recent studies have identified genes involved in high-altitude adaptation in Tibetans. Three of these genes (EPAS1, EGLN1 and PPARA) are associated with decreased haemoglobin levels compared with non-Tibetans living at altitude. Consistent with the phenotype, EGLN1 in Tibetans has a gain-of-function mutation that confers a higher affinity for oxygen, hence less sensitivity to hypoxia. Considering the demands imposed upon metabolism in meeting energy demands despite limitations on fuel oxidation, we hypothesized that other selected genes might alter metabolism to allow adaptation to altitude despite the desensitization of the upstream hypoxia sensing caused by the EGLN1 mutation that results in the failure to sense hypoxia. A shift in fuel preference to glucose oxidation and glycolysis at the expense of fatty acid oxidation would provide adaptation to decreased oxygen availability. Measurements of serum metabolites from Tibetans living at high altitude are consistent with this hypothesis; the EPAS1 haplotypeis significantly associated with increased lactate levels (suggesting increased anaerobic metabolism), and the PPARA haplotype and serum free fatty acids are positively related (suggesting decreased fat oxidation). These data suggest that the high-altitude adaptations may offer protection from diabetes at high altitude but increase the risk of diabetes at lower elevations and/or with adoption of a non-traditional diet. It should also be considered in future work in the field that because iron is a cofactor for EGLN1, there may be significant associations of phenotypes with the significant degrees of variation seen in tissue iron among human populations.
Introduction
The high-altitude environment is challenging in terms of weather, sustenance, ultraviolet irradiation and hypoxia, and over the hundreds of generations in which native populations have occupied such regions, they have evolved to meet these challenges (Ge et al. 2012; Simonson et al. 2012). Tibetan highlanders have been intensively studied in this regard. Recent genome-wide scans of positive selection in Tibetans have identified hypoxia-sensing and –regulated genes as candidates for high-altitude adaptation (Beall et al. 2010; Bigham et al. 2010; Simonson et al. 2010; Yi et al. 2010a,b; Peng et al. 2011; Wang et al. 2011; Xu et al. 2011; Wuren et al. 2014). Many of the selected genes, not surprisingly, encode proteins involved in hypoxia signalling. Members of the hypoxia-inducible factor(HIF) pathway help to orchestrate molecular responses during hypoxic stress that include increasing oxygen delivery to tissues (increasing haemoglobin concentrations and promoting vasculogenesis) and adapting metabolism to the decreased availability of oxygen (Semenza, 2009; Majmundar et al. 2010).
In the absence of adequate oxygen, energy production from oxidative metabolism may be diminished. Furthermore, if oxidative metabolism proceeds in hypoxia, reactive oxidative intermediates will accumulate in mitochondria. Both energy depletion and oxidative stress can result in cell death, so these competing demands need to be balanced and non-oxidative mechanisms for energy production need to be activated in hypoxia. Oxidation of fatty acids yields less ATP per molecule of oxygen consumed than oxidation of carbohydrates, suggesting that decreased fatty acid oxidation should be a particularly favourable adaptation to hypoxia (Holden et al. 1995). Several studies have demonstrated decreased reliance on fat metabolism and increased glucose utilization at high altitude, both in people chronically dwelling at high altitude and in those who have acclimatized (Brooks et al. 1991; Holden et al. 1995; Roberts et al. 1996a,b). Another metabolic change induced by hypoxia is a conversion from oxidative glucose metabolism to glycolysis in order to maintain energy production. This occurs through upregulation of glucose uptake and glycolysis and downregulation of mitochondrial glucose oxidation (Kim et al. 2006; Papandreou et al. 2006).
Recent work at the whole-organism level has revealed that HIF plays a major role in regulating metabolism, highlighting a strong relationship between HIF and metabolic demands in humans (Formenti et al. 2010). Two of the regions consistently identified as targets of high-altitude adaptation in the studies cited above contain the EPAS1 gene, which encodes the HIF-2α subunit, and EGLN1, which encodes proline hydroxylase 2 (PHD2). Hypoxia-inducible factor-2α is a transcription factor that, along with other members of the HIF family and their binding partners, initiate the transcriptional response to hypoxia. Proline hydroxylase 2 is one of the proline hydroxylases that, in the presence of adequate oxygen, iron and α-ketoglutarate, targets HIFs for degradation. When any of these substrates and cofactors is inadequate, HIF is not hydroxylated, escapes degradation and translocates to the nucleus. Recently, the Tibetan mutation in PHD2 has been identified (Lorenzo et al. 2014; Song et al. 2014). The mutation has two effects, increasing affinity of PHD2 for oxygen, which would tend to blunt hypoxia signalling (Lorenzoetal.2014), and decreasing affinity for the HSP90 co-chaperone p23, which would be predicted to augment hypoxia signalling (Song et al. 2014). How these effects are integrated into the overall physiological response of Tibetans to high altitude is not yet clear, although the increased oxygen affinity might explain a unique aspect of Tibetan adaptation, namely their failure to increase haemoglobin levels at high altitude (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010a,b).
