Abstract
Background:
Posttraumatic osteoarthritis (OA) is a common disorder associated with a high socioeconomic burden, particularly in young, physically active, and working patients. Tranexamic acid (TXA) is commonly used in orthopaedic trauma surgery as an antifibrinolytic agent to control excessive bleeding. Previous studies have reported that TXA modulates inflammation and bone cell function, both of which are dysregulated during posttraumatic OA disease progression.
Purpose:
To evaluate the therapeutic effects of systemic and topical TXA treatment on the progression of posttraumatic OA in the knee of mice.
Study Design:
Controlled laboratory study.
Methods:
OA was induced via anterior cruciate ligament (ACL) transection on the right knee of female mice. Mice were treated with TXA or vehicle intraperitoneally daily or intra-articularly weekly for 4 weeks, starting on the day of surgery. Articular cartilage degeneration, synovitis, bone erosion, and osteophyte formation were scored histologically. Micro−computed tomography evaluation was conducted to measure the subchondral bone microstructure and osteophyte volume. Cartilage thickness and bone remodeling were assessed histomorphometrically.
Results:
Both systemic and topical TXA treatment significantly reduced cartilage degeneration, synovitis, and bone erosion scores and increased the ratio of hyaline to calcified cartilage thickness in posttraumatic OA. Systemic TXA reversed ACL transection-induced subchondral bone loss and osteophyte formation, whereas topical treatment had no effect. Systemic TXA decreased the number and surface area of osteoclasts, whereas those of osteoblasts were not affected. No effect of topical TXA on osteoblast or osteoclast parameters was observed.
Conclusion:
Both systemic and topical TXA exerted protective effects on the progression of posttraumatic OA. Drug repurposing of TXA may, therefore, be useful for the prevention or treatment of posttraumatic OA, particularly after ACL surgery.
Clinical Relevance:
TXA might be beneficial in patients with posttraumatic OA of the knee.
Keywords: bone remodeling, cartilage, osteoarthritis, osteophytes, posttraumatic synovitis, tranexamic acid
Tranexamic acid (TXA) is a synthetic analog of the amino acid lysine and represents the most used antifibrinolytic agent to control excessive bleeding. It is applied in various clinical scenarios—including polytrauma and major surgery—to reduce blood loss and transfusion requirements. TXA is listed among the most essential medications by the World Health Organization 35 based on its clinical efficacy, safety, and low cost. TXA functions as a synthetic lysine analog that reversibly forms a complex with plasminogen, preventing it from binding to fibrin and, therefore, inhibiting fibrinolysis. 49 Based on its function as a plasminogen inhibitor, TXA has been postulated to be involved in various processes—including immunomodulation.21,60 Our previous study 5 further showed that TXA stimulation reduced the expression of inflammatory cytokines in murine macrophages in vitro. We also found that TXA treatment increased the proliferation and osteogenic differentiation of bone marrow−derived osteoblasts while it inhibited osteoclastogenesis. 5 Together, these findings suggest that TXA can potentially treat musculoskeletal diseases in which inflammatory responses and bone metabolism are disturbed.
In this regard, osteoarthritis (OA) represents one of the most prevalent chronic diseases, affecting 250 million people worldwide. 27 It is characterized by progressive cartilage degeneration, pathologic subchondral bone remodeling, synovitis, and osteophyte formation. 31 OA most often occurs in the knee joint and is associated with severe pain, disability, and poor quality of life. 45 Despite the high individual and socioeconomic burden, there is no cure for OA. 1 Clinical treatment for OA is primarily limited to pain management with anti-inflammatory and analgesic drugs and, eventually, arthroplasty. 15 Among sex, age, obesity, and metabolic diseases, a major risk factor for OA is previous joint injury. Anterior cruciate ligament (ACL) injury is one of the major causes of OA, as approximately 50% of patients who have experienced an ACL injury develop OA within 5 to 15 years after the initial injury. 13 ACL reconstruction (ACLR) is recommended in young and physically active patients to restore normal joint biomechanics with overall favorable outcomes. However, ACLR does not reduce the risk of developing posttraumatic OA. 37 Based on its manifold biological functions, TXA was proposed as a candidate drug that could attenuate key processes in the progression of OA. An increasing number of clinical trials using TXA in ACLR have thus been conducted. However, most of them only focused on its antifibrinolytic effects on short-term outcomes—including hemarthrosis and drain output.2,29 Currently, a randomized controlled trial (NCT03552705) is investigating the effect of systemic TXA treatment during ACLR surgery on acute joint inflammation and long-term cartilage health in patients with ACL ruptures.
In this study, we preclinically investigated the potential therapeutic effects of systemic and topical TXA on the progression of OA after transction of the ACL in mice.
Methods
Materials
TXA was supplied by Carinopharm GmbH. Clindamycin was provided by Hikma Pharma GmbH. Buprenorphine (Buprenovet) was provided by Richter Pharma AG. Metamizole (Novaminsulfon-ratiopharm) was supplied by Ratiopharm.
Mice and OA Induction
Animal studies are reported in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. 48 All animal experiments were approved by the ethics committee of the University Medical Center Hamburg-Eppendorf and by the Behörde für Justiz und Verbraucherschutz der Freien und Hansestadt Hamburg Lebensmittelsicherheit und Veterinärwesen and were performed adherent to the policies and principles established by the Animal Welfare Act (Federal Law Gazett I; p.1094) and the National Institutes of Health guide for care and use of laboratory animals.
The sample size in this study was calculated based on our laboratory's previous work according to the method provided at http://www.lasec.cuhk.edu.hk/sample-size-calculation.html, with a statistical power of 80% and a significance level of P < .05. Using the mean and standard deviation of bone volume fraction in tibial subchondral bone, we calculated the sample size as n ≥ 5 in each group. However, as processing the knee samples for histologic analyses in the correct plane is challenging and occasionally results in sample destruction, we increased the group size to n ≥ 6 per group to account for the potential loss of samples.
All mice were housed in a specific pathogen-free animal facility, maintained in a standard condition at a 12-hour light/dark cycle, and fed ad libitum. The ACL transection (ACLT) surgery was performed on the right knee joint of all the mice to induce OA as described previously. 25 Briefly, mice were anesthetized by inhaling isoflurane (4% isoflurane for induction, 1%-2% isoflurane for maintenance). The mice preoperatively received 150 mg·kg-1 clindamycin for prevention of infection and 0.1 mg·kg-1 buprenorphine for analgesia. The right knee joint was exposed through a medial parapatellar approach using a longitudinal skin incision. The ACL was transected using a microsurgical knife, and the complete transection was confirmed using a positive anterior drawer test. All the mice had healthy contralateral knees without surgical intervention. For postoperative care, mice were placed in an incubator overnight to recover and given drinking water substituted with 1 mg·ml-1 metamizole for 3 days.
