ABSTRACT
Aims/Introduction
An overrepresentation of epilepsy has been suggested in patients with type 1 diabetes (T1D). This meta‐analysis was conducted to evaluate if type 1 diabetes is associated with a higher incidence of epilepsy.
Materials and Methods
Longitudinal observational studies which are relevant to the purpose of the meta‐analysis were screened and obtained by searching PubMed, Embase, and Web of Science databases. Random‐effects models were used when significant heterogeneity was observed; otherwise, fixed‐effects models were used.
Results
Six observational studies involving 10 datasets of 8,001,899 participants were included, with six datasets including children and only one dataset including older people. Among them, 100,414 (1.25%) had type 1 diabetes. During the follow‐up duration of 5.4–15.2 years (mean: 9.5 years), 98,644 cases (1.23%) of epilepsy were observed. Compared with participants with normoglycemia, those with type 1 diabetes were shown to have a higher incidence of epilepsy (risk ratio [RR]: 2.41, 95% confidence interval 1.69–3.44, P < 0.001; I 2 = 95%) after adjustment of potential confounding variables including age and sex. Subgroup analysis showed consistent results in nested case–control and retrospective cohort studies, and in studies of children, non‐elderly adult, and older participants (P for subgroup difference = 0.42 and 0.07). In addition, a stronger association of type 1 diabetes and epilepsy was suggested in studies with follow‐up duration <10 years compared with those ≥10 years (RR: 3.34 vs 1.61, P for subgroup difference < 0.001).
Conclusion
Patients with type 1 diabetes may have a higher risk of epilepsy, which was mainly driven by datasets including children.
Keywords: Epilepsy, Meta‐analysis, Observational studies
A meta‐analysis of six observational studies showed that type 1 diabetes may be associated with a higher incidence of epilepsy. The association was consistent in children and the adult population.
INTRODUCTION
Type 1 diabetes (T1D) is a prevalent metabolic disorder with autoimmune causes, particularly among children 1 , 2 . The pathophysiology of type 1 diabetes stems from the immune system‐mediated destruction of pancreatic β‐cells, influenced by various genetic and environmental factors 3 , 4 . A growing body of evidence indicates a 3–4% rise in the occurrence of type 1 diabetes over the past three decades 5 . Patients with type 1 diabetes exhibit a range of clinically established complications that can be attributed to impaired glucose metabolism, namely retinopathy, nephropathy, peripheral neuropathy, and cardiovascular disease 6 , 7 . Furthermore, type 1 diabetes has been associated with various immune disorders, including coeliac disease, hypothyroidism, hyperthyroidism, and Addison disease 8 . Notably, recent research indicates a potential heightened susceptibility among patients with type 1 diabetes to several neurocognitive disorders 9 , 10 , including epilepsy 11 . Several observational studies have indicated a potential overrepresentation of epilepsy in individuals with type 1 diabetes 12 , 13 , 14 , 15 , although the findings have not always been consistent 16 . Moreover, the potential elevated risk of the development of epilepsy among individuals with type 1 diabetes in comparison with those with normoglycemia remains uncertain. Due to this knowledge gap, the current study aimed to conduct a comprehensive review and meta‐analysis to evaluate the correlation between type 1 diabetes and the occurrence of epilepsy.
MATERIALS AND METHODS
The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement 17 , 18 , as well as the Cochrane Handbook 19 , during the entirety of the planning, conducting, and reporting phases.
Inclusion and exclusion criteria of studies
The development of inclusion criteria adhered to the PICOS recommendations and aligned with the objective of the meta‐analysis.
P (patients): General population without epilepsy at baseline.
I (exposure): Participants with type 1 diabetes. Methods and criteria used for the diagnosis of type 1 diabetes were consistent with those used in the original study.
C (control): Participants without type 1 diabetes.
O (outcomes): Incidence of epilepsy between participants with and without type 1 diabetes at baseline. The diagnosis and confirmation of epilepsy were also consistent with the criteria used in the original study.
S (study design): Observational studies with longitudinal follow‐up, which included nested case–control studies and cohort studies.
Reviews, editorials, previous meta‐analyses, cross‐sectional studies, studies including participants with type 2 diabetes rather than type 1 diabetes, studies that did not include controls with normoglycemia, or did not report the incidence of epilepsy were excluded. In cases where there was overlap in patient populations, the study with the largest sample size was included in the meta‐analysis.