In addition to the genes in the HIF pathway, other selection candidates have emerged that may have a more direct effect on metabolic adaptation to high altitude. One is in the genomic region containing PPARA, which encodes the nuclear peroxisome proliferator-activated receptor α (PPARα) that regulates fatty acid metabolism and is itself regulated by HIF. This region, like those of EPAS1 and EGLN1, is associated with lower haemoglobin levels in Tibetans (Simonson et al. 2010).
Other candidates with potentially direct metabolic consequences include PKLR, which encodes the liver form of pyruvate kinase, PTEN, a phosphoinoside phosphatase involved in growth and metabolic signalling, and ANGPTL4, which is transcriptionally regulated by both HIFs and PPARs and inhibits lipoprotein lipase (Simonson et al. 2010, 2012). In addition, regions including genes involved in iron and haem metabolism have been selected in some Tibetan regions, including HMOX2, which encodes haem oxygenase, and HFE, which encodes a protein involved in regulating iron absorption in the gut (Wuren et al. 2014). Modulation of tissue iron levels could affect the activity of PHD2, which senses iron as a cofactor in the hydroxylation of HIFs, but in addition could have independent effects on metabolism through multiple mechanisms (Huang et al. 2011; Gabrielsen et al. 2012; Simcox & McClain, 2013).
It is possible that inherited alterations in genes that regulate metabolism, such as PPARA and other downstream targets, may compensate for wide-ranging changes inherent to global alterations in the HIF pathway. The most obvious adaptive metabolic change induced by hypoxia is a conversion from oxidative glucose metabolism to glycolysis to maintain energy production, which has been well described and shown to depend largely on HIF signalling (Denko, 2008; Semenza, 2012). Certain adaptive changes, such as interrupting normal HIF-mediated hypoxic signalling to limit possibly maladaptive increases in erythrocyte mass (Prchal, 2010), may need to be balanced by other changes (e.g. rescuing downstream hypoxia-mediated regulatory changes that would otherwise have been abrogated by any global changes in hypoxia signalling). Alternatively, orchestrated changes in the hypoxia-sensing pathway may allow adaptation through various aspects of tissue-specific and cellular homeostasis, whereby haemoglobin level is a secondary outcome (Storz, 2010).
We have therefore begun to characterize the metabolic consequences of Tibetan adaptation to their extreme environment. The goal of these studies is not only to develop a better understanding of metabolic regulation in general, but also to anticipate possible consequences of changes in the diet or geographical distribution of Tibetans in the modern world. For example, if at low altitude or with higher calorie diets Tibetans were more or less susceptible to disorders such as diabetes or metabolic syndrome, understanding the basis of that could suggest potential new targets to treat those diseases. In addition to the Tibetans, we have also studied other populations with genetic alterations in hypoxia sensing in order to ascribe any observed changes to alterations in HIF versus other implicated pathways. Finally, it is important to emphasize that other high-altitude populations have probably developed different strategies to adapt to the multiple stressors of life in that environment, so the study, for example, of Andean and African populations may yield different insights into the various ways that modulation of these and other pathways impact human phenotypes.