Study Design
To ensure maximum comparability between groups, we designed the study with all groups being operated on the same day and treated with vehicle or TXA in parallel thereafter. Wildtype (C57BL/6J) mouse matings, initiated in parallel and aimed to yield ≥24 age-matched female mice, produced 25 mice. These 25 mice (age, 12-14 weeks; weight, 20-25 g) were randomized into 1 of the 4 groups: systemic vehicle (n = 6), systemic TXA (n = 7), topical vehicle (n = 6), and topical TXA (n = 6), resulting in slightly unbalanced group sizes. Systemic TXA was administered at 100 mg·kg-1 daily by intraperitoneal injections for 4 weeks, starting on the day of surgery. Control mice received vehicle only (0.9% saline). Topical treatment was administered weekly via intra-articular injections (5 μL of 20 mg·mL-1 TXA or vehicle) into the operated knees, starting on the day of surgery. Even though there are some concerns regarding the toxicity of TXA with high local levels against chondrocytes, tenocytes, synoviocytes, and osteoblasts,9,30 concentrations <20 mg·mL are expected to be safe.9,47 Therefore, the systemic and topical doses of TXA were within the safe concentration range in this study. At 4 weeks after the operation, mice were sacrificed under deep anesthesia using isoflurane, and the operated and the contralateral healthy knee joints were harvested for further analysis. The experimental strategy is presented in Appendix Figure A1 (available in the online version of this article).
Micro−Computed Tomography Analysis
Bilateral mouse knee joints were fixed in 10% formalin for 24 hours. Thereafter, micro−computed tomography (μCT) scanning was performed using a Scanco vivaCT 80 (Scanco Medical AG) at 70 kVp, 113 μA, and a 400-ms integration time with a voxel size of 15.6 μm. The representative 3-dimensional images were reconstructed using the µCT Ray V4.0-4 (Scanco Medical AG), and structural parameters were calculated using the μCT Evaluation Program Version 6.6 (Scanco Medical AG). The osteophyte volume was measured, and the tibial subchondral bone was evaluated. The assessed trabecular parameters included the bone volume (mm3), total volume (mm3), bone volume fraction (%), trabecular number (mm-1), trabecular thickness (mm), and trabecular separation (mm). 10
Histological Analysis
The knee joint samples were decalcified in 0.5 M ethylenediaminetetraacetic acid solution (pH 7.4) for 4 days on a roller at 4°C. The decalcified knees were dehydrated and embedded in paraffin. A total of 30 serial coronal sections (4 μm) were cut from the posterior to the anterior plane, and every third section was collected and stained with hematoxylin and eosin (H&E), tartrate-resistant acid phosphatase (TRAP), and Bone-Inflammation-Cartilage (BIC) stain 6 as previously described. Three of the collected sections were chosen from the anterior, central, and posterior levels, and the highest score obtained at 1 of the 3 levels was used for the analysis described previously. 43 The quantitative histomorphometry analysis for osteoblasts and osteoclasts in tibial subchondral bone was performed using an OsteoMeasure system (Osteometrics Inc) connected to a BX50 microscope equipped with a DP72 camera (Olympus Optical Co, Ltd). Osteoblasts were identified in H&E-stained sections, and osteoclasts were visualized using TRAP staining. Measurements of the hyaline cartilage, calcified cartilage, and subchondral bone plate parameters were taken in H&E-stained sections within a 400 μm × 300 μm area, which was centered on the medial tibial plateau 44 (Appendix Figure A2, available online). The assessed histomorphometry parameters included osteoblast surface per bone surface (%), number of osteoblasts per bone perimeter (mm-1), osteoclast surface per bone surface (%), and number of osteoclasts per bone perimeter (mm-1). Cartilage and subchondral parameters included the ratio of hyaline cartilage thickness to calcified cartilage thickness, 42 percentage of hyaline cartilage area per total cartilage area (hyaline cartilage area/total cartilage area; %), and subchondral bone plate thickness (μm).
The semiquantitative histopathological scoring was performed using the BIC-stained sections on all 4 joint quadrants, including the medial tibial plateau, medial femoral condyle, lateral tibial plateau, and lateral femoral condyle. As previously described, the Osteoarthritis Research Society International (OARSI) scoring system 26 was used to grade cartilage degeneration (range, 0-6). The synovitis score 33 was used to evaluate the synovial lining thickness and cellular density in the synovial stroma (range, 0-6). The cortical bone erosion caused by the pannus was scored using the bone erosion score 28 (range, 0-3). Furthermore, the osteophyte score 36 was used to evaluate osteophyte formation—including osteophyte size and maturity (range, 0-6). A detailed description of the scoring approaches is shown in Appendix Table A1 (available online).
Data and Statistical Analysis
The researchers were blinded during treatment sample processing and data analyses. Data analysis and figure plotting were performed using GraphPad Prism Version 9.1.1 (GraphPad Software Inc). Individual data points, mean, and standard deviation are plotted in each graph. Inconsistent sample sizes ranging between 5 and 7 for several parameters were explained by the slightly unbalanced group sizes and loss or destruction of samples during processing. For multiple-group comparisons, data were analyzed via 1-way or 2-way analysis of variance test as indicated, followed by the Tukey post hoc tests. The significance level was set at P < .05.