Search of databases
We conducted a comprehensive search of electronic databases, namely PubMed, Embase, and Web of Science, from their inception until August 15, 2023, in order to identify studies published up to that date. The search was carried out with the terms including (1) “type 1 diabetes” OR “T1D” OR “T1DM” OR “brittle diabetes mellitus” OR “juvenile onset diabetes” OR “insulin dependent diabetes” OR “autoimmune diabetes”; (2) “epilepsy” OR “epileptic” OR “epilepsia” OR “seizure” OR “seizures” OR “convulsion”; and (3) “cohort” OR “cohorts” OR “followed” OR “follow‐up” OR “incidence” OR “occurrence” OR “longitudinal” OR “prospective” OR “retrospective” OR “prospectively” OR “retrospectively”. Only studies of human participants that were published in English language were included. During our manual screening process, we thoroughly examined references from pertinent original and review articles to identify potentially relevant studies.
Data extraction and quality evaluation
Literature searches, data collection, and study quality assessments were carried out independently by the two authors. In case of discrepancies, discussion between the two authors was indicated to reach a consensus. Among the studies included in the analysis, we collected information regarding study information, demographic factors, and participant characteristics, methods for diagnosing type 1 diabetes and epilepsy, follow‐up durations, as well as confounding factors that were adjusted when the association between type 1 diabetes and the incidence of epilepsy was reported. The study's quality was assessed using the Newcastle‐Ottawa Scale (NOS) 20 , which evaluates participant selection, group comparability, and outcome validity. The scale consisted of nine stars, with a higher number indicating a superior study.
Statistics
Risk ratios (RR) and their corresponding 95% confidence intervals (CI) were used to present the association between type 1 diabetes and the incidence of epilepsy during follow‐up. RR data and the corresponding standard error (SE) were computed using either 95% CI or P values, and then subjected to logarithmic transformation to stabilize variance and to normalize distribution 19 . To assess the level of heterogeneity among studies, the Cochrane Q test and the I 2 statistic were employed 21 . An I 2 value exceeding 50% signifies substantial heterogeneity across the studies. In cases where significant heterogeneity was observed, a random‐effects model was utilized; otherwise, a fixed‐effects model was employed 19 . In order to assess the impact of individual studies on the results of the meta‐analysis, a sensitivity analysis was conducted by systematically excluding one dataset at a time 22 . Additionally, subgroup analyses were performed to investigate the influence of various study characteristics on the outcome. These characteristics included study design, age of the participants, mean follow‐up durations, and the adjustment of potential confounding factors. The selection of medians as cutoff values for defining subgroups was employed for continuous variables. The estimation of publication bias is conducted using a funnel plot, which relies on visual assessments of symmetry, in conjunction with Egger's regression asymmetry test 23 . The statistical analyses were performed using RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation).
RESULTS
Database search and study identification
Figure 1 depicts the procedure of conducting a literature search and retrieving relevant studies. Initially, a total of 877 records were acquired from the database, out of which 238 duplicate entries were eliminated. Subsequently, 617 studies were excluded based on the screening of their titles and abstracts, as they did not align with the objectives of the meta‐analysis. After conducting full‐text reviews of 22 studies, 16 were excluded due to the reasons specified in Figure 1. Consequently, six studies were deemed suitable for the subsequent meta‐analysis 24 , 25 , 26 , 27 , 28 , 29 .
Figure 1.
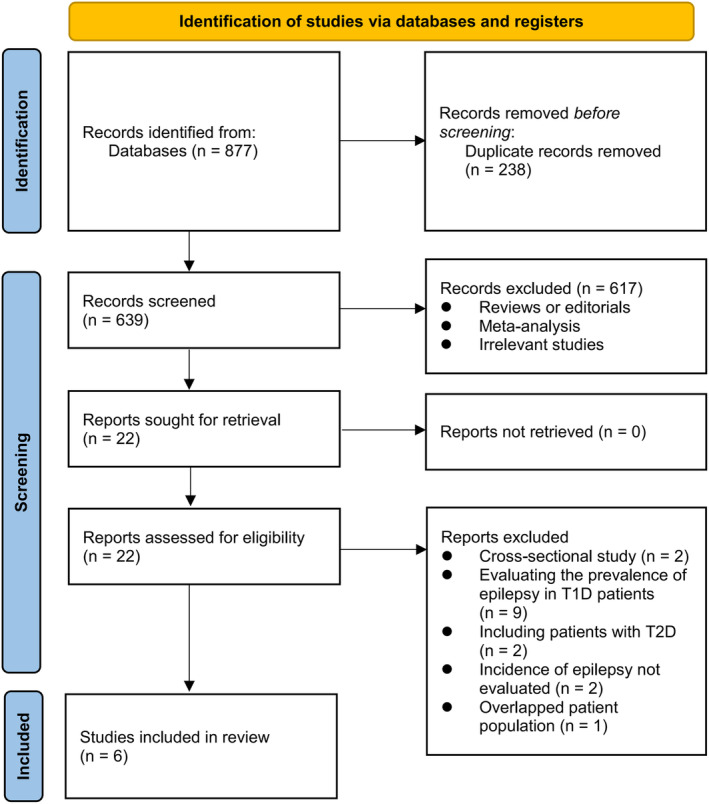
Flowchart of database search and study inclusion.