Metabolic phenotypes associated with genetic selection for high-altitude adaptation in Tibetans
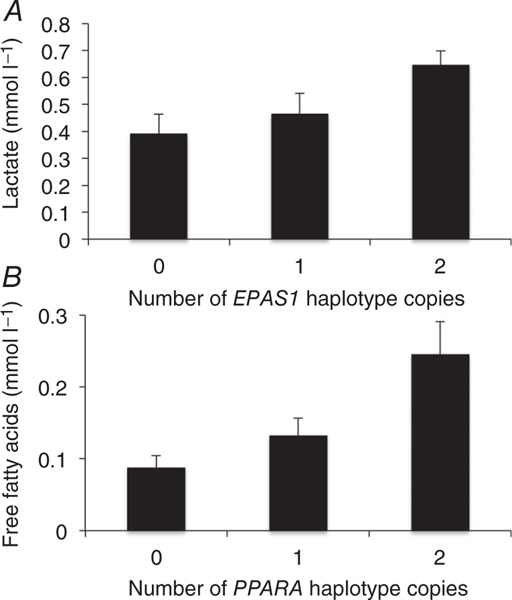
To determine whether the EPAS1, EGLN1 or PPARA regions in Tibetans were associated with their metabolic phenotype, we measured serum levels of triglycerides, free fatty acids, β-hydroxybutyrate and lactate in serum from 36 individuals. Owing to logistical challenges, we were not able to obtain fasted specimens. Spearman rank-order correlation analysis between the serum levels and the selected haplotypes (zero, one or two copies) revealed that lactate was positively associated with the adaptive EPAS1 haplotype (P < 0.003; Fig. 1A and Table 1). The PPARA haplotype was positively correlated with serum free fatty acids (P < 0.01; Fig. 1B and Table 1). The EGLN1 gene region was not associated with any of these rum metabolite levels measured, although there was a trend toward a relationship with lactate (P = 0.07; Table 1). It should be emphasized that these samples were not collected in the fasted state. Given that feeding and fasting are major determinants of the levels of these metabolites, caution in interpretation is warranted, although we assume, given the time of day of collection, that most individuals had eaten before blood sample was obtained and should therefore be comparable and at the least randomized across the different genotypes.
Figure 1. Association of previously identified adaptive haplotypes and metabolites.
A, lactate concentrations are plotted against the group of putatively advantageous haplotypes (zero, one or two) at the EPAS1 locus. B, serum free fatty acids (FFA) concentrations are plotted against the number of putatively advantageous PPARA haplotype copies (zero, one or two).
Table 1.
Haplotype–phenotype significance values for Spearman rank-order correlation analysis of metabolites measured in Tibetans (n = 36) living at 4500 m
| Metabolite |
EGLN1
|
EPAS1
|
PPARA
|
|||
|---|---|---|---|---|---|---|
| P Value | r | P Value | r | P Value | r | |
| Triglycerides | 0.150 | 0.245 | 0.307 | 0.175 | 0.973 | −0.006 |
| Free fatty acids | 0.505 | −0.115 | 0.860 | −0.031 | 0.014* | 0.406 |
| Three hydroxybutyrate | 0.230 | −0.205 | 0.590 | 0.093 | 0.182 | 0.227 |
| Lactate | 0.070 | 0.305 | 0.003* | 0.482 | 0.424 | 0.137 |
P < 0.05, Spearman rank-order correlation
The measurement of static levels of lactate and other metabolites does not allow conclusions to be drawn about production rates, but given the known function of the hypoxia-sensing pathway to increase glycolysis and lactate production, the results are consistent with the haplotypes affecting those pathways. Both EPAS1 and PPARA are involved in hypoxia signalling (Aragones et al. 2008, 2009). Humans acutely exposed to hypoxia consequently exhibit increased anaerobic glucose metabolism (Kelly et al. 2010). We observed that the adaptive EPAS1 haplotypes were associated with increased lactate levels, consistent with decreased glucose oxidation. Metabolic activity of HIF-2α has been shown to be required for the shift to anaerobic metabolism that facilitates adaptation to hypoxia in skeletal muscle of mice (Semenza, 2009; Majmundar et al. 2010). Additionally, individuals with Chuvash polycythaemia, an autosomal recessive disorder in which HIF degradation is impaired, exhibit higher lactate concentrations during exercise than do normal individuals (Formenti et al. 2010). Mice lacking EPAS1, however, also exhibit lactic acidosis (Scortegagna et al. 2003). Thus, inactivation of hypoxia sensing as well as activation may lead to increased lactate through multiple and probably complex mechanisms, implying that if the observed increases in lactate are related to changes in EPAS1, the adaptive Tibetan polymorphism could be associated with either increased or decreased HIF-2α activity.