Results
Both Systemic and Topical TXA Treatment Ameliorate ACLT-Induced Cartilage Degeneration
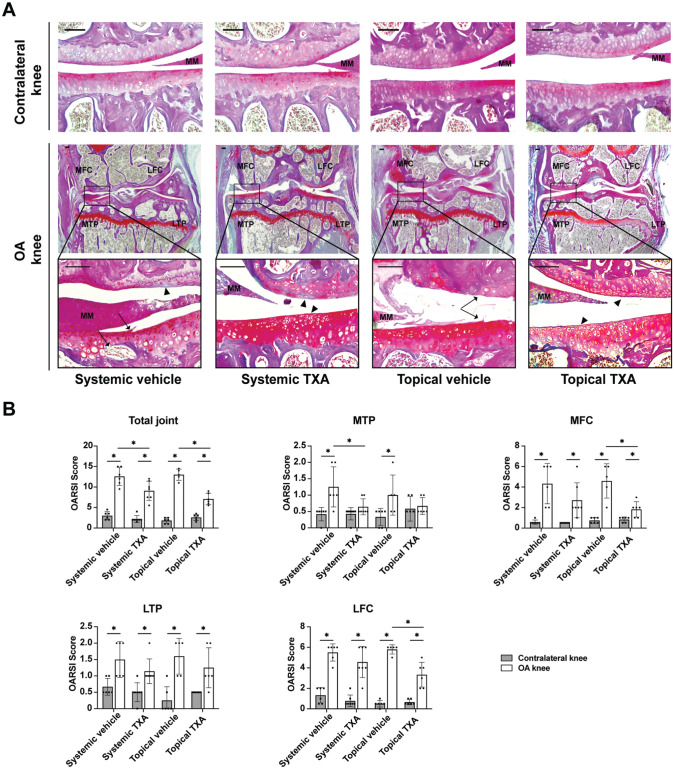
As articular cartilage deterioration is a major consequence of OA, we first investigated whether TXA protected cartilage from degeneration in OA knees. At 4 weeks after surgery, the operated knees from vehicle-treated mice had disrupted integrity and loss of proteoglycans in articular cartilage, which was attenuated by both systemic and topical TXA treatment (Figure 1A). Semiquantitative analysis of cartilage degradation showed that the total OARSI scores of OA knees were significantly decreased in the systemic TXA group (P = .019) and the topical TXA group (P < .001) when compared with the corresponding vehicle groups (Figure 1B) (Appendix Table A1, available online). The increase in OARSI scores of the medial tibial plateau was reversed by systemic and topical TXA treatment (Figure 1B). In addition, the OA knees in the topical TXA group showed significantly lower scores in the medial and lateral femoral compartments than those in the topical vehicle group (P = .005 and P = .002, respectively). No difference in the scores for the medial femoral condyle, lateral tibial plateau, and lateral femoral condyle was observed between the systemic vehicle and TXA groups (P = .140, P = .193, and P = .209, respectively).
Figure 1.
Systemic and topical TXA treatment attenuate articular cartilage degradation after ACLT. (A) Bone-Inflammation-Cartilage staining sections in the medial compartments of OA and contralateral knees at 4 weeks after ACLT (scale bar = 100 μm). Arrowheads indicate areas with loss of Safranin O staining. Arrows indicate clefts or erosion in the articular cartilage and irregular surface. (B) The OARSI scoring of the total joint, MTP, MFC, LTP, and LFC. The data are expressed as means ± SD; n = 5-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). *P < .05 for comparisons denoted by bar. ACLT, anterior cruciate ligament transection; LFC, lateral femoral condyle; LTP, lateral tibial plateau; MFC, medial femoral condyle; MM, medial meniscus; MTP, medial tibial plateau; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International; TXA, tranexamic acid.
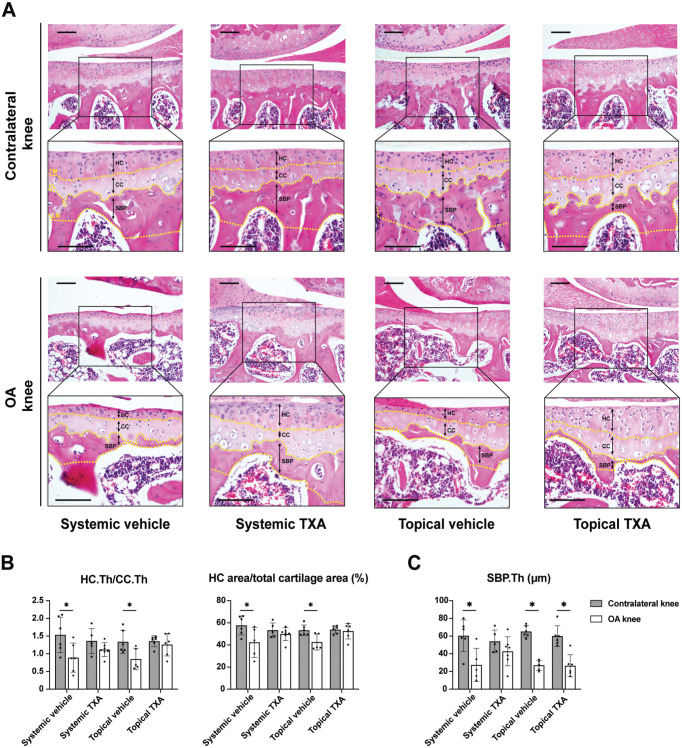
Next, we performed histomorphometric analysis in the medial tibial plateau to further evaluate the changes in the chondro-osseous junctional region, 38 including calcified and noncalcified cartilage layers and subchondral bone plate. The tidemark is a metabolically active front of calcification, separating hyaline and calcified cartilage. 34 With OA progression, the tidemark duplication results in the thickening of the calcified cartilage and the thinning of the hyaline cartilage. As indicated by H&E staining (Figure 2A), the hyaline cartilage/calcified cartilage thickness ratio and the hyaline cartilage/total cartilage area ratio were significantly decreased in the OA knees from both the systemic (P = .035 and P = .048, respectively) and the topical (P = .032 and P = .017, respectively) vehicle groups at 4 weeks after surgery, which were both reversed by systemic and topical TXA (Figure 2B). To assess the microarchitecture of the subchondral bone plate, H&E staining was employed. The subchondral bone plate is a thin cortical lamella between the calcified cartilage and subchondral trabecular bone. In agreement with previous studies,17,22 we found that the subchondral bone plate thickness underwent a remarkable decrease in OA knees at 4 weeks after ACLT in both vehicle groups (P = .016 and P < .001, respectively) (Figure 2C). The reduction was prevented by systemic TXA but not by topical treatment.
Figure 2.
Systemic and topical TXA treatment preserve hyaline cartilage after ACLT. (A) The representative histological images of the medial tibial cartilage stained with hematoxylin and eosin 4 weeks after ACLT (scale bar = 100 μm). A box (400 × 300 μm) was centered on the medial tibial plateau. The TM, CL, and LM of the SBP are marked by yellow dotted lines. The HC, CC, and SBP are labeled with black double-headed arrows. (B) Quantitative analysis of the ratio of HC thickness to CC thickness (HC.Th/CC.Th) and percentage of the HC area out of the total cartilage area. (C) Quantitative analysis of the SBP thickness (SBP.Th). All data are expressed as mean ± SD; n = 5-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). *P < .05 for comparisons denoted by bar. ACLT, anterior cruciate ligament transection; CC, calcified cartilage; CL, cement line; HC, hyaline cartilage; LM, lower margin; OA, osteoarthritis; SBP, subchondral bone plate; TM, tidemark; TXA, tranexamic acid.