Study characteristics
The overview of the included studies is displayed in Table 1. Overall, three retrospective cohort studies 24 , 28 , 29 and the other three nested case–control studies 25 , 26 , 27 were included in the meta‐analysis, which were published from 2014 to 2020 and performed in the United States, the Netherlands, China, the United Kingdom, Finland, and Norway. All of the studies included general populations. A total of 8,001,899 participants were included. Three studies included children only 25 , 26 , 28 , while the other three studies include both children and adults 24 , 27 , 29 , and reported the outcome according to the age group of the participants. Patients with type 1 diabetes were identified with database codes in all of the included studies except for one study 29 , in which patients with type 1 diabetes were solely classified based on their utilization of insulin or its analogs. In this study, the authors mentioned that ‘the Norwegian Directorate of Health did not recommend treating type 2 diabetes mellitus with insulin/insulin analogs in monotherapy during the entire study period. Thus, by excluding all patients who received oral antidiabetics and only including patients who received insulin or insulin analogs in monotherapy the dominating part of our study group should have type 1 diabetes mellitus’ 29 . Accordingly, a total of 100,414 (1.25%) participants had type 1 diabetes at baseline. The mean follow‐up durations of the studies were 5.4–15.2 years (mean: 9.5 years), and 98,644 cases (1.23%) of epilepsy were observed during follow‐up. Potential confounding factors such as age and sex were controlled in all of the included studies, while for two of the included studies 26 , 27 , other variables such as a history of head injury was also adjusted. Among the included studies, all had scores between six and seven stars, indicating that they were of moderate to good quality (Table 2).
Table 1.
Characteristics of the included studies
| Study | Country | Design | Participants | Sample size | Age (years) | Male (%) | Diagnosis of T1D | No. of patients with T1D | Follow‐up duration (years) | Validation of patients with epilepsy | No. of patients with epilepsy | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ong 24 | USA | RC | Nation‐wide population | 2,518,034 | Children (<18) and non‐elderly adults (18–65) | 48.3 | ICD codes | 43,704 | 7 | ICD codes | 10,041 | Age and sex |
| Fazeli Farsani 25 | The Netherlands | NCC | General population | 4,505 | Children (<19), mean: 10.1 | 50.8 | ICD codes | 915 | 5.4 | Hospital records | 19 | Age and sex |
| Chou 26 | China | NCC | Nation‐wide population | 28,248 | Children (<18), mean: 10.4 | 53.5 | ICD codes | 2,568 | 6.8 | ICD codes | 240 | Age, sex, urbanization, intellectual disability, low birth weight and head injury |
| Dafoulas 27 | UK | NCC | Nation‐wide population | 24,610 | Children (<18) and young adults (18–40), mean: 17.9 | 59 | Database codes | 4,922 | 5.4 | Database codes | 81 | Age, sex, social deprivation, cerebral palsy, head injury and learning disability |
| Sillanpaa 28 | Finland | RC | Nation‐wide population | 679,375 | Children (<15) | NR | ICD codes | 6,162 | 15.2 | ICD codes | 8,512 | Age and sex |
| Borsheim 29 | Norway | RC | Nation‐wide population | 4,747,127 | Children (<20), non‐elderly adults (20–60), and older people (60+) | 45.4 | Prescription records | 42,143 | 10 | Prescription records | 79,751 | Age and sex |
ICD, International Classification of Disease; NCC, nested case–control; RC, retrospective cohort; T1D, type 1 diabetes.
Table 2.