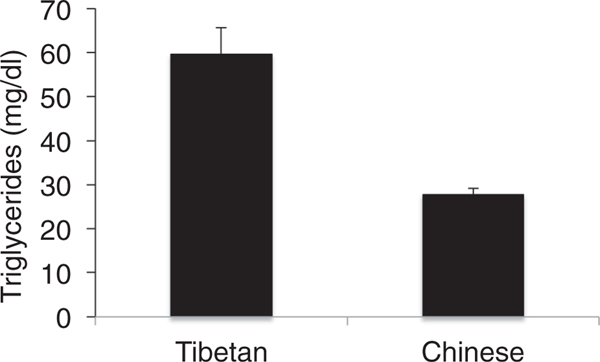
A previous report demonstrates a high prevalence of hypertriglyceridemia in Tibetan highlanders (Sherpa et al. 2011). We measured fasting triglyceride levels in a separate cohort of Tibetans and Han Chinese living in an urban environment near sea level, and confirmed this observation of significantly higher triglycerides in Tibetans compared with the Han, although their average levels in this cohort of younger individuals remain in the normal range (Fig. 2; P = 0.006). Although free fatty acids were associated with the PPARA haplotype (Fig. 1B), serum triglycerides were not. It should be pointed out, however, that the sera from the natives at high altitude were not collected in the fasting state, and serum triglycerides vary acutely with fasting, feeding and dietary composition. PPARA encodes the nuclear receptor protein PPARα, a major regulator of fatty acid oxidation (Narravula & Colgan, 2001; Piguet et al. 2010). Downregulation of several genes involved in fatty acid oxidation, including PPARA, has been observed in rats exposed to hypoxia (Kennedy et al. 2001). Activation of PPARα is associated with lower serum free fatty acids and triglycerides (Barbier et al. 2002). Thus, if the adaptive genotype were responsible for the increased triglyceride levels in Tibetans, it would be consistent with decreased expression or activity of PPARα. We did not, however, observe decreased β-hydroxybutyrate with the adaptive PPARA haplotype (Table 1), so there is no direct evidence of decreased fatty acid oxidation. Another explanation for increased lipids would be increased lipid synthesis. Fat anabolic pathways are upregulated in hypoxia, mediated at least in part by upregulation of PPARγ (Krishnan et al. 2009; Piguet et al. 2010) and sterol response element binding protein 1 (SREBP-1, Li et al. 2006), but we have no data from these studies specifically to implicate either increased synthesis or decreased degradation of fatty acids to the observed phenotype.
Figure 2.
Serum triglycerides measured in a cohort of fasted Tibetan and Han Chinese (n = 14 and 16, respectively; P < 0.01 by Student’s unpaired t test)
Recent studies suggest that some of the PPARα-dependent effects of hypoxia on fat metabolism may be mediated through HIF-2α (Aragones et al. 2008). Paradoxically, mice with either deletion of Epas1 or liver-specific overexpression of Epas1 exhibit hepatic steatosis, and both models show evidence of decreased fatty acid oxidation (Scortegagna et al. 2003; Rankin et al. 2009). Thus, the interrelationships among the status of the primary hypoxia signalling pathways, their downstream metabolic effectors and the final metabolic phenotype of the organism are highly complex. In addition, environmental factors are crucial in determining metabolic status. Thus, determining the specific roles of changes in EPAS1, EGLN1 and PPARA on the observed changes in metabolites will require further study.
Hypoxia and diabetes risk
Hypoxia-induced regulation of metabolism and its alteration in adapted populations may carry implications for the risks of diabetes and obesity. Recent studies point to inverse relationships between residence at altitude and the risks of both diabetes (Woolcott et al. 2014) and obesity (Voss et al. 2014). Furthermore, animal models have demonstrated important roles for HIF signalling in insulin secretion and sensitivity to insulin (Halberg et al. 2009; Regazzetti et al. 2009; Gamboa et al. 2011; Jiang et al. 2011; Bensellam et al. 2012). Other studies have shown that chronic hypoxia of high altitude is associated with decreased serum glucose and insulin concentrations in humans (Lindgarde et al. 2004; Baracco et al. 2007). A variety of mechanisms might explain this phenomenon, including enhanced cellular glucose uptake, glycolysis and glycogenesis related to increased HIF-1 expression and decreased hepatic gluconeogenesis related to increased HIF-2 expression (Hu et al. 2003; Dongiovanni et al. 2008; Rankin et al. 2009; Pescador et al. 2010). For these reasons, we have also examined glucose homeostasis in a human population with chronically activated hypoxia signalling (McClain et al. 2013).