Systemic TXA Treatment Reverses ACLT-Induced Subchondral Bone Loss, Whereas Topical Treatment Does Not
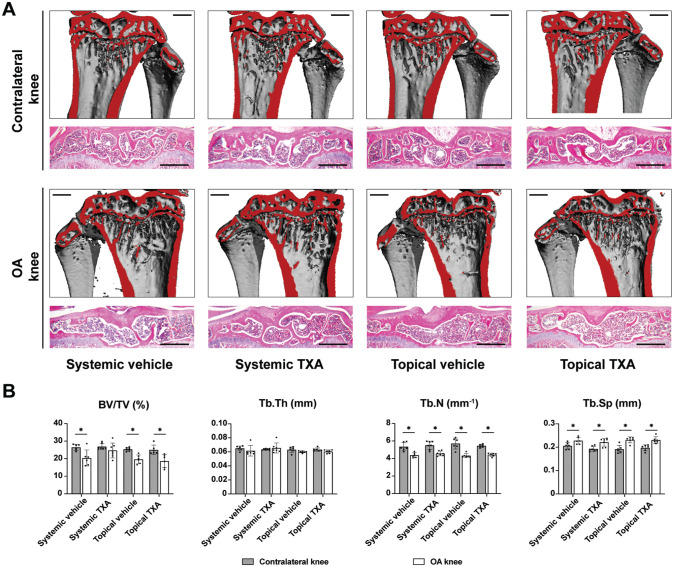
To monitor the effects of TXA on the microarchitecture of the tibial subchondral trabecular bone, μCT and H&E staining were employed (Figure 3A). In both systemic and topical vehicle groups, the bone volume per total volume of subchondral trabecular bone was significantly lower in OA knees at 4 weeks after ACLT compared with contralateral knees (P = .012 and P < .001, respectively) (Figure 3B). Likewise, trabecular numbers decreased (P = .002 and P < .001, respectively), and trabecular separation increased in the OA knees of the vehicle groups (P = .025 and P < .001, respectively). The subchondral bone loss was prevented by systemic TXA treatment but not by topical treatment. Neither systemic nor topical TXA significantly altered trabecular numbers or separation.
Figure 3.
Systemic TXA treatment protects against subchondral bone loss after ACLT. (A) The representative 3-dimensional μCT reconstruction and hematoxylin and eosin staining images of the coronal views of tibial subchondral bone 4 weeks after ACLT (scale bar = 500 μm). Red indicates cut plane. (B) μCT quantitative analysis of tibial subchondral bone for the BV/TV, Tb.N, Tb.Th, and Tb.Sp. The data are expressed as mean ± SD; n = 6-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). *P < .05 for comparisons denoted by bar. μCT, micro−computed tomography; ACLT, anterior cruciate ligament transection; BV/TV, bone volume fraction; OA, osteoarthritis; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness; TXA, tranexamic acid.
Systemic TXA Treatment Inhibits ACLT-Induced Abnormal Bone Remodeling in Subchondral Bone, Whereas Topical Treatment Does Not
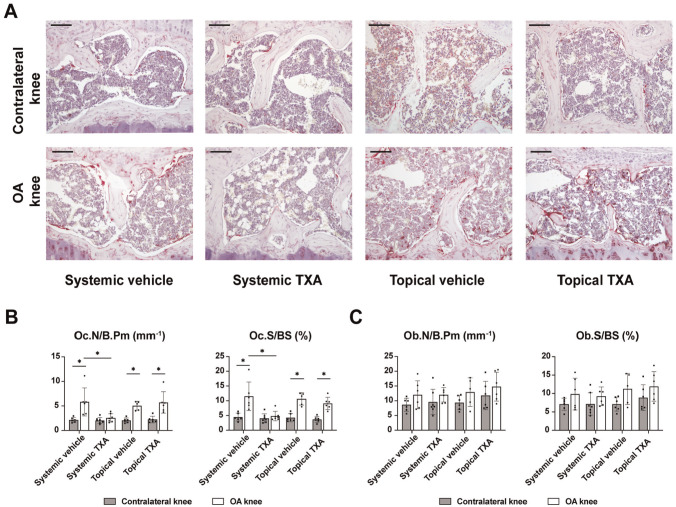
Abnormalities of cellular bone remodeling disrupt the microstructure of subchondral bone, including subchondral bone plate and underlying trabeculae in OA. Thus, osteoclast and osteoblast parameters were assessed using bone histomorphometry to account for the changes in subchondral bone. Previous studies showed elevated bone resorption in mice17,22 and patients 7 with early or progressive OA. Using TRAP-activity staining (Figure 4A), we found that the osteoclast surface and numbers in subchondral bone showed a significant increase in OA knees compared with contralateral knees in the systemic (P = .006 and P = .011, respectively) and topical vehicle (P < .001 and P < .001, respectively) groups (Figure 4B). While topical TXA had no effect, systemic TXA resulted in normalizing excessive osteoclast parameters in OA. Assessing bone formation parameters, we found osteoblast surface and numbers unaltered in OA of both vehicle groups (P = .178 and P = .059, respectively). Likewise, neither topical nor systemic TXA was associated with a change in osteoblast parameters (Figure 4C).
Figure 4.
Systemic TXA treatment inhibits ACLT-induced bone resorption in subchondral bone. (A) Representative images of tibial subchondral bone 4 weeks after ACLT, in which osteoclasts were stained by TRAP. Scale bar = 100 μm. (B) Quantitative analysis of Oc.S/BS and the number of Oc.N/B.Pm. (C) Quantitative analysis of the Ob.S/BS and the number of Ob.N/B.Pm. The data are expressed as mean ± SD; n = 5-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). *P < .05 for comparisons denoted by bar. ACLT, anterior cruciate ligament transection; OA, osteoarthritis; Ob.N/B.Pm, number of osteoblasts per bone perimeter; Ob.S/BS, osteoblast surface per bone surface; Oc.N/B.Pm, number of osteoclasts per bone perimeter; Oc.S/BS, osteoclast surface per bone surface; TRAP, tartrate-resistant acid phosphatase; TXA, tranexamic acid.