Study quality evaluation via the Newcastle‐Ottawa scale
| Study | Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Enough long follow‐up duration | Adequacy of follow‐up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Ong 24 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Fazeli Farsani 25 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Chou 26 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Dafoulas 27 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Sillanpaa 28 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Borsheim 29 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
Meta‐analysis results
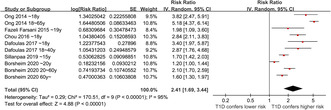
Overall, ten datasets from six observational studies were included in the meta‐analysis 24 , 25 , 26 , 27 , 28 , 29 . A significant heterogeneity was observed among the included studies (P for Cochrane Q test <0.001, I 2 = 95%). Therefore, a random‐effects model was used for the meta‐analysis. Pooled results showed that compared with controls with normoglycemia, those with type 1 diabetes was associated with a higher incidence of epilepsy (RR: 2.41, 95% CI: 1.69–3.44, P < 0.001; Figure 2). Sensitivity analysis was done by excluding one dataset at a time (RR: 2.14–2.63, P all <0.05). Specifically, excluding the three datasets from the study of Borcheim et al. 29 also retrieved similar results (RR: 2.94, 95% CI: 1.91–4.53, P < 0.001; I 2 = 93%). Subgroup analysis showed consistent results in nested case–control and retrospective cohort studies (RR: 2.79 vs 2.25, P for subgroup difference = 0.42; Figure 3a), and in studies of children, non‐elderly adult, and older participants (RR: 2.23, 3.17. and 1.60, P for subgroup difference = 0.07; Figure 3b). In addition, a stronger association between type 1 diabetes and epilepsy was observed in studies with a follow‐up duration ≥10 years compared with those <10 years (RR: 3.34 vs 1.61, P for subgroup difference < 0.001; Figure 4a). Finally, a consistent association was observed in studies only adjusting age and sex, and in studies with adjustment for other factors besides age and sex (RR: 2.21 vs 2.94, P for subgroup difference = 0.27; Figure 4b).
Figure 2.
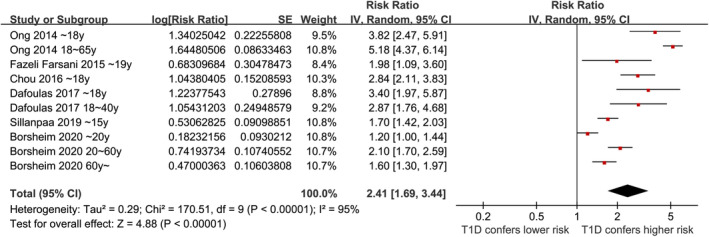
Forest plots for the meta‐analyses regarding the association between type 1 diabetes and the risk of epilepsy.
Figure 3.
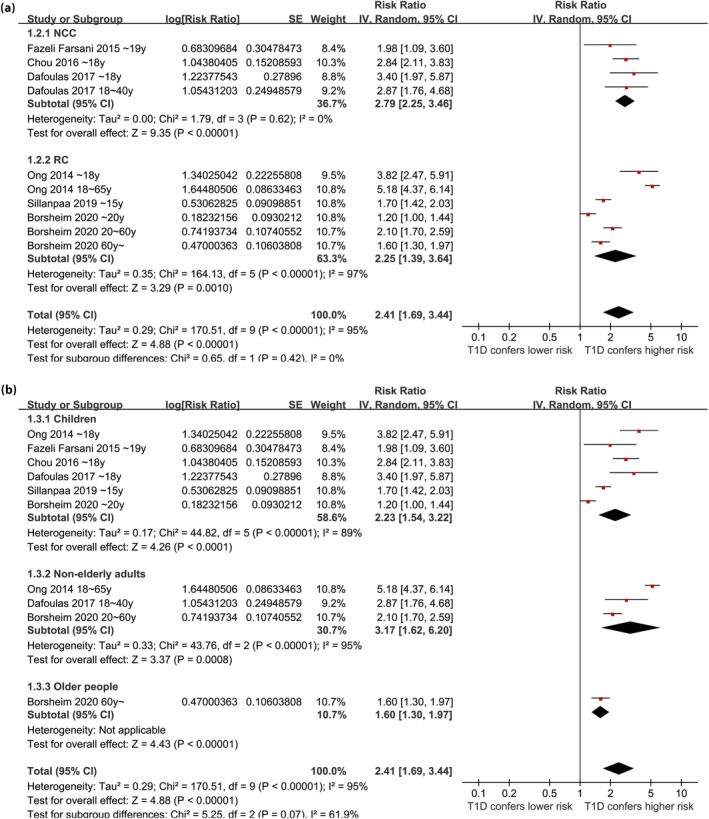
Forest plots for the subgroup analyses regarding the association between type 1 diabetes and the risk of epilepsy; (a) subgroup analysis according to study design; and (b) subgroup analysis according to the age groups.