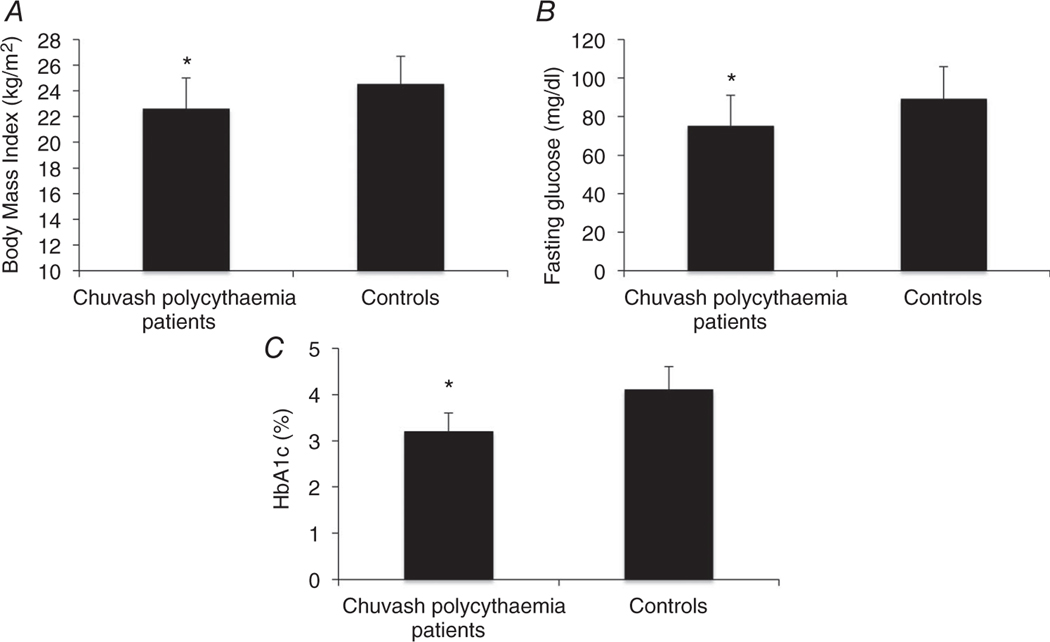
Chuvash polycythaemia is an autosomal recessive congenital disorder characterized by a homozygous 598C>T mutation in the VHL gene that results in an R200W amino acid change in the VHL protein (Ang et al. 2002). It is characterized by augmented HIF-1α and HIF-2α concentrations during normoxia and altered expression of erythropoietin, glucose transporter-1 and a number of other genes (Ang et al. 2002; Gordeuk et al. 2004; Hickey et al. 2007). We found that 88 Chuvash VHLR200W homozygotes had lower body mass indices, random glucose and glycosylated haemoglobin A1c levels than 52 Chuvash subjects with wild-type VHL alleles (Fig. 3). We expanded these observations in VHLR200W homozygote mice and found that they had lower fasting glucose values and lower glucose excursions than wild-type control mice but no change in fasting insulin concentrations (data not shown; McClain et al. 2013). Hepatic expression of Glut2 and G6pc but not Pdk2 was decreased, and skeletal muscle expression of Glut1, Pdk1 and Pdk4 was increased (data not shown; McClain et al. 2013). These results suggest that both decreased hepatic gluconeogenesis and increased skeletal uptake and glycolysis contribute to the decreased glucose concentrations. Further study is needed to determine whether pharmacological manipulation of HIF expression might be beneficial for treatment of diabetic patients.
Figure 3. Body mass index (A), fasting serum glucose (B) and glycosylated haemoglobin A1c concentrations (HbA1c; C) in 88 Chuvash VHLR200W homozygotes compared with 52 Chuvash subjects with wild-type VHL alleles.
Shown are the medians and interquartile ranges (P = 0.004 for body mass index, P = 0.0001 for glucose and P = 0.006 for HbA1c).