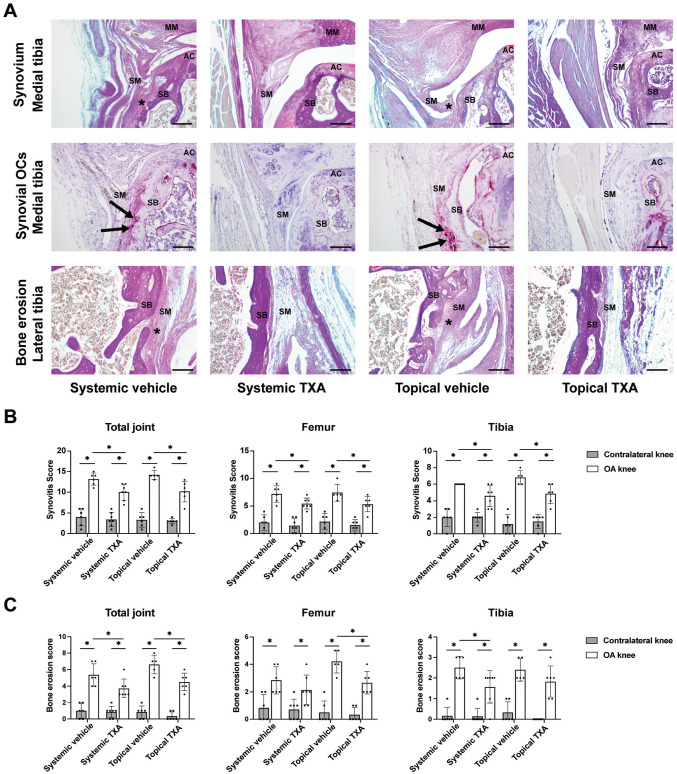
Both Systemic and Topical TXA Treatment Alleviate Synovitis in ACLT Models
We investigated the influence of TXA on synovial inflammation, a hallmark manifestation and promoting factor of OA. BIC staining showed that the synovial tissues in OA knees from vehicle groups displayed noticeable pathological changes such as synovial hyperplasia, pannus-like tissue formation, and bone erosion (Figure 5A) (Appendix Figure A3, available online). Moreover, in OA knees from vehicle groups, we also observed TRAP-positive osteoclasts in the hypertrophic synovium, which contacted with eroded cortical bone. However, in both systemic and topical TXA groups, the pathological changes of synovial tissues in OA knees were alleviated, and fewer synovial osteoclasts could be observed. Next, the synovial hyperplasia and bone erosion were quantified by synovitis and bone erosion scores, respectively (Appendix Table A1, available online). First, the total synovitis scores of OA knees were significantly decreased in systemic and topical TXA groups (P = .007 and P = .009, respectively) compared with the corresponding vehicle groups (Figure 5B). Similar results were found when analyzing the femoral and tibial synovitis scores separately. Second, both systemic and topical TXA treatment significantly decreased the total bone erosion scores (P = .038 and P = .011, respectively) compared with vehicle treatment (Figure 5C). The OA knees in the topical TXA group showed significantly lower scores in the femoral compartment than in the topical vehicle group (P = .013). In contrast, systemic TXA treatment significantly reduced the bone erosion score in the tibial compartment compared with systemic vehicle treatment (P = .034).
Figure 5.
Systemic and topical TXA treatment alleviate synovitis after ACLT. (A) Bone-Inflammation-Cartilage and TRAP staining of the tibial knee joint for synovium, bone erosion, and synovial osteoclasts (scale bar = 100 μm). Asterisks indicate synovial hyperplasia and pannus formation with bone erosion. Black arrows indicate TRAP-positive osteoclasts. (B) Synovitis scoring of the total joint, femoral side, and tibial side. (C) Bone erosion scoring of the total joint, femoral side, and tibial side. The data are expressed as mean ± SD; n = 5-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). *P < .05 for comparisons denoted by bar. AC, articular cartilage; ACLT, anterior cruciate ligament transection; MM, medial meniscus; OA, osteoarthritis; SB, subchondral bone; SM, synovium; TRAP, tartrate-resistant acid phosphatase; TXA, tranexamic acid.
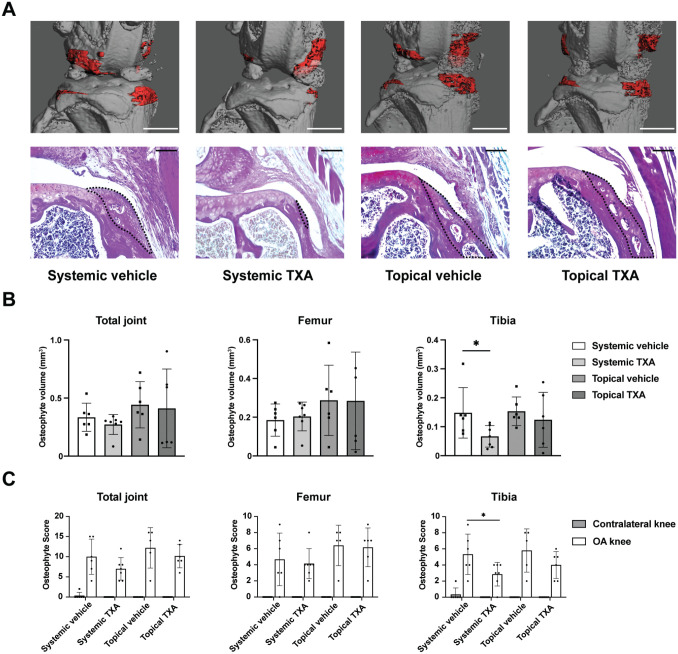
Systemic TXA Treatment Reduces Tibial Osteophyte Formation in ACLT Models, Whereas Topical Treatment Does Not
Osteophyte formation is one of the main radiographic and histological features of OA, which can form early during disease manifestation. 54 Therefore, we measured the osteophyte volume of the OA knees using μCT and assessed osteophyte formation histologically using the osteophyte scoring system (Figure 6A; Appendix Table A1, available online). μCT analysis identified a significant reduction in tibial osteophyte volume in the systemic TXA group compared with the systemic vehicle group (P = .046), while there was no difference in femoral osteophyte volume between these 2 groups (P = .068) (Figure 6B). Topical TXA treatment, on the other hand, affected neither femoral nor tibial osteophyte volume compared with vehicle controls (P = .983 and P = .515, respectively). The histological evaluation confirmed that a significant difference only occurred in tibial osteophyte scores between the systemic TXA and vehicle groups (P = .048) (Figure 6C).