Figure 4.
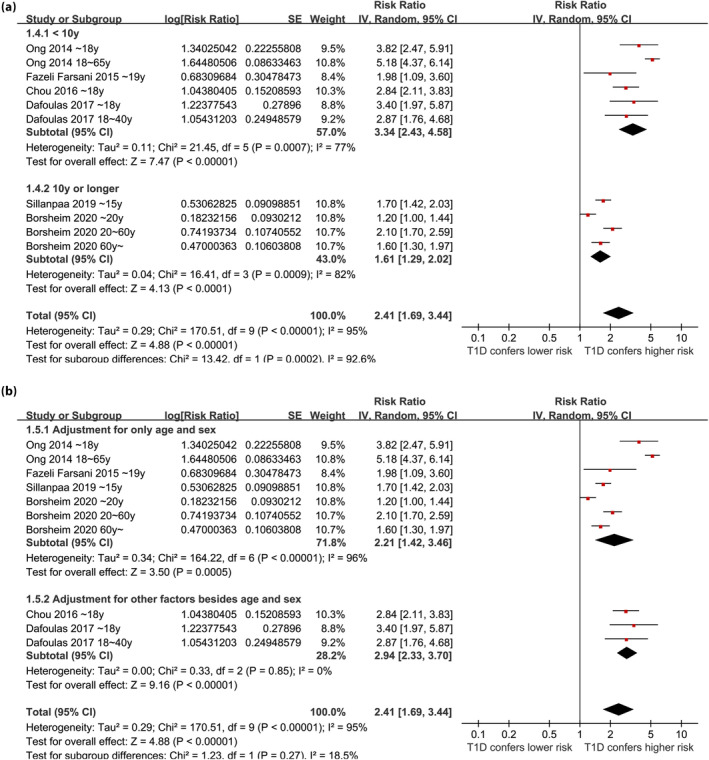
Forest plots for the subgroup analyses regarding the association between type 1 diabetes and the risk of epilepsy; (a) subgroup analysis according to follow‐up durations; and (b) subgroup analysis according to the variables adjusted.
Publication bias
The funnel plots illustrating the meta‐analysis of type 1 diabetes and the incidence of epilepsy are depicted in Figure 5. Upon visual inspection, the plots exhibit symmetry, implying a minimal presence of publication bias. Furthermore, the application of Egger's regression tests yielded a P‐value of 0.72, indicating a low probability of publication bias.
Figure 5.
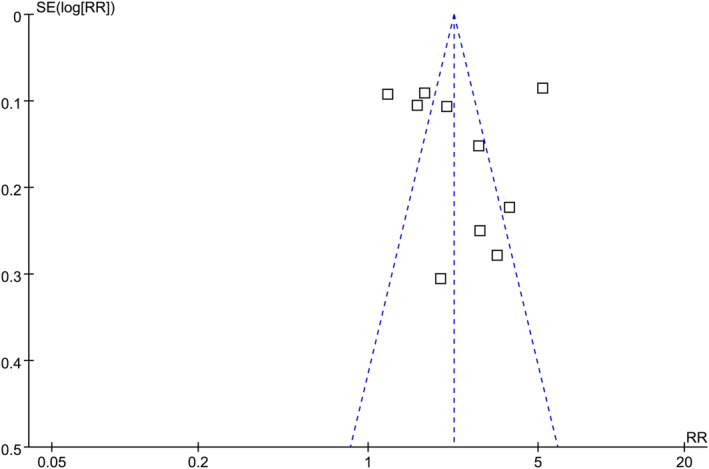
Funnel plots for the publication bias underlying the meta‐analysis regarding the association between type 1 diabetes and the risk of epilepsy.
DISCUSSION
This meta‐analysis involved the aggregation of data from ten datasets obtained from six observational studies. The findings indicated that individuals with type 1 diabetes exhibited a significantly heightened susceptibility to epilepsy when compared with those with normoglycemia. Additionally, consistent outcomes were obtained through sensitivity analysis, which involved the exclusion of one dataset at a time. Furthermore, subgroup analysis revealed that the relationship between type 1 diabetes and epilepsy risk remained unaffected by variables such as study design, patient age groups, and adjusted potential confounding factors. Interestingly, we found that the association between type 1 diabetes and the increased risk of epilepsy was stronger in studies with a follow‐up duration of <10 years compared with those with a follow‐up duration ≥10 years. Taken together, these finding suggest that type 1 diabetes may be a risk factor of epilepsy in children and adult populations.