While altitude may offer protection from diabetes due to activation of HIF signalling, the situation for Tibetans may be more complex. If the adaptive genotype has relatively impaired hypoxia signalling, as is suggested by one analysis of the PHD2 mutation (Lorenzo et al. 2014), and if independent mutations have then rescued advantageous metabolic phenotypes that would otherwise be activated by HIF signalling, then one might predict that Tibetans are genetically locked into a high-altitude phenotype. For example, if the normal high-altitude phenotype of relatively decreased fatty acid oxidation were so determined, they would have that phenotype also at low altitude, and preliminary data (McClain, Ge, Prchal, unpublished observations) suggest that is the case.
Thus, the selected metabolic haplotypes may result in a relative in ability to shift between fat and glucose oxidation, so-called metabolic inflexibility (Storlienetal.2004).Such inflexibility and fatty acid oxidation capacities (Holland et al. 2007; Koves et al. 2008) are both implicated in the pathogenesis of type 2 diabetes mellitus.
Although Tibetan highlanders have a relatively low prevalence of diabetes (Matsubayashi et al. 2009), the diet is also relatively low calorie (Wang et al. 2010), and high altitudes are associated with lower body weights among Tibetans (Sherpaet al.2010). As populations move to lower altitudes and encounter a more industrialized lifestyle and higher calorie diets, however, the metabolic adaptations to altitude could have health implications. For example, increasing total fat and calorie consumption with a metabolic profile that will not support fat oxidation could result in accumulation of lipid intermediates thought to play a role in the pathogenesis of diabetes (Holland et al. 2007; Koves et al. 2008). Further study of the metabolic implications of high-altitude adaptation may allow interventions to ameliorate this risk and also identify potential new targets to treat obesity and diabetes.
Summary
Our results demonstrate increased lactate and free fatty acids in Tibetans with selected haplotypes. This pattern is consistent with the hypothesis that anaerobic glucose metabolism is increased and fatty acid oxidation may be decreased in the high-altitude adapted Tibetans compared with Tibetans without the adapted haplotypes living at the same altitude. The effects of these adaptations on diabetes risk, however, remain undefined. This is particularly true for Tibetans living at lower altitudes and Tibetans exposed to non-traditional diets and lifestyles. Controlled studies including more dynamic metabolic analyses and studies at different altitudes will be required to understand the physiological significance of these patterns better.
New Findings.
-
What is the topic of this review?
The topic of this review is how Tibetans have adapted genetically to high altitude, particularly with reference to altitude-induced changes in metabolism.
-
What advances does it highlight?
It highlights recent work on metabolic phenotyping in Tibetans and demonstrates that selected genetic haplotypes influence their metabolism of fats and glucose.
Acknowledgements
We would like to thank the high-altitude inhabitants for participating in this study. We also thank Drs Xue-Feng, Yong Mei, Zhaxi, Zhuruma, He Long and Dan Ba at the hospitals of Maduo and Tuo Tuo River for their co-operation and hospitality during data collection.
Funding
This work was supported in part by R01HL079912-04 (V.G.) from National Heart, Lung, and Blood Institute, by 1R01 DK081842 (D.A.M.) from National Institute of Diabetes and Digestive and Kidney Diseases, by UL1 RR025764 (D.A.M.) from National Center for the Advancement of Translational Science, by the Research Service of the Veterans Administration (D.A.M.), the National Basic Research Program of China (no. 2006CB504100, R.-L.G.), National Natural Science Foundation of China (no. 30393133, R.-L.G.) and the National Institutes of Health (NIH GM-59290).
Footnotes
Additional information
Competing interests
None declared.