Figure 6.
Systemic TXA treatment reduces tibial osteophyte formation after ACLT. (A) Representative 3-dimensional μCT reconstruction and Bone-Inflammation-Cartilage staining images of osteophytes 4 weeks after ACLT. For the upper panel, osteophytes are shown in red (scale bar = 1 mm); for the lower panel, osteophytes are marked by the black dotted line (scale bar = 100 μm). (B) μCT quantitative analysis of osteophyte volume in the total joint, femoral side, and tibial side. n = 6-7 per group as indicated (1-way ANOVA followed by the Tukey post hoc test). (C) Osteophyte scoring of the total joint, femoral side, and tibial side. n = 5-7 per group as indicated (2-way analysis of variance followed by the Tukey post hoc test). The data are expressed as mean ± SD; *P < .05 for comparisons denoted by bar. μCT, micro−computed tomography; ACLT, anterior cruciate ligament transection; OA, osteoarthritis; TXA, tranexamic acid.
Discussion
In this study, we found beneficial effects of TXA on the course of experimental OA. Both systemic and topical TXA protected cartilage from ACLT-induced degeneration. Moreover, both routes of administration lowered inflammatory responses of the synovial tissue, while only systemic TXA affected subchondral bone remodeling and osteophyte formation.
OA is a leading cause of function loss and disability, with a significant burden on the patients’ quality of life and overall health care costs. Posttraumatic OA accounts for approximately 12% of the overall prevalence of symptomatic OA and mostly occurs in young people. 11 Currently, the only ultimate treatment option is replacement of the affected joint via arthroplasty, which is not an ideal solution for young patients because of higher physical activity and limited life span of prostheses. Thus, effective early intervention attenuating OA progression is essential for patients to delay or even avoid arthroplasty.
TXA is a Food and Drug Administration−approved and inexpensive antifibrinolytic drug listed among the most essential medications by the World Health Organization. 35 TXA functions as a reversible plasminogen inhibitor that prevents the lysis of fibrin clots. Currently, TXA is routinely used to treat or prevent hyperfibrinolysis in patients with severe trauma or undergoing major orthopaedic surgery, with only minimal risk of vascular adverse effects such as thromboembolism. 52 In elective orthopaedic surgeries such as ACLR, TXA may be applied systemically or topically at the site of bleeding. 57 Thus, in the current preclinical study, we also applied these 2 routes of administration in mice with ACLT and tested whether TXA may prevent posttraumatic degenerative joint disease.
A major finding of this study is the observation that systemic and topical TXA significantly reduced cartilage degeneration in the total joint and even normalized cartilage damage in the medial tibial plateau. In this regard, articular cartilage homeostasis during OA is affected by mechanical stress, inflammatory responses, and secondary effects from surrounding tissues. 8 Previous in vitro experiments showed that TXA does not promote chondrocyte function and is cytotoxic to cartilage at concentrations >20 mg·mL-1.3,9,47,56 In turn, at concentrations <20 mg·mL-1, TXA did not affect chondrocytes.3,47 Therefore, TXA may not directly affect chondrocyte homeostasis within the safe concentration range but rather mediate its chondroprotective effect, for instance, by modulating inflammatory responses in plasminogen-dependent or -independent manners.20,55
This notion is strengthened by our observation that systemic and topical TXA markedly reduced synovial hyperplasia and alleviated bone erosion—both indicative of hyperinflammation in the damaged joint. In particular, synovitis is present at the early stage of OA and persists throughout OA development. 4 It usually manifests as synovial membrane thickening, pannus formation, and fluid effusion and was reported to precede the occurrence of cartilaginous and bone pathologies in OA,23,50 suggesting that synovitis may independently augment disease progression in OA. Mechanistically, activated synovial cells and macrophages in the inflamed synovium were reported to produce proinflammatory mediators and proteolytic enzymes responsible for cartilage breakdown. 41 Thus, although a direct effect of TXA on cartilage cannot be excluded, it is also possible that TXA preserves cartilage integrity by reducing synovitis.
Evidence that TXA exerts anti-inflammatory and immunomodulatory actions has been accumulating. Previous clinical studies showed that TXA could attenuate hyperinflammation and reduce immunosuppression after cardiac surgery 21 or primary total knee arthroplasty. 60 In murine burn models, TXA attenuated the release of damage-associated molecular patterns and decreased lung macrophage infiltration. 14 In this regard, synovial macrophages represent the most abundant immune cell type in OA synovium, 53 significantly contributing to proinflammatory cytokines. 19 Our previous study showed that TXA stimulation in vitro reduced inflammatory markers such as interleukin−1α and cytokine interleukin−1β expression in murine bone marrow−derived macrophages. 5 Therefore, TXA may inhibit the inflammatory response in the inflamed synovium by regulating the release of proinflammatory cytokines from macrophages. Likewise, TXA decreased the expression of cluster of differentiation 14 (CD14) in macrophages, which is expressed in macrophages and essential for osteoclastogenesis. 16 In this regard, TRAP-positive osteoclasts in the contact area between inflamed synovium and eroded cortical bone were lower in the TXA groups compared with vehicle groups, indicating that TXA treatment inhibited bone resorption caused by synovitis. Previous studies indicated that osteoclasts in the inflammatory synovial infiltrate are primarily derived from CD14+ monocytes/macrophages.18,58 Thus, a possible interpretation is that TXA suppresses the differentiation of synovial macrophages to osteoclasts—although inhibiting pro-osteoclastogenic factors—thereby reducing bone erosion in OA knees.
Consistent with findings by others, we observed subchondral bone loss—including thinner subchondral bone plates and deteriorated subchondral trabecular bone—in the early stage of OA 4 weeks after ACLT. It is currently considered that subchondral bone provides mechanical and nutritional support for articular cartilage. 12 Previous studies showed that early OA primarily presented as subchondral bone loss,12,32 and restoring the microarchitecture of subchondral bone could slow down cartilage degeneration.17,39,61 After systemic TXA treatment, a marked alleviation of subchondral bone loss was observed. In contrast, topical TXA failed to affect the microarchitecture of subchondral bone. Subchondral bone loss in early OA resulted from increased bone remodeling, with excessive osteoclast activity and increased net bone resorption.24,40 In agreement, we also observed a significant increase in osteoclast number and surface in OA knees with vehicle treatment, while osteoblast parameters were not significantly altered. The systemic TXA treatment resulted in a normalization of excessive osteoclast parameters in OA, whereas no effect on osteoblast parameters was observed. This is in part contrary to our previous report, where TXA inhibited osteoclastogenesis and promoted extracellular matrix mineralization in bone marrow−derived osteoblasts in vitro. 5 Likewise, systemic TXA inhibited osteophyte formation—usually because of abnormal bone remodeling.46,51,61 Together, these data indicate that in vivo, systemic TXA results in normalizing abnormal bone remodeling rather than directly stimulating osteoblast function and bone formation.