To the best of our knowledge, only one meta‐analysis published in 2017 evaluated the association between type 1 diabetes and epilepsy. This meta‐analysis included three observational studies, including one cross‐sectional study and two cohort studies, and showed that type 1 diabetes may be related with epilepsy. Due to the limited study available, sensitivity and subgroup analysis was not able to be performed in the previous meta‐analysis, and the potential influences of study characteristics on the association between type 1 diabetes and epilepsy remain unknown. Compared with the previous meta‐analysis, our meta‐analysis has several advantages. First, an extensive literature search was carried out in three most commonly used electronic databases, which retrieved six up‐to‐date observational studies according to the aim of the meta‐analysis. The sample size of the current meta‐analysis was much larger than the previous one. Second, all of the included studies were with longitudinal follow‐ups, which therefore could establish a potential relationship between type 1 diabetes and the incidence of epilepsy. Moreover, multivariate analyses were performed in all of the included studies when the association between type 1 diabetes and the incidence of epilepsy was evaluated, which suggested that the association was not likely to be confounded by factors such as age, sex, or history of brain injury. Finally, a series of sensitivity and subgroup analyses were performed, which showed that the results were not primarily driven by either of the included dataset or not significantly affected by study characteristics such as study design, age group, and adjustment of potential confounding factors. These findings highlight the stability and robustness of the finding. Taken together, these results suggest that type 1 diabetes may be a risk factor for epilepsy.
Our subgroup analysis suggested that the relationship between type 1 diabetes and an increased risk of epilepsy may be stronger in short‐term studies (<10 years) compared with long‐term studies (≥10 years). This is consistent with the findings of a previous large study in Finland which showed that children with type 1 diabetes had an increased, but slowly declining, risk of developing epilepsy 28 . The potential mechanisms underlying the association between type 1 diabetes and epilepsy remain unknown. Clinical studies have demonstrated the presence of electroencephalogram (EEG) irregularities in individuals diagnosed with type 1 diabetes, particularly among those with inadequate glycemic control 30 . These abnormalities include focal epileptiform abnormalities, reduced relative power of alpha, beta, and gamma frequencies in the temporal regions, as well as diminished EEG coherence 31 , 32 , 33 , 34 . Furthermore, there is a proposition suggesting that the presence of anti‐glutamic acid decarboxylase antibodies (GAD‐Abs) may serve as a significant connection between type 1 diabetes and epilepsy 35 . A significant number of patients with type 1 diabetes exhibited positive results for GAD‐Abs, which may contribute to the development of epilepsy through various mechanisms 36 . These mechanisms include interference with the catalytic site of GAD on neurons through the neutralizing efficacy of GAD‐Abs, activation of a cellular‐mediated response against synaptic vesicles of GABAergic neurons following the formation of a membrane complex, and mimicry of GABA function through a direct interaction between GAD‐Abs and GABA receptors 37 , 38 , 39 , 40 . Additionally, cerebral injury resulting from long‐term persistent hyperglycemia and abrupt hypoglycemia has also been proposed as potential mechanisms for the pathogenesis of epilepsy in patients with type 1 diabetes 11 . However, the predominant molecular signaling pathways remain to be determined in future studies.
This study has limitations. First, only nested case–control and retrospective cohort studies were included, and these studies may be associated with recall and selection biases. The results of the meta‐analysis should be validated in prospective studies. Second, although a subgroup analysis according to the age group failed to show a significant modification of age on the increased risk of epilepsy in patients with type 1 diabetes, the majority (6 out of 10) of datasets used in the meta‐analysis included children with type 1 diabetes rather than adults with type 1 diabetes, and only one dataset included older people with type 1 diabetes. Accordingly, the association between type 1 diabetes and an increased risk of epilepsy in this meta‐analysis was mainly driven by datasets including children, and the association between type 1 diabetes and epilepsy in older people should be further validated in future studies. Furthermore, validation of the diagnosis of type 1 diabetes and epilepsy was mostly achieved by database codes rather than by clinical diagnosis among the included studies, which may be associated with the risk of misclassification. However, due to the low prevalence of type 1 diabetes and the low incidence of epilepsy, a large‐scale prospective cohort study to indicate their relationship based on clinical evaluation was difficult to perform. In addition, in one of the included studies, 29 the classification of patients with type 1 diabetes was based solely on their utilization of insulin or its analogs, which may confound the results of the meta‐analysis. However, sensitivity analyses by excluding the three datasets derived from this study showed consistent results. Moreover, a causative relationship between type 1 diabetes and epilepsy could not be determined based on the current meta‐analysis because all of the included studies were observational. Finally, we could not exclude that there were unadjusted residual factors confounding the association between type 1 diabetes and epilepsy, such as antidiabetic treatments, dietary factors, and the incidence of severe hypoglycemia.