References
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL & Prchal JT (2002). Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32, 614–621. [DOI] [PubMed] [Google Scholar]
- Aragones J, Fraisl P, Baes M & Carmeliet P (2009). Oxygen sensors at the crossroad of metabolism. Cell Metab 9, 11–22. [DOI] [PubMed] [Google Scholar]
- Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P & Carmeliet P (2008). Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40, 170–180. [DOI] [PubMed] [Google Scholar]
- Baracco R, Mohanna S & Seclen S (2007). A comparison of the prevalence of metabolic syndrome and its components in high and low altitude populations in Peru. Metab Syndr Relat Disord 5, 55–62. [DOI] [PubMed] [Google Scholar]
- Barbier O, Torra IP, Duguay Y, Blanquart C, Fruchart JC, Glineur C & Staels B (2002). Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol 22, 717–726. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P & Zheng YT (2010). Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107, 11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensellam M, Duvillié B, Rybachuk G, Laybutt DR, Magnan C, Guiot Y, Pouyssegur J & Jonas JC (2012). Glucose-induced O2 consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic beta-cells. PLoS One 7, e29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, López Herráez D, Brutsaert T, Parra EJ, Moore LG & Shriver MD (2010). Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6, e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE & Reeves JT (1991). Increased dependence on blood glucose after acclimatization to 4300 m. J Appl Physiol 70, 919–927. [DOI] [PubMed] [Google Scholar]
- Denko NC (2008). Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 8, 705–713. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G & Fargion S (2008). Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol 172, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti F, Constantin-Teodosiu D, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM, Lappin TR, McMullin MF, McNamara CJ, Mills W, Murphy JA, O’Connor DF, Percy MJ, Ratcliffe PJ, Smith TG, Treacy M, Frayn KN, Greenhaff PL, Karpe F, Clarke K & Robbins PA (2010). Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA 107, 12722–12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT & McClain DA (2012). Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest 122, 3529–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa JL, Garcia-Cazarin ML & Andrade FH (2011). Chronic hypoxia increases insulin-stimulated glucose uptake in mouse soleus muscle. Am J Physiol Regul Integr Comp Physiol 300, R85–R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge RL, Simonson TS, Cooksey RC, Tanna U, Qin G, Huff CD, Witherspoon DJ, Xing J, Zhengzhong B, Prchal JT, Jorde LB & McClain DA (2012). Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Mol Genet Metab 106, 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT & Polyakova LA (2004). Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood 103, 3924–3932. [DOI] [PubMed] [Google Scholar]
- Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA & Scherer PE (2009). Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29, 4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MM, Lam JC, Bezman NA, Rathmell WK & Simon MC (2007). von Hippel–Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2α signaling and splenic erythropoiesis. J Clin Invest 117, 3879–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Stone CK, Clark CM, Brown WD, Nickles RJ, Stanley C & Hochachka PW (1995). Enhanced cardiac metabolism of plasma glucose in high-altitude natives: adaptation against chronic hypoxia. J Appl Physiol 79, 222–228. [DOI] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ & Summers SA (2007). Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5, 167–179. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B & Simon MC (2003). Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol 23, 9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Jones D, Luo B, Sanderson M, Soto J, Abel ED, Cooksey RC & McClain DA (2011). Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 60, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM & Gonzalez FJ (2011). Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Williamson DL, Fealy CE, Kriz DA, Krishnan RK, Huang H, Ahn J, Loomis JL & Kirwan JP (2010). Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism 59, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SL, Stanley WC, Panchal AR & Mazzeo RS (2001). Alterations in enzymes involved in fat metabolism after acute and chronic altitude exposure. J Appl Physiol 90, 17–22. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL & Dang CV (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3, 177–185. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD & Muoio DM (2008). Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7, 45–56. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T & Krek W (2009). Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9, 512–524. [DOI] [PubMed] [Google Scholar]
- Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL & Polotsky VY (2006). Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1α. Physiol Genomics 25, 450–457. [DOI] [PubMed] [Google Scholar]