Intra-articular injections are commonly used clinically to treat joint diseases. Although associated with the risk of bacterial contamination, topical injections have several advantages compared with systemic administration. For example, they provide initial high local drug concentrations followed by rapid clearance. 59 Thus, lower total drug doses can be used, reducing potential systemic side effects. However, this may also explain why systemic TXA normalized bone remodeling in the subchondral bone whereas topical treatment did not. With topical injections, TXA may not reach the subchondral compartment in sufficient concentration because of short persistence in the joint cavity and/or very low systemic concentration. Together, the bone-protective effects appear to be dependent on the blood supply to the subchondral bone and, therefore, require a systemic route of drug application.
Despite the promising effects of TXA found in this study, several limitations need to be mentioned here. First, the sample size of this study is small, and the results from animal OA models, in general, cannot be directly translated into clinical application. Second, the potential benefits of TXA must be weighed against possible adverse effects, including thromboembolism or bacterial contamination during systemic and topical application, respectively. Here, even though the safety of TXA for routine clinical application has already been validated, long-term and repeated administration of comparably high doses needs careful evaluation. Third, this study used only female mice, and it remains uncertain if the findings can be generalized to male mice. A further limitation of our study is that the assessments were performed at a single time point only. Follow-up studies are required to assess the effects of TXA at both earlier and later time points and whether there is a long-term protective effect after cessation of TXA treatment. In this regard, the results of the ongoing clinical trial (NCT03552705) evaluating the effect of TXA on reducing inflammation and improving joint health in patients undergoing ACL repair are eagerly awaited. Finally, as this was a preclinical study with an in-depth morphologic analysis of tissue integrity during OA progression, we mainly employed radiographic, histological, and histomorphometric approaches. Although we propose several possible explanations for our observations, the molecular mechanisms underlying these findings require further study.
In conclusion, we report the beneficial effects of TXA on the course of posttraumatic OA. In mice with ACLT-induced OA, systemic and topical TXA treatment alleviate cartilage degeneration and synovitis. Systemic TXA results in a normalization of bone remodeling, thereby preserving subchondral bone microstructure and reducing osteophyte formation. This work suggests that TXA may indeed benefit patients with posttraumatic OA.
Supplemental Material
Supplemental material, sj-pdf-1-ajs-10.1177_03635465231220855 for Tranexamic Acid Attenuates the Progression of Posttraumatic Osteoarthritis in Mice by Weixin Xie, Shan Jiang, Antonia Donat, Paul Richard Knapstein, Lilly-Charlotte Albertsen, Judith Luisa Kokot, Cordula Erdmann, Tim Rolvien, Karl-Heinz Frosch, Anke Baranowsky and Johannes Keller in The American Journal of Sports Medicine
Footnotes
Submitted May 17, 2023; accepted October 25, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported in part by the German Research Foundation (KE 2179/9-1). The China Scholarship Council provided funds to W.X. and S.J. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
ORCID iD: Tim Rolvien  https://orcid.org/0000-0003-1058-1307
https://orcid.org/0000-0003-1058-1307
References
- 1. Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293-311. [DOI] [PubMed] [Google Scholar]
- 2. Alkhatib N, AlNouri M, Abdullah ASA, et al. Tranexamic acid use in anterior cruciate ligament reconstruction decreases bleeding complications: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy. 2022;38(2):506-518. [DOI] [PubMed] [Google Scholar]
- 3. Ambra LF, de Girolamo L, Niu W, Phan A, Spector M, Gomoll AH. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):931-935. [DOI] [PubMed] [Google Scholar]
- 4. Atukorala I, Kwoh CK, Guermazi A, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baranowsky A, Appelt J, Tseneva K, et al. Tranexamic acid promotes murine bone marrow-derived osteoblast proliferation and inhibits osteoclast formation in vitro. Int J Mol Sci. 2021;22(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergmann B, Mölne J, Gjertsson I. The bone-inflammation-cartilage (BIC) stain: a novel staining method combining safranin O and Van Gieson’s stains. J Histochem Cytochem. 2015;63(9):737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46(12):3178-3184. [DOI] [PubMed] [Google Scholar]
- 8. Blaney Davidson EN, van de Loo FAJ, van den Berg WB, van der Kraan PM. How to build an inducible cartilage-specific transgenic mouse. Arthritis Res Ther. 2014;16(3):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolam SM, O’Regan-Brown A, Paul Monk A, Musson DS, Cornish J, Munro JT. Toxicity of tranexamic acid (TXA) to intra-articular tissue in orthopaedic surgery: a scoping review. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1862-1871. [DOI] [PubMed] [Google Scholar]
- 10. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25(7):1468-1486. [DOI] [PubMed] [Google Scholar]
- 11. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-744. [DOI] [PubMed] [Google Scholar]
- 12. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665-673. [DOI] [PubMed] [Google Scholar]
- 13. Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397-405. [DOI] [PubMed] [Google Scholar]
- 14. Carter DW, Prudovsky I, Kacer D, et al. Tranexamic acid suppresses the release of mitochondrial DAMPs and reduces lung inflammation in a murine burn model. J Trauma Acute Care Surg. 2019;86(4):617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho H, Walker A, Williams J, Hasty KA. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res Int. 2015;2015:595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costa-Rodrigues J, Fernandes A, Fernandes MH. Spontaneous and induced osteoclastogenic behaviour of human peripheral blood mononuclear cells and their CD14(+) and CD14(-) cell fractions. Cell Prolif. 2011;44(5):410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cui Z, Crane J, Xie H, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. 2016;75(9):1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danks L, Sabokbar A, Gundle R, Athanasou NA. Synovial macrophage-osteoclast differentiation in inflammatory arthritis. Ann Rheum Dis. 2002;61(10):916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484-1499. [DOI] [PubMed] [Google Scholar]