CONCLUSIONS
In conclusion, the results of the meta‐analysis suggest that compared with participants with normoglycemia, patients with type 1 diabetes may be related to a higher risk of epilepsy. The results of the meta‐analysis were primarily driven by studies including children with type 1 diabetes, while only one dataset including older people with type 1 diabetes. Although these results should be validated in prospective cohort studies, the current evidence supports that type 1 diabetes may be a risk factor for epilepsy in children and the adult population. Further studies are warranted to determine the underlying mechanisms and to explore whether optimizing glycemic control could reduce the risk of epilepsy in patients with type 1 diabetes.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Approval date of Registry and the Registration No. of the study/trial: N/A.
Animal studies: N/A.
FUNDING STATEMENT
No funding was received for this study.
ACKNOWLEDGMENTS
None.
REFERENCES
- 1. Vanderniet JA, Jenkins AJ, Donaghue KC. Epidemiology of type 1 diabetes. Curr Cardiol Rep 2022; 24: 1455–1465. [DOI] [PubMed] [Google Scholar]
- 2. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of disease study 2021. Lancet 2023; 402: 203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiMeglio LA, Evans‐Molina C, Oram RA. Type 1 diabetes. Lancet 2018; 391: 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Syed FZ. Type 1 diabetes mellitus. Ann Intern Med 2022; 175: ITC33–ITC48. [DOI] [PubMed] [Google Scholar]
- 5. Norris JM, Johnson RK, Stene LC. Type 1 diabetes‐early life origins and changing epidemiology. Lancet Diabetes Endocrinol 2020; 8: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piona C, Ventrici C, Marcovecchio L, et al. Long‐term complications of type 1 diabetes: What do we know and what do we need to understand? Minerva Pediatr (Torino) 2021; 73: 504–522. [DOI] [PubMed] [Google Scholar]
- 7. Tommerdahl KL, Shapiro ALB, Nehus EJ, et al. Early microvascular complications in type 1 and type 2 diabetes: Recent developments and updates. Pediatr Nephrol 2022; 37: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Popoviciu MS, Kaka N, Sethi Y, et al. Type 1 diabetes mellitus and autoimmune diseases: A critical review of the association and the application of personalized medicine. J Pers Med 2023; 13: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shalimova A, Graff B, Gasecki D, et al. Cognitive dysfunction in type 1 diabetes mellitus. J Clin Endocrinol Metab 2019; 104: 2239–2249. [DOI] [PubMed] [Google Scholar]
- 10. Cameron FJ, Northam EA, Ryan CM. The effect of type 1 diabetes on the developing brain. Lancet Child Adolesc Health 2019; 3: 427–436. [DOI] [PubMed] [Google Scholar]
- 11. Mastrangelo M, Tromba V, Silvestri F, et al. Epilepsy in children with type 1 diabetes mellitus: Pathophysiological basis and clinical hallmarks. Eur J Paediatr Neurol 2019; 23: 240–247. [DOI] [PubMed] [Google Scholar]
- 12. McCorry D, Nicolson A, Smith D, et al. An association between type 1 diabetes and idiopathic generalized epilepsy. Ann Neurol 2006; 59: 204–206. [DOI] [PubMed] [Google Scholar]
- 13. Mancardi MM, Striano P, Giannattasio A, et al. Type 1 diabetes and epilepsy: More than a casual association? Epilepsia 2010; 51: 320–321. [DOI] [PubMed] [Google Scholar]
- 14. Ramakrishnan R, Appleton R. Study of prevalence of epilepsy in children with type 1 diabetes mellitus. Seizure 2012; 21: 292–294. [DOI] [PubMed] [Google Scholar]
- 15. Aguiar TS, Dantas JR, Cabral DB, et al. Association between high titers of glutamic acid decarboxylase antibody and epilepsy in patients with type 1 diabetes mellitus: A cross‐sectional study. Seizure 2019; 71: 318–321. [DOI] [PubMed] [Google Scholar]
- 16. O’Connell MA, Harvey AS, Mackay MT, et al. Does epilepsy occur more frequently in children with type 1 diabetes? J Paediatr Child Health 2008; 44: 586–589. [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. The Cochrane Collaboration . 2021. www.training.cochrane.org/handbook.