- Lindgarde F, Ercilla MB, Correa LR & Ahren B (2004). Body adiposity, insulin, and leptin in subgroups of Peruvian Amerindians. High Alt Med Biol 5, 27–31. [DOI] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S,Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P & Prchal JT (2014). A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46, 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain DA, Abuelgasim KA, Nouraie M, Salomon-Andonie J, Niu X, Miasnikova G, Polyakova LA, Sergueeva A, Okhotin DJ, Cherqaoui R, Okhotin D, Cox JE, Swierczek S, Song J, Simon MC, Huang J, Simcox JA, Yoon D, Prchal JT & Gordeuk VR (2013). Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism. J Mol Med (Berl) 91, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ & Simon MC (2010). Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40, 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi K, Kimura Y, Sakamoto R, Wada T, Ishimoto Y, Hirosaki M, Konno A, Chen W, Ishine M, Kosaka Y, Wada C, Nakatsuka M, Otsuka K, Fujisawa M, Wang H, Dai Q, Yang A, Gao J, Li Z, Qiao H, Zhang Y, Ge RL & Okumiya K (2009). Comprehensive geriatric assessment of elderly highlanders in Qinghai, China I: activities of daily living, quality of life and metabolic syndrome. Geriatr Gerontol Int 9, 333–341. [DOI] [PubMed] [Google Scholar]
- Narravula S & Colgan SP (2001). Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor α expression during hypoxia. J Immunol 166, 7543–7548. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL & Denko NC (2006). HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3, 187–197. [DOI] [PubMed] [Google Scholar]
- Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu Basang, Ciwangsangbu Danzengduojie, Chen H, Shi H & Su B (2011). Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 28, 1075–1081. [DOI] [PubMed] [Google Scholar]
- Pescador N, Villar D, Cifuentes D, Garcia-Rocha M, Ortiz-Barahona A, Vazquez S, Ordonez A, Cuevas Y, Saez-Morales D, Garcia-Bermejo ML, Landazuri MO, Guinovart J & del Peso L (2010). Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One 5, e9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet AC, Stroka D, Zimmermann A & Dufour JF (2010). Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 118, 401–410. [DOI] [PubMed] [Google Scholar]
- Prchal J, ed. (2010). Clinical Manifestations and Classification of Erythrocyte Disorders. McGraw-Hill, New York. [Google Scholar]
- Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q & Haase VH (2009). Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol 29, 4527–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzetti C, Peraldi P, Gremeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, Bost F, LeMarchand-Brustel Y, Tanti JF & Giorgetti-Peraldi S (2009). Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Butterfield GE, Cymerman A, Reeves JT, Wolfel EE & Brooks GA (1996a). Acclimatization to 4300-m altitude decreases reliance on fat as a substrate. J Appl Physiol 81, 1762–1771. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Reeves JT, Butterfield GE, Mazzeo RS, Sutton JR, Wolfel EE & Brooks GA (1996b). Altitude and beta-blockade augment glucose utilization during submaximal exercise. J Appl Physiol 80, 605–615. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ & Garcia JA (2003). Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35, 331–340. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2009). Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 114, 2015–2019. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2012). Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa LY, Deji, Stigum H, Chongsuvivatwong V, Luobu O, Thelle DS, Nafstad P & Bjertness E (2011). Lipid profile and its association with risk factors for coronary heart disease in the highlanders of Lhasa, Tibet. High Alt Med Biol 12, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa LY, Deji, Stigum H, Chongsuvivatwong V, Thelle DS& Bjertness E (2010). Obesity in Tibetans aged 30–70 living at different altitudes under the north and south faces of Mt. Everest. Int J Environ Res Public Health 7, 1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox JA & McClain DA (2013). Iron and diabetes risk. Cell Metab 17, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, McClain DA, Jorde LB & Prchal JT (2012). Genetic determinants of Tibetan high-altitude adaptation. Hum Genet 131, 527–533. [DOI] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT & Ge R (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75. [DOI] [PubMed] [Google Scholar]
- Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR & Lee FS (2014). Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem 289, 14656–14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlien L, Oakes ND & Kelley DE (2004). Metabolic flexibility. Proc Nutr Soc 63, 363–368. [DOI] [PubMed] [Google Scholar]
- Storz JF (2010). Evolution. Genes for high altitudes. Science 329, 40–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JD, Allison DB, Webber, Otto JL & Clark LL (2014). Lower obesity rate during residence at high altitude among a military population with frequent migration: a quasi experimental model for investigating spatial causation. PLoS One 9, e93493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang YB, Zhang F, Lin H, Wang X, Wan N, Ye Z, Weng H, Zhang L, Li X, Yan J, Wang P, Wu T, Cheng L, Wang J, Wang DM, Ma X & Yu J (2011). On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One 6, e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Dang S & Yan H (2010). Nutrient intakes of rural Tibetan mothers: a cross-sectional survey. BMC Public Health 10, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D & Bergman RN (2014). Inverse association between diabetes and altitude: a cross-sectional study in the adult population of the United States. Obesity (Silver Spring) 22, 2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuren T, Simonson TS, Qin G, Xing J, Huff CD, Witherspoon DJ, Jorde LB & Ge RL (2014). Shared and unique signals of high-altitude adaptation in geographically distinct Tibetan populations. PLoS One 9, e88252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H & Jin L (2011). A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol 28, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J & Wang J (2010a). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Jun W, XingBo S, XiaoJun L, Liu D & BinWu Y (2010b). Genetic analysis of 17 Y-chromosomal STRs haplotypes of Chinese Tibetan ethnic minority group. Leg Med (Tokyo) 12, 108–111. [DOI] [PubMed] [Google Scholar]