- 20. Degirmenci E, Ozturan KE, Sahin AA, Yilmaz F, Kaya YE. Effects of tranexamic acid on the recovery of osteochondral defects treated by microfracture and acellular matrix scaffold: an experimental study. J Orthop Surg Res. 2019;14(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Draxler DF, Yep K, Hanafi G, et al. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. 2019;3(10):1598-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang C, Guo J-W, Wang Y-J, et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin. 2022;43(5):1299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Felson DT, Niu J, Neogi T, et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage. 2016;24(3):458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014;12(1):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061-1069. [DOI] [PubMed] [Google Scholar]
- 26. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(suppl 3):S17-S23. [DOI] [PubMed] [Google Scholar]
- 27. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745-1759. [DOI] [PubMed] [Google Scholar]
- 28. Jackson MT, Moradi B, Zaki S, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis Rheumatol. 2014;66(12):3337-3348. [DOI] [PubMed] [Google Scholar]
- 29. Johns WL, Walley KC, Hammoud S, Gonzalez TA, Ciccotti MG, Patel NK. Tranexamic acid in anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2021;49(14):4030-4041. [DOI] [PubMed] [Google Scholar]
- 30. Karaaslan F. Editorial commentary: tranexamic acid: okay, it reduces the bleeding, but are we sure topical use is not harmful to the cartilage? Arthroscopy. 2019;35(7):2133-2135. [DOI] [PubMed] [Google Scholar]
- 31. Klein JC, Keith A, Rice SJ, et al. Functional testing of thousands of osteoarthritis-associated variants for regulatory activity. Nat Commun. 2019;10(1):2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klose-Jensen R, Hartlev LB, Boel LWT, et al. Subchondral bone turnover, but not bone volume, is increased in early stage osteoarthritic lesions in the human hip joint. Osteoarthritis Cartilage. 2015;23(12):2167-2173. [DOI] [PubMed] [Google Scholar]
- 33. Lewis JS, Hembree WC, Furman BD, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19(7):864-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li G, Yin J, Gao J, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lier H, Shander A. Tranexamic acid: the king is dead, long live the king! Br J Anaesth. 2020;124(6):659-662. [DOI] [PubMed] [Google Scholar]
- 36. Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. [DOI] [PubMed] [Google Scholar]
- 38. Lyons TJ, McClure SF, Stoddart RW, McClure J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet Disord. 2006;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma L, Zhao X, Liu Y, Wu J, Yang X, Jin Q. Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone. Int J Mol Med. 2021;47(3):4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol. 2017;232(11):2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuzaki T, Alvarez-Garcia O, Mokuda S, et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci Transl Med. 2018;10(428):eAAN746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maumus M, Noël D, Ea HK, et al. Identification of TGFβ signatures in six murine models mimicking different osteoarthritis clinical phenotypes. Osteoarthritis Cartilage. 2020;28(10):1373-1384. [DOI] [PubMed] [Google Scholar]
- 44. McNulty MA, Loeser RF, Davey C, Callahan MF, Ferguson CM, Carlson CS. A comprehensive histological assessment of osteoarthritis lesions in mice. Cartilage. 2011;2(4):354-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nüesch E, Dieppe P, Reichenbach S, Williams S, Iff S, Jüni P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:D1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panahifar A, Maksymowych WP, Doschak MR. Potential mechanism of alendronate inhibition of osteophyte formation in the rat model of post-traumatic osteoarthritis: evaluation of elemental strontium as a molecular tracer of bone formation. Osteoarthritis Cartilage. 2012;20(7):694-702. [DOI] [PubMed] [Google Scholar]
- 47. Parker JD, Lim KS, Kieser DC, Woodfield TBF, Hooper GJ. Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Joint J. 2018;100(3):404-412. [DOI] [PubMed] [Google Scholar]
- 48. Percie du, Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract Thromb Haemost. 2021;5(5):e12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roemer FW, Guermazi A, Felson DT, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sagar DR, Ashraf S, Xu L, et al. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis. 2014;73(8):1558-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taeuber I, Weibel S, Herrmann E, et al. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg. 2021;156(6):e210884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomson A, Hilkens CMU. Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Front Immunol. 2021;12:678757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237-244. [DOI] [PubMed] [Google Scholar]
- 55. Vignon E, Mathieu P, Bejui J, et al. Study of an inhibitor of plasminogen activator (tranexamic acid) in the treatment of experimental osteoarthritis. J Rheumatol Suppl. 1991;27:131-133. [PubMed] [Google Scholar]
- 56. Wagenbrenner M, Heinz T, Horas K, et al. Impact of tranexamic acid on chondrocytes and osteogenically differentiated human mesenchymal stromal cells (hMSCs) in vitro. J Clin Med. 2020;9(12):3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong J, George RB, Hanley CM, Saliba C, Yee DA, Jerath A. Tranexamic acid: current use in obstetrics, major orthopedic, and trauma surgery. Canadian J Anaesth. 2021;68(6):894-917. [DOI] [PubMed] [Google Scholar]
- 58. Xue J, Xu L, Zhu H, et al. CD14CD16 monocytes are the main precursors of osteoclasts in rheumatoid arthritis via expressing Tyro3TK. Arthritis Res Ther. 2020;22(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu SP, Hunter DJ. Intra-articular therapies for osteoarthritis. Expert Opin Pharmacother. 2016;17(15):2057-2071. [DOI] [PubMed] [Google Scholar]
- 60. Zhang S, Xu H, Xie J, Cao G, Lei Y, Pei F. Tranexamic acid attenuates inflammatory effect and modulates immune response in primary total knee arthroplasty: a randomized, placebo-controlled, pilot trial. Inflammopharmacology. 2020;28(4):839-849. [DOI] [PubMed] [Google Scholar]
- 61. Ziemian SN, Witkowski AM, Wright TM, Otero M, van der Meulen MCH. Early inhibition of subchondral bone remodeling slows load-induced posttraumatic osteoarthritis development in mice. J Bone Mineral Res. 2021;36(10):2027-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajs-10.1177_03635465231220855 for Tranexamic Acid Attenuates the Progression of Posttraumatic Osteoarthritis in Mice by Weixin Xie, Shan Jiang, Antonia Donat, Paul Richard Knapstein, Lilly-Charlotte Albertsen, Judith Luisa Kokot, Cordula Erdmann, Tim Rolvien, Karl-Heinz Frosch, Anke Baranowsky and Johannes Keller in The American Journal of Sports Medicine