- 20. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses . 2010. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 22. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between‐study heterogeneity in meta‐analysis: Proposed metrics and empirical evaluation. Int J Epidemiol 2008; 37: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong MS, Kohane IS, Cai T, et al. Population‐level evidence for an autoimmune etiology of epilepsy. JAMA Neurol 2014; 71: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fazeli Farsani S, Souverein PC, van der Vorst MM, et al. Chronic comorbidities in children with type 1 diabetes: A population‐based cohort study. Arch Dis Child 2015; 100: 763–768. [DOI] [PubMed] [Google Scholar]
- 26. Chou IC, Wang CH, Lin WD, et al. Risk of epilepsy in type 1 diabetes mellitus: A population‐based cohort study. Diabetologia 2016; 59: 1196–1203. [DOI] [PubMed] [Google Scholar]
- 27. Dafoulas GE, Toulis KA, McCorry D, et al. Type 1 diabetes mellitus and risk of incident epilepsy: A population‐based, open‐cohort study. Diabetologia 2017; 60: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sillanpaa M, Saarinen MM, Ronnemaa T, et al. Overrepresentation of epilepsy in children with type 1 diabetes is declining in a longitudinal population study in Finland. Acta Paediatr 2019; 108: 2235–2240. [DOI] [PubMed] [Google Scholar]
- 29. Wie Borsheim A, Engeland A, Gilhus NE. Epilepsy and autoimmune diseases: Comorbidity in a national patient cohort. Seizure 2020; 75: 89–95. [DOI] [PubMed] [Google Scholar]
- 30. Verrotti A, Scaparrotta A, Olivieri C, et al. Seizures and type 1 diabetes mellitus: Current state of knowledge. Eur J Endocrinol 2012; 167: 749–758. [DOI] [PubMed] [Google Scholar]
- 31. Hyllienmark L, Maltez J, Dandenell A, et al. EEG abnormalities with and without relation to severe hypoglycaemia in adolescents with type 1 diabetes. Diabetologia 2005; 48: 412–419. [DOI] [PubMed] [Google Scholar]
- 32. Asvold BO, Sand T, Hestad KA, et al. Quantitative EEG in type 1 diabetic adults with childhood exposure to severe hypoglycaemia: A 16 year follow‐up study. Diabetologia 2011; 54: 2404–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sejling AS, Kjaer TW, Pedersen‐Bjergaard U, et al. Hypoglycemia‐associated EEG changes following antecedent hypoglycemia in type 1 diabetes mellitus. Diabetes Technol Ther 2017; 19: 85–90. [DOI] [PubMed] [Google Scholar]
- 34. Gallardo‐Moreno GB, Gonzalez‐Garrido AA, Villasenor‐Cabrera T, et al. Sustained attention in schoolchildren with type‐1 diabetes. A quantitative EEG study. Clin Neurophysiol 2020; 131: 2469–2478. [DOI] [PubMed] [Google Scholar]
- 35. Vidmar KK, Pollock AJ. Intractable seizures in children with type 1 diabetes: Implications of the ketogenic diet. WMJ 2022; 121: 292–296. [PubMed] [Google Scholar]
- 36. Nakajima H, Nakamura Y, Inaba Y, et al. Neurologic disorders associated with anti‐glutamic acid decarboxylase antibodies: A comparison of anti‐GAD antibody titers and time‐dependent changes between neurologic disease and type I diabetes mellitus. J Neuroimmunol 2018; 317: 84–89. [DOI] [PubMed] [Google Scholar]
- 37. Marcovecchio ML, Petrosino MI, Chiarelli F. Diabetes and epilepsy in children and adolescents. Curr Diab Rep 2015; 15: 21. [DOI] [PubMed] [Google Scholar]
- 38. Baizabal‐Carvallo JF, Alonso‐Juarez M. Cerebellar disease associated with anti‐glutamic acid decarboxylase antibodies: Review. J Neural Transm (Vienna) 2017; 124: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 39. Valinciute J, Juceviciute N, Balnyte R, et al. GAD65 antibody‐associated epilepsy. Medicina (Kaunas) 2023; 59: 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daif A, Lukas RV, Issa NP, et al. Antiglutamic acid decarboxylase 65 (GAD65) antibody‐associated epilepsy. Epilepsy Behav 2018; 80: 331–336. [DOI] [PubMed] [Google Scholar]


